User login
The apricot tree
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
My apricot tree has bloomed. It is a foolish tree planted by a foolish man since it blossoms, with beautiful pink then white flowers, at least 3 weeks too early in Northern Kentucky. Nonetheless, I am hopeful that it will produce fruit, maybe this year.
The apricot tree takes me back to my early childhood in Oklahoma City. We had a small apricot tree in the backyard of our rental house, and my dad would talk about how there was nothing finer than a sun ripened apricot. Those were happy times. My dad was a milkman and was home every day by late afternoon, though he was still taking classes at night to try to finish his degree. My mother was at home and my older brother in first grade down the street. My little sister was small and tried to keep up.
My time was unstructured, and I reveled in the backyard. In retrospect, the backyard was an open display of broken and hoped for dreams. There was a junked car my best friend Alvin and I would sit in, there was a huge tree stump we sat on and played around, we had an old slow dog named Pooch, gifted to us when my mom’s sister moved to Alaska. We ran around with no shirts or shoes, played and pretended, and carefully watched the apricot tree.
I remember one time when the apricots finally ripened. My father climbed up and got me one, and it was so sweet I did not notice that the juice ran down my face and my bare chest. It was the sweetest and most wonderful thing I have ever tasted. All the better for having to wait for it.
. I have had four major meetings canceled and though my livelihood and life are at risk, I feel oddly free and happy. I am no longer under those pressures to research, write, and present, and am spending at lot of time at home with my wife and daughter. I think I will clean out the garage (who knows what I will find?) and work in the backyard – and keep a close watch on the apricot tree.
As many of you have, I have awkwardly embraced telemedicine in the past. It is interesting now, how HIPAA regulations and state licensing requirements have finally been tossed aside, making it possible to practice telemedicine. I suspect things will stay that way if it is demonstrated they are unnecessary.
In my office, we are depopulating the waiting room and autoclaving face masks. I am cleaning out the stockroom and donating extra gloves, gowns, and masks to the local hospital. We may shut down altogether. There is little more I can do unless called to man a ventilator. I hope it doesn’t come to that, but I will serve if called.
I suggest you embrace your current unstructured time and use it to let your mind roam. It is a reprieve from today’s hyperconnected, hurly burly world. I also suggest you check COVID-19 news updates only once a day and turn off television news altogether. Other than following the recommendations and guidance of public health authorities, there is nothing you can do to speed up the resolution of this pandemic.
No matter how awful, this will pass. It is a warm spring and it is possible the apricot tree will not be bitten by frost, and we may have fruit this year. We should know in about 2 months. I am going to keep a close watch on it.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Match Day 2020: Online announcements replace celebrations, champagne
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
Responsible use of breast cancer screening
In this edition of “Applying research to practice,” I examine a study suggesting that annual screening mammography does not reduce the risk of death from breast cancer in women aged 75 years and older. I also highlight a related editorial noting that we should optimize treatment as well as screening for breast cancer.
Regular screening mammography in women aged 50-69 years prevents 21.3 breast cancer deaths among 10,000 women over a 10-year time period (Ann Intern Med. 2016 Feb 16;164[4]:244-55). However, in the published screening trials, few participants were older than 70 years of age.
More than half of women above age 74 receive annual mammograms (Health, United States, 2018. www.cdc.gov/nchs/data/hus/hus18.pdf). And more than a third of breast cancer deaths occur in women aged 70 years or older (CA Cancer J Clin. 2016 Mar-Apr;66[2]:96-114).
Do older women benefit from annual mammography to the same extent as younger women? Is there a point at which benefit ends?
To answer these questions, Xabier García-Albéniz, MD, PhD, of Harvard Medical School in Boston, and colleagues studied 1,058,013 women enrolled in Medicare during 2000-2008 (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M18-1199).
The researchers examined data on patients aged 70-84 years who had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer. The team emulated a prospective trial by examining deaths over an 8-year period for women aged 70 years and older who either continued or stopped screening mammography. The researchers conducted separate analyses for women aged 70-74 years and those aged 75-84 years.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but there were no major reductions in breast cancer–related deaths.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was reduced for women who continued screening versus those who stopped it by one death per 1,000 women (hazard ratio, 0.78). Among women aged 75-84 years, the 8-year risk reduction was 0.07 deaths per 1,000 women (HR, 1.00).
The authors concluded that continuing mammographic screening past age 75 years resulted in no material difference in cancer-specific mortality over an 8-year time period, in comparison with stopping regular screening examinations.
Considering treatment as well as screening
For a variety of reasons (ethical, economic, methodologic), it is unreasonable to expect a randomized, clinical trial examining the value of mammography in older women. An informative alternative would be a well-designed, large-scale, population-based, observational study that takes into consideration potentially confounding variables of the binary strategies of continuing screening versus stopping it.
Although the 8-year risk of breast cancer in older women is not low among screened women – 5.5% in women aged 70-74 years and 5.8% in women aged 75-84 years – and mammography remains an effective screening tool, the effect of screening on breast cancer mortality appears to decline as women age.
In the editorial that accompanies the study by Dr. García-Albéniz and colleagues, Otis Brawley, MD, of Johns Hopkins University, Baltimore, highlighted the role of inadequate, ineffective, inconvenient, or poorly tolerated treatment in older women (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M20-0429).
Dr. Brawley illustrated that focusing too much on screening diverts attention from the major driver of cancer mortality in older women: suboptimal treatment. That certainly has been the case for the dramatic impact of improved lung cancer treatment on mortality, despite a statistically significant impact of screening on lung cancer mortality as well.
