User login
Study finds spironolactone doesn’t boost breast cancer recurrence
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
in a large retrospective study, Chapman Wei said in a in a virtual meeting held by the George Washington University department of dermatology in Washington. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled due to the COVID-19 pandemic.
Spironolactone is an aldosterone antagonist and heart failure medication that, because of its peripheral antiandrogen effects, is often used off-label to treat female androgenetic hair loss. Although it has been available for nigh on half a century and has a well-established favorable safety profile, with no indication of carcinogenic effects, little is known about its use in treating alopecia in breast cancer survivors on endocrine therapies, where there has been a theoretic possibility that the drug’s antiandrogen effects could promote breast cancer recurrence.
Not so, said Mr. Wei, from George Washington University.
He presented a retrospective, propensity score–matched, case-control study that used the Humana Insurance database. The initial comparison was between 746 women who went on spironolactone after their breast cancer diagnosis versus 28,400 female breast cancer patients who didn’t take the drug. The primary outcome was recurrent breast cancer within 2 years after diagnosis.
“We chose 2 years because most breast cancer relapses occur within that time,” Mr. Wei explained.
In the initial unadjusted between-group comparison, the breast cancer recurrence rate was 16.5% in the spironolactone group, significantly higher than the 12.8% rate in more than 28,000 controls. However, in a comparison between the spironolactone group and 746 controls extensively propensity score–matched for acne, hypertension, hirsutism, smoking, illicit drug use, heart failure, primary aldosteronism, and other potential confounding variables, there was no significant difference between spironolactone users and controls, with 2-year breast cancer recurrence rates of 16.5% and 15.8%, respectively.
In a multivariate Cox regression analysis, the stand-out finding was that alcohol abuse was independently associated with a 2.3-fold increased risk of breast cancer recurrence.
Mr. Wei noted that these findings confirm those in a recent literature review by investigators at Memorial Sloan Kettering Cancer Center in New York who found no increase in estrogen levels with spironolactone and no heightened risk of female breast cancer while on the drug in three studies totaling 49,298 patients.
“Spironolactone has the potential to be used as a relatively safe systemic treatment option for the management of [endocrine therapy–induced alopecia] in female breast cancer patients and survivors on endocrine therapies who respond poorly to monotherapy with topical minoxidil,” the Sloan Kettering researchers declared (Breast Cancer Res Treat. 2019 Feb;174[1]:15-26).
Mr. Wei reported having no financial conflicts regarding his unfunded study.
Dr. Douglas Paauw reflects on practicing in the COVID-19 world
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at imnews@mdedge.com.
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at imnews@mdedge.com.
As we are all facing uncertainties in caring for our patients amid the COVID-19 pandemic, I practice at the University of Washington, Seattle, in an area that initially had the highest prevalence of COVID-19 cases in the United States.
I have never felt better about being a part of the medical profession because of the altruism, compassion, and deep caring I have seen displayed by my colleagues, our nurses, our staff, and our students. I am proud to have worked with all of them while trying to figure out how to practice in this environment.
These times are really difficult and challenging as we face new problems every day. Last week, we had to send our students home, and we switched to phone and telehealth visits to keep our patients and staff safer.
I have had some unanticipated electronic messages from patients during this time. Two of my patients with major medical problems and very dependent on their medications were stranded internationally and running out of medications. I had the family of an incarcerated patient contact me for a letter because that patient was moved to a part of a jail where all patients with upper respiratory infection symptoms were being housed. My patient has severe immunosuppression, and they were requesting an exception for him.
Another of my patients, who has sarcoidosis and is immunosuppressed, informed me that her daughter who lives with her was diagnosed with COVID-19. After 3 days, this patient told me she had become febrile and short of breath. I instructed her patient to go to a hospital, where she was also diagnosed with COVID-19 and was admitted. This patient was discharged within 24 hours, because the utilization review department did not feel she should be in the hospital.
The lack of beds is forcing physicians to frequently make tough decisions like the one made for this patient. This unfortunate reality raises the question of: “How do you manage a patient you are worried about from his or her home?”
In this particular case, I sent my patient an oxygen saturation monitor. We touched base frequently, and I felt okay as long as her saturations on room air were above 90%. So far, she has done okay.
More recently, I received a message from a patient recently diagnosed with Mycobacterium avium complex. I learned that this patient and her disabled husband’s caregiver refused to continue to provide care to them, because my patient had a cough, which began 2 months prior. In this case, a COVID-19 test was done for the explicit purpose of getting the caregiver to return to work.
So how do we face this?
