User login
Oral propranolol shown safe in PHACE
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the safety of oral propranolol for treatment of complicated infantile hemangiomas in patients with PHACE syndrome comes from a recent multicenter study.
Oral propranolol is now well-ensconced as first-line therapy for complicated infantile hemangiomas in otherwise healthy children. However, the beta-blocker’s use in PHACE (Posterior fossa malformations, Hemangiomas, Arterial anomalies, Cardiac defects, and Eye abnormalities) syndrome has been controversial, with concerns raised by some that it might raise the risk for arterial ischemic stroke. Not so, Moise L. Levy, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I’m not suggesting you use propranolol with reckless abandon in this population, but this stroke concern is something that should be put to bed based on this study,” advised Dr. Levy, professor of dermatology and pediatrics at Dell Medical School in Austin, Tex., and physician-in-chief at Dell Children’s Medical Center.
PHACE syndrome is characterized by large, thick, plaque-like hemangiomas greater than 5 cm in size, most commonly on the face, although they can be located elsewhere.
“There was concern that if you found severely altered cerebrovascular arterial flow and you put a kid on a beta-blocker you might be causing some harm. But what I will tell you is that in this recently published paper this was not in fact an issue,” he said.
Dr. Levy was not an investigator in the multicenter retrospective study, which included 76 patients with PHACE syndrome treated for infantile hemangioma with oral propranolol at 0.3 mg/kg per dose or more at 11 academic tertiary care pediatric dermatology clinics. Treatment started at a median age of 56 days.
There were no strokes, TIAs, cardiovascular events, or other significant problems associated with treatment. Twenty-nine children experienced mild adverse events: minor gastrointestinal or respiratory symptoms, and sleep disturbances were threefold more frequent than reported with placebo in another study. The investigators noted that the safety experience in their PHACE syndrome population compared favorably with that in 726 infants without PHACE syndrome who received oral propranolol for hemangiomas, where the incidence of serious adverse events on treatment was 0.4% (JAMA Dermatol. 2019 Dec 11. doi: 10.1001/jamadermatol.2019.3839).
‘Hemangiomas – but we were taught that they go away’
Dr. Levy gave a shout-out to the American Academy of Pediatrics for publishing interdisciplinary expert consensus-based practice guidelines for the management of infantile hemangiomas, which he praised as “quite well done” (Pediatrics. 2019 Jan;143[1]. pii: e20183475. doi: 10.1542/peds.2018-3475).
Following release of the guidelines last year, he and other pediatric vascular anomalies experts saw an uptick in referrals from general pediatricians, which has since tapered off.
“It’s probably like for all of us: We read an article, it’s fresh on the mind, then you forget about the article and what you’ve read. So we need a little reinforcement from a learning perspective. This is a great article,” he said.
The guidelines debunk as myth the classic teaching that infantile hemangiomas go away. Explicit information is provided about the high-risk anatomic sites warranting consideration for early referral, including the periocular, lumbosacral, and perineal areas, the lip, and lower face.
“The major point is early identification of those lesions requiring evaluation and intervention. Hemangiomas generally speaking are at their ultimate size by 3-5 months of age. The bottom line is if you think something needs to be done, please send that patient, or act upon that patient, sooner rather than later. I can’t tell you how many cases of hemangiomas I’ve seen when the kid is 18 months of age, 3 years of age, 5 years, with a large area of redundant skin, scarring, or something of that sort, and it would have been really nice to have seen them earlier and acted upon them then,” the pediatric dermatologist said.
The guidelines recommend intervention or referral by 1 month of age, ideally. Guidance is provided about the use of oral propranolol as first-line therapy.
“Propranolol is something that has been a real game changer for us,” he noted. “Many people continue to be worried about side effects in using this, particularly in the young childhood population, but this paper shows pretty clearly that hypotension or bradycardia is not a real concern. I never hospitalize these patients for propranolol therapy except in high-risk populations: very preemie, any history of breathing problems. We check the blood pressure and heart rate at baseline, again at 7-10 days, and at every visit. We’ve never found any significant drop in blood pressure.”
