User login
Get out the inpatient vote
Disenfranchisement undeniably remains a major problem across the United States. While it is challenging for health care providers to find time to vote, hospitalized patients are an underrecognized vulnerable group, often unable to exercise this constitutional right. With the 2020 election approaching, voting is as important as ever.
On morning rounds after the 2018 election, we discussed the impact of a changing majority in the House of Representatives and its potential impact on health care in America. We discussed where, when, and how we voted, and then suddenly considered a question that we were unable to answer: How do our hospitalized patients vote and did any of them vote in this important election?
Inpatients rarely know when or how long they will be hospitalized. They often have no chance to prepare by paying bills, arranging care for loved ones, or finding coverage for employment responsibilities. The sickest patients can do little more than wonder about anything other than their short-term health. As a result of restricted voting laws, they, like too many others, are effectively disenfranchised.
We asked administrators in multiple hospitals across New York City how to help our patients vote. Unfortunately, the process is overwhelmingly complex and varies by state. Absentee ballots, which are easily accessible in New York if it they are requested no later than 7 days before the election, are harder to come by on the same day. Most people struggle to vote in general – with only 61% voting in the 2016 election.1 To combat this, individual hospitals have created initiatives such as Penn Votes, which has helped 65 hospitalized Pennsylvania residents vote in the last three elections2 – a success, but still leaving so many without a voice.
With health care being a major policy issue for the 2020 election, voting has never been more important for patients. With nearly 1 million hospital beds in America,3 hospitalized patients represent a significant number of potential voters who are functionally disenfranchised. Most importantly, these patients are directly under our care, and we are their strongest advocates. Therefore, we ask our fellow health care providers to start planning today how we will help our patients exercise their voices, participate in our health care policy debate, and choose the future leaders of our country.
Dr. Rosenblatt is assistant professor of medicine, Division of Gastroenterology and Hepatology, at Weill Cornell Medicine, New York. Dr. Verna is assistant professor of medicine, Department of Surgery, at Columbia University Irving Medical School, New York. Dr. Rosenblatt and Dr. Verna reported having no relevant conflicts of interest.
References
1. File T. Voting in America: A Look at the 2016 Presidential Election [Internet]. 2017 [cited 2020 Jan 7];Available from: https://www.census.gov/newsroom/blogs/random-samplings/2017/05/voting_in_america.html.
2. Vigodner S. Penn students are helping hospitalized patients cast emergency ballots for Tuesday’s election [Internet]. Dly. Pennsylvanian. 2018;Available from: https://www.thedp.com/article/2018/11/penn-med-votes-emergency-hospital-patients-upenn-philadelphia-elections.
3. Association AH. Fast facts on US hospitals [Internet]. 2019 [cited 2020 Jan 7];Available from: https://www.aha.org/statistics/fast-facts-us-hospitals.
Disenfranchisement undeniably remains a major problem across the United States. While it is challenging for health care providers to find time to vote, hospitalized patients are an underrecognized vulnerable group, often unable to exercise this constitutional right. With the 2020 election approaching, voting is as important as ever.
On morning rounds after the 2018 election, we discussed the impact of a changing majority in the House of Representatives and its potential impact on health care in America. We discussed where, when, and how we voted, and then suddenly considered a question that we were unable to answer: How do our hospitalized patients vote and did any of them vote in this important election?
Inpatients rarely know when or how long they will be hospitalized. They often have no chance to prepare by paying bills, arranging care for loved ones, or finding coverage for employment responsibilities. The sickest patients can do little more than wonder about anything other than their short-term health. As a result of restricted voting laws, they, like too many others, are effectively disenfranchised.
We asked administrators in multiple hospitals across New York City how to help our patients vote. Unfortunately, the process is overwhelmingly complex and varies by state. Absentee ballots, which are easily accessible in New York if it they are requested no later than 7 days before the election, are harder to come by on the same day. Most people struggle to vote in general – with only 61% voting in the 2016 election.1 To combat this, individual hospitals have created initiatives such as Penn Votes, which has helped 65 hospitalized Pennsylvania residents vote in the last three elections2 – a success, but still leaving so many without a voice.
With health care being a major policy issue for the 2020 election, voting has never been more important for patients. With nearly 1 million hospital beds in America,3 hospitalized patients represent a significant number of potential voters who are functionally disenfranchised. Most importantly, these patients are directly under our care, and we are their strongest advocates. Therefore, we ask our fellow health care providers to start planning today how we will help our patients exercise their voices, participate in our health care policy debate, and choose the future leaders of our country.
Dr. Rosenblatt is assistant professor of medicine, Division of Gastroenterology and Hepatology, at Weill Cornell Medicine, New York. Dr. Verna is assistant professor of medicine, Department of Surgery, at Columbia University Irving Medical School, New York. Dr. Rosenblatt and Dr. Verna reported having no relevant conflicts of interest.
