User login
CD4 cells implicated in pathology of CCCA
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
in the lymphocytic inflammatory infiltrate, according to a histopathological study of biopsy specimens.
“Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from lichen planopilaris or frontal fibrosing alopecia in some cases when histopathological features alone cannot be used to definitely distinguish between them,” reported Alexandra Flamm, MD, Ata Moshiri, MD, and coauthors from the departments of dermatology and pathology, University of Pennsylvania, Philadelphia.
The histopathological features of CCCA have been characterized previously, but the goal of this study was to go further in piecing together the pathophysiology, they noted.
Horizontal sections of 4-mm punch biopsy specimens were examined from 18 black women with a known diagnosis of CCCA. Both affected and unaffected follicles were evaluated with attention to the number and percentage of CD1a+ Langerhans cells, CD3+, CD4+, and CD8+ lymphocytes.
In this series, the lymphocytic infiltrate in both the affected and unaffected follicles was predominantly composed of CD4+ cells. The perifollicular ratio for CD4+ to CD8+ cells in affected follicles was 5.3:1. It was only modestly lower in unaffected follicles (4.3:1) and in the intrafollicular space of affected follicles (2.5:1).
Affected follicles had a higher number of CD1a+ Langerhans cells than unaffected follicles. This finding suggests, as others have hypothesized, that the antigen-presenting Langerhans cells draw lymphocytes to the follicle, according to the investigators. Elevated numbers of Langerhans cells have also been reported in other forms of scarring alopecia, such as lichen planopilaris (LPP).
In the case of CCCA, CD1a+ Langerhans cells appear to localize to the hair follicle in response to stimulus such as an injury. The CD4+ cells that follow the Langerhans cells participate in an inflammatory reaction that drives follicle destruction. In addition to this damage and scarring, the inflammatory response is also likely to be disrupting the blood supply.
“Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium,” the authors explained. Ultimately, “the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath.”
Prior studies of scarring alopecia diseases, including LLP, frontal fibrosing alopecia (FFA), and keratosis follicularis spinulosa decalvans (KFSD), have typically described a predominantly CD8+ lymphocytic infiltrate. The evidence from this study that the infiltrate is CD4+ predominant in CCCA supports the conclusion that the pathophysiologic features of this type of alopecia are unique, according to the authors.
Work by others has associated CCCA with mutations in the PAD13 gene, which suggests a defect in the formation of hair shaft structure, but this may speak to susceptibility but not the mechanism of hair follicle damage. Rather, this study suggests that it is the concentration of a CD4+ predominant lymphocytic infiltrate in the perifollicular space that induces the pathological events.
For determining the fundamental cause of CCCA, “it will be important to determine what recruits the Langerhans cells to affected follicles,” the investigators suggested. Meanwhile, they expressed hope that the progress being made into decoding the pathogenesis of CCCA will lead to novel therapeutic strategies.
The authors did not list any disclosures. The funding source was listed as the Center for Scientific Review (Grant/Award).
SOURCE: Flamm A et al. J Cutan Pathol. 2020 Feb 18.doi: 10.1111/cup.13666.
20% of U.S. COVID-19 deaths were aged 20-64 years
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
*Correction, 3/20/2020: An earlier version of this story misstated the age range for COVID-19 deaths. The headline of this story was corrected to read "20% of COVID-19 deaths were aged 20-64 years" and the text was adjusted to reflect the correct age range.
A review of more than 4,000 U.S. patients who were diagnosed with novel coronavirus infection (COVID-19) shows that an unexpected 20% of deaths occurred among adults aged 20-64 years, and 20% of those hospitalized were aged 20-44 years.
The expectation has been that people over 65 are most vulnerable to COVID-19 infection, but this study indicates that, at least in the United States, a significant number of patients under 45 can land in the hospital and can even die of the disease.
To assess rates of hospitalization, admission to an ICU, and death among patients with COVID-19 by age group, the Centers for Disease Control and Prevention analyzed 4,226 COVID-19 cases in the United States that were reported between Feb. 12 and March 16.
