User login
APPRENTICE registry: Wide variation exists in acute pancreatitis treatment, outcomes
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
Etiologies, demographics, management, and outcomes vary widely among patients with acute pancreatitis around the world, according to an analysis of data from the prospective, international APPRENTICE patient registry.
In some cases – particularly in regard to therapeutic interventions – the differences are “strikingly divergent” and demonstrate a “lag behind current evidence,” Bassem Matta, MD, of the University of Pittsburgh Medical Center, and colleagues reported in Clinical Gastroenterology and Hepatology.
Findings of a disproportionately higher rate of opioid prescribing during hospitalization and at discharge at North American sites are especially alarming, the investigators said.
Demographics and etiologies
The most common etiologies among 1,612 patients in the registry, which collects data from individuals with acute pancreatitis at six centers in Europe, three centers in India, five centers in Latin America, and eight centers in North America, were biliary (45%) and alcoholic (21%), and severity was mild in 65% of patients, moderate in 23%, and severe in 12%, they noted.
The predominant etiology in Latin America was biliary (78%), whereas the predominant etiology in India was alcoholic (45%).
The mean age of patients in Europe was 58 years, which is older than the mean age of 46 years for all regions represented in the registry, and comorbid conditions were also more common among patients in Europe (73% vs. 50% overall), the investigators found.
In addition to age differences, significant geographic differences were seen with respect to sex, ethnicity, and race distributions. Patients from Indian sites, for example, were mostly men (75%), were younger in age (median, 39 years), and were more likely to have alcoholic etiology (45% vs. 14% in the other areas). Most of the Latin American patients were women (67%), were young (median, 43 years), and most often had biliary etiology (78% vs. 37% elsewhere).
In contrast, European and North American subjects had a relatively equal sex distribution and an overall older age (median, 58 years).
“Observed differences in etiology and demographics likely reflect a tight interconnection between age, sex, and etiology,” the investigators wrote.
Management
Analgesic utilization was “markedly variable” across the world, they said, explaining that nonsteroidal anti-inflammatory drugs (NSAIDs) were the mainstay of pain management in Europe (68%), whereas Indian sites used tramadol in 91% of patients.
Latin American centers frequently used opioids (59%), NSAIDs (48%), and tramadol (34%).
However, opioid analgesics were used in 93% of patients in North America, compared with 27% of patients in the other regions, and 64% vs. 2.7% of patients in North American vs. the other regions were discharged on opioid analgesics.
This is of particular concern in light of a meta-analysis showing no difference in efficacy between opioids and nonsteroidal anti-inflammatory drugs for pain control in acute pancreatitis, the investigators said, noting that “[i]t is not entirely clear why such divergences exist between North American centers compared to the rest of the world.
“Notably, no clear statements are included in the current societal guidelines addressing optimal strategies for analgesia in [acute pancreatitis],” they added.
Also of note, the rate of endoscopic retrograde cholangiopancreatography (ERCP) – which guidelines based on strong evidence say should be limited to urgent cases among biliary acute pancreatitis patients with suspected cholangitis or biliary obstruction – was much higher at North American sites (44.7% vs. 21.9% overall) and post-ERCP pancreatitis was significantly more common at North American sites (19% vs. 2.8% in the other geographic areas), they said.
However, these differences were mostly driven by two North American sites, which classified 50 out of 90 and 22 out of 62 enrolled patients, respectively, as having post-ERCP pancreatitis.
Further, cholecystectomies were performed at the time of hospital admission in 60% of patients in Latin America, compared with 15% overall.
Another notable difference in management related to intravenous fluid use; similar amounts were administered during the first 24 hours in India and Latin America (3-3.2 liters), but in Europe the average was 2.5 liters, and while lactated Ringers and normal saline were the main types of fluid used, lactated Ringers was the dominant type used in India (92%), but was rarely used in Latin America (7%).
Outcomes
The overall median length of stay was 8 days, and overall mortality during hospitalization was 2.8%. In patients with mild disease, the shortest lengths of stay were in North America (4 vs. 7 days in other regions), and severe disease was more common in India (23% vs. 9% elsewhere).
Intensive care unit admissions were highest at Indian centers, and in-hospital mortality was highest in Europe (5.7%), compared with 3.3% in India, 2.3% in Latin America, and 0.6% in North America, they said.
Mortality during the initial hospital stay among patients with severe acute pancreatitis was 44% in Europe, compared with 15% in the other three regions.
Multivariable regression analyses adjusting for potential confounders such as age, sex, body mass index, Charlson score, etiology, and transfer status showed that the odds of severe acute pancreatitis were 11.2 times higher in Europe, 7 times higher in India, and 5.6 times higher in Latin America, compared with North America.
The odds ratios for mortality during hospitalization among patients with severe disease were 10.4 in Europe, 4.2 in India, and 8.3 in Latin America, compared with North America.
Implications of the findings
Around the world, acute pancreatitis is a leading cause of gastrointestinal-related hospital admissions, and incidence is reportedly increasing in the United States and Europe, the investigators said, noting that about 20% of patients develop severe disease with relatively high morbidity and mortality.
Multiple advances in management have emerged over the last decade, but it is unclear whether those recent advances have gained traction worldwide, they added.
The APPRENTICE registry was created as a response to the lack of prospective, multinational data and the current study aimed to assess the geographic differences in patient characteristics, management, and outcomes across four geographic areas.
The findings, which represent “a bird’s eye view” of regional variation, underscore a need for “adequately powered, multicenter, randomized controlled trials comparing the efficacy of different fluid resuscitation protocols” in acute pancreatitis patients, the investigators said.
Further, “the interventions specific to each region are in certain aspects strikingly divergent, and in many occasions lag behind current evidence,” they wrote, noting the largely variable length-of-stay outcomes and mortality rates.
“In addition to depicting key features of [acute pancreatitis], the results from this study may serve as a reference guide for designing future clinical trials,” they concluded.
The authors reported having no disclosures.
SOURCE: Matt B et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.11.017.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Consider sparing the uterus in prolapse procedures
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Chemo-free induction-consolidation protocol for Ph+ ALL improved survival
ORLANDO – A front-line chemotherapy-free induction-consolidation protocol that combines dasatinib and blinatumomab for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) resulted in high survival and molecular response rates in the phase 2 D-ALBA trial.
At a median follow-up of 14.3 months, 61 of 63 patients enrolled in the multicenter trial had completed induction with the second-generation tyrosine kinase inhibitor (TKI) dasatinib, 60 had received the first cycle of treatment with the bispecific monoclonal antibody blinatumomab, and 56, 45, 36, and 25 had received second, third, fourth, and fifth cycles of blinatumomab, respectively, Sabina Chiaretti, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The molecular response rate at the end of induction on day 85 was 29%, said Dr. Chiaretti of the department of translational and precision medicine, Sapienza University, Rome.
“Even more importantly, at the primary endpoint [the end of the second cycle of blinatumomab], 60% of patients were molecular responders,” she said.
Of note, the molecular response rate continued to increase with additional blinatumomab cycles; the rate was 79% after cycle 4, she said.
The overall survival (OS) and disease-free survival (DFS) rates also were “very exciting and promising” at 92.5% and 89.7%, respectively, she added.
DFS did not differ significantly based on molecular response at day 85 (100% vs. 87.4% in those with vs. without a molecular response; P = .154), but patients with p190 fusion protein had slightly worse DFS, compared with those who had p210 or both p190 and p210 fusion protein (83.5% vs. 100%; P = .48).
Study participants included adult Ph+ ALL patients with a median age of 54.5 years (range of 24.1-81.7 years) who were enrolled between May 2017 and January 2019; 54% were women and the median white blood cell count was 42 x109/L.
The percentage of study subjects with the p190, p210, and both p190/p210 fusion proteins was 65.1%, 27%, and 7.9% respectively, Dr. Chiaretti said.
Treatment included dasatinib at a dose of 140 mg/day as induction for 85 days along with steroids, which were started 7 days prior to induction and continued for a total of 31 days. Those who had a complete hematologic response (CHR) after induction received postinduction consolidation treatment with blinatumomab at a flat dose of 28 mcg/day for at least 2 cycles, and up to 3 additional cycles were allowed at physician discretion based on molecular response.
During the course of the study, 156 adverse events occurred, including 50 serious adverse events. The latter most often involved infections, including 6 cytomegalovirus infections and 6 cases of prolonged fever; one of those cases was likely related to blinatumomab.
Two patients died, including an 80-year-old woman who died during induction, and a patient who was in CHR. Six relapses occurred, including one that involved a major protocol violation; three were extramedullary.