References
1. File T. Voting in America: A Look at the 2016 Presidential Election [Internet]. 2017 [cited 2020 Jan 7];Available from: https://www.census.gov/newsroom/blogs/random-samplings/2017/05/voting_in_america.html.
2. Vigodner S. Penn students are helping hospitalized patients cast emergency ballots for Tuesday’s election [Internet]. Dly. Pennsylvanian. 2018;Available from: https://www.thedp.com/article/2018/11/penn-med-votes-emergency-hospital-patients-upenn-philadelphia-elections.
3. Association AH. Fast facts on US hospitals [Internet]. 2019 [cited 2020 Jan 7];Available from: https://www.aha.org/statistics/fast-facts-us-hospitals.
Disenfranchisement undeniably remains a major problem across the United States. While it is challenging for health care providers to find time to vote, hospitalized patients are an underrecognized vulnerable group, often unable to exercise this constitutional right. With the 2020 election approaching, voting is as important as ever.
On morning rounds after the 2018 election, we discussed the impact of a changing majority in the House of Representatives and its potential impact on health care in America. We discussed where, when, and how we voted, and then suddenly considered a question that we were unable to answer: How do our hospitalized patients vote and did any of them vote in this important election?
Inpatients rarely know when or how long they will be hospitalized. They often have no chance to prepare by paying bills, arranging care for loved ones, or finding coverage for employment responsibilities. The sickest patients can do little more than wonder about anything other than their short-term health. As a result of restricted voting laws, they, like too many others, are effectively disenfranchised.
We asked administrators in multiple hospitals across New York City how to help our patients vote. Unfortunately, the process is overwhelmingly complex and varies by state. Absentee ballots, which are easily accessible in New York if it they are requested no later than 7 days before the election, are harder to come by on the same day. Most people struggle to vote in general – with only 61% voting in the 2016 election.1 To combat this, individual hospitals have created initiatives such as Penn Votes, which has helped 65 hospitalized Pennsylvania residents vote in the last three elections2 – a success, but still leaving so many without a voice.
With health care being a major policy issue for the 2020 election, voting has never been more important for patients. With nearly 1 million hospital beds in America,3 hospitalized patients represent a significant number of potential voters who are functionally disenfranchised. Most importantly, these patients are directly under our care, and we are their strongest advocates. Therefore, we ask our fellow health care providers to start planning today how we will help our patients exercise their voices, participate in our health care policy debate, and choose the future leaders of our country.
Dr. Rosenblatt is assistant professor of medicine, Division of Gastroenterology and Hepatology, at Weill Cornell Medicine, New York. Dr. Verna is assistant professor of medicine, Department of Surgery, at Columbia University Irving Medical School, New York. Dr. Rosenblatt and Dr. Verna reported having no relevant conflicts of interest.
References
1. File T. Voting in America: A Look at the 2016 Presidential Election [Internet]. 2017 [cited 2020 Jan 7];Available from: https://www.census.gov/newsroom/blogs/random-samplings/2017/05/voting_in_america.html.
2. Vigodner S. Penn students are helping hospitalized patients cast emergency ballots for Tuesday’s election [Internet]. Dly. Pennsylvanian. 2018;Available from: https://www.thedp.com/article/2018/11/penn-med-votes-emergency-hospital-patients-upenn-philadelphia-elections.
3. Association AH. Fast facts on US hospitals [Internet]. 2019 [cited 2020 Jan 7];Available from: https://www.aha.org/statistics/fast-facts-us-hospitals.
CVH in pregnant women: Ample room for improvement
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Genomic prostate score does not improve risk assessment
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
A genomic prostate score (GPS) has little value in predicting adverse outcomes in men who have undergone a period of active surveillance before having a radical prostatectomy, according to a study published in the Journal of Clinical of Oncology.
The hazard ratio for adverse pathology using the 17-gene Oncotype DX Genomic Prostate Score did not reach statistical significance in a multivariate model (HR, 1.17; P = .066). This model took into account factors such as the prostate-specific antigen density (PSAD) and the Gleason grade group at diagnosis.
“In our study, the independent association of GPS with adverse pathology after initial active surveillance was not statistically significant,” Daniel W. Lin, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues wrote.
There was also no association between the GPS and having upgraded biopsy findings during active surveillance.
Active surveillance is the “preferred management strategy” for men with low-risk prostate cancer, observed Dr. Lin and colleagues, but its use is often tempered by the worry that there may be underlying pathology that is not detected using routine clinical measures such as prostate-specific antigen testing. In their study, the investigators looked to see if using the GPS could help risk-stratify men undergoing active surveillance.
They noted that the biopsy-based genomic test had been shown to predict adverse surgical pathology and recurrence in men with low- and intermediate-risk prostate cancer who had undergone immediate radical prostatectomy. The team therefore wanted to clarify the test’s role in men who had been initially managed with a period of active surveillance.
