User login
9/11 responders show increased risk of leukemia, other cancers
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
New data suggest an increased risk of leukemia among responders who worked at the World Trade Center site after the attacks on Sept. 11, 2001.
Previous studies have shown that 9/11 responders have a higher incidence of cancers than does the general population. The current study is the first to show a higher incidence of leukemia among responders. It also shows a higher incidence of thyroid and prostate cancers as well as all cancer types combined.
These findings were published in JNCI Cancer Spectrum.
“This study showed increased incidence of several cancer types compared to previously conducted studies with shorter follow-up periods,” study author Susan L, Teitelbaum, PhD, of the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Because of the long latency period of many types of cancer, it is possible that increased rates of other cancers, as well as World Trade Center exposure health issues, may emerge after longer periods of study.”
Dr. Teitelbaum and colleagues evaluated responders enrolled in the World Trade Center Health Program General Responder Cohort from when it was established in July 2002 through the end of follow-up, which was Dec. 31, 2013, for New York residents and Dec. 31, 2012, for residents of other states.
To be eligible for the cohort, responders must have worked on the World Trade Center rescue and recovery effort a minimum of 4 hours in the first 4 days from Sept. 11, 2001, 24 hours in September 2001, or 80 hours from September through December 2001. Responders also had to complete at least one monitoring visit.
Responders’ data were linked to data from cancer registries in New York, New Jersey, Pennsylvania, and Connecticut (where most responders lived at the time of the attacks), as well as Florida and North Carolina (where responders were known to retire). The responders were linked to the registries using probabilistic matching algorithms, which made use of information such as patient name, address, social security number, sex, race, and birth date.
The researchers noted that patients who enrolled in the General Responder Cohort had their cancer certified for federally funded treatment, and this factor might result in “sicker members disproportionately self-selecting into the program.” To reduce this potential bias, the researchers conducted a restricted analysis in which counts of cancer cases and person-years of observation began 6 months after responder enrollment.
The researchers analyzed data on 28,729 responders who primarily worked in protective services (49.0%) and construction (20.8%). Responders spent a median of 52 days on the rescue and recovery effort, and 44.4% of them had some exposure to the dust cloud caused by the collapse of the towers.
In the restricted analysis, there were 1,072 cancers observed in 999 responders. Compared with the general population, responders had a significantly higher incidence of all cancers combined, with a standardized incidence ratio (SIR) of 1.09.
Responders had a significantly higher incidence of prostate cancer (SIR,1.25), thyroid cancer (SIR, 2.19), and leukemia (SIR, 1.41). The leukemia category included acute myeloid leukemia (SIR,1.58) and chronic lymphocytic leukemia (SIR, 1.08).
“Although other studies have revealed elevated SIRs for other hematologic malignancies, this is the first reported, statistically significant, elevated SIR for leukemia,” the researchers wrote. “Leukemia is known to occur after exposure to occupational carcinogens, including benzene (burning jet fuel and other sources at the [World Trade Center] site), possibly at low levels of exposure and with a latency of several years from exposure.”
A multivariate analysis showed no association between cancer incidence and the length of time responders spent on the rescue and recovery effort or the intensity of their exposure to the dust cloud or debris pile.
The analysis did show an elevated risk of all cancers combined with each 1-year increase in responder age (hazard ratio, 1.09), among male responders (HR, 1.21), and among responders who smoked at baseline (HR, 1.29).
This research was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The researchers disclosed no conflicts of interest.
SOURCE: Shapiro MZ et al. JNCI Cancer Spectr. 2020 Jan 14. doi: 10.1093/jncics/pkz090.
FROM JNCI CANCER SPECTRUM
Gout rates reduced with SGLT2 inhibitors
The incidence of gout was approximately 40% lower in diabetes patients who were prescribed sodium-glucose cotransporter 2 inhibitors (SGLT2) than it was in those who were prescribed glucagonlike peptide–1 receptor (GLP-1) agonists in a population-based new-user cohort study.
Hyperuricemia is a known cause of gout and common in type 2 diabetes patients. SGLT2 inhibitors may reduce the risk of gout by preventing the reabsorption of glucose and lowering serum uric acid levels; however, the impact on gout risk remains uncertain, wrote Michael Fralick, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in the Annals of Internal Medicine, the researchers compared SGLT2 inhibitors and GLP-1 agonists in patients with type 2 diabetes to assess protection against gout.
The study population included adults with type 2 diabetes mellitus who had a new prescription for an SGTL2 inhibitor or GLP-1 agonist. The average age of the patients was 54 years; approximately half were women. Baseline characteristics were similar between the groups.
Overall, the researchers found a relative risk reduction of approximately 40% and an absolute risk reduction of approximately three fewer cases per 1,000 person-years in patients who received SGLT2 inhibitors, compared with those who received GLP-1 agonists. The incidence rate for gout in the SGLT2 and GLP-1 groups were 4.9 per 1,000 person-years and 7.8 per 1,000 person-years, respectively.