As with lung cancer screening, Dr. Brawley describes the goal of defining “personalized screening recommendations” in breast cancer, or screening that is targeted to the highest-risk women and those who stand a high chance of benefiting from treatment if they are diagnosed with breast cancer.
As our population ages and health care expenditures continue to rise, there can be little disagreement that responsible use of cancer diagnostics will be as vital as judicious application of treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I examine a study suggesting that annual screening mammography does not reduce the risk of death from breast cancer in women aged 75 years and older. I also highlight a related editorial noting that we should optimize treatment as well as screening for breast cancer.
Regular screening mammography in women aged 50-69 years prevents 21.3 breast cancer deaths among 10,000 women over a 10-year time period (Ann Intern Med. 2016 Feb 16;164[4]:244-55). However, in the published screening trials, few participants were older than 70 years of age.
More than half of women above age 74 receive annual mammograms (Health, United States, 2018. www.cdc.gov/nchs/data/hus/hus18.pdf). And more than a third of breast cancer deaths occur in women aged 70 years or older (CA Cancer J Clin. 2016 Mar-Apr;66[2]:96-114).
Do older women benefit from annual mammography to the same extent as younger women? Is there a point at which benefit ends?
To answer these questions, Xabier García-Albéniz, MD, PhD, of Harvard Medical School in Boston, and colleagues studied 1,058,013 women enrolled in Medicare during 2000-2008 (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M18-1199).
The researchers examined data on patients aged 70-84 years who had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer. The team emulated a prospective trial by examining deaths over an 8-year period for women aged 70 years and older who either continued or stopped screening mammography. The researchers conducted separate analyses for women aged 70-74 years and those aged 75-84 years.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but there were no major reductions in breast cancer–related deaths.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was reduced for women who continued screening versus those who stopped it by one death per 1,000 women (hazard ratio, 0.78). Among women aged 75-84 years, the 8-year risk reduction was 0.07 deaths per 1,000 women (HR, 1.00).
The authors concluded that continuing mammographic screening past age 75 years resulted in no material difference in cancer-specific mortality over an 8-year time period, in comparison with stopping regular screening examinations.
Considering treatment as well as screening
For a variety of reasons (ethical, economic, methodologic), it is unreasonable to expect a randomized, clinical trial examining the value of mammography in older women. An informative alternative would be a well-designed, large-scale, population-based, observational study that takes into consideration potentially confounding variables of the binary strategies of continuing screening versus stopping it.
Although the 8-year risk of breast cancer in older women is not low among screened women – 5.5% in women aged 70-74 years and 5.8% in women aged 75-84 years – and mammography remains an effective screening tool, the effect of screening on breast cancer mortality appears to decline as women age.
In the editorial that accompanies the study by Dr. García-Albéniz and colleagues, Otis Brawley, MD, of Johns Hopkins University, Baltimore, highlighted the role of inadequate, ineffective, inconvenient, or poorly tolerated treatment in older women (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M20-0429).
Dr. Brawley illustrated that focusing too much on screening diverts attention from the major driver of cancer mortality in older women: suboptimal treatment. That certainly has been the case for the dramatic impact of improved lung cancer treatment on mortality, despite a statistically significant impact of screening on lung cancer mortality as well.
As with lung cancer screening, Dr. Brawley describes the goal of defining “personalized screening recommendations” in breast cancer, or screening that is targeted to the highest-risk women and those who stand a high chance of benefiting from treatment if they are diagnosed with breast cancer.
As our population ages and health care expenditures continue to rise, there can be little disagreement that responsible use of cancer diagnostics will be as vital as judicious application of treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I examine a study suggesting that annual screening mammography does not reduce the risk of death from breast cancer in women aged 75 years and older. I also highlight a related editorial noting that we should optimize treatment as well as screening for breast cancer.
Regular screening mammography in women aged 50-69 years prevents 21.3 breast cancer deaths among 10,000 women over a 10-year time period (Ann Intern Med. 2016 Feb 16;164[4]:244-55). However, in the published screening trials, few participants were older than 70 years of age.
More than half of women above age 74 receive annual mammograms (Health, United States, 2018. www.cdc.gov/nchs/data/hus/hus18.pdf). And more than a third of breast cancer deaths occur in women aged 70 years or older (CA Cancer J Clin. 2016 Mar-Apr;66[2]:96-114).
Do older women benefit from annual mammography to the same extent as younger women? Is there a point at which benefit ends?
To answer these questions, Xabier García-Albéniz, MD, PhD, of Harvard Medical School in Boston, and colleagues studied 1,058,013 women enrolled in Medicare during 2000-2008 (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M18-1199).
The researchers examined data on patients aged 70-84 years who had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer. The team emulated a prospective trial by examining deaths over an 8-year period for women aged 70 years and older who either continued or stopped screening mammography. The researchers conducted separate analyses for women aged 70-74 years and those aged 75-84 years.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but there were no major reductions in breast cancer–related deaths.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was reduced for women who continued screening versus those who stopped it by one death per 1,000 women (hazard ratio, 0.78). Among women aged 75-84 years, the 8-year risk reduction was 0.07 deaths per 1,000 women (HR, 1.00).
The authors concluded that continuing mammographic screening past age 75 years resulted in no material difference in cancer-specific mortality over an 8-year time period, in comparison with stopping regular screening examinations.
