User login
Product update: Neuromodulation device, cystoscopy simplified, hysteroscopy seal, next immunization frontier
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
Evidence builds for bariatric surgery’s role in cancer prevention
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
LAS VEGAS – The ability of bariatric surgery and substantial subsequent weight loss to cut the incidence of a variety of obesity-related cancers and other malignancies received further confirmation in results from two studies reported at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
In one study, 2,107 adults enrolled in the Longitudinal Assessment of Bariatric Surgery (LABS-2) study showed a statistically significant halving of the cancer incidence during 7 years of follow-up in patients who underwent bariatric surgery and had a reduction of at least 20% in their presurgical body mass index (BMI), compared with patients in the study who underwent bariatric surgery but lost less weight, reported Andrea M. Stroud, MD, a bariatric surgeon at the Oregon Health & Science University, Portland.
In the second study, analysis of about 1.7 million hospitalized U.S. patients in the National Inpatient Sample showed that the incidence of an obesity-related cancer was 21% higher in more than 1.4 million obese individuals (BMI, 35 kg/m2 or greater) with no history of bariatric surgery, compared with nearly 247,000 people in the same database with a history of both obesity and bariatric surgery, said Juliana Henrique, MD, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
The study reported by Dr. Henrique focused specifically on the 13 cancer types identified by the Centers for Disease Control and Prevention as having an incidence that links with overweight and obesity (Morb Mortal Wkly Rep. 2017;66[39]:1052-8), whereas the study presented by Dr. Stroud included all incident cancers during follow-up, but which were predominantly obesity related, with breast cancer – an obesity-related malignancy – having the highest incidence. Overall, 40% of all U.S. cancers in 2014 were obesity related, according to the CDC’s report.
“A number of studies have shown decreases in cancer rates after bariatric surgery, especially female cancers like breast and ovarian,” commented John Scott, MD, director of metabolic and bariatric surgery for Prism Health–Upstate in Greenville, S.C. “These two reports build on that.”
The evidence for weight loss after bariatric surgery as a means to cut the risk of a first or recurrent cancer has become strong enough for some patients to see cancer prophylaxis as a prime reason to undergo the procedure, said surgeons at the meeting.
Bariatric surgery and subsequent weight loss “is a substantial preventive factor for cancer, especially in patients who have obesity and diabetes,” commented Theresa LaMasters, MD, a bariatric surgeon in West Des Moines, Iowa. “It might not just be weight loss. It’s likely a multifactorial effect, including reduced inflammation after bariatric surgery, but weight loss is a component” of the effect, Dr. LaMasters said in an interview. It is now common for her to see patients seeking bariatric surgery because of a family or personal history of cancer. “Patients are trying to reduce their future risk” for cancer with bariatric surgery, she added.
The LABS-2 study enrolled 2,458 patients who were part of the first LABS cohort, LABS-1, but followed them longer term. The data Dr. Stroud reported came from 2,107 of the LABS-2 patients without a history of cancer, no cancer diagnosed in the first year after bariatric surgery, and longer-term follow-up of 7 years. About three-quarters of the patients underwent gastric bypass, with the rest undergoing laparoscopic gastric band placement. Nearly half of those included had diabetes. Their average BMI was 45-50 kg/m2.
Dr. Stroud and associates ran an analysis that divided the populations into tertiles based on percentage of baseline body mass lost at 12 months after surgery and cancer-free survival during the 7 years after the 12-month follow-up. The incidence of cancer was 51% lower in patients who lost 20%-34% of their BMI, compared with those who lost less than 20%, a statistically significant difference, and patients who lost 35% or more of their BMI had a 31% reduced cancer rate, compared with those who lost less than 20%, a difference that was not statistically significant, Dr. Stroud reported. The patients who lost less weight after surgery mostly underwent gastric banding, whereas those who lost more mostly underwent gastric bypass.
The analysis reported by Dr. Henrique used data collected in the U.S. National Inpatient Sample during 2010-2014, which totaled more than 7 million patients hospitalized for cancer, including 1,423,367 with a history of obesity and 246,668 with obesity who had undergone bariatric surgery. Those without bariatric surgery had a 21% higher rate of developing obesity-related cancers after adjustment for many baseline demographic and clinical features, Dr. Henrique said. The cancer protection after bariatric surgery was especially notable in the subset of patients in the sample with a genetic predisposition to developing cancer.
LABS-1 and LABS-2 were funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Stroud and Dr. Henrique had no disclosures.
SOURCES: Stroud AM et al. Obesity Week, Abstract A107; Henrique J et al. Obesity Week, Abstract A108.
REPORTING FROM OBESITY WEEK 2019
HRQoL deteriorates during chemoradiotherapy for bladder cancer
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
Chemoradiotherapy impairs health-related quality of life (HRQoL) after the initiation of treatment in patients with muscle-invasive bladder cancer, according to an exploratory analysis of trial data.