To calculate the GPS, the investigators retrospectively analyzed diagnostic biopsy samples that had been prospectively collected from 432 men in the Canary Prostate Active Surveillance Study. The primary endpoint was adverse pathology in men who underwent radical prostatectomy after initial surveillance. Adverse pathology was defined as a Gleason grade of 3 or greater, a staging of pT3a or higher (with or without N1), or both.
After a median follow-up of 4.6 years, 167 (39%) men experienced upgrading of their prostate cancer at a surveillance biopsy, with 51 (12%) being upgraded to a Gleason grade group of 3 or higher. A total of 101 (23%) men had radical prostatectomy at a median of 2.1 years after their diagnostic biopsy, and just over half (n = 52; 51%) had adverse pathology at this time point.
GPS was associated with adverse pathology when the diagnostic Gleason grade group was taken into account (HR, 1.18; P = .030) but not when the investigators adjusted for both PSAD and diagnostic Gleason grade group. By contrast, PSAD (HR, 1.75; P = .025) was significantly associated with adverse pathology.
“Adding GPS to a model containing PSAD and diagnostic [Gleason grade group] did not significantly improve stratification of risk for [adverse pathology] over the clinical variables alone,” Dr. Lin and colleagues concluded.
This work was supported by the Canary Foundation, the Department of Defense, the National Institutes of Health, and Genomic Health. The authors disclosed relationships with Genomic Health and other companies.
SOURCE: Lin DW et al. J Clin Oncol. 2020 Mar 4. doi: 10.1200/JCO.19.02267.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Study: Delays filling biologic prescriptions have consequences
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
Insurance and specialty pharmacy delays in authorizing new biologic prescriptions for severe allergies leave waiting patients at risk of asthma attacks, hospitalizations, emergency department visits and prednisone shots and their known side effects, according to a single-center study that was to have been presented at the annual meeting of the American Academy of Allergy, Asthma and Immunology.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The study of 80 patients in State College, Pa., found that they waited an average of 44 days from when their doctor submitted the preauthorization request to the insurance company until the practice received the shipment for dispensing to the patient, investigator Faoud Ishmael, MD, PhD, of Mount Nittany Medical Group said in an interview. “The implication here is that these are really the most severe patients who, you would argue, need their medications the quickest, and it’s taking longer to get them than it would an inhaler,” Dr. Ishmael said.
The study focused on patients with severe asthma (n = 60) or urticarial (n = 20) who received a new prescription of monoclonal antibody therapy from March 2014 to August 2019. For asthma treatments, the average time was 45.8 days; for urticaria, 40.6 days (P = .573), Dr. Ishmael said. The researchers divided the total amount of time into two components: insurance plan review and approval (P = .654, and specialty pharmacy review and dispensing of the medicine, each of which averaged 22.8 days (P = .384), he said.
He also noted wide disparity in the range of approval times. “The shortest approval time was 1 day, and the longest 97 days,” Dr. Ishmael said. “It’s interesting that we had this really broad spread.”
What’s more, the study found no trend for the delays among insurers and specialty pharmacies, Dr. Ishmael added. “When these prescriptions get submitted, it’s like a black box,” he said. “It really seems arbitrary why some of them take so long and some of them don’t.” The findings were independent of type of coverage, whether commercial or government, or even specific insurance plans. “It’s more the process that is flawed rather than one insurance company being the bad guy,” he said.
The study also looked at what happened to patients while they were waiting for their prescriptions to be delivered. “What we found is that over half of asthmatics had an exacerbation – 51% had at least one asthma attack where they needed prednisone,” Dr. Ishmael said (P = .0015), “and we had three patients admitted to the hospital over that time frame when they were waiting for the drugs.” One of those patients had been admitted twice, making four total hospitalizations. Preliminary data analysis showed that about 40% of the patients who had attacks went to the emergency department.
For asthmatics who needed prednisone, the average dose was 480 mg (P = .284) – “a pretty substantial number,” in Dr. Ishmael’s words. He noted that a large portion of the study patients were obese, with a mean body mass index of 33 kg/m2. Other comorbidities prevalent in the study population were hypertension and type 2 diabetes. “Prednisone is something that could worsen all of those conditions, so it’s not a trivial issue,” he said.
The study, however, didn’t evaluate costs of the interventions during the delay period vs. the costs of the medications themselves. Of the 80 prescriptions Dr. Ishmael and coauthors submitted, only one was rejected, that person being a smoker, he said. “I understand these are expensive medicines, but it’s counterproductive to delay them because in the long run the insurance company ends up paying for the hospitalization and the drug rather than just the drug,” he said.
Timothy Craig, DO, of Penn State Health Allergy, Asthma, and Immunology and professor of medicine and pediatrics at Penn State College of Medicine, both in Hershey, said he was surprised at the brevity of the delays reported in Dr. Ishmael’s study. “They do much better than we do with preauthorization,” he said, noting that, in his experience, these approvals take much longer. He added that his own research has found faulty insurance plan algorithms are at the heart of these delays. “We need more studies to clarify how much this is interfering with patient care and how much risk they’re putting patients in,” he said.