The study findings were limited by the investigators’ inability to measure potential confounding variables such as body mass index, alcohol use, and high purine diet; incomplete lab data on creatinine and hemoglobin A; and a low baseline risk for gout in the study population, the researchers noted. However, the results persisted across sensitivity analysis and, if replicated, suggest that “SGLT2 inhibitors might be an effective class of medication for the prevention of gout for patients with diabetes or metabolic disorders,” they wrote.
The study was supported in part by Brigham and Women’s Hospital; lead author Dr. Fralick disclosed funding from the Eliot Phillipson Clinician-Scientist Training Program at the University of Toronto and the Canadian Institutes of Health Research.
SOURCE: Fralick M et al. Ann Intern Med. 2020 Jan 14. doi: 10.7326/M19-2610.
The incidence of gout was approximately 40% lower in diabetes patients who were prescribed sodium-glucose cotransporter 2 inhibitors (SGLT2) than it was in those who were prescribed glucagonlike peptide–1 receptor (GLP-1) agonists in a population-based new-user cohort study.
Hyperuricemia is a known cause of gout and common in type 2 diabetes patients. SGLT2 inhibitors may reduce the risk of gout by preventing the reabsorption of glucose and lowering serum uric acid levels; however, the impact on gout risk remains uncertain, wrote Michael Fralick, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in the Annals of Internal Medicine, the researchers compared SGLT2 inhibitors and GLP-1 agonists in patients with type 2 diabetes to assess protection against gout.
The study population included adults with type 2 diabetes mellitus who had a new prescription for an SGTL2 inhibitor or GLP-1 agonist. The average age of the patients was 54 years; approximately half were women. Baseline characteristics were similar between the groups.
Overall, the researchers found a relative risk reduction of approximately 40% and an absolute risk reduction of approximately three fewer cases per 1,000 person-years in patients who received SGLT2 inhibitors, compared with those who received GLP-1 agonists. The incidence rate for gout in the SGLT2 and GLP-1 groups were 4.9 per 1,000 person-years and 7.8 per 1,000 person-years, respectively.
The study findings were limited by the investigators’ inability to measure potential confounding variables such as body mass index, alcohol use, and high purine diet; incomplete lab data on creatinine and hemoglobin A; and a low baseline risk for gout in the study population, the researchers noted. However, the results persisted across sensitivity analysis and, if replicated, suggest that “SGLT2 inhibitors might be an effective class of medication for the prevention of gout for patients with diabetes or metabolic disorders,” they wrote.
The study was supported in part by Brigham and Women’s Hospital; lead author Dr. Fralick disclosed funding from the Eliot Phillipson Clinician-Scientist Training Program at the University of Toronto and the Canadian Institutes of Health Research.
SOURCE: Fralick M et al. Ann Intern Med. 2020 Jan 14. doi: 10.7326/M19-2610.
The incidence of gout was approximately 40% lower in diabetes patients who were prescribed sodium-glucose cotransporter 2 inhibitors (SGLT2) than it was in those who were prescribed glucagonlike peptide–1 receptor (GLP-1) agonists in a population-based new-user cohort study.
Hyperuricemia is a known cause of gout and common in type 2 diabetes patients. SGLT2 inhibitors may reduce the risk of gout by preventing the reabsorption of glucose and lowering serum uric acid levels; however, the impact on gout risk remains uncertain, wrote Michael Fralick, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in the Annals of Internal Medicine, the researchers compared SGLT2 inhibitors and GLP-1 agonists in patients with type 2 diabetes to assess protection against gout.
The study population included adults with type 2 diabetes mellitus who had a new prescription for an SGTL2 inhibitor or GLP-1 agonist. The average age of the patients was 54 years; approximately half were women. Baseline characteristics were similar between the groups.
Overall, the researchers found a relative risk reduction of approximately 40% and an absolute risk reduction of approximately three fewer cases per 1,000 person-years in patients who received SGLT2 inhibitors, compared with those who received GLP-1 agonists. The incidence rate for gout in the SGLT2 and GLP-1 groups were 4.9 per 1,000 person-years and 7.8 per 1,000 person-years, respectively.
The study findings were limited by the investigators’ inability to measure potential confounding variables such as body mass index, alcohol use, and high purine diet; incomplete lab data on creatinine and hemoglobin A; and a low baseline risk for gout in the study population, the researchers noted. However, the results persisted across sensitivity analysis and, if replicated, suggest that “SGLT2 inhibitors might be an effective class of medication for the prevention of gout for patients with diabetes or metabolic disorders,” they wrote.
The study was supported in part by Brigham and Women’s Hospital; lead author Dr. Fralick disclosed funding from the Eliot Phillipson Clinician-Scientist Training Program at the University of Toronto and the Canadian Institutes of Health Research.
SOURCE: Fralick M et al. Ann Intern Med. 2020 Jan 14. doi: 10.7326/M19-2610.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Sodium-glucose cotransporter 2 (SGLT2) inhibitor use was associated with lower rates of gout in type 2 diabetes patients compared with glucagonlike peptide–1 (GLP-1) agonist use.
