User login
Low P values shouldn’t always impress you
SAN DIEGO – Even if a P value hints at statistical significance by dipping under .05, it might not tell you anything worthwhile. Effect sizes are hugely important – as long as accompanying P values measure up. And pharmaceutical companies often keep revealing numbers under wraps unless you know what – and whom – to ask.
Those lessons come courtesy of Leslie Citrome, MD, MPH, who spoke to colleagues about study numbers at Psych Congress 2019.
Dr. Citrome, clinical professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, offered several tips about interpreting medical statistics as you make clinical decisions.
Don’t get hung up on the P value.
The P value helps you understand how likely it is that a difference in a study is statistically significant. In medical research, P values under .05 are considered especially desirable. They suggest that an outcome – drug A performed better than drug B, for example – didn’t happen purely by chance.
Here’s the hitch: The P value might not matter at all. “Clinicians often assume that if the P value is less than.05, the result must be important. But even a P value of less than .05 is meaningless outside of the context of how big the treatment effect is,” Dr. Citrome said. “If a clinical trial result shows us a small effect size, then who cares?”
Understand what effect sizes tell you.
Effect size measurements evaluate clinical impact and include number needed to treat (NNT) and number needed to harm (NNH). NNT refers to the number of patients needed to treat with an intervention in order to get a positive effect in one additional patient; NNH is the reverse and examines negative effects that can range from the minor (mild dry mouth) to the devastating (death).
What’s a good size for an NNT? “I respect any NNT versus placebo of less than 10,” Dr. Citrome said. “It’s something I’ll probably consider in day-to-day practice.” Double-digit and triple-digit NNTs “are usually irrelevant unless we’re dealing with very specific outcomes that have long-term consequences.”
As for the opposite side of the picture – NNH – values higher than 10 are ideal.
He cautioned that NNT and NNH, like P values, cannot stand alone. In fact, they work together. In order to have value, NNT or NNH must be statistically significant, and P values provide this crucial insight.
Consider Dr. Citrome’s blood pressure.
As Dr. Citrome noted, research suggests that, among patients with diastolic BP from 90 to 109 mm Hg, 1 additional person will avoid death, stroke, or heart attack for every 141 people who take an antihypertensive medication, compared to those who do not, over a 5-year period. That’s a lot of people taking medication for a long time, with potential side effects, for a fairly small effect size. However, the outcomes are dire, so it is still worth it.
Then there’s Dr. Citrome himself, who has had diastolic BP in the range of 115 to 129 mm Hg. The NNT is 3. For every 3 people who take an antihypertensive vs. not over a 5-year period, 1 additional person will avoid a potentially catastrophic cardiovascular event.
“Guess who’s pretty adherent to taking his antihypertensive medication?” he asked. “I am.”
Ask for effect sizes if you don’t see them.
It’s not unusual for pharmaceutical representatives to avoid providing information about medication effect sizes. “The sales representatives as well as speakers at [Food and Drug Administration]–regulated promotional speaking events can only speak to basically what’s on the label. NNT and NNH are not currently found on product labels. But they are very relevant, and we need to know this information.”
What to do? “There is a workaround here,” he said. “Ask the sales rep to talk to a medical science liaison, who is free to come to your office and talk about all the data that they have.”
Dr. Citrome reported multiple disclosures, including various relationships with pharmaceutical companies.
SAN DIEGO – Even if a P value hints at statistical significance by dipping under .05, it might not tell you anything worthwhile. Effect sizes are hugely important – as long as accompanying P values measure up. And pharmaceutical companies often keep revealing numbers under wraps unless you know what – and whom – to ask.
Those lessons come courtesy of Leslie Citrome, MD, MPH, who spoke to colleagues about study numbers at Psych Congress 2019.
Dr. Citrome, clinical professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, offered several tips about interpreting medical statistics as you make clinical decisions.
Don’t get hung up on the P value.
The P value helps you understand how likely it is that a difference in a study is statistically significant. In medical research, P values under .05 are considered especially desirable. They suggest that an outcome – drug A performed better than drug B, for example – didn’t happen purely by chance.
Here’s the hitch: The P value might not matter at all. “Clinicians often assume that if the P value is less than.05, the result must be important. But even a P value of less than .05 is meaningless outside of the context of how big the treatment effect is,” Dr. Citrome said. “If a clinical trial result shows us a small effect size, then who cares?”
Understand what effect sizes tell you.
Effect size measurements evaluate clinical impact and include number needed to treat (NNT) and number needed to harm (NNH). NNT refers to the number of patients needed to treat with an intervention in order to get a positive effect in one additional patient; NNH is the reverse and examines negative effects that can range from the minor (mild dry mouth) to the devastating (death).
What’s a good size for an NNT? “I respect any NNT versus placebo of less than 10,” Dr. Citrome said. “It’s something I’ll probably consider in day-to-day practice.” Double-digit and triple-digit NNTs “are usually irrelevant unless we’re dealing with very specific outcomes that have long-term consequences.”
As for the opposite side of the picture – NNH – values higher than 10 are ideal.
He cautioned that NNT and NNH, like P values, cannot stand alone. In fact, they work together. In order to have value, NNT or NNH must be statistically significant, and P values provide this crucial insight.
Consider Dr. Citrome’s blood pressure.
As Dr. Citrome noted, research suggests that, among patients with diastolic BP from 90 to 109 mm Hg, 1 additional person will avoid death, stroke, or heart attack for every 141 people who take an antihypertensive medication, compared to those who do not, over a 5-year period. That’s a lot of people taking medication for a long time, with potential side effects, for a fairly small effect size. However, the outcomes are dire, so it is still worth it.
Then there’s Dr. Citrome himself, who has had diastolic BP in the range of 115 to 129 mm Hg. The NNT is 3. For every 3 people who take an antihypertensive vs. not over a 5-year period, 1 additional person will avoid a potentially catastrophic cardiovascular event.
“Guess who’s pretty adherent to taking his antihypertensive medication?” he asked. “I am.”
Ask for effect sizes if you don’t see them.
It’s not unusual for pharmaceutical representatives to avoid providing information about medication effect sizes. “The sales representatives as well as speakers at [Food and Drug Administration]–regulated promotional speaking events can only speak to basically what’s on the label. NNT and NNH are not currently found on product labels. But they are very relevant, and we need to know this information.”
What to do? “There is a workaround here,” he said. “Ask the sales rep to talk to a medical science liaison, who is free to come to your office and talk about all the data that they have.”
Dr. Citrome reported multiple disclosures, including various relationships with pharmaceutical companies.
SAN DIEGO – Even if a P value hints at statistical significance by dipping under .05, it might not tell you anything worthwhile. Effect sizes are hugely important – as long as accompanying P values measure up. And pharmaceutical companies often keep revealing numbers under wraps unless you know what – and whom – to ask.