However, HRQoL scores improved to pretreatment levels within 6 months, and improvements were maintained at 5-year follow up.
“[We] planned a prospective assessment of patient-reported outcomes within BC2001, using the Functional Assessment of Cancer Therapy–Bladder (FACT-BL) questionnaire,” wrote Robert A. Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research, England, and colleagues. Their report is in European Urology.
The exploratory analyses of HRQoL data included 452 patients from the phase 3 BC2001 trial. The objective of the analysis was to evaluate the impact of chemoradiotherapy on HRQoL in trial participants.
The BC2001 trial randomized patients to either radiotherapy (standard or reduced high-dose volume radiotherapy) and/or chemotherapy (radiotherapy or chemoradiotherapy) comparison groups. The primary outcome was the change in bladder cancer subscale (BLCS) scores from baseline to 12 months.
At trial baseline, study subjects were enrolled into the optional substudy, which involved completion of the FACT-BL questionnaire. Follow-up assessment was conducted at various time points after the start of radiotherapy.
After analysis, the researchers found that HRQoL scores deteriorated at end of treatment (BLCS: –5.06; 99% confidence interval, –6.12 to –4.00; overall FACT-BL: –8.22; 99% CI, –10.76 to –5.68), but improved to baseline levels at 6 months, and were maintained thereafter.
“Two-thirds of patients report stable or improved HRQoL on long-term follow-up,” they reported. “There [was] no evidence of impairment in HRQoL resulting from the addition of chemotherapy,” the researchers said.
In addition, the team found that pretrial administration of neoadjuvant chemotherapy had no significant impact on HRQoL outcomes.
One key limitation of the analysis was incomplete follow-up with HRQoL questionnaires. Over time, response rates declined, with a 60% expected response rate at 5 years.
“Addition of concomitant chemotherapy or use of neoadjuvant chemotherapy has no significant impact on HRQoL, further supporting the routine use of 5-FU and mitomycin C in this setting,” Dr. Huddart and coauthors concluded.
Cancer Research UK funded the study. The authors reported having no conflicts of interest.
SOURCE: Huddart RA et al. Eur Urol. 2019 Dec 13. doi: 10.1016/j.eururo.2019.11.001.
FROM EUROPEAN UROLOGY
Delayed hospital admission after hip fracture raises mortality risk
a retrospective, observational study suggests.
Among 867 elderly patients who underwent hip fracture surgery at a university hospital in China and who were available for follow-up, the proportion hospitalized on the day of injury was 25.4%, and the proportion hospitalized on days 1, 2, and 7 after injury were 54.7%, 66.3%, and 12.6%, respectively, reported Wei He, MD, of the Second Affiliated Hospital of Zhejiang University, Hangzhou, China, and colleagues in the World Journal of Emergency Medicine.
The mean time from admission to surgery was 5.2 days. Mortality rates at 1 year, 3 months, and 1 month after surgery were 10.5%, 5.4%, and 3.3%, respectively. Hospitalization at 7 or more days after injury was an independent risk factor for 1-year mortality (odds ratio, 1.76), the authors found.
Although the influence of surgical delay on mortality and morbidity among hip fracture patients has been widely studied, most data focus on surgery timing among hospitalized patients and fail to consider preadmission waiting time, they noted.
The current study aimed to assess outcomes based on “actual preadmission waiting time” through an analysis of data and surgical outcomes from a hospital electronic medical record system and from postoperative telephone interviews. Study subjects were patients aged over 65 years who underwent hip fracture surgery between Jan. 1, 2014, and Dec. 31, 2017. The mean age was 81.4 years, 74.7% of the patients were women, 67.1% had femoral neck fracture, and 56.1% had hip replacement surgery.
The findings, though limited by the retrospective nature of the study and the single-center design, suggest that, under the current conditions in China, admission delay may increase 1-year mortality, they wrote, concluding that “[i]n addition to early surgery highlighted in the guidelines, we also advocate early admission.”
The authors reported having no disclosures.
SOURCE: He W et al. World J Emerg Med. 2020;11(1):27-32.
a retrospective, observational study suggests.
Among 867 elderly patients who underwent hip fracture surgery at a university hospital in China and who were available for follow-up, the proportion hospitalized on the day of injury was 25.4%, and the proportion hospitalized on days 1, 2, and 7 after injury were 54.7%, 66.3%, and 12.6%, respectively, reported Wei He, MD, of the Second Affiliated Hospital of Zhejiang University, Hangzhou, China, and colleagues in the World Journal of Emergency Medicine.
The mean time from admission to surgery was 5.2 days. Mortality rates at 1 year, 3 months, and 1 month after surgery were 10.5%, 5.4%, and 3.3%, respectively. Hospitalization at 7 or more days after injury was an independent risk factor for 1-year mortality (odds ratio, 1.76), the authors found.