Major finding: The incidence of gout was 4.9 per 1,000 person-years in patients on SGLT2 inhibitors and 7.8 per 1,000 person-years in patients on GLP1 agonists.
Study details: The data come from a population-based cohort study of 295,907 adults with type 2 diabetes.
Disclosures: The study was supported in part by Brigham and Women’s Hospital; lead author Dr. Fralick disclosed funding from the Eliot Phillipson Clinician-Scientist Training Program at the University of Toronto and the Canadian Institutes of Health Research.
Source: Fralick M et al. Ann Intern Med. 2020 Jan 14. doi: 10.7326/M19-2610.
Draft ACR Takayasu’s guidelines: Surgery is the last resort
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
REPORTING FROM ACR 2019
Drop in flu activity may not signal seasonal peak
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.
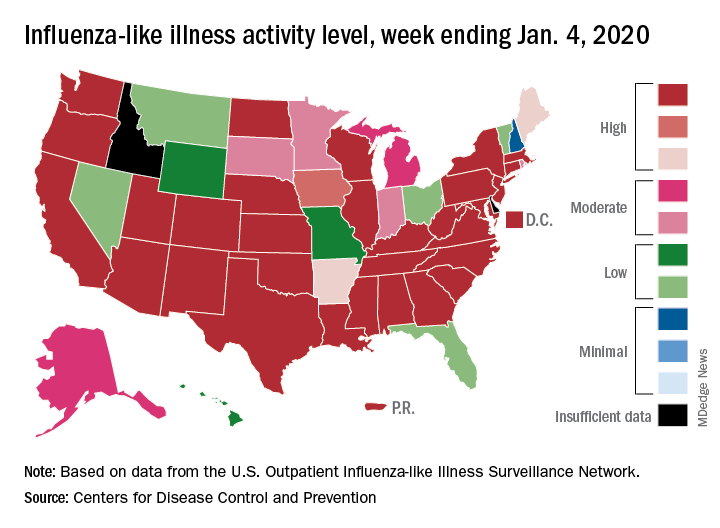
Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
For everything there is a season
2020 SoHM Survey ready to launch
Wow, the last 2 years have just flown by! I can’t believe it’s already time to launch the Society of Hospital Medicine State of Hospital Medicine survey again! Right now is the season for you to roll up your sleeves and get to work helping SHM develop the nation’s definitive resource on the current state of hospital medicine practice.
I’m really excited about this year’s survey. SHM’s Practice Analysis Committee has redesigned it to eliminate some out-of-date or little-used questions and to add a few new, more relevant questions. Even more exciting, we have a new survey platform that should massively improve your experience of submitting data for the survey and also make the back-end data tabulation and analysis much quicker and more accurate. Multisite groups will now have two options for submitting data – a redesigned, more user-friendly Excel tool, or a new pathway to submit data in the reporting platform by replicating responses.
In addition, our new survey platform should help us produce the final report a little more quickly and improve its usability.
New-for-2020 survey topics will include:
- Expanded information on nurse practitioner/physician assistant roles
- Diversity in hospital medicine physician leadership
- Specific questions for hospital medicine groups (HMGs) serving children that will better capture unique attributes of these hospital medicine practices
Why participate?
I can’t emphasize enough that each and every survey submission matters a lot. The State of Hospital Medicine report claims to be the authoritative resource for information about the specialty of hospital medicine. But the report can’t fulfill this claim if the underlying data is skimpy because people were too busy, couldn’t be bothered to participate, or if participation is not broadly representative of the amazing diversity of hospital medicine practices out there.
Your participation will help ensure that you are contributing to a robust hospital medicine database, and that your own group’s information is represented in the survey results. By doing so you will be helping to ensure hospital medicine’s place as perhaps the crucial specialty for U.S. health care in the coming decade.
In addition, participants will receive free access to the survey results, so there’s a direct benefit to you and your HMG as well.
How can you participate?
Here’s what you need to know:
1. The survey opens on Jan.6, 2020, and closes on Feb. 14, 2020.
2. You can find general information about the survey at this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/, and register to participate by using this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/sohm-survey/.
3. To participate, you’ll want to collect the following general types of information for your hospital medicine group:
- Basic group descriptive information (for example, types of patients seen, number of hospitals covered, teaching status, etc.)
- Scope of clinical services
- Nurse practitioners and physician assistants in the HMG
- Full-time equivalent (FTE) information
- Information about the physician leader(s)
- Staffing/scheduling arrangements, including backup plans, paid time off, unfilled positions, predominant scheduling pattern, night coverage arrangements, dedicated admitters, unit-based assignments, etc.
- Compensation model (but not specific amounts)
- Value of employee benefits and CME
- Total work relative value units generated by the HMG, and number of times the following CPT codes were billed: 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239
- Information about financial support provided to the HMG
- Specific questions for academic HMGs, including financial support for nonclinical work, and allocation of FTEs
- Specific questions for HMGs serving children, including the hospital settings served, proportion of part-time staff, FTE definition, and information about board certification in pediatric hospital medicine
I’m hoping that all of you will join me in working to make the 2020 State of Hospital Medicine survey and report the best one yet!
