User login
AGA News
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
Accelerating the careers of future hospitalists
Grant program provides funding, research support
When it comes to what future hospitalists should be doing to accelerate their careers, is there such a thing as a “no-brainer” opportunity? Aram Namavar, MD, MS, thinks so.
Dr. Namavar is a first-year internal medicine resident at UC San Diego pursuing a career as an academic hospitalist. He is passionate about building interdisciplinary platforms for patient care enhancement and serving disadvantaged and underserved communities.
Membership in the Society of Hospital Medicine is free for medical students and offers a diverse array of resources specifically curated for the ever-expanding needs of the specialty and its aspiring leaders. An active member of SHM since 2015, Dr. Namavar has looked to the organization for leading career-enhancing opportunities and resources in hospital medicine to help him achieve his altruistic career goals.
For Dr. Namavar, a few of these professional development–focused opportunities include becoming an active member of the Physicians-in-Training Committee, a founding member of the Resident and Student Special Interest Group, and a recipient of the Student Hospitalist Scholar Grant.
“I applied for the Student Hospitalist Scholar Grant to have a dedicated summer of learning quality improvement through being in meetings with hospital medicine leaders and leading my research initiatives alongside my team,” Dr. Namavar said. He described the experience as pivotal to his growth within hospital medicine and as a medical student.
The key component to SHM’s Student Hospitalist Scholar Grant opportunity is the ability for first- and second-year medical students to work alongside leading hospital medicine professionals in scholarly projects to help interested students gain perspective on working within the specialty.
“As a young, interested trainee in hospital medicine, working with a mentor who is established in the field allows one to learn what steps to take in the future to become a leader,” he said. “[It allowed me to] gain insight into leadership style and develop a strong network for the future.”
In addition to the program’s mentorship benefits, grant recipients also receive complimentary registration to SHM’s Annual Conference with the added perks of funding and research support, accommodation expenses, and acceptance into SHM’s RIV Poster Competition.
“I attended the SHM Annual Conference previously,” Dr. Namavar said. “However, as a grant recipient, you have the chance to connect with faculty who will come to your poster presentation and want to learn about your project. This platform allows you to meet individuals from across the nation and connect with those interested in helping trainees thrive within hospital medicine.”
With the grant funding, Dr. Namavar completed his project, “Evaluation of Decisional Conflict as a Simple Tool to Assess Risk of Readmission.” He described this endeavor as a multidimensional project that took on a holistic view of patient-centered readmissions. “We evaluated patient conflict in posthospitalization resources as a marker of readmission, social determinants of health, and health literacy as risk factors for hospital readmission.”
Described by Dr. Namavar as a “no-brainer” opportunity, SHM’s Student Hospitalist Scholar Grant “offers some of the best benefits overall – funding for your project, automatic acceptance at the Annual Conference, the chance to have your work highlighted in blog posts, networking opportunities with faculty across the nation, and travel reimbursement for the conference.”
Building your networks or establishing your professional career path does not stop at individual networking events or scholarship programs, Dr. Namavar said. It’s about piecing together the building blocks to set yourself up for success.
“My long-term involvement in SHM through working on a committee, leading a special interest group, attending annual meetings, and receiving the grant from SHM has helped me to build new, long-lasting connections in the field,” he said. “Because of this, I plan to continue to serve within SHM in multiple capacities throughout my career in hospital medicine.”
Are you a first- or second-year medical student interested in taking the next step in your hospital medicine career? Apply to SHM’s Student Hospitalist Scholar Grant program through late January 2020 at hospitalmedicine.org/scholargrant.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Grant program provides funding, research support
Grant program provides funding, research support
When it comes to what future hospitalists should be doing to accelerate their careers, is there such a thing as a “no-brainer” opportunity? Aram Namavar, MD, MS, thinks so.
Dr. Namavar is a first-year internal medicine resident at UC San Diego pursuing a career as an academic hospitalist. He is passionate about building interdisciplinary platforms for patient care enhancement and serving disadvantaged and underserved communities.