Although the influence of surgical delay on mortality and morbidity among hip fracture patients has been widely studied, most data focus on surgery timing among hospitalized patients and fail to consider preadmission waiting time, they noted.
The current study aimed to assess outcomes based on “actual preadmission waiting time” through an analysis of data and surgical outcomes from a hospital electronic medical record system and from postoperative telephone interviews. Study subjects were patients aged over 65 years who underwent hip fracture surgery between Jan. 1, 2014, and Dec. 31, 2017. The mean age was 81.4 years, 74.7% of the patients were women, 67.1% had femoral neck fracture, and 56.1% had hip replacement surgery.
The findings, though limited by the retrospective nature of the study and the single-center design, suggest that, under the current conditions in China, admission delay may increase 1-year mortality, they wrote, concluding that “[i]n addition to early surgery highlighted in the guidelines, we also advocate early admission.”
The authors reported having no disclosures.
SOURCE: He W et al. World J Emerg Med. 2020;11(1):27-32.
a retrospective, observational study suggests.
Among 867 elderly patients who underwent hip fracture surgery at a university hospital in China and who were available for follow-up, the proportion hospitalized on the day of injury was 25.4%, and the proportion hospitalized on days 1, 2, and 7 after injury were 54.7%, 66.3%, and 12.6%, respectively, reported Wei He, MD, of the Second Affiliated Hospital of Zhejiang University, Hangzhou, China, and colleagues in the World Journal of Emergency Medicine.
The mean time from admission to surgery was 5.2 days. Mortality rates at 1 year, 3 months, and 1 month after surgery were 10.5%, 5.4%, and 3.3%, respectively. Hospitalization at 7 or more days after injury was an independent risk factor for 1-year mortality (odds ratio, 1.76), the authors found.
Although the influence of surgical delay on mortality and morbidity among hip fracture patients has been widely studied, most data focus on surgery timing among hospitalized patients and fail to consider preadmission waiting time, they noted.
The current study aimed to assess outcomes based on “actual preadmission waiting time” through an analysis of data and surgical outcomes from a hospital electronic medical record system and from postoperative telephone interviews. Study subjects were patients aged over 65 years who underwent hip fracture surgery between Jan. 1, 2014, and Dec. 31, 2017. The mean age was 81.4 years, 74.7% of the patients were women, 67.1% had femoral neck fracture, and 56.1% had hip replacement surgery.
The findings, though limited by the retrospective nature of the study and the single-center design, suggest that, under the current conditions in China, admission delay may increase 1-year mortality, they wrote, concluding that “[i]n addition to early surgery highlighted in the guidelines, we also advocate early admission.”
The authors reported having no disclosures.
SOURCE: He W et al. World J Emerg Med. 2020;11(1):27-32.
FROM THE WORLD JOURNAL OF EMERGENCY MEDICINE
Carbohydrate restriction a viable choice for reversal of type 2 diabetes, expert says
LOS ANGELES – according to Sarah Hallberg, DO.
“Nutritional ketosis supports diabetes reversal by reducing insulin resistance while providing an alternative fuel to glucose with favorable signaling properties,” she said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease.
Low-carbohydrate nutritional patterns including ketosis have extensive clinical trial evidence for improvement of type 2 diabetes, including preliminary results from a 5-year study of 465 patients enrolled in the Indiana Type 2 Diabetes Reversal Trial that Dr. Hallberg is overseeing in her role as medical director and founder of the medically supervised weight-loss program at Indiana University Health Arnett, Lafayette.
“The ketogenic diet is not a fad diet, it’s what we used to treat people with before the advent of insulin,” said Dr. Hallberg, who has been recommending and counseling patients with type 2 diabetes to follow a ketogenic diet for nearly 10 years. “Of course, insulin has been wonderful. It’s saved so many people with type 1 diabetes. But we also misused it in type 2 diabetes. Instead of counseling people the way we used to about the food that they’re taking in to control their blood sugar, we’ve just been putting [them] on medication, including insulin.”
The American Diabetes Association and other organizations have updated their guidelines to include low-carbohydrate eating patterns for type 2 diabetes treatment, she continued. Veterans Affairs/Department of Defense recommend carbohydrate levels as low as 14%.
Dr. Hallberg, who is also medical director for Virta Health, defined a very-low-carbohydrate or ketogenic diet as less than 50 g of carbohydrates per day, or fewer than 10% of calories consumed. A low-carbohydrate diet is 51-130 g of carbohydrates per day, or 25% or fewer calories consumed, whereas anything above 25% calories consumed is a not a low-carbohydrate diet. A well-formulated ketogenic diet, she continued, consists of 5%-10% carbohydrates (or less than 50 g), 15%-20% protein, and 70%-80% fat. The carbohydrates include 5-10 g per day of protein-based food, 10-15 g of vegetables, 5-10 g of nuts/seeds, 5-10 g of fruits, and 5-10 g of miscellaneous nutrients. “When we’re talking about a total carbohydrate intake per day of under 50 g, you can get a lot of vegetables and nuts in,” she said. “I like to tell my patients they’re not eating GPS: no grains, no potatoes, and no sugar.”