Dr. Levy reported financial relationships with half a dozen pharmaceutical companies, none relevant to his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
What I Learned From SARS in 2003 That Will Help Me Cope With COVID-19 in 2020
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
On March 25, 2003, I was in Vancouver at my niece’s bat mitzvah when I saw a picture of my hospital in Toronto on the television news; a story about SARS patients in Toronto. Until then, SARS had been a distant event happening in mainland China and Hong Kong; it had been something that seemed very far away and theoretical. When I returned to Toronto, we had clusters of cases in several hospitals and healthcare workers were falling ill. I was the Physician in Chief at one of those hospitals and was responsible for the clinical care delivered by physicians in the Department of Medicine. So the burden of figuring out what we were going to do fell on me and the other members of the hospital leadership team.
SARS IN 2003
As the outbreak evolved, we only knew a few things. It was a respiratory infection, likely viral, with a very high mortality rate, compared with most other viral respiratory infections. We learned the hard way that, while it was mostly transmitted by droplets, some patients were able to widely transmit it through the air, and therefore likely through ventilation systems. We knew that most infections were occurring in hospitals but there was also community spread at events like funerals. We had no test to confirm the presence of the virus and, indeed, only figured out it was a coronavirus well into the outbreak. Diagnoses were made using clinical criteria; this uncertainty was a major source of anxiety about potential community spread without direct links to known cases. We had no idea how long it was going to last, nor did we know how it would end. We were entering uncharted territory.
Decisions had to be made. Which patients needed isolation, and which did not? We made mistakes early on that caused hundreds of healthcare workers and people to be quarantined (complete isolation) for 10 days; this was a difficult situation for them, their families, and the people who had to replace them in the workplace.
Within a very short period we changed our way of life in hospitals. We screened everyone who entered with questionnaires and measured their temperatures. Once entering the hospital, we all wore N95 masks in public spaces and when in a room with another person—not just patients. We all got sore throats from wearing the masks 10 hours a day. All patients were placed in respiratory precautions, which meant that, any time we entered their rooms, we had to don all the personal protective equipment (PPE). Yet we didn’t run out of supplies. When a member of a provincial leadership team fell ill with SARS shortly after attending an in-person meeting of the committee, all the other members went into quarantine. As a result, we stopped having leadership team meetings in person, and mostly stayed in our own offices, communicating by phone and email.
The hospital took on a bizarre atmosphere: everyone in masks and little face-to-face contact. Yet outside the hospital, life went on mostly as normal. Some people wore masks on the street, but public events and businesses stayed open. Some healthcare workers were shunned in the community out of fear. But I went to another bat mitzvah and even a Stanley Cup playoff game at the height of the outbreak. Only healthcare workers were asked to stop meeting in large groups. The contrast for me was striking.
The Ontario Ministry of Health started a daily noon hour phone conference call; one physician and one administrator from every hospital in the province were on the call. I attended those for my hospital and, because I knew or taught many of the people on the line, was quickly asked to chair the calls. They were incredibly important and were a source of information exchange and emotional support for all of us. Before each call, I spoke with a person from Toronto Public Health who updated me on the number of cases and deaths. I needed to absorb that information before the calls to maintain my composure when she told the rest of the group. At times I could hear the fear in people’s voices as they described the clinical course of their patients.
Because I chaired the calls, I was asked to coordinate the study that documented the clinical outcomes of all the patients in the hopes that we could distinguish it from other common respiratory syndromes. With the help of my colleagues in the 11 hospitals that treated SARS patients, the ethics review boards, medical records personnel who copied the charts, Christopher Booth, MD, (a second- year resident at the time who headed the study), and a few medical students we were able to go from the idea to do the study to electronic publication in JAMA in 30 days.1 It was JAMA’s first experience with rapid review, and the editors there were very helpful. Working on this study was very therapeutic; it allowed me to feel I was doing something that could help.
I was scared—both for my own health and the health of my family, but also terribly frightened for the health of the people who worked here. When I went home every night, I looked at the people on the street and wondered how many would still be there a few months later. And then it all ended. (Actually, it ended twice; we let up a bit too early because we so wanted it to be over.)
COVID-19 IN 2020
The COVID-19 pandemic has many similarities, but there are also significant differences. The most obvious is that because there is more community spread, life outside the hospital is much more severely disrupted. Countries have responded by sliding into more and more practices that try to limit person-to-person spread. First travel restrictions from other countries, then moral suasion to promote social distancing (which is really just physical distancing), then closing schools and nonessential businesses, and finally complete lock downs.
These events have spurred panic buying of some items (hand sanitizer, toilet paper, masks), and the fear of major disruptions of the supply chain for things like food. SARS was much more limited in its overall economic effect, though the WHO precautionary travel advisory against nonessential travel to Toronto, which lasted for only 1 week, resulted in a long-lasting reduction in tourism and a hit to the theatre business in our city.