Additional analyses in this study showed that the most frequent copy number aberration was, as expected based on the available literature, IKZF1 deletion, which was present in 25 of 46 available samples (54%). Of those, 11 (23.9%) were found to have the IKZF1-plus signature, defined as IKZF1 and/or PAX5 and/or CDKN2A/B deletions, she said.
Further, ABL1 mutational analysis conducted in 15 patients with evidence of MRD increase showed that 8 were wild type and 7 were mutated – with 6 of the 7 represented by the gatekeeper mutation T315I, and one by an E255K mutation. All but 1 mutation occurred in p190 cases prior to the start of blinatumomab.
Of note, and in line with prior findings, blinatumomab was effective for reducing or eradicating the MRD levels in these difficult-to-treat patients, Dr. Chiaretti said.
An analysis of the immunologic compartment carried out in 12 patients who completed all 5 cycles of blinatumomab showed a significant increase in the rate of CD8+ T cells (29% vs. 19.8% before the start of blinatumomab; P = .04) and a significant reduction in the rate of Tregs (3.7% vs. 11% before blinatumomab; P = .02), she added.
The findings of this study to date – with some patients having more than 2 years of follow-up – are notable given the high rates of molecular response and survival, Dr. Chiaretti said.
Outcomes in patients with Ph+ ALL were generally poor before the introduction of TKIs, but “the scenario completely changed,” she explained.
“In general, all TKI-based treatments – with or without chemotherapy – have led to overall survival rates in the range of 50% ... which means that we still need to improve our clinical management,” she said. “Another finding that became clear is the fact that patients who achieve MRD-negative status have a significantly better outcome than those who do not.”
The D-ALBA trial was designed with the aim of increasing the rate of MRD negativity in newly diagnosed patients using dasatinib and blinatumomab, and the results demonstrate that this chemotherapy-free induction/consolidation approach is feasible in the front-line setting for adult Ph+ ALL patients, she said, adding that “it is strongly effective in inducing high rates of MRD negativity, and it results in much better survival rates.”
The findings with respect to IKZF1-plus cases and ABL1 mutations underscore the need for further work, she said.
“We still have to face some challenging cases,” she explained. “This study, as others before, really proves that IKZF1-plus cases are very difficult to treat; they require intensification and probably alternative strategies.”
Dr. Chiaretti reported membership on a board of directors or advisory committee for Pfizer, Incyte, Amgen, and Shire.
SOURCE: Chiaretti S et al. ASH 2019, Abstract 740.
ORLANDO – A front-line chemotherapy-free induction-consolidation protocol that combines dasatinib and blinatumomab for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) resulted in high survival and molecular response rates in the phase 2 D-ALBA trial.
At a median follow-up of 14.3 months, 61 of 63 patients enrolled in the multicenter trial had completed induction with the second-generation tyrosine kinase inhibitor (TKI) dasatinib, 60 had received the first cycle of treatment with the bispecific monoclonal antibody blinatumomab, and 56, 45, 36, and 25 had received second, third, fourth, and fifth cycles of blinatumomab, respectively, Sabina Chiaretti, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The molecular response rate at the end of induction on day 85 was 29%, said Dr. Chiaretti of the department of translational and precision medicine, Sapienza University, Rome.
“Even more importantly, at the primary endpoint [the end of the second cycle of blinatumomab], 60% of patients were molecular responders,” she said.
Of note, the molecular response rate continued to increase with additional blinatumomab cycles; the rate was 79% after cycle 4, she said.
The overall survival (OS) and disease-free survival (DFS) rates also were “very exciting and promising” at 92.5% and 89.7%, respectively, she added.
DFS did not differ significantly based on molecular response at day 85 (100% vs. 87.4% in those with vs. without a molecular response; P = .154), but patients with p190 fusion protein had slightly worse DFS, compared with those who had p210 or both p190 and p210 fusion protein (83.5% vs. 100%; P = .48).
Study participants included adult Ph+ ALL patients with a median age of 54.5 years (range of 24.1-81.7 years) who were enrolled between May 2017 and January 2019; 54% were women and the median white blood cell count was 42 x109/L.
The percentage of study subjects with the p190, p210, and both p190/p210 fusion proteins was 65.1%, 27%, and 7.9% respectively, Dr. Chiaretti said.
Treatment included dasatinib at a dose of 140 mg/day as induction for 85 days along with steroids, which were started 7 days prior to induction and continued for a total of 31 days. Those who had a complete hematologic response (CHR) after induction received postinduction consolidation treatment with blinatumomab at a flat dose of 28 mcg/day for at least 2 cycles, and up to 3 additional cycles were allowed at physician discretion based on molecular response.
During the course of the study, 156 adverse events occurred, including 50 serious adverse events. The latter most often involved infections, including 6 cytomegalovirus infections and 6 cases of prolonged fever; one of those cases was likely related to blinatumomab.
Two patients died, including an 80-year-old woman who died during induction, and a patient who was in CHR. Six relapses occurred, including one that involved a major protocol violation; three were extramedullary.
Additional analyses in this study showed that the most frequent copy number aberration was, as expected based on the available literature, IKZF1 deletion, which was present in 25 of 46 available samples (54%). Of those, 11 (23.9%) were found to have the IKZF1-plus signature, defined as IKZF1 and/or PAX5 and/or CDKN2A/B deletions, she said.
Further, ABL1 mutational analysis conducted in 15 patients with evidence of MRD increase showed that 8 were wild type and 7 were mutated – with 6 of the 7 represented by the gatekeeper mutation T315I, and one by an E255K mutation. All but 1 mutation occurred in p190 cases prior to the start of blinatumomab.
Of note, and in line with prior findings, blinatumomab was effective for reducing or eradicating the MRD levels in these difficult-to-treat patients, Dr. Chiaretti said.
An analysis of the immunologic compartment carried out in 12 patients who completed all 5 cycles of blinatumomab showed a significant increase in the rate of CD8+ T cells (29% vs. 19.8% before the start of blinatumomab; P = .04) and a significant reduction in the rate of Tregs (3.7% vs. 11% before blinatumomab; P = .02), she added.
The findings of this study to date – with some patients having more than 2 years of follow-up – are notable given the high rates of molecular response and survival, Dr. Chiaretti said.
Outcomes in patients with Ph+ ALL were generally poor before the introduction of TKIs, but “the scenario completely changed,” she explained.
“In general, all TKI-based treatments – with or without chemotherapy – have led to overall survival rates in the range of 50% ... which means that we still need to improve our clinical management,” she said. “Another finding that became clear is the fact that patients who achieve MRD-negative status have a significantly better outcome than those who do not.”
The D-ALBA trial was designed with the aim of increasing the rate of MRD negativity in newly diagnosed patients using dasatinib and blinatumomab, and the results demonstrate that this chemotherapy-free induction/consolidation approach is feasible in the front-line setting for adult Ph+ ALL patients, she said, adding that “it is strongly effective in inducing high rates of MRD negativity, and it results in much better survival rates.”
The findings with respect to IKZF1-plus cases and ABL1 mutations underscore the need for further work, she said.
“We still have to face some challenging cases,” she explained. “This study, as others before, really proves that IKZF1-plus cases are very difficult to treat; they require intensification and probably alternative strategies.”
Dr. Chiaretti reported membership on a board of directors or advisory committee for Pfizer, Incyte, Amgen, and Shire.
SOURCE: Chiaretti S et al. ASH 2019, Abstract 740.
ORLANDO – A front-line chemotherapy-free induction-consolidation protocol that combines dasatinib and blinatumomab for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) resulted in high survival and molecular response rates in the phase 2 D-ALBA trial.
At a median follow-up of 14.3 months, 61 of 63 patients enrolled in the multicenter trial had completed induction with the second-generation tyrosine kinase inhibitor (TKI) dasatinib, 60 had received the first cycle of treatment with the bispecific monoclonal antibody blinatumomab, and 56, 45, 36, and 25 had received second, third, fourth, and fifth cycles of blinatumomab, respectively, Sabina Chiaretti, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The molecular response rate at the end of induction on day 85 was 29%, said Dr. Chiaretti of the department of translational and precision medicine, Sapienza University, Rome.
“Even more importantly, at the primary endpoint [the end of the second cycle of blinatumomab], 60% of patients were molecular responders,” she said.
Of note, the molecular response rate continued to increase with additional blinatumomab cycles; the rate was 79% after cycle 4, she said.
The overall survival (OS) and disease-free survival (DFS) rates also were “very exciting and promising” at 92.5% and 89.7%, respectively, she added.
DFS did not differ significantly based on molecular response at day 85 (100% vs. 87.4% in those with vs. without a molecular response; P = .154), but patients with p190 fusion protein had slightly worse DFS, compared with those who had p210 or both p190 and p210 fusion protein (83.5% vs. 100%; P = .48).