Those lessons come courtesy of Leslie Citrome, MD, MPH, who spoke to colleagues about study numbers at Psych Congress 2019.
Dr. Citrome, clinical professor of psychiatry and behavioral sciences at New York Medical College, Valhalla, offered several tips about interpreting medical statistics as you make clinical decisions.
Don’t get hung up on the P value.
The P value helps you understand how likely it is that a difference in a study is statistically significant. In medical research, P values under .05 are considered especially desirable. They suggest that an outcome – drug A performed better than drug B, for example – didn’t happen purely by chance.
Here’s the hitch: The P value might not matter at all. “Clinicians often assume that if the P value is less than.05, the result must be important. But even a P value of less than .05 is meaningless outside of the context of how big the treatment effect is,” Dr. Citrome said. “If a clinical trial result shows us a small effect size, then who cares?”
Understand what effect sizes tell you.
Effect size measurements evaluate clinical impact and include number needed to treat (NNT) and number needed to harm (NNH). NNT refers to the number of patients needed to treat with an intervention in order to get a positive effect in one additional patient; NNH is the reverse and examines negative effects that can range from the minor (mild dry mouth) to the devastating (death).
What’s a good size for an NNT? “I respect any NNT versus placebo of less than 10,” Dr. Citrome said. “It’s something I’ll probably consider in day-to-day practice.” Double-digit and triple-digit NNTs “are usually irrelevant unless we’re dealing with very specific outcomes that have long-term consequences.”
As for the opposite side of the picture – NNH – values higher than 10 are ideal.
He cautioned that NNT and NNH, like P values, cannot stand alone. In fact, they work together. In order to have value, NNT or NNH must be statistically significant, and P values provide this crucial insight.
Consider Dr. Citrome’s blood pressure.
As Dr. Citrome noted, research suggests that, among patients with diastolic BP from 90 to 109 mm Hg, 1 additional person will avoid death, stroke, or heart attack for every 141 people who take an antihypertensive medication, compared to those who do not, over a 5-year period. That’s a lot of people taking medication for a long time, with potential side effects, for a fairly small effect size. However, the outcomes are dire, so it is still worth it.
Then there’s Dr. Citrome himself, who has had diastolic BP in the range of 115 to 129 mm Hg. The NNT is 3. For every 3 people who take an antihypertensive vs. not over a 5-year period, 1 additional person will avoid a potentially catastrophic cardiovascular event.
“Guess who’s pretty adherent to taking his antihypertensive medication?” he asked. “I am.”
Ask for effect sizes if you don’t see them.
It’s not unusual for pharmaceutical representatives to avoid providing information about medication effect sizes. “The sales representatives as well as speakers at [Food and Drug Administration]–regulated promotional speaking events can only speak to basically what’s on the label. NNT and NNH are not currently found on product labels. But they are very relevant, and we need to know this information.”
What to do? “There is a workaround here,” he said. “Ask the sales rep to talk to a medical science liaison, who is free to come to your office and talk about all the data that they have.”
Dr. Citrome reported multiple disclosures, including various relationships with pharmaceutical companies.
REPORTING FROM PSYCH CONGRESS 2019
Rituximab bests mycophenolate in pemphigus vulgaris
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
MADRID – Pascal Joly, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Not only did rituximab prove superior in terms of efficacy in the PEMPHIX trial, with a five times greater likelihood of achieving a complete remission lasting for at least 16 weeks while off oral corticosteroids than with mycophenolate mofetil in the 52-week study, but the total number of disease flares in the mycophenolate mofetil group was five times higher. Moreover, rituximab-treated patients received a markedly lower cumulative dose of prednisone as well.
“Rituximab has a superior overall benefit-risk profile, compared to mycophenolate mofetil, in patients with moderate to severe pemphigus vulgaris,” concluded Dr. Joly, professor of dermatology at the University of Rouen (France) and president of the French Society of Dermatology.
The study was undertaken because mycophenolate mofetil is commonly used as a corticosteroid-sparing drug in patients with pemphigus vulgaris, even though its efficacy for the treatment of this rare, severe autoimmune blistering disease is unproven, he explained.
In contrast, rituximab was approved by the Food and Drug Administration and European regulators for treatment of pemphigus vulgaris on the strength of the pivotal phase 3 Ritux 3 trial – also led by Dr. Joly – which demonstrated the superiority of this intravenously administered anti-CD20 monoclonal antibody plus short-term prednisone over high-dose corticosteroid monotherapy, which for decades had been the standard treatment despite its considerable toxicity burden (Lancet. 2017 May 20;389[10083]:2031-40).
An independently conducted analysis of Ritux 3 recently concluded that rituximab plus short-term prednisone was more effective than prednisone alone, with a lower risk of life-threatening, corticosteroid-related adverse events and less cumulative corticosteroid exposure (Br J Dermatol. 2019 Sep 5. doi: 10.1111/bjd.18482).
Also, an international panel of 93 pemphigus experts has declared that rituximab should be considered an evidence-based first-line therapy for moderate to-severe pemphigus (J Am Acad Dermatol. 2018 Feb 10. doi: 10.1016/j.jaad.2018.02.021).
The phase 3, placebo-controlled PEMPHIX trial randomized 135 patients with moderate or severe pemphigus at 49 academic medical centers in the United States and nine other countries to double-blind rituximab or mycophenolate mofetil on top of background oral prednisone at 1.0-1.5 mg/kg per day, with the steroid to be tapered and discontinued within 4-6 months.
The primary endpoint of the study was the proportion of patients in each study arm at week 52 who had achieved a sustained complete remission lasting for at least 16 weeks while off prednisone. The rate was 40.3% in the rituximab group and 9.5% with mycophenolate mofetil, for a 383% increased likelihood of sustained complete remission in the rituximab group.
In addition, all of the study’s secondary endpoints significantly favored rituximab. The median cumulative dose of corticosteroid was 2.7 g through 52 weeks in the rituximab arm, compared with 4 g with mycophenolate mofetil. The total number of disease flares over 52 weeks was 6 in the rituximab group and 44 in the mycophenolate arm. Five rituximab-treated patients experienced disease flares, as did 26 on mycophenolate. Thus, the likelihood of a flare was seven times lower with rituximab.
Scores on the Dermatology Life Quality Index improved by an average of 8.87 points from baseline to week 52 in the rituximab group versus 6 points with mycophenolate. And 62.7% of rituximab-treated patients had a week-52 Dermatology Life Quality Index score of 0, meaning no impact of the disease on their quality of life, compared with 25% of the mycophenolate group.
Dr. Joly characterized the safety profile of rituximab as “manageable, with acceptable tolerability.” About 9% of the rituximab group and 7.4% of mycophenolate-treated patients had one or more treatment-related adverse events, a nonsignificant difference. The rate of treatment-related serious infections was 3.0% with rituximab and 2.9% with mycophenolate. Serious infusion reactions leading to study withdrawal occurred in three patients on rituximab and one on mycophenolate. The rate of grade 3 or worse corticosteroid-related adverse events was 1.5% with rituximab and significantly greater at 7.4% with mycophenolate.