The internet and social media have made it easier to disseminate valuable information and instructions, while at the same time easier to spread false information. But we had a lot of false information during SARS, too. One of the biggest differences for the United States (which was almost unaffected by SARS) is that the current extreme political divide creates two separate tracks of information and beliefs. A united message is very important.
Finally, the shortage of PPE in some jurisdictions, which was not an issue in Toronto during SARS, has severely heightened the fear for healthcare workers. In 2003, we also had lots of discussion about the tension between our professional duty and the safety of healthcare workers and their families (many of us separated ourselves from our families in our own homes while working clinically). To my recollection, two nurses and one physician died of SARS in Toronto. But when hospitals actually run out of PPE—something that is happening with COVID-19—those discussions take on a much more ominous tone.
LESSONS LEARNED
In my opinion, SARS was a dry run for us in Toronto and the other places in the world that it affected (Taiwan, Hong Kong, Singapore); one that helped us prepare in advance and will help us cope with COVID-19. But what did I personally learn from my SARS experience?
First, I learned that accurate information in these kinds of situations is hard to come by. We heard lots of rumors from people all over the world. But when I found that it was very difficult for me to figure out exactly what was going on in my own hospital (eg, who was in contact with people who fell ill or went into quarantine, how patients were faring), I realized that figuring out what was happening half way around the world from news reports was near impossible. I learned to wait for official announcements.
Second, I learned that talking to my colleagues was both therapeutic—providing emotional support and an outlet for feelings—and anxiety provoking when we overreacted to rumors.
Third, I learned that, like others, I was susceptible to exhibiting obsessive behaviors in an attempt to establish control over uncertainty. Constantly washing my hands, checking my temperature, and seeking reassuring facts from others only worked to calm me for a few minutes. And then I felt the need to do it again. This time I find myself checking my twitter account constantly; half afraid I will see something frightening, half looking for good news from people I trust. I now recognize this behavior and it helps me contain it.
Fourth, I learned that events that occurred remotely had much less effect on everyone than those that occurred close by. Having two people I knew get SARS, and then learning they recovered was perhaps the most meaningful event for me during the entire episode.
Finally, I learned that in the end I and the people I care about survived—nothing bad happened to us. The world did not end after SARS. It took me about a year, including some time with a terrific psychiatrist, to realize I was safe after all. And that realization is what I am most hanging on to today.
Acknowledgments
Sanjay Saint (University of Michigan), Christopher Booth (Queens University), and Sagar Rohailla (University of Toronto) provided comments on an earlier draft. None were compensated for doing so.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
1. Booth C, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801-2809. https://doi.org/10.1001/jama.289.21.JOC30885.
© 2020 Society of Hospital Medicine
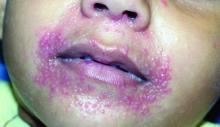
Don’t call it perioral dermatitis
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – , according to Jessica Sprague, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital.
Years ago, some of her senior colleagues at the children’s hospital carried out a retrospective study of 79 patients, aged 6 months to 18 years, who were treated for what’s typically called perioral dermatitis. Of note, only 40% of patients had isolated perioral involvement, while 30% of the patients had no perioral lesions at all. Perinasal lesions were present in 43%, and 25% had periocular involvement, she noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The peak incidence of periorificial dermatitis in this series was under age 5 years. At presentation, the rash had been present for an average of 8 months. Seventy-two percent of patients had a history of exposure to corticosteroids, most often in the form of topical steroids, but in some cases inhaled or systemic steroids.
“Obviously you want to discontinue the topical steroid. Sometimes you need to taper them off, or you can switch to a topical calcineurin inhibitor [TCI] because they tend to flare a lot when you stop their topical steroid, although there are cases of TCIs precipitating periorificial dermatitis, so keep that in mind,” Dr. Sprague said.
If a patient is on inhaled steroids by mask for asthma, switching to a tube can sometimes limit the exposure, she continued.
Her first-line therapy for mild to moderate periorificial dermatitis, and the one supported by the strongest evidence base, is metronidazole cream. Other topical agents shown to be effective include azelaic acid, sulfacetamide, clindamycin, and topical calcineurin inhibitors.
Oral therapy is a good option for more extensive or recalcitrant cases.
