User login
Guidelines on delaying cancer surgery during COVID-19
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
White House expands seniors’ telehealth for COVID-19
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more,” said CMS Administrator Seema Verma during a March 17 White House press briefing on administration actions to contain the spread of COVID-19.
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health & Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states.Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She added that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
AGA has released a guide to commercial telehealth COVID-19 coding policies (http://ow.ly/8CIH30qsU0B) that supplements their guide to public payors.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more,” said CMS Administrator Seema Verma during a March 17 White House press briefing on administration actions to contain the spread of COVID-19.
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health & Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states.Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She added that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
AGA has released a guide to commercial telehealth COVID-19 coding policies (http://ow.ly/8CIH30qsU0B) that supplements their guide to public payors.
“Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patients’ places of residence, starting March 6, 2020,” the Centers for Medicare & Medicaid Services said in a fact sheet issued March 17.
Some of the existing benefits were previously limited to rural communities.
“These services can also be provided in a variety of settings, including nursing homes, hospital outpatient departments, and more,” said CMS Administrator Seema Verma during a March 17 White House press briefing on administration actions to contain the spread of COVID-19.
That means that seniors can continue to receive their routine care without having to leave the home and risk infection, or they can get medical guidance if they have mild symptoms, which would help mitigate the spread to others.
“This shift is very important for clinicians and providers who, over the coming weeks, will face considerable strain on their time and resources,” Dr. Verma said. “[It] allows the health care system to prioritize care for those who have more needs or who are in dire need, and it also preserves protective equipment.”
A range of providers will be able to deliver telehealth services, including doctors, nurse practitioners, clinical psychologists, and licensed clinical social workers. Visits using telehealth services will be considered the same as in-person visits and will be paid as if the patient were seen in the office.
This expansion of Medicare telehealth services will continue for the duration of the COVID-19 public health emergency.
“In addition, the [Health & Human Services’] office of inspector general is providing flexibility for health care providers to reduce or waive cost-sharing for telehealth visits paid by federal health care programs,” the fact sheet states.Key to the expansion is that it will cover the entire United States and will not be limited to rural areas.
Dr. Verma also noted that the administration “will be temporarily suspending certain HIPAA requirements so that doctors can provide telehealth with their own phones.”
She added that state Medicaid agencies can expand their telehealth services without the approval of CMS during this emergency.
AGA has released a guide to commercial telehealth COVID-19 coding policies (http://ow.ly/8CIH30qsU0B) that supplements their guide to public payors.
Cervical pessary didn’t prevent preterm birth in selected women
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
GRAPEVINE, TEX. – or a composite measure of adverse neonatal outcomes, according to a randomized, open-label study.
“The Arabin pessary should not be used to prevent preterm birth in women with twin pregnancy,” Jane E. Norman, MD, dean of health sciences at the University of Bristol (England), said at the Pregnancy Meeting.
“Preterm birth is very common in twin pregnancy” and leads to “excess neonatal mortality amongst twins,” Dr. Norman said at the meeting, which is sponsored by the Society for Maternal-Fetal Medicine. “Preventing preterm birth is important for both singletons and twins, but it could have even more benefits in twins.”
Emerging evidence has suggested that the Arabin cervical pessary may be useful for the prevention of preterm birth in women with singleton pregnancy and a short cervix. In twin pregnancy, data are more limited.
The ProTwin study randomized 813 women with twin pregnancy to a cervical pessary or standard care. Although the pessary had no impact on preterm birth overall, among women with cervical length of less than 38 mm, those who received a pessary were less likely to have preterm birth. The sample size was small, however, and the average length of the cervix in ProTwin differed from that in a previous U.K. study, Dr. Norman said.