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
2020 SoHM Survey ready to launch
2020 SoHM Survey ready to launch
Wow, the last 2 years have just flown by! I can’t believe it’s already time to launch the Society of Hospital Medicine State of Hospital Medicine survey again! Right now is the season for you to roll up your sleeves and get to work helping SHM develop the nation’s definitive resource on the current state of hospital medicine practice.
I’m really excited about this year’s survey. SHM’s Practice Analysis Committee has redesigned it to eliminate some out-of-date or little-used questions and to add a few new, more relevant questions. Even more exciting, we have a new survey platform that should massively improve your experience of submitting data for the survey and also make the back-end data tabulation and analysis much quicker and more accurate. Multisite groups will now have two options for submitting data – a redesigned, more user-friendly Excel tool, or a new pathway to submit data in the reporting platform by replicating responses.
In addition, our new survey platform should help us produce the final report a little more quickly and improve its usability.
New-for-2020 survey topics will include:
- Expanded information on nurse practitioner/physician assistant roles
- Diversity in hospital medicine physician leadership
- Specific questions for hospital medicine groups (HMGs) serving children that will better capture unique attributes of these hospital medicine practices
Why participate?
I can’t emphasize enough that each and every survey submission matters a lot. The State of Hospital Medicine report claims to be the authoritative resource for information about the specialty of hospital medicine. But the report can’t fulfill this claim if the underlying data is skimpy because people were too busy, couldn’t be bothered to participate, or if participation is not broadly representative of the amazing diversity of hospital medicine practices out there.
Your participation will help ensure that you are contributing to a robust hospital medicine database, and that your own group’s information is represented in the survey results. By doing so you will be helping to ensure hospital medicine’s place as perhaps the crucial specialty for U.S. health care in the coming decade.
In addition, participants will receive free access to the survey results, so there’s a direct benefit to you and your HMG as well.
How can you participate?
Here’s what you need to know:
1. The survey opens on Jan.6, 2020, and closes on Feb. 14, 2020.
2. You can find general information about the survey at this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/, and register to participate by using this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/sohm-survey/.
3. To participate, you’ll want to collect the following general types of information for your hospital medicine group:
- Basic group descriptive information (for example, types of patients seen, number of hospitals covered, teaching status, etc.)
- Scope of clinical services
- Nurse practitioners and physician assistants in the HMG
- Full-time equivalent (FTE) information
- Information about the physician leader(s)
- Staffing/scheduling arrangements, including backup plans, paid time off, unfilled positions, predominant scheduling pattern, night coverage arrangements, dedicated admitters, unit-based assignments, etc.
- Compensation model (but not specific amounts)
- Value of employee benefits and CME
- Total work relative value units generated by the HMG, and number of times the following CPT codes were billed: 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239
- Information about financial support provided to the HMG
- Specific questions for academic HMGs, including financial support for nonclinical work, and allocation of FTEs
- Specific questions for HMGs serving children, including the hospital settings served, proportion of part-time staff, FTE definition, and information about board certification in pediatric hospital medicine
I’m hoping that all of you will join me in working to make the 2020 State of Hospital Medicine survey and report the best one yet!
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Wow, the last 2 years have just flown by! I can’t believe it’s already time to launch the Society of Hospital Medicine State of Hospital Medicine survey again! Right now is the season for you to roll up your sleeves and get to work helping SHM develop the nation’s definitive resource on the current state of hospital medicine practice.
I’m really excited about this year’s survey. SHM’s Practice Analysis Committee has redesigned it to eliminate some out-of-date or little-used questions and to add a few new, more relevant questions. Even more exciting, we have a new survey platform that should massively improve your experience of submitting data for the survey and also make the back-end data tabulation and analysis much quicker and more accurate. Multisite groups will now have two options for submitting data – a redesigned, more user-friendly Excel tool, or a new pathway to submit data in the reporting platform by replicating responses.
In addition, our new survey platform should help us produce the final report a little more quickly and improve its usability.
New-for-2020 survey topics will include:
- Expanded information on nurse practitioner/physician assistant roles
- Diversity in hospital medicine physician leadership
- Specific questions for hospital medicine groups (HMGs) serving children that will better capture unique attributes of these hospital medicine practices
Why participate?
I can’t emphasize enough that each and every survey submission matters a lot. The State of Hospital Medicine report claims to be the authoritative resource for information about the specialty of hospital medicine. But the report can’t fulfill this claim if the underlying data is skimpy because people were too busy, couldn’t be bothered to participate, or if participation is not broadly representative of the amazing diversity of hospital medicine practices out there.
Your participation will help ensure that you are contributing to a robust hospital medicine database, and that your own group’s information is represented in the survey results. By doing so you will be helping to ensure hospital medicine’s place as perhaps the crucial specialty for U.S. health care in the coming decade.