Burnout had been high before this difficult time. But now physicians are being called to care for more and sicker patients without the necessary personal protective gear. Our physicians have demonstrated strength and commitment to patients in their response to this challenge, but they need help from others, including regulators.
I think a first step that needs to be taken is to decrease the volume of documentation physicians are required to make in this time where we are forced to triage to what is most important and drop what isn’t. How is spending so much time documenting instead of seeing the high volumes of patients who need to be seen a good thing? Documentation to the level that Medicare has required isn’t going to work. In fact, it has never been a good thing and is a big driver of burnout.
Our health care system was broken and badly injured before this crisis, and I think now might be a time when positive changes for the future occur. In fact, COVID-19 has resulted in some temporary changes in medicine that I would like to see outlast this outbreak. The telehealth option is now available, for example, and this kind of care is covered much more broadly by Medicare under the 1135 waiver – this has been needed for years. Being able to conduct regular clinic visits via telehealth without the marked restrictions that were previously in place is a big advance. It is currently in place for this emergency only, but this is the time to start pushing hard to make sure this option will be permanent.
I invite you to help me fight for long-term change. Write a letter to the editor of your local newspaper or blog, share your thoughts on social media, and tweet. (I suggest using #documentationordoctors or, although a bit long, #excessivedocumentationcostslives.) This is an unprecedented time in modern medicine. Traumatic times are when the greatest changes occur. Let’s hope for the better.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He frequently contributes Pearl of the Month and Myth of the Month columns to MDedge, and he serves on the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact Dr. Paauw at imnews@mdedge.com.
Due to the COVID-19 pandemic, the AAN urges feds to further expand telehealth benefits
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
On March 17, the Trump administration announced an expansion of telehealth benefits to help stop the spread of COVID-19 and allow more Medicare patients to receive virtual care without having to visit a healthcare center or physician office.
Under the expansion, Medicare will pay for office, hospital, and other visits furnished via telehealth across the country and including in the patient’s home, delivered by a range of providers, such as physicians, nurse practitioners, clinical psychologists, and licensed clinical social workers.
Prior to this waiver, Medicare would only pay for telehealth on a limited basis, such as when the patient receiving the service was in a designated rural area.
However, in a letter to Alex Azar, secretary of the U.S. Department of Health & Human Services (HHS), the AAN says the easing of restrictions on telehealth should be extended beyond Medicare fee-for-service to both Medicare Advantage and Medicaid patients.
Practice changing?
“It is very heartening that the government is stepping up to the plate” and lifting many telemedicine restrictions, Neil Busis, MD, member of the AAN Health Policy Subcommittee, said in an interview.
Dr. Busis, who leads the telemedicine program for the department of neurology at NYU Langone Health in New York, said the global pandemic has “heightened, focused, and sharpened” attention to the need for telehealth services, particularly for neurology.
“By definition, a lot of neurology patients have mobility problems, traveling is a burden, making it difficult to see a neurologist,” he said.
Dr. Busis hopes these waivers in telehealth, made on a temporary and emergency basis, will become permanent once the COVID-19 pandemic has passed.
“What we hope is that the usefulness of various virtual technologies tested in the crucible of this pandemic will stimulate people to think about it once the pandemic is over and not rescind these loosening of restrictions, and that this will be the beginning of a new era for telemedicine,” he said.
The COVID-19 pandemic may be a “catalyst to accelerate the incorporation of non-face-to-face care into our armamentarium,” he added.
“What we have discovered in recent years is non-face-to-face care with enabling communication technologies is as effective in many clinical situations as face-to-face care. Now is the time to really focus on making the virtual experience as good as possible and to make it as available to as many people as possible,” said Dr. Busis.
Reduce regulatory burdens
The AAN also calls on the federal government to urge states to take action to ensure access to telehealth services and allow telehealth companies to provide telehealth technology and education free of charge to providers who don’t currently use telehealth in their practices.
“The AAN notes that doing so may implicate provisions of the Anti-Kickback Statute. We believe during the current emergency that HHS should issue guidance making it clear to providers that accepting free access to telehealth platforms and education does not put them at risk of violating fraud and abuse laws,” the letter, signed by AAN President James Stevens, MD, stated.
The AAN also wants the government to reduce regulatory burdens during this public health emergency to allow physicians more time to focus on patient care. “This is especially true for providers that are self-quarantining or are in a practice that is experiencing staffing shortages due to self-quarantines,” he wrote.
Specifically, the AAN asked the Centers for Medicare & Medicaid Services to extend the March 31 deadline for physicians to submit their data for the Merit-based Incentive Payment System program for calendar year 2019 (and other compliance deadlines) by at least 30 days.