An additional 48-week safety assessment beyond the 52-week primary outcome is ongoing.
Asked what future role he sees for mycophenolate in pemphigus vulgaris, Dr. Joly replied that the only study in the literature that shows the drug outperforms placebo was seriously flawed. “In the future, it’s very likely that the indications for use of mycophenolate in pemphigus vulgaris will be fewer and fewer,” the dermatologist added.
In reply to a question about the merits of routine antibiotic prophylaxis against pneumocystis carinii pneumonia in patients taking rituximab for pemphigus vulgaris, Dr. Joly said the incidence isn’t sufficiently high to justify such practice. After all, he noted, there were no cases of pneumocystis carinii pneumonia in rituximab-treated patients in PEMPHIX and only one in Ritux 3.
EADV Scientific Programming Committee Chair Brigitte Dreno, MD, PhD, professor and head of dermatology at University Hospital in Nantes, France, inquired as to whether there’s a role for maintenance therapy in a potent rituximab-based treatment strategy such as utilized in PEMPHIX.
Definitely, Dr. Joly replied. However, further study is required to work out the best maintenance program.
“There are many arguments for maintenance therapy in these patients. For one, the frequency of relapses increases with the length of follow-up. Also, anti–desmoglein-specific T cells can still be detected after rituximab therapy, even in patients in complete remission. So there is a need for maintenance therapy, perhaps at months 6, 12, and 18, but the optimal regimen isn’t determined yet,” according to Dr. Joly.
PEMPHIX was sponsored by F. Hoffmann-La Roche. Dr. Joly reported serving as a consultant to Roche, Amgen, Principia Biopharma, and Argenx.
REPORTING FROM THE EADV CONGRESS
Making and using guidelines
Modern medicine increasingly relies on the adoption and use of guidelines.
Forty years ago, medicine was like free-form, rhythmic gymnastics in which physicians would develop an artisanal treatment plan for each patient. Now, medicine frequently involves recognizing when we need to do a triple-twisting, double-back somersault (the Biles II) and then performing it. The belief is that better outcomes flow from reduced variability in diagnostic and treatment plans, based on guidelines developed from evidence-based medicine from large meta-analyses. This dogma, still unproven in real life, probably works best for 95% of patients. The physician must not omit a step of deciding whether their particular patient is one of the 5% of patients to whom the guideline does not apply.
To be useful, the guidelines must be based on accurate science, produce a significantly positive cost-benefit-risk analysis, be wisely constructed, and be clearly written.
Alas, many guidelines fall far short of this ideal, and when they fail, they impugn all of medical care, they lower the credibility of the organizations that issue them, and they lower the public’s trust in medicine, which thereby impedes improving the public health. Don’t sweat the small stuff for public health guidelines.
The science matters. Nutritional guidelines have been particularly rickety, as John P.A. Ioannidis, MD, wrote in a JAMA op-ed 1 year ago.1 For instance, previous dietary recommendations to reduce cholesterol by avoiding eggs have since been shown to be wrong. The recommendation for reducing salt intake has been heavily criticized. Now the decades-long condemnation of red meat has been challenged. New “guidelines,” suggested by one group (let’s view it as a minority report that contradicts many official guidelines) in the October 1, 2019, issue of Annals of Internal Medicine, say that red meat and processed meats aren’t the boogeyman.2 The authors of the accompanying editorial are from the Center for Pediatric and Adolescent Comparative Effectiveness Research at Indiana University, Indianapolis.3 The editorial supports the new study, criticizing past recommendations because “the field of nutritional epidemiology is plagued by observational studies that have conducted inappropriate analyses, accompanied by likely erroneous conclusions.”
Clarity also matters. One factor in the current opiate epidemic was guidance in the mid-1990s making pain the “fifth vital sign.” This certainly was not the only factor nor was it necessarily the primary one. Most disasters, like most codes on the ward, proceed from multiple smaller failures and missteps. An emphasis on assessing pain in hospitalized patients did not intend to be interpreted as requiring that all pain be eliminated with strong medication, but that was the practical consequence. In response to the epidemic of overdose deaths, guidelines were promulgated in 2016 recommending reducing doses used for chronic opiate regimens. Some patients with chronic pain feared, and soon experienced, the consequences of those changes. In October 2019, those guidelines were revised telling physicians to go slower.4 In explaining the revision, one government official is quoted as saying: “Clearly we believe that there has been misinterpretation of the guidelines, which were very clear.”5 F. Scott Fitzgerald once wrote that “the test of a first-rate intelligence is the ability to hold two opposed ideas in mind at the same time and still retain the ability to function.” I reread that governmental doublespeak three times and my brain broke.
Clinical practice guidelines are an important part of modern medicine. But we need to be wiser about their creation. The science needs to be rigorous. The committees need to contain skeptics rather than just research scientists and clinicians with a vested interest in the field. The purported benefits of the guideline must be weighed against costs, risks, and unintended consequences. Humility is important. All physicians are taught the principle: “First, do no harm.” In explaining medical ethics to students, I rephrase that principle as: “Be cautious and humble. You are not as smart as you think you are.” Consider this food for thought the next time you read or create a guideline.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. JAMA. 2018;320(10):969-70.
2. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
3. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620.
4. U.S. Department of Health & Human Services. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of opioid analgesics. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf.
5. “New guidelines on opioid tapering tell doctors to go slow.” Washington Post. 2019 Oct 10.
Modern medicine increasingly relies on the adoption and use of guidelines.
Forty years ago, medicine was like free-form, rhythmic gymnastics in which physicians would develop an artisanal treatment plan for each patient. Now, medicine frequently involves recognizing when we need to do a triple-twisting, double-back somersault (the Biles II) and then performing it. The belief is that better outcomes flow from reduced variability in diagnostic and treatment plans, based on guidelines developed from evidence-based medicine from large meta-analyses. This dogma, still unproven in real life, probably works best for 95% of patients. The physician must not omit a step of deciding whether their particular patient is one of the 5% of patients to whom the guideline does not apply.
To be useful, the guidelines must be based on accurate science, produce a significantly positive cost-benefit-risk analysis, be wisely constructed, and be clearly written.
Alas, many guidelines fall far short of this ideal, and when they fail, they impugn all of medical care, they lower the credibility of the organizations that issue them, and they lower the public’s trust in medicine, which thereby impedes improving the public health. Don’t sweat the small stuff for public health guidelines.
