User login
FDA OKs durvalumab combo for extensive-stage SCLC
The US Food and Drug Administration has approved the immunotherapy durvalumab (Imfinzi, AstraZeneca) in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC).
Durvalumab plus chemotherapy “can be considered a new standard in ES-SCLC,” said Myung-Ju Ahn, MD, Sungkyunkwan University, Seoul, South Korea, last year at the European Society of Medical Oncology (ESMO) annual meeting, where he discussed results from the phase 3 trial known as CASPIAN.
The new approval is based on efficacy and safety data from that trial, conducted in patients with previously untreated ES-SCLC. In the experimental group (n = 268), durvalumab plus etoposide and a platinum agent (EP) was followed by maintenance durvalumab, and in the control group (n = 269) patients received the EP regimen alone.
Median overall survival (OS) was 13 months in the durvalumab plus chemotherapy group compared with 10.3 months in the chemotherapy alone group (hazard ratio 0.73; 95% confidence interval, 0.59-0.91; P = .0047).
Reporting these results, trial investigator Luis Paz-Ares, MD, Hospital Universitario 12 de Octubre, Madrid, put the new survival benefit in the context of standard treatments at the ESMO meeting last year.
“Initial response rates to etoposide plus a platinum are high, but responses are not durable and patients treated with EP typically relapse within 6 months of starting treatment with a median OS of approximately 10 months,” he said.
In addition to the primary endpoint of OS, additional efficacy outcome measures were investigator-assessed progression-free survival (PFS) and objective response rate (ORR).
Median PFS was not statistically significant with immunotherapy; it was 5.1 months (95% CI, 4.7-6.2) in the durvalumab plus chemotherapy group and 5.4 months (95% CI, 4.8-6.2) in the chemotherapy alone group (HR, 0.78; 95% CI, 0.65-0.94).
The investigator-assessed ORR was 68% in the durvalumab plus chemotherapy group and 58% in the chemotherapy alone group.
The most common adverse reactions (≥ 20%) in patients with ES-SCLC were nausea, fatigue/asthenia, and alopecia, according to the FDA.
At ESMO, Paz-Ares reported that rates of serious adverse events (AEs) were comparable at 30.9% and 36.1% for the durvalumab plus EP group vs. EP alone, respectively; rates of AEs leading to discontinuation were identical in both groups at 9.4%. Unsurprisingly, immune-mediated AEs were higher at 19.6% in the durvalumab combination group vs. 2.6% in the EP alone group.
In this setting, durvalumab is administered prior to chemotherapy on the same day. The recommended durvalumab dose (when administered with etoposide and carboplatin or cisplatin) is 1,500 mg every 3 weeks prior to chemotherapy and then every 4 weeks as a single-agent maintenance therapy.
Durvalumab is already approved for metastatic non–small cell lung cancer in patients whose tumors have only spread in the chest, and is also approved for use in urothelial cancer.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved the immunotherapy durvalumab (Imfinzi, AstraZeneca) in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC).
Durvalumab plus chemotherapy “can be considered a new standard in ES-SCLC,” said Myung-Ju Ahn, MD, Sungkyunkwan University, Seoul, South Korea, last year at the European Society of Medical Oncology (ESMO) annual meeting, where he discussed results from the phase 3 trial known as CASPIAN.
The new approval is based on efficacy and safety data from that trial, conducted in patients with previously untreated ES-SCLC. In the experimental group (n = 268), durvalumab plus etoposide and a platinum agent (EP) was followed by maintenance durvalumab, and in the control group (n = 269) patients received the EP regimen alone.
Median overall survival (OS) was 13 months in the durvalumab plus chemotherapy group compared with 10.3 months in the chemotherapy alone group (hazard ratio 0.73; 95% confidence interval, 0.59-0.91; P = .0047).
Reporting these results, trial investigator Luis Paz-Ares, MD, Hospital Universitario 12 de Octubre, Madrid, put the new survival benefit in the context of standard treatments at the ESMO meeting last year.
“Initial response rates to etoposide plus a platinum are high, but responses are not durable and patients treated with EP typically relapse within 6 months of starting treatment with a median OS of approximately 10 months,” he said.
In addition to the primary endpoint of OS, additional efficacy outcome measures were investigator-assessed progression-free survival (PFS) and objective response rate (ORR).
Median PFS was not statistically significant with immunotherapy; it was 5.1 months (95% CI, 4.7-6.2) in the durvalumab plus chemotherapy group and 5.4 months (95% CI, 4.8-6.2) in the chemotherapy alone group (HR, 0.78; 95% CI, 0.65-0.94).
The investigator-assessed ORR was 68% in the durvalumab plus chemotherapy group and 58% in the chemotherapy alone group.
The most common adverse reactions (≥ 20%) in patients with ES-SCLC were nausea, fatigue/asthenia, and alopecia, according to the FDA.
At ESMO, Paz-Ares reported that rates of serious adverse events (AEs) were comparable at 30.9% and 36.1% for the durvalumab plus EP group vs. EP alone, respectively; rates of AEs leading to discontinuation were identical in both groups at 9.4%. Unsurprisingly, immune-mediated AEs were higher at 19.6% in the durvalumab combination group vs. 2.6% in the EP alone group.
In this setting, durvalumab is administered prior to chemotherapy on the same day. The recommended durvalumab dose (when administered with etoposide and carboplatin or cisplatin) is 1,500 mg every 3 weeks prior to chemotherapy and then every 4 weeks as a single-agent maintenance therapy.
Durvalumab is already approved for metastatic non–small cell lung cancer in patients whose tumors have only spread in the chest, and is also approved for use in urothelial cancer.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved the immunotherapy durvalumab (Imfinzi, AstraZeneca) in combination with etoposide and either carboplatin or cisplatin as first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC).
Durvalumab plus chemotherapy “can be considered a new standard in ES-SCLC,” said Myung-Ju Ahn, MD, Sungkyunkwan University, Seoul, South Korea, last year at the European Society of Medical Oncology (ESMO) annual meeting, where he discussed results from the phase 3 trial known as CASPIAN.
The new approval is based on efficacy and safety data from that trial, conducted in patients with previously untreated ES-SCLC. In the experimental group (n = 268), durvalumab plus etoposide and a platinum agent (EP) was followed by maintenance durvalumab, and in the control group (n = 269) patients received the EP regimen alone.
Median overall survival (OS) was 13 months in the durvalumab plus chemotherapy group compared with 10.3 months in the chemotherapy alone group (hazard ratio 0.73; 95% confidence interval, 0.59-0.91; P = .0047).