The AAN also calls on the CMS to delay implementation of the Appropriate Use Criteria program by 1 year, saying that many providers will not have the capacity to “meaningfully” participate in the current testing year for the program.
A version of this article originally appeared on Medscape.com.
‘Larger-than-life’ physician Stephen Schwartz dies of COVID-19 at 78
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
Stephen M. Schwartz, MD, PhD, a pioneer in the field of vascular biology and a longtime professor of pathology at the University of Washington, Seattle, died March 17, 2020, after being hospitalized with COVID-19. He was 78.
“This has become all too real,” UW president Ana Mari Cauce said on Facebook, where she described Dr. Schwartz as “larger than life,” and superimposed a photo of him in front of Mount Rainier, according to a report in the Seattle Times.
Dr. Schwartz is “rightfully considered a giant among investigators of the biology of smooth muscle cells and the structure of blood vessels,” Paul Ramsey, MD, CEO of UW Medicine, said in a statement. He will be remembered for his “vigorous advocacy for research and for the field of vascular biology as well as for his many trainees who have gone on to great success as independent investigators in the field of vascular pathobiology,” Dr. Ramsey said.
Dr. Schwartz received a BA in biology from Harvard University in 1963 and an MD from Boston University in 1967. Dr. Schwartz started a residency in the UW department of pathology in 1967 and received his PhD from the institution in 1973. From 1974 to 1979, he was an assistant professor of pathology and became a full professor in 1984.
Dr. Schwartz was also an adjunct professor in the UW departments of bioengineering and medicine, “reflective of his many collaborative relationships with faculty in other departments in our medical school and in the world,” Dr. Ramsey said.
“Dr. Schwartz left a lasting imprint on the UW School of Medicine and the broader scientific community. He will be greatly missed,” he added.
‘A great loss’
Dr. Schwartz chaired numerous national and international meetings in the field of vascular biology. He was the founding chair of the Gordon Research Conference on Vascular Biology and a cofounder and second president of the North American Vascular Biology Organization (NAVBO). He created NAVBO’s flagship summer course, Vasculata.
“The NAVBO community has suffered a great loss,” Bernadette Englert, executive officer for the organization, said in a statement. He will be “sorely missed by generations of vascular biologists and pathologists.”
News of Dr. Schwartz’s passing lit up Twitter. Here are just a few comments:
UW lost to COVID-19 “beloved professor Stephen Schwartz, a pioneer in vascular biology and a larger-than-life scientist. Steve, you will be missed!” Rong Tian, MD, PhD, with the bioengineering department, wrote in a tweet.
“Stephen Schwartz was a giant in vascular biology and a mentor to countless faculty and trainees, including myself. He will be deeply missed,” said Kelly Stevens, PhD, also from the bioengineering department.
A version of this article originally appeared on Medscape.com.
AAP adds specifics to policy on abusive head trauma
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
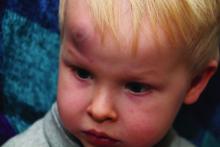
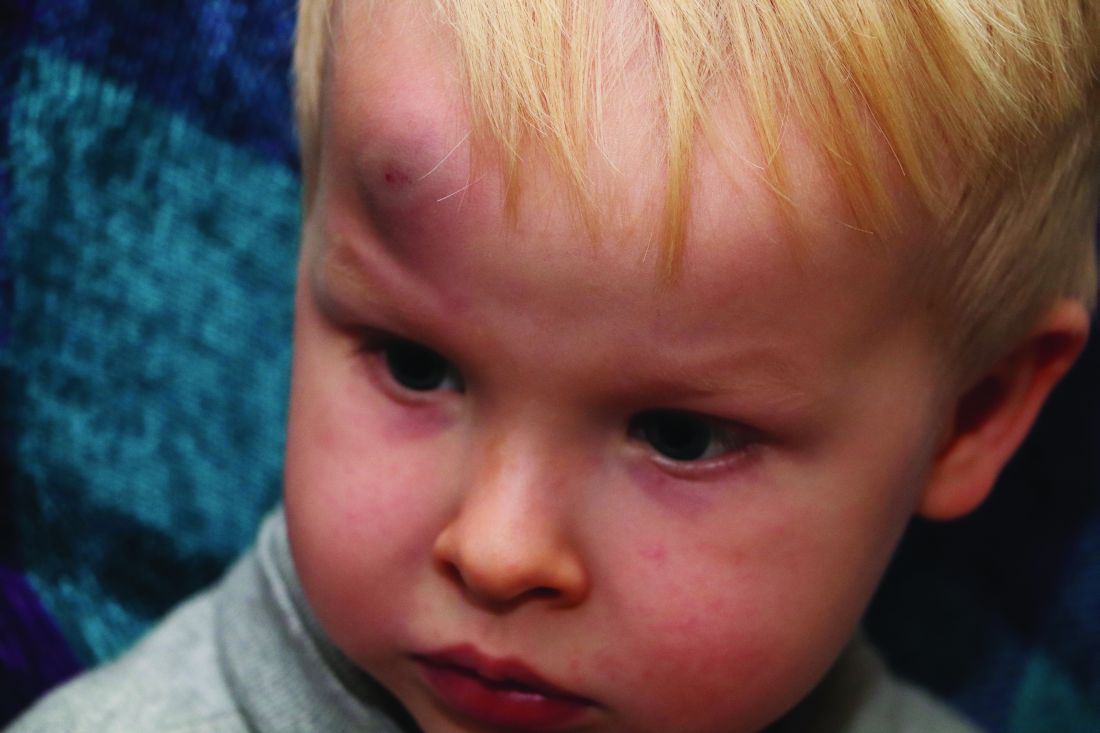
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
the American Academy of Pediatrics said in an updated policy statement.