The science matters. Nutritional guidelines have been particularly rickety, as John P.A. Ioannidis, MD, wrote in a JAMA op-ed 1 year ago.1 For instance, previous dietary recommendations to reduce cholesterol by avoiding eggs have since been shown to be wrong. The recommendation for reducing salt intake has been heavily criticized. Now the decades-long condemnation of red meat has been challenged. New “guidelines,” suggested by one group (let’s view it as a minority report that contradicts many official guidelines) in the October 1, 2019, issue of Annals of Internal Medicine, say that red meat and processed meats aren’t the boogeyman.2 The authors of the accompanying editorial are from the Center for Pediatric and Adolescent Comparative Effectiveness Research at Indiana University, Indianapolis.3 The editorial supports the new study, criticizing past recommendations because “the field of nutritional epidemiology is plagued by observational studies that have conducted inappropriate analyses, accompanied by likely erroneous conclusions.”
Clarity also matters. One factor in the current opiate epidemic was guidance in the mid-1990s making pain the “fifth vital sign.” This certainly was not the only factor nor was it necessarily the primary one. Most disasters, like most codes on the ward, proceed from multiple smaller failures and missteps. An emphasis on assessing pain in hospitalized patients did not intend to be interpreted as requiring that all pain be eliminated with strong medication, but that was the practical consequence. In response to the epidemic of overdose deaths, guidelines were promulgated in 2016 recommending reducing doses used for chronic opiate regimens. Some patients with chronic pain feared, and soon experienced, the consequences of those changes. In October 2019, those guidelines were revised telling physicians to go slower.4 In explaining the revision, one government official is quoted as saying: “Clearly we believe that there has been misinterpretation of the guidelines, which were very clear.”5 F. Scott Fitzgerald once wrote that “the test of a first-rate intelligence is the ability to hold two opposed ideas in mind at the same time and still retain the ability to function.” I reread that governmental doublespeak three times and my brain broke.
Clinical practice guidelines are an important part of modern medicine. But we need to be wiser about their creation. The science needs to be rigorous. The committees need to contain skeptics rather than just research scientists and clinicians with a vested interest in the field. The purported benefits of the guideline must be weighed against costs, risks, and unintended consequences. Humility is important. All physicians are taught the principle: “First, do no harm.” In explaining medical ethics to students, I rephrase that principle as: “Be cautious and humble. You are not as smart as you think you are.” Consider this food for thought the next time you read or create a guideline.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. JAMA. 2018;320(10):969-70.
2. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
3. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620.
4. U.S. Department of Health & Human Services. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of opioid analgesics. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf.
5. “New guidelines on opioid tapering tell doctors to go slow.” Washington Post. 2019 Oct 10.
Modern medicine increasingly relies on the adoption and use of guidelines.
Forty years ago, medicine was like free-form, rhythmic gymnastics in which physicians would develop an artisanal treatment plan for each patient. Now, medicine frequently involves recognizing when we need to do a triple-twisting, double-back somersault (the Biles II) and then performing it. The belief is that better outcomes flow from reduced variability in diagnostic and treatment plans, based on guidelines developed from evidence-based medicine from large meta-analyses. This dogma, still unproven in real life, probably works best for 95% of patients. The physician must not omit a step of deciding whether their particular patient is one of the 5% of patients to whom the guideline does not apply.
To be useful, the guidelines must be based on accurate science, produce a significantly positive cost-benefit-risk analysis, be wisely constructed, and be clearly written.
Alas, many guidelines fall far short of this ideal, and when they fail, they impugn all of medical care, they lower the credibility of the organizations that issue them, and they lower the public’s trust in medicine, which thereby impedes improving the public health. Don’t sweat the small stuff for public health guidelines.
The science matters. Nutritional guidelines have been particularly rickety, as John P.A. Ioannidis, MD, wrote in a JAMA op-ed 1 year ago.1 For instance, previous dietary recommendations to reduce cholesterol by avoiding eggs have since been shown to be wrong. The recommendation for reducing salt intake has been heavily criticized. Now the decades-long condemnation of red meat has been challenged. New “guidelines,” suggested by one group (let’s view it as a minority report that contradicts many official guidelines) in the October 1, 2019, issue of Annals of Internal Medicine, say that red meat and processed meats aren’t the boogeyman.2 The authors of the accompanying editorial are from the Center for Pediatric and Adolescent Comparative Effectiveness Research at Indiana University, Indianapolis.3 The editorial supports the new study, criticizing past recommendations because “the field of nutritional epidemiology is plagued by observational studies that have conducted inappropriate analyses, accompanied by likely erroneous conclusions.”
Clarity also matters. One factor in the current opiate epidemic was guidance in the mid-1990s making pain the “fifth vital sign.” This certainly was not the only factor nor was it necessarily the primary one. Most disasters, like most codes on the ward, proceed from multiple smaller failures and missteps. An emphasis on assessing pain in hospitalized patients did not intend to be interpreted as requiring that all pain be eliminated with strong medication, but that was the practical consequence. In response to the epidemic of overdose deaths, guidelines were promulgated in 2016 recommending reducing doses used for chronic opiate regimens. Some patients with chronic pain feared, and soon experienced, the consequences of those changes. In October 2019, those guidelines were revised telling physicians to go slower.4 In explaining the revision, one government official is quoted as saying: “Clearly we believe that there has been misinterpretation of the guidelines, which were very clear.”5 F. Scott Fitzgerald once wrote that “the test of a first-rate intelligence is the ability to hold two opposed ideas in mind at the same time and still retain the ability to function.” I reread that governmental doublespeak three times and my brain broke.
Clinical practice guidelines are an important part of modern medicine. But we need to be wiser about their creation. The science needs to be rigorous. The committees need to contain skeptics rather than just research scientists and clinicians with a vested interest in the field. The purported benefits of the guideline must be weighed against costs, risks, and unintended consequences. Humility is important. All physicians are taught the principle: “First, do no harm.” In explaining medical ethics to students, I rephrase that principle as: “Be cautious and humble. You are not as smart as you think you are.” Consider this food for thought the next time you read or create a guideline.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. JAMA. 2018;320(10):969-70.
2. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-1621.
3. Ann Intern Med. 2019 Oct 1. doi: 10.7326/M19-2620.
4. U.S. Department of Health & Human Services. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of opioid analgesics. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf.
5. “New guidelines on opioid tapering tell doctors to go slow.” Washington Post. 2019 Oct 10.
Policymakers must invest in health care innovation
Affordable pharma tops consumer list
In 2017, the United States spent $3.5 trillion on health care, and that number is projected to be close 20% of our GDP over the next 10 years. For consumers, prescription drugs feel like the biggest contributor.
“Although pharmaceutical spending accounts for less than 10% of health care spending, consumers bear much more of the out-of-pocket cost of the prescription drugs through copays or coinsurance at the pharmacy counter than they pay for hospital or physician costs,” said Tanisha Carino, PhD, author of a Health Affairs blog post about directions for innovation in health care. “This experience has led to rising concerns among Americans about the cost of prescription drugs.”