The COVID-19 pandemic poses a double-edged sword for physicians managing patients with severe asthma, Dr. Craig noted. “Their asthma care is important, especially if they do test for COVID-19,” he said. On the other hand, doctors and nurses attending to COVID-19 patients will have less time to haggle with payers to expedite coverage for biologics for their severe asthma patients, he said. “I hope the flexibility is there, especially at this time to allow people to get on the biologics and stay on them,” he said.
Dr. Ishmael said these findings have serious implications because biologics are getting prescribed ever more frequently for asthma and hives. Steps his practice has taken to streamline the process include following the payer’s approval guidelines as closely as possible. This sometimes can mean making sure a patient with severe asthma has been maximized on controller medications before submitting the biologic prescription, he said. Another step is to use drug company programs to remove barriers to coverage.
Nonetheless, the approval process can be daunting even when taking those steps, he said. “Those guidelines that constitute approval may vary a lot from one insurer to another; and sometimes those guidelines are different from the criteria that studies may have used when these drugs were being evaluated in clinical trials,” he said. It would be helpful, he said, if payers used the National Heart, Lung and Blood institute and the Global Initiative for Asthma guidelines for biologics.
One of the goals of the researchers is to present their findings to payers, “to let them know, here are some of the hang-ups and the real risks associated with delaying these medications,” Dr. Ishmael said.
“When specialists especially prescribe these therapies, there’s usually a valid reason,” he said. “We really need to do something about the current process – if there are ways to make it more transparent, faster.”
Dr. Ishmael has no relevant financial relationships to disclose.
SOURCE: Ishmael F et al. AAAAI 2020. Session 3609, Presentation 558.
REPORTING FROM AAAAI
How long is it safe to delay gynecologic cancer surgery?
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at obnews@mdedge.com.
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
Liver cancer increase driven mainly by NASH in men over 60
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.
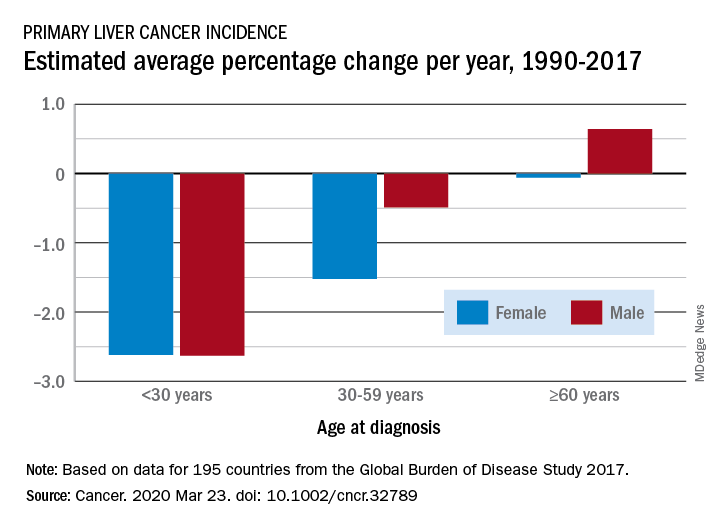
The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Liver cancer rates have been increasing, but a new analysis finds that the increase has occurred primarily in men older than 60 years in developed countries.

The findings come from an analysis of data from the Global Burden of Disease (GBD) Study 2017, published online March 23 in Cancer.
From 1990 to 2017, the number of cancer cases increased nearly threefold in older men and more than twofold in older women (aged 60 years or more). This increase was driven mainly by an increase in liver cancer caused by nonalcoholic steatohepatitis (NASH), also termed fatty liver disease, note the authors.
In contrast, the incidence of liver cancer among men and women who are younger than 30 years and those aged 30 to 59 years declined during this period.
The decreases seen in younger adults were largely ascribed to hepatitis B virus (HBV) vaccination and were consistent in most regions except in developed countries, where liver cancer rates increased irrespective of sex and age.
“Our findings suggest a lack of attention for older people in current liver cancer prevention efforts and highlight the emerging concern of obesity as a risk factor for liver cancer,” lead author Xingdong Chen, MD, PhD, Fudan University, China, said in a statement.
“Liver cancer prevention strategies in both developing and developed countries should be tailored and updated,” he added.
The authors point out that liver cancer was previously considered to be rare in the Western hemisphere.
“However, we found a significant increase in primary liver cancer incidence – regardless of etiology, sex, or age – in most of these countries over the last few decades,” they observe.
The fact that the most pronounced increase in liver cancer was caused by NASH suggests that more attention should be paid to weight management and obesity control as primary prevention strategies in these regions, they suggest.
Study design
Annual incidence data were collected from 1990 to 2017 and were categorized by sex, region, country, age group, and etiology.