Overall, older patients in this group were the most likely to be hospitalized, to be admitted to ICU, and to die of COVID-19. A total of 31% of the cases, 45% of hospitalizations, 53% of ICU admissions, and 80% of deaths occurred in patients aged 65 years and older. “Similar to reports from other countries, this finding suggests that the risk for serious disease and death from COVID-19 is higher in older age groups,” said the investigators. “In contrast, persons aged [19 years and younger] appear to have milder COVID-19 illness, with almost no hospitalizations or deaths reported to date in the United States in this age group.”
But compared with the under-19 group, patients aged 20-44 years appeared to be at higher risk for hospitalization and ICU admission, according to the data published March 18 in Morbidity and Mortality Weekly Report.
The researchers excluded from their analysis patients who repatriated to the United States from Wuhan, China, and from Japan, including patients repatriated from cruise ships. Data on serious underlying health conditions were not available, and many cases were missing key data, they noted.
Among 508 patients known to have been hospitalized, 9% were aged 85 years or older, 36% were aged 65-84 years, 17% were aged 55-64 years, 18% were 45-54 years, and 20% were aged 20-44 years.
Among 121 patients admitted to an ICU, 7% were aged 85 years or older, 46% were aged 65-84 years, 36% were aged 45-64 years, and 12% were aged 20-44 years. Between 11% and 31% of patients with COVID-19 aged 75-84 years were admitted to an ICU.
Of 44 deaths, more than a third occurred among adults aged 85 years and older, and 46% occurred among adults aged 65-84 years, and 20% occurred among adults aged 20-64 years.
More follow-up time is needed to determine outcomes among active cases, the researchers said. These results also might overestimate the prevalence of severe disease because the initial approach to testing for COVID-19 focused on people with more severe disease. “These preliminary data also demonstrate that severe illness leading to hospitalization, including ICU admission and death, can occur in adults of any age with COVID-19,” according to the CDC.
SOURCE: CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep. 2020 Mar 18. doi: 10.15585/mmwr.mm6912e2.
New sickle cell drugs give hope, but access remains a barrier
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
New melanoma treatments linked to mortality decline
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.
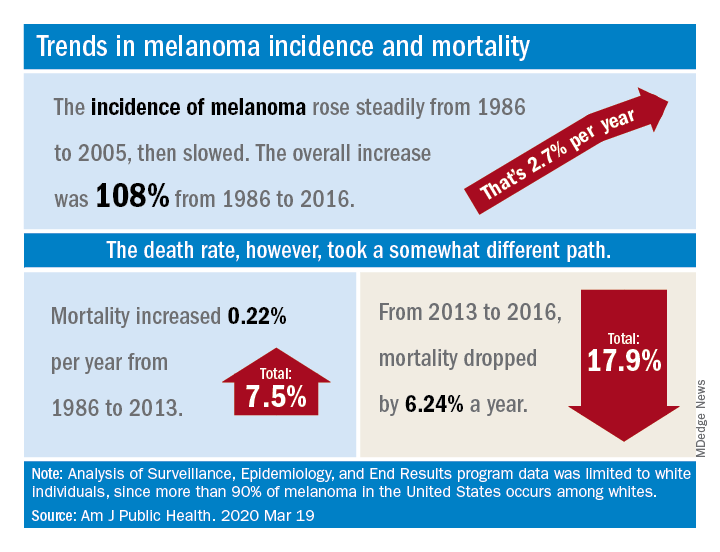
The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
Recent advances in treatment appear to have reversed the course of melanoma mortality since 2013, according to data published in the American Journal of Public Health.

The U.S. death rate for melanoma, which had been rising at a rate of 0.22% a year for more than 2 decades, dropped by 17.9%, or 6.24% per year, during 2013-2016. That decline “coincides with the introduction of multiple new and efficacious treatments for metastatic melanoma,” such as BRAF inhibitors and immune checkpoint inhibitors, study author Juliana Berk-Krauss, MD, of the State University of New York Downstate Medical Center in Brooklyn and colleagues wrote.