In fact, a December 2018 Politico-Harvard poll showed Americans from both political parties overwhelmingly agreed that taking action to lower drug prices should have been the top priority of the new Congress that took office in January of this year.
“Addressing the affordability of prescription drugs will require investing in medical research and policies that speed new products to the market that will promote competition and, hopefully, will hold down prices and offer greater choice to patients,” said Dr. Carino, who is executive director of FasterCures, a center of the Milken Institute devoted to improving the biomedical innovation ecosystem. “Policymakers have an opportunity to address the immediate concerns patients have in affording their medication.”
According to Dr. Carino, policymakers can also continue to encourage health-improving medical innovation through the following:
- Boosting investment in research and development.
- Increasing safety and coordination of health data for biomedical research.
- Incentivizing innovation in underinvested areas.
- Building the capacity of patient organizations.
Hospitalists, she added, will play a critical role in participating in the clinical research that will lead to the next generation of treatments.
Reference
1. Carino T. “To get more bang for your health-care buck, invest in innovation.” Health Affairs Blog. 2019 Jan 24. doi: 10.1377/hblog20190123.483080. Accessed Feb. 6, 2019.
Affordable pharma tops consumer list
Affordable pharma tops consumer list
In 2017, the United States spent $3.5 trillion on health care, and that number is projected to be close 20% of our GDP over the next 10 years. For consumers, prescription drugs feel like the biggest contributor.
“Although pharmaceutical spending accounts for less than 10% of health care spending, consumers bear much more of the out-of-pocket cost of the prescription drugs through copays or coinsurance at the pharmacy counter than they pay for hospital or physician costs,” said Tanisha Carino, PhD, author of a Health Affairs blog post about directions for innovation in health care. “This experience has led to rising concerns among Americans about the cost of prescription drugs.”
In fact, a December 2018 Politico-Harvard poll showed Americans from both political parties overwhelmingly agreed that taking action to lower drug prices should have been the top priority of the new Congress that took office in January of this year.
“Addressing the affordability of prescription drugs will require investing in medical research and policies that speed new products to the market that will promote competition and, hopefully, will hold down prices and offer greater choice to patients,” said Dr. Carino, who is executive director of FasterCures, a center of the Milken Institute devoted to improving the biomedical innovation ecosystem. “Policymakers have an opportunity to address the immediate concerns patients have in affording their medication.”
According to Dr. Carino, policymakers can also continue to encourage health-improving medical innovation through the following:
- Boosting investment in research and development.
- Increasing safety and coordination of health data for biomedical research.
- Incentivizing innovation in underinvested areas.
- Building the capacity of patient organizations.
Hospitalists, she added, will play a critical role in participating in the clinical research that will lead to the next generation of treatments.
Reference
1. Carino T. “To get more bang for your health-care buck, invest in innovation.” Health Affairs Blog. 2019 Jan 24. doi: 10.1377/hblog20190123.483080. Accessed Feb. 6, 2019.
In 2017, the United States spent $3.5 trillion on health care, and that number is projected to be close 20% of our GDP over the next 10 years. For consumers, prescription drugs feel like the biggest contributor.
“Although pharmaceutical spending accounts for less than 10% of health care spending, consumers bear much more of the out-of-pocket cost of the prescription drugs through copays or coinsurance at the pharmacy counter than they pay for hospital or physician costs,” said Tanisha Carino, PhD, author of a Health Affairs blog post about directions for innovation in health care. “This experience has led to rising concerns among Americans about the cost of prescription drugs.”
In fact, a December 2018 Politico-Harvard poll showed Americans from both political parties overwhelmingly agreed that taking action to lower drug prices should have been the top priority of the new Congress that took office in January of this year.
“Addressing the affordability of prescription drugs will require investing in medical research and policies that speed new products to the market that will promote competition and, hopefully, will hold down prices and offer greater choice to patients,” said Dr. Carino, who is executive director of FasterCures, a center of the Milken Institute devoted to improving the biomedical innovation ecosystem. “Policymakers have an opportunity to address the immediate concerns patients have in affording their medication.”
According to Dr. Carino, policymakers can also continue to encourage health-improving medical innovation through the following:
- Boosting investment in research and development.
- Increasing safety and coordination of health data for biomedical research.
- Incentivizing innovation in underinvested areas.
- Building the capacity of patient organizations.
Hospitalists, she added, will play a critical role in participating in the clinical research that will lead to the next generation of treatments.
Reference
1. Carino T. “To get more bang for your health-care buck, invest in innovation.” Health Affairs Blog. 2019 Jan 24. doi: 10.1377/hblog20190123.483080. Accessed Feb. 6, 2019.
HIV-Positive Kidney Transplantations Offer Hope
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
People with HIV could be a safe kidney donation source for other people with HIV, according to researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and from the University of Cape Town, South Africa.
Their study followed 51 study participants with HIV who received kidney transplants from deceased donors with HIV in South Africa.
Five years after kidney transplantation, 83% of the South African cohort survived; 79% still had a functioning transplanted kidney. Those findings are similar to findings from a 2010 US NIAID-funded study, with kidneys from both living and deceased donors that reported an 88% survival rate and 74% kidney graft survival rate after 3 years.
All participants in the South African cohort were virally suppressed at the time of transplantation. The researchers did not observe any increases in viral load among those who adhered to antiretroviral therapy (ART). While 10 participants changed their ART regimens during the study, none did so because of drug resistance.
Deceased donors had strains of HIV genetically distinct from those of the transplant recipients. The investigators watched closely for signs of possible superinfections with strains of HIV that might be resistant to the recipient’s ART regimen. They identified only 1 potential case of transient superinfection, but further analyses determined that it was most likely residual virus carried over from the donor during the transplant and not a true sustained superinfection. “By using the most advanced laboratory techniques available, our team showed that HIV superinfection is of limited risk in these patients,” said study author Andrew Redd, PhD, of the NIAID Laboratory of Immunoregulation.
Such studies were illegal in the US before the HIV Organ Policy Equity (HOPE) Act passed in 2013. The law intended to increase the availability of organs for transplantation for people with HIV and permits US transplant teams with an approved research protocol to transplant organs from donors with HIV into qualified recipients with HIV and end-stage organ failure.
Two ongoing NIAID-funded clinical trials, the HOPE in Action Multicenter Kidney Study and the HOPE in Action Multicenter Liver Study, are comparing clinical outcomes among people living with HIV who receive organs from deceased donors with HIV to those who receive HIV-negative organs.
Eureka Challenge Winners Help Lighten the Load of Life With Alzheimer Disease
Mobile applications and other tech solutions that target the specific needs of patients with Alzheimer and their caregivers won the day in the first Eureka prize competition, sponsored by the National Institute on Aging.
The Improving Care for People with Alzheimer’s Disease and Related Dementias Using Technology (iCare-AD/ADRD) Challenge received 33 applications for mobile device applications or web-based methods to help users coordinate and navigate life with dementia. The judging was based on creativity and innovation, rationale and potential impact, value to relevant stakeholders, usability, and functionality and feasibility.