Reporting these results, trial investigator Luis Paz-Ares, MD, Hospital Universitario 12 de Octubre, Madrid, put the new survival benefit in the context of standard treatments at the ESMO meeting last year.
“Initial response rates to etoposide plus a platinum are high, but responses are not durable and patients treated with EP typically relapse within 6 months of starting treatment with a median OS of approximately 10 months,” he said.
In addition to the primary endpoint of OS, additional efficacy outcome measures were investigator-assessed progression-free survival (PFS) and objective response rate (ORR).
Median PFS was not statistically significant with immunotherapy; it was 5.1 months (95% CI, 4.7-6.2) in the durvalumab plus chemotherapy group and 5.4 months (95% CI, 4.8-6.2) in the chemotherapy alone group (HR, 0.78; 95% CI, 0.65-0.94).
The investigator-assessed ORR was 68% in the durvalumab plus chemotherapy group and 58% in the chemotherapy alone group.
The most common adverse reactions (≥ 20%) in patients with ES-SCLC were nausea, fatigue/asthenia, and alopecia, according to the FDA.
At ESMO, Paz-Ares reported that rates of serious adverse events (AEs) were comparable at 30.9% and 36.1% for the durvalumab plus EP group vs. EP alone, respectively; rates of AEs leading to discontinuation were identical in both groups at 9.4%. Unsurprisingly, immune-mediated AEs were higher at 19.6% in the durvalumab combination group vs. 2.6% in the EP alone group.
In this setting, durvalumab is administered prior to chemotherapy on the same day. The recommended durvalumab dose (when administered with etoposide and carboplatin or cisplatin) is 1,500 mg every 3 weeks prior to chemotherapy and then every 4 weeks as a single-agent maintenance therapy.
Durvalumab is already approved for metastatic non–small cell lung cancer in patients whose tumors have only spread in the chest, and is also approved for use in urothelial cancer.
This article first appeared on Medscape.com.
Dawn of new lupus nephritis treatment after AURORA trial
The calcineurin inhibitor is the first novel agent to demonstrate effectiveness in the treatment of people with lupus nephritis, a disease that can lead to irreversible kidney damage, kidney failure, and even death.
“We believe that voclosporin – as an add-on therapy – will help more patients achieve remission,” said Keisha Gibson, MD, MPH, chief of pediatric nephrology at the UNC Kidney Center in Chapel Hill, North Carolina, who led the study and presented the findings during a virtual session at the National Kidney Foundation 2020 Spring Clinical Meetings.
The burden that lupus places on patients and the health system is high, she reported. Patients with lupus and uncontrolled kidney disease frequently have complications that require hospitalization, can suffer complete or partial loss of income, and can become temporarily disabled. And up to 50% of lupus patients will develop lupus nephritis.
“When we are lucky and see early treatment responses in our patients, we know that their long-term outcomes, measured by proteinuria reduction, are quite good,” said Gibson. “Unfortunately, in 10% to 30% of patients, despite standard-of-care therapies, we see progression to end-stage kidney disease within 15 years of diagnosis. This confers a huge cost to overall health and quality of life, and is an excess burden to the healthcare system.
“It is clear that one of the biggest risk factors for these patients who have kidney disease that is progressing to kidney failure is an inability to control the inflammation and chronic damage from uncontrolled disease,” she told Medscape Medical News.
“While there have certainly been a few advances in the care of patients with lupus disease, I believe that this therapy may help move the needle, specifically for patients with kidney involvement from their lupus disease,” she added.
Renal Response Much Higher With Voclosporin
The typical standard of care for patients with active lupus and kidney disease is either a combination of cyclophosphamide plus steroids or a combination of mycophenolate mofetil plus steroids.
The global, double-blind, randomized, placebo-controlled trial compared the effectiveness and safety of twice-daily oral voclosporin 23.7 mg with placebo. All 357 study participants also received mycophenolate 2 g daily and rapidly tapered low-dose oral corticosteroids.
At 1 year, the renal response rate was higher in the voclosporin group than in the placebo group (40.8% vs 22.5%; odds ratio, 2.65; P < .001).
“AURORA patients achieved low levels of protein twice as quickly as patients on standard of care,” the researchers write in their abstract. Median time to the achievement of a urine protein to creatinine ratio below 0.5 mg/mg was significantly and clinically better with voclosporin than with placebo (169 vs 372 days; log rank P < .001).
And voclosporin was well tolerated. The percentage of serious adverse events was similar in the voclosporin and placebo groups (20.8% vs 21.3%), with infection being the most common serious event (10.1% vs 11.2%).
In the voclosporin group, there were no significant differences between baseline and 1 year estimated glomerular filtration rates (eGFR), blood pressure, lipid levels, or sugar levels.
And there were fewer deaths in the voclosporin group than in the placebo group (1 vs 5).
Benefits were seen in all subgroups, including age, sex, race, biopsy class, geographic region of origin, and mycophenolate exposure at screening, Gibson reported.
Promise of a New Treatment
The promise of a new treatment is exciting for the field, said Joseph Vassalotti, MD, chief medical officer of the National Kidney Foundation.
However, it’s important to note that “this study is applicable only to patients with lupus nephritis with preserved kidney function,” he added.
An “eGFR less than 45 mL/min per 1.73 m2 was implied as an exclusion criteria, so I would say it certainly doesn’t apply to patients with impaired kidney function,” he said, pointing out that this will be clearer when the final data are published.
The study cohort was heterogeneous; 86% of participants had class 3 or 4 biopsy findings and 14% had class 5, so the superiority of voclosporin is convincing, he said.
“We look forward to full publication and more details,” he told Medscape Medical News. “They do have an extension study, and it will be interesting to see how that plays out.”
Voclosporin was granted Fast Track designation by the US Food and Drug Administration in 2016, and these positive phase 3 results mean that a New Drug Application will likely be filed in the next few months. If approved, the drug could be on the market by 2021, according to a press release from Aurinia.
Nephrologists are familiar with calcineurin inhibitors, so it will be interesting to see, given the success of this trial, if voclosporin has a potential role in other kidney diseases, “such as minimal change disease,” said Vassalotti.
He added that there are concerns, unrelated to the AURORA trial, about the supply of hydroxychloroquine, which is also used for the treatment of lupus nephritis. “An overarching concern is that the supply will be limited now that the drug is being studied and used as prophylaxis after exposure to SARS-CoV-2 and as a treatment for COVID-19, creating challenges for patients with lupus nephritis trying to obtain their needed medication,” he said.
Gibson reports no relevant financial relationships. Two coauthors work for the drugmaker, Aurinia. Vassalotti reports no relevant financial relationships.