Recently, Dr. Hallberg and colleagues published a review in which they sought to evaluate the appropriateness of sources cited in the ADA’s guidelines on eating patterns for the management of type 2 diabetes, identify additional relevant sources, and evaluate the evidence (Diabetes Obes Metab. 2019;21[8]:1769-79). “We looked at how much evidence there is for the low-carb diet, the Mediterranean diet, the DASH [Dietary Approaches to Stop Hypertension] diet, and a plant-based diet,” she said. “We found a wide variation in the evidence for each eating pattern, but the low-carb eating pattern for diabetes has so much more evidence than any of the other eating patterns.”
In an earlier study, researchers followed 10 inpatients with diabetes in a metabolic ward for 3 weeks. Their mean age was 51 years, and their mean body mass index was 40.3 kg/m2. The patients were fed a standard diet for 7 days, then a low-carbohydrate diet (21 g per day) for 14 days (Ann Intern Med 2005; 142[6]:403-11). After 2 weeks of the low-carbohydrate diet, their mean fasting blood glucose dropped from 7.5 to 6.3 mmol/L, and their mean hemoglobin A1c (HbA1c) fell from 7.3% to 6.8%. “The levels came down very fast,” said Dr. Hallberg, who was not involved with the study. “This is an important part of the intervention, because when you get a patient who’s tried everything, who’s injecting hundreds of units of insulin every day, you can make a huge difference in the first couple of weeks. It is not unusual for us to pull patients off of 200-plus units of insulin. This is as motivating as all get out. It also affects their pocketbook right away. This is one of the reasons our patients are able to sustain a ketogenic diet along with support: early motivation and satisfaction.”
In a longer-term trial, researchers evaluated the impact of a ketogenic diet in 64 obese patients with diabetes over the course of 56 weeks (Moll Cell Biochem. 2007;302[1-2]:249-56). The body weight, body mass index, and levels of blood glucose, total cholesterol, LDL cholesterol, triglycerides, and urea showed a significant decrease from week 1 to week 56 (P less than .0001), while the level of HDL cholesterol increased significantly (P less than .0001).
A separate trial conducted in Israel evaluated the effects of a low-carbohydrate diet, compared with a Mediterranean or low-fat diet in 322 moderately obese patients over the course of 2 years (N Engl J Med. 2008;359:229-41). The rate of adherence to a study diet was 85% at 2 years. The mean weight change was greatest for those on the low-carbohydrate diet, followed by the Mediterranean and low-fat diets. Fasting glucose was best for those on the Mediterranean diet at the end of 2 years, whereas change in HbA1c was best among those on the low-carbohydrate diet.
Another study randomized patients to a low-carbohydrate ketogenic diet (less than 20 g per day with no calorie restriction) or to a low–glycemic index diet (55% carbohydrate restriction of 500 kcal from baseline) over the course of 24 weeks (Nutr Metab [Lond]. 2008 Dec 19. doi:10.1186/1743-7075-5-36). Between baseline and week 24, the mean HbA1c fell from 8.8% to 7.3% in the very-low-carbohydrate diet group, and from 8.3% to 7.8% in the low–glycemic diet group, for a between-group comparison P value of .03. In addition, 95% of patients in the low-carbohydrate diet group were able to reduce or eliminate the number of medications they were taking, compared with 62% of patients in the low–glycemic diet group (P less than .01).
Dr. Hallberg and colleagues are currently in year 4 of the 5-year Indiana Type 2 Diabetes Reversal Study, a prospective, nonrandomized, controlled trial of carbohydrate restriction in 465 patients, making it the largest and longest study of its kind. Of the 465 patients, 387 are in the continuous-care arm, which consists of a diet from Virta Health based on principles of nutritional ketosis, and 87 patients in a usual care arm who are followed for 2 years. The trial includes patients who have been prescribed insulin and who have been diagnosed with diabetes for an average of 8 years.
At the meeting, Dr. Hallberg presented preliminary results based on 2 years of data collection. The retention rate was 83% at 1 year and 74% at 2 years. In the treatment arm, the researchers observed that the level of beta hydroxybutyrate, or evidence of ketogenesis, was the same at 2 years as it had been at 1 year. “So, people were still following the diet, as well as being engaged,” she said.
