User login
Treating pain with virtual reality
Pilot studies are underway
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
Pilot studies are underway
Pilot studies are underway
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
Physicians may soon have another tool to help patients deal with pain: virtual reality (VR) therapy. A New York Times article earlier this year described this new treatment option and the way immersive VR experiences seem to crowd pain sensations out of the brain.
Jeffrey I. Gold, PhD, director of the Children’s Outcomes, Research, and Evaluation program at Children’s Hospital Los Angeles, told the newspaper that VR was “like an endogenous narcotic providing a physiological and chemical burst that causes you to feel good.”
So far, VR has been most successfully used in cases of acute pain. “But it can also enhance the effectiveness of established techniques like physical therapy, hypnosis and cognitive behavioral therapy to treat debilitating chronic pain,” the New York Times reported.
“Using VR as an adjunct, we can teach coping skills, techniques patients can use on their own that will help diminish chronic pain,” said Hunter Hoffman, PhD, principal investigator at the Human Photonics Laboratory of the University of Washington, Seattle. “Learning changes the brain and gives patients something that continues to work when they take the helmet off. When patients realize their pain isn’t inevitable, they’re more receptive to doing physical therapy exercises and more likely to move on their own.”
Others with experience in VR say the technique can foster mindfulness, which teaches the mind how to quiet the body and nervous system through breathing.
Pilot studies of VR and pain management are underway, and software companies are developing programs that create therapeutic VR environments.
Reference
1. “Virtual Reality as Therapy for Pain.” Jane E. Brody, New York Times. 2019 Apr 29. https://www.nytimes.com/2019/04/29/well/live/virtual-reality-as-therapy-for-pain.html.
Don’t neglect urinary tract in gynecologic procedures
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Beware the dangers of nerve injury in vaginal surgery