In addition, participants will receive free access to the survey results, so there’s a direct benefit to you and your HMG as well.
How can you participate?
Here’s what you need to know:
1. The survey opens on Jan.6, 2020, and closes on Feb. 14, 2020.
2. You can find general information about the survey at this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/, and register to participate by using this link: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/sohm-survey/.
3. To participate, you’ll want to collect the following general types of information for your hospital medicine group:
- Basic group descriptive information (for example, types of patients seen, number of hospitals covered, teaching status, etc.)
- Scope of clinical services
- Nurse practitioners and physician assistants in the HMG
- Full-time equivalent (FTE) information
- Information about the physician leader(s)
- Staffing/scheduling arrangements, including backup plans, paid time off, unfilled positions, predominant scheduling pattern, night coverage arrangements, dedicated admitters, unit-based assignments, etc.
- Compensation model (but not specific amounts)
- Value of employee benefits and CME
- Total work relative value units generated by the HMG, and number of times the following CPT codes were billed: 99221, 99222, 99223, 99231, 99232, 99233, 99238, 99239
- Information about financial support provided to the HMG
- Specific questions for academic HMGs, including financial support for nonclinical work, and allocation of FTEs
- Specific questions for HMGs serving children, including the hospital settings served, proportion of part-time staff, FTE definition, and information about board certification in pediatric hospital medicine
I’m hoping that all of you will join me in working to make the 2020 State of Hospital Medicine survey and report the best one yet!
Ms. Flores is a partner at Nelson Flores Hospital Medicine Consultants in La Quinta, Calif. She serves on SHM’s Practice Analysis and Annual Meeting Committees, and helps to coordinate SHM’s biannual State of Hospital Medicine survey.
Pharmacist BP telemonitoring cut cardiovascular events, turned profit
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
PHILADELPHIA – A home blood pressure telemonitoring program featuring pharmacist management of patients with uncontrolled hypertension reduced cardiovascular events by half and was cost saving over the course of 5 years, even though the intervention ended after year 1, Karen L. Margolis, MD, reported at the American Heart Association scientific sessions.
“The return on investment was 126%. That means that for every dollar spent on the intervention, that dollar was recouped by $1.00 plus another $1.26,” explained Dr. Margolis, a general internist who serves as executive director for research at the HealthPartners Institute in Bloomington, Minn., and professor of medicine at the University of Minnesota, Minneapolis.
She presented 5-year follow-up data from the Hyperlink (Home Blood Pressure Telemonitoring and Case Management to Control Hypertension) study, a cluster randomized controlled trial involving 16 primary care clinics. Half of the clinics were randomized to the intervention, which entailed home blood pressure telemonitoring and pharmacist-led case management in collaboration with the primary care team. The other eight clinics provided usual care. The intervention portion of the trial, which lasted for 12 months, included 450 adults with uncontrolled hypertension as defined by repeated on-treatment blood pressure readings of 140/90 mm Hg or more. Participants’ baseline mean blood pressure was 148/85 mm Hg while on an average of one and a half antihypertensive drug classes. On average, pharmacists ended up adding one additional drug from a different antihypertensive drug class to achieve improved blood pressure control.
The details of the intervention and the short-term blood pressure results have previously been reported (JAMA. 2013 Jul 3;310[1]:46-56). Briefly, 6 months into the study, patients in the intervention arm averaged 11/6 mm Hg lower blood pressure than did the usual care controls. At 12 months – when the intervention ended – the between-group difference was similar at 10/5 mm Hg. At 18 months, the difference, while attenuated, remained significant at 7/3 mm Hg in favor of the intervention group. However, at 54 months, the intervention group’s advantage – a 3–mm Hg lower SBP and a 1–mm Hg lower DBP than in controls – was no longer significant.
The exciting new findings Dr. Margolis presented at the AHA scientific sessions focused on 5-year outcomes. Since HealthPartners is an integrated health care system, follow-up was essentially complete.
“None of the other telemetry studies I’m aware of have published anything on cardiovascular events. And we were somewhat surprised when we looked at our data to see fairly substantial differences in our primary outcome,” she noted.
That outcome was a composite of MI, stroke, heart failure, or cardiovascular death occurring over 5 years. The rate was 4.4% in the intervention group and nearly double at 8.6% in controls. That translated to a 51% relative risk reduction. The biggest difference was in stroke: 4 cases in the intervention arm, 12 in usual care controls.
The 5-year coronary revascularization rate was 5.3% in the intervention arm and 10.4% in controls, for a 52% relative risk reduction.
A major caveat regarding the Hyperlink trial was that, even at 450 patients and 5 years of follow-up, the study was underpowered to show significant differences in event rates, with P =.09 for the primary endpoint.
That being said, the financial results were striking. The intervention cost $1,511 per patient in 2017 U.S. dollars. The cost of treatment for major adverse cardiovascular events totaled $758,000 in the intervention group and $1,538,000 in usual care controls. That works out to $3,420 less per patient in the intervention arm. Offset by the cost of the intervention, that spells a net savings of $1,908 per patient achieved by implementing the year-long intervention. It’s a rare instance in health care of an intervention that actually makes money.