At the end of 2 years, the mean HbA1c reduction was 0.9, the mean reduction for the Homeostatic Model Assessment of Insulin Resistance was 32%, and 55% of completers experienced reversal of their diabetes. Overall, 91% of insulin users reduced or eliminated their use of insulin, and the average weight loss was 10% of baseline weight. “Medication reduction was across the board,” she added. “This is huge from a cost-savings and a patient-satisfaction standpoint. We were improving A1c levels in patients who have had diabetes for an average of over 8 years while we were getting [them] off medication, including insulin. Low carb is now the standard of care.”
Even patients who did not experience a reversal of their diabetes were conferred a benefit. They had an average reduction of 1.2 in HbA1c level, to 7%; their average weight loss was 9.8%; 45% of patients eliminated their diabetes prescriptions; 81% reduced or eliminated their use of insulin; there was an average reduction of 27% in triglyceride levels; and they had a 17% reduction in their 10-year risk score for atherosclerotic cardiovascular disease.
In the overall cohort, the 10-year Atherosclerotic Cardiovascular Disease risk score improved by 12%; almost all markers for cardiovascular disease improved at 1 year. “We were giving these patients appropriate support, which I think is key,” Dr. Hallberg said. “No matter what you do, you have to have a high-touch intervention, and supply that through technology. We do better than medication adherence. Putting patients on a carbohydrate-restricted diet with the appropriate support works for sustainability.”
Dr. Hallberg disclosed that she is an employee of Virta Health and that she is an adviser for Simply Good Foods.
LOS ANGELES – according to Sarah Hallberg, DO.
“Nutritional ketosis supports diabetes reversal by reducing insulin resistance while providing an alternative fuel to glucose with favorable signaling properties,” she said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease.
Low-carbohydrate nutritional patterns including ketosis have extensive clinical trial evidence for improvement of type 2 diabetes, including preliminary results from a 5-year study of 465 patients enrolled in the Indiana Type 2 Diabetes Reversal Trial that Dr. Hallberg is overseeing in her role as medical director and founder of the medically supervised weight-loss program at Indiana University Health Arnett, Lafayette.
“The ketogenic diet is not a fad diet, it’s what we used to treat people with before the advent of insulin,” said Dr. Hallberg, who has been recommending and counseling patients with type 2 diabetes to follow a ketogenic diet for nearly 10 years. “Of course, insulin has been wonderful. It’s saved so many people with type 1 diabetes. But we also misused it in type 2 diabetes. Instead of counseling people the way we used to about the food that they’re taking in to control their blood sugar, we’ve just been putting [them] on medication, including insulin.”
The American Diabetes Association and other organizations have updated their guidelines to include low-carbohydrate eating patterns for type 2 diabetes treatment, she continued. Veterans Affairs/Department of Defense recommend carbohydrate levels as low as 14%.
Dr. Hallberg, who is also medical director for Virta Health, defined a very-low-carbohydrate or ketogenic diet as less than 50 g of carbohydrates per day, or fewer than 10% of calories consumed. A low-carbohydrate diet is 51-130 g of carbohydrates per day, or 25% or fewer calories consumed, whereas anything above 25% calories consumed is a not a low-carbohydrate diet. A well-formulated ketogenic diet, she continued, consists of 5%-10% carbohydrates (or less than 50 g), 15%-20% protein, and 70%-80% fat. The carbohydrates include 5-10 g per day of protein-based food, 10-15 g of vegetables, 5-10 g of nuts/seeds, 5-10 g of fruits, and 5-10 g of miscellaneous nutrients. “When we’re talking about a total carbohydrate intake per day of under 50 g, you can get a lot of vegetables and nuts in,” she said. “I like to tell my patients they’re not eating GPS: no grains, no potatoes, and no sugar.”
Recently, Dr. Hallberg and colleagues published a review in which they sought to evaluate the appropriateness of sources cited in the ADA’s guidelines on eating patterns for the management of type 2 diabetes, identify additional relevant sources, and evaluate the evidence (Diabetes Obes Metab. 2019;21[8]:1769-79). “We looked at how much evidence there is for the low-carb diet, the Mediterranean diet, the DASH [Dietary Approaches to Stop Hypertension] diet, and a plant-based diet,” she said. “We found a wide variation in the evidence for each eating pattern, but the low-carb eating pattern for diabetes has so much more evidence than any of the other eating patterns.”
In an earlier study, researchers followed 10 inpatients with diabetes in a metabolic ward for 3 weeks. Their mean age was 51 years, and their mean body mass index was 40.3 kg/m2. The patients were fed a standard diet for 7 days, then a low-carbohydrate diet (21 g per day) for 14 days (Ann Intern Med 2005; 142[6]:403-11). After 2 weeks of the low-carbohydrate diet, their mean fasting blood glucose dropped from 7.5 to 6.3 mmol/L, and their mean hemoglobin A1c (HbA1c) fell from 7.3% to 6.8%. “The levels came down very fast,” said Dr. Hallberg, who was not involved with the study. “This is an important part of the intervention, because when you get a patient who’s tried everything, who’s injecting hundreds of units of insulin every day, you can make a huge difference in the first couple of weeks. It is not unusual for us to pull patients off of 200-plus units of insulin. This is as motivating as all get out. It also affects their pocketbook right away. This is one of the reasons our patients are able to sustain a ketogenic diet along with support: early motivation and satisfaction.”