Membership in the Society of Hospital Medicine is free for medical students and offers a diverse array of resources specifically curated for the ever-expanding needs of the specialty and its aspiring leaders. An active member of SHM since 2015, Dr. Namavar has looked to the organization for leading career-enhancing opportunities and resources in hospital medicine to help him achieve his altruistic career goals.
For Dr. Namavar, a few of these professional development–focused opportunities include becoming an active member of the Physicians-in-Training Committee, a founding member of the Resident and Student Special Interest Group, and a recipient of the Student Hospitalist Scholar Grant.
“I applied for the Student Hospitalist Scholar Grant to have a dedicated summer of learning quality improvement through being in meetings with hospital medicine leaders and leading my research initiatives alongside my team,” Dr. Namavar said. He described the experience as pivotal to his growth within hospital medicine and as a medical student.
The key component to SHM’s Student Hospitalist Scholar Grant opportunity is the ability for first- and second-year medical students to work alongside leading hospital medicine professionals in scholarly projects to help interested students gain perspective on working within the specialty.
“As a young, interested trainee in hospital medicine, working with a mentor who is established in the field allows one to learn what steps to take in the future to become a leader,” he said. “[It allowed me to] gain insight into leadership style and develop a strong network for the future.”
In addition to the program’s mentorship benefits, grant recipients also receive complimentary registration to SHM’s Annual Conference with the added perks of funding and research support, accommodation expenses, and acceptance into SHM’s RIV Poster Competition.
“I attended the SHM Annual Conference previously,” Dr. Namavar said. “However, as a grant recipient, you have the chance to connect with faculty who will come to your poster presentation and want to learn about your project. This platform allows you to meet individuals from across the nation and connect with those interested in helping trainees thrive within hospital medicine.”
With the grant funding, Dr. Namavar completed his project, “Evaluation of Decisional Conflict as a Simple Tool to Assess Risk of Readmission.” He described this endeavor as a multidimensional project that took on a holistic view of patient-centered readmissions. “We evaluated patient conflict in posthospitalization resources as a marker of readmission, social determinants of health, and health literacy as risk factors for hospital readmission.”
Described by Dr. Namavar as a “no-brainer” opportunity, SHM’s Student Hospitalist Scholar Grant “offers some of the best benefits overall – funding for your project, automatic acceptance at the Annual Conference, the chance to have your work highlighted in blog posts, networking opportunities with faculty across the nation, and travel reimbursement for the conference.”
Building your networks or establishing your professional career path does not stop at individual networking events or scholarship programs, Dr. Namavar said. It’s about piecing together the building blocks to set yourself up for success.
“My long-term involvement in SHM through working on a committee, leading a special interest group, attending annual meetings, and receiving the grant from SHM has helped me to build new, long-lasting connections in the field,” he said. “Because of this, I plan to continue to serve within SHM in multiple capacities throughout my career in hospital medicine.”
Are you a first- or second-year medical student interested in taking the next step in your hospital medicine career? Apply to SHM’s Student Hospitalist Scholar Grant program through late January 2020 at hospitalmedicine.org/scholargrant.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
When it comes to what future hospitalists should be doing to accelerate their careers, is there such a thing as a “no-brainer” opportunity? Aram Namavar, MD, MS, thinks so.
Dr. Namavar is a first-year internal medicine resident at UC San Diego pursuing a career as an academic hospitalist. He is passionate about building interdisciplinary platforms for patient care enhancement and serving disadvantaged and underserved communities.
Membership in the Society of Hospital Medicine is free for medical students and offers a diverse array of resources specifically curated for the ever-expanding needs of the specialty and its aspiring leaders. An active member of SHM since 2015, Dr. Namavar has looked to the organization for leading career-enhancing opportunities and resources in hospital medicine to help him achieve his altruistic career goals.
For Dr. Namavar, a few of these professional development–focused opportunities include becoming an active member of the Physicians-in-Training Committee, a founding member of the Resident and Student Special Interest Group, and a recipient of the Student Hospitalist Scholar Grant.