Considering treatment as well as screening
For a variety of reasons (ethical, economic, methodologic), it is unreasonable to expect a randomized, clinical trial examining the value of mammography in older women. An informative alternative would be a well-designed, large-scale, population-based, observational study that takes into consideration potentially confounding variables of the binary strategies of continuing screening versus stopping it.
Although the 8-year risk of breast cancer in older women is not low among screened women – 5.5% in women aged 70-74 years and 5.8% in women aged 75-84 years – and mammography remains an effective screening tool, the effect of screening on breast cancer mortality appears to decline as women age.
In the editorial that accompanies the study by Dr. García-Albéniz and colleagues, Otis Brawley, MD, of Johns Hopkins University, Baltimore, highlighted the role of inadequate, ineffective, inconvenient, or poorly tolerated treatment in older women (Ann Intern Med. 2020 Feb 25. doi: 10.7326/M20-0429).
Dr. Brawley illustrated that focusing too much on screening diverts attention from the major driver of cancer mortality in older women: suboptimal treatment. That certainly has been the case for the dramatic impact of improved lung cancer treatment on mortality, despite a statistically significant impact of screening on lung cancer mortality as well.
As with lung cancer screening, Dr. Brawley describes the goal of defining “personalized screening recommendations” in breast cancer, or screening that is targeted to the highest-risk women and those who stand a high chance of benefiting from treatment if they are diagnosed with breast cancer.
As our population ages and health care expenditures continue to rise, there can be little disagreement that responsible use of cancer diagnostics will be as vital as judicious application of treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
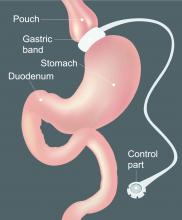
The hospitalized postbariatric surgery patient
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
What every hospitalist should know
What every hospitalist should know
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
With the prevalence of obesity worldwide topping 650 million people1 and nearly 40% of U.S. adults having obesity,2 bariatric surgery is increasingly used to treat this disease and its associated comorbidities.
The American Society for Metabolic & Bariatric Surgery estimates that 228,000 bariatric procedures were performed on Americans in 2017, up from 158,000 in 2011.3 Despite lowering the risks of diabetes, stroke, myocardial infarction, cancer, and all-cause mortality,4 bariatric surgery is associated with increased health care use. Neovius et al. found that people who underwent bariatric surgery used 54 mean cumulative hospital days in the 20 years following their procedures, compared with just 40 inpatient days used by controls.5
Although hospitalists are caring for increasing numbers of patients who have undergone bariatric surgery, many of us may not be aware of some of the things that can lead to hospitalization or otherwise affect inpatient medical care. Here are a few points to keep in mind the next time you care for an inpatient with prior bariatric surgery.
Pharmacokinetics change after surgery
Gastrointestinal anatomy necessarily changes after bariatric surgery and can affect the oral absorption of drugs. Because gastric motility may be impaired and the pH in the stomach is increased after bariatric surgery, the disintegration and dissolution of immediate-release solid pills or caps may be compromised.
It is therefore prudent to crush solid forms or switch to liquid or chewable formulations of immediate-release drugs for the first few weeks to months after surgery. Enteric-coated or long-acting drug formulations should not be crushed and should generally be avoided in patients who have undergone bypass procedures such as Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion with duodenal switch (BPD/DS), as they can demonstrate either enhanced or diminished absorption (depending on the drug).
Reduced intestinal transit times and changes in intestinal pH can alter the absorption of certain drugs as well, and the expression of some drug transporter proteins and enzymes such as the CYP3A4 variant of cytochrome P450 – which is estimated to metabolize up to half of currently available drugs – varies between the upper and the lower small intestine, potentially leading to increased bioavailability of medications metabolized by this enzyme in patients who have undergone bypass surgeries.
Interestingly, longer-term studies have reexamined drug absorption in patients 2-4 years after RYGB and found that initially-increased drug plasma levels often return to preoperative levels or even lower over time,6 likely because of adaptive changes in the GI tract. Because research on the pharmacokinetics of individual drugs after bariatric surgery is lacking, the hospitalist should be aware that the bioavailability of oral drugs is often altered and should monitor patients for the desired therapeutic effect as well as potential toxicities for any drug administered to postbariatric surgery patients.
Finally, note that nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, and corticosteroids should be avoided after bariatric surgery unless the benefit clearly outweighs the risk, as they increase the risk of ulcers even in patients without underlying surgical disruptions to the gastric mucosa.
Micronutrient deficiencies are common and can occur at any time
While many clinicians recognize that vitamin deficiencies can occur after weight loss surgeries which bypass the duodenum, such as the RYGB or the BPD/DS, it is important to note that vitamin and mineral deficiencies occur commonly even in patients with intact intestinal absorption such as those who underwent sleeve gastrectomy (SG) and even despite regained weight due to greater volumes of food (and micronutrient) intake over time.
The most common vitamin deficiencies include iron, vitamin B12, thiamine (vitamin B1), and vitamin D, but deficiencies in other vitamins and minerals may found as well. Anemia, bone fractures, heart failure, and encephalopathy can all be related to postoperative vitamin deficiencies. Most bariatric surgery patients should have micronutrient levels monitored on a yearly basis and should be taking at least a multivitamin with minerals (including zinc, copper, selenium and iron), a form of vitamin B12, and vitamin D with calcium supplementation. Additional supplements may be appropriate depending on the type of surgery the patient had or whether a deficiency is found.
