User login
GALACTIC: Early vasodilation strategy no help in acute heart failure
PARIS – A practical strategy of early and aggressive vasodilation and optimization of long-term medication for acute heart failure did not budge all-cause mortality or 180-day readmission rates, according to results of a pragmatic trial presented at the annual congress of the European Society of Cardiology.
“To our great disappointment, the curves were superimposable” between intervention and control arms in the GALACTIC (Goal-directed Afterload Reduction in Acute Congestive Cardiac Decompensation) trial, said lead investigator Christian Eugen Mueller, MD. “There was no signal of a benefit” for those receiving the targeted intervention: the adjusted hazard ratio was 1.07 for the composite primary endpoint of all-cause mortality or 6-month readmission for acute heart failure (P = 0.59).
GALACTIC, explained Dr. Mueller, was the largest investigator-initiated, randomized, controlled trial of pharmacologic therapy for acute heart failure (AHF).
“It is different in that it did not investigate a single drug, but a strategy of early, intensive, and sustained vasodilation. It is also unique in that it used individual doses of well-characterized, widely available, and mostly inexpensive drugs,” said Dr. Mueller, director of the Cardiovascular Research Institute at the University Hospital, Basel, Switzerland. “So this would have the beauty that, if it has a positive finding, you – in whatever country you come from – would be immediately able to apply it once you’re back home in your institution.”
The study attempted to address the gap between symptom amelioration and long-term outcomes when patients arrive in the ED with AHF. “Despite symptomatic improvement achieved from loop diuretics, mortality and morbidity remain unacceptably high,” said Dr. Mueller, with 40%-50% of AHF patients experiencing rehospitalization or death within 180 days of discharge.
Much remains unknown about the optimal treatment strategy for AHF. Aggressive vasodilation has been shown to improve outcomes in less-severe AHF, and intravenous nitrates are known to improve outcomes in AHF where severe pulmonary edema is present – “a phenotype representing only about 5% of patients,” noted Dr. Mueller. Still, “it is unknown whether aggressive vasodilation also improves outcomes in the much more common less-severe phenotype.”
Also, previous trials that ran intravenous vasodilators at a fixed dose for 48 hours did not improve AHF outcomes, so a one-size-fits-all strategy was not one the GALACTIC investigators sought to pursue.
In addition to a flexible regimen, “any strategy applied needs to take into consideration that the vast majority of patients with acute heart failure, after initial treatment in the ED, are then treated in a general cardiology ward,” added Dr. Mueller.
This meant that intravenous nitrate infusion was not part of the GALACTIC trial; rather, sublingual and transdermal nitrates were used, explained Dr. Mueller. “Transdermal application has the beauty that if you have an adverse effect – and hypotension is the most dangerous one – you can immediately remove the patch, and thereby avoid any further harm.”
The two-part strategy tested in GALACTIC involved reducing cardiac filling pressures by maintaining or increasing organ perfusion, while also increasing “long-term lifesaving therapy” targeting the renin-angiotensin-aldosterone system during hospitalization, with a goal to continue optimal treatment long term.
ACE inhibitors or angiotensin receptor blockers were added on the second day of hospitalization for the intervention group, said Dr. Mueller, and “in the ideal setting, up-titrated very aggressively from day to day.
“However, as you know, up-titration to target dose is sometimes wishful thinking in this frail population,” he said, so the GALACTIC trial protocol included a scheme to back dosing off for hypotension, hypokalemia, or worsening renal function. Systolic BP guided how aggressively vasodilation and ACE inhibitor/angiotensin receptor blocker therapy were escalated.
In the end, 382 patients randomized to the intervention arm received early, intensive, and sustained vasodilation, and the 399 patients in the control arm received standard-of-care treatment according to ESC guidelines. These figures omit two patients in the standard-of-care arm who withdrew consent, but follow-up was otherwise complete, said Dr. Mueller. Physicians treating patients in both study arms had discretion to use such other therapies as loop diuretics, beta-blockers, aldosterone antagonists, and cardiac devices.
Adult patients coming to the ED with acute dyspnea classified as New York Heart Association class III or IV were eligible if they had brain natriuretic peptide (BNP) levels of at least 500 ng/L, or N-terminal of the prohormone BNP (NT-proBNP) levels of at least 2,000 ng/L.
Overall, patients enrolled in GALACTIC were in their late 70s, and women made up 37% of the population.
The actual median BNP for enrollees was about 1,250 ng/L, and the median NT-proBNP was just under 6,000 ng/L. The median left ventricular ejection fraction was 37%. About a third of patients had diabetes, and 85% had hypertension. Over half had known chronic heart failure, about a third had prior history of MI, and half of patients had atrial fibrillation at baseline.
“Signs of congestion were present in all patients, and over 90% had rales on physical examination,” said Dr. Mueller.
Patients who were destined for the ICU, those who had systolic BP below 100 mm Hg or marked creatinine elevation, or who required cardiopulmonary resuscitation were excluded. Also excluded were patients with known structural defects such as severe valvular stenosis, congenital heart disease, or hypertrophic obstructive cardiomyopathy. GALACTIC also excluded patients with isolated right ventricular failure caused by pulmonary hypertension.
Prespecified subgroup analyses compared women with men, and those younger than 75 years with older participants. Women saw a significantly higher hazard ratio for readmission or death, indicating a potential harm from the intervention, said Dr. Mueller. An additional analysis stratified patients by left ventricular ejection fraction. Aside from the intervention’s negative effect on women participating in the trial, no other subgroups benefited or were harmed by an early vasodilation strategy.
Alexandre Mebazaa, MD, the designated discussant for the presentation, said that, although the GALACTIC trial was neutral, it represents “an important step forward in acute heart failure.
“Congratulations: First, because we know that in the critically ill condition it’s very difficult to do trials,” and the GALACTIC investigators succeeded in enrolling patients within the first 5 hours of presentation to EDs, noted Dr. Mebazaa, professor of anesthesiology and critical care medicine at the Paris Diderot School of Medicine.
He added that GALACTIC succeeded in continuing vasodilator use beyond the 48-hour mark. “For the first time, you had the courage to go a little bit further down, and we see that patients got the drug with vasodilator properties for 2 days or more.”
However, the long recruitment period for GALACTIC – first enrollment began in 2007 – meant that the study design reflected a thought process about AHF that doesn’t necessarily reflect current practice, noted Dr. Mebazaa. “The trial was designed many years ago, and at that time, we were still thinking that giving very aggressive treatment in the first hours could have an impact.
“Now, when we will be treating patients with vasodilators with acute heart failure – at least myself and my group – I would really wonder whether there is still evidence in the world to support the use of those agents.”