Inspired by the study, Dr. Norman and coinvestigators conducted STOPPIT-2, a multicenter, open-label, randomized, controlled trial to further study whether a certain cervical length threshold was associated with benefit of a cervical pessary in preventing preterm birth. The trial included women with twin pregnancy who had a cervical length ultrasound between 18 and 20 weeks and 6 days of gestation. Women with a cervical length of 35 mm or less were eligible for randomization. Patients received an Arabin pessary plus standard care or standard care alone.
The primary obstetric outcome was spontaneous onset of labor leading to delivery before 34 weeks and 6 days of gestation. The primary neonatal outcome was a composite of outcomes – stillbirth, neonatal death, periventricular leukomalacia, respiratory morbidity, intraventricular hemorrhage, necrotizing enterocolitis, or sepsis – measured up to 28 days after the expected date of delivery.
The investigators randomized 503 women in all, including 250 to the pessary and 253 to standard care. Both groups had similar baseline characteristics, Dr. Norman said. The average age was about 33 years, and the average cervical length was about 29 mm. A total of 20% had monochorionic diamniotic pregnancies, and 80% had dichorionic pregnancies. The researchers excluded women with monochorionic monoamniotic pregnancies. In the pessary group, 16 patients declined the intervention, and 4 were unable to have the pessary inserted.
Spontaneous preterm birth occurred in 18% of patients in the Arabin pessary group, compared with 21% in the standard treatment group. The adjusted odds ratio, 0.87, was not statistically significant. In subgroups of patients with monochorionic pregnancy, cervical length less than 28 mm, or cervical length less than 25 mm, there was no significant benefit.
The composite measure of adverse neonatal outcomes also did not significantly differ between the groups. None of the individual components indicated benefit of the pessary either, Dr. Norman said.
In subgroup analyses, odds ratios for adverse neonatal outcomes were “tending towards harm for the Arabin pessary group ... although clearly none of them conferring statistical significance,” she said. Among women with cervical length less than 28 mm, a primary neonatal outcome – at least one of the adverse outcomes – occurred in 23% of patients in the Arabin pessary group, compared with 20% of patients in the standard care group.
Approximately two-thirds of patients found pessary insertion painless or slightly uncomfortable, whereas about 10% described the experience of device fitting as very uncomfortable, and about 1% described it as the worse pain imaginable.
“Since we started STOPPIT-2, in addition to ProTwin, another three studies have been published on the efficacy of the Arabin pessary in twins,” said Dr. Norman. Combined data show no significant effect of the pessary on preventing preterm birth in twin pregnancy. Still, the meta-analysis does not rule out the possibility that there could subgroups of patients who may benefit from the intervention, Dr. Norman said.
STOPPIT-2 was funded by the National Institute for Health Research (NIHR) in the United Kingdom. Dr. Norman chaired the UK National Institute for Health and Care Excellence guidelines on preterm labor and birth in 2015. In addition, Dr. Norman was a member of a GlaxoSmithKline data safety and monitoring group for a trial of a preterm birth prevention agent, has consulted for Dilafor, and has received research grants for preterm birth prevention from the U.K. Medical Research Council, NIHR, and Tommy’s: Together, for every baby charity.
SOURCE: Norman JE et al. Am J Obstet Gynecol. 2020 Jan;222(1):S756. Abstract LB 1.
REPORTING FROM THE PREGNANCY MEETING
Cardiac symptoms can be first sign of COVID-19
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
FROM CIRCULATION
FDA approves ozanimod for relapsing and secondary progressive forms of MS
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
At U.S. Ground Zero for coronavirus, a hospital is transformed
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.