This article first appeared on Medscape.com.
The calcineurin inhibitor is the first novel agent to demonstrate effectiveness in the treatment of people with lupus nephritis, a disease that can lead to irreversible kidney damage, kidney failure, and even death.
“We believe that voclosporin – as an add-on therapy – will help more patients achieve remission,” said Keisha Gibson, MD, MPH, chief of pediatric nephrology at the UNC Kidney Center in Chapel Hill, North Carolina, who led the study and presented the findings during a virtual session at the National Kidney Foundation 2020 Spring Clinical Meetings.
The burden that lupus places on patients and the health system is high, she reported. Patients with lupus and uncontrolled kidney disease frequently have complications that require hospitalization, can suffer complete or partial loss of income, and can become temporarily disabled. And up to 50% of lupus patients will develop lupus nephritis.
“When we are lucky and see early treatment responses in our patients, we know that their long-term outcomes, measured by proteinuria reduction, are quite good,” said Gibson. “Unfortunately, in 10% to 30% of patients, despite standard-of-care therapies, we see progression to end-stage kidney disease within 15 years of diagnosis. This confers a huge cost to overall health and quality of life, and is an excess burden to the healthcare system.
“It is clear that one of the biggest risk factors for these patients who have kidney disease that is progressing to kidney failure is an inability to control the inflammation and chronic damage from uncontrolled disease,” she told Medscape Medical News.
“While there have certainly been a few advances in the care of patients with lupus disease, I believe that this therapy may help move the needle, specifically for patients with kidney involvement from their lupus disease,” she added.
Renal Response Much Higher With Voclosporin
The typical standard of care for patients with active lupus and kidney disease is either a combination of cyclophosphamide plus steroids or a combination of mycophenolate mofetil plus steroids.
The global, double-blind, randomized, placebo-controlled trial compared the effectiveness and safety of twice-daily oral voclosporin 23.7 mg with placebo. All 357 study participants also received mycophenolate 2 g daily and rapidly tapered low-dose oral corticosteroids.
At 1 year, the renal response rate was higher in the voclosporin group than in the placebo group (40.8% vs 22.5%; odds ratio, 2.65; P < .001).
“AURORA patients achieved low levels of protein twice as quickly as patients on standard of care,” the researchers write in their abstract. Median time to the achievement of a urine protein to creatinine ratio below 0.5 mg/mg was significantly and clinically better with voclosporin than with placebo (169 vs 372 days; log rank P < .001).
And voclosporin was well tolerated. The percentage of serious adverse events was similar in the voclosporin and placebo groups (20.8% vs 21.3%), with infection being the most common serious event (10.1% vs 11.2%).
In the voclosporin group, there were no significant differences between baseline and 1 year estimated glomerular filtration rates (eGFR), blood pressure, lipid levels, or sugar levels.
And there were fewer deaths in the voclosporin group than in the placebo group (1 vs 5).
Benefits were seen in all subgroups, including age, sex, race, biopsy class, geographic region of origin, and mycophenolate exposure at screening, Gibson reported.
Promise of a New Treatment
The promise of a new treatment is exciting for the field, said Joseph Vassalotti, MD, chief medical officer of the National Kidney Foundation.
However, it’s important to note that “this study is applicable only to patients with lupus nephritis with preserved kidney function,” he added.
An “eGFR less than 45 mL/min per 1.73 m2 was implied as an exclusion criteria, so I would say it certainly doesn’t apply to patients with impaired kidney function,” he said, pointing out that this will be clearer when the final data are published.
The study cohort was heterogeneous; 86% of participants had class 3 or 4 biopsy findings and 14% had class 5, so the superiority of voclosporin is convincing, he said.
“We look forward to full publication and more details,” he told Medscape Medical News. “They do have an extension study, and it will be interesting to see how that plays out.”
Voclosporin was granted Fast Track designation by the US Food and Drug Administration in 2016, and these positive phase 3 results mean that a New Drug Application will likely be filed in the next few months. If approved, the drug could be on the market by 2021, according to a press release from Aurinia.
Nephrologists are familiar with calcineurin inhibitors, so it will be interesting to see, given the success of this trial, if voclosporin has a potential role in other kidney diseases, “such as minimal change disease,” said Vassalotti.
He added that there are concerns, unrelated to the AURORA trial, about the supply of hydroxychloroquine, which is also used for the treatment of lupus nephritis. “An overarching concern is that the supply will be limited now that the drug is being studied and used as prophylaxis after exposure to SARS-CoV-2 and as a treatment for COVID-19, creating challenges for patients with lupus nephritis trying to obtain their needed medication,” he said.
Gibson reports no relevant financial relationships. Two coauthors work for the drugmaker, Aurinia. Vassalotti reports no relevant financial relationships.
This article first appeared on Medscape.com.
The calcineurin inhibitor is the first novel agent to demonstrate effectiveness in the treatment of people with lupus nephritis, a disease that can lead to irreversible kidney damage, kidney failure, and even death.
“We believe that voclosporin – as an add-on therapy – will help more patients achieve remission,” said Keisha Gibson, MD, MPH, chief of pediatric nephrology at the UNC Kidney Center in Chapel Hill, North Carolina, who led the study and presented the findings during a virtual session at the National Kidney Foundation 2020 Spring Clinical Meetings.
The burden that lupus places on patients and the health system is high, she reported. Patients with lupus and uncontrolled kidney disease frequently have complications that require hospitalization, can suffer complete or partial loss of income, and can become temporarily disabled. And up to 50% of lupus patients will develop lupus nephritis.
“When we are lucky and see early treatment responses in our patients, we know that their long-term outcomes, measured by proteinuria reduction, are quite good,” said Gibson. “Unfortunately, in 10% to 30% of patients, despite standard-of-care therapies, we see progression to end-stage kidney disease within 15 years of diagnosis. This confers a huge cost to overall health and quality of life, and is an excess burden to the healthcare system.
“It is clear that one of the biggest risk factors for these patients who have kidney disease that is progressing to kidney failure is an inability to control the inflammation and chronic damage from uncontrolled disease,” she told Medscape Medical News.
“While there have certainly been a few advances in the care of patients with lupus disease, I believe that this therapy may help move the needle, specifically for patients with kidney involvement from their lupus disease,” she added.
Renal Response Much Higher With Voclosporin
The typical standard of care for patients with active lupus and kidney disease is either a combination of cyclophosphamide plus steroids or a combination of mycophenolate mofetil plus steroids.
The global, double-blind, randomized, placebo-controlled trial compared the effectiveness and safety of twice-daily oral voclosporin 23.7 mg with placebo. All 357 study participants also received mycophenolate 2 g daily and rapidly tapered low-dose oral corticosteroids.