Dr. Mueller noted limitations of the GALACTIC trial, including the lack of generalizability to patients with systolic hypotension or severe renal dysfunction, since these populations were excluded. Also, “the open-label design, which was mandated by the aim to test a strategy, not a single drug, may have introduced a bias in the unblinded assessment of dyspnea” during inpatient stay.
The study was funded by several Swiss research institutions and had no industry support. Dr. Mueller reported no relevant conflicts of interest. Dr. Mebazaa reported financial relationships with Roche, Service, Novartis, AstraZeneca, S-Form Pharma, 4Teen$4, Adrenomed, and Sphingotec.
SOURCE: Mueller C. ESC 2019, Hot Line Session 3.
PARIS – A practical strategy of early and aggressive vasodilation and optimization of long-term medication for acute heart failure did not budge all-cause mortality or 180-day readmission rates, according to results of a pragmatic trial presented at the annual congress of the European Society of Cardiology.
“To our great disappointment, the curves were superimposable” between intervention and control arms in the GALACTIC (Goal-directed Afterload Reduction in Acute Congestive Cardiac Decompensation) trial, said lead investigator Christian Eugen Mueller, MD. “There was no signal of a benefit” for those receiving the targeted intervention: the adjusted hazard ratio was 1.07 for the composite primary endpoint of all-cause mortality or 6-month readmission for acute heart failure (P = 0.59).
GALACTIC, explained Dr. Mueller, was the largest investigator-initiated, randomized, controlled trial of pharmacologic therapy for acute heart failure (AHF).
“It is different in that it did not investigate a single drug, but a strategy of early, intensive, and sustained vasodilation. It is also unique in that it used individual doses of well-characterized, widely available, and mostly inexpensive drugs,” said Dr. Mueller, director of the Cardiovascular Research Institute at the University Hospital, Basel, Switzerland. “So this would have the beauty that, if it has a positive finding, you – in whatever country you come from – would be immediately able to apply it once you’re back home in your institution.”
The study attempted to address the gap between symptom amelioration and long-term outcomes when patients arrive in the ED with AHF. “Despite symptomatic improvement achieved from loop diuretics, mortality and morbidity remain unacceptably high,” said Dr. Mueller, with 40%-50% of AHF patients experiencing rehospitalization or death within 180 days of discharge.
Much remains unknown about the optimal treatment strategy for AHF. Aggressive vasodilation has been shown to improve outcomes in less-severe AHF, and intravenous nitrates are known to improve outcomes in AHF where severe pulmonary edema is present – “a phenotype representing only about 5% of patients,” noted Dr. Mueller. Still, “it is unknown whether aggressive vasodilation also improves outcomes in the much more common less-severe phenotype.”
Also, previous trials that ran intravenous vasodilators at a fixed dose for 48 hours did not improve AHF outcomes, so a one-size-fits-all strategy was not one the GALACTIC investigators sought to pursue.
In addition to a flexible regimen, “any strategy applied needs to take into consideration that the vast majority of patients with acute heart failure, after initial treatment in the ED, are then treated in a general cardiology ward,” added Dr. Mueller.
This meant that intravenous nitrate infusion was not part of the GALACTIC trial; rather, sublingual and transdermal nitrates were used, explained Dr. Mueller. “Transdermal application has the beauty that if you have an adverse effect – and hypotension is the most dangerous one – you can immediately remove the patch, and thereby avoid any further harm.”
The two-part strategy tested in GALACTIC involved reducing cardiac filling pressures by maintaining or increasing organ perfusion, while also increasing “long-term lifesaving therapy” targeting the renin-angiotensin-aldosterone system during hospitalization, with a goal to continue optimal treatment long term.
ACE inhibitors or angiotensin receptor blockers were added on the second day of hospitalization for the intervention group, said Dr. Mueller, and “in the ideal setting, up-titrated very aggressively from day to day.
“However, as you know, up-titration to target dose is sometimes wishful thinking in this frail population,” he said, so the GALACTIC trial protocol included a scheme to back dosing off for hypotension, hypokalemia, or worsening renal function. Systolic BP guided how aggressively vasodilation and ACE inhibitor/angiotensin receptor blocker therapy were escalated.
In the end, 382 patients randomized to the intervention arm received early, intensive, and sustained vasodilation, and the 399 patients in the control arm received standard-of-care treatment according to ESC guidelines. These figures omit two patients in the standard-of-care arm who withdrew consent, but follow-up was otherwise complete, said Dr. Mueller. Physicians treating patients in both study arms had discretion to use such other therapies as loop diuretics, beta-blockers, aldosterone antagonists, and cardiac devices.
Adult patients coming to the ED with acute dyspnea classified as New York Heart Association class III or IV were eligible if they had brain natriuretic peptide (BNP) levels of at least 500 ng/L, or N-terminal of the prohormone BNP (NT-proBNP) levels of at least 2,000 ng/L.
Overall, patients enrolled in GALACTIC were in their late 70s, and women made up 37% of the population.
The actual median BNP for enrollees was about 1,250 ng/L, and the median NT-proBNP was just under 6,000 ng/L. The median left ventricular ejection fraction was 37%. About a third of patients had diabetes, and 85% had hypertension. Over half had known chronic heart failure, about a third had prior history of MI, and half of patients had atrial fibrillation at baseline.
“Signs of congestion were present in all patients, and over 90% had rales on physical examination,” said Dr. Mueller.
Patients who were destined for the ICU, those who had systolic BP below 100 mm Hg or marked creatinine elevation, or who required cardiopulmonary resuscitation were excluded. Also excluded were patients with known structural defects such as severe valvular stenosis, congenital heart disease, or hypertrophic obstructive cardiomyopathy. GALACTIC also excluded patients with isolated right ventricular failure caused by pulmonary hypertension.
Prespecified subgroup analyses compared women with men, and those younger than 75 years with older participants. Women saw a significantly higher hazard ratio for readmission or death, indicating a potential harm from the intervention, said Dr. Mueller. An additional analysis stratified patients by left ventricular ejection fraction. Aside from the intervention’s negative effect on women participating in the trial, no other subgroups benefited or were harmed by an early vasodilation strategy.
Alexandre Mebazaa, MD, the designated discussant for the presentation, said that, although the GALACTIC trial was neutral, it represents “an important step forward in acute heart failure.
“Congratulations: First, because we know that in the critically ill condition it’s very difficult to do trials,” and the GALACTIC investigators succeeded in enrolling patients within the first 5 hours of presentation to EDs, noted Dr. Mebazaa, professor of anesthesiology and critical care medicine at the Paris Diderot School of Medicine.
He added that GALACTIC succeeded in continuing vasodilator use beyond the 48-hour mark. “For the first time, you had the courage to go a little bit further down, and we see that patients got the drug with vasodilator properties for 2 days or more.”