The hospital adapts to a new normal
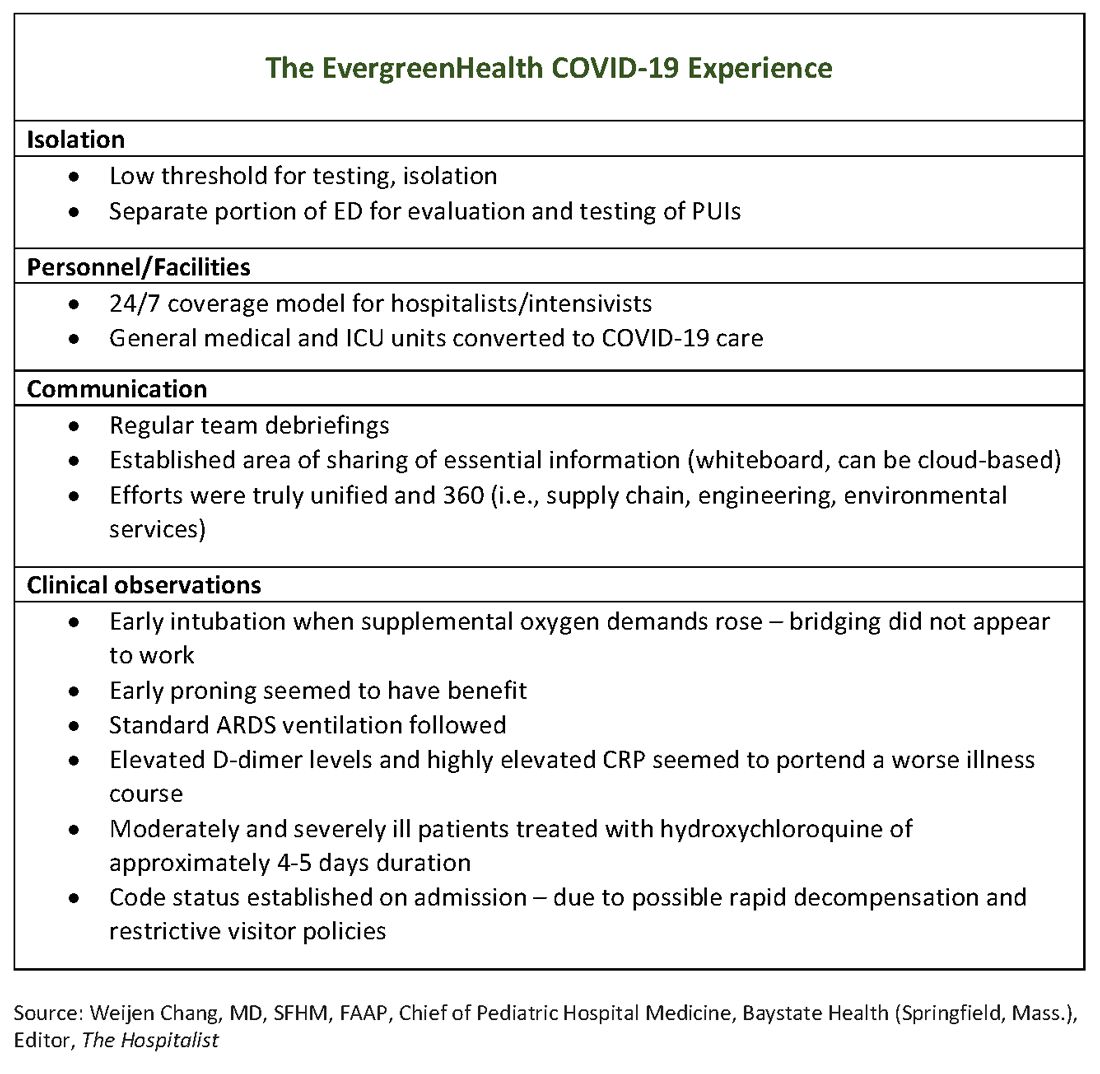
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
New topicals coming for pediatric atopic dermatitis
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
LAHAINA, HAWAII – Novel topical medications are in the works that will address the longstanding unmet need for a Food and Drug Administration–approved noncorticosteroid topical for use in pediatric atopic dermatitis, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
These new agents will be embraced by clinicians for use in delicate skin areas, as well as in the common clinical scenario involving steroid-averse parents, predicted Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
First up is crisaborole (Eucrisa), which is approved for atopic dermatitis (AD) in children aged two years and older and has been under review at the Food and Drug Administration for use in infantile AD. (On March 24, several weeks after the meeting, the FDA approved crisaborole down to aged three months for treatment of mild to moderate AD). Agents earlier in the developmental pipeline include two topical Janus kinase (JAK) inhibitors, ruxolitinib and delgocitinib, as well as tapinarof.