At 1 year, the renal response rate was higher in the voclosporin group than in the placebo group (40.8% vs 22.5%; odds ratio, 2.65; P < .001).
“AURORA patients achieved low levels of protein twice as quickly as patients on standard of care,” the researchers write in their abstract. Median time to the achievement of a urine protein to creatinine ratio below 0.5 mg/mg was significantly and clinically better with voclosporin than with placebo (169 vs 372 days; log rank P < .001).
And voclosporin was well tolerated. The percentage of serious adverse events was similar in the voclosporin and placebo groups (20.8% vs 21.3%), with infection being the most common serious event (10.1% vs 11.2%).
In the voclosporin group, there were no significant differences between baseline and 1 year estimated glomerular filtration rates (eGFR), blood pressure, lipid levels, or sugar levels.
And there were fewer deaths in the voclosporin group than in the placebo group (1 vs 5).
Benefits were seen in all subgroups, including age, sex, race, biopsy class, geographic region of origin, and mycophenolate exposure at screening, Gibson reported.
Promise of a New Treatment
The promise of a new treatment is exciting for the field, said Joseph Vassalotti, MD, chief medical officer of the National Kidney Foundation.
However, it’s important to note that “this study is applicable only to patients with lupus nephritis with preserved kidney function,” he added.
An “eGFR less than 45 mL/min per 1.73 m2 was implied as an exclusion criteria, so I would say it certainly doesn’t apply to patients with impaired kidney function,” he said, pointing out that this will be clearer when the final data are published.
The study cohort was heterogeneous; 86% of participants had class 3 or 4 biopsy findings and 14% had class 5, so the superiority of voclosporin is convincing, he said.
“We look forward to full publication and more details,” he told Medscape Medical News. “They do have an extension study, and it will be interesting to see how that plays out.”
Voclosporin was granted Fast Track designation by the US Food and Drug Administration in 2016, and these positive phase 3 results mean that a New Drug Application will likely be filed in the next few months. If approved, the drug could be on the market by 2021, according to a press release from Aurinia.
Nephrologists are familiar with calcineurin inhibitors, so it will be interesting to see, given the success of this trial, if voclosporin has a potential role in other kidney diseases, “such as minimal change disease,” said Vassalotti.
He added that there are concerns, unrelated to the AURORA trial, about the supply of hydroxychloroquine, which is also used for the treatment of lupus nephritis. “An overarching concern is that the supply will be limited now that the drug is being studied and used as prophylaxis after exposure to SARS-CoV-2 and as a treatment for COVID-19, creating challenges for patients with lupus nephritis trying to obtain their needed medication,” he said.
Gibson reports no relevant financial relationships. Two coauthors work for the drugmaker, Aurinia. Vassalotti reports no relevant financial relationships.
This article first appeared on Medscape.com.
In the Phoenix area, we are in a lull before the coronavirus storm
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
“There is no sound save the throb of the blowers and the vibration of the hard-driven engines. There is little motion as the gun crews man their guns and the fire-control details stand with heads bent and their hands clapped over their headphones. Somewhere out there are the enemy planes.”
That’s from one of my favorite WW2 histories, “Torpedo Junction,” by Robert J. Casey. He was a reporter stationed on board the cruiser USS Salt Lake City. The entry is from a day in February 1942 when the ship was part of a force that bombarded the Japanese encampment on Wake Island. The excerpt describes the scene later that afternoon, as they awaited a counterattack from Japanese planes.
For some reason that paragraph kept going through my mind this past Sunday afternoon, in the comparatively mundane situation of sitting in the hospital library signing off on my dictations and reviewing test results. I certainly was in no danger of being bombed or strafed, yet ...
Around me, the hospital was preparing for battle. As I rounded, most of the beds were empty and many of the floors above me were shut down and darkened. Waiting rooms were empty. If you hadn’t read the news you’d think there was a sudden lull in the health care world.
But the real truth is that it’s the calm before an anticipated storm. The elective procedures have all been canceled. Nonurgent outpatient tests are on hold. Only the sickest are being admitted, and they’re being sent out as soon as possible. Every bed possible is being kept open for the feared onslaught of coronavirus patients in the coming weeks. Protective equipment, already in short supply, is being stockpiled as it becomes available. Plans have been made to erect triage tents in the parking lots.
I sit in the library and think of this. It’s quiet except for the soft hum of the air conditioning blowers as Phoenix starts to warm up for another summer. The muted purr of the computer’s hard drive as I click away on the keys. On the floors above me the nurses and respiratory techs and doctors go about their daily business of patient care, wondering when the real battle will begin (probably 2-3 weeks from the time of this writing, if not sooner).
These are scary times. I’d be lying if I said I wasn’t frightened about what might happen to me, my family, my friends, my coworkers, my patients.
The people working in the hospital above me are in the same boat, all nervous about what’s going to happen. None of them is any more immune to coronavirus than the people they’ll be treating.
But, like the crew of the USS Salt Lake City, they’re ready to do their jobs. Because it’s part of what drove each of us into our own part of this field. Because we care and want to help. And health care doesn’t work unless the whole team does.
I respect them all for it. I always have and always will, and now more than ever.
Good luck.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
FDA approves ixekizumab for pediatric plaque psoriasis
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
cpalmer@mdedge.com
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
cpalmer@mdedge.com
The according to an announcement from Lilly.
Patients need to be candidates for systemic therapy or phototherapy and have no known hypersensitivity to the biologic.
The safety, tolerability, and efficacy of the interleukin-17a antagonist were demonstrated in a phase 3 study that included 171 patients aged 6-17 years with moderate to severe plaque psoriasis. At 12 weeks, 89% those on ixekizumab achieved a 75% improvement on Psoriasis Area and Severity Index score, compared with 25% of those on placebo, and 81% achieved a static Physician’s Global Assessment of clear or almost clear, compared with 11% of those on placebo, according to the Lilly statement.
The safety profile seen with ixekizumab (Taltz) among the pediatric patients with plaque psoriasis is consistent with what has been observed among adult patients, although there were higher rates of conjunctivitis, influenza, and urticaria among the pediatric patients, the statement noted. The biologic may increase the risk of infection, and patients should be evaluated for tuberculosis, hypersensitivity, and inflammatory bowel disease. It is also recommended that routine immunizations be completed before initiating treatment.
Ixekizumab was initially approved for treating adults with moderate to severe plaque psoriasis in 2016, followed by approvals for treatment of adults with active psoriatic arthritis in 2017, and for adults with ankylosing spondylitis in August 2019.