The other possible explanation for the decline in deaths, “education and early detection resulting in migration toward earlier stage melanomas with a greater chance of surgical cure,” is unlikely, according to the investigators. That’s because the small decrease in median tumor thickness that occurred during 1989-2009 “is not associated with changes in prognosis.”
The investigators’ analysis encompassed data from the Surveillance, Epidemiology, and End Results registry recorded during 1986-2016. Nine registry areas were included (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), which covered about 9.4% of the U.S. population. The analysis was limited to the white population, which accounts for more than 90% of melanoma cases in the United States.
The data showed a slight decline in annual percent change in melanoma incidence, from 3.24% for 1986-2005 to 1.72% for 2006-2016. However, over the whole period studied (1986-2016), melanoma incidence increased by 108%, or about 2.7% per year.
“Given the increased incidence of melanoma throughout this period and the lack of stage migration, these data strongly suggest that the mortality decline is due to the extended survival associated with these [newer] treatments,” the investigators wrote.
This study was funded by NYU Langone. Two investigators disclosed potential conflicts of interest, including relationships with Bio-Rad Laboratories, Novartis, Merck, and several other companies.
SOURCE: Berk-Krauss J et al. Am J Public Health. 2020 Mar 19. doi: 10.2105/AJPH.2020.305567.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
April 2020
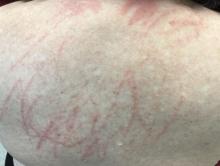
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Carboplatin plus pemetrexed should be ‘a standard option’ in elderly patients with NSCLC
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
For patients age 75 and older with nonsquamous non–small cell lung cancer (NSCLC) not previously treated with chemotherapy, combination carboplatin and pemetrexed followed by pemetrexed maintenance is both effective and tolerable, suggest the results of a phase 3 trial.
The median overall survival was 18.7 months for patients randomized to carboplatin/pemetrexed and 15.5 months for patients randomized to docetaxel monotherapy.
The combination met the prespecified endpoint for noninferiority to docetaxel but was not shown to be superior in terms of overall survival, investigator Isamu Okamoto, MD, of Kyushu University in Fukuoka, Japan, and colleagues reported in JAMA Oncology.
Still, progression-free survival was significantly longer in the carboplatin/pemetrexed arm, and dose reductions were less frequent with the combination than with docetaxel.
“The combination of carboplatin and pemetrexed followed by pemetrexed maintenance ... provides a clinically significant benefit with regard to its effectiveness and tolerability,” the investigators wrote. “This combination should therefore be considered as a standard option for treatment in this setting.”
Dr. Okamoto and colleagues noted that the lung cancer incidence in elderly patients – 75 years and older – is increasing, and cytotoxic chemotherapy remains the standard treatment for patients whose tumors do not carry targetable mutations or are resistant to immunotherapy.
“In anticipation of a further increase in the number of elderly individuals with advanced NSCLC, it will be important to develop more optimal chemotherapeutic regimens for this patient group,” the investigators wrote.
To that end, they conducted a phase 3 trial of carboplatin/pemetrexed in patients aged 75 and older with NSCLC who had not been exposed to cytotoxic chemotherapy.
Patients and treatment
There were 433 patients enrolled in the trial. Their median age was 78 years (range, 75-88 years), and 57.7% were men. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1.
Patients were stratified by clinical stage (III, IV, or recurrence), sex, epidermal growth factor receptor variant status (wild-type, exon 19 deletion, L858R variant of exon 21, or unknown), and treatment center.
The patients were then randomly assigned on a 1:1 basis to receive either intravenous docetaxel at 60 mg/m2 for 60 minutes on day 1 every 3 weeks or pemetrexed at 500 mg/m2 for 10 minutes followed by an infusion of carboplatin at an area under the curve of 5 for 30 minutes on day 1 every 3 weeks. The combination therapy was repeated for up to four courses and followed by 3-week courses of maintenance therapy with the same dose of pemetrexed.