Crisaborole: This phosphodiesterase 4 inhibitor is FDA approved down to 2 years of age. In the phase 4, open-label CrisADe CARE 1 study, crisaborole was studied in 137 children ages 3 months to under 24 months. CrisADe CARE 1, presented at the 2019 annual conference of the Pediatric Dermatology Research Alliance (PeDRA), showed close to a 60% reduction from baseline in Eczema Area and Severity Index (EASI) scores after 28 days of twice-daily therapy in the youngsters, 61% of who had moderate AD, the rest mild disease.
Tolerability and safety were reassuring in the phase 4 study. Although about 3% of subjects each experienced application site pain, discomfort, or erythema, the rate of study discontinuation was impressively low at 2.9%, Dr. Eichenfield observed.
Delgocitinib: Japanese investigators have reported positive results in a phase 2 study of delgocitinib ointment in 98 children and adolescents aged 2-15 years, with AD. After 4 weeks of twice-daily treatment, modified EASI scores improved by a mean of 54% with delgocitinib 0.25% and by 62% with 0.5%, compared with less than a 5% improvement with the vehicle control (J Allergy Clin Immunol. 2019 Dec;144[6]:1575-83). The ointment formulation is being developed specifically for the Japanese market.
Studies of an alternative formulation of the JAK inhibitor as a cream rather than ointment, intended for the U.S. and European markets, are in the early stages, conducted by Leo Pharma. Delgocitinib cream, under study in adults and children down to age 2 years with AD, is also under study for chronic hand dermatitis, a program Dr. Eichenfield is enthusiastic about.
“Hand eczema is something you’re going to hear a lot about in the next 2 years. In the U.S., we have no drug approved specifically for hand eczema. And we actually see a lot of hand eczema in pediatric and adolescent patients. I’d say 75%-80% of the ones I see also have atopic dermatitis,” he said.
Ruxolitinib: Incyte, which is developing the topical JAK inhibitor, recently announced positive results in the first of four phase 3 randomized trials, this one conducted in AD patients aged 12 years and older. The efficacy appears to be comparable to that of topical steroids. Studies in younger children are also planned. Ruxolitinib cream is in advanced clinical trials for treatment of vitiligo.
Tapinarof: This topical aryl hydrocarbon receptor agonist downregulates Th17 cytokines, an attribute desirable for treatment of psoriasis. But it also downregulates Th2 cytokines and improves the damaged skin barrier characteristic of AD via upregulation of the filaggrin and involucrin genes in keratinocytes. In a phase 2b, double-blind clinical trial conducted in 247 adults and adolescents with moderate to severe AD, 12 weeks of once-daily tapinarof 1% enabled 51% of patients to achieve a 75% or greater improvement in EASI scores, compared with 18% in controls on vehicle (J Am Acad Dermatol. 2019 Jan;80[1]:89-98.e3).
Dermavant, which is developing the drug, plans to seek an initial indication for treatment of psoriasis, where a phase 3 study is underway, before pursuing regulatory approval in AD.