The biologic therapies – etanercept, a tumor necrosis factor blocker, and ustekinumab (Stelara), an IL-12/23 antagonist – were previously approved by the FDA for pediatric psoriasis, in children ages 4 years and older and 12 years and older, respectively.
Updated prescribing information for ixekizumab can be found on the Lilly website.
cpalmer@mdedge.com
Physician couples draft wills, face tough questions amid COVID-19
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”
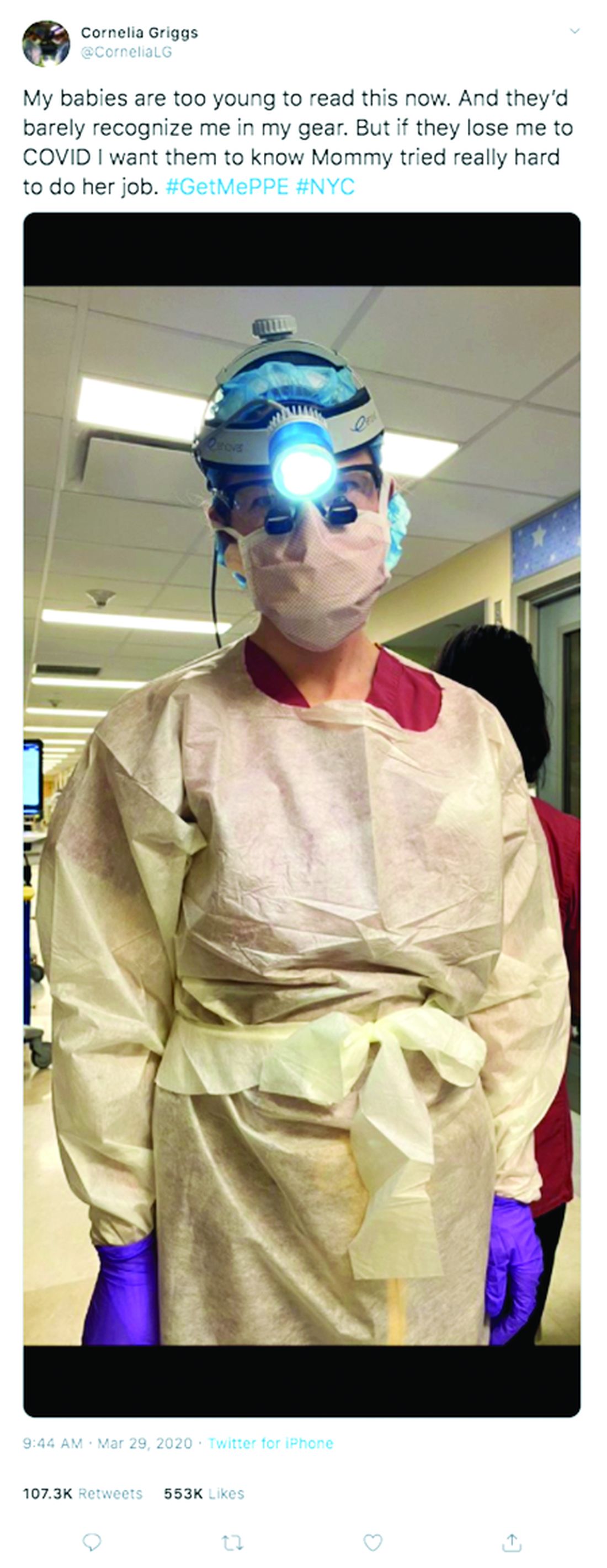
“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Dapagliflozin trial in CKD halted because of high efficacy
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
Flu activity measures continue COVID-19–related divergence
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
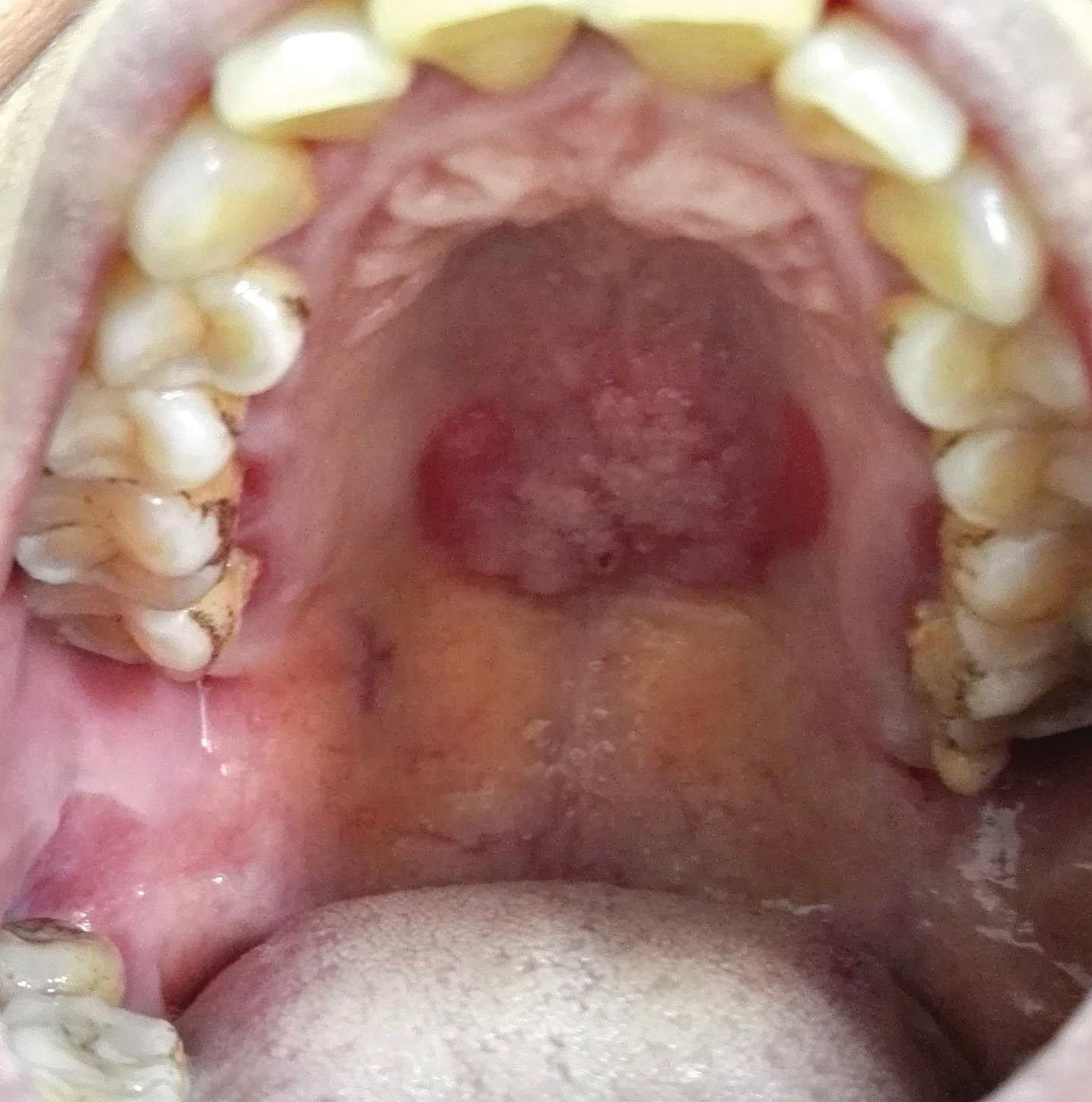
Solitary Warty Mucosal Lesion on the Hard Palate
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
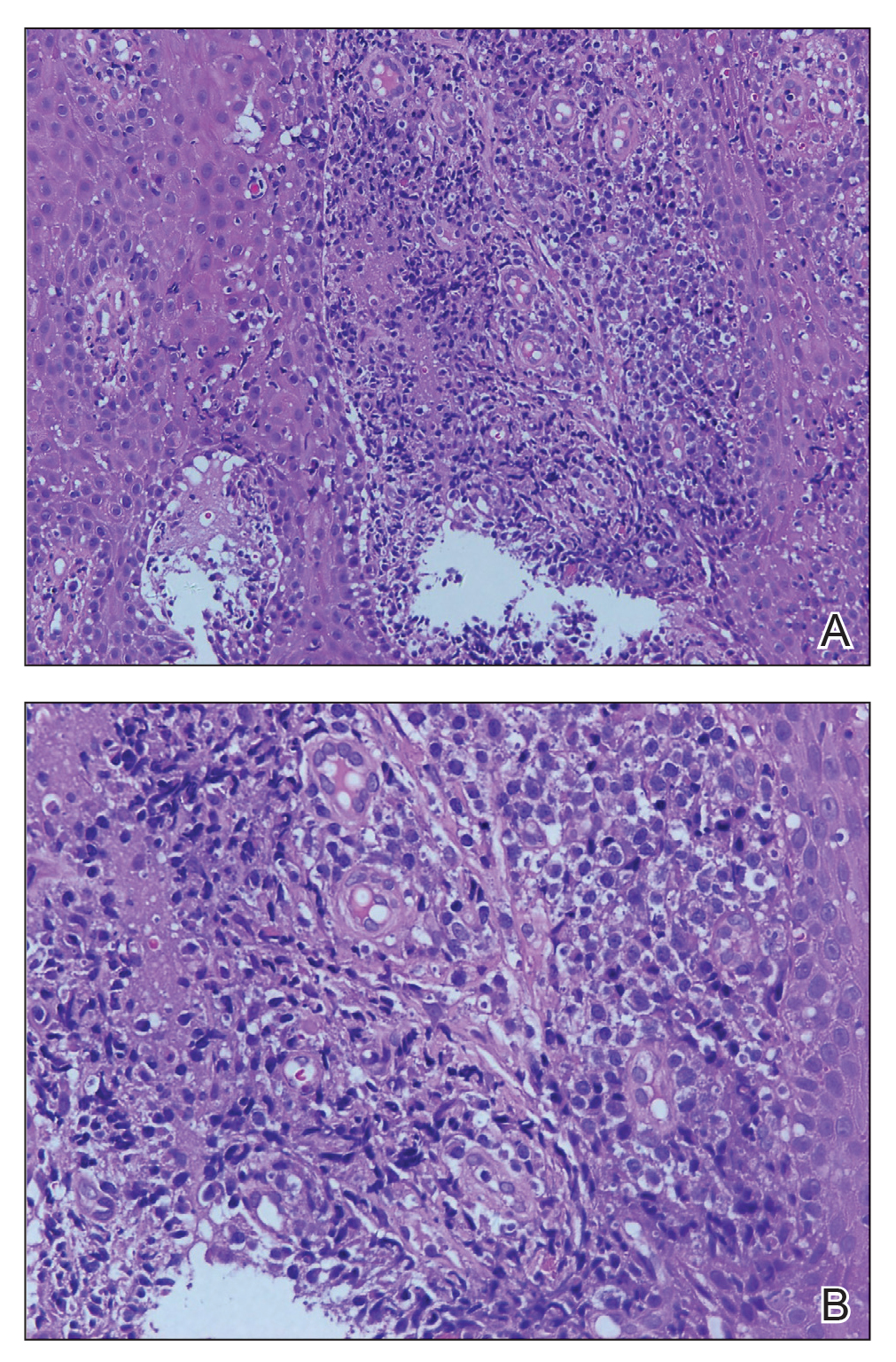
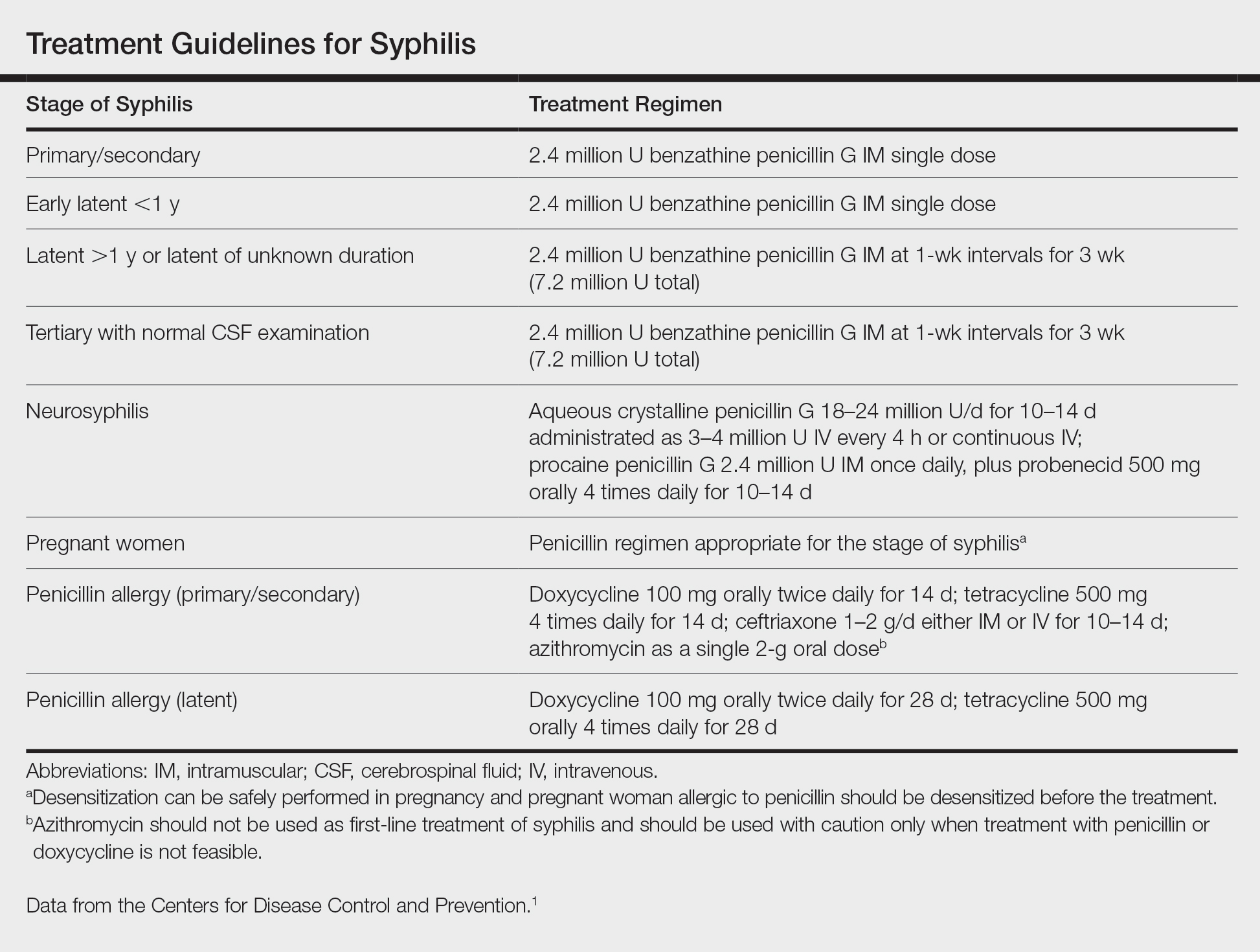
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
The Diagnosis: Solitary Oral Condyloma Lata of Secondary Syphilis
A punch biopsy of the lesion revealed acanthosis with elongation of rete ridges; interface dermatitis; and a moderately dense, predominantly lymphoid dermal infiltration (Figure). Based on a serologic toluidine red unheated serum test (TRUST) titer of 1:64 and positive Treponema antibodies, a diagnosis of secondary syphilitic infection was made. A test for human immunodeficiency virus infection was negative, and the patient was not immunocompromised. Due to allergy to benzathine penicillin G, she was prescribed oral minocycline 100 mg twice daily for 15 days. (See the Table for current recommended regimens from the Centers for Disease Control and Prevention for the treatment of syphilis.1) The hard palate plaque began to fade after 2 days of treatment and completely regressed 2 weeks later. The TRUST titer decreased to 1:4 after 6 months.
