User login
Psychodiagnostic testing services: The elusive quest for clinicians
Assessment psychologists should be colocated in specialty practices
Imagine the clinical care consequences if patients seen in specialty or primary care practices did not have ready access to laboratory, other medical tests, and/or consultative services deemed critical to quickly establishing diagnostic status and the development of an appropriate treatment plan. For instance, what would be the implications if a dentistry practice did not employ a dental hygienist; an otolaryngology group was not staffed with an audiologist; or a gastroenterology practice had no one available for digestive/nutritional consultation support.
Consider a neurologist who suspects that a patient has a potentially life-threatening brain condition, but the patient has to wait months for brain imaging or – even worse – is tasked to find their own provider for this diagnostic test only to be told that the neuroimaging service does not take their insurance and/or there are no available appointments for several months.
Situations of this kind would not be – and should not be – tolerated by medical professionals or their patients.
A common “real-world” scenario: After evaluation, a psychiatrist needs clarification regarding a possible subtle psychotic process, or, in another instance, suspects that there is an early degenerative cognitive change underlying recent changes in mood and personality. However, the psychiatrist has no dependable access to an assessment psychologist to assist in cases of this kind.
Patients are frequently told by psychiatrists and other physicians that they should have psychodiagnostic testing to arrive at a clearer picture of their clinical status and treatment needs. However, most medical practices, in particular, psychiatry, pediatrics, neurology, and neurosurgery, who see substantial numbers of patients who could benefit from testing, do not employ psychologists. When they do, many do not possess the requisite assessment skills to address the reason(s) for referral.
If the patient needing testing services is fortunate enough, he/she is referred to a well-trained psychologist within commuting distance who takes the patient’s insurance and is able to set up a timely appointment – an unlikely set of circumstances in today’s health care environment.
Some state psychological associations allow for a “matching service” of sorts in the form of announcements in the organization’s listserv, which reviews the referral and includes a back channel for psychologists to contact the patient regarding their availability for testing.
Over the past 2 decades, significant advancements have been made in the integration of primary and mental health care. Those need to continue to include colocating assessment psychologists in medical specialty practices, such as psychiatry, which make frequent referrals for psychodiagnostic testing or would like to but have no place to turn.
Dr. Pollak is affiliated with the Seacoast Mental Health Center in Portsmouth, N.H.
Assessment psychologists should be colocated in specialty practices
Assessment psychologists should be colocated in specialty practices
Imagine the clinical care consequences if patients seen in specialty or primary care practices did not have ready access to laboratory, other medical tests, and/or consultative services deemed critical to quickly establishing diagnostic status and the development of an appropriate treatment plan. For instance, what would be the implications if a dentistry practice did not employ a dental hygienist; an otolaryngology group was not staffed with an audiologist; or a gastroenterology practice had no one available for digestive/nutritional consultation support.
Consider a neurologist who suspects that a patient has a potentially life-threatening brain condition, but the patient has to wait months for brain imaging or – even worse – is tasked to find their own provider for this diagnostic test only to be told that the neuroimaging service does not take their insurance and/or there are no available appointments for several months.
Situations of this kind would not be – and should not be – tolerated by medical professionals or their patients.
A common “real-world” scenario: After evaluation, a psychiatrist needs clarification regarding a possible subtle psychotic process, or, in another instance, suspects that there is an early degenerative cognitive change underlying recent changes in mood and personality. However, the psychiatrist has no dependable access to an assessment psychologist to assist in cases of this kind.
Patients are frequently told by psychiatrists and other physicians that they should have psychodiagnostic testing to arrive at a clearer picture of their clinical status and treatment needs. However, most medical practices, in particular, psychiatry, pediatrics, neurology, and neurosurgery, who see substantial numbers of patients who could benefit from testing, do not employ psychologists. When they do, many do not possess the requisite assessment skills to address the reason(s) for referral.
If the patient needing testing services is fortunate enough, he/she is referred to a well-trained psychologist within commuting distance who takes the patient’s insurance and is able to set up a timely appointment – an unlikely set of circumstances in today’s health care environment.
Some state psychological associations allow for a “matching service” of sorts in the form of announcements in the organization’s listserv, which reviews the referral and includes a back channel for psychologists to contact the patient regarding their availability for testing.