The differential diagnosis for abdominal pain after bariatric surgery is unique
While the usual suspects such as diverticulitis or gastritis should be considered in postbariatric surgery patients just as in others, a few specific complications can arise after weight loss surgery.
Marginal ulcerations (ulcers at the surgical anastomotic sites) have been reported in up to a third of patients complaining of abdominal pain or dysphagia after RYGB, with tobacco, alcohol, or NSAID use conferring even greater risk.7 Early upper endoscopy may be warranted in symptomatic patients.
Small bowel obstruction (SBO) may occur due to surgical adhesions as in other patients, but catastrophic internal hernias with associated volvulus can occur due to specific anatomical defects that are created by the RYGB and BPD/DS procedures. CT imaging is insensitive and can miss up to 30% of these cases,8 and nasogastric tubes placed blindly for decompression of an SBO can lead to perforation of the end of the alimentary limb at the gastric pouch outlet, so post-RYGB or BPD/DS patients presenting with signs of small bowel obstruction should have an early surgical consult for expeditious surgical management rather than a trial of conservative medical management.9
Cholelithiasis is a very common postoperative complication, occurring in about 25% of SG patients and 32% of RYGB patients in the first year following surgery. The risk of gallstone formation can be significantly reduced with the postoperative use of ursodeoxycholic acid.10
Onset of abdominal cramping, nausea and diarrhea (sometimes accompanied by vasomotor symptoms) within 15-60 minutes of eating may be due to early dumping syndrome. Rapid delivery of food from the gastric pouch into the small intestine causes the release of gut peptides and an osmotic fluid shift into the intestinal lumen that can trigger these symptoms even in patients with a preserved pyloric sphincter, such as those who underwent SG. Simply eliminating sugars and simple carbohydrates from the diet usually resolves the problem, and eliminating lactose can often be helpful as well.
Postprandial hyperinsulinemic hypoglycemia (“late dumping syndrome”) can develop years after surgery
Vasomotor symptoms such as flushing/sweating, shaking, tachycardia/palpitations, lightheadedness, or difficulty concentrating occurring 1-3 hours after a meal should prompt blood glucose testing, as delayed hypoglycemia can occur after a large insulin surge.
Most commonly seen after RYGB, late dumping syndrome, like early dumping syndrome, can often be managed by eliminating sugars and simple carbohydrates from the diet. The onset of late dumping syndrome has been reported as late as 8 years after surgery,11 so the etiology of symptoms can be elusive. If the diagnosis is unclear, an oral glucose tolerance test may be helpful.
Alcohol use disorder is more prevalent after weight loss surgery
Changes to the gastrointestinal anatomy allow for more rapid absorption of ethanol into the bloodstream, making the drug more potent in postop patients. Simultaneously, many patients who undergo bariatric surgery have a history of using food to buffer negative emotions. Abruptly depriving them of that comfort in the context of the increased potency of alcohol could potentially leave bariatric surgery patients vulnerable to the development of alcohol use disorder, even when they did not misuse alcohol preoperatively.
Of note, alcohol misuse becomes more prevalent after the first postoperative year.12 Screening for alcohol misuse on admission to the hospital is wise in all cases, but perhaps even more so in the postbariatric surgery patient. If a patient does report excessive alcohol use, keep possible thiamine deficiency in mind.
The risk of suicide and self-harm increases after bariatric surgery
While all-cause mortality rates decrease after bariatric surgery compared with matched controls, the risk of suicide and nonfatal self-harm increases.
About half of bariatric surgery patients with nonfatal events have substance misuse.13 Notably, several studies have found reduced plasma levels of SSRIs in patients after RYGB,6 so pharmacotherapy for mood disorders could be less effective after bariatric surgery as well. The hospitalist could positively impact patients by screening for both substance misuse and depression and by having a low threshold for referral to a mental health professional.
As we see ever-increasing numbers of inpatients who have a history of bariatric surgery, being aware of these common and important complications can help today’s hospitalist provide the best care possible.
Dr. Kerns is a hospitalist and codirector of bariatric surgery at the Washington DC VA Medical Center.
References
1. Obesity and overweight. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published Feb 16, 2018.
2. Hales CM et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017.
3. Estimate of Bariatric Surgery Numbers, 2011-2018. ASMBS.org. Published June 2018.
4. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013 Mar;273(3):219-34. doi: 10.1111/joim.12012.
5. Neovius M et al. Health care use during 20 years following bariatric surgery. JAMA. 2012 Sep 19; 308(11):1132-41. doi: 10.1001/2012.jama.11792.
6. Azran C. et al. Oral drug therapy following bariatric surgery: An overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016 Nov;17(11):1050-66. doi: 10.1111/obr.12434.
7. El-hayek KM et al. Marginal ulcer after Roux-en-Y gastric bypass: What have we really learned? Surg Endosc. 2012 Oct;26(10):2789-96. Epub 2012 Apr 28. (Abstract presented at Society of American Gastrointestinal and Endoscopic Surgeons 2012 annual meeting, San Diego.) 8. Iannelli A et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2006;16:1265-71. doi: 10.1381/096089206778663689.
9. Lim R et al. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018 Oct 9;3(1): e000219. doi: 10.1136/tsaco-2018-000219.
10. Coupaye M et al. Evaluation of incidence of cholelithiasis after bariatric surgery in subjects treated or not treated with ursodeoxycholic acid. Surg Obes Relat Dis. 2017;13(4):681-5. doi: 10.1016/j.soard.2016.11.022.
11. Eisenberg D et al. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017 Mar;13(3):371-8. doi: 10.1016/j.soard.2016.12.005.