First place, with $250,000, went to MapHabit for mobile software that provides behavior prompts with customizable picture-and-keyword visual maps to help people with activities of daily living. The platform has different interfaces for users: people with impaired memory, caregiver, or long-term care community manager. Simple commands, such as “take a shower,” are scheduled, with feedback provided to caregivers. The system takes advantage of the brain’s habit (procedural) memory system, the researchers say, rather than the hippocampal (declarative) memory system that is damaged early in AD.
A team from University of California, Los Angeles, won second prize of $100,000 for their web-based Dementia Care Software system, which helps coordinate complex medical and social services. The software, which can be integrated into any of the leading electronic health record systems, has already been used in support of the UCLA Alzheimer’s and Dementia Care Program, a nurse-practitioner co-management approach that includes structured needs assessment, individualized dementia care plans, and round-the-clock access for assistance and advice. The researchers say more than 2,600 patients with dementia have participated in the program so far.
Caregiver411, developed by researchers from North Carolina Agricultural and Technical State University, won the third-place prize of $50,000. The mobile app helps people make informed decisions by, for instance, capturing the care recipient’s stage of AD in order to provide targeted recommendations. Dementia caregivers can obtain tailored resources related to mental, emotional, physical, social, legal, and financial concerns. The app also gives them a forum for social connections and family chat groups through a messaging center.
The Eureka Challenge is part of the 21st Century Cures Act, signed into law in 2016, designed to help accelerate medical product development with innovations incorporating the perspectives of patients. More detailed project descriptions are available at www.nia.nih.gov/challenge-prize.
Mobile applications and other tech solutions that target the specific needs of patients with Alzheimer and their caregivers won the day in the first Eureka prize competition, sponsored by the National Institute on Aging.
The Improving Care for People with Alzheimer’s Disease and Related Dementias Using Technology (iCare-AD/ADRD) Challenge received 33 applications for mobile device applications or web-based methods to help users coordinate and navigate life with dementia. The judging was based on creativity and innovation, rationale and potential impact, value to relevant stakeholders, usability, and functionality and feasibility.
First place, with $250,000, went to MapHabit for mobile software that provides behavior prompts with customizable picture-and-keyword visual maps to help people with activities of daily living. The platform has different interfaces for users: people with impaired memory, caregiver, or long-term care community manager. Simple commands, such as “take a shower,” are scheduled, with feedback provided to caregivers. The system takes advantage of the brain’s habit (procedural) memory system, the researchers say, rather than the hippocampal (declarative) memory system that is damaged early in AD.
A team from University of California, Los Angeles, won second prize of $100,000 for their web-based Dementia Care Software system, which helps coordinate complex medical and social services. The software, which can be integrated into any of the leading electronic health record systems, has already been used in support of the UCLA Alzheimer’s and Dementia Care Program, a nurse-practitioner co-management approach that includes structured needs assessment, individualized dementia care plans, and round-the-clock access for assistance and advice. The researchers say more than 2,600 patients with dementia have participated in the program so far.
Caregiver411, developed by researchers from North Carolina Agricultural and Technical State University, won the third-place prize of $50,000. The mobile app helps people make informed decisions by, for instance, capturing the care recipient’s stage of AD in order to provide targeted recommendations. Dementia caregivers can obtain tailored resources related to mental, emotional, physical, social, legal, and financial concerns. The app also gives them a forum for social connections and family chat groups through a messaging center.
The Eureka Challenge is part of the 21st Century Cures Act, signed into law in 2016, designed to help accelerate medical product development with innovations incorporating the perspectives of patients. More detailed project descriptions are available at www.nia.nih.gov/challenge-prize.
Mobile applications and other tech solutions that target the specific needs of patients with Alzheimer and their caregivers won the day in the first Eureka prize competition, sponsored by the National Institute on Aging.
The Improving Care for People with Alzheimer’s Disease and Related Dementias Using Technology (iCare-AD/ADRD) Challenge received 33 applications for mobile device applications or web-based methods to help users coordinate and navigate life with dementia. The judging was based on creativity and innovation, rationale and potential impact, value to relevant stakeholders, usability, and functionality and feasibility.
First place, with $250,000, went to MapHabit for mobile software that provides behavior prompts with customizable picture-and-keyword visual maps to help people with activities of daily living. The platform has different interfaces for users: people with impaired memory, caregiver, or long-term care community manager. Simple commands, such as “take a shower,” are scheduled, with feedback provided to caregivers. The system takes advantage of the brain’s habit (procedural) memory system, the researchers say, rather than the hippocampal (declarative) memory system that is damaged early in AD.
A team from University of California, Los Angeles, won second prize of $100,000 for their web-based Dementia Care Software system, which helps coordinate complex medical and social services. The software, which can be integrated into any of the leading electronic health record systems, has already been used in support of the UCLA Alzheimer’s and Dementia Care Program, a nurse-practitioner co-management approach that includes structured needs assessment, individualized dementia care plans, and round-the-clock access for assistance and advice. The researchers say more than 2,600 patients with dementia have participated in the program so far.
Caregiver411, developed by researchers from North Carolina Agricultural and Technical State University, won the third-place prize of $50,000. The mobile app helps people make informed decisions by, for instance, capturing the care recipient’s stage of AD in order to provide targeted recommendations. Dementia caregivers can obtain tailored resources related to mental, emotional, physical, social, legal, and financial concerns. The app also gives them a forum for social connections and family chat groups through a messaging center.
The Eureka Challenge is part of the 21st Century Cures Act, signed into law in 2016, designed to help accelerate medical product development with innovations incorporating the perspectives of patients. More detailed project descriptions are available at www.nia.nih.gov/challenge-prize.
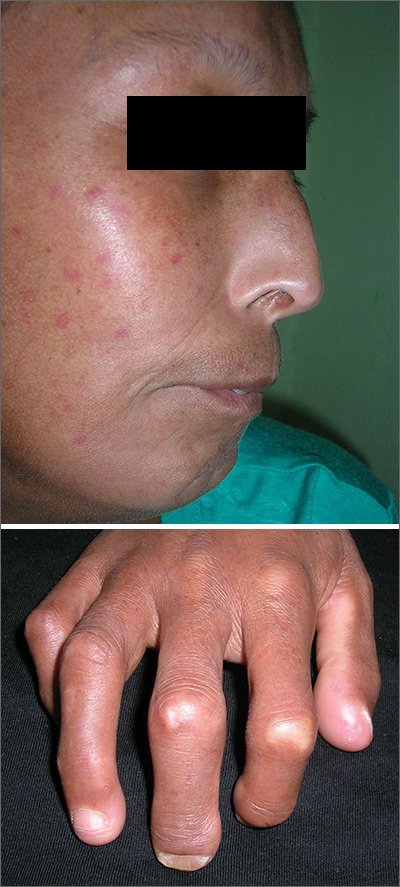
Telangiectasias with tight skin
The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that the patient had CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasias) syndrome also known as limited cutaneous systemic sclerosis (LcSSc). He examined her arms and found that she had firm nodules around the elbows consistent with calcinosis. Further history revealed Raynaud's phenomenon. This clinched the diagnosis of CREST syndrome. The FP ordered blood tests, a chest x-ray (CXR), and pulmonary function tests to determine if there was pulmonary involvement and to learn more about possible systemic effects of her disease.