Abusive head trauma (AHT) is fatal in approximately one-quarter of cases in infants during the first year of life, and less-obvious clinical signs such as vomiting and fussiness often are missed, wrote Sandeep K. Narang, MD, JD, of Northwestern University, Chicago, and colleagues on the AAP Council on Child Abuse and Neglect.
In a policy statement published in Pediatrics, the AAP cautioned physicians to remain vigilant for signs that are common in AHT cases. In particular, bruising on the torso, ears, and neck in children aged younger than 4 years, or any bruising in infants younger than 4 months should be a red flag. In addition, the most recent data indicate that apnea and retinal hemorrhages are more common in cases of abuse than in accidental injuries. The AAP also recommends a skeletal survey in suspected AHT for children younger than 2 years to identify occult fractures.
“Oral injuries in infants, such as frenulum tears, may also accompany or precede AHT,” Dr. Narang and associates said.
In addition, secondary brain injury as a result of AHT can lead to poor outcomes that may be observed. “Almost 70% of survivors of AHT have some degree of lasting neurologic impairment, including static encephalopathy, intellectual disability, cerebral palsy, cortical blindness, seizure disorders, behavior problems, and learning disabilities,” according to the statement.
Endocrine dysfunction also is common in children with a history of AHT, but might not present until years later, the authors noted.
When AHT is suspected in a patient, the policy statement recommends that a subspecialist in child abuse pediatrics or in related areas including radiology, ophthalmology, neurosurgery, neurology, and general pediatric surgery “should also be consulted when necessary to ensure a complete and accurate evaluation.”
Although falls from a height of 1.5 m or 5 feet often are used as an explanation for AHT injuries, “numerous lines of clinical research have clarified the extreme rarity of short falls as a cause of severe neurologic injury or death in young infants,” Dr. Narang and associates wrote.
Other recommendations in the updated policy encourage use of the term “abusive head trauma” in medical communications, as well as encourage caregivers to serve as a medical home for survivors of AHT or refer them to medical homes for rehabilitation and monitoring. Parents and caregivers may need to be educated about the dangers of shaking or striking an infant, shown safe ways to manage a crying baby, and given tools to manage their own stress and frustration.
Physicians are legally required to report suspected cases of child abuse or neglect, and should be prepared to educate stakeholders if you are called on to work with legal and child protective services about the science behind AHT.
“The role of the pediatric practitioner is not to apportion blame or investigate potential criminal activity but to identify the medical problem, evaluate and treat the child’s injuries, and offer honest medical information to parents, families, investigators, and attorneys and/or judges,” Dr. Narang and associates wrote.
This policy statement updates the previous policy statement issued in 2009 and affirmed in 2013. The policy had no external funding, and the authors had no financial conflicts to disclose. Dr. Narang, Amanda Fingarson, DO, and James Lukefahr, MD, have served as paid expert witnesses/consultants in cases of abusive head trauma in infants and children.
SOURCE: Narang SK et al. Pediatrics. 2020 Mar 23. doi: 10.1542/peds.2020-0203.
FROM PEDIATRICS
Treatment options for COVID-19: Dr. Annie Luetkemeyer
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Week-old COVID-19 urology guidelines already outdated
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
Recommendations to help clinicians triage surgical procedures during the COVID-19 pandemic, developed quickly by a team of urology experts from around the world and shared last week, are already out of date.