“If parents are very anxious, like before school photos or holiday photos, sometimes I’ll use oral therapy as well. In younger kids, I prefer erythromycin at 30 mg/kg per day t.i.d. for 3-6 weeks. In kids 8 years old and up you can use doxycycline at 50-100 mg b.i.d., again for 3-6 weeks. And you have to tell them it’s going to take a while for this to go away,” Dr. Sprague said.
She reported having no financial conflicts regarding her presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
What is seronegative rheumatoid arthritis, anyway?
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
MAUI, HAWAII – Viewing seronegative rheumatoid arthritis as something akin to RA-lite would be a big mistake, John J. Cush, MD, asserted at the 2020 Rheumatology Winter Clinical Symposium.
“It’s not a benign subtype of RA. And then again, it may not be RA,” Dr. Cush observed,
“Seronegative RA means that either you need to get serious about what is probably badass disease or you need to reevaluate whether this really is RA and your need for DMARDs [disease-modifying antirheumatic drugs] in an ongoing fashion,” the rheumatologist said. “Always reconsider whether they need less therapy or maybe no therapy at all. Maybe they had inflammation at one point and now they’re left with degenerative and mechanical changes that don’t require a DMARD or biologic.”
He highlighted a Finnish 10-year, prospective, observational study that sheds light on the subject. The study demonstrated that seronegative RA is seldom what it at first seems. The Finnish rheumatologists followed 435 consecutive patients initially diagnosed as having seronegative early RA. The structured follow-up entailed four or five interdisciplinary clinic visits within the first 2 years after diagnosis and again at 5 and 10 years.
By the 10-year mark only 4 of the 435 initially seronegative RA patients had been reclassified as having seropositive RA, while another 9 were reclassified as having erosive RA based upon the development of pathognomonic joint lesions. That’s a paltry 3% reclassification rate to classic RA.
Nearly two-thirds of patients were ultimately reclassified within 10 years as they evolved into diagnoses other than their original seronegative RA. The most common included nonerosive polymyalgia rheumatica in 16% of participants, psoriatic arthritis in 11%, osteoarthritis in 10%, spondyloarthritis in 8.7%, gout in 2.3%, and pseudogout in 3.9%.
“I think that’s sobering for you if you’re taking care of these patients, that maybe you need to rethink the diagnosis at every visit or at periodic intervals, especially if you’re going to change therapy,” advised Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The Finnish rheumatologists observed that their findings have important implications both for clinical practice and for research, since RA clinical trials typically include a substantial proportion of seronegative patients.
“If seronegative patients are treated according to the treatment guidelines for progressive RA, a substantial proportion of patients is exposed to unnecessary long-term medication,” the investigators wrote, adding that their “results suggest that it may not be reasonable to study seronegative arthritis patients as a homogeneous entity in RA studies.”
The best recent data suggests about 15% of RA patients are seronegative, Dr. Cush said.
Delay in diagnosis is common in seronegative RA, as highlighted in a recent population-based study by Mayo Clinic rheumatologists. They reported that the median time from first joint swelling to diagnosis of seronegative RA using the 2010 American College of Rheumatology/European League Against Rheumatism criteria was 187 days, compared with a mere 11 days for seropositive RA. The median time to DMARD initiation was longer, too. Half of seropositive RA patients achieved remission within 5 years, as did 28% of seronegative patients, prompting the investigators to conclude “the window of opportunity for intervention may be more frequently missed in this group.”
Choosing the best treatment
Several medications appear to have greater efficacy in seropositive than seronegative RA patients. For example, a meta-analysis of four randomized trials including a collective 2,177 RA patients assigned 2:1 to rituximab (Rituxan) or placebo concluded that 75% of seropositive RA patients had a EULAR moderate or good response at week 24 on the biologic, compared with 44% of seronegative patients.
“Would you not use rituximab in someone who’s seronegative? No, I actually would use it. I may not rush to use it as much, maybe give it earlier in someone who’s seropositive, but I’ve used rituximab in seronegative patients who’ve done just fine,” according to Dr. Cush.
The published experience with abatacept (Orencia) is mixed, most of it coming from European observational datasets. On balance though, 80% of the articles addressing the issue have concluded that response rates to the biologic are better in seropositive RA, he continued.