12. King WC et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012 Jun 20;307(23):2516-25. doi: 10.1001/jama.2012.6147.
13. Neovius M et al. Risk of suicide and non-fatal self-harm after bariatric surgery: Results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018 Mar;6(3):197-207. doi: 10.1016/S2213-8587(17)30437-0.
Disruptions in cancer care in the era of COVID-19
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
What to know about CFTR modulator therapy for cystic fibrosis
Cystic fibrosis transmembrane conductance regulator modulators are bringing new hope to many patients with CF. But what do physicians and patients need to know about the latest CFTR modulator therapies?
Susan M. Millard, MD, is a pediatric pulmonologist at Helen DeVos Children's Hospital in Grand Rapids, Mich. In an audio interview, Dr. Millard discusses the new Food and Drug Administration-approved combination therapy of elexacaftor, tezacaftor, and ivacaftor (Trikafta). It's a trio that could make a significant difference for the roughly 90% of patients with at least one F508del mutation.
Dr. Millard outlines which patients are candidates for the combination therapy, what physicians and patients can expect with Trikafta use, and how the drug affects patients' use of other CF therapies. She also explains the steps physicians should take before starting patients on the therapy, and what side effects to watch for during treatment.
Dr. Millard is the local principal investigator for CF research at Helen DeVos Children’s Hospital, including Mylan, Therapeutic Development Network, and Vertex clinical studies.
Cystic fibrosis transmembrane conductance regulator modulators are bringing new hope to many patients with CF. But what do physicians and patients need to know about the latest CFTR modulator therapies?
Susan M. Millard, MD, is a pediatric pulmonologist at Helen DeVos Children's Hospital in Grand Rapids, Mich. In an audio interview, Dr. Millard discusses the new Food and Drug Administration-approved combination therapy of elexacaftor, tezacaftor, and ivacaftor (Trikafta). It's a trio that could make a significant difference for the roughly 90% of patients with at least one F508del mutation.
Dr. Millard outlines which patients are candidates for the combination therapy, what physicians and patients can expect with Trikafta use, and how the drug affects patients' use of other CF therapies. She also explains the steps physicians should take before starting patients on the therapy, and what side effects to watch for during treatment.
Dr. Millard is the local principal investigator for CF research at Helen DeVos Children’s Hospital, including Mylan, Therapeutic Development Network, and Vertex clinical studies.
Cystic fibrosis transmembrane conductance regulator modulators are bringing new hope to many patients with CF. But what do physicians and patients need to know about the latest CFTR modulator therapies?
Susan M. Millard, MD, is a pediatric pulmonologist at Helen DeVos Children's Hospital in Grand Rapids, Mich. In an audio interview, Dr. Millard discusses the new Food and Drug Administration-approved combination therapy of elexacaftor, tezacaftor, and ivacaftor (Trikafta). It's a trio that could make a significant difference for the roughly 90% of patients with at least one F508del mutation.
Dr. Millard outlines which patients are candidates for the combination therapy, what physicians and patients can expect with Trikafta use, and how the drug affects patients' use of other CF therapies. She also explains the steps physicians should take before starting patients on the therapy, and what side effects to watch for during treatment.
Dr. Millard is the local principal investigator for CF research at Helen DeVos Children’s Hospital, including Mylan, Therapeutic Development Network, and Vertex clinical studies.
TAVR for low-risk bicuspid aortic stenosis appears safe, effective
NATIONAL HARBOR, MD – Data presented at the 2020 CRT meeting, sponsored by MedStar Heart & Vascular Institute, show that transcatheter aortic valve replacement (TAVR) is safe and effective, at least in the short term, for low-risk patients with bicuspid aortic stenosis, which addresses a data gap.
In an investigator-driven prospective study, called LRT, there was no mortality, no myocardial infarctions (MI), and no disabling strokes 30 days after the procedure, according to Ronald Waksman, MD, associate director of cardiology at Medstar Heart Institute in Washington.
TAVR was approved in 2019 for low-risk patients with symptomatic severe aortic stenosis regardless of aortic valve morphology, but the pivotal industry-led trials only enrolled patients with tricuspid valves, excluding the bicuspid population, according to Dr. Waksman.
However, bicuspid patients were enrolled in the investigator-led LRT study. The 1-year results with tricuspid values have been published previously (JACC Cardiovasc Interv. 2019;12:901-7), but new data from this same study provides the first prospective evaluation in low-risk bicuspid patients.
The LRT enrollment criteria were the same for the bicuspid patients as they were for those enrolled with tricuspid valves. These included a low surgical risk, defined as a Society of Thoracic Surgeons (STS) score of 3 or less; no high-risk criteria independent of STS score; and eligibility for a transfemoral percutaneous procedure. Patients were permitted to undergo TAVR with any commercially available device.
The 61 patients enrolled from August 2016 to September 2019 were compared at 30 days of follow-up with 211 low-risk bicuspid patients undergoing surgical aortic valve replacement (SAVR). Propensity matched, the two groups had generally similar baseline characteristics with some exceptions. Relative to the SAVR group, the TAVR group had an older median age (68.6 vs. 63.4 years) and a lower proportion of males (42.6% vs. 65.7%) and a lower proportion with New York Heart Association heart failure of class III or higher (15.8% vs. 24.6%).