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Laparoscopic techniques for Essure device removal
ODYSSEY Outcomes: Alirocumab cut stroke, PAD, VTE
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
REPORTING FROM THE AHA 2019
Cartilage Sutures for a Large Nasal Defect
Practice Gap
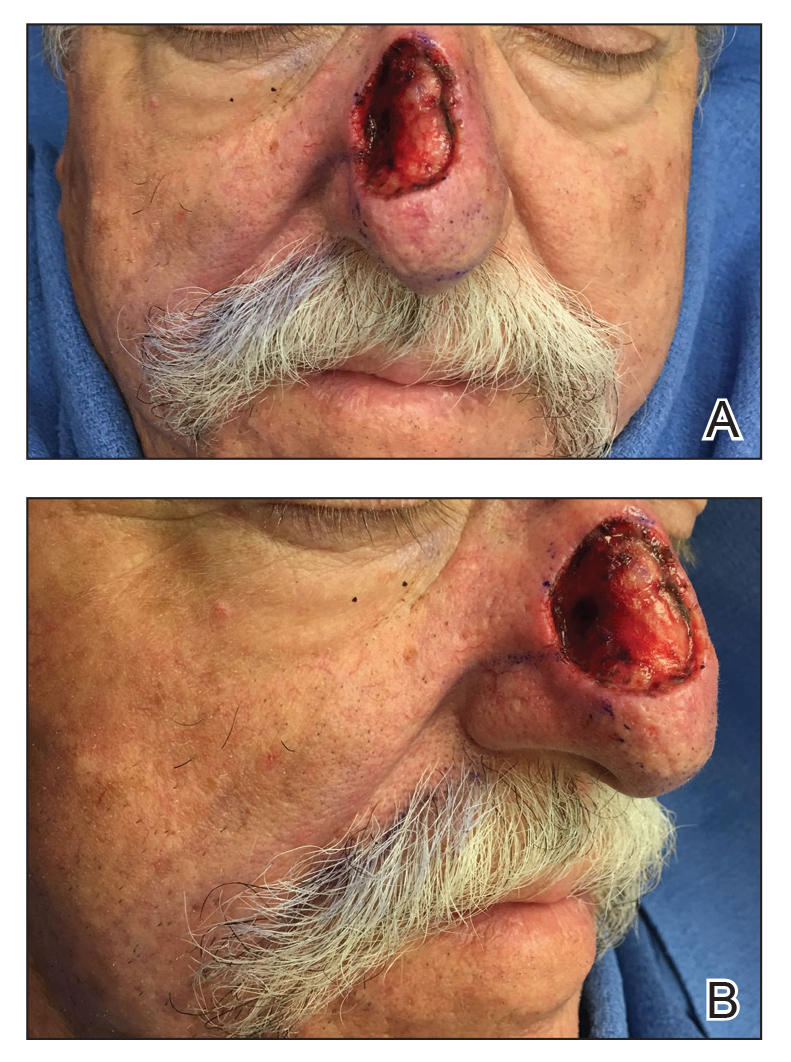
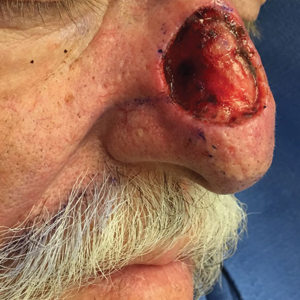
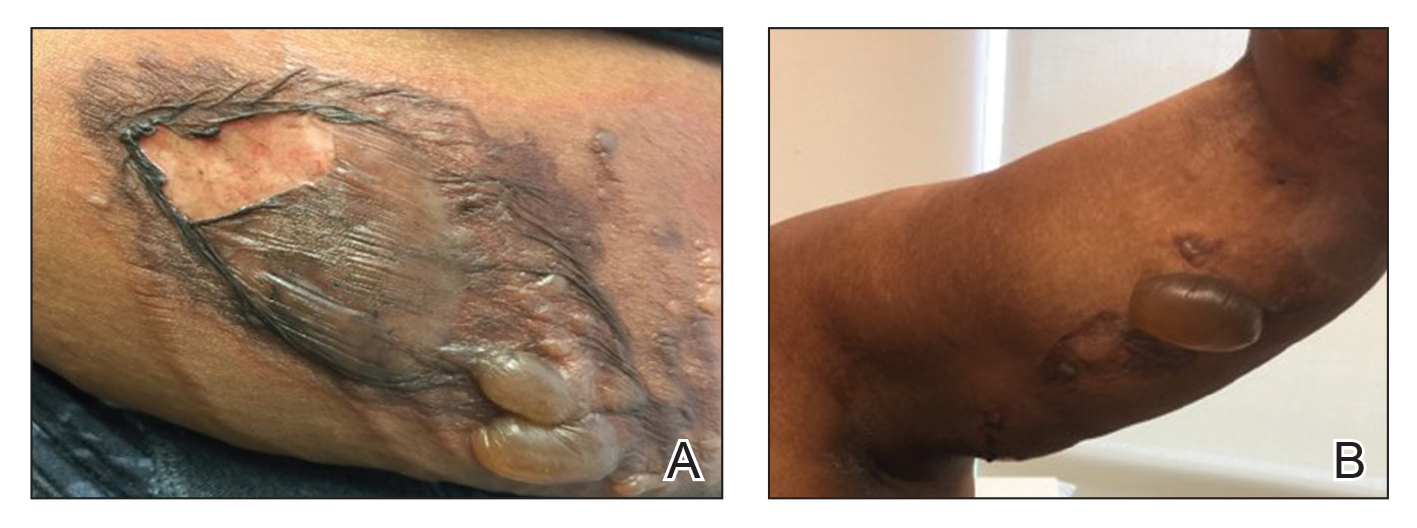
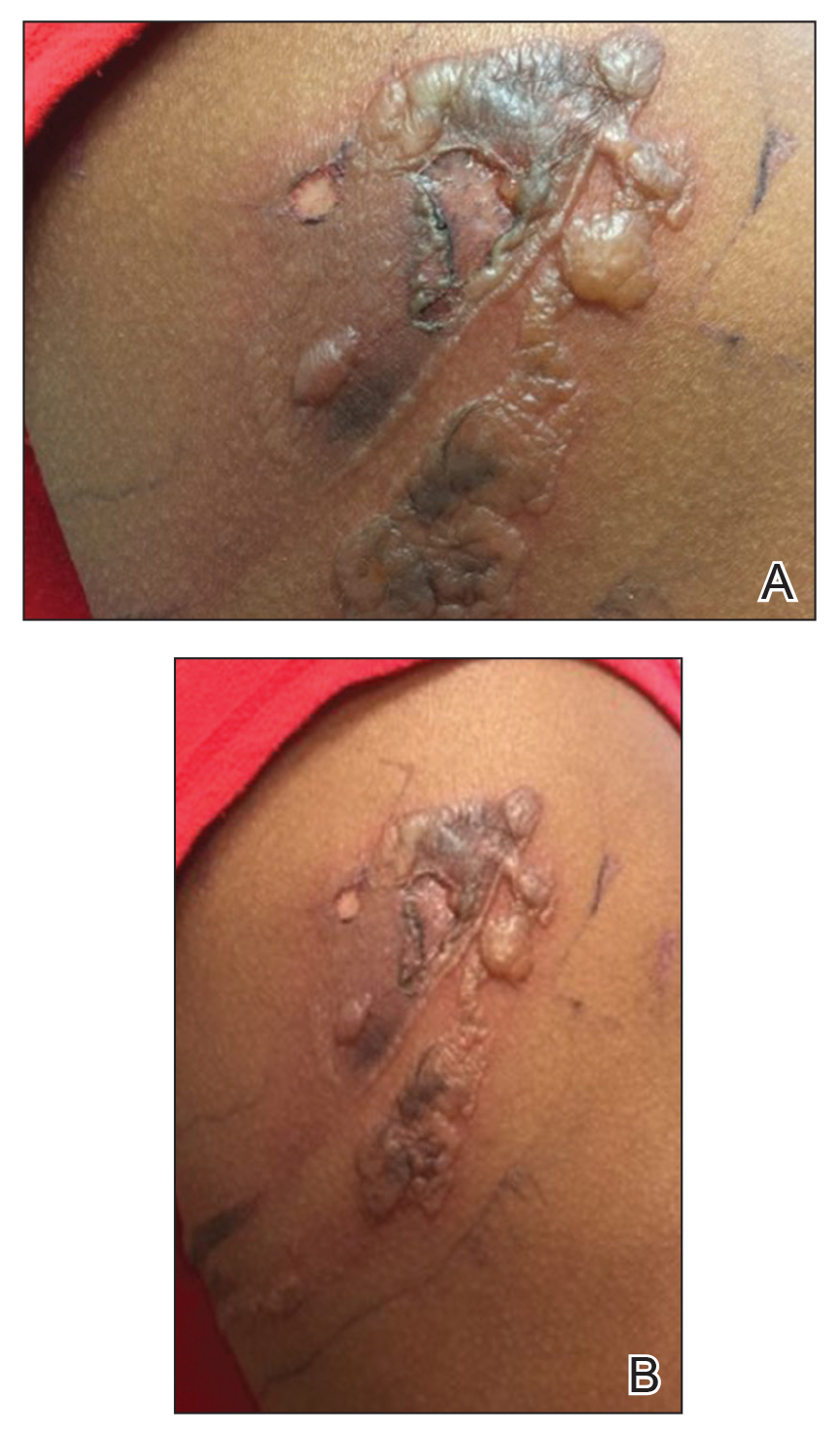
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
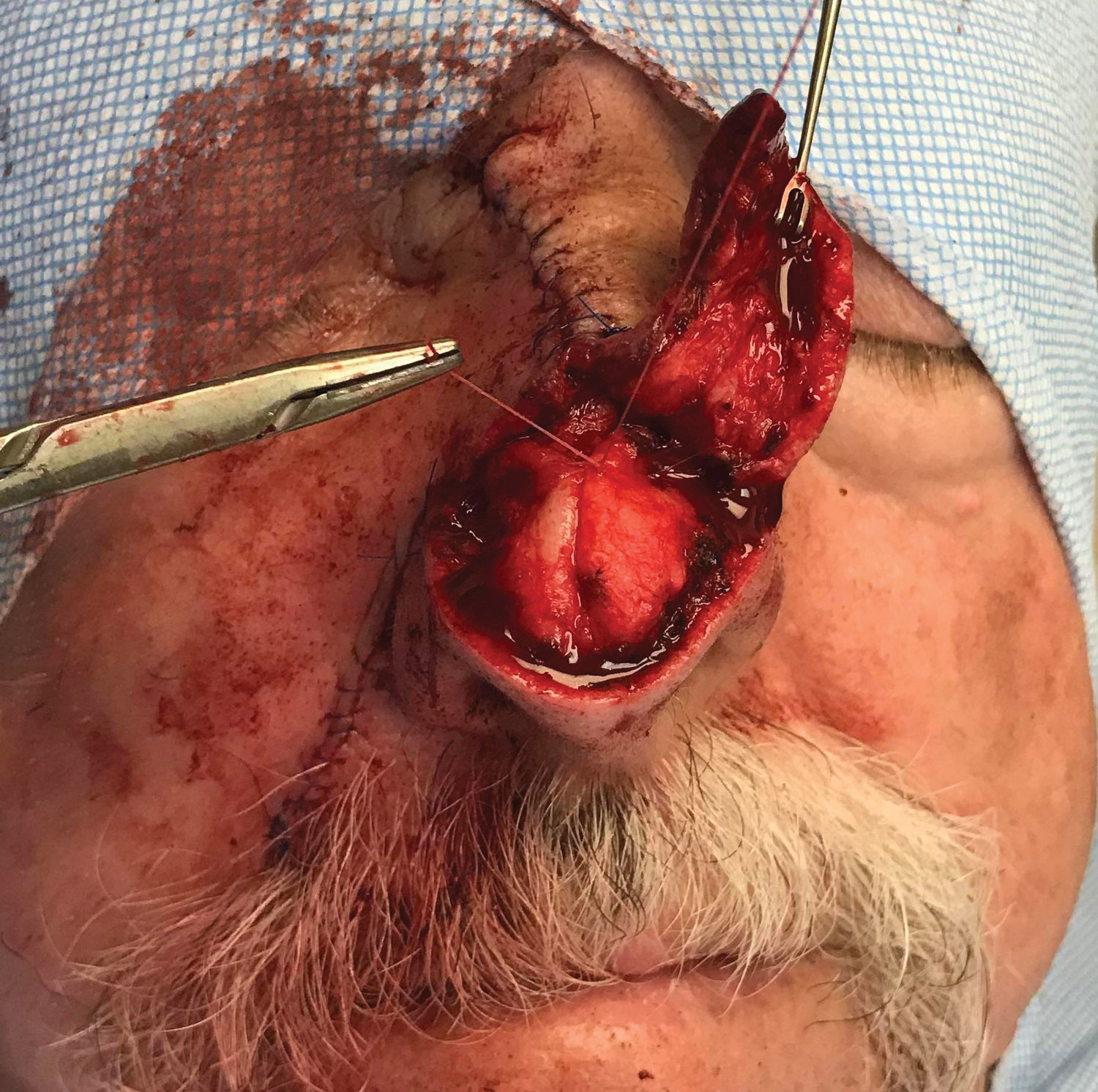
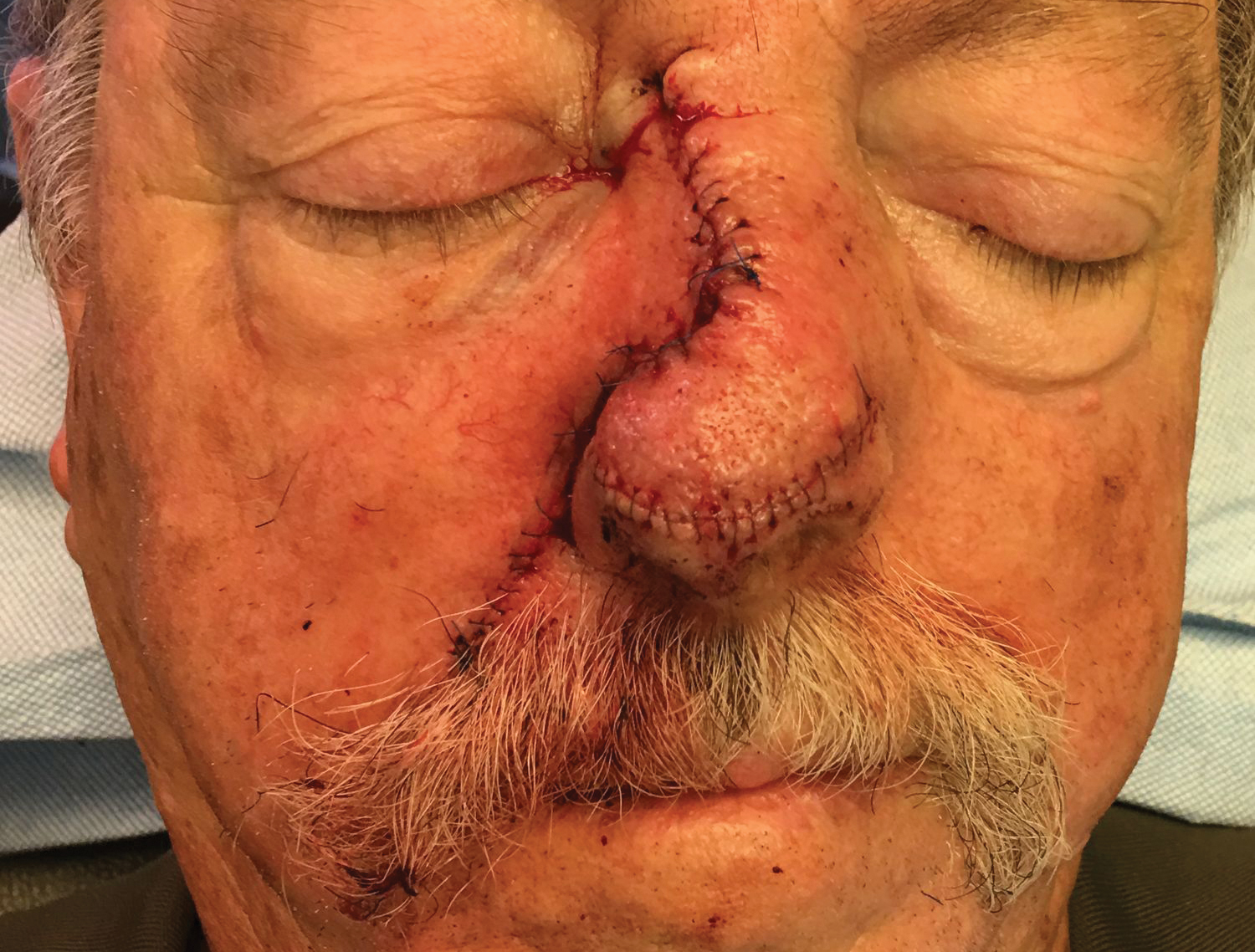
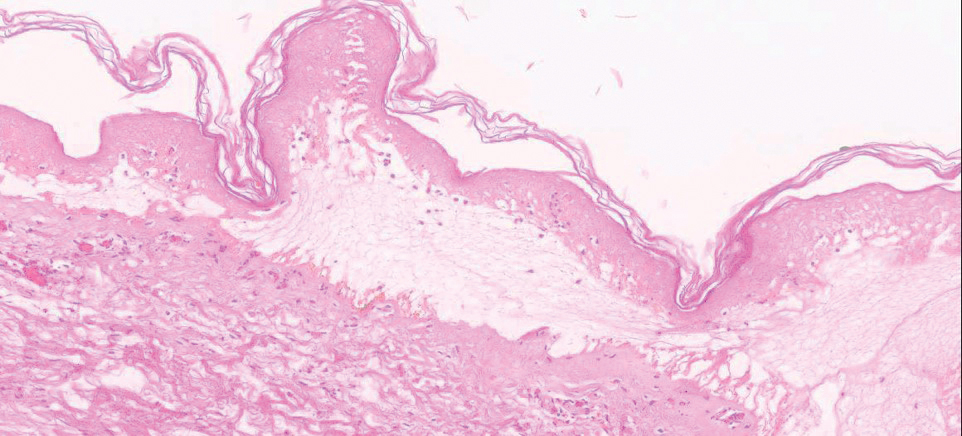
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
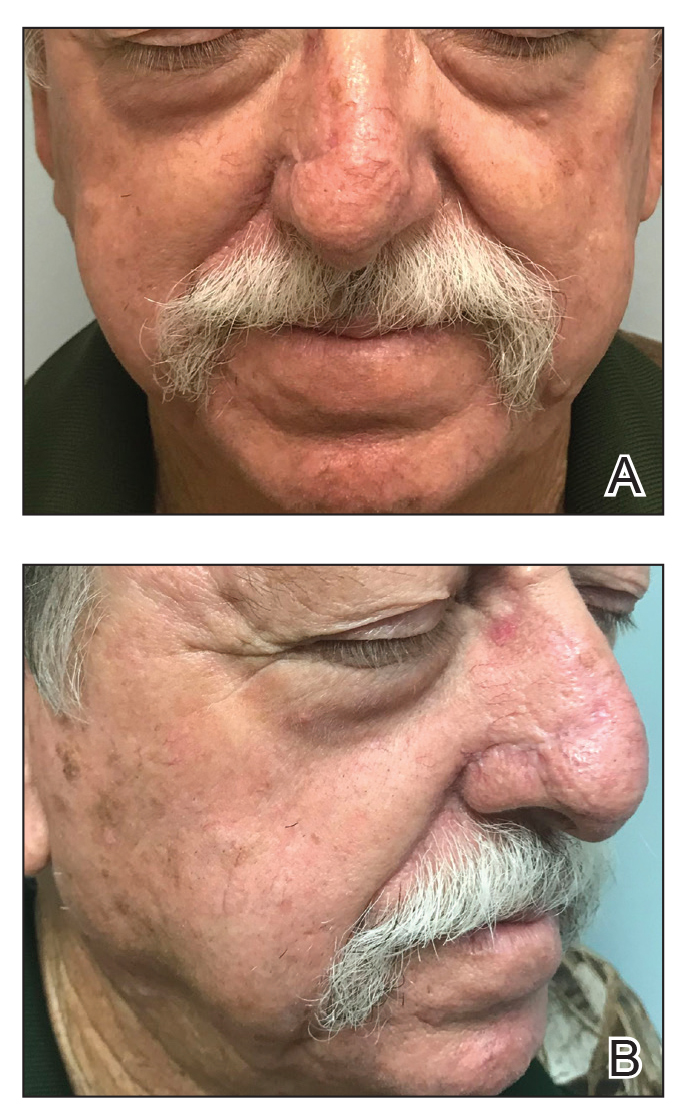
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