Australian investigators who pooled data from five phase 3 randomized clinical trials of tofacitinib (Xeljanz) in RA concluded that double-positive patients – that is, those who were seropositive for both rheumatoid factor and anti–citrullinated protein antibody (ACPA) – were roughly twice as likely to achieve ACR20 and ACR50 responses to the oral Janus kinase inhibitor at either 5 or 10 mg twice daily than patients who were double negative.
“Double positivity is very important in prognosis and severity, compared to single positivity,” the rheumatologist observed. “I think you should worry most about the patients who have the highest titers of rheumatoid factor and ACPA.”
Asked about the merits of supplemental laboratory testing for serum 14-3-3 eta, a proposed novel biomarker in RA, as well as for anti–carbamylated protein antibodies (anti-CarP), Dr. Cush replied that it’s unclear that the additional testing is really worthwhile.
“Ordering more tests doesn’t make us smarter,” he commented. “Quite simply, with rheumatoid factor and ACPA, adding one on top of the other, you just gain maybe 10% more certainty in the diagnosis. Adding anti-CarP antibodies or serum 14-3-3 eta doesn’t add more than a few percentage points, but now you’ve quadrupled the cost of testing.”
Dr. Cush reported receiving research funding from and/or serving as a consultant to numerous pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2020
CLEOPATRA: Pertuzumab has long-term benefit in HER2+ breast cancer
, with nearly 40% of patients achieving long-term survival, the CLEOPATRA end-of-study analysis shows.
The regimen, combining dual HER2 targeting with chemotherapy, became standard of care in this population as a result of its good efficacy and safety relative to placebo, first established in the phase 3, randomized trial 8 years ago (N Engl J Med. 2012;366:109-19).
Trial updates since then, most recently at a median follow-up of 50 months (N Engl J Med. 2015;372:724-34), have shown clear progression-free and overall survival benefits, with acceptable cardiac and other toxicity.
Investigators led by Sandra M. Swain, MD, of Georgetown University, Washington, performed a final analysis of data from the 808 patients in CLEOPATRA, now at a median follow-up of 99.9 months.
Results reported in The Lancet Oncology showed that, compared with placebo, pertuzumab prolonged investigator-assessed progression-free survival by 6.3 months (the same as that seen in the previous update) and prolonged overall survival by 16.3 months (up from 15.7 months in the previous update).
At 8 years, 37% of patients in the pertuzumab group were still alive, and 16% were still alive without progression.
“The combination of pertuzumab, trastuzumab, and docetaxel remains the standard of care for the first-line treatment of HER2-positive metastatic breast cancer, owing to its overall survival benefits and maintained long-term overall and cardiac safety,” Dr. Swain and coinvestigators concluded. “Prospective identification of patients who will be long-term responders to treatment is an area for future research.”
In an accompanying comment, Matteo Lambertini, MD, PhD, of IRCCS Ospedale Policlinico San Martino in Genova, Italy, and Ines Vaz-Luis, MD, of Institut Gustave Roussy in Villejuif, France, contended that these results, “which are also observed in real-world datasets, challenge the concept of HER2-positive metastatic breast cancer being an incurable disease and open the path to several interconnected clinical and research questions.”
Those questions include the optimal duration of anti-HER2 maintenance therapy in patients without disease progression, best strategies for combining this systemic therapy with local treatment to further improve survival, and new markers to better identify patients likely to be long-term responders, who might benefit from a curative approach, the authors elaborated. They noted that more than half of CLEOPATRA patients had de novo stage IV disease.
“The performance of the current standard pertuzumab-based first-line treatment in patients previously exposed to adjuvant or neoadjuvant anti-HER2 therapy remains to be clarified,” the authors wrote. “Results from several ongoing prospective cohort studies investigating real-world patterns of care and outcomes of patients with HER2-positive metastatic breast cancer will help to clarify this important issue and optimize treatment sequencing.”
Study details
The end-of-study analysis showed that median progression-free survival was 18.7 months with pertuzumab and 12.4 months with placebo (hazard ratio, 0.69; 95% confidence interval, 0.59-0.81). The 8-year landmark progression-free survival rate was 16% with the former and 10% with the latter.
The median overall survival was 57.1 months with pertuzumab and 40.8 months with placebo (HR, 0.69; 95% CI, 0.58-0.82). The 8-year landmark overall survival rate was 37% with the former and 23% with the latter.
A comparison of patients who did and did not achieve long-term response showed that, in both treatment groups, the former more often had tumors that were 3+ positive by HER2 immunohistochemistry and PIK3CA wild-type tumors. The leading grade 3 or 4 adverse event was neutropenia, seen in 49% of patients in the pertuzumab group and 46% of those in the placebo group. The rate of treatment-related death was 1% and 2%, respectively.