“I applied for the Student Hospitalist Scholar Grant to have a dedicated summer of learning quality improvement through being in meetings with hospital medicine leaders and leading my research initiatives alongside my team,” Dr. Namavar said. He described the experience as pivotal to his growth within hospital medicine and as a medical student.
The key component to SHM’s Student Hospitalist Scholar Grant opportunity is the ability for first- and second-year medical students to work alongside leading hospital medicine professionals in scholarly projects to help interested students gain perspective on working within the specialty.
“As a young, interested trainee in hospital medicine, working with a mentor who is established in the field allows one to learn what steps to take in the future to become a leader,” he said. “[It allowed me to] gain insight into leadership style and develop a strong network for the future.”
In addition to the program’s mentorship benefits, grant recipients also receive complimentary registration to SHM’s Annual Conference with the added perks of funding and research support, accommodation expenses, and acceptance into SHM’s RIV Poster Competition.
“I attended the SHM Annual Conference previously,” Dr. Namavar said. “However, as a grant recipient, you have the chance to connect with faculty who will come to your poster presentation and want to learn about your project. This platform allows you to meet individuals from across the nation and connect with those interested in helping trainees thrive within hospital medicine.”
With the grant funding, Dr. Namavar completed his project, “Evaluation of Decisional Conflict as a Simple Tool to Assess Risk of Readmission.” He described this endeavor as a multidimensional project that took on a holistic view of patient-centered readmissions. “We evaluated patient conflict in posthospitalization resources as a marker of readmission, social determinants of health, and health literacy as risk factors for hospital readmission.”
Described by Dr. Namavar as a “no-brainer” opportunity, SHM’s Student Hospitalist Scholar Grant “offers some of the best benefits overall – funding for your project, automatic acceptance at the Annual Conference, the chance to have your work highlighted in blog posts, networking opportunities with faculty across the nation, and travel reimbursement for the conference.”
Building your networks or establishing your professional career path does not stop at individual networking events or scholarship programs, Dr. Namavar said. It’s about piecing together the building blocks to set yourself up for success.
“My long-term involvement in SHM through working on a committee, leading a special interest group, attending annual meetings, and receiving the grant from SHM has helped me to build new, long-lasting connections in the field,” he said. “Because of this, I plan to continue to serve within SHM in multiple capacities throughout my career in hospital medicine.”
Are you a first- or second-year medical student interested in taking the next step in your hospital medicine career? Apply to SHM’s Student Hospitalist Scholar Grant program through late January 2020 at hospitalmedicine.org/scholargrant.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Letter to the Editor
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
Editor’s note: This is one of the many emails readers sent to Dermatology News in response to Dr. Alan Rockoff’s last “Under My Skin” column in the December issue.
Dr. Rockoff,
I was deeply heartbroken to read that this would be your last column in Dermatology News today. I am a medical dermatologist with 19 years of experience in a small town in North Carolina and have always looked forward to reading your columns. No matter what the topic for the article you chose, I would always glean something worthwhile from it, be it a poignant insight, a relevant practice tip on treatment or patient management, and nearly always a hearty laugh.
Being somewhat rural and without a lot of competition, my very busy practice consists mainly of salt of the earth patients needing straight dermatologic care, mostly skin cancer, psoriasis, acne, the general stuff. Your ability to capture the essence of what it is like to be one of us in the trenches, to expose and eloquently define the most common and frustrating issues we face, has been a source of pleasure for years. On more than one occasion, I put down the magazine and attempted to write a thank you letter for one article or another you wrote, to say that you are doing a great job and please continue to enlighten us with your insight and that your efforts are greatly valued. I am embarrassed to admit, I never could complete those emails, thinking “Why bother the man? He is clearly as busy as me, and why would he want to hear from me anyway?”
Well, at the risk of bothering you, sir, please do accept my apologies for not writing you before you retired your article, and please know that your articles have personally given me years of immense happiness. They will be sorely missed, and likely not ever replaced.