In a longer-term trial, researchers evaluated the impact of a ketogenic diet in 64 obese patients with diabetes over the course of 56 weeks (Moll Cell Biochem. 2007;302[1-2]:249-56). The body weight, body mass index, and levels of blood glucose, total cholesterol, LDL cholesterol, triglycerides, and urea showed a significant decrease from week 1 to week 56 (P less than .0001), while the level of HDL cholesterol increased significantly (P less than .0001).
A separate trial conducted in Israel evaluated the effects of a low-carbohydrate diet, compared with a Mediterranean or low-fat diet in 322 moderately obese patients over the course of 2 years (N Engl J Med. 2008;359:229-41). The rate of adherence to a study diet was 85% at 2 years. The mean weight change was greatest for those on the low-carbohydrate diet, followed by the Mediterranean and low-fat diets. Fasting glucose was best for those on the Mediterranean diet at the end of 2 years, whereas change in HbA1c was best among those on the low-carbohydrate diet.
Another study randomized patients to a low-carbohydrate ketogenic diet (less than 20 g per day with no calorie restriction) or to a low–glycemic index diet (55% carbohydrate restriction of 500 kcal from baseline) over the course of 24 weeks (Nutr Metab [Lond]. 2008 Dec 19. doi:10.1186/1743-7075-5-36). Between baseline and week 24, the mean HbA1c fell from 8.8% to 7.3% in the very-low-carbohydrate diet group, and from 8.3% to 7.8% in the low–glycemic diet group, for a between-group comparison P value of .03. In addition, 95% of patients in the low-carbohydrate diet group were able to reduce or eliminate the number of medications they were taking, compared with 62% of patients in the low–glycemic diet group (P less than .01).
Dr. Hallberg and colleagues are currently in year 4 of the 5-year Indiana Type 2 Diabetes Reversal Study, a prospective, nonrandomized, controlled trial of carbohydrate restriction in 465 patients, making it the largest and longest study of its kind. Of the 465 patients, 387 are in the continuous-care arm, which consists of a diet from Virta Health based on principles of nutritional ketosis, and 87 patients in a usual care arm who are followed for 2 years. The trial includes patients who have been prescribed insulin and who have been diagnosed with diabetes for an average of 8 years.
At the meeting, Dr. Hallberg presented preliminary results based on 2 years of data collection. The retention rate was 83% at 1 year and 74% at 2 years. In the treatment arm, the researchers observed that the level of beta hydroxybutyrate, or evidence of ketogenesis, was the same at 2 years as it had been at 1 year. “So, people were still following the diet, as well as being engaged,” she said.
At the end of 2 years, the mean HbA1c reduction was 0.9, the mean reduction for the Homeostatic Model Assessment of Insulin Resistance was 32%, and 55% of completers experienced reversal of their diabetes. Overall, 91% of insulin users reduced or eliminated their use of insulin, and the average weight loss was 10% of baseline weight. “Medication reduction was across the board,” she added. “This is huge from a cost-savings and a patient-satisfaction standpoint. We were improving A1c levels in patients who have had diabetes for an average of over 8 years while we were getting [them] off medication, including insulin. Low carb is now the standard of care.”
Even patients who did not experience a reversal of their diabetes were conferred a benefit. They had an average reduction of 1.2 in HbA1c level, to 7%; their average weight loss was 9.8%; 45% of patients eliminated their diabetes prescriptions; 81% reduced or eliminated their use of insulin; there was an average reduction of 27% in triglyceride levels; and they had a 17% reduction in their 10-year risk score for atherosclerotic cardiovascular disease.
In the overall cohort, the 10-year Atherosclerotic Cardiovascular Disease risk score improved by 12%; almost all markers for cardiovascular disease improved at 1 year. “We were giving these patients appropriate support, which I think is key,” Dr. Hallberg said. “No matter what you do, you have to have a high-touch intervention, and supply that through technology. We do better than medication adherence. Putting patients on a carbohydrate-restricted diet with the appropriate support works for sustainability.”
Dr. Hallberg disclosed that she is an employee of Virta Health and that she is an adviser for Simply Good Foods.
LOS ANGELES – according to Sarah Hallberg, DO.
“Nutritional ketosis supports diabetes reversal by reducing insulin resistance while providing an alternative fuel to glucose with favorable signaling properties,” she said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease.