Since the last update, only two additional serious adverse events were reported: one case of heart failure and one case of symptomatic left ventricular systolic dysfunction in patients given pertuzumab.
The CLEOPATRA trial was funded by F. Hoffmann-La Roche and Genentech. Dr. Swain and coauthors disclosed relationships with these and other companies. Dr. Lambertini disclosed relationships with Roche, Theramex, and Takeda. Dr. Vaz-Luis disclosed relationships with AstraZeneca, Kephren, and Novartis.
SOURCE: Swain SM et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(19)30863-0; Lambertini M et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(20)30058-9.
, with nearly 40% of patients achieving long-term survival, the CLEOPATRA end-of-study analysis shows.
The regimen, combining dual HER2 targeting with chemotherapy, became standard of care in this population as a result of its good efficacy and safety relative to placebo, first established in the phase 3, randomized trial 8 years ago (N Engl J Med. 2012;366:109-19).
Trial updates since then, most recently at a median follow-up of 50 months (N Engl J Med. 2015;372:724-34), have shown clear progression-free and overall survival benefits, with acceptable cardiac and other toxicity.
Investigators led by Sandra M. Swain, MD, of Georgetown University, Washington, performed a final analysis of data from the 808 patients in CLEOPATRA, now at a median follow-up of 99.9 months.
Results reported in The Lancet Oncology showed that, compared with placebo, pertuzumab prolonged investigator-assessed progression-free survival by 6.3 months (the same as that seen in the previous update) and prolonged overall survival by 16.3 months (up from 15.7 months in the previous update).
At 8 years, 37% of patients in the pertuzumab group were still alive, and 16% were still alive without progression.
“The combination of pertuzumab, trastuzumab, and docetaxel remains the standard of care for the first-line treatment of HER2-positive metastatic breast cancer, owing to its overall survival benefits and maintained long-term overall and cardiac safety,” Dr. Swain and coinvestigators concluded. “Prospective identification of patients who will be long-term responders to treatment is an area for future research.”
In an accompanying comment, Matteo Lambertini, MD, PhD, of IRCCS Ospedale Policlinico San Martino in Genova, Italy, and Ines Vaz-Luis, MD, of Institut Gustave Roussy in Villejuif, France, contended that these results, “which are also observed in real-world datasets, challenge the concept of HER2-positive metastatic breast cancer being an incurable disease and open the path to several interconnected clinical and research questions.”
Those questions include the optimal duration of anti-HER2 maintenance therapy in patients without disease progression, best strategies for combining this systemic therapy with local treatment to further improve survival, and new markers to better identify patients likely to be long-term responders, who might benefit from a curative approach, the authors elaborated. They noted that more than half of CLEOPATRA patients had de novo stage IV disease.
“The performance of the current standard pertuzumab-based first-line treatment in patients previously exposed to adjuvant or neoadjuvant anti-HER2 therapy remains to be clarified,” the authors wrote. “Results from several ongoing prospective cohort studies investigating real-world patterns of care and outcomes of patients with HER2-positive metastatic breast cancer will help to clarify this important issue and optimize treatment sequencing.”
Study details
The end-of-study analysis showed that median progression-free survival was 18.7 months with pertuzumab and 12.4 months with placebo (hazard ratio, 0.69; 95% confidence interval, 0.59-0.81). The 8-year landmark progression-free survival rate was 16% with the former and 10% with the latter.
The median overall survival was 57.1 months with pertuzumab and 40.8 months with placebo (HR, 0.69; 95% CI, 0.58-0.82). The 8-year landmark overall survival rate was 37% with the former and 23% with the latter.
A comparison of patients who did and did not achieve long-term response showed that, in both treatment groups, the former more often had tumors that were 3+ positive by HER2 immunohistochemistry and PIK3CA wild-type tumors. The leading grade 3 or 4 adverse event was neutropenia, seen in 49% of patients in the pertuzumab group and 46% of those in the placebo group. The rate of treatment-related death was 1% and 2%, respectively.
Since the last update, only two additional serious adverse events were reported: one case of heart failure and one case of symptomatic left ventricular systolic dysfunction in patients given pertuzumab.