“Data from a total of 195 countries and territories were available,” the investigators note, “and these countries and territories were categorized into 5 regions in terms of sociodemographic index (SDI),” they add.
Data were also retrieved regarding five etiologies of liver cancer: HBV infection, hepatitis C virus (HCV) infection, alcohol use, NASH, and others.
The authors note that age-standardized incidence rates of primary liver cancer caused by those five etiologies increased significantly in Australasia, Western Europe, and high-income regions of North America. The most significant increase was found in liver cancer caused by NASH in the Netherlands (in men) and in Finland (in women).
An increasing trend was observed in most countries for primary liver cancer among people aged 60 years or older, the authors note. They suggest that population expansion, aging, and increasing prevalence of obesity and diabetes might partly explain the marked increase, especially the dramatic increase in the number of cases among older people. Additionally, the “lag effect” of the large HBV infection reservoir in several countries might also contribute to the increase, the authors state. They explain that people infected with HBV early in life may experience progression to liver cancer as they age.
Primary prevention
Prevention of HBV infection – the primary cause of liver cancer – has been possible since the introduction of the HBV vaccine in 1982.
“By the end of 2017, 187 countries had introduced the HBV vaccine into their national immunization schedules, with global coverage with 3 doses of the hepatitis B vaccine ... estimated at 84%,” the authors point out.
This has “dramatically” reduced both the prevalence of HBV infection and the incidence of liver cancer caused by it among younger people in high-risk countries, they comment.
The investigators also observed a significant decrease in the incidence of liver cancer caused by HBV infection in people aged 30 to 59 years, although the decline was smaller than it was for those younger than 30.
Moreover, HCV infection has emerged as a concerning cause of liver cancer among those who used to be at low risk for HCV infection.
Although there is optimism that global control of HCV infection can be achieved through direct-acting antiviral agents, “the high cost, drug resistance, and reinfection rates are still major obstacles to fulfilling this ambitious goal,” Chen and colleagues point out.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sleep-disordered breathing linked with Alzheimer’s disease biomarkers in cognitively normal older adults
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
investigators have found.
Among 127 adults enrolled in a randomized clinical trial of interventions to promote mental well-being in older adults, those with sleep-disordered breathing had significantly greater amyloid burden and gray-matter volume, as well as increased perfusion and metabolism in parietal-occipital regions, reported Claire André, PhD, from the French Institute of Health and Medical Research (INSERM) unit in Caen, and colleagues.
“Our findings highlight the need to treat sleep disorders in the older population, even in the absence of cognitive or behavioral manifestations,” they wrote in a study published in JAMA Neurology.
Previous studies of the possible association between sleep-disordered breathing and dementia risk have shown conflicting or inconsistent results, the authors noted.
“These discrepancies may be explained by the characteristics of patients with sleep-disordered breathing (e.g., recruited from sleep clinics versus from the community, differences in age and disease duration), the scoring criteria of respiratory events, sample sizes, or the lack of controls for possibly biasing covariates,” they wrote.
To see whether they could clear up the confusion, the investigators conducted a retrospective analysis of 127 patients who were enrolled in the Age-Well randomized, controlled trial of the Medit-Ageing European project. The participants were community-dwelling adults (mean age, 69.1 years; 63% women), who were enrolled in the trial and underwent evaluation from 2016 to 2018 at the Cyceron Cancer Center in Caen.
The participants, all of whom were cognitively unimpaired at baseline, underwent neuropsychological assessment, polysomnography, MRI, plus florbetapir- and fluorodeoxyglucose-labeled PET.
The investigators defined sleep-disordered breathing as 15 apnea-hypopnea index events per hour or higher, and compared results between those with sleep-disordered breathing and those without for each imaging modality.
Participants with sleep-disordered breathing has significantly greater amyloid burden (P = .04), gray-matter volume (P = .04), perfusion (P = .04), and metabolism (P = .001), primarily overlapping the posterior cingulate cortex and precuneus, areas known to be significantly involved in Alzheimer’s disease.
When the investigators looked for behavioral and cognitive correlates of sleep-disordered breathing severity with associated brain changes, however, they found no associations with either cognitive performance, self-reported cognitive or sleep difficulties, or symptoms of daytime sleepiness.
“Importantly, to the best of our knowledge, our results show in vivo for the first time that greater amyloid burden colocalizes with greater gray-matter volume, perfusion, and metabolism in older participants with sleep-disordered breathing who are cognitively unimpaired. We believe that these overlapping patterns reinforce the likelihood of common underlying mechanisms,” they wrote.
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is funded through the European Union’s Horizon 2020 Research and Innovation Program, INSERM, and Fondation d’ Entreprise MMA des Entrepreneurs du Futur. Dr. André reported no conflicts of interest to disclose.
SOURCE: André C et al. JAMA Neurol. 2020 Mar 23. doi: 10.1001/jamaneurol.2020.0311.