Low-carbohydrate nutritional patterns including ketosis have extensive clinical trial evidence for improvement of type 2 diabetes, including preliminary results from a 5-year study of 465 patients enrolled in the Indiana Type 2 Diabetes Reversal Trial that Dr. Hallberg is overseeing in her role as medical director and founder of the medically supervised weight-loss program at Indiana University Health Arnett, Lafayette.
“The ketogenic diet is not a fad diet, it’s what we used to treat people with before the advent of insulin,” said Dr. Hallberg, who has been recommending and counseling patients with type 2 diabetes to follow a ketogenic diet for nearly 10 years. “Of course, insulin has been wonderful. It’s saved so many people with type 1 diabetes. But we also misused it in type 2 diabetes. Instead of counseling people the way we used to about the food that they’re taking in to control their blood sugar, we’ve just been putting [them] on medication, including insulin.”
The American Diabetes Association and other organizations have updated their guidelines to include low-carbohydrate eating patterns for type 2 diabetes treatment, she continued. Veterans Affairs/Department of Defense recommend carbohydrate levels as low as 14%.
Dr. Hallberg, who is also medical director for Virta Health, defined a very-low-carbohydrate or ketogenic diet as less than 50 g of carbohydrates per day, or fewer than 10% of calories consumed. A low-carbohydrate diet is 51-130 g of carbohydrates per day, or 25% or fewer calories consumed, whereas anything above 25% calories consumed is a not a low-carbohydrate diet. A well-formulated ketogenic diet, she continued, consists of 5%-10% carbohydrates (or less than 50 g), 15%-20% protein, and 70%-80% fat. The carbohydrates include 5-10 g per day of protein-based food, 10-15 g of vegetables, 5-10 g of nuts/seeds, 5-10 g of fruits, and 5-10 g of miscellaneous nutrients. “When we’re talking about a total carbohydrate intake per day of under 50 g, you can get a lot of vegetables and nuts in,” she said. “I like to tell my patients they’re not eating GPS: no grains, no potatoes, and no sugar.”
Recently, Dr. Hallberg and colleagues published a review in which they sought to evaluate the appropriateness of sources cited in the ADA’s guidelines on eating patterns for the management of type 2 diabetes, identify additional relevant sources, and evaluate the evidence (Diabetes Obes Metab. 2019;21[8]:1769-79). “We looked at how much evidence there is for the low-carb diet, the Mediterranean diet, the DASH [Dietary Approaches to Stop Hypertension] diet, and a plant-based diet,” she said. “We found a wide variation in the evidence for each eating pattern, but the low-carb eating pattern for diabetes has so much more evidence than any of the other eating patterns.”
In an earlier study, researchers followed 10 inpatients with diabetes in a metabolic ward for 3 weeks. Their mean age was 51 years, and their mean body mass index was 40.3 kg/m2. The patients were fed a standard diet for 7 days, then a low-carbohydrate diet (21 g per day) for 14 days (Ann Intern Med 2005; 142[6]:403-11). After 2 weeks of the low-carbohydrate diet, their mean fasting blood glucose dropped from 7.5 to 6.3 mmol/L, and their mean hemoglobin A1c (HbA1c) fell from 7.3% to 6.8%. “The levels came down very fast,” said Dr. Hallberg, who was not involved with the study. “This is an important part of the intervention, because when you get a patient who’s tried everything, who’s injecting hundreds of units of insulin every day, you can make a huge difference in the first couple of weeks. It is not unusual for us to pull patients off of 200-plus units of insulin. This is as motivating as all get out. It also affects their pocketbook right away. This is one of the reasons our patients are able to sustain a ketogenic diet along with support: early motivation and satisfaction.”
In a longer-term trial, researchers evaluated the impact of a ketogenic diet in 64 obese patients with diabetes over the course of 56 weeks (Moll Cell Biochem. 2007;302[1-2]:249-56). The body weight, body mass index, and levels of blood glucose, total cholesterol, LDL cholesterol, triglycerides, and urea showed a significant decrease from week 1 to week 56 (P less than .0001), while the level of HDL cholesterol increased significantly (P less than .0001).
A separate trial conducted in Israel evaluated the effects of a low-carbohydrate diet, compared with a Mediterranean or low-fat diet in 322 moderately obese patients over the course of 2 years (N Engl J Med. 2008;359:229-41). The rate of adherence to a study diet was 85% at 2 years. The mean weight change was greatest for those on the low-carbohydrate diet, followed by the Mediterranean and low-fat diets. Fasting glucose was best for those on the Mediterranean diet at the end of 2 years, whereas change in HbA1c was best among those on the low-carbohydrate diet.