Dr. Eichenfield disclosed serving as a consultant or investigator for various pharmaceutical companies, including Pfizer, and Dermavant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
This article was updated 3/27/20.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Coronavirus and Dermatology: A Resident’s Perspective
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
On January 30, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a public health emergency of international concern.1 Severe acute respiratory syndrome–associated coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is an enveloped, single-stranded RNA virus. It is the seventh known coronavirus to infect humans and third zoonotic Coronaviridae to cause fatal respiratory illness, along with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).2 There has been a rapid shift in the geographic center of the outbreak as well as the numbers of confirmed cases and deaths. Although the first cases in late 2019 and early 2020 were in China, by mid-March Italy became the center of the pandemic, with a steep increase in the number of cases in other European countries and the United States.3 Although COVID-19 does not have known dermatologic manifestations, it has the potential for wide-reaching impact on our field.
Strained Resources
In the United States, COVID-19 initially was associated with international travel but is now rapidly spreading throughout the community. I am currently a dermatology resident at New York-Presbyterian, Columbia campus, in New York, New York, a city that unfortunately finds itself underprepared to handle this unprecedented crisis. As of Monday, March 16, 2020, New York-Presbyterian made the decision to postpone all elective procedures, including Mohs micrographic surgery, to preserve hospital resources, including trained personnel, personal protective equipment, ventilators, and hospital beds. There have not been clear-cut guidelines regarding how to approach other dermatologic care for our patients, including routine clinic visits and inpatient dermatology consultations, leaving decisions up to individual departments and providers.
It would be prudent to learn from our colleagues in China who report steps that have been successful in preventing nosocomial spread of COVID-19 in the dermatologic setting. Tao et al4 described their protocols in both the outpatient and inpatient dermatologic setting, beginning with strict triage before patients can even enter a clinic building for their outpatient appointment. Those who screen positive are sent to a fever clinic for further evaluation, which may include a rapid computed tomography scan (using a machine that may perform 200 chest computed tomography scans per day) and SARS-CoV-2 polymerase chain reaction.5 For inpatient dermatologic consultation of patients with known COVID-19, telehealth and multidisciplinary meetings are first and second line, respectively, with bedside dermatologic consultation as a last resort.4 Chen et al6 described similarly strict triage protocols as well as physician use of full-body personal protective equipment during all patient encounters. These measures are taken in light of the well-documented phenomenon of asymptomatic carriage and transmission, as all patients entering their dermatologic clinics have screened negative for symptomatic SARS-CoV-2 infection.6
Conferences and Education
Coronavirus is impacting the education of millions of individuals worldwide, including that of dermatology residents. The Annual Meeting of the American Academy of Dermatology, which was scheduled to take place in March 2020, was canceled due to COVID-19.7 The American Board of Dermatology has released a statement indicating that for all dermatology residents, time spent in COVID-19–mandated quarantine will count as clinical education if residents are able to work with their program to complete independent structured academic activity during that time.8 We also must consider the possibility that dermatology residents are reassigned to work outside of our specialty, resulting in less time and experience caring for patients with dermatologic conditions. Dermatologists in other countries have been called upon to care for COVID-19 patients, even reported to be working in intensive care units in Italy.9 Virtual technologies may be used in novel ways to support dermatology resident education throughout this process.
Final Thoughts
As physicians, dermatologists are in the position to educate their patients regarding prevention strategies, especially given that the lay press disseminates confusing and inaccurate information. The World Health Organization provides specific guidance, focusing on handwashing, respiratory hygiene, social distancing, and encouraging symptomatic patients to seek remote care whenever possible.10 Many of our patients are at high risk of complications due to COVID-19, whether due to their age or because they are immunocompromised. As the situation unfolds, the impact on and role of dermatology in this crisis will continue to evolve.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
- Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)[news release]. Geneva, Switzerland: World Health Organization; January 30, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29. Accessed March 16, 2020.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- World Health Organization. Coronavirus disease 2019: Situation Report—58. March 18, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2. Accessed March 19, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- McNeil DG. Jr. Inside China’s all out war on the coronavirus. The New York Times. March 4, 2020. https://www.nytimes.com/2020/03/04/health/coronavirus-china-aylward.html?smid=tw-nytimes&smtyp=cur. Accessed March 19, 2020.
- Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol. 2020;82:1034.