FROM JAMA NEUROLOGY
Intracranial artery stenting shows promising 1-year results
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
LOS ANGELES –
One-year after 129 patients received an intracranial stent that’s already on the U.S. market for treating severe atherosclerotic disease following an ischemic stroke, their incidence of death or repeat stroke was 9%, low enough to suggest benefit compared with historic control patients who were medically managed and had a 12% 1-year rate of death or stroke. This signal of incremental benefit from intracranial stenting may help spark renewed interest in an intervention that was largely forsaken in recent years because of safety concerns.
Assessing restenosis frequency
“Stenting seemed to confer some protection against severe or fatal strokes” in a study that provided “the largest 1-year follow-up of stenting” for intracranial atherosclerotic disease, the trigger for roughly 10% of all U.S. stroke cases, Michael J. Alexander, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
What 1-year follow-up highlighted was the restenosis frequency, based on imaging follow-up for 107 of these 129 patients who had received a Wingspan nitinol, self-expanding stent. Seven patients developed symptomatic restenosis in the region of stent placement, and another 11 patients had asymptomatic restenosis that occluded at least 70% of the stented artery, a total 1-year restenosis rate of 18/107 (17%), reported Dr. Alexander, professor of neurosurgery and director of the Neurovascular Center at Cedars-Sinai Medical Center in Los Angeles. The mean time to detection of restenosis was 5 months, with a range of 1-11 months.
Intracranial stenting falls out of favor
The tested Wingspan stent first received Food and Drug Administration approval for intracranial artery placement in 2005, and then in August 2012 the agency tightened the labeled indication to a much smaller, more specifically defined group of patients: those 22-80 years old, with 70%-99% stenosis in a cerebral artery, with a history of at least two strokes, with stent placement timed more than 7 days following the most recent stroke, and refractory to medical therapy. This 2012 label change came in response to a concerning rate of periprocedural complications in patients who received intracrania artery stents as part of a study reported in 2011 that included many patients with clinical characteristics that fell outside the limits the agency later set in 2012. Results from the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial showed that among 451 randomized patients the 224 assigned to stenting had a 15% 30-day rate of death or stroke, compared with a 6% rate among control patients who did not receive a stent, a statistically significant difference that led to early stopping of the trial (New Engl J Med. 2011 Sep 15;365[11]:993-1003). At least half of the patients who received a stent in SAMMPRIS were 7 or fewer days out from their index event, a majority had either a single prior stroke or transient ischemic attack as their index event, and many had not been established as refractory to medical management.
The SAMMPRIS results and the subsequent relabeling of the Wingspan stent by the FDA had two consequences. First came a steep drop in the use of intracranial stenting. After SAMMPRIS, vascular neurologists “abandoned the stent; no one does intracranial stenting” today, commented Louise D. McCullough, MD, professor and chair of neurology at the University of Texas Health Science Center at Houston. The second consequence was an FDA mandate to run a new randomized study to reassess the periprocedural complications when clinicians placed the Wingspan intracranial stent in patients who fully matched the revised 2012 labeling.
That mandated study was the WEAVE (Post Market Surveillance Study of the Wingspan Stent System) trial, which enrolled 152 patients at 24 U.S. sites in a single-arm study, and found a 2.6% rate of death or stroke during the 30 days following intervention that beat the 4% benchmark rate prespecified in the trial’s design (Stroke. 2019 Apr;50[4]:889-94). The WEAVE findings provided even more evidence of the need for the tight labeling the device received in 2012. A safety communication from the FDA in April 2019 noted that an additional 46 patients received an intracranial artery stent during WEAVE despite falling outside the 2012 labeled indications, and this off-label group had a 24% incidence of periprocedural complications, compared with the 2.6% rate in the on-label group. The FDA’s statement reaffirmed the labeling restrictions and highlighted additional cautions and recommendations for using the device.
The WOVEN study
The 1-year follow-up of the WEAVE patients, an extension called the WOVEN (Wingspan One Year Vascular Imaging Events and Neurologic Outcomes) study, was investigator initiated with no commercial funding and included 129 of the original on-label patients (85%) at 15 of the original 24 participating centers.
In addition to the data collected in WOVEN on restenosis rates, follow-up tallied seven patients with a stroke in the vascular territory of the stent during the period that began 30 days after the procedure (when the WEAVE follow-up finished) and continued through 12 months, with no neurologic deaths. When combined with the 4 periprocedural events that occurred during WEAVE, the final WOVEN tally was 11 total events in 129 patients followed for 1 year (9%). Because WEAVE and WOVEN included no control patients, Dr. Alexander compared this 1-year incidence rate with the 12% rate among medically managed control patients in SAMMPRIS.
According to Dr. Alexander, the next step in the path to rehabilitating a clinical role for intracranial stenting is a new randomized study that compares stenting used exclusively to the 2012 labeling with medical management in high-risk patients, those with hemodynamic compromise.