Study participants included adult Ph+ ALL patients with a median age of 54.5 years (range of 24.1-81.7 years) who were enrolled between May 2017 and January 2019; 54% were women and the median white blood cell count was 42 x109/L.
The percentage of study subjects with the p190, p210, and both p190/p210 fusion proteins was 65.1%, 27%, and 7.9% respectively, Dr. Chiaretti said.
Treatment included dasatinib at a dose of 140 mg/day as induction for 85 days along with steroids, which were started 7 days prior to induction and continued for a total of 31 days. Those who had a complete hematologic response (CHR) after induction received postinduction consolidation treatment with blinatumomab at a flat dose of 28 mcg/day for at least 2 cycles, and up to 3 additional cycles were allowed at physician discretion based on molecular response.
During the course of the study, 156 adverse events occurred, including 50 serious adverse events. The latter most often involved infections, including 6 cytomegalovirus infections and 6 cases of prolonged fever; one of those cases was likely related to blinatumomab.
Two patients died, including an 80-year-old woman who died during induction, and a patient who was in CHR. Six relapses occurred, including one that involved a major protocol violation; three were extramedullary.
Additional analyses in this study showed that the most frequent copy number aberration was, as expected based on the available literature, IKZF1 deletion, which was present in 25 of 46 available samples (54%). Of those, 11 (23.9%) were found to have the IKZF1-plus signature, defined as IKZF1 and/or PAX5 and/or CDKN2A/B deletions, she said.
Further, ABL1 mutational analysis conducted in 15 patients with evidence of MRD increase showed that 8 were wild type and 7 were mutated – with 6 of the 7 represented by the gatekeeper mutation T315I, and one by an E255K mutation. All but 1 mutation occurred in p190 cases prior to the start of blinatumomab.
Of note, and in line with prior findings, blinatumomab was effective for reducing or eradicating the MRD levels in these difficult-to-treat patients, Dr. Chiaretti said.
An analysis of the immunologic compartment carried out in 12 patients who completed all 5 cycles of blinatumomab showed a significant increase in the rate of CD8+ T cells (29% vs. 19.8% before the start of blinatumomab; P = .04) and a significant reduction in the rate of Tregs (3.7% vs. 11% before blinatumomab; P = .02), she added.
The findings of this study to date – with some patients having more than 2 years of follow-up – are notable given the high rates of molecular response and survival, Dr. Chiaretti said.
Outcomes in patients with Ph+ ALL were generally poor before the introduction of TKIs, but “the scenario completely changed,” she explained.
“In general, all TKI-based treatments – with or without chemotherapy – have led to overall survival rates in the range of 50% ... which means that we still need to improve our clinical management,” she said. “Another finding that became clear is the fact that patients who achieve MRD-negative status have a significantly better outcome than those who do not.”
The D-ALBA trial was designed with the aim of increasing the rate of MRD negativity in newly diagnosed patients using dasatinib and blinatumomab, and the results demonstrate that this chemotherapy-free induction/consolidation approach is feasible in the front-line setting for adult Ph+ ALL patients, she said, adding that “it is strongly effective in inducing high rates of MRD negativity, and it results in much better survival rates.”
The findings with respect to IKZF1-plus cases and ABL1 mutations underscore the need for further work, she said.
“We still have to face some challenging cases,” she explained. “This study, as others before, really proves that IKZF1-plus cases are very difficult to treat; they require intensification and probably alternative strategies.”
Dr. Chiaretti reported membership on a board of directors or advisory committee for Pfizer, Incyte, Amgen, and Shire.
SOURCE: Chiaretti S et al. ASH 2019, Abstract 740.
REPORTING FROM ASH 2019
COACT: No benefit of immediate PCI for non–ST-elevation cardiac arrest at 1 year
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.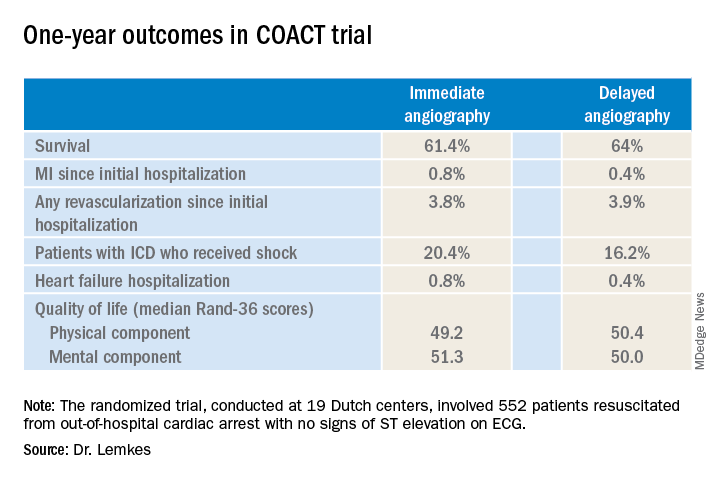
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
REPORTING FROM AHA 2019
State of Practice: Unresectable Stage III NSCLC
In this issue of CHEST Clinical Perspectives, CHEST is undertaking primary research with pulmonologists to assess perceptions regarding curative intent when it comes to treating patients diagnosed with stage III NSCLC. The objectives of this research are to:
- Understand the role of the pulmonologist in diagnostic process, including diagnosis, cell type, staging.
- Understand the process of referral for treatment of patients with stage III NSCLC diagnosed in pulmonary practices, including frequency of referral to oncology and barriers to referral.
- Understand knowledge levels about stage III NSCLC, including differences between patients with stage III and stage IV and how that impacts referral for treatment.
- Understand the extent to which pulmonologists consider stage III patients to be in a curative state.
In this issue of CHEST Clinical Perspectives, CHEST is undertaking primary research with pulmonologists to assess perceptions regarding curative intent when it comes to treating patients diagnosed with stage III NSCLC. The objectives of this research are to:
- Understand the role of the pulmonologist in diagnostic process, including diagnosis, cell type, staging.
- Understand the process of referral for treatment of patients with stage III NSCLC diagnosed in pulmonary practices, including frequency of referral to oncology and barriers to referral.
- Understand knowledge levels about stage III NSCLC, including differences between patients with stage III and stage IV and how that impacts referral for treatment.
- Understand the extent to which pulmonologists consider stage III patients to be in a curative state.
In this issue of CHEST Clinical Perspectives, CHEST is undertaking primary research with pulmonologists to assess perceptions regarding curative intent when it comes to treating patients diagnosed with stage III NSCLC. The objectives of this research are to:
- Understand the role of the pulmonologist in diagnostic process, including diagnosis, cell type, staging.
- Understand the process of referral for treatment of patients with stage III NSCLC diagnosed in pulmonary practices, including frequency of referral to oncology and barriers to referral.
- Understand knowledge levels about stage III NSCLC, including differences between patients with stage III and stage IV and how that impacts referral for treatment.
- Understand the extent to which pulmonologists consider stage III patients to be in a curative state.
Comorbidity rates remain stable over 10 years in childhood-onset epilepsy
BALTIMORE – , according to research presented at the annual meeting of the American Epilepsy Society. Compared with controls, however, young adults with childhood-onset epilepsy have higher rates of psychiatric comorbidity.
The findings suggest that “diagnoses that are identified at baseline continue to be a problem over time,” said Jana E. Jones, PhD, associate professor of neuropsychology at the University of Wisconsin in Madison. Although neurologists understand that comorbidities are common among patients with childhood-onset epilepsy, “it would be good for us to continue to learn what factors are influencing this,” she added.
Investigators sought predictors of outcomes at 10 years
Since 2004, Dr. Jones and her colleagues at the University of Wisconsin have been conducting a study of patients with childhood-onset epilepsy. After the population had completed 10 years of follow-up, the researchers analyzed the data to identify potential patterns of medical and psychiatric comorbidities. One question that they sought to answer was whether any baseline factors could predict outcomes at 10 years.
The researchers analyzed data for 53 patients with childhood-onset epilepsy and 55 controls without epilepsy. At baseline, participants were between ages 8 years and 18 years and had no intellectual disability or neurologic impairment. Within 1 year of epilepsy diagnosis, each participant underwent a psychiatric interview based on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS). Ten years later, participants underwent the Composite International Diagnostic Interview (CIDI), a psychiatric interview for adults. Information about medical comorbidities was collected through interviews and record review at baseline and through an online survey at the 10-year follow-up.
Participants’ mean age at baseline was 12 years. Mean IQ was 105 for the epilepsy group and 109 for the control group. At 10 years, participants’ mean age was about 23 years. Among patients with epilepsy, 55% had focal epilepsy, and 42% had generalized epilepsy. About 40% of participants with epilepsy were in remission at 10 years, which Dr. Jones and colleagues defined as having achieved 5 years without taking medications and without having seizures. At 10 years after diagnosis, 51% of patients with epilepsy were not taking any seizure medication, including approximately 11% of patients with epilepsy who were not categorized as in remission. Most patients taking medication were on monotherapy.