Both regimens were continued until disease progression or the development of unacceptable toxicities.
Efficacy and safety
All 433 randomized patients were included in the efficacy analysis, but the safety analysis included 428 patients. Three patients assigned to docetaxel and two assigned to carboplatin/pemetrexed did not receive protocol treatment.
The respective median overall survival for the docetaxel and carboplatin/pemetrexed arms was 15.5 months and 18.7 months, which translated to a stratified hazard ratio for death of 0.85, meeting the prespecified noninferiority endpoint (P = .003).
However, the upper limit of the 95% confidence interval was 1.056, exceeding the prespecified superiority margin of 1.000. Therefore, the combination could not be proven superior to docetaxel with regard to overall survival.
On the other hand, progression-free survival was significantly longer with carboplatin/pemetrexed. The median progression-free survival was 6.4 months in the combination arm and 4.3 months in the docetaxel arm (unstratified HR, 0.739; P < .001).
The overall response rate with carboplatin/pemetrexed was 36.8%, compared with 28.2% for docetaxel, but this difference was not statistically significant.
Adverse events that were more common in the docetaxel arm than in the combination arm included grade 3/4 decreases in white blood cell count (68.7% and 28%, respectively), grade 3/4 decreases in neutrophil count (86% and 46.3%, respectively), and febrile neutropenia (17.8% and 4.2%, respectively).
Adverse events more frequently seen with the combination than with docetaxel included anemia (29.4% and 1.9%, respectively) and decreased platelet counts (25.7% and 1.4%, respectively).
Two patients in each arm died from treatment-related causes. In the docetaxel arm, the deaths were caused by acute respiratory distress syndrome and pneumonitis. In the combination arm, the deaths were caused by dyspnea and pneumonitis.
Approximately 29% of patients in each arm reported improvement in quality of life at 18 weeks, compared with baseline.
Performance status is key
A lung cancer specialist who was not involved in the study agreed with the authors that age should not be the primary determinant for choice of a treatment regimen.
“There’s a convergence of data over the last decade or so that has really clearly shown that our treatment decisions should be based on performance status much more than chronologic age, certainly for our patients who are in their 70s, and even potentially into their early 80s,” Howard (Jack) West, MD, of City of Hope Comprehensive Cancer Center in Duarte, Calif., said in an interview.
“The available data really say that patients with a good performance status who are in their 70s should be treated just like patients in their 60s and 50s,” he said.
He added, however, that for patients such as those in the study without targetable driver mutations, the best treatment would likely be immunotherapy or immunotherapy combined with chemotherapy.
“If there were a patient with a nonsquamous non–small cell lung cancer where we would be thinking about carboplatin and pemetrexed, I would go further than just carbo and pemetrexed; I would give carbo and pemetrexed with pembrolizumab for most of these patients,” he said.
Dr. West said the study primarily offers reassurances about the efficacy and tolerability of the carboplatin/pemetrexed combination in patients aged 75 years and older.
The study was funded by agencies of the Japanese government. The investigators disclosed relationships with Boehringer Ingelheim, AstraZeneca, Eli Lilly Japan KK, and many other companies. Dr. West disclosed consulting for Merck.
SOURCE: Okamoto I et al. JAMA Oncol. 2020 Mar 12. doi: 10.1001/jamaoncol.2019.6828.
FROM JAMA ONCOLOGY
N-acetyl cysteine may positively affect cerebral glucose metabolism in MS
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Key clinical point: In patients with multiple sclerosis (MS), N-acetyl cysteine (NAC) positively affects cerebral glucose metabolism, and this appears to be associated with improved symptoms related to cognition and attention.
Major finding: Based on fluorodeoxyglucose (FDG) positron emission tomography (PET) data, NAC group vs. the control group demonstrated significant increases in cerebral glucose metabolism after therapy in several brain regions including the lateral and middle temporal lobes, inferior frontal lobe, and caudate (P less than .05). NAC group had significant improvements in self-reported scores related to cognition and attention.