“I would change some things we said a week ago,” said David Canes, MD, from Lahey Hospital and Medical Center in Burlington, Massachusetts, and Derry, New Hampshire, who was one of those experts.
“We now know it’s not possible to create a cookbook in the face of a rapidly evolving pandemic,” he told Medscape Medical News.
“It’s heartening that we could do it so fast, but now it’s a snapshot in time, a starting point. People have to have conversations locally, in their community, taking into account where they are in relation to a surge of COVID patients, to make good decisions,” Canes said.
Long-thought-out guidance can no longer come from societies. “As the pace of information changes so rapidly,” Canes said he has changed the way he disseminates information and searches for guidance. “I’m even looking to nontraditional channels, like Twitter.”
As the COVID-19 pandemic evolves, informal discussions on social media are helping specialists make decisions. “Threads about various cancers and how people are handling them are helpful,” he said.
He described, for example, a thoughtful discussion on the use of androgen-deprivation therapy, a hormone therapy that can block the effects of androgens and can slow the growth of prostate cancer. “This is not a standard-of-care treatment,” he said, but now it’s being discussed very seriously to treat patients whose care might get delayed.
A multiple-choice survey was posted on Twitter by Ashish Kamat, MD, MBBS, from the MD Anderson Cancer Center in Houston, asking respondents what they would do for a patient with stage T2 high-grade muscle invasive bladder cancer and normal glomerular filtration during the pandemic.
In less than 20 hours, his post received 290 votes in response.
And when Badar Mian, MD, from the Albany Medical Center in New York, asked 23 urologists whether they would recommend radiotherapy (20 fractions) without any chemotherapy, he quickly got two responses: one yes and one no, with explanations.
People are responding to posts quickly. “With the COVID pandemic, we can’t wait for consensus guidelines from the American Urology Association or European Association of Urology,” Canes said.
One Week Changed Everything
When Canes and his coauthors said last week that prostatectomies should be delayed, they didn’t know the extent to which surgery was going to be halted. “When we wrote this statement, most facilities were still allowing elective surgeries or were just on the cusp of shutting down.”
Today, if you’re in an area where elective surgeries are still allowed or it is early in the crisis, “you might still take a patient with a Gleason 9 and a PSA of 25 and judiciously get the surgery done.”
As of March 23, however, surgery in New York City is entirely off the table. “No cancer surgery is happening anymore,” Canes reported.
The recommendations suggested using “shared decision-making” to guide radiation therapy choices. “But now, bringing a patient in for daily radiation treatment may not even be feasible, with the effort it takes to clean, the consumption of PPEs, etc,” he added.
When the dust settles, there will be a lot of assessment of current decision-making. “We’ll see if there are blips in mortality according to decisions being made,” Canes said.
The bottom line is that “we’re running on a 24-hour news cycle,” he pointed out. “It’s humbling to see how quickly decision-making changes and how nimble we have to be in making these very difficult decisions that we’ve never had to make before.”
For his own patients, Canes said he is doing consultations by phone or video at this point. “My patients have been very gracious; everyone has a general feeling we’re all in this together.”
And so far, “I haven’t had a situation where I thought the patient wasn’t going to survive,” he added.
This article first appeared on Medscape.com.