However, the long recruitment period for GALACTIC – first enrollment began in 2007 – meant that the study design reflected a thought process about AHF that doesn’t necessarily reflect current practice, noted Dr. Mebazaa. “The trial was designed many years ago, and at that time, we were still thinking that giving very aggressive treatment in the first hours could have an impact.
“Now, when we will be treating patients with vasodilators with acute heart failure – at least myself and my group – I would really wonder whether there is still evidence in the world to support the use of those agents.”
Dr. Mueller noted limitations of the GALACTIC trial, including the lack of generalizability to patients with systolic hypotension or severe renal dysfunction, since these populations were excluded. Also, “the open-label design, which was mandated by the aim to test a strategy, not a single drug, may have introduced a bias in the unblinded assessment of dyspnea” during inpatient stay.
The study was funded by several Swiss research institutions and had no industry support. Dr. Mueller reported no relevant conflicts of interest. Dr. Mebazaa reported financial relationships with Roche, Service, Novartis, AstraZeneca, S-Form Pharma, 4Teen$4, Adrenomed, and Sphingotec.
SOURCE: Mueller C. ESC 2019, Hot Line Session 3.
PARIS – A practical strategy of early and aggressive vasodilation and optimization of long-term medication for acute heart failure did not budge all-cause mortality or 180-day readmission rates, according to results of a pragmatic trial presented at the annual congress of the European Society of Cardiology.
“To our great disappointment, the curves were superimposable” between intervention and control arms in the GALACTIC (Goal-directed Afterload Reduction in Acute Congestive Cardiac Decompensation) trial, said lead investigator Christian Eugen Mueller, MD. “There was no signal of a benefit” for those receiving the targeted intervention: the adjusted hazard ratio was 1.07 for the composite primary endpoint of all-cause mortality or 6-month readmission for acute heart failure (P = 0.59).
GALACTIC, explained Dr. Mueller, was the largest investigator-initiated, randomized, controlled trial of pharmacologic therapy for acute heart failure (AHF).
“It is different in that it did not investigate a single drug, but a strategy of early, intensive, and sustained vasodilation. It is also unique in that it used individual doses of well-characterized, widely available, and mostly inexpensive drugs,” said Dr. Mueller, director of the Cardiovascular Research Institute at the University Hospital, Basel, Switzerland. “So this would have the beauty that, if it has a positive finding, you – in whatever country you come from – would be immediately able to apply it once you’re back home in your institution.”
The study attempted to address the gap between symptom amelioration and long-term outcomes when patients arrive in the ED with AHF. “Despite symptomatic improvement achieved from loop diuretics, mortality and morbidity remain unacceptably high,” said Dr. Mueller, with 40%-50% of AHF patients experiencing rehospitalization or death within 180 days of discharge.
Much remains unknown about the optimal treatment strategy for AHF. Aggressive vasodilation has been shown to improve outcomes in less-severe AHF, and intravenous nitrates are known to improve outcomes in AHF where severe pulmonary edema is present – “a phenotype representing only about 5% of patients,” noted Dr. Mueller. Still, “it is unknown whether aggressive vasodilation also improves outcomes in the much more common less-severe phenotype.”
Also, previous trials that ran intravenous vasodilators at a fixed dose for 48 hours did not improve AHF outcomes, so a one-size-fits-all strategy was not one the GALACTIC investigators sought to pursue.
In addition to a flexible regimen, “any strategy applied needs to take into consideration that the vast majority of patients with acute heart failure, after initial treatment in the ED, are then treated in a general cardiology ward,” added Dr. Mueller.
This meant that intravenous nitrate infusion was not part of the GALACTIC trial; rather, sublingual and transdermal nitrates were used, explained Dr. Mueller. “Transdermal application has the beauty that if you have an adverse effect – and hypotension is the most dangerous one – you can immediately remove the patch, and thereby avoid any further harm.”
The two-part strategy tested in GALACTIC involved reducing cardiac filling pressures by maintaining or increasing organ perfusion, while also increasing “long-term lifesaving therapy” targeting the renin-angiotensin-aldosterone system during hospitalization, with a goal to continue optimal treatment long term.
ACE inhibitors or angiotensin receptor blockers were added on the second day of hospitalization for the intervention group, said Dr. Mueller, and “in the ideal setting, up-titrated very aggressively from day to day.
“However, as you know, up-titration to target dose is sometimes wishful thinking in this frail population,” he said, so the GALACTIC trial protocol included a scheme to back dosing off for hypotension, hypokalemia, or worsening renal function. Systolic BP guided how aggressively vasodilation and ACE inhibitor/angiotensin receptor blocker therapy were escalated.
In the end, 382 patients randomized to the intervention arm received early, intensive, and sustained vasodilation, and the 399 patients in the control arm received standard-of-care treatment according to ESC guidelines. These figures omit two patients in the standard-of-care arm who withdrew consent, but follow-up was otherwise complete, said Dr. Mueller. Physicians treating patients in both study arms had discretion to use such other therapies as loop diuretics, beta-blockers, aldosterone antagonists, and cardiac devices.
Adult patients coming to the ED with acute dyspnea classified as New York Heart Association class III or IV were eligible if they had brain natriuretic peptide (BNP) levels of at least 500 ng/L, or N-terminal of the prohormone BNP (NT-proBNP) levels of at least 2,000 ng/L.
Overall, patients enrolled in GALACTIC were in their late 70s, and women made up 37% of the population.
The actual median BNP for enrollees was about 1,250 ng/L, and the median NT-proBNP was just under 6,000 ng/L. The median left ventricular ejection fraction was 37%. About a third of patients had diabetes, and 85% had hypertension. Over half had known chronic heart failure, about a third had prior history of MI, and half of patients had atrial fibrillation at baseline.
“Signs of congestion were present in all patients, and over 90% had rales on physical examination,” said Dr. Mueller.
Patients who were destined for the ICU, those who had systolic BP below 100 mm Hg or marked creatinine elevation, or who required cardiopulmonary resuscitation were excluded. Also excluded were patients with known structural defects such as severe valvular stenosis, congenital heart disease, or hypertrophic obstructive cardiomyopathy. GALACTIC also excluded patients with isolated right ventricular failure caused by pulmonary hypertension.
Prespecified subgroup analyses compared women with men, and those younger than 75 years with older participants. Women saw a significantly higher hazard ratio for readmission or death, indicating a potential harm from the intervention, said Dr. Mueller. An additional analysis stratified patients by left ventricular ejection fraction. Aside from the intervention’s negative effect on women participating in the trial, no other subgroups benefited or were harmed by an early vasodilation strategy.