Trends in psychiatric and medical comorbidities
At baseline, approximately 75% of children with epilepsy had a psychiatric or medical diagnosis, compared with 40% of controls. At the 10-year follow-up, 62% of children with epilepsy had a psychiatric diagnosis, compared with 35% of controls. Among controls, 4% had a medical comorbidity (i.e., asthma) alone at baseline. Asthma was the most common medical comorbidity at baseline among patients with epilepsy, and other comorbidities included sleep disorder, head injury, and scoliosis. Six percent of patients had a medical comorbidity alone at baseline. The proportion of patients with both psychiatric and medical comorbidity was 8% at baseline. Patients with epilepsy at baseline had an increased risk of psychiatric comorbidity.
At 10 years, the most common medical comorbidity among patients with epilepsy was head injury (18.9%), followed by allergies and asthma. The rate of migraine was about 13% among controls and slightly less in the epilepsy group. Dr. Jones and colleagues found no significant differences in medical comorbidities between groups at 10 years. At that point, the rate of medical comorbidity was 4% among patients and 11% among controls.
The rate of psychiatric comorbidity remained relatively stable over 10 years, said Dr. Jones. Approximately 47% of patients with epilepsy had a psychiatric diagnosis at 10 years, compared with 29% of controls. In addition, 38% of patients with epilepsy had both psychiatric and medical diagnoses, compared with 29% of controls. Epilepsy increased the risk of psychiatric comorbidity at the 10-year follow-up. Neither medications, remission status, nor seizure type predicted any comorbidity at 10 years.
Dr. Jones and colleagues compared comorbidity rates between the study sample and the National Comorbidity Survey Replication (NCS-R), which reported population-based data that included an epilepsy sample. About 47% of the epilepsy group had an anxiety disorder, compared with 40.7% in the NCS-R. The rate of anxiety disorders was higher in the control group (45.5%) than in the control group (30.8%) in the NCS-R. Approximately 26.4% of the population in Dr. Jones’s study had a mood disorder, compared with 25.9% in the National Comorbidity Survey.
Dr. Jones and colleagues are conducting 15-year follow-up of their original population. One question they will examine is whether medical comorbidities will increase in patients with childhood-onset epilepsy as they approach age 30 years.
Two of the investigators received funding in the form of a grant from the National Institutes of Health.
SOURCE: Kesselmayer RF et al. AES 2019. Abstract 1.288.
BALTIMORE – , according to research presented at the annual meeting of the American Epilepsy Society. Compared with controls, however, young adults with childhood-onset epilepsy have higher rates of psychiatric comorbidity.
The findings suggest that “diagnoses that are identified at baseline continue to be a problem over time,” said Jana E. Jones, PhD, associate professor of neuropsychology at the University of Wisconsin in Madison. Although neurologists understand that comorbidities are common among patients with childhood-onset epilepsy, “it would be good for us to continue to learn what factors are influencing this,” she added.
Investigators sought predictors of outcomes at 10 years
Since 2004, Dr. Jones and her colleagues at the University of Wisconsin have been conducting a study of patients with childhood-onset epilepsy. After the population had completed 10 years of follow-up, the researchers analyzed the data to identify potential patterns of medical and psychiatric comorbidities. One question that they sought to answer was whether any baseline factors could predict outcomes at 10 years.
The researchers analyzed data for 53 patients with childhood-onset epilepsy and 55 controls without epilepsy. At baseline, participants were between ages 8 years and 18 years and had no intellectual disability or neurologic impairment. Within 1 year of epilepsy diagnosis, each participant underwent a psychiatric interview based on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS). Ten years later, participants underwent the Composite International Diagnostic Interview (CIDI), a psychiatric interview for adults. Information about medical comorbidities was collected through interviews and record review at baseline and through an online survey at the 10-year follow-up.
Participants’ mean age at baseline was 12 years. Mean IQ was 105 for the epilepsy group and 109 for the control group. At 10 years, participants’ mean age was about 23 years. Among patients with epilepsy, 55% had focal epilepsy, and 42% had generalized epilepsy. About 40% of participants with epilepsy were in remission at 10 years, which Dr. Jones and colleagues defined as having achieved 5 years without taking medications and without having seizures. At 10 years after diagnosis, 51% of patients with epilepsy were not taking any seizure medication, including approximately 11% of patients with epilepsy who were not categorized as in remission. Most patients taking medication were on monotherapy.
Trends in psychiatric and medical comorbidities
At baseline, approximately 75% of children with epilepsy had a psychiatric or medical diagnosis, compared with 40% of controls. At the 10-year follow-up, 62% of children with epilepsy had a psychiatric diagnosis, compared with 35% of controls. Among controls, 4% had a medical comorbidity (i.e., asthma) alone at baseline. Asthma was the most common medical comorbidity at baseline among patients with epilepsy, and other comorbidities included sleep disorder, head injury, and scoliosis. Six percent of patients had a medical comorbidity alone at baseline. The proportion of patients with both psychiatric and medical comorbidity was 8% at baseline. Patients with epilepsy at baseline had an increased risk of psychiatric comorbidity.
At 10 years, the most common medical comorbidity among patients with epilepsy was head injury (18.9%), followed by allergies and asthma. The rate of migraine was about 13% among controls and slightly less in the epilepsy group. Dr. Jones and colleagues found no significant differences in medical comorbidities between groups at 10 years. At that point, the rate of medical comorbidity was 4% among patients and 11% among controls.
The rate of psychiatric comorbidity remained relatively stable over 10 years, said Dr. Jones. Approximately 47% of patients with epilepsy had a psychiatric diagnosis at 10 years, compared with 29% of controls. In addition, 38% of patients with epilepsy had both psychiatric and medical diagnoses, compared with 29% of controls. Epilepsy increased the risk of psychiatric comorbidity at the 10-year follow-up. Neither medications, remission status, nor seizure type predicted any comorbidity at 10 years.
Dr. Jones and colleagues compared comorbidity rates between the study sample and the National Comorbidity Survey Replication (NCS-R), which reported population-based data that included an epilepsy sample. About 47% of the epilepsy group had an anxiety disorder, compared with 40.7% in the NCS-R. The rate of anxiety disorders was higher in the control group (45.5%) than in the control group (30.8%) in the NCS-R. Approximately 26.4% of the population in Dr. Jones’s study had a mood disorder, compared with 25.9% in the National Comorbidity Survey.
Dr. Jones and colleagues are conducting 15-year follow-up of their original population. One question they will examine is whether medical comorbidities will increase in patients with childhood-onset epilepsy as they approach age 30 years.
Two of the investigators received funding in the form of a grant from the National Institutes of Health.
SOURCE: Kesselmayer RF et al. AES 2019. Abstract 1.288.
BALTIMORE – , according to research presented at the annual meeting of the American Epilepsy Society. Compared with controls, however, young adults with childhood-onset epilepsy have higher rates of psychiatric comorbidity.
The findings suggest that “diagnoses that are identified at baseline continue to be a problem over time,” said Jana E. Jones, PhD, associate professor of neuropsychology at the University of Wisconsin in Madison. Although neurologists understand that comorbidities are common among patients with childhood-onset epilepsy, “it would be good for us to continue to learn what factors are influencing this,” she added.
Investigators sought predictors of outcomes at 10 years
Since 2004, Dr. Jones and her colleagues at the University of Wisconsin have been conducting a study of patients with childhood-onset epilepsy. After the population had completed 10 years of follow-up, the researchers analyzed the data to identify potential patterns of medical and psychiatric comorbidities. One question that they sought to answer was whether any baseline factors could predict outcomes at 10 years.
The researchers analyzed data for 53 patients with childhood-onset epilepsy and 55 controls without epilepsy. At baseline, participants were between ages 8 years and 18 years and had no intellectual disability or neurologic impairment. Within 1 year of epilepsy diagnosis, each participant underwent a psychiatric interview based on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS). Ten years later, participants underwent the Composite International Diagnostic Interview (CIDI), a psychiatric interview for adults. Information about medical comorbidities was collected through interviews and record review at baseline and through an online survey at the 10-year follow-up.
Participants’ mean age at baseline was 12 years. Mean IQ was 105 for the epilepsy group and 109 for the control group. At 10 years, participants’ mean age was about 23 years. Among patients with epilepsy, 55% had focal epilepsy, and 42% had generalized epilepsy. About 40% of participants with epilepsy were in remission at 10 years, which Dr. Jones and colleagues defined as having achieved 5 years without taking medications and without having seizures. At 10 years after diagnosis, 51% of patients with epilepsy were not taking any seizure medication, including approximately 11% of patients with epilepsy who were not categorized as in remission. Most patients taking medication were on monotherapy.