A Comparison of Knowledge Acquisition and Perceived Efficacy of a Traditional vs Flipped Classroom–Based Dermatology Residency Curriculum
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).
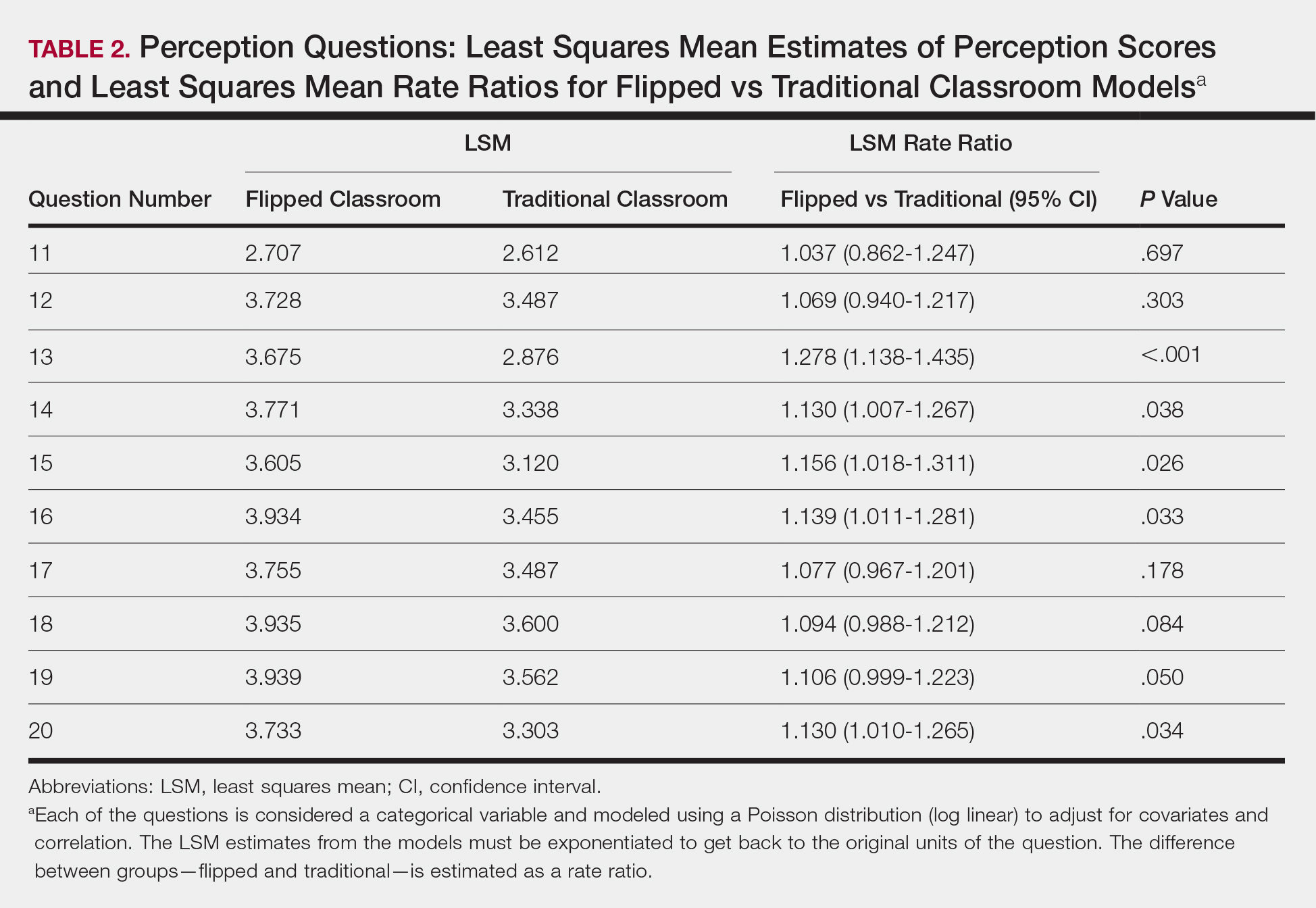
Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
Practice Points
- There was not a significant difference in dermatology resident factual quiz scores when comparing flipped vs traditional classroom teaching sessions.
- There was a significant difference between the flipped vs traditional teaching models, with dermatology residents favoring the flipped classroom, for co-resident lecture participation and individual and co-resident enjoyment of the lecture.
- Residents also perceived that the flipped classroom sessions were more efficient, better prepared them for boards, and better prepared them for clinical practice.
Mystery Burns and Nocturnal Seizure Safety
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Practice Points
- Burns and scars from burns can lead to both life-threatening consequences and lifelong psychological effects.
- Many epileptic patients who present with thermal burn injuries do not remember getting burned.
- Clinicians should be aware of all the potential dangers that patients with epilepsy may encounter both during the day and night.
Is Artificial Intelligence Going to Replace Dermatologists?
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Practice Points
- The use of computer-assisted diagnosis in medicine dates back to the 1960s in radiology.
- New techniques in machine learning, also known as deep learning, were introduced around 2010. Compared to the predecessor forms of computing, these new methods are dynamically changing systems that improve with continuous data exposure and therefore performance is dependent on the quality and generalizability of the training data sets.
- Standardized large data sets and prospective real-life clinical trials are lacking in radiology and subsequently dermatology for diagnosis.
- Artificial intelligence is helpful with triaging and is improving workflow efficiency for radiologists by helping prioritize tasks, which is the current direction for dermatology.