Alexandre Mebazaa, MD, the designated discussant for the presentation, said that, although the GALACTIC trial was neutral, it represents “an important step forward in acute heart failure.
“Congratulations: First, because we know that in the critically ill condition it’s very difficult to do trials,” and the GALACTIC investigators succeeded in enrolling patients within the first 5 hours of presentation to EDs, noted Dr. Mebazaa, professor of anesthesiology and critical care medicine at the Paris Diderot School of Medicine.
He added that GALACTIC succeeded in continuing vasodilator use beyond the 48-hour mark. “For the first time, you had the courage to go a little bit further down, and we see that patients got the drug with vasodilator properties for 2 days or more.”
However, the long recruitment period for GALACTIC – first enrollment began in 2007 – meant that the study design reflected a thought process about AHF that doesn’t necessarily reflect current practice, noted Dr. Mebazaa. “The trial was designed many years ago, and at that time, we were still thinking that giving very aggressive treatment in the first hours could have an impact.
“Now, when we will be treating patients with vasodilators with acute heart failure – at least myself and my group – I would really wonder whether there is still evidence in the world to support the use of those agents.”
Dr. Mueller noted limitations of the GALACTIC trial, including the lack of generalizability to patients with systolic hypotension or severe renal dysfunction, since these populations were excluded. Also, “the open-label design, which was mandated by the aim to test a strategy, not a single drug, may have introduced a bias in the unblinded assessment of dyspnea” during inpatient stay.
The study was funded by several Swiss research institutions and had no industry support. Dr. Mueller reported no relevant conflicts of interest. Dr. Mebazaa reported financial relationships with Roche, Service, Novartis, AstraZeneca, S-Form Pharma, 4Teen$4, Adrenomed, and Sphingotec.
SOURCE: Mueller C. ESC 2019, Hot Line Session 3.
REPORTING FROM THE ESC CONGRESS 2019
Ibrutinib linked to hypertension in B-cell malignancies
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
FROM BLOOD
Adverse cytogenetics trump molecular risk in NPM1-mutated AML
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
REPORTING FROM THE JOURNAL OF CLINICAL ONCOLOGY
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
Don’t let knowledge gaps hold back fracture prevention efforts, experts say
ORLANDO – Clinicians shouldn't let the lack of research on osteoporosis treatment compliance and barriers to care keep them from acting on what's already known about drug therapies' effectiveness in preventing fractures.
That's the advice from leaders at the American Society for Bone and Mineral Research, who discussed recent recommendations issued from a National Institutes of Health Pathways to Prevention Workshop on the “Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention.”
Speakers at a special session of the annual meeting were in agreement on the need for more research into current barriers of osteoporosis care and new methods for increasing patient and physician compliance with recommendations.
But the focus should also be on what researchers in bone health have already learned about treating osteoporosis, “which is quite a bit,” said Benjamin Z. Leder, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, who gave the ASBMR’s perspective at the session. Dr. Leder was also first author on a perspective paper that outlined the society’s concerns about the recommendations coming from the workshop (J Bone Miner Res. 2019;34[9]:1549-51).
Gaps in knowledge and treatment
“We thought that [not focusing on what is already known about osteoporosis treatment] perhaps would have the potential to exacerbate the treatment gap that exists in osteoporosis therapy,” Dr. Leder said in his presentation. “We also felt that any knowledge deficits must be understood in the context of what has been unequivocally demonstrated in terms of our knowledge of osteoporosis therapies.”
That includes knowing how to identify patients at the highest risk of fracture, which medications reduce the risk of osteoporotic and osteopenic fractures, and that using these medications saves lives, Dr. Leder noted.
With limited resources in osteoporosis and the absence of any large, randomized, controlled trials or new therapies because of lack of interest from industry, stakeholders should be examining what can be done outside of trials. In osteoporosis, as is the case with any other therapeutic area or disease state, the results of randomized, placebo-controlled trials will not address every patient subgroup of interest, he said.
“Some extrapolation is always required,” Dr. Leder said. “We have to – as we treat, as we practice medicine – use the evidence we have and then try to use that to guide us when the randomized, controlled trial doesn’t answer that specific question.”
Dr. Leder also emphasized that fear of side effects such as osteonecrosis of the jaw and atypical femoral fracture are not the only barriers in osteoporosis care. Adherence is generally poor with osteoporosis treatment, and physicians usually have competing priorities when seeing their patients. “Even in the absence of the idea that these side effects are potentially paralyzing patients and physicians, we have to remember that we started from a fairly low baseline,” Dr. Leder said.
Other issues that complicate the issue of osteoporosis care are misinformation available online that may cause patients to not use medications, overestimations of the benefits of not using osteoporosis medication in favor of nondrug interventions, and conflicting, faulty, or unhelpful guidelines from medical societies, he said.
“There are a lot of issues that need to be addressed. The [Pathways to Prevention] has really done us a great service, and the ASBMR hopes to continue supporting their efforts moving forward,” he said.
Recommendations for secondary fracture prevention
Although there are gaps in osteoporosis treatment, there is still room to act, which the ASBMR aimed to accomplish in its Secondary Fracture Prevention Initiative, Douglas P. Kiel, MD, MPH, said in his presentation.
In 2016, the ASBMR put out a Call to Action to intensify screening for high-risk patients to prevent fractures, and 39 organizations signed on to the effort. The target population for the initiative is men and women aged 65 years or older who have experienced a vertebral or hip fracture who would ideally be appropriately evaluated, managed, and treated in a multidisciplinary clinical system with case management through systems like a fracture liaison service.
“The bottom line is, if you have a patient in this age group who has already experienced one of these fractures, they should be treated,” said Dr. Kiel, director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston and a professor of medicine at Harvard Medical School. “It doesn’t mean we should ignore other populations, but we need to start somewhere, and this was a well-defined, at-risk population.”
Consensus clinical recommendations for the initiative were recently published in the Journal of Bone and Mineral Research (J Bone Miner Res. 2019 Sep 20. doi: 10.1002/jbmr.3877), and the ASBMR plans to spread the recommendations in a wide number of areas, including through stakeholder organizations, social media, webinars, educational sessions, and in other guidelines.
The next step for the initiative is to execute the ASBMR’s Action Plan, which prioritizes challenges such as reimbursement for fracture liaison services and care coordination, establishment of a national fracture registry, and sharing the society’s messaging on osteoporosis fracture–prevention in guidelines and education services. The ASBMR is also examining whether it could take advantage of a new National Institutes of Health grant for dissemination and implementation research in health to help fund the Secondary Fracture Prevention Initiative, Dr. Kiel said.
Dr. Leder and Dr. Kiel reported no relevant financial disclosures.