Trends in psychiatric and medical comorbidities
At baseline, approximately 75% of children with epilepsy had a psychiatric or medical diagnosis, compared with 40% of controls. At the 10-year follow-up, 62% of children with epilepsy had a psychiatric diagnosis, compared with 35% of controls. Among controls, 4% had a medical comorbidity (i.e., asthma) alone at baseline. Asthma was the most common medical comorbidity at baseline among patients with epilepsy, and other comorbidities included sleep disorder, head injury, and scoliosis. Six percent of patients had a medical comorbidity alone at baseline. The proportion of patients with both psychiatric and medical comorbidity was 8% at baseline. Patients with epilepsy at baseline had an increased risk of psychiatric comorbidity.
At 10 years, the most common medical comorbidity among patients with epilepsy was head injury (18.9%), followed by allergies and asthma. The rate of migraine was about 13% among controls and slightly less in the epilepsy group. Dr. Jones and colleagues found no significant differences in medical comorbidities between groups at 10 years. At that point, the rate of medical comorbidity was 4% among patients and 11% among controls.
The rate of psychiatric comorbidity remained relatively stable over 10 years, said Dr. Jones. Approximately 47% of patients with epilepsy had a psychiatric diagnosis at 10 years, compared with 29% of controls. In addition, 38% of patients with epilepsy had both psychiatric and medical diagnoses, compared with 29% of controls. Epilepsy increased the risk of psychiatric comorbidity at the 10-year follow-up. Neither medications, remission status, nor seizure type predicted any comorbidity at 10 years.
Dr. Jones and colleagues compared comorbidity rates between the study sample and the National Comorbidity Survey Replication (NCS-R), which reported population-based data that included an epilepsy sample. About 47% of the epilepsy group had an anxiety disorder, compared with 40.7% in the NCS-R. The rate of anxiety disorders was higher in the control group (45.5%) than in the control group (30.8%) in the NCS-R. Approximately 26.4% of the population in Dr. Jones’s study had a mood disorder, compared with 25.9% in the National Comorbidity Survey.
Dr. Jones and colleagues are conducting 15-year follow-up of their original population. One question they will examine is whether medical comorbidities will increase in patients with childhood-onset epilepsy as they approach age 30 years.
Two of the investigators received funding in the form of a grant from the National Institutes of Health.
SOURCE: Kesselmayer RF et al. AES 2019. Abstract 1.288.
REPORTING FROM AES 2019
Patient-reported outcomes highlight long-term impacts of prostatectomy
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
For men with prostate cancer, prostatectomy is more likely to cause long-term, clinically meaningful incontinence than are other treatment modalities, based on patient-reported outcomes from more than 2,000 men.
Patients with high-risk disease who underwent prostatectomy also reported worse sexual function at every time point over 5 years, reported Karen E. Hoffman, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues. Other functional differences between treatments faded over time, they said.
“These estimates of the long-term bowel, bladder, and sexual function after localized prostate cancer treatment may clarify expectations and enable men to make informed choices about care,” the investigators wrote. Their report is in JAMA.
The prospective, population-based cohort study involved 1,386 men with favorable-risk prostate cancer and 619 men with unfavorable-risk disease. For men with favorable-risk disease, treatments included low-dose brachytherapy, external beam radiation therapy (EBRT), nerve-sparing prostatectomy, and active surveillance. Men with unfavorable-risk disease were treated with either prostatectomy or EBRT plus androgen deprivation therapy (ADT).
At multiple time points for 5 years, patients completed the 26-item Expanded Prostate Index Composite questionnaire (EPIC; range, 0-100). Clinically significant functional differences between treatment modalities were defined for bowel and hormonal function (4-6), urinary irritative symptoms (5-7), urinary incontinence (6-9), and sexual function (10-12). All 2,005 men completed at least one questionnaire, with about 3 out of 4 men (77%) still responding after 5 years.
Across all patients, prostatectomy was associated with a relatively higher risk of long-term, clinically meaningful urinary incontinence.
For men with favorable-risk disease, the adjusted mean difference for incontinence between nerve-sparing prostatectomy and active surveillance was –10.9 (P less than .001). Patients in this group who elected prostatectomy over active surveillance had a higher rate of urinary leakage (10% vs. 7%; P = .04); however, there was no significant difference in rates of moderate or big problems with urinary function, which suggests that incontinence issues were typically mild.
For men with unfavorable risk disease, prostatectomy carried a higher risk of more severe incontinence. The adjusted mean difference between prostatectomy and EBRT plus ADT was –23.2 (P less than .001), and patients treated with prostatectomy were more likely to report moderate or big problems with urinary function (17% vs. 13%; P = .005). In addition to these urinary issues, men with unfavorable-risk disease who underwent prostatectomy were more likely to report worse sexual function at 5 years, compared with those who were treated with EBRT plus ADT (adjusted mean difference, –12.5).
While other clinically meaningful differences were found between treatments for other functional categories, these differences lost statistical significance by the 5-year time point.
The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
SOURCE: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
FROM JAMA
Key clinical point: For men with prostate cancer, prostatectomy is significantly more likely to cause long-term incontinence than are other treatment modalities.
Major finding: For each time point through 5 years, men with unfavorable-risk prostate cancer who underwent prostatectomy had significantly worse incontinence scores than did those who underwent external beam radiation therapy (EBRT) and androgen deprivation therapy (ADT) (adjusted mean difference, –23.2).
Study details: A prospective, population-based cohort study involving 2,005 men prostate cancer.
Disclosures: The Agency for Healthcare Research and Quality, the Patient-Centered Outcomes Research Institute, and the National Cancer Institute funded the study. The investigators reported additional relationships with Dendreon, Bayer, AstraZeneca, and others.
Source: Hoffman et al. JAMA. 2020 Jan 14. doi: 10.1001/jama.2019.20675.
BET inhibitor exhibits activity in myelofibrosis
ORLANDO – The BET inhibitor CPI-0610, given alone or in combination with ruxolitinib, has demonstrated activity in a phase 2 trial of patients with relapsed/refractory myelofibrosis (MF).
Responses were best among transfusion-dependent patients who received CPI-0610 and ruxolitinib. All but one of these patients experienced symptom improvement.
In the monotherapy group, results were best among transfusion-independent patients. All of these patients had an improvement in Patient Global Impression of Change (PGIC) score, and most had a 50% or greater improvement in total symptom score (TSS).
John Mascarenhas, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these results from the phase 2 MANIFEST trial (NCT02158858) at the annual meeting of the American Society of Hematology.
Dr. Mascarenhas presented data on 90 patients – 59 with primary MF, 16 with post–polycythemia vera MF, 13 with post–essential thrombocythemia MF, and 2 whose type of MF was unknown. At baseline, the patients’ median age was 69 years and 76.7% of patients had received at least 6 months of ruxolitinib treatment.
Of the 36 patients who received CPI-0610 monotherapy, 34 were still receiving the treatment as of Oct. 17, 2019. Of the 54 patients treated with CPI-0610 and ruxolitinib, 41 were still receiving the combination at that time. The median duration of treatment was 11.3 weeks in the monotherapy arm and 25.9 weeks in the combination arm. Responses were assessed at 24 weeks.
“CPI-0610 monotherapy or added on to ruxolitinib in a relapsed/refractory MF population demonstrated antitumor activity, as evidenced by spleen and symptom improvement,” Dr. Mascarenhas said. “Symptom responses were observed in a majority of patients. We treated these patients, and they felt much better. It was impressive.”
Efficacy of monotherapy
There were two evaluable patients who had been transfusion dependent at baseline and received CPI-0610 monotherapy for at least 24 weeks. Neither patient achieved transfusion independence, and neither had a spleen volume reduction of at least 35% (SVR35). One patient had a 50% or greater improvement in TSS, and one had an improvement in PGIC score.
There were seven evaluable patients who were transfusion independent at baseline and received CPI-0610 for at least 24 weeks. None of these patients had an SVR35 response, three of five evaluable patients had a 50% or greater improvement in TSS, and all seven had an improvement in PGIC score.
Efficacy of the combination
There were 14 patients who had been transfusion dependent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. Six of these patients had become transfusion independent at week 24.
Among the patients who were transfusion dependent at baseline, 25% (3/12) had an SVR35 response at week 24, 54% (7/13) had a 50% or greater improvement in TSS, and 75% (9/12) had an improvement in PGIC score.
There were 13 evaluable patients who were transfusion independent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. None of these patients had an SVR35 response, 38% had a 50% or greater improvement in TSS, and 69% had an improvement in PGIC score.