Over the past 2 decades, significant advancements have been made in the integration of primary and mental health care. Those need to continue to include colocating assessment psychologists in medical specialty practices, such as psychiatry, which make frequent referrals for psychodiagnostic testing or would like to but have no place to turn.
Dr. Pollak is affiliated with the Seacoast Mental Health Center in Portsmouth, N.H.
Imagine the clinical care consequences if patients seen in specialty or primary care practices did not have ready access to laboratory, other medical tests, and/or consultative services deemed critical to quickly establishing diagnostic status and the development of an appropriate treatment plan. For instance, what would be the implications if a dentistry practice did not employ a dental hygienist; an otolaryngology group was not staffed with an audiologist; or a gastroenterology practice had no one available for digestive/nutritional consultation support.
Consider a neurologist who suspects that a patient has a potentially life-threatening brain condition, but the patient has to wait months for brain imaging or – even worse – is tasked to find their own provider for this diagnostic test only to be told that the neuroimaging service does not take their insurance and/or there are no available appointments for several months.
Situations of this kind would not be – and should not be – tolerated by medical professionals or their patients.
A common “real-world” scenario: After evaluation, a psychiatrist needs clarification regarding a possible subtle psychotic process, or, in another instance, suspects that there is an early degenerative cognitive change underlying recent changes in mood and personality. However, the psychiatrist has no dependable access to an assessment psychologist to assist in cases of this kind.
Patients are frequently told by psychiatrists and other physicians that they should have psychodiagnostic testing to arrive at a clearer picture of their clinical status and treatment needs. However, most medical practices, in particular, psychiatry, pediatrics, neurology, and neurosurgery, who see substantial numbers of patients who could benefit from testing, do not employ psychologists. When they do, many do not possess the requisite assessment skills to address the reason(s) for referral.
If the patient needing testing services is fortunate enough, he/she is referred to a well-trained psychologist within commuting distance who takes the patient’s insurance and is able to set up a timely appointment – an unlikely set of circumstances in today’s health care environment.
Some state psychological associations allow for a “matching service” of sorts in the form of announcements in the organization’s listserv, which reviews the referral and includes a back channel for psychologists to contact the patient regarding their availability for testing.
Over the past 2 decades, significant advancements have been made in the integration of primary and mental health care. Those need to continue to include colocating assessment psychologists in medical specialty practices, such as psychiatry, which make frequent referrals for psychodiagnostic testing or would like to but have no place to turn.
Dr. Pollak is affiliated with the Seacoast Mental Health Center in Portsmouth, N.H.
DNA methylation changes: An early biomarker for methotrexate response?
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
FROM RHEUMATOLOGY
Research on pediatric firearms deaths is underfunded
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
FROM HEALTH AFFAIRS
New mechanisms, therapies for acne considered
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – It used to be thought that acne begins with microcomedones, which go on to develop either inflammatory lesions or noninflammatory lesions, but more recent evidence has changed that perception, according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital, Detroit.
Dr. Stein Gold said at the annual Coastal Dermatology Symposium. “All acne is inflammation acne,” and inflammation also persists, she added. Biopsies of scarred lesions, once considered postinflammatory, also have revealed persistent inflammation, she noted.
One study found that persistent scars can evolve from closed comedones, papules, and pustules, but the most common was a papule that turned into a postinflammatory lesion (J Drugs Dermatol 2017 Jun 1;16[6]:566-72). “So when patients come in and they have these red spots on their face, it’s not over. There’s still time to be aggressive because those inflammatory lesions are more likely to lead to scars than anything else,” Dr. Stein Gold said. “And we also know that papules that develop into scars do so because they’re there for a longer period of time. Those that develop scars are present about 10.5 days, compared with 6.6 days for those that don’t develop into scars.”
She went on to review some of the new treatments for acne that can be brought to bear in such cases. These include developments with topical retinoids that are aimed at improving delivery and reducing skin irritation.
A new topical retinoid, trifarotene cream, 0.005%, showed efficacy and tolerability for both the face and trunk in a recent phase 3 trial of patients with moderate facial and truncal acne and was recently approved for patients aged 9 years and older. In the study, about 30%-40% of people aged 9 years and older treated with once-daily trifarotene cream (Aklief) achieved clear or almost-clear status of the face at 12 weeks, vs. about 20% and 26%, of those on the vehicle cream (J Am Acad Dermatol. 2019 Jun;80[6]:1691-9).
The drug can also treat papules and pustules, nearly as well as it treats blackheads and whiteheads, according to Dr. Stein Gold. Like other retinoids, it produces some redness and scaling and rather than letting these adverse events discourage patients, she leans in. “I tell patients they’re going to have some sloughing of the skin the first 2 weeks. I tell them that people pay money for that. It’s called a chemical peel,” said Dr. Stein Gold, noting that patients respond well to this information.
If patients find the treatments too irritating, she advises them to avoid applying it to wet skin. They can also apply it every other night, or even less frequently, and then work up to more frequent use, she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
Tazarotene is another topical retinoid that can be very irritating. A new lotion formulation of tazarotene 0.045% contains a lower dose than the 0.1% typically used in creams, and has similar efficacy but reduced irritation, Dr. Stein Gold said. In August, the manufacturer submitted an application for approval with the Food and Drug Administration for treatment of acne.
Dr. Stein Gold also talked about using retinoids to minimize scarring, referring to a study of patients with moderate and severe facial acne, and atrophic acne scars, comparing adapalene 0.3% plus benzoyl peroxide 2.5% gel on one side of the face and vehicle on the other side of the face for 24 weeks, followed by application of the active treatment to both sides of the face for 24 weeks. Treatment was associated with a reduction of atrophic acne scars at 24 weeks, which was maintained for up to 48 weeks (Am J Clin Dermatol. 2019 Oct[5];20:725-32).
“We can now say to patients, ‘Not only can I help you with your acne, but I can potentially even improve your atrophic scarring,’ ” she said.
Finally, she discussed clascoterone, a novel androgen receptor antagonist, which inhibits sebum production and prevents colonization by Cutibacterium acnes (formerly called Propionibacterium acnes) and subsequent inflammation. “It does a lot of good things in terms of the pathogenesis of acne, but more importantly, it is one of the first drugs that topically has been shown to decrease the production of sebum,” Dr. Stein Gold said. A 1% cream formulation is being studied for acne.
Dr. Stein Gold is a consultant, investigator, and/or speaker for Galderma, Ortho Derm, Sol Gel, Foamix, Cassiopea, and Almirall.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Take drug, patient-level factors into account for when to end antiresorptive therapy
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – according to an overview presented by Marcy B. Bolster, MD.
Recently published studies may help guide decisions about initiating and discontinuing treatment with bisphosphonates or denosumab (Prolia), the antiresorptive therapies. Understanding the ideal duration of bisphosphonate drug holidays “is a work in progress,” Dr. Bolster, from Harvard Medical School in Boston, said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
No holiday with denosumab
Data indicate that twice yearly denosumab remains safe at 10 years, but studies have found a rapid loss of bone mineral density and an increased risk for vertebral fractures after treatment is discontinued (J Bone Miner Res. 2018 Feb;33[2]:190-8).
“Therefore, it is not appropriate for denosumab to be utilized with a drug holiday. If a patient is placed on denosumab, then consideration needs to be given for what to follow the course of denosumab,” Dr. Bolster said. “It is important to review with our patients the essential scheduled dosing of every 6 months, that the patient should not miss doses, and that we are not going to be able to initiate a drug holiday without starting another medicine.”
Patients likely to require hospitalization may not be good candidates for denosumab therapy because they may not be able to adhere to the dosing regimen, she said.
Denosumab vs. bisphosphonates: Real-world data
Trials have found greater increases in bone mineral density with denosumab, compared with the bisphosphonate drug alendronate, but that finding does not necessarily equate with reduced fracture risk, Dr. Bolster said. A recent population-based study examined fracture risk in approximately 92,000 people over age 50 years. Most were women, and their mean age was 71 years (JAMA Netw Open. 2019 Apr 5;2[4]:e192416).
The researchers compared the incidence of hospitalization for hip fracture among new denosumab users and new alendronate users during the 3 years after starting treatment. At 3 years, hip fractures occurred in 3.7% of the denosumab group and in 3.1% of the alendronate group. The rate of any fracture was 9% for each group. Although the study design had limitations, the analysis found “no difference between denosumab and alendronate in terms of fracture-risk reduction,” Dr. Bolster said. “Both agents are good agents.”
A recent meta-analysis compared fracture risk with denosumab and any bisphosphonate treatment using data from 10 trials that included more than 5,000 patients (J Clin Endocrinol Metab. 2019 May 1;104[5]:1753-65).
At 12 and 24 months, denosumab produced greater increases in bone mineral density at the spine, hip, and femoral neck. “In fact, there was a greater increase in bone density seen in those on denosumab who had had prior bisphosphonate use,” Dr. Bolster said. In 9 out of 10 trials, however, fracture rate did not differ between patients who received denosumab or any bisphosphonate at 12 or 24 months.
Bisphosphonate drug holidays
An increased risk of atypical femoral fracture with long-term bisphosphonate therapy has driven research on the effects of bisphosphonate drug holidays. “When we start a drug holiday, it requires continued close monitoring of the patient’s risk factors,” as well as monitoring whether a new fracture occurs during the holiday, Dr. Bolster said.
“We have very little data to guide the duration of a drug holiday,” she said. One study examined changes in bone density and bone turnover markers during a drug holiday after treatment with oral alendronate or intravenous zoledronic acid (J Bone Miner Res. 2019 May;34[5]:810-6).
The investigators conducted a post hoc analysis of data from the FLEX and HORIZON trials. Although alendronate was used for a longer duration, compared with zoledronic acid (5 years vs. 3 years), alendronate had a more rapid offset of drug effect after 3 years. The difference may relate to compliance rates with oral therapy during the treatment period, Dr. Bolster said.
The study did not examine fracture rates, which is the outcome that ultimately matters at the end of the day, she said.
Data suggest that bisphosphonate holidays are associated with increased risk of hip fracture. An analysis of Medicare data by Curtis et al. found that “hip fracture rates were lowest among those who remained on bisphosphonates,” Dr. Bolster said. Hip fracture rates increased with the length of the drug holiday, and a drug holiday of between 2 and 3 years was associated with 39% increased risk. The analysis included data from more than 156,000 women, about 40% of whom stopped bisphosphonates for more than 6 months. A total of 3,745 hip fractures occurred during follow-up.
Individualize treatment
“Duration of therapy should be individualized to the patient,” Dr. Bolster said. Physicians should assess the patient’s risk factors and take into account fragility fractures before and during treatment, bone density, and comorbidities.
“In terms of duration for drug holiday, does the patient now have osteopenia after treatment?” she said. “It is uncommon for bone density to change significantly during treatment, but occasionally we have a patient who goes from osteoporosis to osteopenia.”
The resumption of treatment should be based on established guidelines and individual patient factors, she said. For some postmenopausal woman, estrogen or raloxifene may not be ideal treatments when resuming therapy because these medications may increase cardiovascular or thrombotic risks. Denosumab may not be a good option for some patients because of the limitations surrounding its ability to be discontinued. The anabolic agents teriparatide and abaloparatide “may be good options to consider after a drug holiday, or even to give to patients during the drug holiday,” Dr. Bolster said. “The drug holiday does not have to be a treatment holiday. It really just needs to be an antiresorptive holiday.”
Dr. Bolster owns stock in Johnson & Johnson and is on an advisory board for Gilead.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Role of Psoriasis in the Development of Merkel Cell Carcinoma
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
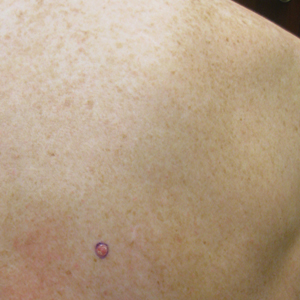
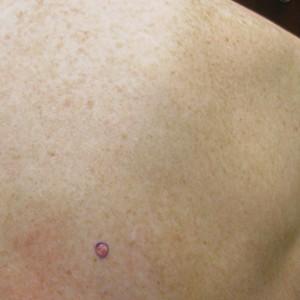
Merkel Cell Carcinoma in a Patient With a History of Psoriasis
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
Background
Merkel cell carcinoma (MCC) is a rare, aggressive cutaneous malignancy.1 Immunosuppression, advanced age, and UV light exposure of fair skin are major risk factors; additionally, polyomavirus infection is detected in as many as 80% of cases.2-5 Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis.6 Approximately half of patients present with localized disease, in which surgical resection with or without adjuvant radiotherapy is usually indicated. However, overall prognosis is poor with MCC due to high recurrence rates.3
This neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes; distant metastasis also can occur, most commonly to the liver, lungs, bones, and brain.7,8 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. The frequency of metastasis at diagnosis as well as high recurrence rates after treatment contribute to the overall poor prognosis of MCC. Local recurrence rates have been reported at 25%, with lymph node involvement in 52% and metastasis in 34% of cases; most recurrences occur within 2 years of diagnosis.8 The aggressiveness of the tumor determines patient mortality; in cases without lymph node involvement, the 5-year survival rate is 83.3%. However, the 5-year survival rate drops to 58.3% with lymph node involvement; in patients with metastatic disease, it is just 31.3%.8,9 Although MCC is a chemosensitive disease, durable responses are rarely seen in the advanced setting.3 Most patients with metastatic disease have a median progression-free survival of only 3 months.1
Presentation and Diagnosis
An MCC tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface, most often on the sun-exposed head and neck region.3,9-11 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% present on the extremities, and 12% to 14% on the trunk.6 Unfortunately, this nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC can only be achieved with a skin biopsy, which allows for distinction from other clinically similar-appearing neoplasms.
Specific guidelines for an MCC diagnostic evaluation have been proposed by the National Comprehensive Cancer Network (NCCN) based on a framework of clinical presentation, preliminary workup, diagnosis, and additional workup.12 The simplified algorithm for evaluation includes the following12,13:
- Examine skin and lymph nodes
- Obtain a biopsy specimen stained with hematoxylin and eosin (including Breslow thickness and evidence of lymphovascular invasion) and immunostaining (including but not limited to cytokeratin (CK) 20 and thyroid transcription factor 1)
- Obtain a sentinel lymph node biopsy specimen from patients with negative clinical nodes, prior to excision when possible
- Perform fine-needle aspiration or core biopsy first for patients with positive clinical nodes; if negative, consider open biopsy, but if positive, proceed to next step
- Perform imaging as clinically indicated with magnetic resonance imaging, computed tomography, or positron emission tomography–computed tomography
- Consider consultation with a multidisciplinary tumor board
Histologic Features of MCC
Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.8,10 Immunohistochemical features include CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and CK7 negativity, which allows it to be differentiated from other neoplasms. Chromogranin and synaptophysin positivity provide further histologic confirmation. The absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.4,8
Merkel Cell Polyomavirus and Other Risk Factors
Merkel cell carcinoma is frequently associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases.8 Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.10
Immunosuppression and UV-exposed fair skin also are considered major risk factors, which may be explained by the increase in MCPyV small T-antigen transcripts induced by UV irradiation.10 Moreover, as is seen in other cancers induced by viruses, host immunity can hinder tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development as well as the potential for a worse prognosis.4,8 The precise incidence of MCC in immunosuppressed patients appears unclear; it is possible that chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.4,8
Impact of Comorbidities on MCC
These risk factors were all observed in the case patient. However, it also is possible that his associated comorbidities also had an impact on the presentation of his disease.8 For example, patients with rheumatoid arthritis have been shown to have an elevated risk for the development of MCC.14 Moreover, inflammatory monocytes infected with MCPyV, as would be expected in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by migrating to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.8,15 Testing for MCPyV was never performed in the case patient; however, it certainly would be prudent to do so in such a presentation. Additionally, further studies to determine the correlation of MCC to these disease processes are warranted.8
Given that MCC tends to affect an older population in which other notable comorbidities are not uncommon, the cost, side effects, and convenience for patients of any treatment plan are important considerations.8 In the case study, a combined regimen of carboplatin and VP-16 (etoposide) was utilized. This regimen was well tolerated by the patient and successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination has been shown to prolong survival in patients with distant metastasis compared to those patients not receiving chemotherapy.6 In all high-risk patients, close clinical monitoring is essential to help optimize outcomes.