ORLANDO – Clinicians shouldn't let the lack of research on osteoporosis treatment compliance and barriers to care keep them from acting on what's already known about drug therapies' effectiveness in preventing fractures.
That's the advice from leaders at the American Society for Bone and Mineral Research, who discussed recent recommendations issued from a National Institutes of Health Pathways to Prevention Workshop on the “Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention.”
Speakers at a special session of the annual meeting were in agreement on the need for more research into current barriers of osteoporosis care and new methods for increasing patient and physician compliance with recommendations.
But the focus should also be on what researchers in bone health have already learned about treating osteoporosis, “which is quite a bit,” said Benjamin Z. Leder, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, who gave the ASBMR’s perspective at the session. Dr. Leder was also first author on a perspective paper that outlined the society’s concerns about the recommendations coming from the workshop (J Bone Miner Res. 2019;34[9]:1549-51).
Gaps in knowledge and treatment
“We thought that [not focusing on what is already known about osteoporosis treatment] perhaps would have the potential to exacerbate the treatment gap that exists in osteoporosis therapy,” Dr. Leder said in his presentation. “We also felt that any knowledge deficits must be understood in the context of what has been unequivocally demonstrated in terms of our knowledge of osteoporosis therapies.”
That includes knowing how to identify patients at the highest risk of fracture, which medications reduce the risk of osteoporotic and osteopenic fractures, and that using these medications saves lives, Dr. Leder noted.
With limited resources in osteoporosis and the absence of any large, randomized, controlled trials or new therapies because of lack of interest from industry, stakeholders should be examining what can be done outside of trials. In osteoporosis, as is the case with any other therapeutic area or disease state, the results of randomized, placebo-controlled trials will not address every patient subgroup of interest, he said.
“Some extrapolation is always required,” Dr. Leder said. “We have to – as we treat, as we practice medicine – use the evidence we have and then try to use that to guide us when the randomized, controlled trial doesn’t answer that specific question.”
Dr. Leder also emphasized that fear of side effects such as osteonecrosis of the jaw and atypical femoral fracture are not the only barriers in osteoporosis care. Adherence is generally poor with osteoporosis treatment, and physicians usually have competing priorities when seeing their patients. “Even in the absence of the idea that these side effects are potentially paralyzing patients and physicians, we have to remember that we started from a fairly low baseline,” Dr. Leder said.
Other issues that complicate the issue of osteoporosis care are misinformation available online that may cause patients to not use medications, overestimations of the benefits of not using osteoporosis medication in favor of nondrug interventions, and conflicting, faulty, or unhelpful guidelines from medical societies, he said.
“There are a lot of issues that need to be addressed. The [Pathways to Prevention] has really done us a great service, and the ASBMR hopes to continue supporting their efforts moving forward,” he said.
Recommendations for secondary fracture prevention
Although there are gaps in osteoporosis treatment, there is still room to act, which the ASBMR aimed to accomplish in its Secondary Fracture Prevention Initiative, Douglas P. Kiel, MD, MPH, said in his presentation.
In 2016, the ASBMR put out a Call to Action to intensify screening for high-risk patients to prevent fractures, and 39 organizations signed on to the effort. The target population for the initiative is men and women aged 65 years or older who have experienced a vertebral or hip fracture who would ideally be appropriately evaluated, managed, and treated in a multidisciplinary clinical system with case management through systems like a fracture liaison service.
“The bottom line is, if you have a patient in this age group who has already experienced one of these fractures, they should be treated,” said Dr. Kiel, director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston and a professor of medicine at Harvard Medical School. “It doesn’t mean we should ignore other populations, but we need to start somewhere, and this was a well-defined, at-risk population.”
Consensus clinical recommendations for the initiative were recently published in the Journal of Bone and Mineral Research (J Bone Miner Res. 2019 Sep 20. doi: 10.1002/jbmr.3877), and the ASBMR plans to spread the recommendations in a wide number of areas, including through stakeholder organizations, social media, webinars, educational sessions, and in other guidelines.
The next step for the initiative is to execute the ASBMR’s Action Plan, which prioritizes challenges such as reimbursement for fracture liaison services and care coordination, establishment of a national fracture registry, and sharing the society’s messaging on osteoporosis fracture–prevention in guidelines and education services. The ASBMR is also examining whether it could take advantage of a new National Institutes of Health grant for dissemination and implementation research in health to help fund the Secondary Fracture Prevention Initiative, Dr. Kiel said.
Dr. Leder and Dr. Kiel reported no relevant financial disclosures.
ORLANDO – Clinicians shouldn't let the lack of research on osteoporosis treatment compliance and barriers to care keep them from acting on what's already known about drug therapies' effectiveness in preventing fractures.
That's the advice from leaders at the American Society for Bone and Mineral Research, who discussed recent recommendations issued from a National Institutes of Health Pathways to Prevention Workshop on the “Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention.”
Speakers at a special session of the annual meeting were in agreement on the need for more research into current barriers of osteoporosis care and new methods for increasing patient and physician compliance with recommendations.
But the focus should also be on what researchers in bone health have already learned about treating osteoporosis, “which is quite a bit,” said Benjamin Z. Leder, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, who gave the ASBMR’s perspective at the session. Dr. Leder was also first author on a perspective paper that outlined the society’s concerns about the recommendations coming from the workshop (J Bone Miner Res. 2019;34[9]:1549-51).
Gaps in knowledge and treatment
“We thought that [not focusing on what is already known about osteoporosis treatment] perhaps would have the potential to exacerbate the treatment gap that exists in osteoporosis therapy,” Dr. Leder said in his presentation. “We also felt that any knowledge deficits must be understood in the context of what has been unequivocally demonstrated in terms of our knowledge of osteoporosis therapies.”
That includes knowing how to identify patients at the highest risk of fracture, which medications reduce the risk of osteoporotic and osteopenic fractures, and that using these medications saves lives, Dr. Leder noted.
With limited resources in osteoporosis and the absence of any large, randomized, controlled trials or new therapies because of lack of interest from industry, stakeholders should be examining what can be done outside of trials. In osteoporosis, as is the case with any other therapeutic area or disease state, the results of randomized, placebo-controlled trials will not address every patient subgroup of interest, he said.
“Some extrapolation is always required,” Dr. Leder said. “We have to – as we treat, as we practice medicine – use the evidence we have and then try to use that to guide us when the randomized, controlled trial doesn’t answer that specific question.”
Dr. Leder also emphasized that fear of side effects such as osteonecrosis of the jaw and atypical femoral fracture are not the only barriers in osteoporosis care. Adherence is generally poor with osteoporosis treatment, and physicians usually have competing priorities when seeing their patients. “Even in the absence of the idea that these side effects are potentially paralyzing patients and physicians, we have to remember that we started from a fairly low baseline,” Dr. Leder said.