For the primary outcome of all-cause mortality at 30 days, there were no deaths in the TAVR group versus one death in the SAVR group. TAVR was associated with a shorter length of stay (2.0 vs. 5.8 days) and a lower risk of new-onset atrial fibrillation (1.6% vs. 42.6%). However, pacemaker implantations were more common in the TAVR group (11.5% vs. 5.6%). There was one stroke in the TAVR group, but it was not disabling.
Following TAVR, there was one case of paravalvular leak, but it was of moderate severity, according to Dr. Waksman. Leaflet thrombosis in the form of hypoattenuated leaflet thickening (HALT), which occurred in 10.2% of patients; reduced leaflet motion (RELM), which occurred in 6.9%; and hypoattenuation affecting motion (HAM), which also occurred in 6.9%, was observed at 30 days following TAVR, but these have not so far been associated with any clinical consequences.
At baseline, only 4.9% of the bicuspid patients met criteria for NYHA class I function and 23% were in NYHA class III or higher. By 30 days, the proportion in NYHA class I had risen to 78.3%, and no patient was in NYHA class III or higher. Dr. Waksman characterized the improvement in hemodynamics – measured by mean aortic valve gradient and aortic valve area – as “excellent” at 30 days.
Further follow-up of bicuspid patients in the LRT study is needed to confirm long-term safety and efficacy, but Dr. Waksman indicated that the 30-day results are encouraging, in part because they show no greater risk of periprocedural complications than that observed in the tricuspid population.
NATIONAL HARBOR, MD – Data presented at the 2020 CRT meeting, sponsored by MedStar Heart & Vascular Institute, show that transcatheter aortic valve replacement (TAVR) is safe and effective, at least in the short term, for low-risk patients with bicuspid aortic stenosis, which addresses a data gap.
In an investigator-driven prospective study, called LRT, there was no mortality, no myocardial infarctions (MI), and no disabling strokes 30 days after the procedure, according to Ronald Waksman, MD, associate director of cardiology at Medstar Heart Institute in Washington.
TAVR was approved in 2019 for low-risk patients with symptomatic severe aortic stenosis regardless of aortic valve morphology, but the pivotal industry-led trials only enrolled patients with tricuspid valves, excluding the bicuspid population, according to Dr. Waksman.
However, bicuspid patients were enrolled in the investigator-led LRT study. The 1-year results with tricuspid values have been published previously (JACC Cardiovasc Interv. 2019;12:901-7), but new data from this same study provides the first prospective evaluation in low-risk bicuspid patients.
The LRT enrollment criteria were the same for the bicuspid patients as they were for those enrolled with tricuspid valves. These included a low surgical risk, defined as a Society of Thoracic Surgeons (STS) score of 3 or less; no high-risk criteria independent of STS score; and eligibility for a transfemoral percutaneous procedure. Patients were permitted to undergo TAVR with any commercially available device.
The 61 patients enrolled from August 2016 to September 2019 were compared at 30 days of follow-up with 211 low-risk bicuspid patients undergoing surgical aortic valve replacement (SAVR). Propensity matched, the two groups had generally similar baseline characteristics with some exceptions. Relative to the SAVR group, the TAVR group had an older median age (68.6 vs. 63.4 years) and a lower proportion of males (42.6% vs. 65.7%) and a lower proportion with New York Heart Association heart failure of class III or higher (15.8% vs. 24.6%).
For the primary outcome of all-cause mortality at 30 days, there were no deaths in the TAVR group versus one death in the SAVR group. TAVR was associated with a shorter length of stay (2.0 vs. 5.8 days) and a lower risk of new-onset atrial fibrillation (1.6% vs. 42.6%). However, pacemaker implantations were more common in the TAVR group (11.5% vs. 5.6%). There was one stroke in the TAVR group, but it was not disabling.
Following TAVR, there was one case of paravalvular leak, but it was of moderate severity, according to Dr. Waksman. Leaflet thrombosis in the form of hypoattenuated leaflet thickening (HALT), which occurred in 10.2% of patients; reduced leaflet motion (RELM), which occurred in 6.9%; and hypoattenuation affecting motion (HAM), which also occurred in 6.9%, was observed at 30 days following TAVR, but these have not so far been associated with any clinical consequences.
At baseline, only 4.9% of the bicuspid patients met criteria for NYHA class I function and 23% were in NYHA class III or higher. By 30 days, the proportion in NYHA class I had risen to 78.3%, and no patient was in NYHA class III or higher. Dr. Waksman characterized the improvement in hemodynamics – measured by mean aortic valve gradient and aortic valve area – as “excellent” at 30 days.
Further follow-up of bicuspid patients in the LRT study is needed to confirm long-term safety and efficacy, but Dr. Waksman indicated that the 30-day results are encouraging, in part because they show no greater risk of periprocedural complications than that observed in the tricuspid population.
NATIONAL HARBOR, MD – Data presented at the 2020 CRT meeting, sponsored by MedStar Heart & Vascular Institute, show that transcatheter aortic valve replacement (TAVR) is safe and effective, at least in the short term, for low-risk patients with bicuspid aortic stenosis, which addresses a data gap.
In an investigator-driven prospective study, called LRT, there was no mortality, no myocardial infarctions (MI), and no disabling strokes 30 days after the procedure, according to Ronald Waksman, MD, associate director of cardiology at Medstar Heart Institute in Washington.
TAVR was approved in 2019 for low-risk patients with symptomatic severe aortic stenosis regardless of aortic valve morphology, but the pivotal industry-led trials only enrolled patients with tricuspid valves, excluding the bicuspid population, according to Dr. Waksman.