Safety
“CPI-0610 monotherapy or as an add-on to [ruxolitinib] was generally well tolerated,” Dr. Mascarenhas said. “Thrombocytopenia was asymptomatic, generally reversible, and manageable. There were no other unanticipated safety concerns.”
All 90 patients were evaluable for safety. Hematologic adverse events included thrombocytopenia (23.3%) and anemia (8.9%).
The most common nonhematologic adverse events were diarrhea (32.2%), nausea (22.2%), cough (16.7%), fatigue (14.4%), vomiting (14.4%), and upper respiratory tract infection (14.4%).
Eight patients (8.9%) experienced grade 4 adverse events, but all events resolved. Four events occurred in the monotherapy arm, and one (rash) required dose interruption. Of the four events in the combination arm, one (anemia) was considered treatment related.
There were three fatal adverse events – acute kidney injury, traumatic subdural hematoma, and brain stem hemorrhage. None of these events were considered related to CPI-0610.
Based on these preliminary results, the cohort of transfusion-dependent patients receiving CPI-0610 and ruxolitinib has been expanded. A cohort of ruxolitinib-naive patients receiving CPI-0610 and ruxolitinib has been expanded as well.
The MANIFEST trial is funded by Constellation Pharmaceuticals in collaboration with the Leukemia & Lymphoma Society. Dr. Mascarenhas reported relationships with Incyte, Janssen, CTI Biopharma, Novartis, Roche, Merck, Celgene, Promedior, Merus, and PharmaEssentia.
SOURCE: Mascarenhas J et al. ASH 2019, Abstract 670.
ORLANDO – The BET inhibitor CPI-0610, given alone or in combination with ruxolitinib, has demonstrated activity in a phase 2 trial of patients with relapsed/refractory myelofibrosis (MF).
Responses were best among transfusion-dependent patients who received CPI-0610 and ruxolitinib. All but one of these patients experienced symptom improvement.
In the monotherapy group, results were best among transfusion-independent patients. All of these patients had an improvement in Patient Global Impression of Change (PGIC) score, and most had a 50% or greater improvement in total symptom score (TSS).
John Mascarenhas, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these results from the phase 2 MANIFEST trial (NCT02158858) at the annual meeting of the American Society of Hematology.
Dr. Mascarenhas presented data on 90 patients – 59 with primary MF, 16 with post–polycythemia vera MF, 13 with post–essential thrombocythemia MF, and 2 whose type of MF was unknown. At baseline, the patients’ median age was 69 years and 76.7% of patients had received at least 6 months of ruxolitinib treatment.
Of the 36 patients who received CPI-0610 monotherapy, 34 were still receiving the treatment as of Oct. 17, 2019. Of the 54 patients treated with CPI-0610 and ruxolitinib, 41 were still receiving the combination at that time. The median duration of treatment was 11.3 weeks in the monotherapy arm and 25.9 weeks in the combination arm. Responses were assessed at 24 weeks.
“CPI-0610 monotherapy or added on to ruxolitinib in a relapsed/refractory MF population demonstrated antitumor activity, as evidenced by spleen and symptom improvement,” Dr. Mascarenhas said. “Symptom responses were observed in a majority of patients. We treated these patients, and they felt much better. It was impressive.”
Efficacy of monotherapy
There were two evaluable patients who had been transfusion dependent at baseline and received CPI-0610 monotherapy for at least 24 weeks. Neither patient achieved transfusion independence, and neither had a spleen volume reduction of at least 35% (SVR35). One patient had a 50% or greater improvement in TSS, and one had an improvement in PGIC score.
There were seven evaluable patients who were transfusion independent at baseline and received CPI-0610 for at least 24 weeks. None of these patients had an SVR35 response, three of five evaluable patients had a 50% or greater improvement in TSS, and all seven had an improvement in PGIC score.
Efficacy of the combination
There were 14 patients who had been transfusion dependent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. Six of these patients had become transfusion independent at week 24.
Among the patients who were transfusion dependent at baseline, 25% (3/12) had an SVR35 response at week 24, 54% (7/13) had a 50% or greater improvement in TSS, and 75% (9/12) had an improvement in PGIC score.
There were 13 evaluable patients who were transfusion independent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. None of these patients had an SVR35 response, 38% had a 50% or greater improvement in TSS, and 69% had an improvement in PGIC score.
Safety
“CPI-0610 monotherapy or as an add-on to [ruxolitinib] was generally well tolerated,” Dr. Mascarenhas said. “Thrombocytopenia was asymptomatic, generally reversible, and manageable. There were no other unanticipated safety concerns.”
All 90 patients were evaluable for safety. Hematologic adverse events included thrombocytopenia (23.3%) and anemia (8.9%).
The most common nonhematologic adverse events were diarrhea (32.2%), nausea (22.2%), cough (16.7%), fatigue (14.4%), vomiting (14.4%), and upper respiratory tract infection (14.4%).
Eight patients (8.9%) experienced grade 4 adverse events, but all events resolved. Four events occurred in the monotherapy arm, and one (rash) required dose interruption. Of the four events in the combination arm, one (anemia) was considered treatment related.
There were three fatal adverse events – acute kidney injury, traumatic subdural hematoma, and brain stem hemorrhage. None of these events were considered related to CPI-0610.
Based on these preliminary results, the cohort of transfusion-dependent patients receiving CPI-0610 and ruxolitinib has been expanded. A cohort of ruxolitinib-naive patients receiving CPI-0610 and ruxolitinib has been expanded as well.
The MANIFEST trial is funded by Constellation Pharmaceuticals in collaboration with the Leukemia & Lymphoma Society. Dr. Mascarenhas reported relationships with Incyte, Janssen, CTI Biopharma, Novartis, Roche, Merck, Celgene, Promedior, Merus, and PharmaEssentia.
SOURCE: Mascarenhas J et al. ASH 2019, Abstract 670.
ORLANDO – The BET inhibitor CPI-0610, given alone or in combination with ruxolitinib, has demonstrated activity in a phase 2 trial of patients with relapsed/refractory myelofibrosis (MF).
Responses were best among transfusion-dependent patients who received CPI-0610 and ruxolitinib. All but one of these patients experienced symptom improvement.
In the monotherapy group, results were best among transfusion-independent patients. All of these patients had an improvement in Patient Global Impression of Change (PGIC) score, and most had a 50% or greater improvement in total symptom score (TSS).
John Mascarenhas, MD, of the Icahn School of Medicine at Mount Sinai, New York, presented these results from the phase 2 MANIFEST trial (NCT02158858) at the annual meeting of the American Society of Hematology.
Dr. Mascarenhas presented data on 90 patients – 59 with primary MF, 16 with post–polycythemia vera MF, 13 with post–essential thrombocythemia MF, and 2 whose type of MF was unknown. At baseline, the patients’ median age was 69 years and 76.7% of patients had received at least 6 months of ruxolitinib treatment.
Of the 36 patients who received CPI-0610 monotherapy, 34 were still receiving the treatment as of Oct. 17, 2019. Of the 54 patients treated with CPI-0610 and ruxolitinib, 41 were still receiving the combination at that time. The median duration of treatment was 11.3 weeks in the monotherapy arm and 25.9 weeks in the combination arm. Responses were assessed at 24 weeks.
“CPI-0610 monotherapy or added on to ruxolitinib in a relapsed/refractory MF population demonstrated antitumor activity, as evidenced by spleen and symptom improvement,” Dr. Mascarenhas said. “Symptom responses were observed in a majority of patients. We treated these patients, and they felt much better. It was impressive.”
Efficacy of monotherapy
There were two evaluable patients who had been transfusion dependent at baseline and received CPI-0610 monotherapy for at least 24 weeks. Neither patient achieved transfusion independence, and neither had a spleen volume reduction of at least 35% (SVR35). One patient had a 50% or greater improvement in TSS, and one had an improvement in PGIC score.
There were seven evaluable patients who were transfusion independent at baseline and received CPI-0610 for at least 24 weeks. None of these patients had an SVR35 response, three of five evaluable patients had a 50% or greater improvement in TSS, and all seven had an improvement in PGIC score.
Efficacy of the combination
There were 14 patients who had been transfusion dependent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. Six of these patients had become transfusion independent at week 24.
Among the patients who were transfusion dependent at baseline, 25% (3/12) had an SVR35 response at week 24, 54% (7/13) had a 50% or greater improvement in TSS, and 75% (9/12) had an improvement in PGIC score.
There were 13 evaluable patients who were transfusion independent at baseline and received CPI-0610 and ruxolitinib for at least 24 weeks. None of these patients had an SVR35 response, 38% had a 50% or greater improvement in TSS, and 69% had an improvement in PGIC score.