Systemic sclerosis (scleroderma) is characterized by skin induration and thickening accompanied by variable tissue fibrosis and inflammatory infiltration in numerous visceral organs. Systemic sclerosis can be diffuse or limited to the skin and adjacent tissues (LcSSc). Patients with LcSSc usually have skin sclerosis that is restricted to the hands and, to a lesser extent, the face and neck.
The patient’s antinuclear antibody test was positive with a speckled nucleolar staining pattern, which is a common finding in systemic sclerosis. Her CXR showed evidence of interstitial lung disease with a ground glass pattern. Her pulmonary function test showed a diminished diffusion capacity. Pulmonary disease is seen in all types of systemic sclerosis and not diagnostic for one type. The patient was referred to Rheumatology for further diagnosis and management.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Scleroderma and morphea. In: Usatine R, Smith M, Mayeaux EJ, et al. eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1204-1212.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Late response to eculizumab may occur in minority of myasthenia gravis patients
AUSTIN – according to a secondary analysis presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine.
Evidence for the sustained effectiveness of eculizumab, a terminal complement inhibitor, in adult patients with antiacetylcholine receptor antibody-positive refractory generalized myasthenia gravis was provided by the 6-month, double-blind, placebo-controlled REGAIN study and its open-label extension. James F. Howard Jr., MD, distinguished professor of neuromuscular disease at the University of North Carolina in Chapel Hill and colleagues sought to analyze response profiles in REGAIN and its open-label extension.
The findings raise the possibility that complement inhibition with eculizumab should not be abandoned rapidly. “We accept that [eculizumab] works quickly,” Dr. Howard said. “There is an impression that if you don’t respond within the first 3 months, you’re not going to respond. I think these data would suggest otherwise.”
The investigators analyzed participants’ Myasthenia Gravis–Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores, which had been recorded throughout REGAIN and the extension. They defined early and late responses as improvement in MG-ADL score (i.e., a decrease of three or more points) or QMG score (i.e., a decrease of five or more points) occurring at 12 weeks or earlier or after 12 weeks, respectively, after initiation of eculizumab therapy. Patients randomized to eculizumab in REGAIN initially were treated with an IV induction dose of 900 mg/week before receiving 1,200 mg every 2 weeks thereafter.
Dr. Howard and colleagues included 98 patients in their analysis. Approximately 32% of patients achieved their first response within the first week of treatment, and 15% responded at week 2. About 16% of patients had a late response.
Responses to treatment on the MG-ADL scale had occurred in 67.3% by week 12 and in 84.7% by the end of the extension. Treatment with eculizumab resulted in QMG responses in 56.1% by week 12 and 71.4% by the end of the extension. The investigators observed response over multiple consecutive assessments for the vast majority of patients.
At week 130, the least-squares mean percentage changes from baseline in MG-ADL score were −61.9% and −47.5% in early and late MG-ADL responders, respectively. The least-squares mean percentage changes from baseline in QMG score were −40.8% and −55.5% in early and late QMG responders, respectively.
The investigators observed significant baseline differences between early versus late QMG responders in mean duration of myasthenia gravis (10.46 years vs. 5.46 years) and mean QMG score (18.6 vs. 15.1).
“I can’t explain [this finding]. It may simply be due to the low numbers of patients,” Dr. Howard said. “Whether this is going to hold up in postmarketing analysis remains to be seen. I’m not convinced that that is meaningful.”
Study funding was provided by Alexion Pharmaceuticals (the developer of eculizumab), the Centers for Disease Control and Prevention, and the National Institutes of Health. Dr. Howard reported receiving research support and consulting fees or honoraria from Alexion and several other pharmaceutical companies. Several other authors reported financial relationships with Alexion and other pharmaceutical companies; two authors are employees of Alexion.
Howard J et al. AANEM 2019. Unnumbered Abstract.
AUSTIN – according to a secondary analysis presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine.
Evidence for the sustained effectiveness of eculizumab, a terminal complement inhibitor, in adult patients with antiacetylcholine receptor antibody-positive refractory generalized myasthenia gravis was provided by the 6-month, double-blind, placebo-controlled REGAIN study and its open-label extension. James F. Howard Jr., MD, distinguished professor of neuromuscular disease at the University of North Carolina in Chapel Hill and colleagues sought to analyze response profiles in REGAIN and its open-label extension.
The findings raise the possibility that complement inhibition with eculizumab should not be abandoned rapidly. “We accept that [eculizumab] works quickly,” Dr. Howard said. “There is an impression that if you don’t respond within the first 3 months, you’re not going to respond. I think these data would suggest otherwise.”
The investigators analyzed participants’ Myasthenia Gravis–Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores, which had been recorded throughout REGAIN and the extension. They defined early and late responses as improvement in MG-ADL score (i.e., a decrease of three or more points) or QMG score (i.e., a decrease of five or more points) occurring at 12 weeks or earlier or after 12 weeks, respectively, after initiation of eculizumab therapy. Patients randomized to eculizumab in REGAIN initially were treated with an IV induction dose of 900 mg/week before receiving 1,200 mg every 2 weeks thereafter.
Dr. Howard and colleagues included 98 patients in their analysis. Approximately 32% of patients achieved their first response within the first week of treatment, and 15% responded at week 2. About 16% of patients had a late response.
Responses to treatment on the MG-ADL scale had occurred in 67.3% by week 12 and in 84.7% by the end of the extension. Treatment with eculizumab resulted in QMG responses in 56.1% by week 12 and 71.4% by the end of the extension. The investigators observed response over multiple consecutive assessments for the vast majority of patients.
At week 130, the least-squares mean percentage changes from baseline in MG-ADL score were −61.9% and −47.5% in early and late MG-ADL responders, respectively. The least-squares mean percentage changes from baseline in QMG score were −40.8% and −55.5% in early and late QMG responders, respectively.
The investigators observed significant baseline differences between early versus late QMG responders in mean duration of myasthenia gravis (10.46 years vs. 5.46 years) and mean QMG score (18.6 vs. 15.1).
“I can’t explain [this finding]. It may simply be due to the low numbers of patients,” Dr. Howard said. “Whether this is going to hold up in postmarketing analysis remains to be seen. I’m not convinced that that is meaningful.”