- Hruza GJ. 2020 annual AAD meeting is canceled due to COVID-19 outbreak. American Academy of Dermatology website. https://www.aad.org/member/meetings/am2020/faqs/coronavirus. Accessed March 16, 2020.
- American Board of Dermatology. Impact of COVID-19 on dermatology resident education. March 6, 2020. https://www.abderm.org/2978.aspx. Accessed March 16, 2020.
- “It’s Like a War” [podcast]. The Daily. March 17, 2020. https://www.nytimes.com/2020/03/17/podcasts/the-daily/italy-coronavirus.html?action=click&module=audio-series-bar®ion=header&pgtype=Article. Accessed March 20, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for public. March 18, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed March 19, 2020.
Perspective from the heartland: Cancer care and research during a public health crisis
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Skin Burns From Transcranial Electrical Stimulation
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
To the Editor:
In recent years, noninvasive brain stimulation techniques have gained growing importance in the treatment of psychiatric1 and neurologic disorders as well as in neurologic rehabilitation (eg, after a stroke).2 One of these techniques is transcranial electrical stimulation (tES), which includes transcranial direct current stimulation (tDCS), transcranial random noise stimulation, and transcranial alternating current stimulation. The current is administered through rubber electrodes covered by saline-soaked sponges that are attached to the skull over the dysfunctional brain areas using broad rubber bands.
Transcranial direct current stimulation ameliorates brain function by anodal stimulation after a series of 5 to 10 stimulations.1 Recently, commercially available brain stimulation devices have been developed to improve working memory for online/video gaming3; however, application of a direct current (eg, 1–2 mA) over longer periods of time (15–20 minutes) can cause skin burns due to drying out of the electrode.4 Inhomogeneities in skin-electrode contact can lead to current bridges, resulting in quick evaporation of the contact medium (sodium chloride solution) and subsequent thermic damage of the skin. Another possible cause of skin lesions associated with tES is skin contact with the rubber electrode due to incorrect positioning of the electrode in the sponge covering. We report 2 cases of burns caused by tDCS.
A 55-year-old woman who was treated with tDCS (2 mA; 20 minutes) for recurrent depressive disorder developed a 0.5-cm, round, erosive, second-degree burn with hemorrhagic crust on the skin in the middle of the area where a 5×7-cm electrode (cathode) was positioned over the right orbit (Figure 1). It was the fifth stimulation with tDCS. The anode was positioned over the left dorsolateral prefrontal cortex. It was determined that the saline-soaked sponge covering the rubber electrode dried out during treatment and caused the burn. Transcranial direct current stimulation subsequently was stopped, and the lesion healed without intervention within 4 to 5 weeks, resulting in a small scar.
A 20-year-old man who was treated with tDCS (2 mA; 20 minutes) for schizophrenia developed a superficial stripe-shaped burn on the skin over the right orbit after the eighth stimulation because the 5×7-cm rubber electrode (cathode) was not fully covered by the saline-soaked sponge and the skin came into direct contact with the short side of the electrode (Figure 2). Transcranial direct current stimulation was stopped, and the skin lesion healed without intervention within 4 to 5 weeks with no scar.
The main factor associated with thermic skin damage in tES is incorrect application of the electrodes; therefore, a high standard should be applied when soaking sponges and placing and fixing the electrodes but likely is only guaranteed in specialized services and not when utilizing tES at home. Dermatologists may be confronted with an increasing number of burns due to the widespread use of tES for modulating neuropsychiatric disorders but also due to the use of tES as a lifestyle brain tuning instrument.3
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
- Mondino M, Bennabi D, Poulet E, et al. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15:261-275.
- Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database Syst Rev. 2013;11:CD009645.
- Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637-643.
- Palm U, Keeser D, Schiller C, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimul. 2008;1:386-387.
Practice Point
- Cranial skin burns can point to misuse of electrical stimulation.