Safety
“CPI-0610 monotherapy or as an add-on to [ruxolitinib] was generally well tolerated,” Dr. Mascarenhas said. “Thrombocytopenia was asymptomatic, generally reversible, and manageable. There were no other unanticipated safety concerns.”
All 90 patients were evaluable for safety. Hematologic adverse events included thrombocytopenia (23.3%) and anemia (8.9%).
The most common nonhematologic adverse events were diarrhea (32.2%), nausea (22.2%), cough (16.7%), fatigue (14.4%), vomiting (14.4%), and upper respiratory tract infection (14.4%).
Eight patients (8.9%) experienced grade 4 adverse events, but all events resolved. Four events occurred in the monotherapy arm, and one (rash) required dose interruption. Of the four events in the combination arm, one (anemia) was considered treatment related.
There were three fatal adverse events – acute kidney injury, traumatic subdural hematoma, and brain stem hemorrhage. None of these events were considered related to CPI-0610.
Based on these preliminary results, the cohort of transfusion-dependent patients receiving CPI-0610 and ruxolitinib has been expanded. A cohort of ruxolitinib-naive patients receiving CPI-0610 and ruxolitinib has been expanded as well.
The MANIFEST trial is funded by Constellation Pharmaceuticals in collaboration with the Leukemia & Lymphoma Society. Dr. Mascarenhas reported relationships with Incyte, Janssen, CTI Biopharma, Novartis, Roche, Merck, Celgene, Promedior, Merus, and PharmaEssentia.
SOURCE: Mascarenhas J et al. ASH 2019, Abstract 670.
REPORTING FROM ASH 2019
NCCN guidelines highlight ‘complicated’ treatment for pediatric lymphomas
The National Comprehensive Cancer Network (NCCN) has released its first set of guidelines for managing pediatric aggressive mature B-cell lymphomas.
The guidelines highlight the complexities of treating pediatric Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL), as recommendations include a range of multiagent regimens for different patient groups at various time points.
“The treatment of this disease is relatively complicated,” said Kimberly J. Davies, MD, a pediatric hematologist/oncologist at Dana-Farber Cancer Institute in Boston and chair of the guidelines panel. “The chemotherapy regimens have a lot of drugs, a lot of nuances to how they’re supposed to be given. These guidelines delineate that treatment and help the provider … make sure they are delivering the treatment a patient needs.”
The guidelines recommend different regimens according to a patient’s risk group, but the same treatment approach should be used for patients with BL and those with DLBCL.
“The biggest difference between pediatric and adult patients is that pediatric patients are more uniformly treated, regardless of what type of aggressive B-cell lymphoma they have,” said Matthew Barth, MD, a pediatric hematologist/oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and vice chair of the NCCN guidelines panel.
“Adults with diffuse large B-cell lymphoma and Burkitt lymphoma are generally treated with different chemotherapy regimens, but, in pediatrics, we use the same treatment regimens for both diffuse large B-cell lymphoma and Burkitt lymphoma,” he added.
As an example, the new guidelines recommend that pediatric patients with low-risk BL/DLBCL receive the POG9219 regimen (N Engl J Med. 1997 Oct 30;337[18]:1259-66) or FAB/LMB96 regimen A (Br J Haematol. 2008 Jun;141[6]:840-7) as induction, or they should be enrolled in a clinical trial.
On the other hand, induction for high-risk pediatric BL/DLBCL patients should consist of rituximab and a chemotherapy regimen used in the COG ANHL1131 trial. The recommendation to incorporate rituximab in high-risk pediatric patients is based on results from that trial (J Clin Oncol. 2016 May 20. doi: 10.1200/JCO.2016.34.15_suppl.10507).
“Until recent clinical trial data was available, we weren’t really sure how to incorporate rituximab into the treatment of pediatric patients with mature B-cell lymphomas,” Dr. Barth said. “We now have evidence that rituximab is clearly beneficial for patients who are in higher-risk groups.”
Dr. Barth and Dr. Davies both noted that pediatric BL and DLBCL have high cure rates. Long-term survival rates range from about 80% to more than 90%, according to the American Cancer Society. However, the patients who do relapse or progress can be difficult to treat.
“We have quite good cure rates at this point in time, which is a great success, but that means that a very small population of patients don’t respond to initial therapy, and … it’s hard to know what the best treatment for those patients is,” Dr. Davies said.
She noted that studies are underway to determine if immunotherapies, including chimeric antigen receptor T-cell therapy, might improve outcomes in patients with relapsed or refractory disease.
For now, the NCCN guidelines recommend clinical trial enrollment for relapsed/refractory patients. Alternatively, these patients can receive additional chemotherapy, and responders can proceed to transplant. Patients who don’t achieve at least a partial response may go on to a clinical trial or receive best supportive care.
Dr. Davies and Dr. Barth reported having no conflicts of interest.
The National Comprehensive Cancer Network (NCCN) has released its first set of guidelines for managing pediatric aggressive mature B-cell lymphomas.
The guidelines highlight the complexities of treating pediatric Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL), as recommendations include a range of multiagent regimens for different patient groups at various time points.
“The treatment of this disease is relatively complicated,” said Kimberly J. Davies, MD, a pediatric hematologist/oncologist at Dana-Farber Cancer Institute in Boston and chair of the guidelines panel. “The chemotherapy regimens have a lot of drugs, a lot of nuances to how they’re supposed to be given. These guidelines delineate that treatment and help the provider … make sure they are delivering the treatment a patient needs.”
The guidelines recommend different regimens according to a patient’s risk group, but the same treatment approach should be used for patients with BL and those with DLBCL.
“The biggest difference between pediatric and adult patients is that pediatric patients are more uniformly treated, regardless of what type of aggressive B-cell lymphoma they have,” said Matthew Barth, MD, a pediatric hematologist/oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and vice chair of the NCCN guidelines panel.
“Adults with diffuse large B-cell lymphoma and Burkitt lymphoma are generally treated with different chemotherapy regimens, but, in pediatrics, we use the same treatment regimens for both diffuse large B-cell lymphoma and Burkitt lymphoma,” he added.
As an example, the new guidelines recommend that pediatric patients with low-risk BL/DLBCL receive the POG9219 regimen (N Engl J Med. 1997 Oct 30;337[18]:1259-66) or FAB/LMB96 regimen A (Br J Haematol. 2008 Jun;141[6]:840-7) as induction, or they should be enrolled in a clinical trial.
On the other hand, induction for high-risk pediatric BL/DLBCL patients should consist of rituximab and a chemotherapy regimen used in the COG ANHL1131 trial. The recommendation to incorporate rituximab in high-risk pediatric patients is based on results from that trial (J Clin Oncol. 2016 May 20. doi: 10.1200/JCO.2016.34.15_suppl.10507).
“Until recent clinical trial data was available, we weren’t really sure how to incorporate rituximab into the treatment of pediatric patients with mature B-cell lymphomas,” Dr. Barth said. “We now have evidence that rituximab is clearly beneficial for patients who are in higher-risk groups.”
Dr. Barth and Dr. Davies both noted that pediatric BL and DLBCL have high cure rates. Long-term survival rates range from about 80% to more than 90%, according to the American Cancer Society. However, the patients who do relapse or progress can be difficult to treat.
“We have quite good cure rates at this point in time, which is a great success, but that means that a very small population of patients don’t respond to initial therapy, and … it’s hard to know what the best treatment for those patients is,” Dr. Davies said.
She noted that studies are underway to determine if immunotherapies, including chimeric antigen receptor T-cell therapy, might improve outcomes in patients with relapsed or refractory disease.
For now, the NCCN guidelines recommend clinical trial enrollment for relapsed/refractory patients. Alternatively, these patients can receive additional chemotherapy, and responders can proceed to transplant. Patients who don’t achieve at least a partial response may go on to a clinical trial or receive best supportive care.
Dr. Davies and Dr. Barth reported having no conflicts of interest.
The National Comprehensive Cancer Network (NCCN) has released its first set of guidelines for managing pediatric aggressive mature B-cell lymphomas.
The guidelines highlight the complexities of treating pediatric Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL), as recommendations include a range of multiagent regimens for different patient groups at various time points.
“The treatment of this disease is relatively complicated,” said Kimberly J. Davies, MD, a pediatric hematologist/oncologist at Dana-Farber Cancer Institute in Boston and chair of the guidelines panel. “The chemotherapy regimens have a lot of drugs, a lot of nuances to how they’re supposed to be given. These guidelines delineate that treatment and help the provider … make sure they are delivering the treatment a patient needs.”
The guidelines recommend different regimens according to a patient’s risk group, but the same treatment approach should be used for patients with BL and those with DLBCL.
“The biggest difference between pediatric and adult patients is that pediatric patients are more uniformly treated, regardless of what type of aggressive B-cell lymphoma they have,” said Matthew Barth, MD, a pediatric hematologist/oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and vice chair of the NCCN guidelines panel.