The CLEOPATRA trial was funded by F. Hoffmann-La Roche and Genentech. Dr. Swain and coauthors disclosed relationships with these and other companies. Dr. Lambertini disclosed relationships with Roche, Theramex, and Takeda. Dr. Vaz-Luis disclosed relationships with AstraZeneca, Kephren, and Novartis.
SOURCE: Swain SM et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(19)30863-0; Lambertini M et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(20)30058-9.
, with nearly 40% of patients achieving long-term survival, the CLEOPATRA end-of-study analysis shows.
The regimen, combining dual HER2 targeting with chemotherapy, became standard of care in this population as a result of its good efficacy and safety relative to placebo, first established in the phase 3, randomized trial 8 years ago (N Engl J Med. 2012;366:109-19).
Trial updates since then, most recently at a median follow-up of 50 months (N Engl J Med. 2015;372:724-34), have shown clear progression-free and overall survival benefits, with acceptable cardiac and other toxicity.
Investigators led by Sandra M. Swain, MD, of Georgetown University, Washington, performed a final analysis of data from the 808 patients in CLEOPATRA, now at a median follow-up of 99.9 months.
Results reported in The Lancet Oncology showed that, compared with placebo, pertuzumab prolonged investigator-assessed progression-free survival by 6.3 months (the same as that seen in the previous update) and prolonged overall survival by 16.3 months (up from 15.7 months in the previous update).
At 8 years, 37% of patients in the pertuzumab group were still alive, and 16% were still alive without progression.
“The combination of pertuzumab, trastuzumab, and docetaxel remains the standard of care for the first-line treatment of HER2-positive metastatic breast cancer, owing to its overall survival benefits and maintained long-term overall and cardiac safety,” Dr. Swain and coinvestigators concluded. “Prospective identification of patients who will be long-term responders to treatment is an area for future research.”
In an accompanying comment, Matteo Lambertini, MD, PhD, of IRCCS Ospedale Policlinico San Martino in Genova, Italy, and Ines Vaz-Luis, MD, of Institut Gustave Roussy in Villejuif, France, contended that these results, “which are also observed in real-world datasets, challenge the concept of HER2-positive metastatic breast cancer being an incurable disease and open the path to several interconnected clinical and research questions.”
Those questions include the optimal duration of anti-HER2 maintenance therapy in patients without disease progression, best strategies for combining this systemic therapy with local treatment to further improve survival, and new markers to better identify patients likely to be long-term responders, who might benefit from a curative approach, the authors elaborated. They noted that more than half of CLEOPATRA patients had de novo stage IV disease.
“The performance of the current standard pertuzumab-based first-line treatment in patients previously exposed to adjuvant or neoadjuvant anti-HER2 therapy remains to be clarified,” the authors wrote. “Results from several ongoing prospective cohort studies investigating real-world patterns of care and outcomes of patients with HER2-positive metastatic breast cancer will help to clarify this important issue and optimize treatment sequencing.”
Study details
The end-of-study analysis showed that median progression-free survival was 18.7 months with pertuzumab and 12.4 months with placebo (hazard ratio, 0.69; 95% confidence interval, 0.59-0.81). The 8-year landmark progression-free survival rate was 16% with the former and 10% with the latter.
The median overall survival was 57.1 months with pertuzumab and 40.8 months with placebo (HR, 0.69; 95% CI, 0.58-0.82). The 8-year landmark overall survival rate was 37% with the former and 23% with the latter.
A comparison of patients who did and did not achieve long-term response showed that, in both treatment groups, the former more often had tumors that were 3+ positive by HER2 immunohistochemistry and PIK3CA wild-type tumors. The leading grade 3 or 4 adverse event was neutropenia, seen in 49% of patients in the pertuzumab group and 46% of those in the placebo group. The rate of treatment-related death was 1% and 2%, respectively.
Since the last update, only two additional serious adverse events were reported: one case of heart failure and one case of symptomatic left ventricular systolic dysfunction in patients given pertuzumab.
The CLEOPATRA trial was funded by F. Hoffmann-La Roche and Genentech. Dr. Swain and coauthors disclosed relationships with these and other companies. Dr. Lambertini disclosed relationships with Roche, Theramex, and Takeda. Dr. Vaz-Luis disclosed relationships with AstraZeneca, Kephren, and Novartis.
SOURCE: Swain SM et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(19)30863-0; Lambertini M et al. Lancet Oncol. 2020 Mar 12. doi: 10.1016/S1470-2045(20)30058-9.