These results were unusual enough that Dr. Margolis and her coinvestigators decided to feed their wealth of SBP readings into a microsimulation model, which they ran 1,000 times. The model predicted – in light of the fact that patients in the intervention group were on average 2 years older than the controls were – that the expected reduction in the primary endpoint was 12% rather than the observed 51% relative risk reduction.
How to explain the discrepancy? The Hyperlink results could have been due to chance. Or it could be, Dr. Margolis surmised, that the pharmacists helped accomplish improvements in other cardiovascular risk factors, such as hyperlipidemia, smoking, or sedentary behavior. That’s unknown, since the investigators focused on changes in blood pressure only. Future studies of home telemonitoring and pharmacist case management of uncontrolled hypertension should be powered to detect significant differences in cardiovascular events and should track additional risk factors, she concluded.
She reported having no financial conflicts regarding the study.
SOURCE: Margolis KL. AHA 2019. Abstract MDP232.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Consider hyperbaric oxygen for inflammatory ileal pouchitis
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
SAN ANTONIO – , Hamna Fahad, MD, reported at the annual meeting of the American College of Gastroenterology.
Dr. Fahad, of the Cleveland Clinic, presented a retrospective case series of 21 consecutive clinic patients who presented with inflammatory bowel disease, a surgically created ileal pouch–anal anastomosis, and medically refractory pouchitis. All patients received 30 hyperbaric oxygen treatment sessions, each an hour long, over the course of 2 months. This intensive regimen worked out to 3-5 sessions per week involving 100% oxygen pressurized to 2.4-3.0 ATA.
Overall, 19 of 21 patients experienced improvement in their modified Pouchitis Disease Activity Index (mPDAI) score. The mean total mPDAI at baseline was 8.71, improving significantly to 5 post treatment. The mPDAI symptoms subscore also showed significant improvement in response to a course of hyperbaric oxygen therapy, decreasing from 4 points to 2. The cuff subscore fell from 3 to 0, and the pouch body subscore improved from 3 to 2.
Thirteen of 21 patients reported subjective symptomatic improvement in stool frequency, bleeding, urgency, and fevers, including 6 with complete symptomatic remission. Seventeen patients demonstrated significant endoscopic improvement upon blinded assessment. Seven of 9 patients with fistulae experienced healing of the fistula tract.
The treatment entailed no side effects. However, the benefits weren’t uniformly durable. Several patients underwent a second 30-session round of hyperbaric oxygen therapy within a year because of recurrent pouchitis symptoms refractory to corticosteroids, biologics, and other medications.
Dr. Fahad said the mechanism of benefit for hyperbaric oxygen in the treatment of chronic inflammatory pouchitis is probably severalfold: reversal of a disordered microbiome through inhibition of the growth of anaerobes, reduced production of tumor necrosis factor–alpha and other inflammatory cytokines, and increased plasma oxygen, which reduces ischemia at the tissue level, thereby promoting tissue healing.
Audience members had a practical question: How can they get this treatment paid for? One gastroenterologist said she has encountered considerable payer resistance when she has sought coverage of hyperbaric oxygen for patients with ulcerative colitis and fistulae, even though there is already published evidence of benefit. But Dr. Fahad’s groundbreaking study provides the first such evidence in pouchitis. So how did she and her coworkers do it? Eighty percent of the pouchitis patients obtained payer approval only upon appeal, which was readily granted, she explained.
Dr. Fahad reported having no financial conflicts regarding her study, conducted without commercial support.
SOURCE: Fahad H. ACG 2019 Abstract 38.
REPORTING FROM ACG 2019
FDA approves diazepam nasal spray for seizure clusters
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
The drug may be administered by a care partner outside of a medical setting for the treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern. The formulation is the first nasal spray approved by the FDA as a rescue treatment for people with epilepsy aged 6 years and older, according to Neurelis, the developer of the drug. Midazolam nasal spray, approved in May 2019, is indicated for patients with epilepsy aged 12 years and older.
Investigators evaluated the safety of diazepam nasal spray in a long-term, open-label, repeat-dose, clinical trial. The study enrolled 130 patients aged 6 years and older; more than 2,000 seizures were treated. The drug generally was safe and well tolerated, and the most common adverse reactions were somnolence, headache, and nasal discomfort.
The FDA has granted Valtoco 7 years of orphan drug exclusivity. In the United States, about 170,000 patients with epilepsy are at risk of cluster or acute repetitive seizures, the company said. Until recently, approved rescue medications had been rectally administered.
Patients may receive a second dose of diazepam nasal spray at least 4 hours after an initial dose if needed, but caregivers should not use more than two doses to treat a single episode, according to the prescribing information. In addition, the prescribing information recommends that diazepam nasal spray be used for no more than one episode every 5 days and no more than five episodes per month.