“Adults with diffuse large B-cell lymphoma and Burkitt lymphoma are generally treated with different chemotherapy regimens, but, in pediatrics, we use the same treatment regimens for both diffuse large B-cell lymphoma and Burkitt lymphoma,” he added.
As an example, the new guidelines recommend that pediatric patients with low-risk BL/DLBCL receive the POG9219 regimen (N Engl J Med. 1997 Oct 30;337[18]:1259-66) or FAB/LMB96 regimen A (Br J Haematol. 2008 Jun;141[6]:840-7) as induction, or they should be enrolled in a clinical trial.
On the other hand, induction for high-risk pediatric BL/DLBCL patients should consist of rituximab and a chemotherapy regimen used in the COG ANHL1131 trial. The recommendation to incorporate rituximab in high-risk pediatric patients is based on results from that trial (J Clin Oncol. 2016 May 20. doi: 10.1200/JCO.2016.34.15_suppl.10507).
“Until recent clinical trial data was available, we weren’t really sure how to incorporate rituximab into the treatment of pediatric patients with mature B-cell lymphomas,” Dr. Barth said. “We now have evidence that rituximab is clearly beneficial for patients who are in higher-risk groups.”
Dr. Barth and Dr. Davies both noted that pediatric BL and DLBCL have high cure rates. Long-term survival rates range from about 80% to more than 90%, according to the American Cancer Society. However, the patients who do relapse or progress can be difficult to treat.
“We have quite good cure rates at this point in time, which is a great success, but that means that a very small population of patients don’t respond to initial therapy, and … it’s hard to know what the best treatment for those patients is,” Dr. Davies said.
She noted that studies are underway to determine if immunotherapies, including chimeric antigen receptor T-cell therapy, might improve outcomes in patients with relapsed or refractory disease.
For now, the NCCN guidelines recommend clinical trial enrollment for relapsed/refractory patients. Alternatively, these patients can receive additional chemotherapy, and responders can proceed to transplant. Patients who don’t achieve at least a partial response may go on to a clinical trial or receive best supportive care.
Dr. Davies and Dr. Barth reported having no conflicts of interest.
Half of SLE patients have incident neuropsychiatric events
Neuropsychiatric events occurred in just over half of all patients recently diagnosed with systemic lupus erythematosus and followed for an average of nearly 8 years in an international study of more than 1,800 patients.
Up to 30% of these neuropsychiatric (NP) events in up to 20% of the followed cohort were directly attributable to systemic lupus erythematosus (SLE) in a representative patient population, wrote John G. Hanly, MD, and associates in Annals of the Rheumatic Diseases. Their findings were consistent with prior reports, they added.
Another notable finding from follow-up of these 1,827 SLE patients was that among those without a history of SLE-related NP events at baseline, 74% remained free of NP events during the subsequent 10 years, wrote Dr. Hanly, professor of medicine and director of the lupus clinic at Dalhousie University, Halifax, N.S., and coauthors. Among patients free from SLE-associated NP events after 2 years, 84% remained event free during their remaining follow-up. SLE patients with a history of an NP event that subsequently resolved had a 72% rate of freedom from another NP event during 10 years of follow-up.
These findings came from patients recently diagnosed with SLE (within the preceding 15 months) and enrolled at any of 31 participating academic medical centers in North America, Europe, and Asia. The investigators considered preenrollment NP events to include those starting from 6 months prior to diagnosis of SLE until the time patients entered the study. They used case definitions for 19 SLE-associated NP events published by the American College of Rheumatology (Arthritis Rheum. 1999 Apr;42[4]:599-608). All enrolled patients underwent annual assessment for NP events, with follow-up continuing as long as 18 years.
The researchers identified NP events in 955 of the 1,827 enrolled patients, a 52% incidence, including 1,910 unique NP events that included episodes from each of the 19 NP event types, with 92% involving the central nervous system and 8% involving the peripheral nervous system. The percentage of NP events attributable to SLE ranged from 17% to 31%, and they occurred in 14%-21% of the studied patients, with the range reflecting various attribution models used in the analyses. Some patients remained in the same NP state, while others progressed through more than one state.
The study did not receive commercial funding. Dr. Hanly had no disclosures.
SOURCE: Hanly JG et al. Ann Rheum Dis. 2020 Jan 8. doi: 10.1136/annrheumdis-2019-216150.
Neuropsychiatric events occurred in just over half of all patients recently diagnosed with systemic lupus erythematosus and followed for an average of nearly 8 years in an international study of more than 1,800 patients.
Up to 30% of these neuropsychiatric (NP) events in up to 20% of the followed cohort were directly attributable to systemic lupus erythematosus (SLE) in a representative patient population, wrote John G. Hanly, MD, and associates in Annals of the Rheumatic Diseases. Their findings were consistent with prior reports, they added.
Another notable finding from follow-up of these 1,827 SLE patients was that among those without a history of SLE-related NP events at baseline, 74% remained free of NP events during the subsequent 10 years, wrote Dr. Hanly, professor of medicine and director of the lupus clinic at Dalhousie University, Halifax, N.S., and coauthors. Among patients free from SLE-associated NP events after 2 years, 84% remained event free during their remaining follow-up. SLE patients with a history of an NP event that subsequently resolved had a 72% rate of freedom from another NP event during 10 years of follow-up.
These findings came from patients recently diagnosed with SLE (within the preceding 15 months) and enrolled at any of 31 participating academic medical centers in North America, Europe, and Asia. The investigators considered preenrollment NP events to include those starting from 6 months prior to diagnosis of SLE until the time patients entered the study. They used case definitions for 19 SLE-associated NP events published by the American College of Rheumatology (Arthritis Rheum. 1999 Apr;42[4]:599-608). All enrolled patients underwent annual assessment for NP events, with follow-up continuing as long as 18 years.
The researchers identified NP events in 955 of the 1,827 enrolled patients, a 52% incidence, including 1,910 unique NP events that included episodes from each of the 19 NP event types, with 92% involving the central nervous system and 8% involving the peripheral nervous system. The percentage of NP events attributable to SLE ranged from 17% to 31%, and they occurred in 14%-21% of the studied patients, with the range reflecting various attribution models used in the analyses. Some patients remained in the same NP state, while others progressed through more than one state.
The study did not receive commercial funding. Dr. Hanly had no disclosures.
SOURCE: Hanly JG et al. Ann Rheum Dis. 2020 Jan 8. doi: 10.1136/annrheumdis-2019-216150.
Neuropsychiatric events occurred in just over half of all patients recently diagnosed with systemic lupus erythematosus and followed for an average of nearly 8 years in an international study of more than 1,800 patients.
Up to 30% of these neuropsychiatric (NP) events in up to 20% of the followed cohort were directly attributable to systemic lupus erythematosus (SLE) in a representative patient population, wrote John G. Hanly, MD, and associates in Annals of the Rheumatic Diseases. Their findings were consistent with prior reports, they added.
Another notable finding from follow-up of these 1,827 SLE patients was that among those without a history of SLE-related NP events at baseline, 74% remained free of NP events during the subsequent 10 years, wrote Dr. Hanly, professor of medicine and director of the lupus clinic at Dalhousie University, Halifax, N.S., and coauthors. Among patients free from SLE-associated NP events after 2 years, 84% remained event free during their remaining follow-up. SLE patients with a history of an NP event that subsequently resolved had a 72% rate of freedom from another NP event during 10 years of follow-up.
These findings came from patients recently diagnosed with SLE (within the preceding 15 months) and enrolled at any of 31 participating academic medical centers in North America, Europe, and Asia. The investigators considered preenrollment NP events to include those starting from 6 months prior to diagnosis of SLE until the time patients entered the study. They used case definitions for 19 SLE-associated NP events published by the American College of Rheumatology (Arthritis Rheum. 1999 Apr;42[4]:599-608). All enrolled patients underwent annual assessment for NP events, with follow-up continuing as long as 18 years.
The researchers identified NP events in 955 of the 1,827 enrolled patients, a 52% incidence, including 1,910 unique NP events that included episodes from each of the 19 NP event types, with 92% involving the central nervous system and 8% involving the peripheral nervous system. The percentage of NP events attributable to SLE ranged from 17% to 31%, and they occurred in 14%-21% of the studied patients, with the range reflecting various attribution models used in the analyses. Some patients remained in the same NP state, while others progressed through more than one state.
The study did not receive commercial funding. Dr. Hanly had no disclosures.
SOURCE: Hanly JG et al. Ann Rheum Dis. 2020 Jan 8. doi: 10.1136/annrheumdis-2019-216150.
FROM ANNALS OF THE RHEUMATIC DISEASES