The patient's husband was examined following confirmation of his wife's infection; his TRUST titer was 1:64 and Treponema antibodies were positive. No skin lesions were detected. A test for human immunodeficiency virus infection also was negative. Further inquiry revealed that he had had sexual intercourse with a prostitute about 3 months prior. He was diagnosed with latent syphilis and prescribed the same medication regimen as his wife. However, after 6 months, his TRUST titer was still 1:64, possibly due to irregular medication use.
Secondary syphilis often is preceded by flulike symptoms of fever, sore throat, headache, malaise, generalized painless lymphadenopathy, and myalgia 4 to 10 weeks after onset of infection.2-5 Condyloma lata can be one of the characteristic mucosal signs of secondary syphilis; however, it is typically located in the anogenital area or less commonly in atypical areas such as the umbilicus, axillae, inframammary folds, and toe web spaces.6 Condyloma lata in the oral cavity is rare. In fact, this unusual manifestation prompted the patient to suspect cancer and she initially presented to a local tumor hospital. However, oral computed tomography did not detect any tumor cells, and subsequent testing yielded the diagnosis of secondary syphilis.
The differential diagnosis for a warty oral mass includes squamous cell carcinoma, condyloma acuminatum, oral submucous fibrosis, and Wegener granulomatosis.
Similar to other nontreponemal tests, TRUST is a flocculation-based quantitative test that can be used to follow treatment response, as its antibody titers may correlate with disease activity.7 Clinically, a 4-fold change in titer (equivalent to a change of 2 dilutions) is considered necessary to demonstrate a notable difference between 2 nontreponemal test results obtained using the same serologic test. The TRUST titers for the case patient decreased from 1:64 to 1:4, indicating a good response to minocycline. In contrast, the TRUST of her husband remained as high at 6-month follow-up as it had been at initial examination. This serofast state was most likely related to his irregular medication use; however, other possibilities should be considered, including confounding nontreponemal inflammatory conditions in the host, the variability of host response to infection, or even persistent low-level infection with Treponema pallidum.8 Because treponemal antibodies typically remain positive for life and most patients who have a reactive treponemal test will have a reactive report for the remainder of their lives, regardless of treatment or disease activity, treponemal antibody titers should not be used to monitor treatment response.9
China has experienced a resurgence in the incidence and prevalence of syphilis in recent decades. According to the national reporting database, the annual rate of syphilis in China has increased 14.3% since 2009 (6.5 cases per 100,000 population in 1999 vs 24.66 cases per 100,000 population in 2009).10 This re-emergence is truly remarkable, given this infection was virtually eradicated in the country 60 years ago. Recognizing this syphilis epidemic as a public health threat, the Ministry of Health of the People's Republic of China in 2010 announced a 10-year plan for syphilis control and prevention to curb the spread of syphilis and other sexually transmitted diseases. Currently, the syphilis burden is still great, with 25.54 cases per 100,000 population in 2016,11 but the situation has been stabilized and the annual increase is less than 1% since the plan's introduction.
Globally, there has been a marked resurgence of syphilis in the last decade, largely attributed to changing social and behavioral factors, especially among the population of men who have sex with men. Despite the availability of effective treatments and previously reliable prevention strategies, there are an estimated 6 million new cases of syphilis in those aged 15 to 49 years, and congenital syphilis causes more than 300,000 fetal and neonatal deaths each year.12 Continued vigilance and investment is needed to combat syphilis worldwide, and recognition of syphilis, with its versatile presentations, is of vital importance today.13
The presentation of secondary syphilis can be highly variable and requires a high level of awareness.4-6 Solitary oral involvement in secondary syphilis is rare and can lead to misdiagnosis; therefore, a high level of suspicion for syphilis should be maintained when evaluating oral lesions.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Centers for Disease Control and Prevention. 2015 SexuallyTransmitted Diseases Treatment Guidelines: Syphilis. https://www.cdc.gov/std/tg2015/syphilis.htm. Accessed March 25, 2020.