Prednisolone scores for hand OA
ATLANTA – Dutch investigators at the annual meeting of the American College of Rheumatology made a good case for 6 weeks of low-dose prednisolone to help people with hand osteoarthritis get over a particularly bad spell.
A total of 42 patients randomized to prednisolone 10 mg/day fell a mean of 21.5 mm at 6 weeks from a baseline visual analog hand pain score of 54 mm (out of a possible 100 mm), versus a drop of 5.2 mm from a baseline score of 53 mm among 46 randomized to placebo; the mean group difference was 16.5 mm. Patients taking prednisolone did better on function, quality of life, and physician global assessments, too.
“This trial provides evidence that local inflammation is a suitable target for drug treatment in hand OA. We think this study provides clinicians with a short-term treatment option for patients who have a flare of their disease,” said lead investigator Féline Kroon, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center.
“The large beneficial effect size exceeded that of all available therapeutic options for hand osteoarthritis,” including NSAIDs, she and her team noted in the study write-up, which was published to coincide with the meeting (Lancet. 2019 Nov 30;394[10213]:1993-2001).
Many physicians already use short-course prednisolone for hand OA, but there was no clinical evidence that it helped until now. The study also adds weight to the idea that OA has an inflammatory component – an idea that has been building for a while, Dr. Kroon said.
Leiden investigators and others have previously shown that synovial inflammation is often present in hand OA and a main determinant of pain and radiographic progression.
The 92 patients in the Low-Dose Prednisolone in Patients with Painful Hand Osteoarthritis (HOPE) trial had to have at least four interphalangeal joints (IPJs) with osteoarthritic nodes, at least one IPJ with soft swelling or erythema, and at least one with a positive power Doppler signal or grade 2 or higher synovitis on ultrasound. They also had to have flared at least 20 mm on the pain scale with NSAID washout.
There were more responders in the prednisolone group at 6 weeks (72% versus 33%), and significantly greater improvement in synovial thickening. There was no difference in power Doppler score or synovitis score per joint on MRI, but bone marrow lesions appeared less severe with prednisolone.
All the between-group differences disappeared when prednisolone was tapered after 6 weeks.
Four patients discontinued the study because of an adverse event: a myocardial infarction in the prednisolone group, and, in the control arm, a bowel surgery, an infected leg hematoma, and a case of Lyme arthritis of the knee. Adverse events were otherwise mild and similar in both arms.
The mean age in the study was 64 years, and 79% of the subjects were women. Exclusion criteria included chronic inflammatory rheumatic diseases, psoriasis, use of immune-modulating drugs within 90 days of baseline, and predominantly thumb base pain instead of finger pain.
The approach “is for short course. Long-term steroids can have important side effects, like osteoporosis. We do not think this study should be used to encourage prolonged prescribing of glucocorticoids in patients with hand OA,” Dr. Kroon said.
Two previous trials of glucocorticoids for hand OA were inconclusive. A dose of prednisone 5 mg/day for 4 weeks did not separate from placebo in one, and the second showed pain improvements with a combination of prednisolone and dipyridamole versus placebo, but with more adverse events, particularly dipyridamole headaches.
The work was funded by the Dutch Arthritis Society. Dr. Kroon did not have any disclosures.
SOURCE: Kroon F et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1760.
ATLANTA – Dutch investigators at the annual meeting of the American College of Rheumatology made a good case for 6 weeks of low-dose prednisolone to help people with hand osteoarthritis get over a particularly bad spell.
A total of 42 patients randomized to prednisolone 10 mg/day fell a mean of 21.5 mm at 6 weeks from a baseline visual analog hand pain score of 54 mm (out of a possible 100 mm), versus a drop of 5.2 mm from a baseline score of 53 mm among 46 randomized to placebo; the mean group difference was 16.5 mm. Patients taking prednisolone did better on function, quality of life, and physician global assessments, too.
“This trial provides evidence that local inflammation is a suitable target for drug treatment in hand OA. We think this study provides clinicians with a short-term treatment option for patients who have a flare of their disease,” said lead investigator Féline Kroon, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center.
“The large beneficial effect size exceeded that of all available therapeutic options for hand osteoarthritis,” including NSAIDs, she and her team noted in the study write-up, which was published to coincide with the meeting (Lancet. 2019 Nov 30;394[10213]:1993-2001).
Many physicians already use short-course prednisolone for hand OA, but there was no clinical evidence that it helped until now. The study also adds weight to the idea that OA has an inflammatory component – an idea that has been building for a while, Dr. Kroon said.
Leiden investigators and others have previously shown that synovial inflammation is often present in hand OA and a main determinant of pain and radiographic progression.
The 92 patients in the Low-Dose Prednisolone in Patients with Painful Hand Osteoarthritis (HOPE) trial had to have at least four interphalangeal joints (IPJs) with osteoarthritic nodes, at least one IPJ with soft swelling or erythema, and at least one with a positive power Doppler signal or grade 2 or higher synovitis on ultrasound. They also had to have flared at least 20 mm on the pain scale with NSAID washout.
There were more responders in the prednisolone group at 6 weeks (72% versus 33%), and significantly greater improvement in synovial thickening. There was no difference in power Doppler score or synovitis score per joint on MRI, but bone marrow lesions appeared less severe with prednisolone.
All the between-group differences disappeared when prednisolone was tapered after 6 weeks.
Four patients discontinued the study because of an adverse event: a myocardial infarction in the prednisolone group, and, in the control arm, a bowel surgery, an infected leg hematoma, and a case of Lyme arthritis of the knee. Adverse events were otherwise mild and similar in both arms.
The mean age in the study was 64 years, and 79% of the subjects were women. Exclusion criteria included chronic inflammatory rheumatic diseases, psoriasis, use of immune-modulating drugs within 90 days of baseline, and predominantly thumb base pain instead of finger pain.
The approach “is for short course. Long-term steroids can have important side effects, like osteoporosis. We do not think this study should be used to encourage prolonged prescribing of glucocorticoids in patients with hand OA,” Dr. Kroon said.
Two previous trials of glucocorticoids for hand OA were inconclusive. A dose of prednisone 5 mg/day for 4 weeks did not separate from placebo in one, and the second showed pain improvements with a combination of prednisolone and dipyridamole versus placebo, but with more adverse events, particularly dipyridamole headaches.
The work was funded by the Dutch Arthritis Society. Dr. Kroon did not have any disclosures.
SOURCE: Kroon F et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1760.
ATLANTA – Dutch investigators at the annual meeting of the American College of Rheumatology made a good case for 6 weeks of low-dose prednisolone to help people with hand osteoarthritis get over a particularly bad spell.
A total of 42 patients randomized to prednisolone 10 mg/day fell a mean of 21.5 mm at 6 weeks from a baseline visual analog hand pain score of 54 mm (out of a possible 100 mm), versus a drop of 5.2 mm from a baseline score of 53 mm among 46 randomized to placebo; the mean group difference was 16.5 mm. Patients taking prednisolone did better on function, quality of life, and physician global assessments, too.
“This trial provides evidence that local inflammation is a suitable target for drug treatment in hand OA. We think this study provides clinicians with a short-term treatment option for patients who have a flare of their disease,” said lead investigator Féline Kroon, MD, a rheumatologist at Leiden (the Netherlands) University Medical Center.
“The large beneficial effect size exceeded that of all available therapeutic options for hand osteoarthritis,” including NSAIDs, she and her team noted in the study write-up, which was published to coincide with the meeting (Lancet. 2019 Nov 30;394[10213]:1993-2001).
Many physicians already use short-course prednisolone for hand OA, but there was no clinical evidence that it helped until now. The study also adds weight to the idea that OA has an inflammatory component – an idea that has been building for a while, Dr. Kroon said.
Leiden investigators and others have previously shown that synovial inflammation is often present in hand OA and a main determinant of pain and radiographic progression.
The 92 patients in the Low-Dose Prednisolone in Patients with Painful Hand Osteoarthritis (HOPE) trial had to have at least four interphalangeal joints (IPJs) with osteoarthritic nodes, at least one IPJ with soft swelling or erythema, and at least one with a positive power Doppler signal or grade 2 or higher synovitis on ultrasound. They also had to have flared at least 20 mm on the pain scale with NSAID washout.
There were more responders in the prednisolone group at 6 weeks (72% versus 33%), and significantly greater improvement in synovial thickening. There was no difference in power Doppler score or synovitis score per joint on MRI, but bone marrow lesions appeared less severe with prednisolone.
All the between-group differences disappeared when prednisolone was tapered after 6 weeks.
Four patients discontinued the study because of an adverse event: a myocardial infarction in the prednisolone group, and, in the control arm, a bowel surgery, an infected leg hematoma, and a case of Lyme arthritis of the knee. Adverse events were otherwise mild and similar in both arms.
The mean age in the study was 64 years, and 79% of the subjects were women. Exclusion criteria included chronic inflammatory rheumatic diseases, psoriasis, use of immune-modulating drugs within 90 days of baseline, and predominantly thumb base pain instead of finger pain.
The approach “is for short course. Long-term steroids can have important side effects, like osteoporosis. We do not think this study should be used to encourage prolonged prescribing of glucocorticoids in patients with hand OA,” Dr. Kroon said.
Two previous trials of glucocorticoids for hand OA were inconclusive. A dose of prednisone 5 mg/day for 4 weeks did not separate from placebo in one, and the second showed pain improvements with a combination of prednisolone and dipyridamole versus placebo, but with more adverse events, particularly dipyridamole headaches.
The work was funded by the Dutch Arthritis Society. Dr. Kroon did not have any disclosures.
SOURCE: Kroon F et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1760.
REPORTING FROM ACR 2019
Methotrexate gives durable remission from idiopathic granulomatous mastitis
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
REPORTING FROM ACR 2019