Another study randomized patients to a low-carbohydrate ketogenic diet (less than 20 g per day with no calorie restriction) or to a low–glycemic index diet (55% carbohydrate restriction of 500 kcal from baseline) over the course of 24 weeks (Nutr Metab [Lond]. 2008 Dec 19. doi:10.1186/1743-7075-5-36). Between baseline and week 24, the mean HbA1c fell from 8.8% to 7.3% in the very-low-carbohydrate diet group, and from 8.3% to 7.8% in the low–glycemic diet group, for a between-group comparison P value of .03. In addition, 95% of patients in the low-carbohydrate diet group were able to reduce or eliminate the number of medications they were taking, compared with 62% of patients in the low–glycemic diet group (P less than .01).
Dr. Hallberg and colleagues are currently in year 4 of the 5-year Indiana Type 2 Diabetes Reversal Study, a prospective, nonrandomized, controlled trial of carbohydrate restriction in 465 patients, making it the largest and longest study of its kind. Of the 465 patients, 387 are in the continuous-care arm, which consists of a diet from Virta Health based on principles of nutritional ketosis, and 87 patients in a usual care arm who are followed for 2 years. The trial includes patients who have been prescribed insulin and who have been diagnosed with diabetes for an average of 8 years.
At the meeting, Dr. Hallberg presented preliminary results based on 2 years of data collection. The retention rate was 83% at 1 year and 74% at 2 years. In the treatment arm, the researchers observed that the level of beta hydroxybutyrate, or evidence of ketogenesis, was the same at 2 years as it had been at 1 year. “So, people were still following the diet, as well as being engaged,” she said.
At the end of 2 years, the mean HbA1c reduction was 0.9, the mean reduction for the Homeostatic Model Assessment of Insulin Resistance was 32%, and 55% of completers experienced reversal of their diabetes. Overall, 91% of insulin users reduced or eliminated their use of insulin, and the average weight loss was 10% of baseline weight. “Medication reduction was across the board,” she added. “This is huge from a cost-savings and a patient-satisfaction standpoint. We were improving A1c levels in patients who have had diabetes for an average of over 8 years while we were getting [them] off medication, including insulin. Low carb is now the standard of care.”
Even patients who did not experience a reversal of their diabetes were conferred a benefit. They had an average reduction of 1.2 in HbA1c level, to 7%; their average weight loss was 9.8%; 45% of patients eliminated their diabetes prescriptions; 81% reduced or eliminated their use of insulin; there was an average reduction of 27% in triglyceride levels; and they had a 17% reduction in their 10-year risk score for atherosclerotic cardiovascular disease.
In the overall cohort, the 10-year Atherosclerotic Cardiovascular Disease risk score improved by 12%; almost all markers for cardiovascular disease improved at 1 year. “We were giving these patients appropriate support, which I think is key,” Dr. Hallberg said. “No matter what you do, you have to have a high-touch intervention, and supply that through technology. We do better than medication adherence. Putting patients on a carbohydrate-restricted diet with the appropriate support works for sustainability.”
Dr. Hallberg disclosed that she is an employee of Virta Health and that she is an adviser for Simply Good Foods.
EXPERT ANALYSIS FROM WCIRDC 2019
2020 Update on obstetrics
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
Papules on hands and soles
Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.
Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.
FDA approves pembrolizumab for BCG-unresponsive NMIBC with CIS
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, non–muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or have elected not to undergo cystectomy.
The approval was based on response in a single-arm trial of 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors, the FDA said in a statement.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% confidence interval, 31-51) and median response duration was 16.2 months.
The most common adverse reactions in patients who received pembrolizumab in the trial were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism, and nasopharyngitis.
The recommended dose is 200 mg every 3 weeks, the FDA said.
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
What is optimal hormonal treatment for women with polycystic ovary syndrome?
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
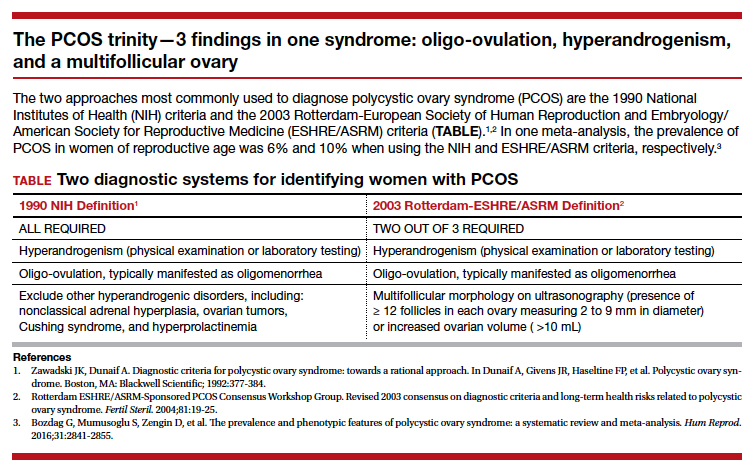
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.