Other issues that complicate the issue of osteoporosis care are misinformation available online that may cause patients to not use medications, overestimations of the benefits of not using osteoporosis medication in favor of nondrug interventions, and conflicting, faulty, or unhelpful guidelines from medical societies, he said.
“There are a lot of issues that need to be addressed. The [Pathways to Prevention] has really done us a great service, and the ASBMR hopes to continue supporting their efforts moving forward,” he said.
Recommendations for secondary fracture prevention
Although there are gaps in osteoporosis treatment, there is still room to act, which the ASBMR aimed to accomplish in its Secondary Fracture Prevention Initiative, Douglas P. Kiel, MD, MPH, said in his presentation.
In 2016, the ASBMR put out a Call to Action to intensify screening for high-risk patients to prevent fractures, and 39 organizations signed on to the effort. The target population for the initiative is men and women aged 65 years or older who have experienced a vertebral or hip fracture who would ideally be appropriately evaluated, managed, and treated in a multidisciplinary clinical system with case management through systems like a fracture liaison service.
“The bottom line is, if you have a patient in this age group who has already experienced one of these fractures, they should be treated,” said Dr. Kiel, director of the Musculoskeletal Research Center at the Institute for Aging Research at Hebrew SeniorLife in Boston and a professor of medicine at Harvard Medical School. “It doesn’t mean we should ignore other populations, but we need to start somewhere, and this was a well-defined, at-risk population.”
Consensus clinical recommendations for the initiative were recently published in the Journal of Bone and Mineral Research (J Bone Miner Res. 2019 Sep 20. doi: 10.1002/jbmr.3877), and the ASBMR plans to spread the recommendations in a wide number of areas, including through stakeholder organizations, social media, webinars, educational sessions, and in other guidelines.
The next step for the initiative is to execute the ASBMR’s Action Plan, which prioritizes challenges such as reimbursement for fracture liaison services and care coordination, establishment of a national fracture registry, and sharing the society’s messaging on osteoporosis fracture–prevention in guidelines and education services. The ASBMR is also examining whether it could take advantage of a new National Institutes of Health grant for dissemination and implementation research in health to help fund the Secondary Fracture Prevention Initiative, Dr. Kiel said.
Dr. Leder and Dr. Kiel reported no relevant financial disclosures.
EXPERT ANALYSIS FROM ASBMR 2019
Coronary Artery Calcification Scores in Migraine Patients
No significant differences were demonstrated in the amount of coronary calcifications in patients with and without migraine, a new study found. Researchers evaluated if the increased cardiovascular (CV) risk in migraineurs is attributed to an increased coronary artery calcification (CAC). They found:
- The CAC score was assessed by computed tomography of the heart in 1,437 patients, of which 337 were migraineurs.
- All patients had a similar CV risk profile, so that the risk for CAC could be considered similar between migraineurs and non-migraineurs.
- There were no significant differences in the amount of CAC in patients with or without migraine.
Filippopulos FM, et al. Coronary artery calcification score in migraine patients. [Published online ahead of print October 1, 2019]. Sci Rep. doi: 10.1038/s41598-019-50660-9.
No significant differences were demonstrated in the amount of coronary calcifications in patients with and without migraine, a new study found. Researchers evaluated if the increased cardiovascular (CV) risk in migraineurs is attributed to an increased coronary artery calcification (CAC). They found:
- The CAC score was assessed by computed tomography of the heart in 1,437 patients, of which 337 were migraineurs.
- All patients had a similar CV risk profile, so that the risk for CAC could be considered similar between migraineurs and non-migraineurs.
- There were no significant differences in the amount of CAC in patients with or without migraine.
Filippopulos FM, et al. Coronary artery calcification score in migraine patients. [Published online ahead of print October 1, 2019]. Sci Rep. doi: 10.1038/s41598-019-50660-9.
No significant differences were demonstrated in the amount of coronary calcifications in patients with and without migraine, a new study found. Researchers evaluated if the increased cardiovascular (CV) risk in migraineurs is attributed to an increased coronary artery calcification (CAC). They found:
- The CAC score was assessed by computed tomography of the heart in 1,437 patients, of which 337 were migraineurs.
- All patients had a similar CV risk profile, so that the risk for CAC could be considered similar between migraineurs and non-migraineurs.
- There were no significant differences in the amount of CAC in patients with or without migraine.
Filippopulos FM, et al. Coronary artery calcification score in migraine patients. [Published online ahead of print October 1, 2019]. Sci Rep. doi: 10.1038/s41598-019-50660-9.
Abnormal CV Response to Nitroglycerin in Migraine
Patients with migraine who developed a migraine-like attack in response to nitroglycerin demonstrated stronger systemic cardiovascular (CV) responses compared to non-headache controls, a new study found. In 16 women with migraine without aura and 10 age- and gender-matched controls, intravenous nitroglycerin was administered. Researchers found:
- Nitroglycerin provoked a migraine-like attack in 81.2% of migraineurs but not in controls.
- Migraineurs who later developed a migraine-like attack showed different responses in all parameters vs controls.
- The decreases in cardiac output and stroke volume were more rapid and longer lasting, heart rate increased, mean arterial pressure and total peripheral resistance were higher and decreased after an initial increase.
van Oosterhout WPJ, et al. Abnormal cardiovascular response to nitroglycerin in migraine. [Published online ahead of print October 9, 2019]. Cephalalgia. doi: 10.1177/0333102419881657.
Patients with migraine who developed a migraine-like attack in response to nitroglycerin demonstrated stronger systemic cardiovascular (CV) responses compared to non-headache controls, a new study found. In 16 women with migraine without aura and 10 age- and gender-matched controls, intravenous nitroglycerin was administered. Researchers found:
- Nitroglycerin provoked a migraine-like attack in 81.2% of migraineurs but not in controls.
- Migraineurs who later developed a migraine-like attack showed different responses in all parameters vs controls.
- The decreases in cardiac output and stroke volume were more rapid and longer lasting, heart rate increased, mean arterial pressure and total peripheral resistance were higher and decreased after an initial increase.
van Oosterhout WPJ, et al. Abnormal cardiovascular response to nitroglycerin in migraine. [Published online ahead of print October 9, 2019]. Cephalalgia. doi: 10.1177/0333102419881657.
Patients with migraine who developed a migraine-like attack in response to nitroglycerin demonstrated stronger systemic cardiovascular (CV) responses compared to non-headache controls, a new study found. In 16 women with migraine without aura and 10 age- and gender-matched controls, intravenous nitroglycerin was administered. Researchers found:
- Nitroglycerin provoked a migraine-like attack in 81.2% of migraineurs but not in controls.