With gratitude,
Jeff Suchniak, MD
Rocky Mount, N.C.
ERAS for cesarean delivery: Antenatal and preoperative care
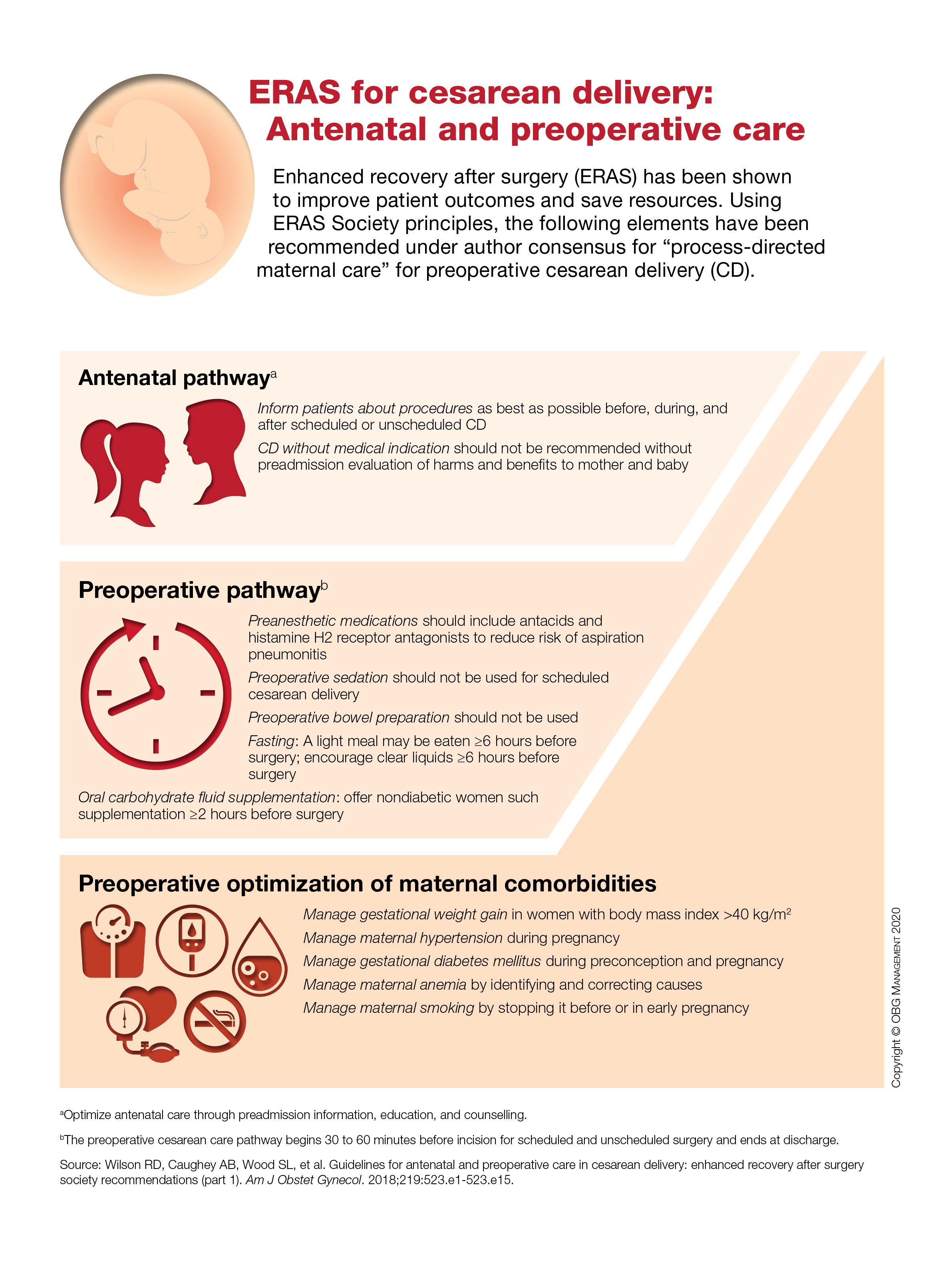


Firm Tumor Encasing the Left Second Toe of an Infant
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).
Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).
Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF), or recurring digital fibrous tumor of childhood, is a benign juvenile myofibromatosis that presents as a firm, flesh-colored or slightly erythematous, dome-shaped papule or nodule on the dorsolateral aspects of the digits, usually sparing the thumbs and great toes.1 The tumor appears most commonly at birth and in infants younger than 1 year. It grows slowly over the first month, then rapidly over the next 10 to 14 months.1,2
Although lesions usually regress spontaneously within a few years, excision may be necessary when functional impairment and joint deformity occur. Tumors, however, may recur locally.1,2
Histologically, IDFs are composed of spindled myofibroblasts with characteristic round eosinophilic intracytoplasmic inclusion bodies, which represent actin and vimentin filaments.1 In our case, histopathologic evaluation showed a proliferation of fibrous spindle cells with pathognomonic eosinophilic intracytoplasmic inclusions consistent with IDF (Figure).
Fibrosarcomas are high-grade and low-grade soft-tissue neoplasms comprised of atypical spindle cells in a herringbone pattern with mitotic figures on pathology.3 They typically present as a slowly growing subcutaneous tumor on the lower extremities of young to middle-aged adults that may progress to become a palpable tender nodule. Infantile hemangiomas, the most common benign soft-tissue tumors of childhood, are vascular proliferations more commonly seen in low-birth-weight female white infants of multiple gestation pregnancies. Superficial hemangiomas present as bright red and lobular nodules or plaques. Deep hemangiomas present as ill-defined, blue-violaceous nodules with no overlying skin changes. Mixed hemangiomas present with features of both superficial and deep hemangiomas. Infantile hemangiomas experience a proliferative phase until 9 to 12 months of age, followed by a gradual involutional phase ending with possible residual telangiectases or fibrofatty change. Unlike IDFs, infantile hemangiomas favor the head and neck over other areas of the body. Keloids are firm, smooth, variably colored papules or plaques of haphazardly arranged thick dermal collagen bundles. They usually develop within a year of skin injury and extend beyond the original injury margin into normal tissue. Supernumerary digits present as small fleshy papules or larger nodules with a vestigial nail, most commonly on the ulnar side of the fifth finger or the radial side of the thumb. Histologically, they are composed of fascicles of nerve fibers.3
Treatment in our patient included partial amputation of the left second toe and excision with local tissue rearrangement by a plastic surgeon. His postoperative course was complicated by a minor wound infection, which resolved with a 7-day course of cephalexin. Since then, the patient has healed well, with gradual toe tissue and nail growth. No recurrence was reported 11 months after surgery.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
- Heymann WR. Infantile digital fibromatosis. J Am Acad Dermatol. 2008;59:122-123.
- Niamba P, Léauté-Labrèze C, Boralevi F, et al. Further documentation of spontaneous regression of infantile digital fibromatosis. Pediatr Dermatol. 2007;24:280-284.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Philadelphia, PA: Elsevier Limited; 2018.
A 10-month-old infant boy presented to the dermatology clinic with a firm, nonpainful, 5.5.×5.6-cm tumor encasing the left second toe, with associated skin breakdown, gait impairment, and lateral displacement of the third toe. The lesion began as a small bump under the toenail at 2 months of age; it then grew rapidly without bleeding or ulceration. It was diagnosed as a hemangioma at 4 months of age, and oral propranolol was initiated for 3 months, without suppression of tumor growth. The patient was referred to the pediatric dermatology department where a clinical diagnosis was made, and the patient was subsequently referred to plastic surgery for excision.
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
FDA approves avapritinib for adults with GIST with PDGFRA mutation
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
FUEL trial: Post-Fontan udenafil shows mixed results
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM AHA 2019