Study funding was provided by Alexion Pharmaceuticals (the developer of eculizumab), the Centers for Disease Control and Prevention, and the National Institutes of Health. Dr. Howard reported receiving research support and consulting fees or honoraria from Alexion and several other pharmaceutical companies. Several other authors reported financial relationships with Alexion and other pharmaceutical companies; two authors are employees of Alexion.
Howard J et al. AANEM 2019. Unnumbered Abstract.
AUSTIN – according to a secondary analysis presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine.
Evidence for the sustained effectiveness of eculizumab, a terminal complement inhibitor, in adult patients with antiacetylcholine receptor antibody-positive refractory generalized myasthenia gravis was provided by the 6-month, double-blind, placebo-controlled REGAIN study and its open-label extension. James F. Howard Jr., MD, distinguished professor of neuromuscular disease at the University of North Carolina in Chapel Hill and colleagues sought to analyze response profiles in REGAIN and its open-label extension.
The findings raise the possibility that complement inhibition with eculizumab should not be abandoned rapidly. “We accept that [eculizumab] works quickly,” Dr. Howard said. “There is an impression that if you don’t respond within the first 3 months, you’re not going to respond. I think these data would suggest otherwise.”
The investigators analyzed participants’ Myasthenia Gravis–Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores, which had been recorded throughout REGAIN and the extension. They defined early and late responses as improvement in MG-ADL score (i.e., a decrease of three or more points) or QMG score (i.e., a decrease of five or more points) occurring at 12 weeks or earlier or after 12 weeks, respectively, after initiation of eculizumab therapy. Patients randomized to eculizumab in REGAIN initially were treated with an IV induction dose of 900 mg/week before receiving 1,200 mg every 2 weeks thereafter.
Dr. Howard and colleagues included 98 patients in their analysis. Approximately 32% of patients achieved their first response within the first week of treatment, and 15% responded at week 2. About 16% of patients had a late response.
Responses to treatment on the MG-ADL scale had occurred in 67.3% by week 12 and in 84.7% by the end of the extension. Treatment with eculizumab resulted in QMG responses in 56.1% by week 12 and 71.4% by the end of the extension. The investigators observed response over multiple consecutive assessments for the vast majority of patients.
At week 130, the least-squares mean percentage changes from baseline in MG-ADL score were −61.9% and −47.5% in early and late MG-ADL responders, respectively. The least-squares mean percentage changes from baseline in QMG score were −40.8% and −55.5% in early and late QMG responders, respectively.
The investigators observed significant baseline differences between early versus late QMG responders in mean duration of myasthenia gravis (10.46 years vs. 5.46 years) and mean QMG score (18.6 vs. 15.1).
“I can’t explain [this finding]. It may simply be due to the low numbers of patients,” Dr. Howard said. “Whether this is going to hold up in postmarketing analysis remains to be seen. I’m not convinced that that is meaningful.”
Study funding was provided by Alexion Pharmaceuticals (the developer of eculizumab), the Centers for Disease Control and Prevention, and the National Institutes of Health. Dr. Howard reported receiving research support and consulting fees or honoraria from Alexion and several other pharmaceutical companies. Several other authors reported financial relationships with Alexion and other pharmaceutical companies; two authors are employees of Alexion.
Howard J et al. AANEM 2019. Unnumbered Abstract.
REPORTING FROM AANEM 2019
Surgery better than medical therapy in some GERD patients
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Surgery may be more effective than medical therapy in some patients with treatment-refractory gastroesophageal reflux disease.
Major finding: Patients with treatment-refractory gastroesophageal reflux disease treated with surgery were 2.38 times more likely to respond to surgery than medical treatment.
Study details: A randomized, controlled trial in 78 patients with gastroesophageal reflux disease.
Disclosures: The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
Source: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Hyperhidrosis treatment options update
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – , David Pariser, MD, said at the annual Coastal Dermatology Symposium.
Hyperhidrosis is among the dermatological conditions that have the greatest impact on quality of life, and it can be particularly concerning to teens, said Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk. He referred to some new developments for an old, often misunderstood, standby: antiperspirants. “I am amazed that many people do not know the difference between an antiperspirant and a deodorant,” he said, pointing out that antiperspirants contain active ingredients – aluminum and zirconium salts – that block sweat glands, while deodorants contain a masking fragrance.
There are new-generation topical antiperspirants available over the counter, with descriptions that include “clinical strength” or “clinical protection” on the labels; they come in a box and cost about twice as much as standard products. “But they are better, and they work just as well as some of the commercial preparations,” Dr. Pariser said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
One issue he highlighted was that antiperspirants are often misapplied. They shouldn’t be applied on wet skin because they react with water to create hydrochloric acid, which can irritate the skin, he said. The best time to apply an antiperspirant is right before bedtime, since it gives the salts time to clog sweat pores before sweat or water can interfere. “The plugs last for a couple of days,” so there’s no need to worry about rinsing the product off during a morning shower, he noted.
Additional therapeutic options include agents like oral glycopyrrolate, starting at a low dose (1 mg twice per day), increasing by 1 mg/day weekly until efficacy is achieved or limited by adverse events. There is also a glycopyrrolate oral solution 1mg/5ml (Cuvposa) that can be used in children.
A topical version of the anticholinergic glycopyrronium tosylate, applied using an infused cloth, was approved for treating axillary hyperhidrosis in June2018 and offers the potential for an enhanced local anticholinergic effect. Dr. Pariser, one of the authors, discussed the recently published results of two pivotal studies that found good improvement in a specially-designed quality of life endpoint (J Am Acad Derm. 2019; Jan;80[1]:128-138.e2).
Efficacy in a subanalysis of 44 pediatric subjects (ages 9-16 years) was similar as those reported in adults, and the rate of those reporting dry mouth (24% in both age groups) was similar. Of concern was a 16% rate of mydriasis in the pediatric group, compared with 6% in the older group. One patient even wound up in the emergency room for a stroke work-up as a result, said Dr. Pariser, who is confident that the problem was caused by inadvertent exposure to the eye during application. He advises patients to avoid contact with the eyes.
Other approaches to treatment of hyperhidrosis include oxybutynin, iontophoresis, an microwave thermolysis (which may also reduce odor and hair). Endoscopic thoracic sympathectomy is effective but is the most invasive option; botulinum toxin is a minimally invasive alternative to surgery.
For those who sweat when they experience anxiety, propranolol 5-10 mg taken about 1 hour before an event that could cause hyperhydrosis can be effective, said Dr. Pariser, who recommends a test dose. “I don’t normally tell patients to try something at home. But they should try this at home” before using it prior to an important event, he added.
Dr. Pariser is a consultant and/or investigator for Dermira, Brickell Biotech, TheraVida, Atacama, TDI Surgitech, Dermavant, and Revance Therapeutics. He has not done commercial speaking, has not been on speaker’s bureaus, and has no stock or options in any company.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM