User login
Vaping-linked lung injuries near 1,500
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
High burden of mental health symptoms in teens with insomnia
Adolescents diagnosed with insomnia have a high prevalence of concurrent mental health disorders and should be screened for them, according to new research.
For a study published in the Journal of Clinical Sleep Medicine, Tori R. Van Dyk, PhD, of Loma Linda (Calif.) University, and colleagues, enrolled 376 adolescents aged 11-18 years (mean age 14.5, 55% female) diagnosed with primary insomnia and referred to a sleep clinic. Subjects were evaluated using two validated questionnaires used to measure sleep disorders in adolescents, while caregivers reported and mental health diagnoses and symptoms using a standard behavioral checklist for adolescents.
Dr. Van Dyk and colleagues found that 75% of subjects had at least one or more parent-reported mental health diagnosis, most commonly anxiety, mood disorders, and ADHD. Some 64% had a clinical elevation of mental health symptoms on evaluation, most commonly affective disorders, with 40% of the cohort having two or more elevations. Specific mental health symptoms were seen linked with particular sleep symptoms. A greater burden of ADHD symptoms, for example, was significantly associated with more difficulties falling asleep, maintaining sleep, and reinitiating sleep after waking at night.
A total of 15% of subjects were reported by caregivers to engage in deliberate self-harming behaviors or talking about or attempting suicide – a higher rate than in the general adolescent population. “Because youth presenting for insomnia treatment may be even more likely to engage in self-harm behavior or to be suicidal, particular attention should be paid to directly assessing for these high-risk behaviors within the context of behavioral sleep medicine evaluations,” Dr. Van Dyk and colleagues wrote in their analysis.
Although mental health symptoms have been linked to sleep problems in other studies of children and adults, “associations identified in younger youths and/or adults should not be assumed to hold true among adolescents,” the researchers wrote, adding that adolescence “is a distinctive developmental period characterized by increases in both psychopathology and sleep problems, changing biology, increasing independence, and unique social and societal demands.” The investigators noted that because pediatric sleep specialists are relatively rare, the management of adolescent sleep problems and related mental health symptoms is likely to fall on primary care and other providers who “would benefit in recognizing the relationship between sleep problems and mental health symptoms in this population.”
Dr. Van Dyk and colleagues noted among the weaknesses of their study its cross-sectional design, use of parent-reported mental health symptoms only, lack of information on medication use or mental health treatment, and the potential for selection bias toward more severe cases.
The authors disclosed no outside funding or conflicts of interest related to their study.
SOURCE: Van Dyk TR et al. J Clin Sleep Med. 2019 Sep 6. doi: 10.5664/jcsm.7970.
Adolescents diagnosed with insomnia have a high prevalence of concurrent mental health disorders and should be screened for them, according to new research.
For a study published in the Journal of Clinical Sleep Medicine, Tori R. Van Dyk, PhD, of Loma Linda (Calif.) University, and colleagues, enrolled 376 adolescents aged 11-18 years (mean age 14.5, 55% female) diagnosed with primary insomnia and referred to a sleep clinic. Subjects were evaluated using two validated questionnaires used to measure sleep disorders in adolescents, while caregivers reported and mental health diagnoses and symptoms using a standard behavioral checklist for adolescents.
Dr. Van Dyk and colleagues found that 75% of subjects had at least one or more parent-reported mental health diagnosis, most commonly anxiety, mood disorders, and ADHD. Some 64% had a clinical elevation of mental health symptoms on evaluation, most commonly affective disorders, with 40% of the cohort having two or more elevations. Specific mental health symptoms were seen linked with particular sleep symptoms. A greater burden of ADHD symptoms, for example, was significantly associated with more difficulties falling asleep, maintaining sleep, and reinitiating sleep after waking at night.
A total of 15% of subjects were reported by caregivers to engage in deliberate self-harming behaviors or talking about or attempting suicide – a higher rate than in the general adolescent population. “Because youth presenting for insomnia treatment may be even more likely to engage in self-harm behavior or to be suicidal, particular attention should be paid to directly assessing for these high-risk behaviors within the context of behavioral sleep medicine evaluations,” Dr. Van Dyk and colleagues wrote in their analysis.
Although mental health symptoms have been linked to sleep problems in other studies of children and adults, “associations identified in younger youths and/or adults should not be assumed to hold true among adolescents,” the researchers wrote, adding that adolescence “is a distinctive developmental period characterized by increases in both psychopathology and sleep problems, changing biology, increasing independence, and unique social and societal demands.” The investigators noted that because pediatric sleep specialists are relatively rare, the management of adolescent sleep problems and related mental health symptoms is likely to fall on primary care and other providers who “would benefit in recognizing the relationship between sleep problems and mental health symptoms in this population.”
Dr. Van Dyk and colleagues noted among the weaknesses of their study its cross-sectional design, use of parent-reported mental health symptoms only, lack of information on medication use or mental health treatment, and the potential for selection bias toward more severe cases.
The authors disclosed no outside funding or conflicts of interest related to their study.
SOURCE: Van Dyk TR et al. J Clin Sleep Med. 2019 Sep 6. doi: 10.5664/jcsm.7970.
Adolescents diagnosed with insomnia have a high prevalence of concurrent mental health disorders and should be screened for them, according to new research.
For a study published in the Journal of Clinical Sleep Medicine, Tori R. Van Dyk, PhD, of Loma Linda (Calif.) University, and colleagues, enrolled 376 adolescents aged 11-18 years (mean age 14.5, 55% female) diagnosed with primary insomnia and referred to a sleep clinic. Subjects were evaluated using two validated questionnaires used to measure sleep disorders in adolescents, while caregivers reported and mental health diagnoses and symptoms using a standard behavioral checklist for adolescents.
Dr. Van Dyk and colleagues found that 75% of subjects had at least one or more parent-reported mental health diagnosis, most commonly anxiety, mood disorders, and ADHD. Some 64% had a clinical elevation of mental health symptoms on evaluation, most commonly affective disorders, with 40% of the cohort having two or more elevations. Specific mental health symptoms were seen linked with particular sleep symptoms. A greater burden of ADHD symptoms, for example, was significantly associated with more difficulties falling asleep, maintaining sleep, and reinitiating sleep after waking at night.
A total of 15% of subjects were reported by caregivers to engage in deliberate self-harming behaviors or talking about or attempting suicide – a higher rate than in the general adolescent population. “Because youth presenting for insomnia treatment may be even more likely to engage in self-harm behavior or to be suicidal, particular attention should be paid to directly assessing for these high-risk behaviors within the context of behavioral sleep medicine evaluations,” Dr. Van Dyk and colleagues wrote in their analysis.
Although mental health symptoms have been linked to sleep problems in other studies of children and adults, “associations identified in younger youths and/or adults should not be assumed to hold true among adolescents,” the researchers wrote, adding that adolescence “is a distinctive developmental period characterized by increases in both psychopathology and sleep problems, changing biology, increasing independence, and unique social and societal demands.” The investigators noted that because pediatric sleep specialists are relatively rare, the management of adolescent sleep problems and related mental health symptoms is likely to fall on primary care and other providers who “would benefit in recognizing the relationship between sleep problems and mental health symptoms in this population.”
Dr. Van Dyk and colleagues noted among the weaknesses of their study its cross-sectional design, use of parent-reported mental health symptoms only, lack of information on medication use or mental health treatment, and the potential for selection bias toward more severe cases.
The authors disclosed no outside funding or conflicts of interest related to their study.
SOURCE: Van Dyk TR et al. J Clin Sleep Med. 2019 Sep 6. doi: 10.5664/jcsm.7970.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Primary CNS lymphoma: R-CHOP hits back
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive form of non-Hodgkin lymphoma solely confined to the CNS. The majority of PCNSL histologically presents as diffuse large B-cell lymphoma (DLBCL). However, outcomes in these patients are notably inferior, compared with nodal or other extranodal DLBCL. In order to achieve long-term progression-free survival, high-dose methotrexate (HD-MTX)–based chemotherapy followed by consolidation is needed. However, this treatment is associated with high toxicity burden and it is restricted to a select patient population – the young and fit – and requires administration at specialized hematological centers.
In the 1990s, the conventional DLBCL treatment regimen with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was tested in PCNSL patients. The results were rather disappointing. The addition of CHOP to whole brain radiation or HD-MTX could not improve survival.1-3 The reason for CHOP failure was poor CNS penetration of doxorubicin and cyclophosphamide because of their high molecular weight. Consequently, it was concluded that there is no role for CHOP-like chemotherapy in the treatment of PCNSL.4
But is this really the case? Twenty years later, this traditional view has been challenged by Andres J.M. Ferreri, MD, and colleagues in the INGRID trial.5 Dr. Ferreri presented findings from the trial at the International Conference on Malignant Lymphoma in Lugano, Switzerland, which was greeted with much excitement.6
INGRID is a phase 2 trial conducted on patients with refractory/relapsed PCNSL. It consisted of a CHOP plus rituximab (R-CHOP) regimen, which was upgraded by engineered tumor necrosis factor–alpha (TNF-alpha). The idea was to enhance the blood-brain barrier (BBB) permeability and consequently improve the efficacy of R-CHOP in PCNSL. The use of human TNF-alpha is limited by relevant toxicities. In order to avoid that, a fusion of human TNF-alpha and CNGRCG peptide (called NGR-TNF) was developed.
CNGRCG peptide is a ligand of CD13, an aminopeptidase that is expressed almost exclusively on tumor blood vessels. Preclinical data showed that binding of CNGRCG to CD13 results in targeted – local, not systemic – delivery of TNF-alpha to the tumor blood vessels. Consequently, TNF-alpha led to increased vascular permeability in tumor tissue and enabled higher penetration of chemotherapeutic agents.7,8
Altogether, 12 heavily pretreated PCNSL patients were included in the INGRID trial. Seven patients had two or more previous treatment regimens. Within this trial, patients received R-CHOP with NGR-TNF (0.8 mcg/m2) applied 2 hours prior to R-CHOP. The great majority of grade 3/4 adverse events were hematological toxicities. Importantly, no neurological side effects of any grade occurred.
The primary aim of this study was to investigate the CD13 expression on tumor tissue and provide a proof of concept for the use of NGR-TNF/R-CHOP. Indeed, CD13 expression was observed on tumor vessels in all patients. Consequently, increased BBB permeability in tumor tissue after NGR-TNF infusion was observed using dynamic contrast-enhanced MRI and by brain scintigraphy (SPECT). This was assessed 1 day after NGR-TNF/R-CHOP treatment. More importantly, this effect on BBB seems to be sustained because it was also observed after the last cycle of NGR-TNF/R-CHOP. The fact that there was no change of drug concentrations of R-CHOP components in plasma or cerebrospinal fluid suggests that the effect of NGR-TNF is restricted to tumor vessels.
The authors also reported preliminary results regarding response rates to NGR-TNF/R-CHOP. The overall response rate was 75%. Of note, six patients achieved complete remission and one patient achieved a partial remission. The median duration of response was 10 months (range, 7-14 months), and nine patients were able to proceed to consolidation treatment.
These preliminary results are encouraging and open a new window for the treatment strategies in PCNSL patients. NGR-TNF/R-CHOP treatment induced responses in 75% of these heavily pretreated patients. The low toxicity profile and feasibility of this regimen could allow clinicians to carry out this treatment approach in outpatient settings, as well as in older and comorbid patients. Extensive supportive therapy – such as intensive hydration or leucovorin-rescue by HD-MTX – is not needed.
These results will need to be confirmed through testing in a larger patient population. Dr. Ferreri and colleagues are currently conducting the extended phase of this study and aim to recruit 28 patients. If they report positive results from that study, evaluation of NGR-TNF/R-CHOP as a first-line treatment of PCNSL seems to be the next reasonable step.
Dr. Zeremski and Dr. Fischer are both in the department of hematology/oncology and affiliated with the Health Campus Immunology, Infectiology and Inflammation at Otto-von-Guericke University Magdeburg (Germany). Dr. Fischer is a member of the editorial advisory board of Hematology News. The authors reported having no conflicts of interest.
References
1. J Clin Oncol. 1996;14:556-64.
2. Cancer. 2000;89:1359-70.
3. J Neurooncol. 1996;30:257-65.
4. Guidelines on the diagnosis and management of adult patients with primary CNS lymphoma (PCNSL) and primary intra-ocular lymphoma (PIOL). British Society for Haematology/British Committee for Standards in Haematology; HO/016, 2009.
5. Blood. 2019;134:252-62.
6. Hematol Oncol. 2019; 37:159.
7. BioDrugs. 2013;27:591-603.
8. J Clin Invest. 2002;110:475-82.
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive form of non-Hodgkin lymphoma solely confined to the CNS. The majority of PCNSL histologically presents as diffuse large B-cell lymphoma (DLBCL). However, outcomes in these patients are notably inferior, compared with nodal or other extranodal DLBCL. In order to achieve long-term progression-free survival, high-dose methotrexate (HD-MTX)–based chemotherapy followed by consolidation is needed. However, this treatment is associated with high toxicity burden and it is restricted to a select patient population – the young and fit – and requires administration at specialized hematological centers.
In the 1990s, the conventional DLBCL treatment regimen with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was tested in PCNSL patients. The results were rather disappointing. The addition of CHOP to whole brain radiation or HD-MTX could not improve survival.1-3 The reason for CHOP failure was poor CNS penetration of doxorubicin and cyclophosphamide because of their high molecular weight. Consequently, it was concluded that there is no role for CHOP-like chemotherapy in the treatment of PCNSL.4
But is this really the case? Twenty years later, this traditional view has been challenged by Andres J.M. Ferreri, MD, and colleagues in the INGRID trial.5 Dr. Ferreri presented findings from the trial at the International Conference on Malignant Lymphoma in Lugano, Switzerland, which was greeted with much excitement.6
INGRID is a phase 2 trial conducted on patients with refractory/relapsed PCNSL. It consisted of a CHOP plus rituximab (R-CHOP) regimen, which was upgraded by engineered tumor necrosis factor–alpha (TNF-alpha). The idea was to enhance the blood-brain barrier (BBB) permeability and consequently improve the efficacy of R-CHOP in PCNSL. The use of human TNF-alpha is limited by relevant toxicities. In order to avoid that, a fusion of human TNF-alpha and CNGRCG peptide (called NGR-TNF) was developed.
CNGRCG peptide is a ligand of CD13, an aminopeptidase that is expressed almost exclusively on tumor blood vessels. Preclinical data showed that binding of CNGRCG to CD13 results in targeted – local, not systemic – delivery of TNF-alpha to the tumor blood vessels. Consequently, TNF-alpha led to increased vascular permeability in tumor tissue and enabled higher penetration of chemotherapeutic agents.7,8
Altogether, 12 heavily pretreated PCNSL patients were included in the INGRID trial. Seven patients had two or more previous treatment regimens. Within this trial, patients received R-CHOP with NGR-TNF (0.8 mcg/m2) applied 2 hours prior to R-CHOP. The great majority of grade 3/4 adverse events were hematological toxicities. Importantly, no neurological side effects of any grade occurred.
The primary aim of this study was to investigate the CD13 expression on tumor tissue and provide a proof of concept for the use of NGR-TNF/R-CHOP. Indeed, CD13 expression was observed on tumor vessels in all patients. Consequently, increased BBB permeability in tumor tissue after NGR-TNF infusion was observed using dynamic contrast-enhanced MRI and by brain scintigraphy (SPECT). This was assessed 1 day after NGR-TNF/R-CHOP treatment. More importantly, this effect on BBB seems to be sustained because it was also observed after the last cycle of NGR-TNF/R-CHOP. The fact that there was no change of drug concentrations of R-CHOP components in plasma or cerebrospinal fluid suggests that the effect of NGR-TNF is restricted to tumor vessels.
The authors also reported preliminary results regarding response rates to NGR-TNF/R-CHOP. The overall response rate was 75%. Of note, six patients achieved complete remission and one patient achieved a partial remission. The median duration of response was 10 months (range, 7-14 months), and nine patients were able to proceed to consolidation treatment.
These preliminary results are encouraging and open a new window for the treatment strategies in PCNSL patients. NGR-TNF/R-CHOP treatment induced responses in 75% of these heavily pretreated patients. The low toxicity profile and feasibility of this regimen could allow clinicians to carry out this treatment approach in outpatient settings, as well as in older and comorbid patients. Extensive supportive therapy – such as intensive hydration or leucovorin-rescue by HD-MTX – is not needed.
These results will need to be confirmed through testing in a larger patient population. Dr. Ferreri and colleagues are currently conducting the extended phase of this study and aim to recruit 28 patients. If they report positive results from that study, evaluation of NGR-TNF/R-CHOP as a first-line treatment of PCNSL seems to be the next reasonable step.
Dr. Zeremski and Dr. Fischer are both in the department of hematology/oncology and affiliated with the Health Campus Immunology, Infectiology and Inflammation at Otto-von-Guericke University Magdeburg (Germany). Dr. Fischer is a member of the editorial advisory board of Hematology News. The authors reported having no conflicts of interest.
References
1. J Clin Oncol. 1996;14:556-64.
2. Cancer. 2000;89:1359-70.
3. J Neurooncol. 1996;30:257-65.
4. Guidelines on the diagnosis and management of adult patients with primary CNS lymphoma (PCNSL) and primary intra-ocular lymphoma (PIOL). British Society for Haematology/British Committee for Standards in Haematology; HO/016, 2009.
5. Blood. 2019;134:252-62.
6. Hematol Oncol. 2019; 37:159.
7. BioDrugs. 2013;27:591-603.
8. J Clin Invest. 2002;110:475-82.
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive form of non-Hodgkin lymphoma solely confined to the CNS. The majority of PCNSL histologically presents as diffuse large B-cell lymphoma (DLBCL). However, outcomes in these patients are notably inferior, compared with nodal or other extranodal DLBCL. In order to achieve long-term progression-free survival, high-dose methotrexate (HD-MTX)–based chemotherapy followed by consolidation is needed. However, this treatment is associated with high toxicity burden and it is restricted to a select patient population – the young and fit – and requires administration at specialized hematological centers.
In the 1990s, the conventional DLBCL treatment regimen with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) was tested in PCNSL patients. The results were rather disappointing. The addition of CHOP to whole brain radiation or HD-MTX could not improve survival.1-3 The reason for CHOP failure was poor CNS penetration of doxorubicin and cyclophosphamide because of their high molecular weight. Consequently, it was concluded that there is no role for CHOP-like chemotherapy in the treatment of PCNSL.4
But is this really the case? Twenty years later, this traditional view has been challenged by Andres J.M. Ferreri, MD, and colleagues in the INGRID trial.5 Dr. Ferreri presented findings from the trial at the International Conference on Malignant Lymphoma in Lugano, Switzerland, which was greeted with much excitement.6
INGRID is a phase 2 trial conducted on patients with refractory/relapsed PCNSL. It consisted of a CHOP plus rituximab (R-CHOP) regimen, which was upgraded by engineered tumor necrosis factor–alpha (TNF-alpha). The idea was to enhance the blood-brain barrier (BBB) permeability and consequently improve the efficacy of R-CHOP in PCNSL. The use of human TNF-alpha is limited by relevant toxicities. In order to avoid that, a fusion of human TNF-alpha and CNGRCG peptide (called NGR-TNF) was developed.
CNGRCG peptide is a ligand of CD13, an aminopeptidase that is expressed almost exclusively on tumor blood vessels. Preclinical data showed that binding of CNGRCG to CD13 results in targeted – local, not systemic – delivery of TNF-alpha to the tumor blood vessels. Consequently, TNF-alpha led to increased vascular permeability in tumor tissue and enabled higher penetration of chemotherapeutic agents.7,8
Altogether, 12 heavily pretreated PCNSL patients were included in the INGRID trial. Seven patients had two or more previous treatment regimens. Within this trial, patients received R-CHOP with NGR-TNF (0.8 mcg/m2) applied 2 hours prior to R-CHOP. The great majority of grade 3/4 adverse events were hematological toxicities. Importantly, no neurological side effects of any grade occurred.
The primary aim of this study was to investigate the CD13 expression on tumor tissue and provide a proof of concept for the use of NGR-TNF/R-CHOP. Indeed, CD13 expression was observed on tumor vessels in all patients. Consequently, increased BBB permeability in tumor tissue after NGR-TNF infusion was observed using dynamic contrast-enhanced MRI and by brain scintigraphy (SPECT). This was assessed 1 day after NGR-TNF/R-CHOP treatment. More importantly, this effect on BBB seems to be sustained because it was also observed after the last cycle of NGR-TNF/R-CHOP. The fact that there was no change of drug concentrations of R-CHOP components in plasma or cerebrospinal fluid suggests that the effect of NGR-TNF is restricted to tumor vessels.
The authors also reported preliminary results regarding response rates to NGR-TNF/R-CHOP. The overall response rate was 75%. Of note, six patients achieved complete remission and one patient achieved a partial remission. The median duration of response was 10 months (range, 7-14 months), and nine patients were able to proceed to consolidation treatment.
These preliminary results are encouraging and open a new window for the treatment strategies in PCNSL patients. NGR-TNF/R-CHOP treatment induced responses in 75% of these heavily pretreated patients. The low toxicity profile and feasibility of this regimen could allow clinicians to carry out this treatment approach in outpatient settings, as well as in older and comorbid patients. Extensive supportive therapy – such as intensive hydration or leucovorin-rescue by HD-MTX – is not needed.
These results will need to be confirmed through testing in a larger patient population. Dr. Ferreri and colleagues are currently conducting the extended phase of this study and aim to recruit 28 patients. If they report positive results from that study, evaluation of NGR-TNF/R-CHOP as a first-line treatment of PCNSL seems to be the next reasonable step.
Dr. Zeremski and Dr. Fischer are both in the department of hematology/oncology and affiliated with the Health Campus Immunology, Infectiology and Inflammation at Otto-von-Guericke University Magdeburg (Germany). Dr. Fischer is a member of the editorial advisory board of Hematology News. The authors reported having no conflicts of interest.
References
1. J Clin Oncol. 1996;14:556-64.
2. Cancer. 2000;89:1359-70.
3. J Neurooncol. 1996;30:257-65.
4. Guidelines on the diagnosis and management of adult patients with primary CNS lymphoma (PCNSL) and primary intra-ocular lymphoma (PIOL). British Society for Haematology/British Committee for Standards in Haematology; HO/016, 2009.
5. Blood. 2019;134:252-62.
6. Hematol Oncol. 2019; 37:159.
7. BioDrugs. 2013;27:591-603.
8. J Clin Invest. 2002;110:475-82.
Low disease activity a valid target in early SLE, investigators say
, investigators in a small retrospective study have concluded.
Both complete remission (CR) and achievement of a lupus low disease activity state (LLDAS) were independently associated with lower accrual of early damage after 6 months of treatment, according to results of the study, reported in Arthritis Care & Research.
Patients maintaining LLDAS over the next 12 months developed considerably less damage than did those who never achieved LLDAS or who did not maintain LLDAS over time, according to the investigators, led by Alberto Floris, MD, and Matteo Piga, MD, of the AOU University Clinic of Cagliari (Italy).
Based on these findings, maintenance of both CR and LLDAS should be targeted for damage prevention, according to the authors. “Significant differences did not emerge between persistent LLDAS and CR over 12 months in preventing early damage,” they said in their report.
Their retrospective, single-center analysis included 116 adult patients with newly diagnosed SLE who had an SLE Disease Activity Index 2000 (SLEDAI-2K) score of at least 6 at baseline and started treatment at enrollment. The mean age of this patient cohort was approximately 35 years, and about 90% were female.
After 6 months of follow-up, 42% of the cohort achieved LLDAS, defined in part as a SLEDAI-2K of 4 or less with no major organ activity or new disease activity, while 21.6% met CR criteria, which included a SLEDAI-2K of 0.
Achieving LLDAS at that 6-month time point was independently associated with lower damage accrual at 18 months (odds ratio, 0.25; 95% confidence interval, 0.06-0.99; P = .049); likewise, a 6-month CR translated into less damage at 18 months, according to multivariate analysis (OR, 0.07; 95% CI, 0.01-0.59; P = .015).
Those patients in LLDAS at 6 months who maintained that target for another 12 months had significantly less damage when compared with those who lost response or never achieved it, the investigators added.
“Losing the early attained LLDAS and CR may frustrate prevention of damage, and therefore, LLDAS and CR maintenance should be targeted since the first stage of SLE management,” the investigators said in their report.
About 54% of the patients in LLDAS at 6 months achieved CR at an 18-month follow-up time point, while about 21% remained in LLDAS and 25% went on to develop active disease, the investigators said. Among those in CR at 6 months, 60% maintained it at 18 months, while 40% worsened to either LLDAS or active disease.
Dr. Floris, Dr. Piga, and coauthors reported no funding or competing interests related to their research.
SOURCE: Floris A et al. Arthritis Care Res. 2019 Oct 10. doi: 10.1002/acr.24086.
, investigators in a small retrospective study have concluded.
Both complete remission (CR) and achievement of a lupus low disease activity state (LLDAS) were independently associated with lower accrual of early damage after 6 months of treatment, according to results of the study, reported in Arthritis Care & Research.
Patients maintaining LLDAS over the next 12 months developed considerably less damage than did those who never achieved LLDAS or who did not maintain LLDAS over time, according to the investigators, led by Alberto Floris, MD, and Matteo Piga, MD, of the AOU University Clinic of Cagliari (Italy).
Based on these findings, maintenance of both CR and LLDAS should be targeted for damage prevention, according to the authors. “Significant differences did not emerge between persistent LLDAS and CR over 12 months in preventing early damage,” they said in their report.
Their retrospective, single-center analysis included 116 adult patients with newly diagnosed SLE who had an SLE Disease Activity Index 2000 (SLEDAI-2K) score of at least 6 at baseline and started treatment at enrollment. The mean age of this patient cohort was approximately 35 years, and about 90% were female.
After 6 months of follow-up, 42% of the cohort achieved LLDAS, defined in part as a SLEDAI-2K of 4 or less with no major organ activity or new disease activity, while 21.6% met CR criteria, which included a SLEDAI-2K of 0.
Achieving LLDAS at that 6-month time point was independently associated with lower damage accrual at 18 months (odds ratio, 0.25; 95% confidence interval, 0.06-0.99; P = .049); likewise, a 6-month CR translated into less damage at 18 months, according to multivariate analysis (OR, 0.07; 95% CI, 0.01-0.59; P = .015).
Those patients in LLDAS at 6 months who maintained that target for another 12 months had significantly less damage when compared with those who lost response or never achieved it, the investigators added.
“Losing the early attained LLDAS and CR may frustrate prevention of damage, and therefore, LLDAS and CR maintenance should be targeted since the first stage of SLE management,” the investigators said in their report.
About 54% of the patients in LLDAS at 6 months achieved CR at an 18-month follow-up time point, while about 21% remained in LLDAS and 25% went on to develop active disease, the investigators said. Among those in CR at 6 months, 60% maintained it at 18 months, while 40% worsened to either LLDAS or active disease.
Dr. Floris, Dr. Piga, and coauthors reported no funding or competing interests related to their research.
SOURCE: Floris A et al. Arthritis Care Res. 2019 Oct 10. doi: 10.1002/acr.24086.
, investigators in a small retrospective study have concluded.
Both complete remission (CR) and achievement of a lupus low disease activity state (LLDAS) were independently associated with lower accrual of early damage after 6 months of treatment, according to results of the study, reported in Arthritis Care & Research.
Patients maintaining LLDAS over the next 12 months developed considerably less damage than did those who never achieved LLDAS or who did not maintain LLDAS over time, according to the investigators, led by Alberto Floris, MD, and Matteo Piga, MD, of the AOU University Clinic of Cagliari (Italy).
Based on these findings, maintenance of both CR and LLDAS should be targeted for damage prevention, according to the authors. “Significant differences did not emerge between persistent LLDAS and CR over 12 months in preventing early damage,” they said in their report.
Their retrospective, single-center analysis included 116 adult patients with newly diagnosed SLE who had an SLE Disease Activity Index 2000 (SLEDAI-2K) score of at least 6 at baseline and started treatment at enrollment. The mean age of this patient cohort was approximately 35 years, and about 90% were female.
After 6 months of follow-up, 42% of the cohort achieved LLDAS, defined in part as a SLEDAI-2K of 4 or less with no major organ activity or new disease activity, while 21.6% met CR criteria, which included a SLEDAI-2K of 0.
Achieving LLDAS at that 6-month time point was independently associated with lower damage accrual at 18 months (odds ratio, 0.25; 95% confidence interval, 0.06-0.99; P = .049); likewise, a 6-month CR translated into less damage at 18 months, according to multivariate analysis (OR, 0.07; 95% CI, 0.01-0.59; P = .015).
Those patients in LLDAS at 6 months who maintained that target for another 12 months had significantly less damage when compared with those who lost response or never achieved it, the investigators added.
“Losing the early attained LLDAS and CR may frustrate prevention of damage, and therefore, LLDAS and CR maintenance should be targeted since the first stage of SLE management,” the investigators said in their report.
About 54% of the patients in LLDAS at 6 months achieved CR at an 18-month follow-up time point, while about 21% remained in LLDAS and 25% went on to develop active disease, the investigators said. Among those in CR at 6 months, 60% maintained it at 18 months, while 40% worsened to either LLDAS or active disease.
Dr. Floris, Dr. Piga, and coauthors reported no funding or competing interests related to their research.
SOURCE: Floris A et al. Arthritis Care Res. 2019 Oct 10. doi: 10.1002/acr.24086.
FROM ARTHRITIS CARE & RESEARCH
‘Clean’ and ‘natural’ beauty products
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Clean beauty products have taken over the skin care market. A wave of new indie brands has entered the skin care market, some of which have garnered fame from bloggers and celebrities and via social media. There has also been a shift towards larger, more-established brands developing and marketing cleaner alternatives to their established skin care lines.
As consumers, physicians, and parents, we all want nontoxic products. However, as highlighted in a recent editorial by Bruce Brod, MD, and Courtney Blair Rubin, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, the Food and Drug Administration has “failed to define clean and natural, leaving these labels open to interpretation by nondermatologist retailers, bloggers, and celebrities who have set out to define clean beauty for themselves” (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2724). This vague interpretation has given rise to a billion-dollar industry of products that is unregulated and may, in fact, not be safer than other products.
For the last decade, to skin care products have also been on the rise. Some of the ingredients deemed toxic include petrolatum and parabens, which have good safety profiles and clinically, are among the least allergenic ingredients in skin products, particularly among patients with the most sensitive skin. In contrast, botanical oils, essential oils, and plant-based natural fragrances are chronic culprits of contact sensitivities and severe skin allergies.
I encourage all dermatologists to read this viewpoint as this topic will inevitably be a point of discussion with many patients. Large studies and expert consensus of safety profiles of chemicals – particularly those deemed carcinogenic, endocrine disruptors, and environmental hazards – are often lacking, leading to confusion for consumers. Our professional organizations and industry should be leading the efforts to establish standardized definitions and FDA regulations of skin care products deemed clean and natural so that the differentiation between marketing taglines and true, substantiated FDA-supported claims are clearer for consumers.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
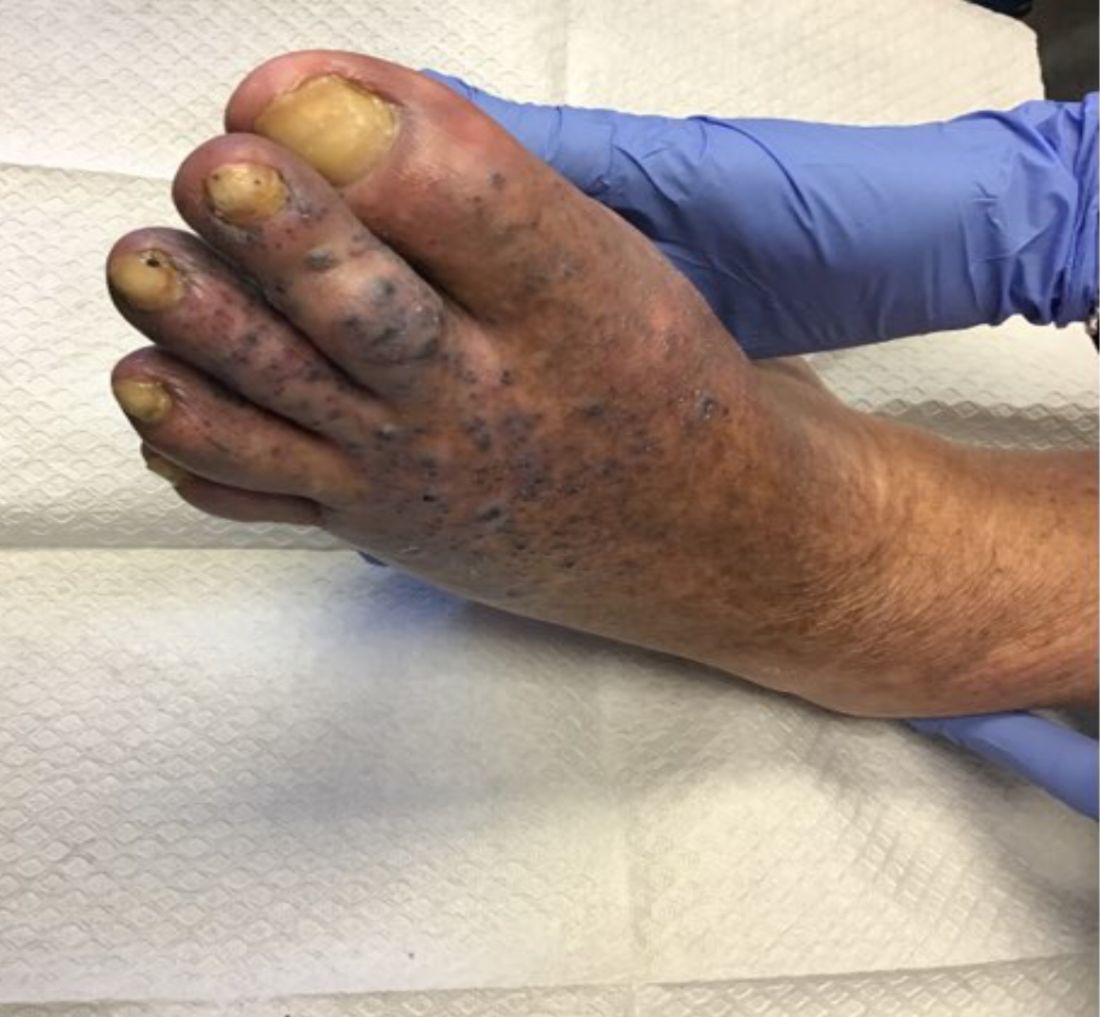
Violaceous papules on calf & foot
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Vitamin D deficiency appears to worsen survival in Hodgkin lymphoma
Vitamin D deficiency is associated with worse progression-free and overall survival among patients with Hodgkin lymphoma, according to new study findings.
Sven Borchmann, MD, of the University of Cologne (Germany) and German Hodgkin Study Group and coauthors conducted a case-control study of 351 patients enrolled in the German Hodgkin Study Group trials who had available baseline serum samples. Pretreatment vitamin D levels were assessed and categorized as deficient (less than 30 nmol/L), insufficient (30-49 nmol/L), or sufficient (50 nmol/L or greater). The findings were published in the Journal of Clinical Oncology.
The researchers found that before starting treatment, 50% of patients were vitamin D deficient.
Patients with baseline vitamin D deficiency had significantly lower progression-free survival – 10.2% lower at 5 years and 17.6% lower at 10 years – compared with patients with either sufficient or insufficient vitamin D levels (P less than .001). They also had 2% lower overall survival at 5 years and 11.1% lower overall survival at 10 years (P less than .001).
The researchers also conducted preclinical studies in effort to understand the effect of vitamin D on Hodgkin lymphoma cells and in Hodgkin lymphoma tumor models.
They explored the effect of vitamin D on cultured Hodgkin lymphoma cell lines and saw a dose-response effect of calcitriol in reducing cell proliferation rates. They then looked at the effect of calcitriol on cell lines that were also exposed to doxorubicin or etoposide, and found calcitriol improved the cytotoxicity of these chemotherapy agents, especially at lower doses.
Finally, they conducted an in-vivo mouse study using Hodgkin lymphoma xenografts, and looked at whether vitamin D supplementation increased the effect of doxorubicin or etoposide. This revealed that chemotherapy and vitamin D supplementation together were significantly better at controlling tumor growth, compared with monotherapy with either vitamin D or doxorubicin and compared with placebo.
“On the basis of our study results and the limited toxicity of vitamin D replacement therapy, we would advocate for vitamin D deficiency screening and replacement to be incorporated into future randomized clinical trials to properly clarify the role of vitamin D replacement in HL [Hodgkin lymphoma],” the researchers wrote. “The goal of these trials should be to determine whether vitamin D replacement in HL improves outcome.”
No study funding information was reported. Dr. Borchmann reported honoraria and research funding from Takeda. Other authors reported financial disclosures related to Takeda, Roche, Bristol-Myers Squibb, and other companies.
SOURCE: Borchmann S et al. J Clin Oncol. 2019 Oct 17. doi:10.1200/JCO.19.00985.
Vitamin D deficiency is associated with worse progression-free and overall survival among patients with Hodgkin lymphoma, according to new study findings.
Sven Borchmann, MD, of the University of Cologne (Germany) and German Hodgkin Study Group and coauthors conducted a case-control study of 351 patients enrolled in the German Hodgkin Study Group trials who had available baseline serum samples. Pretreatment vitamin D levels were assessed and categorized as deficient (less than 30 nmol/L), insufficient (30-49 nmol/L), or sufficient (50 nmol/L or greater). The findings were published in the Journal of Clinical Oncology.
The researchers found that before starting treatment, 50% of patients were vitamin D deficient.
Patients with baseline vitamin D deficiency had significantly lower progression-free survival – 10.2% lower at 5 years and 17.6% lower at 10 years – compared with patients with either sufficient or insufficient vitamin D levels (P less than .001). They also had 2% lower overall survival at 5 years and 11.1% lower overall survival at 10 years (P less than .001).
The researchers also conducted preclinical studies in effort to understand the effect of vitamin D on Hodgkin lymphoma cells and in Hodgkin lymphoma tumor models.
They explored the effect of vitamin D on cultured Hodgkin lymphoma cell lines and saw a dose-response effect of calcitriol in reducing cell proliferation rates. They then looked at the effect of calcitriol on cell lines that were also exposed to doxorubicin or etoposide, and found calcitriol improved the cytotoxicity of these chemotherapy agents, especially at lower doses.
Finally, they conducted an in-vivo mouse study using Hodgkin lymphoma xenografts, and looked at whether vitamin D supplementation increased the effect of doxorubicin or etoposide. This revealed that chemotherapy and vitamin D supplementation together were significantly better at controlling tumor growth, compared with monotherapy with either vitamin D or doxorubicin and compared with placebo.
“On the basis of our study results and the limited toxicity of vitamin D replacement therapy, we would advocate for vitamin D deficiency screening and replacement to be incorporated into future randomized clinical trials to properly clarify the role of vitamin D replacement in HL [Hodgkin lymphoma],” the researchers wrote. “The goal of these trials should be to determine whether vitamin D replacement in HL improves outcome.”
No study funding information was reported. Dr. Borchmann reported honoraria and research funding from Takeda. Other authors reported financial disclosures related to Takeda, Roche, Bristol-Myers Squibb, and other companies.
SOURCE: Borchmann S et al. J Clin Oncol. 2019 Oct 17. doi:10.1200/JCO.19.00985.
Vitamin D deficiency is associated with worse progression-free and overall survival among patients with Hodgkin lymphoma, according to new study findings.
Sven Borchmann, MD, of the University of Cologne (Germany) and German Hodgkin Study Group and coauthors conducted a case-control study of 351 patients enrolled in the German Hodgkin Study Group trials who had available baseline serum samples. Pretreatment vitamin D levels were assessed and categorized as deficient (less than 30 nmol/L), insufficient (30-49 nmol/L), or sufficient (50 nmol/L or greater). The findings were published in the Journal of Clinical Oncology.
The researchers found that before starting treatment, 50% of patients were vitamin D deficient.
Patients with baseline vitamin D deficiency had significantly lower progression-free survival – 10.2% lower at 5 years and 17.6% lower at 10 years – compared with patients with either sufficient or insufficient vitamin D levels (P less than .001). They also had 2% lower overall survival at 5 years and 11.1% lower overall survival at 10 years (P less than .001).
The researchers also conducted preclinical studies in effort to understand the effect of vitamin D on Hodgkin lymphoma cells and in Hodgkin lymphoma tumor models.
They explored the effect of vitamin D on cultured Hodgkin lymphoma cell lines and saw a dose-response effect of calcitriol in reducing cell proliferation rates. They then looked at the effect of calcitriol on cell lines that were also exposed to doxorubicin or etoposide, and found calcitriol improved the cytotoxicity of these chemotherapy agents, especially at lower doses.
Finally, they conducted an in-vivo mouse study using Hodgkin lymphoma xenografts, and looked at whether vitamin D supplementation increased the effect of doxorubicin or etoposide. This revealed that chemotherapy and vitamin D supplementation together were significantly better at controlling tumor growth, compared with monotherapy with either vitamin D or doxorubicin and compared with placebo.
“On the basis of our study results and the limited toxicity of vitamin D replacement therapy, we would advocate for vitamin D deficiency screening and replacement to be incorporated into future randomized clinical trials to properly clarify the role of vitamin D replacement in HL [Hodgkin lymphoma],” the researchers wrote. “The goal of these trials should be to determine whether vitamin D replacement in HL improves outcome.”
No study funding information was reported. Dr. Borchmann reported honoraria and research funding from Takeda. Other authors reported financial disclosures related to Takeda, Roche, Bristol-Myers Squibb, and other companies.
SOURCE: Borchmann S et al. J Clin Oncol. 2019 Oct 17. doi:10.1200/JCO.19.00985.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Patients with Hodgkin lymphoma and vitamin D deficiency had a 17.6% lower progression-free survival at 10 years, compared with patients who were not vitamin D deficient (P less than .001).
Study details: A case-control study in 351 patients with Hodgkin lymphoma.
Disclosures: No study funding information was reported. Dr. Borchmann reported honoraria and research funding from Takeda. Other authors reported financial disclosures related to Takeda, Roche, Bristol-Myers Squibb, and other companies.
Source: Borchmann S et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00985.
Cell culture–based flu vaccine maintains immunogenicity
WASHINGTON – Influenza vaccines that substitute flu grown in cell-culture for the standard formulation of flu grown in eggs recently came onto the U.S. market, and new evidence confirmed that cell-grown flu works at least as well as its egg-grown counterpart for triggering immune responses.
Results from a randomized study with 148 evaluable subjects that directly compared the immune response of individuals aged 4-20 years old to the 2018-2019 commercial formulation of a mostly cell-based influenza vaccine with a commercially marketed, fully egg-based vaccine from the same vintage showed “no difference” between the two vaccines for inducing serologic titers on both the hemagluttination inhibition assay and by microneutralization, Richard K. Zimmerman, MD, said at an annual scientific meeting on infectious diseases.
The question addressed by the study was whether the primarily cell culture–grown vaccine would perform differently in children than a standard, egg-grown vaccine. “We thought that we might find something different, but we didn’t,” said Dr. Zimmerman, a professor of family medicine at the University of Pittsburgh who studies vaccines. The finding gave further support to using flu vaccines made without eggs because of their advantages over egg-based vaccines, he said in an interview.
Dr. Zimmerman cited two major, potential problems with egg-grown influenza vaccines. First, they require a big supply of eggs to manufacture, which can pose logistical challenges that are absent with cell culture–grown vaccine once the bioreactor capacity exists to produce the necessary amount of cells. This means that egg-free vaccine production can ramp up faster when a pandemic starts, he noted.
Second, over time, egg-grown vaccine strains of influenza have become increasingly adapted to grow in eggs with the result that “in some years the egg-grown virus is so different as to not work as well [Proc Natl Acad Sci. 2017 Nov;114[44]:12578-83]. With cell culture you bypass” issues of glycosylation mismatch or other antigenic problems caused by egg passage, he explained.
Dr. Zimmerman feels so strongly about the superiority of the cell-culture vaccine that “I am personally going to get a vaccine that’s not egg based,” and he advised the University of Pittsburgh Medical Center to focus its 2019-2020 flu vaccine purchase primarily on formulations made by cell culture. For the 2019-2020 season, that specifically is Flucelvax, an inactivated influenza vaccine licensed for people aged at least 4 years old, and Flublok, a recombinant flu vaccine also produced entirely in cell culture and licensed for people aged at least 18 years old. The 2019-2020 season is the first one during which the quadravalent Flucelvax vaccine has all four component strains (one H1N1, one H3N2, and two B strains) grown in cell culture.
The study run by Dr. Zimmerman and associates at the start of the 2018-2019 season used that season’s formulation of Flucelvax, which had only three of its four component strains grown in cell culture plus one strain (H1N1) grown in eggs. The Pittsburgh researchers randomized 168 individuals to receive the 2018-2019 Flucelvax vaccine or Fluzone, an entirely egg-made quadravelent vaccine, and they had analyzable results from 148 of the enrolled participants, more than 85% of whom were 9-20 years old. The study’s primary endpoint was the extent of seropositivity and seroconversion 28 days after immunization measured with both a hemagglutination inhibition assay and by a microneutralization assay. The results showed similar rates in the 75 children who received Flucelvax and the 73 who received Fluzone. For example, seropositivity against B Victoria lineage strains by the hemagglutination inhibition assay 28 days after vaccination was 76% in children who received Flucelvax, and it was 79% among those who got Fluzone, with a seroconversion rate of 34% in each of the two study subgroups.
“These findings do not say that egg-free is better, but it was certainly no worse. My guess is that in some years vaccines that are egg-free will make a big difference. In other years it may not. But you don’t know ahead of time,” Dr. Zimmerman said.
The study received no commercial funding but received free Fluzone vaccine from Sanofi Pasteur. Dr. Zimmerman had no disclosures.
WASHINGTON – Influenza vaccines that substitute flu grown in cell-culture for the standard formulation of flu grown in eggs recently came onto the U.S. market, and new evidence confirmed that cell-grown flu works at least as well as its egg-grown counterpart for triggering immune responses.
Results from a randomized study with 148 evaluable subjects that directly compared the immune response of individuals aged 4-20 years old to the 2018-2019 commercial formulation of a mostly cell-based influenza vaccine with a commercially marketed, fully egg-based vaccine from the same vintage showed “no difference” between the two vaccines for inducing serologic titers on both the hemagluttination inhibition assay and by microneutralization, Richard K. Zimmerman, MD, said at an annual scientific meeting on infectious diseases.
The question addressed by the study was whether the primarily cell culture–grown vaccine would perform differently in children than a standard, egg-grown vaccine. “We thought that we might find something different, but we didn’t,” said Dr. Zimmerman, a professor of family medicine at the University of Pittsburgh who studies vaccines. The finding gave further support to using flu vaccines made without eggs because of their advantages over egg-based vaccines, he said in an interview.
Dr. Zimmerman cited two major, potential problems with egg-grown influenza vaccines. First, they require a big supply of eggs to manufacture, which can pose logistical challenges that are absent with cell culture–grown vaccine once the bioreactor capacity exists to produce the necessary amount of cells. This means that egg-free vaccine production can ramp up faster when a pandemic starts, he noted.
Second, over time, egg-grown vaccine strains of influenza have become increasingly adapted to grow in eggs with the result that “in some years the egg-grown virus is so different as to not work as well [Proc Natl Acad Sci. 2017 Nov;114[44]:12578-83]. With cell culture you bypass” issues of glycosylation mismatch or other antigenic problems caused by egg passage, he explained.
Dr. Zimmerman feels so strongly about the superiority of the cell-culture vaccine that “I am personally going to get a vaccine that’s not egg based,” and he advised the University of Pittsburgh Medical Center to focus its 2019-2020 flu vaccine purchase primarily on formulations made by cell culture. For the 2019-2020 season, that specifically is Flucelvax, an inactivated influenza vaccine licensed for people aged at least 4 years old, and Flublok, a recombinant flu vaccine also produced entirely in cell culture and licensed for people aged at least 18 years old. The 2019-2020 season is the first one during which the quadravalent Flucelvax vaccine has all four component strains (one H1N1, one H3N2, and two B strains) grown in cell culture.
The study run by Dr. Zimmerman and associates at the start of the 2018-2019 season used that season’s formulation of Flucelvax, which had only three of its four component strains grown in cell culture plus one strain (H1N1) grown in eggs. The Pittsburgh researchers randomized 168 individuals to receive the 2018-2019 Flucelvax vaccine or Fluzone, an entirely egg-made quadravelent vaccine, and they had analyzable results from 148 of the enrolled participants, more than 85% of whom were 9-20 years old. The study’s primary endpoint was the extent of seropositivity and seroconversion 28 days after immunization measured with both a hemagglutination inhibition assay and by a microneutralization assay. The results showed similar rates in the 75 children who received Flucelvax and the 73 who received Fluzone. For example, seropositivity against B Victoria lineage strains by the hemagglutination inhibition assay 28 days after vaccination was 76% in children who received Flucelvax, and it was 79% among those who got Fluzone, with a seroconversion rate of 34% in each of the two study subgroups.
“These findings do not say that egg-free is better, but it was certainly no worse. My guess is that in some years vaccines that are egg-free will make a big difference. In other years it may not. But you don’t know ahead of time,” Dr. Zimmerman said.
The study received no commercial funding but received free Fluzone vaccine from Sanofi Pasteur. Dr. Zimmerman had no disclosures.
WASHINGTON – Influenza vaccines that substitute flu grown in cell-culture for the standard formulation of flu grown in eggs recently came onto the U.S. market, and new evidence confirmed that cell-grown flu works at least as well as its egg-grown counterpart for triggering immune responses.
Results from a randomized study with 148 evaluable subjects that directly compared the immune response of individuals aged 4-20 years old to the 2018-2019 commercial formulation of a mostly cell-based influenza vaccine with a commercially marketed, fully egg-based vaccine from the same vintage showed “no difference” between the two vaccines for inducing serologic titers on both the hemagluttination inhibition assay and by microneutralization, Richard K. Zimmerman, MD, said at an annual scientific meeting on infectious diseases.
The question addressed by the study was whether the primarily cell culture–grown vaccine would perform differently in children than a standard, egg-grown vaccine. “We thought that we might find something different, but we didn’t,” said Dr. Zimmerman, a professor of family medicine at the University of Pittsburgh who studies vaccines. The finding gave further support to using flu vaccines made without eggs because of their advantages over egg-based vaccines, he said in an interview.
Dr. Zimmerman cited two major, potential problems with egg-grown influenza vaccines. First, they require a big supply of eggs to manufacture, which can pose logistical challenges that are absent with cell culture–grown vaccine once the bioreactor capacity exists to produce the necessary amount of cells. This means that egg-free vaccine production can ramp up faster when a pandemic starts, he noted.
Second, over time, egg-grown vaccine strains of influenza have become increasingly adapted to grow in eggs with the result that “in some years the egg-grown virus is so different as to not work as well [Proc Natl Acad Sci. 2017 Nov;114[44]:12578-83]. With cell culture you bypass” issues of glycosylation mismatch or other antigenic problems caused by egg passage, he explained.
Dr. Zimmerman feels so strongly about the superiority of the cell-culture vaccine that “I am personally going to get a vaccine that’s not egg based,” and he advised the University of Pittsburgh Medical Center to focus its 2019-2020 flu vaccine purchase primarily on formulations made by cell culture. For the 2019-2020 season, that specifically is Flucelvax, an inactivated influenza vaccine licensed for people aged at least 4 years old, and Flublok, a recombinant flu vaccine also produced entirely in cell culture and licensed for people aged at least 18 years old. The 2019-2020 season is the first one during which the quadravalent Flucelvax vaccine has all four component strains (one H1N1, one H3N2, and two B strains) grown in cell culture.
The study run by Dr. Zimmerman and associates at the start of the 2018-2019 season used that season’s formulation of Flucelvax, which had only three of its four component strains grown in cell culture plus one strain (H1N1) grown in eggs. The Pittsburgh researchers randomized 168 individuals to receive the 2018-2019 Flucelvax vaccine or Fluzone, an entirely egg-made quadravelent vaccine, and they had analyzable results from 148 of the enrolled participants, more than 85% of whom were 9-20 years old. The study’s primary endpoint was the extent of seropositivity and seroconversion 28 days after immunization measured with both a hemagglutination inhibition assay and by a microneutralization assay. The results showed similar rates in the 75 children who received Flucelvax and the 73 who received Fluzone. For example, seropositivity against B Victoria lineage strains by the hemagglutination inhibition assay 28 days after vaccination was 76% in children who received Flucelvax, and it was 79% among those who got Fluzone, with a seroconversion rate of 34% in each of the two study subgroups.
“These findings do not say that egg-free is better, but it was certainly no worse. My guess is that in some years vaccines that are egg-free will make a big difference. In other years it may not. But you don’t know ahead of time,” Dr. Zimmerman said.
The study received no commercial funding but received free Fluzone vaccine from Sanofi Pasteur. Dr. Zimmerman had no disclosures.
REPORTING FROM ID WEEK 2019
Spotting immunodeficiency in the pediatric dermatology clinic
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Psilocybin research at Johns Hopkins
A new center opens to study novel treatment uses for psychedelics
Psychiatry Grand Rounds started on Monday, Oct. 7, 2019, as they do every Monday at Johns Hopkins, with the department chair interviewing a patient.
The patient told James Potash, MD, that he had suffered with depression for many years and that medications were only a partial fix. His participation in a psilocybin trial at Johns Hopkins in Baltimore was different, and 3 months after the second, and final, treatment, he felt remarkably better. The treatment had quieted the self-deprecating script that had looped through his thoughts for years. “It was nothing like what I was expecting,” the patient said. “I didn’t feel out of control. And now I’m not fighting with myself all day.”
Grand Rounds at Hopkins are different from those at other institutions in that the rounds are given by departments’ full-time faculty members; psychiatrists from other institutions are invited to come speak at other times during the week. But Grand Rounds are reserved for the faculty to present their own research. The patient interview was followed by a lecture by Roland Griffiths, PhD, on “Psilocybin: A potentially promising treatment for depression.” Dr. Griffiths has been studying the effects of psychedelic drugs for the past 40 years; he’s been at Hopkins since 1972. The Grand Rounds presentation came just weeks after it was announced that Dr. Griffiths would be heading the Johns Hopkins Center for Psychedelic & Consciousness Research, funded with $17 million in private donations.
Dr. Griffiths began by talking about the history of psychedelic drug research and the fact that research had been dormant for several decades. “There was – and still is – concern about potential adverse reactions, including panic reactions and the possibility of precipitating psychosis,” he said.
Originally, he conducted several studies in healthy volunteers with no history of psychedelic use. Participants had one or more day-long sessions with staff members who would then serve as monitors when psilocybin was administered; these preliminary meetings were designed to build trust and rapport with the team and to decrease the risk of adverse reactions. On session days when psilocybin was administered, participants were told to eat a low-fat breakfast and then were given a high dose of psilocybin in a capsule. The next 6 hours were spent with the volunteer lying on a couch with an eye mask, headphones with soft music, and two monitors in the room. “The room is decorated like a living room. We created a container for people to explore their inner experience.”
Dr. Griffiths talked about the experiences people reported.
“There were large increases in personally meaningful and insight-type experiences. People reported a sense of unity and interconnectedness of all things, accompanied by a sense of preciousness or reverence, and by the sense that ... the experience was more real or true than everyday waking consciousness. Although the acute effects of psilocybin resolved by the end of the session day, the memories remain and people reported enduring changes in mood, attitude, and behavior.”
The data were remarkable. One month after these high-dose psilocybin sessions, 78% of the volunteers rated the experience as among the top five most personally meaningful experiences of their lives, 94% said they had an increased sense of well-being with improved life satisfaction, and nearly 90% endorsed positive behavior change. Six months after the last session, participants still endorsed having more positive relationships, feeling more love, tolerance, empathy and compassion, friends, family members, and others. Family members and work colleagues who were interviewed noted these positive changes as well.
At this point in Grand Rounds, I was ready to volunteer. My best estimate (tongue in cheek, with no corroboration) is that about half of the audience was waiting on free psilocybin samples, while the other half was remembering Timothy Leary, PhD, bad trips, and psychosis. “We’ve been down this road before,” the gentleman next to me commented.
Dr. Griffiths went on to talk about trials of psilocybin in clinical populations. In one study, the effects of psilocybin were examined in cancer patients with depression and anxiety. Of the 51 cancer patients, half previously had been treated with psychotropic medications before coming to the study. This randomized, double-blind crossover study compared high-dose psilocybin with an extremely low dose of psilocybin used as the placebo.
Another study looked at patients with major depression who, like the patient interviewed, had not had full resolution of symptoms with conventional antidepressants. Of the 24 participants in that group, 69% reported clinically significant improvement 12 weeks after the treatment. Just over half of participants reported a full remission to normal.
Dr. Griffiths ended by concluding that the positive experiences people have with psilocybin are associated with “enduring positive trait changes in attitudes, mood and behavior, spirituality, and altruism ... and that such experiences are now amenable to systematic prospective scientific study.”
J. Raymond DePaulo, MD, chair of the National Network of Depression Centers and former chair of psychiatry at Johns Hopkins, noted that he used to be skeptical. He has been impressed that Dr. Griffiths has listened to criticism and addressed concerns others have raised. “When it was suggested that this was the result of delirium, he started giving people cognitive tests while they were using psilocybin and he showed it wasn’t the case.”
“I have always objected to words like ‘psychedelic’ and ‘hallucinogen,’ ” said Dr. DePaulo. “They are confusing and inaccurate. No one takes these medications because they want to hallucinate. They take them to change their mood. That said, I am thrilled that we are on the precipice of being able to use this to treat depression.”
Since 1999, the Johns Hopkins Psilocybin Research Project has conducted more than 700 sessions with 369 participants. When might psilocybin be available to patients outside of a research protocol? Two companies have obtained approval from the Food and Drug Administration to initiate registration in trials for treatment of depression. Dr. Griffiths estimates that it could be another 5 years before psilocybin will be available for medical use.
The new center with its generous funding will allow for more studies with more patient populations. The center’s projects will look at using psilocybin as a treatment for opioid use disorder, comorbid depression and alcohol use disorder, anorexia nervosa, depression in Alzheimer’s disease, posttreatment Lyme disease syndrome, and PTSD.
Scott Aaronson, MD, is a mood disorder expert and director of clinical research at the Sheppard Pratt Health System in Towson/Baltimore. Dr. Aaronson, who also studies psilocybin as a treatment for depression, noted: “The problem with the development of psychedelics is that we are not really prepared to capture the improvement we see with their use, as the main changes are not in the classic markers of depression like the Montgomery-Åsberg or the Hamilton Depression rating scales, but [rather] in the patient’s perception of who they are, how they fit in with the world, and what is most important. We need to develop ways to capture those critical changes and work with the FDA to develop an entirely new vision of what constitutes improvement in psychiatric illness. My overall take is that it is a terrific time to specialize in treatment-resistant mood disorders!”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
A new center opens to study novel treatment uses for psychedelics
A new center opens to study novel treatment uses for psychedelics
Psychiatry Grand Rounds started on Monday, Oct. 7, 2019, as they do every Monday at Johns Hopkins, with the department chair interviewing a patient.
The patient told James Potash, MD, that he had suffered with depression for many years and that medications were only a partial fix. His participation in a psilocybin trial at Johns Hopkins in Baltimore was different, and 3 months after the second, and final, treatment, he felt remarkably better. The treatment had quieted the self-deprecating script that had looped through his thoughts for years. “It was nothing like what I was expecting,” the patient said. “I didn’t feel out of control. And now I’m not fighting with myself all day.”
Grand Rounds at Hopkins are different from those at other institutions in that the rounds are given by departments’ full-time faculty members; psychiatrists from other institutions are invited to come speak at other times during the week. But Grand Rounds are reserved for the faculty to present their own research. The patient interview was followed by a lecture by Roland Griffiths, PhD, on “Psilocybin: A potentially promising treatment for depression.” Dr. Griffiths has been studying the effects of psychedelic drugs for the past 40 years; he’s been at Hopkins since 1972. The Grand Rounds presentation came just weeks after it was announced that Dr. Griffiths would be heading the Johns Hopkins Center for Psychedelic & Consciousness Research, funded with $17 million in private donations.
Dr. Griffiths began by talking about the history of psychedelic drug research and the fact that research had been dormant for several decades. “There was – and still is – concern about potential adverse reactions, including panic reactions and the possibility of precipitating psychosis,” he said.
Originally, he conducted several studies in healthy volunteers with no history of psychedelic use. Participants had one or more day-long sessions with staff members who would then serve as monitors when psilocybin was administered; these preliminary meetings were designed to build trust and rapport with the team and to decrease the risk of adverse reactions. On session days when psilocybin was administered, participants were told to eat a low-fat breakfast and then were given a high dose of psilocybin in a capsule. The next 6 hours were spent with the volunteer lying on a couch with an eye mask, headphones with soft music, and two monitors in the room. “The room is decorated like a living room. We created a container for people to explore their inner experience.”
Dr. Griffiths talked about the experiences people reported.
“There were large increases in personally meaningful and insight-type experiences. People reported a sense of unity and interconnectedness of all things, accompanied by a sense of preciousness or reverence, and by the sense that ... the experience was more real or true than everyday waking consciousness. Although the acute effects of psilocybin resolved by the end of the session day, the memories remain and people reported enduring changes in mood, attitude, and behavior.”
The data were remarkable. One month after these high-dose psilocybin sessions, 78% of the volunteers rated the experience as among the top five most personally meaningful experiences of their lives, 94% said they had an increased sense of well-being with improved life satisfaction, and nearly 90% endorsed positive behavior change. Six months after the last session, participants still endorsed having more positive relationships, feeling more love, tolerance, empathy and compassion, friends, family members, and others. Family members and work colleagues who were interviewed noted these positive changes as well.
At this point in Grand Rounds, I was ready to volunteer. My best estimate (tongue in cheek, with no corroboration) is that about half of the audience was waiting on free psilocybin samples, while the other half was remembering Timothy Leary, PhD, bad trips, and psychosis. “We’ve been down this road before,” the gentleman next to me commented.
Dr. Griffiths went on to talk about trials of psilocybin in clinical populations. In one study, the effects of psilocybin were examined in cancer patients with depression and anxiety. Of the 51 cancer patients, half previously had been treated with psychotropic medications before coming to the study. This randomized, double-blind crossover study compared high-dose psilocybin with an extremely low dose of psilocybin used as the placebo.
Another study looked at patients with major depression who, like the patient interviewed, had not had full resolution of symptoms with conventional antidepressants. Of the 24 participants in that group, 69% reported clinically significant improvement 12 weeks after the treatment. Just over half of participants reported a full remission to normal.
Dr. Griffiths ended by concluding that the positive experiences people have with psilocybin are associated with “enduring positive trait changes in attitudes, mood and behavior, spirituality, and altruism ... and that such experiences are now amenable to systematic prospective scientific study.”
J. Raymond DePaulo, MD, chair of the National Network of Depression Centers and former chair of psychiatry at Johns Hopkins, noted that he used to be skeptical. He has been impressed that Dr. Griffiths has listened to criticism and addressed concerns others have raised. “When it was suggested that this was the result of delirium, he started giving people cognitive tests while they were using psilocybin and he showed it wasn’t the case.”
“I have always objected to words like ‘psychedelic’ and ‘hallucinogen,’ ” said Dr. DePaulo. “They are confusing and inaccurate. No one takes these medications because they want to hallucinate. They take them to change their mood. That said, I am thrilled that we are on the precipice of being able to use this to treat depression.”
Since 1999, the Johns Hopkins Psilocybin Research Project has conducted more than 700 sessions with 369 participants. When might psilocybin be available to patients outside of a research protocol? Two companies have obtained approval from the Food and Drug Administration to initiate registration in trials for treatment of depression. Dr. Griffiths estimates that it could be another 5 years before psilocybin will be available for medical use.
The new center with its generous funding will allow for more studies with more patient populations. The center’s projects will look at using psilocybin as a treatment for opioid use disorder, comorbid depression and alcohol use disorder, anorexia nervosa, depression in Alzheimer’s disease, posttreatment Lyme disease syndrome, and PTSD.
Scott Aaronson, MD, is a mood disorder expert and director of clinical research at the Sheppard Pratt Health System in Towson/Baltimore. Dr. Aaronson, who also studies psilocybin as a treatment for depression, noted: “The problem with the development of psychedelics is that we are not really prepared to capture the improvement we see with their use, as the main changes are not in the classic markers of depression like the Montgomery-Åsberg or the Hamilton Depression rating scales, but [rather] in the patient’s perception of who they are, how they fit in with the world, and what is most important. We need to develop ways to capture those critical changes and work with the FDA to develop an entirely new vision of what constitutes improvement in psychiatric illness. My overall take is that it is a terrific time to specialize in treatment-resistant mood disorders!”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Psychiatry Grand Rounds started on Monday, Oct. 7, 2019, as they do every Monday at Johns Hopkins, with the department chair interviewing a patient.
The patient told James Potash, MD, that he had suffered with depression for many years and that medications were only a partial fix. His participation in a psilocybin trial at Johns Hopkins in Baltimore was different, and 3 months after the second, and final, treatment, he felt remarkably better. The treatment had quieted the self-deprecating script that had looped through his thoughts for years. “It was nothing like what I was expecting,” the patient said. “I didn’t feel out of control. And now I’m not fighting with myself all day.”
Grand Rounds at Hopkins are different from those at other institutions in that the rounds are given by departments’ full-time faculty members; psychiatrists from other institutions are invited to come speak at other times during the week. But Grand Rounds are reserved for the faculty to present their own research. The patient interview was followed by a lecture by Roland Griffiths, PhD, on “Psilocybin: A potentially promising treatment for depression.” Dr. Griffiths has been studying the effects of psychedelic drugs for the past 40 years; he’s been at Hopkins since 1972. The Grand Rounds presentation came just weeks after it was announced that Dr. Griffiths would be heading the Johns Hopkins Center for Psychedelic & Consciousness Research, funded with $17 million in private donations.
Dr. Griffiths began by talking about the history of psychedelic drug research and the fact that research had been dormant for several decades. “There was – and still is – concern about potential adverse reactions, including panic reactions and the possibility of precipitating psychosis,” he said.
Originally, he conducted several studies in healthy volunteers with no history of psychedelic use. Participants had one or more day-long sessions with staff members who would then serve as monitors when psilocybin was administered; these preliminary meetings were designed to build trust and rapport with the team and to decrease the risk of adverse reactions. On session days when psilocybin was administered, participants were told to eat a low-fat breakfast and then were given a high dose of psilocybin in a capsule. The next 6 hours were spent with the volunteer lying on a couch with an eye mask, headphones with soft music, and two monitors in the room. “The room is decorated like a living room. We created a container for people to explore their inner experience.”
Dr. Griffiths talked about the experiences people reported.
“There were large increases in personally meaningful and insight-type experiences. People reported a sense of unity and interconnectedness of all things, accompanied by a sense of preciousness or reverence, and by the sense that ... the experience was more real or true than everyday waking consciousness. Although the acute effects of psilocybin resolved by the end of the session day, the memories remain and people reported enduring changes in mood, attitude, and behavior.”
The data were remarkable. One month after these high-dose psilocybin sessions, 78% of the volunteers rated the experience as among the top five most personally meaningful experiences of their lives, 94% said they had an increased sense of well-being with improved life satisfaction, and nearly 90% endorsed positive behavior change. Six months after the last session, participants still endorsed having more positive relationships, feeling more love, tolerance, empathy and compassion, friends, family members, and others. Family members and work colleagues who were interviewed noted these positive changes as well.
At this point in Grand Rounds, I was ready to volunteer. My best estimate (tongue in cheek, with no corroboration) is that about half of the audience was waiting on free psilocybin samples, while the other half was remembering Timothy Leary, PhD, bad trips, and psychosis. “We’ve been down this road before,” the gentleman next to me commented.
Dr. Griffiths went on to talk about trials of psilocybin in clinical populations. In one study, the effects of psilocybin were examined in cancer patients with depression and anxiety. Of the 51 cancer patients, half previously had been treated with psychotropic medications before coming to the study. This randomized, double-blind crossover study compared high-dose psilocybin with an extremely low dose of psilocybin used as the placebo.
Another study looked at patients with major depression who, like the patient interviewed, had not had full resolution of symptoms with conventional antidepressants. Of the 24 participants in that group, 69% reported clinically significant improvement 12 weeks after the treatment. Just over half of participants reported a full remission to normal.
Dr. Griffiths ended by concluding that the positive experiences people have with psilocybin are associated with “enduring positive trait changes in attitudes, mood and behavior, spirituality, and altruism ... and that such experiences are now amenable to systematic prospective scientific study.”
J. Raymond DePaulo, MD, chair of the National Network of Depression Centers and former chair of psychiatry at Johns Hopkins, noted that he used to be skeptical. He has been impressed that Dr. Griffiths has listened to criticism and addressed concerns others have raised. “When it was suggested that this was the result of delirium, he started giving people cognitive tests while they were using psilocybin and he showed it wasn’t the case.”
“I have always objected to words like ‘psychedelic’ and ‘hallucinogen,’ ” said Dr. DePaulo. “They are confusing and inaccurate. No one takes these medications because they want to hallucinate. They take them to change their mood. That said, I am thrilled that we are on the precipice of being able to use this to treat depression.”
Since 1999, the Johns Hopkins Psilocybin Research Project has conducted more than 700 sessions with 369 participants. When might psilocybin be available to patients outside of a research protocol? Two companies have obtained approval from the Food and Drug Administration to initiate registration in trials for treatment of depression. Dr. Griffiths estimates that it could be another 5 years before psilocybin will be available for medical use.
The new center with its generous funding will allow for more studies with more patient populations. The center’s projects will look at using psilocybin as a treatment for opioid use disorder, comorbid depression and alcohol use disorder, anorexia nervosa, depression in Alzheimer’s disease, posttreatment Lyme disease syndrome, and PTSD.
Scott Aaronson, MD, is a mood disorder expert and director of clinical research at the Sheppard Pratt Health System in Towson/Baltimore. Dr. Aaronson, who also studies psilocybin as a treatment for depression, noted: “The problem with the development of psychedelics is that we are not really prepared to capture the improvement we see with their use, as the main changes are not in the classic markers of depression like the Montgomery-Åsberg or the Hamilton Depression rating scales, but [rather] in the patient’s perception of who they are, how they fit in with the world, and what is most important. We need to develop ways to capture those critical changes and work with the FDA to develop an entirely new vision of what constitutes improvement in psychiatric illness. My overall take is that it is a terrific time to specialize in treatment-resistant mood disorders!”
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.





