- Migraineurs who later developed a migraine-like attack showed different responses in all parameters vs controls.
- The decreases in cardiac output and stroke volume were more rapid and longer lasting, heart rate increased, mean arterial pressure and total peripheral resistance were higher and decreased after an initial increase.
van Oosterhout WPJ, et al. Abnormal cardiovascular response to nitroglycerin in migraine. [Published online ahead of print October 9, 2019]. Cephalalgia. doi: 10.1177/0333102419881657.
Three free apps for urogynecology providers
Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.
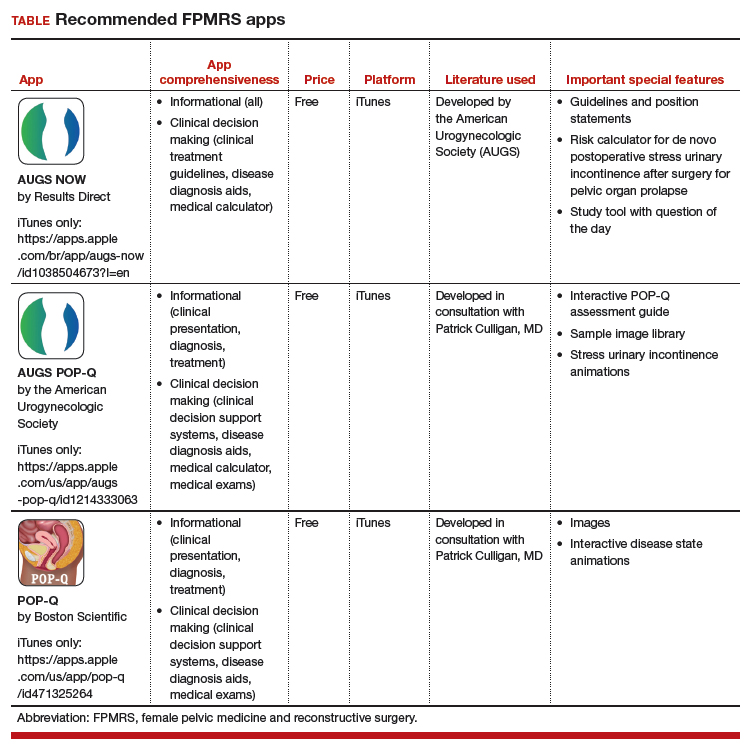
1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.

Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.

1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Poor Patient Awareness Common in Migraine
Poor awareness of migraine is common among patients in several countries, a new study found. The multicenter study was conducted in 12 headache centers in 7 countries with each center recruiting 100 or less patients referred for a first visit and diagnosed with migraine. Participants were given a structured clinical questionnaire-based interview about perceptions of the type of headache they suffer from, its cause, previous diagnoses, investigations, and treatments. Researchers found:
- Of the 1,161 patients who completed the study, 28% of participants were aware they suffer from migraine.
- 64% of participants called their migraine “headache,” less commonly used terms such as “cervical pain,” “tension headache,” and “sinusitis.”
- 8% of general practitioners and 35% of specialists consulted for migraine formulated the correct diagnosis.
Viana M, et al. Poor patient awareness and frequent misdiagnosis of migraine: findings from a large transcontinental cohort. [Published online ahead of print October 1, 2019]. Eur J Neurol. doi: 10.1111/ene.14098.
Poor awareness of migraine is common among patients in several countries, a new study found. The multicenter study was conducted in 12 headache centers in 7 countries with each center recruiting 100 or less patients referred for a first visit and diagnosed with migraine. Participants were given a structured clinical questionnaire-based interview about perceptions of the type of headache they suffer from, its cause, previous diagnoses, investigations, and treatments. Researchers found:
- Of the 1,161 patients who completed the study, 28% of participants were aware they suffer from migraine.
- 64% of participants called their migraine “headache,” less commonly used terms such as “cervical pain,” “tension headache,” and “sinusitis.”
- 8% of general practitioners and 35% of specialists consulted for migraine formulated the correct diagnosis.
Viana M, et al. Poor patient awareness and frequent misdiagnosis of migraine: findings from a large transcontinental cohort. [Published online ahead of print October 1, 2019]. Eur J Neurol. doi: 10.1111/ene.14098.
Poor awareness of migraine is common among patients in several countries, a new study found. The multicenter study was conducted in 12 headache centers in 7 countries with each center recruiting 100 or less patients referred for a first visit and diagnosed with migraine. Participants were given a structured clinical questionnaire-based interview about perceptions of the type of headache they suffer from, its cause, previous diagnoses, investigations, and treatments. Researchers found:
- Of the 1,161 patients who completed the study, 28% of participants were aware they suffer from migraine.
- 64% of participants called their migraine “headache,” less commonly used terms such as “cervical pain,” “tension headache,” and “sinusitis.”
- 8% of general practitioners and 35% of specialists consulted for migraine formulated the correct diagnosis.
Viana M, et al. Poor patient awareness and frequent misdiagnosis of migraine: findings from a large transcontinental cohort. [Published online ahead of print October 1, 2019]. Eur J Neurol. doi: 10.1111/ene.14098.
Minimize blood pressure peaks, variability after stroke reperfusion
ST. LOUIS – Albuquerque. Investigators found that every 10–mm Hg increase in peak systolic pressure boosted the risk of in-hospital death 24% (P = .01) and reduced the chance of being discharged home or to a inpatient rehabilitation facility 13% (P = .03). Results were even stronger for peak mean arterial pressure, at 76% (P = .01) and 29% (P = .04), respectively; trends in the same direction for peak diastolic pressure were not statistically significant.
Also, every 10–mm Hg increase in blood pressure variability again increased the risk of dying in the hospital, whether it was systolic (33%; P = .002), diastolic (33%; P = .03), or mean arterial pressure variability (58%; P = .02). Higher variability also reduced the chance of being discharged home or to a rehab 10%-20%, but the findings, although close, were not statistically significant.
Neurologists generally do what they can to control blood pressure after stroke, and the study confirms the need to do that. What’s new is that the work was limited to reperfusion patients – intravenous thrombolysis with alteplase in 83.5%, mechanical thrombectomy in 60%, with some having both – which has not been the specific focus of much research.
“Be much more aggressive in terms of making sure the variability is limited and limiting the peaks,” especially within 24 hours of reperfusion, said lead investigator and stroke neurologist Dinesh Jillella, MD, of Emory University, Atlanta, at the annual meeting of the American Neurological Association. “We want to be much more aggressive [with these patients]; it might limit our worse outcomes,” Dr. Jillella said. He conducted the review while in training at the University of New Mexico.