However, bicuspid patients were enrolled in the investigator-led LRT study. The 1-year results with tricuspid values have been published previously (JACC Cardiovasc Interv. 2019;12:901-7), but new data from this same study provides the first prospective evaluation in low-risk bicuspid patients.
The LRT enrollment criteria were the same for the bicuspid patients as they were for those enrolled with tricuspid valves. These included a low surgical risk, defined as a Society of Thoracic Surgeons (STS) score of 3 or less; no high-risk criteria independent of STS score; and eligibility for a transfemoral percutaneous procedure. Patients were permitted to undergo TAVR with any commercially available device.
The 61 patients enrolled from August 2016 to September 2019 were compared at 30 days of follow-up with 211 low-risk bicuspid patients undergoing surgical aortic valve replacement (SAVR). Propensity matched, the two groups had generally similar baseline characteristics with some exceptions. Relative to the SAVR group, the TAVR group had an older median age (68.6 vs. 63.4 years) and a lower proportion of males (42.6% vs. 65.7%) and a lower proportion with New York Heart Association heart failure of class III or higher (15.8% vs. 24.6%).
For the primary outcome of all-cause mortality at 30 days, there were no deaths in the TAVR group versus one death in the SAVR group. TAVR was associated with a shorter length of stay (2.0 vs. 5.8 days) and a lower risk of new-onset atrial fibrillation (1.6% vs. 42.6%). However, pacemaker implantations were more common in the TAVR group (11.5% vs. 5.6%). There was one stroke in the TAVR group, but it was not disabling.
Following TAVR, there was one case of paravalvular leak, but it was of moderate severity, according to Dr. Waksman. Leaflet thrombosis in the form of hypoattenuated leaflet thickening (HALT), which occurred in 10.2% of patients; reduced leaflet motion (RELM), which occurred in 6.9%; and hypoattenuation affecting motion (HAM), which also occurred in 6.9%, was observed at 30 days following TAVR, but these have not so far been associated with any clinical consequences.
At baseline, only 4.9% of the bicuspid patients met criteria for NYHA class I function and 23% were in NYHA class III or higher. By 30 days, the proportion in NYHA class I had risen to 78.3%, and no patient was in NYHA class III or higher. Dr. Waksman characterized the improvement in hemodynamics – measured by mean aortic valve gradient and aortic valve area – as “excellent” at 30 days.
Further follow-up of bicuspid patients in the LRT study is needed to confirm long-term safety and efficacy, but Dr. Waksman indicated that the 30-day results are encouraging, in part because they show no greater risk of periprocedural complications than that observed in the tricuspid population.
REPORTING FROM CRT 2020
Spotlight on SMA, Part 2: The Spinal Muscular Atrophy Treatment Landscape
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
Tense Bullae on the Hands
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
The Diagnosis: Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita (EBA) is a rare autoimmune blistering disorder characterized by tense bullae, skin fragility, atrophic scarring, and milia formation.1 Blisters occur on a noninflammatory base in the classic variant and are trauma induced, hence the predilection for the extensor surfaces.2 Mucosal involvement also has been described.1 The characteristic findings in EBA are IgG autoantibodies directed at the N-terminal collagenous domain of type VII collagen, which composes the anchoring fibrils in the basement membrane zone.1 Differentiating EBA from other subepidermal bullous diseases, especially bullous pemphigoid (BP), can be difficult, necessitating specialized tests.
Biopsy of the perilesional skin can help identify the location of the blister formation. Our patient's biopsy showed a subepidermal blister with granulocytes. The differential diagnosis of a subepidermal blister includes BP, herpes gestationis, cicatricial pemphigoid, EBA, bullous systemic lupus erythematosus, dermatitis herpetiformis, linear IgA disease, and porphyria cutanea tarda.
Direct immunofluorescence (DIF) was performed on the biopsy from our patient, which showed linear/particulate IgG, C3, and IgA deposits in the basement membrane zone, narrowing the differential diagnosis to BP or EBA. To differentiate EBA from BP, DIF of perilesional skin using a salt-split preparation was performed. This test distinguishes the location of the immunoreactants at the basement membrane zone. The antibody complexes in BP are found on the epidermal side of the split, while the antibody complexes in EBA are found on the dermal side of the split. Indirect immunofluorescence on salt-split skin also has been used to distinguish EBA from BP but is only conclusive if there are circulating autoantibodies to the basement membrane zone in the serum, which occurs in approximately 50% of patients with EBA and 15% of patients with BP.3 The immune complexes in our patient were found to be on the dermal side of the split after DIF on salt-split skin, confirming the diagnosis of EBA (Figure).
Differentiating EBA from BP has great value, as the diagnosis affects treatment options. Bullous pemphigoid is fairly easy to treat, with most patients responding to prednisone.3 Epidermolysis bullosa acquisita usually is resistant to therapy. The disease course is chronic with exacerbations and remissions. Dapsone often is used to control the disease, though this therapy for EBA is not currently approved by the US Food and Drug Administration. The recommended initial dose of dapsone is 50 mg daily and should be increased by 50 mg each week until remission, usually 100 to 250 mg.4 We prescribed dapsone for our patient upon clinical suspicion of EBA before the DIF on salt-split skin was completed. A trial of prednisone may be warranted for EBA if there is no response to dapsone or colchicine, but the response is unpredictable. Cyclosporine usually results in a quick response and may be considered if there is clinically severe disease and other treatment alternatives have failed.4
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
- Ishii N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what's new. J Dermatol. 2010;37:220-230.