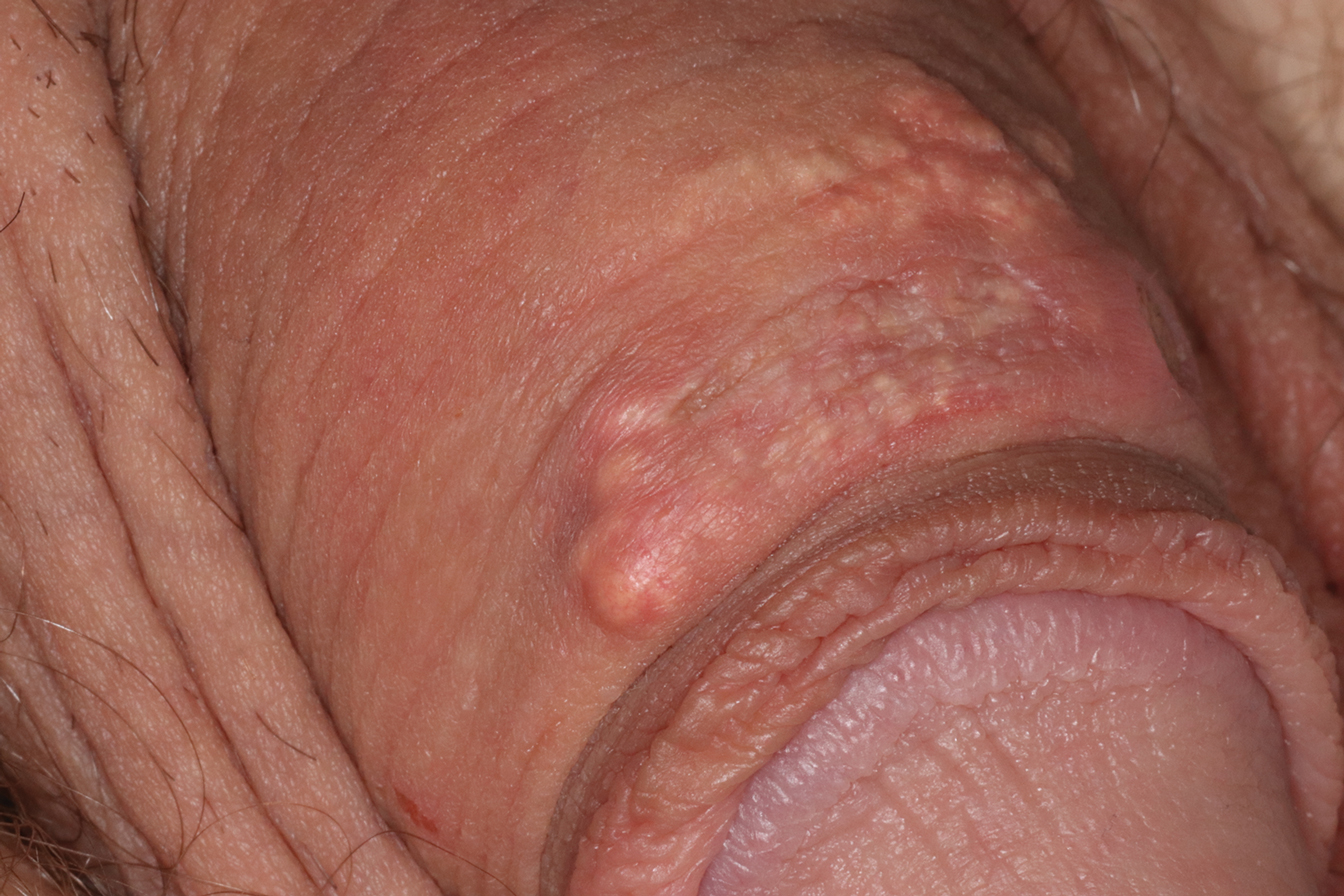
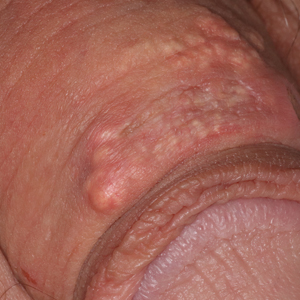
Multinodular Plaque on the Penis
The Diagnosis: Tophaceous Gout
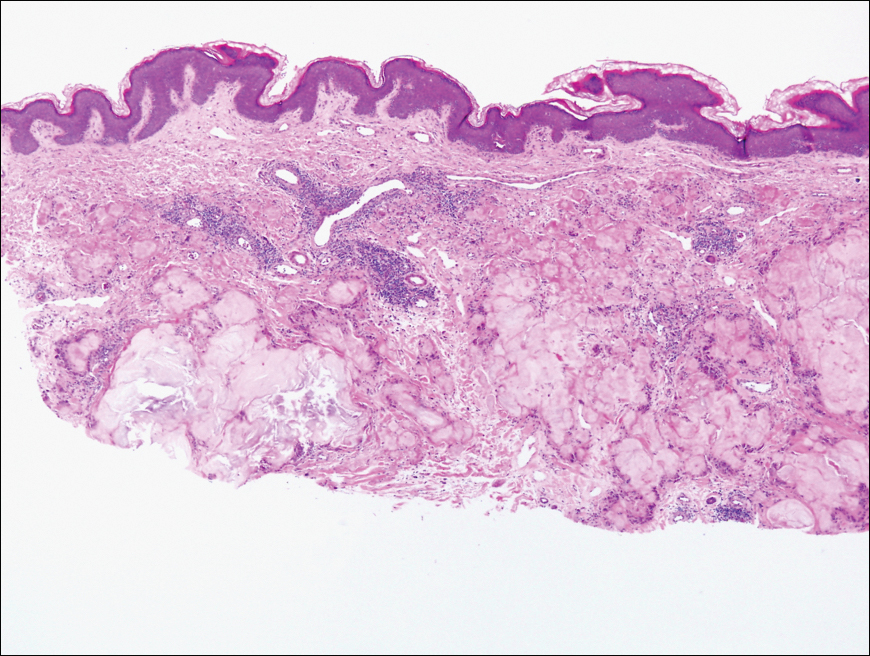
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
The Diagnosis: Tophaceous Gout
Biopsy revealed amorphous pink material within the center of palisading granulomas lined by histiocytes and giant cells. Scattered crystal remnants also were identified within the center of the granulomas; however, the majority of the crystals were dissolved during the formalin processing of the tissue to become the amorphous material. A perivascular mixed inflammatory infiltrate composed of lymphocytes, histiocytes, and plasma cells surrounded the tophi nodules. A biopsy confirmed the diagnosis of tophaceous gout (Figure).
Gout is a systemic metabolic disease characterized by the supersaturation of monosodium urate (MSU) crystals in joints and bursae. Peripheral joints most commonly are affected due to the poor solubility of MSU crystals at low temperatures.1 It is one of the most common forms of inflammatory arthritis, with an estimated prevalence of 4% of adults in the United States.2 An estimated $1 billion is spent each year on ambulatory care for gout.3 Gout occurs most commonly in men and usually manifests in the fifth or sixth decades of life.4 Risk factors for the development of gout include obesity, hypertension, poor dietary habits and kidney function, excessive alcohol intake, and diuretic use.3
Disease manifestations range from asymptomatic hyperuricemia to acute gouty arthritis and chronic tophaceous gout. Patients may present with chronic tophaceous gout without a prior clinically apparent acute gout episode.5,6 Uncontrolled gout may result in large accumulations of MSU crystals, leading to well-circumscribed masses (known as tophi), as demonstrated in our patient.1 Tophi are pathognomonic features of gout and are the sine qua non of advanced gout (also known as chronic tophaceous gout).2 Clinically, these tophi appear as subcutaneous, yellowish white, firm and smooth nodules that are highlighted on the skin.4 Tophi most commonly are found on the helix, articular and periarticular tissue, and the tissue of the hands and feet. They usually are visible on physical examination but also may be detected on imaging studies.2,4
Gouty tophi have been reported in extraordinary locations, such as in sclerae; vocal cords; heart valves; abdominal striae; nerves; axial skeleton4,7; and the penis, as in our patient and one other case.2 These gouty deposits can appear similarly to lipomas, rheumatoid and osteoarthritic nodules, and infectious and malignant processes.1,5 When tophi present in unusual locations, tissue biopsy often is necessary to confirm the diagnosis. Tissue preservation in alcohol is required to preserve the urate crystals. Microscopically, urate crystals appear as tightly packed, brown, needle-shaped crystals surrounded by granulomatous inflammation with foreign body giant cells, macrophages, and possibly some fibrosis. When examined under polarized light, the MSU crystals are negatively birefringent. However, when clinical suspicion for gout is low and the tissue is instead formalin fixed, as was performed in our case, the crystals dissolve into fibrillary amorphous deposits within the center of the granulomatous inflammation, which is another characteristic histologic finding in tophaceous gout.8
Management of gout focuses on urate-lowering therapy including lifestyle changes. Lower serum urate levels are associated with a decreased incidence of acute gout attacks and chronic tophaceous gout.2 Urate-lowering drugs often are combined with anti-inflammatory drugs during acute attacks. Lifestyle changes, such as weight loss, exercise, reduced alcohol consumption, high fluid intake, and a low-purine diet also are beneficial.3,4 Although gout cannot be cured, it can be effectively managed, and appropriate treatment can improve quality of life and reduce the risk for permanent joint damage and structural deformities. If medical treatment and lifestyle changes fail to adequately control tophaceous gout or if tophi become symptomatic, surgical removal of tophi is appropriate.4
At follow-up, our patient opted for surgical removal of the penile tophi. Using local anesthesia, surgical debulking via curettage was performed. Open defects were closed with fine absorbable sutures, and prophylactic antibiotics were given. Allopurinol also was started. Six weeks following extraction, the patient reported no complications and the area was continuing to heal.
Tophaceous gout would be distinguished from conditions in the differential diagnosis based on histologic findings from hematoxylin and eosin (H&E)-stained sections. Actinomycotic mycetoma is rare in the United States and is characterized by a seropurulent or stringy exudate with grains, ulcerations, melicerous scabs, and retractable scarring.9 On H&E-stained sections, actinomyces appear filamentous with deeply basophilic staining and radially oriented acidophilic projections.10 Calcinosis cutis of the penis has been reported to appear as asymptomatic papules; however, microscopic sections reveal deeply basophilic calcium deposits within the tissue.11 Multinodular syphilis shows characteristic histology with lichenoid or vacuolar interface dermatitis, slender acanthosis, plasma cells, and endothelial swelling of the small vessels. A Treponema pallidum immunoperoxidase stain shows numerous organisms. Planar xanthoma shows xanthomatous or foamy histiocytes throughout the dermis on H&E-stained sections.12
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
- Ragab G, Elshahaly M, Bardin T. Gout: an old disease in new perspective--a review. J Adv Res. 2007;8:495-511.
- Flores Martín JF, Vázquez Alonso F, Puche Sanz I, et al. Gouty tophi in the penis: a case report and review of the literature. Case Rep Urol. 2012;2012:594905.
- Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:58-68.
- Forbess LJ, Fields TR. The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum. 2012;42:146-154.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431-1446.
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447-1461.
- Gaviria JL, Ortega VG, Gaona J. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Patterson JW, Hosler GA, Weedon D. Weedon's Skin Pathology. Edinburgh, Scotland: Churchill Livingstone/Elsevier; 2016.
- Guerra-Leal JD, Medrano-Danés LA, Montemayor-Martinez A, et al. The importance of diagnostic imaging of mycetoma in the foot [published online December 18, 2018]. Int J Dermatol. 2019;58:600-604.
- Fazeli MS, Bateni H. Actinomycosis: a rare soft tissue infection. Dermatol Online J. 2005;11:18.
- Cohen PR, Tschen JA. Idiopathic calcinosis cutis of the penis. J Clin Aesthet Dermatol. 2012;5:23-30.
- Ko C, Elston DM, Ferringer T. Dermatopathology. 3rd ed. Philadelphia, PA: Elsevier; 2019.
A 34-year-old man presented for evaluation of a slowly growing group of firm white bumps on the penis. The lesions were nontender and asymptomatic. Medical and family history was notable for gout, though he was not being treated. Physical examination revealed a 3-cm, firm, multinodular, chalky white plaque on the dorsal aspect of the penile shaft. A tangential biopsy was performed and sent for hematoxylin and eosin staining.
FDA to allow alternative respiratory devices to treat COVID-19
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
“Whenever possible, health care facilities should use FDA-cleared conventional/standard full-featured ventilators when necessary to support patients with respiratory failure, or a device subject to an Emergency Use Authorization (EUA), if any,” FDA stated in a guidance document issued March 22.
“However, to help ensure the availability of the greatest possible number of devices for this purpose, ... FDA does not intend to object to limited modifications to indications, claims, functionality, or to the hardware, software, or materials of FDA-cleared devices used to support patients with respiratory failure or respiratory insufficiency, without prior submission of a premarket notification” for the duration of the declared national emergency related to the COVID-19 pandemic.
FDA Commissioner Stephen Hahn, MD, said in a statement that the agency is doing everything it can to support patients, health care professionals, and others during this pandemic.
“One of the most impactful steps we can take is to help with access and availability to life-saving medical treatments,” he said. “Our policy issued today demonstrates our ability to react and adapt quickly during this pandemic and help very ill patients access the lifesaving ventilator support they need. To do that, we are providing maximum regulatory flexibility to facilitate an increase in ventilator inventory, while still providing crucial FDA oversight. We believe this action will immediately increase ventilator availability.”
The document identified examples of where modifications would not create undue risk, including the use of powered emergency ventilators and anesthesia gas machines for patients needing mechanical ventilation; the use of ventilators outside of their cleared environment; the use of devices used to treat patients with sleep apnea, such as CPAPs and BiPAPs, to treat respiratory insufficiency when appropriate design mitigations are in place to minimize aerosolization; and the use of oxygen concentrators for primary supply when medically necessary and clinically appropriate.
The agency also is allowing for changes to the hardware, software, and materials to FDA-cleared ventilators and anesthesia gas machines, such as modifications to motors, batteries, or other electrical components; material changes to components in the gas pathways or with other patient tissue contact; the introduction of filtration to minimize aerosolization; and other hardware and software modifications.
FDA is also allowing for products to be used past their indicated shelf life.
Live zoster vaccine confers limited protection during tofacitinib therapy
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
FROM ANNALS OF THE RHEUMATIC DISEASES