What led to the study is that Dr. Jillella and colleagues noticed that similar reperfusion patients can have very different outcomes, and he wanted to find modifiable risk factors that could account for the differences. The study did not address why high peaks and variability lead to worse outcomes, but he said hemorrhagic conversion might play a role.
It is also possible that higher pressures could be a marker of bad outcomes, as opposed to a direct cause, but the findings were adjusted for two significant confounders: age and the National Institutes of Health Stroke Scale score, which were both significantly higher in patients who did not do well. But after adjustment, “we [still] found an independent association with blood pressures and worse outcomes,” he said.
Higher peak systolic pressures and variability were also associated with about a 15% lower odds of leaving the hospital with a modified Rankin Scale score of 3 or less, which means the patient has some moderate disability but is still able to walk without assistance.
Patients were 69 years old on average, and about 60% were men. The majority were white. About a third had a modified Rankin Scale score at or below 3 at discharge, and about two-thirds were discharged home or to a rehabilitation facility; 17% of patients died in the hospital.
Differences in antihypertensive regimens were not associated with outcomes on univariate analysis. Dr. Jillella said that, ideally, he would like to run a multicenter, prospective trial of blood pressure reduction targets after reperfusion.
There was no external funding, and Dr. Jillella didn’t have any relevant disclosures.
ST. LOUIS – Albuquerque. Investigators found that every 10–mm Hg increase in peak systolic pressure boosted the risk of in-hospital death 24% (P = .01) and reduced the chance of being discharged home or to a inpatient rehabilitation facility 13% (P = .03). Results were even stronger for peak mean arterial pressure, at 76% (P = .01) and 29% (P = .04), respectively; trends in the same direction for peak diastolic pressure were not statistically significant.
Also, every 10–mm Hg increase in blood pressure variability again increased the risk of dying in the hospital, whether it was systolic (33%; P = .002), diastolic (33%; P = .03), or mean arterial pressure variability (58%; P = .02). Higher variability also reduced the chance of being discharged home or to a rehab 10%-20%, but the findings, although close, were not statistically significant.
Neurologists generally do what they can to control blood pressure after stroke, and the study confirms the need to do that. What’s new is that the work was limited to reperfusion patients – intravenous thrombolysis with alteplase in 83.5%, mechanical thrombectomy in 60%, with some having both – which has not been the specific focus of much research.
“Be much more aggressive in terms of making sure the variability is limited and limiting the peaks,” especially within 24 hours of reperfusion, said lead investigator and stroke neurologist Dinesh Jillella, MD, of Emory University, Atlanta, at the annual meeting of the American Neurological Association. “We want to be much more aggressive [with these patients]; it might limit our worse outcomes,” Dr. Jillella said. He conducted the review while in training at the University of New Mexico.
What led to the study is that Dr. Jillella and colleagues noticed that similar reperfusion patients can have very different outcomes, and he wanted to find modifiable risk factors that could account for the differences. The study did not address why high peaks and variability lead to worse outcomes, but he said hemorrhagic conversion might play a role.
It is also possible that higher pressures could be a marker of bad outcomes, as opposed to a direct cause, but the findings were adjusted for two significant confounders: age and the National Institutes of Health Stroke Scale score, which were both significantly higher in patients who did not do well. But after adjustment, “we [still] found an independent association with blood pressures and worse outcomes,” he said.
Higher peak systolic pressures and variability were also associated with about a 15% lower odds of leaving the hospital with a modified Rankin Scale score of 3 or less, which means the patient has some moderate disability but is still able to walk without assistance.
Patients were 69 years old on average, and about 60% were men. The majority were white. About a third had a modified Rankin Scale score at or below 3 at discharge, and about two-thirds were discharged home or to a rehabilitation facility; 17% of patients died in the hospital.
Differences in antihypertensive regimens were not associated with outcomes on univariate analysis. Dr. Jillella said that, ideally, he would like to run a multicenter, prospective trial of blood pressure reduction targets after reperfusion.
There was no external funding, and Dr. Jillella didn’t have any relevant disclosures.
ST. LOUIS – Albuquerque. Investigators found that every 10–mm Hg increase in peak systolic pressure boosted the risk of in-hospital death 24% (P = .01) and reduced the chance of being discharged home or to a inpatient rehabilitation facility 13% (P = .03). Results were even stronger for peak mean arterial pressure, at 76% (P = .01) and 29% (P = .04), respectively; trends in the same direction for peak diastolic pressure were not statistically significant.
Also, every 10–mm Hg increase in blood pressure variability again increased the risk of dying in the hospital, whether it was systolic (33%; P = .002), diastolic (33%; P = .03), or mean arterial pressure variability (58%; P = .02). Higher variability also reduced the chance of being discharged home or to a rehab 10%-20%, but the findings, although close, were not statistically significant.
Neurologists generally do what they can to control blood pressure after stroke, and the study confirms the need to do that. What’s new is that the work was limited to reperfusion patients – intravenous thrombolysis with alteplase in 83.5%, mechanical thrombectomy in 60%, with some having both – which has not been the specific focus of much research.
“Be much more aggressive in terms of making sure the variability is limited and limiting the peaks,” especially within 24 hours of reperfusion, said lead investigator and stroke neurologist Dinesh Jillella, MD, of Emory University, Atlanta, at the annual meeting of the American Neurological Association. “We want to be much more aggressive [with these patients]; it might limit our worse outcomes,” Dr. Jillella said. He conducted the review while in training at the University of New Mexico.
What led to the study is that Dr. Jillella and colleagues noticed that similar reperfusion patients can have very different outcomes, and he wanted to find modifiable risk factors that could account for the differences. The study did not address why high peaks and variability lead to worse outcomes, but he said hemorrhagic conversion might play a role.
It is also possible that higher pressures could be a marker of bad outcomes, as opposed to a direct cause, but the findings were adjusted for two significant confounders: age and the National Institutes of Health Stroke Scale score, which were both significantly higher in patients who did not do well. But after adjustment, “we [still] found an independent association with blood pressures and worse outcomes,” he said.
Higher peak systolic pressures and variability were also associated with about a 15% lower odds of leaving the hospital with a modified Rankin Scale score of 3 or less, which means the patient has some moderate disability but is still able to walk without assistance.
Patients were 69 years old on average, and about 60% were men. The majority were white. About a third had a modified Rankin Scale score at or below 3 at discharge, and about two-thirds were discharged home or to a rehabilitation facility; 17% of patients died in the hospital.
Differences in antihypertensive regimens were not associated with outcomes on univariate analysis. Dr. Jillella said that, ideally, he would like to run a multicenter, prospective trial of blood pressure reduction targets after reperfusion.
There was no external funding, and Dr. Jillella didn’t have any relevant disclosures.
REPORTING FROM ANA 2019