Study details: Twenty-four patients with a diagnosis of MS were randomly assigned to NAC plus standard of care or standard of care only (waitlist control group). FDG PET was used to assess cerebral glucose metabolism at baseline and after 2 months in all patients.
Disclosures: This study was supported by a grant from the Coors Foundation. The authors declared no potential conflicts of interest.
Citation: Monti DA et al. Front Neurol. 2020 Feb 14. doi: 10.3389/fneur.2020.00088.
Does diet quality influence late-onset MS risk?
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Key clinical point: Diet quality is not associated with the risk for late-onset multiple sclerosis (MS) in middle-aged individuals.
Major finding: Diet quality, assessed by means of the Alternative Healthy Eating Index-2010, was not statistically significantly associated with late-onset MS diagnosis risk (hazard ratio highest vs. lowest tertile, 0.79; test for trend: P = .22). Smoking did not modify the association.
Study details: Prospective cohort study based on the Danish cohort “Diet, Cancer and Health” evaluated the association between diet quality and the hazards of late-onset MS diagnosis in 56,867 individuals (age, 50-64 years) who were followed for a median of 20.4 years; 124 patients with MS were identified.
Disclosures: This study did not receive any specific funding. The data collection for the Diet, Cancer and Health cohort was funded by the Danish Cancer Society. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. The other authors reported no conflicts of interest.
Citation: Pommerich UM et al. Mult Scler Relat Disord. 2020 Jan 26. doi: 10.1016/j.msard.2020.101968.
Elevated leptin levels linked with MS risk
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Key clinical point: Increase in leptin concentration is associated with an elevated multiple sclerosis (MS) risk among young individuals.
Major finding: A 1-unit increase in leptin z-score was associated with higher MS risk in individuals younger than 20 years (odds ratio [OR], 1.4; 95% confidence interval, 1.1-1.9) and in all men (OR, 1.4; 95% confidence interval, 1.0-2.0). In contrast, MS risk reduced with increased leptin levels in women aged 30-39 years after adjustment for insulin levels (OR, 0.74; 95% confidence interval, 0.54-1.0).
Study details: This nested case-control study used blood samples from Swedish biobanks and compared leptin and insulin concentrations in 649 individuals who later developed relapsing-remitting MS and 649 matched controls.
Disclosures: This study was supported by the Swedish Research Council. Lucia Alonso-Magdalena has received speaking fees from Merck Serono and served on advisory board for Merck Serono and Biogen. Magnus Vrethem has received honoraria for lectures from Genzyme and for advisory boards from Roche and Novartis.
Citation: Biström M et al. Mult Scler. 2020 Feb 7. doi: 10.1177/1352458520905033.
Concussion in adolescents tied to MS risk
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.
Key clinical point: Concussions in adolescents correlate to an elevated risk for multiple sclerosis (MS).
Major finding: The risk for MS was higher among patients exposed to a concussion in adolescence (hazard ratio [HR], 1.29; P = .03). Sex-specific analysis revealed a higher risk for MS only in males who sustained a concussion in adolescence (HR, 1.41; P = 0.04).
Study details: Retrospective study included 97,965 patients (age, 11-18 years) exposed to a concussion who were matched to 293,895 unexposed patients; primary outcome was MS diagnosis.
Disclosures: This study was funded by an unrestricted investigator-initiated trial grant from Roche Canada and supported by ICES. The corresponding author has served on advisory boards for Biogen Idec, EMD Serono, Genzyme Canada, Novartis, and Roche; has received Investigator Initiated Grant Funds from Biogen Idec, Novartis, and Roche; and has acted as site PI for multicenter trials funded by Novartis, Genzyme, Roche, and AbbVie. All other authors declared no conflicts of interest.
Citation: Povolo CA et al. Mult Scler. 2020 Feb 24. doi: 10.1177/1352458520908037.