Encouraging data, but is it compelling?
“There may be a benefit” from intracranial stenting, but “we need a larger trial to convince people” said Dr. McCullough. The WEAVE and new WOVEN findings provide a “signal that stenting may be better than medical therapy, but this was only in just over 100 patients. We’ll need a larger study,” she said in an interview. The findings also reinforced that restenosis remains a challenge for intracranial artery stenting.
“Intracranial atherosclerosis is very difficult to treat, and we need new strategies for these patients.” The WEAVE and WOVEN results “suggest that while the restenosis rate may be high, it may also be manageable.” Delaying stent placement to no sooner than 8 days after a stroke may be a key step for improving safety, but new approaches are also need to minimize the restenosis risk, Dr. McCullough noted.
WEAVE was sponsored by Stryker Neurovascular, the company that markets the Wingspan intracranial artery stent. WOVEN received no commercial funding. Dr. Alexander has been a consultant to Stryker Neurovascular. Dr. McCullough had no disclosures.
SOURCE: Alexander MJ et al. International Stroke Conference, Abstract LB4.
REPORTING FROM ISC 2020
AMA offers resources for front-line physicians
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
.
The literature include news, advocacy, and other information to help front-line physicians provide care to patients and keep themselves safe “in a rapidly changing environment,” the organization said in a statement.
“The AMA continues to forcefully advocate for [personal protective equipment] and critical policy and regulatory changes needed to address our public health and health system needs. Because so many of the challenges of the pandemic are felt at a practice level, we are also providing new tools and information to help physicians respond,” AMA President Patrice A. Harris, MD, said in the statement.
The COVID-19 physician and practice resources released by the AMA include:
- A Physicians Guide to COVID-19 .
- An AMA COVID-19 online resource center and a COVID-19 FAQ.
- A Quick Guide to Telemedicine in Practice.
- Ethical guidance for physicians .
- Evidence-based resources from the The JAMA Network COVID-19 Resource Center.
- CME for physicians through the JAMA Network’s JN Learning website.
Hand washing and hand sanitizer on the skin and COVID-19 infection risk
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.
As we deal with the effects of the COVID-19 pandemic, hand washing and the use of hand sanitizers have been key for infection prevention. With drier, colder weather in many of the communities initially affected by COVID-19, skin was already prone to dryness and a skin barrier compromised, and hand eczema was more prevalent because of these factors alone. This article explores the while maintaining the maximum possible degree of infection prevention.
With many viruses, including coronavirus, the virus is a self-assembled nanoparticle in which the most vulnerable structure is the outer lipid bilayer. Soaps dissolve the lipid membrane and the virus breaks apart, inactivating it; they are also alkaline surfactants that pick up particles – including dirt, bacteria, and viruses – which are removed from the surface of the skin when the soaps are rinsed off. In the process of washing, the alkalinity of the soap (pH approximately 9-10), compared with the normal outer skin pH of approximately 5.5 or lower, also can affect the skin barrier as well as the resident skin microflora. In a study by Lambers et al., it was found that an acid skin pH (4-4.5) keeps the resident bacterial flora attached to the skin, whereas an alkaline pH (8-9) promotes the dispersal from the skin in assessments of the volar forearm.
With regard to the effectiveness of hand washing against viruses, the length of time spent hand washing has been shown to have an impact on influenza-like illness. In a recent study of 2,082 participants by Bin Abdulrahman et al., those who spent only 5-10 seconds hand washing with soap and hand rubbing were at a higher risk of more frequent influenza-like illness (odds ratio, 1.37; 95% confidence interval, 1.08-1.75), compared with those who washed their hands for 15 seconds or longer. Moreover, hand washing with soap and rubbing after shaking hands was found to be an independent protective factor against frequent influenza-like illness (adjusted OR, 0.59; 95% confidence interval, 0.37-0.94). Previous studies on the impact of hand washing on bacterial and parasitic illnesses also found similar results: Hand washing for 15-20 seconds or longer reduces infection.
Alcohol, long known as a disinfectant, has been recommended for disinfecting the hands since the late 1800s. Most alcohol-based hand antiseptics contain isopropanol, ethanol, N-propanol, or a combination of two of these products. The antimicrobial activity of alcohols can be attributed to their ability to denature and coagulate proteins, thereby lysing microorganisms’ cells, and disrupting their cellular metabolism. Alcohol solutions containing 60%-95% alcohol are the most effective. Notably, very high concentrations of alcohol are less potent because less water is found in higher concentrations of alcohol and proteins are not denatured easily in the absence of water. Alcohol-based hand sanitizers also often contain humectants, such as glycerin and/or aloe vera, to help prevent skin dryness and replace water content that is stripped by the use of alcohol on the skin surface.
Other topical disinfectants can also be used to inactivate coronaviruses from surfaces, including the skin. A recently published analysis of 22 studies found that human coronaviruses – such as severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, or endemic human coronaviruses (HCoV) – can persist on inanimate surfaces such as metal, glass, or plastic for up to 9 days (COVID-19 was found in a study to persist on metal for up to 2-3 days), but can be efficiently inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate, are less effective.
In the case of SARS, treatment of SARS-CoV with povidone-iodine products for 2 minutes reduced virus infectivity to below the detectable level, equivalent to the effect of ethanol, in one study. Formalin fixation of the infected cells and heating the virus to 56° C, as used in routine tissue processing, were found to inactivate several coronaviruses as well. Based on this information, ethanol-based hand sanitizers, typically containing ethanol content of 60% or higher, can be used to inactivate coronaviruses on the skin, including COVID-19.
In patients with influenza-virus infections, whether pathogens were in wet or dried mucus played a role in whether hand washing or rubbing with hand sanitizer was more effective. In a study that examined the effects of hand washing versus antiseptic hand rubbing with an ethanol-based hand disinfectant on inactivation of influenza A virus adhered to the hands, the investigators showed that the effectiveness of the ethanol-based disinfectant against influenza A virus in mucus was reduced, compared with influenza A virus in saline. Influenza A in mucus remained active, despite 120 seconds of hand rubbing with hand sanitizer; however, influenza A in saline was completely inactivated within 30 seconds. Interestingly, rubbing hands with an ethanol-based disinfectant inactivated influenza A virus in mucus within 30 seconds with mucus that had dried completely because the hydrogel characteristics had been eliminated. Hand washing rapidly inactivated influenza A virus whether in mucus form, saline, or dried mucous.
It is important to note that in COVID-19 infections, a productive cough or rhinorrhea are not as common compared with dry cough. Regardless, the findings of the study described above should be considered if mucous symptoms develop during a COVID-19 infection when determining infection control. Luckily, with COVID-19, both hand washing and use of an ethanol-based hand sanitizer are seemingly effective in inactivating the virus or removing it from the skin surface.
After frequent hand washing, we all can experience dryness and potentially cracked skin as well. With hand sanitizer, the alcohol content can also cause burning of skin, especially compromised skin.
Vanilloid receptor-1 (VR1), a heat-gated ion channel, is responsible for the burning sensation caused by capsaicin. Ethanol lowers the amount of heat needed to turn on VR1 nocioceptive pain receptors by almost ten degrees, resulting in a potential burning sensation when applied.
Nails are affected as well with frequent hand washing and/or application of hand sanitizer and can become cracked or brittle. Contact dermatitis, both irritant and allergic, can occur with increased use of disinfectants, particularly household cleaners without proper barrier protection.
We’ve previously mentioned the effect of hand washing disrupting the resident skin microflora. Maintaining the skin microflora and barrier is an important component of skin health for preventing both dermatitis and infection. Hand washing or use of hand sanitizer is of paramount importance and effective in infection control for COVID-19. To maintain skin health and the skin barrier, applying lotion or cream after hand washing is recommended. It is recommended to avoid scrubbing hands while washing, since this causes breaks in the skin. Using water that is too hot is not recommended as it can inflame the skin further and disrupt the skin barrier.
Wearing gloves, if possible, is recommended when using household disinfectant products to further decrease skin irritation, barrier disruption, and risk of contact dermatitis. I have found hand emollients that contain ceramides or ingredients higher in omega 6 fatty acids, such as borage seed oil or other oils high in linoleic acid content, to be helpful. In addition to improving the skin barrier, emollients and perhaps those with topical pre- or probiotics, may help restore the skin microflora, potentially improving infection control further. Application of hand moisturizer each time after hand washing to maintain better infection control and barrier protection was also recommended by the recent consensus statement of Chinese experts on protection of skin and mucous membrane barrier for health care workers fighting against COVID-19.
We and our patients have remarked how it seems like our hands have aged 20-50 years in the previous 2 weeks. No one is complaining, everyone understands that protecting themselves and others against a potentially lethal virus is paramount. Maintaining skin health is of secondary concern, but maintaining healthy skin may also protect the skin barrier, another important component of potential infection control.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. They had no relevant disclosures. Write to them at dermnews@mdedge.com.
Resources
Lambers H et al. Int J Cosmet Sci. 2006 Oct;28(5):359-70.
Bin Abdulrahman AK et al. BMC Public Health. 2019 Oct 22;19(1):1324. doi: 10.1186/s12889-019-77.
Kariwa H et al. Dermatology. 2006;212 Suppl 1:119-23.
HIrose R et al. mSphere. 2019 Sep 18;4(5). pii: e00474-19. doi: 10.1128/mSphere.00474-19.
Trevisani M et al. Nat Neurosci. 2002 Jun;5(6):546-51.
Yan Y et al. Dermatol Ther. 2020 Mar 13:e13310. doi: 10.1111/dth.13310.