- Lombardo J, Alhashim M. Secondary syphilis: an atypical presentation complicated by a false negative rapid plasma reagin test. Cutis. 2018;101:E11-E13.
- Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283-290.
- Dourmishev LA, Assen L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Martin DH, Mroczkowski TF. Dermatological manifestations of sexually transmitted diseases other than HIV. Infect Dis Clin North Am. 1994;8:533-583.
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277.
- Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22:137-147.
- Seña AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420-422.
- Rhoads DD, Genzen JR, Bashleben CP, et al. Prevalence of traditional and reverse-algorithm syphilis screening in laboratory practice: a survey of participants in the College of American Pathologists syphilis serology proficiency testing program. Arch Pathol Lab Med. 2017;141:93-97.
- Tucker JD, Cohen MS. China's syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis. 2011;24:50-55.
- Yang S, Wu J, Ding C, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2016;17:716-725.
- Noah K, Jeffrey DK. An update on the global epidemiology of syphilis. Curr Epidemiol Rep. 2018;5:24-38.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
A 50-year-old Chinese woman presented with a painless, well-demarcated, nontender, elevated, flat-topped verrucous plaque on the hard palate of 1 month's duration. The lesion measured 2 cm in diameter. The patient reported no other dermatologic or systemic concerns, and no other skin or genital lesions were observed.
Study identifies risk factors for infection after transbronchial biopsy
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
FROM CHEST
PARP inhibitors not cost effective for platinum-resistant ovarian cancer
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
For patients with platinum-resistant ovarian cancer with BRCA1/2 mutations, third- or fourth-line therapy with poly (ADP-ribose) polymerase (PARP) inhibitors is less cost effective than non–platinum-based chemotherapy or bevacizumab-containing regimens, according to a study published in Gynecologic Oncology.
Compared with PARP inhibitors, intravenous chemotherapy regimens tend to produce lower response rates and shorter median progression-free survival (PFS) in this difficult-to-treat population, according to study author Juliet E. Wolford, MD, of the University of California, Irvine, and colleagues.
PARP inhibitors also have the advantages of oral administration and being well tolerated, the researchers noted. However, they found the expense of PARP inhibitors remains substantially greater per month of PFS, even after accounting for the costs of infusion and toxicity management related to chemotherapy.
“We initially wanted to do this study because we suspected that, when including the costs of infusions and costs of managing toxicities, even though the PARP [inhibitors] were more expensive, they would ultimately be more cost effective because they were well tolerated, oral, and more effective,” Dr. Wolford said in an interview. “Surprisingly, the high costs of the PARP [inhibitors] outweighs any other factors so much so that the costs of receiving infusions or managing the adverse events becomes negligible.”
Dr. Wolford and colleagues developed a model using median PFS and toxicity data from regulatory trials to show patient response, complications (hematologic and nonhematologic), progression, and death.
The researchers compared olaparib, rucaparib, and niraparib individually to non–platinum-based chemotherapy regimens and to regimens containing bevacizumab. The team then estimated the costs of intravenous drugs, infusions, toxicity management, and supportive care, based on 2017 Medicare data.
The cost of non–platinum-based intravenous chemotherapy was $6,412 per quality-adjusted month of PFS, a little more than half the cost of bevacizumab-containing regimens, which was $12,187 per month of PFS.
The cost of PARP inhibitors was much higher: $18,970 per month of PFS for niraparib, $16,637 per month of PFS for rucaparib, and $16,327 per month of PFS for olaparib.
“An interesting, albeit not unexpected, phenomenon we observed in our analyses was that, with the relatively higher response rates and/or duration of response associated with PARP [inhibitor] treatment, higher drug costs are incurred,” Dr. Wolford and colleagues wrote.
“The longer patients remain progression free, the longer they remain on treatment and accumulate treatment-related cost,” the authors wrote, noting that complete responses are rare during recurrence treatment, so patients tend to receive salvage therapy until their disease progresses.
However, Dr. Wolford pointed out that using a model requires making assumptions and that “clinical decisions are not derived from a simulation.
“This type of simulation can facilitate the recognition of the financial burden the use of these novel treatments can place on our patients but, more importantly, can highlight the importance of identifying predictive biomarkers,” she said. “We need to be able to distinguish those patients who will benefit the most from the treatment in order to circumvent patients from experiencing financial toxicity from a therapy they will not derive benefit from.”
In their paper, Dr. Wolford and colleagues also pointed out that the new drugs’ cost-effectiveness could substantially improve with minimal reductions in cost, according to many models.
“Such reductions to improve the affordability of many novel molecules can be achieved through mechanisms which result in more widespread use and increased awareness and accessibility of the targeted agent in clinical practice,” the authors wrote.
Further, this study focused on platinum-resistant patients, who are particularly difficult to treat. Expanding the use of PARP inhibitors or identifying the most clinically meaningful uses of them could improve their cost-effectiveness, including possibly using them earlier in the disease course, the authors noted.
“We know from SOLO-1, PRIMA, and PAOLA-1 studies that using the PARP [inhibitors] as frontline maintenance therapy can have a significant benefit, so likely the trend will be to use the PARP [inhibitors] earlier in the disease course and utilizing the antiangiogenic therapy for recurrences when patients begin to develop platinum resistance,” Dr. Wolford said. “It is important to note, however, that, for the frontline trials, we only have PFS data, as the overall survival data is not yet mature.”
The high current costs of PARP inhibitors also follow a common trend with new oncologic agents, Dr. Wolford noted. “When they are first introduced, the high costs are reflective of the high developmental costs. As use of the novel therapies becomes more pervasive, with the approval of additional indications, the costs will eventually decrease over time.”
Dr. Wolford and colleagues did not report any external funding for this study. Some authors disclosed relationships with a range of pharmaceutical, device, and cancer-related businesses.
SOURCE: Wolford JE et al. Gynecol Oncol. 2020 Mar 13. doi: 10.1016/j.ygyno.2020.02.030.
FROM GYNECOLOGIC ONCOLOGY




![&quot;The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” said Dr. Bethany Agusala, who is married to Dr. Kartik Agusala.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/Agusala_B%20and%20K_Tex_web.jpg?itok=v9HARCAN)