- Lehman JS, Camilleri MJ, Gibsom LE. Epidermolysis bullosa acquisita: concise review and practical considerations. Int J Dermatol. 2009;48:227-236.
- Woodley D. Immunofluorescence on the salt-split skin for the diagnosis of epidermolysis bullosa acquisita. Arch Dermatol. 1990;126:229-231.
- Mutasim DF. Bullous diseases. In: Kellerman RD, Rakel DP, eds. Conn's Current Therapy. Philadelphia, PA: Elsevier; 2020:978-982.
A 75-year-old man presented to our clinic with nonpainful, nonpruritic, tense bullae and erosions on the dorsal aspects of the hands and extensor surfaces of the elbows of 1 month's duration. The patient also had erythematous erosions and crusted papules on the left cheek and surrounding the left eye. He denied any new medications, history of liver or kidney disease, or history of hepatitis or human immunodeficiency virus. There were no obvious exacerbating factors, including exposure to sunlight. Direct immunofluorescence using a salt-split preparation was performed on a biopsy of the perilesional skin.
Feds tout drug candidates to treat COVID-19
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”
Therapeutics could be available in the near term to help treat COVID-19 patients, according to President Donald Trump.
During a March 19 press briefing, the president highlighted two drugs that could be put into play in the battle against the virus.
The first product is hydroxychloroquine (Plaquenil), a drug used to treat malaria and severe arthritis, is showing promise as a possible treatment for COVID-19.
“The nice part is it’s been around for a long time, so we know that if things go as planned, it’s not going to kill anybody,” President Trump said. “When you go with a brand-new drug, you don’t know that that’s going to happen,” adding that it has shown “very, very encouraging” results as a potential treatment for COVID-19.
He said this drug will be made available “almost immediately.” During the press conference, Food and Drug Administration Commissioner Stephen M. Hahn, MD, suggested the drug would be made available in the context of a large pragmatic clinical trial, enabling the FDA to collect data on it and make a long-term decision on its viability to treat COVID-19.
Dr. Hahn also pointed to the Gilead drug remdesivir – a drug originally developed to fight Ebola and currently undergoing clinical trials – as another possible candidate for a near-term therapeutic to help treat patients while vaccine development occurs.
Dr. Hahn noted that, while the agency is striving to provide regulatory flexibility, safety is paramount. “Let me make one thing clear: FDA’s responsibility to the American people is to ensure that products are safe and effective and that we are continuing to do that.”
He noted that if these and other experimental drugs show promise, physicians can request them under “compassionate use” provisions.
“We have criteria for that, and very speedy approval for that,” Dr. Hahn said. “The important thing about compassionate use ... this is even beyond ‘right to try.’ [We] get to collect the information about that.”
He noted that the FDA is looking at other drugs that are approved for other indications. The examinations of existing therapies are meant to be a bridge as companies work to develop new therapeutics as well as vaccines.
Dr. Hahn also highlighted a cross-agency effort on convalescent plasma, which uses the plasma from a patient who has recovered from COVID-19 infection to help patients fight the virus. “This is a possible treatment; this is not a proven treatment, “ Dr. Hahn said.
Takeda is working on an immunoglobulin treatment based on its intravenous immunoglobulin product Gammagard Liquid.
Julie Kim, president of plasma-derived therapies at Takeda, said the company should be able to go straight into testing efficacy of this approach, given the known safety profile of the treatment. She made the comments during a March 18 press briefing hosted by Pharmaceutical Research and Manufacturers of America (PhRMA). Ms. Kim did caution that this would not be a mass market kind of treatment, as supply would depend on plasma donations from individuals who have fully recovered from a COVID-19 infection. She estimated that the treatment could be available to a targeted group of high-risk patients in 9-18 months.
PhRMA president and CEO Stephen Ubl said the industry is “literally working around the clock” on four key areas: development of new diagnostics, identification of potential existing treatments to make available through trials and compassionate use, development of novel therapies, and development of a vaccine.
There are more than 80 clinical trials underway on existing treatments that could have approval to treat COVID-19 in a matter of months, he said.
Mikael Dolsten, MD, PhD, chief scientific officer at Pfizer, said that the company is working with Germany-based BioNTech SE to develop an mRNA-based vaccine for COVID-19, with testing expected to begin in Germany, China, and the United States by the end of April. The company also is screening antiviral compounds that were previously in development against other coronavirus diseases.
Clement Lewin, PhD, associate vice president of R&D strategy for vaccines at Sanofi, said the company has partnered with Regeneron to launch a trial of sarilumab (Kevzara), a drug approved to treat moderate to severe rheumatoid arthritis, to help treat COVID-19.
Meanwhile, Lilly Chief Scientific Officer Daniel Skovronsky, MD, PhD, noted that his company is collaborating with AbCellera to develop therapeutics using monoclonal antibodies isolated from one of the first U.S. patients who recovered from COVID-19. He said the goal is to begin testing within the next 4 months.
Separately, World Health Organization Director General Tedros Adhanom Ghebreyesus announced during a March 18 press conference that it is spearheading a large international study examining a number of different treatments in what has been dubbed the SOLIDARITY trial. Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand have signed on to be a part of the trial, with more countries expected to participate.
“I continue to be inspired by the many demonstrations of solidarity from all over the world,” he said. “These and other efforts give me hope that together, we can and will prevail. This virus is presenting us with an unprecedented threat. But it’s also an unprecedented opportunity to come together as one against a common enemy, an enemy against humanity.”