FROM LANCET ONCOLOGY
Should the practice of counseling patients to present to the office for a string check after IUD insertion be halted?
[polldaddy:10527068]
[polldaddy:10527068]
[polldaddy:10527068]
Wuhan data link COVID-19 with myocardial damage
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
FROM JAMA CARDIOLOGY
Low plasma sodium predicts complications in SCD acute pain episodes
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
FROM THE AMERICAN JOURNAL OF MEDICINE
HCV screening risk factors in pregnant women need updating
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
“Because risk-factor screening has obvious limitations, universal screening in pregnancy has been suggested to allow for linkage to postpartum care and identification of children for future testing and treatment,” wrote Mona Prasad, DO, of Ohio State University, Columbus, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers reviewed data from women with singleton pregnancies presenting for prenatal care prior to 23 weeks’ gestation during 2012-2015. Of these, 254 tested positive for the hepatitis C virus (HCV) antibody, for a seroprevalence rate of 2.4 cases per 1,000 women.
The researchers conducted a case-control analysis of 131 women who tested positive and 251 controls to identify HCV infection risk factors based on interviews and chart reviews. They found that risk factors significantly associated with positive HCV antibodies included injection drug use (adjusted odds ratio, 22.9), a history of blood transfusion (aOR, 3.7), having an HCV-infected partner (aOR, 6.3), having had more than three sexual partners (aOR, 5.3), and smoking during pregnancy (aOR, 2.4).
In an unadjusted analysis, the researchers confirmed two of the risk factors currently recommended by the Centers for Disease Control and Prevention for screening for HCV: injection drug use and being born to a mother with HCV infection, but not dialysis, organ transplantation, or HIV infection.
“Our results demonstrate that current risk factors could be contemporized,” Dr. Prasad and colleagues noted. “The currently accepted risk factors such as exposure to clotting factors, dialysis, and organ transplants are unlikely to be found. A thorough assessment of injection drug use history, smoking, transfusions, number of sexual partners, and partners with HCV infection is more sensitive in an obstetric population.”
The study findings were limited by several factors including possible selection bias and inclusion of only 65% of eligible women who were HCV positive, as well as a lack of screening data from 2016 to the present, which may not reflect the impact of the recent opioid epidemic, the researchers noted. However, the results were strengthened by the large sample size, and the generalizability of the study population.
“Our results regarding prevalence rates and risk factors of HCV antibody among pregnant women in the United States will be valuable to policymakers as they weigh the costs and benefits of universal screening,” Dr. Prasad and associates concluded.
Although universal screening has the potential to be more cost effective, given the small population of pregnant women eligible for treatment and lack of an available treatment, “the rationale is weaker for unique universal HCV screening recommendations for pregnant women,” they said.
By contrast, Sammy Saab, MD, MPH, of the University of California, Los Angeles; Ravina Kullar, PharmD, MPH, of Gilead Sciences, Foster City, Calif.; and Prabhu Gounder, MD, MPH, of the Los Angeles Department of Public Health, wrote an accompanying commentary in favor of universal HCV screening for pregnant women, in part because of the increase in HCV in the younger population overall.
“For many women of reproductive age, pregnancy is one of their few points of contact with their health care provider; therefore, pregnancy could provide a crucial time for targeting this population,” they noted.
Risk-based screening is of limited effectiveness because patients are not identified by way of current screening tools or they decline to reveal risk factors that providers might miss, the editorialists said. Pregnancy has not been shown to affect the accuracy of HCV tests, and identifying infections in mothers allows for screening in children as well.
“The perinatal hepatitis B virus infection program, which has been implemented in several state and local public health departments, could serve as an example for how to conduct surveillance for mothers with HCV infection and to ensure that HCV-exposed children receive appropriate follow-up testing and linkage to care,” the editorialists concluded.
The study was supported in part by multiple grants from the National Institute of Child Health and Human Development. Dr. Prasad disclosed funding from Ohio State University and from Gilead. Coauthors had links with pharmaceutical companies, associations, and organizations – most unrelated to this study. The editorialists had no financial conflicts to disclose.
SOURCES: Prasad M et al. Obstet Gynecol. 2020;135:778-88; Saab S et al. Obstet Gynecol. 2020;135:773-7.
FROM OBSTETRICS & GYNECOLOGY
In older HIV patients, B/F/TAF regimen was noninferior to others
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
FROM CROI 2020