Bottom Line
Merkel cell carcinoma is a rare but aggressive cutaneous neoplasm that most frequently affects elderly patients, immunosuppressed patients, and individuals with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases such as psoriasis and rheumatoid arthritis also may play a role. In this case, the patient’s history of chronic immunosuppressive therapy for treatment of psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor. This potential risk should be an important point of discussion with any patient requiring this type of long-term management for disease control. This unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission following treatment with surgery and chemotherapy.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
- O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
- Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
- Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
- Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
- National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
- Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
The Case
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of the lesion, but his wife had recently noticed it; he denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. The patient had been on numerous treatment regimens for the control of psoriasis over the last 20 years, including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept at presentation of this new skin lesion. The use of immunosuppressive agents for his psoriasis provided an additional benefit of controlling the inflammatory arthritic disease.
Physical examination revealed a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
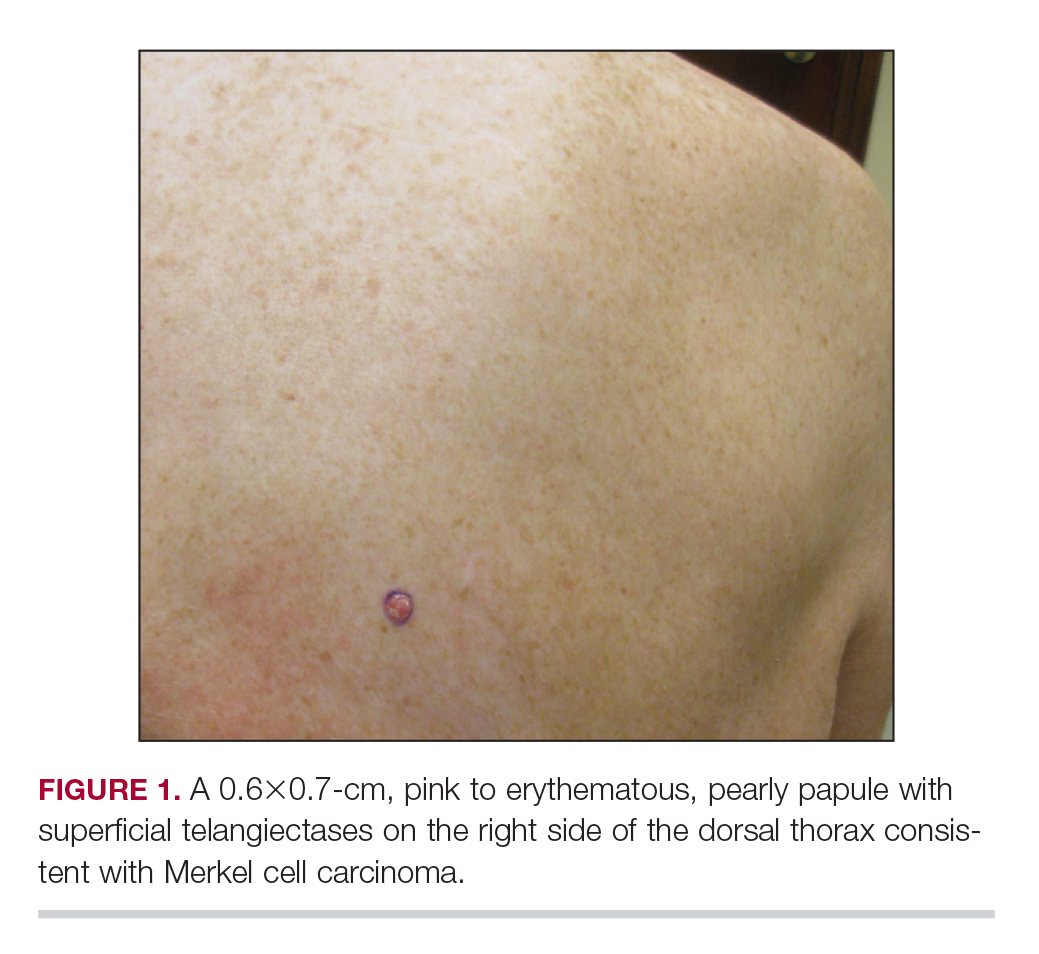
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later, the patient returned to discuss the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and the patient was referred to a tertiary referral center for further evaluation and treatment.
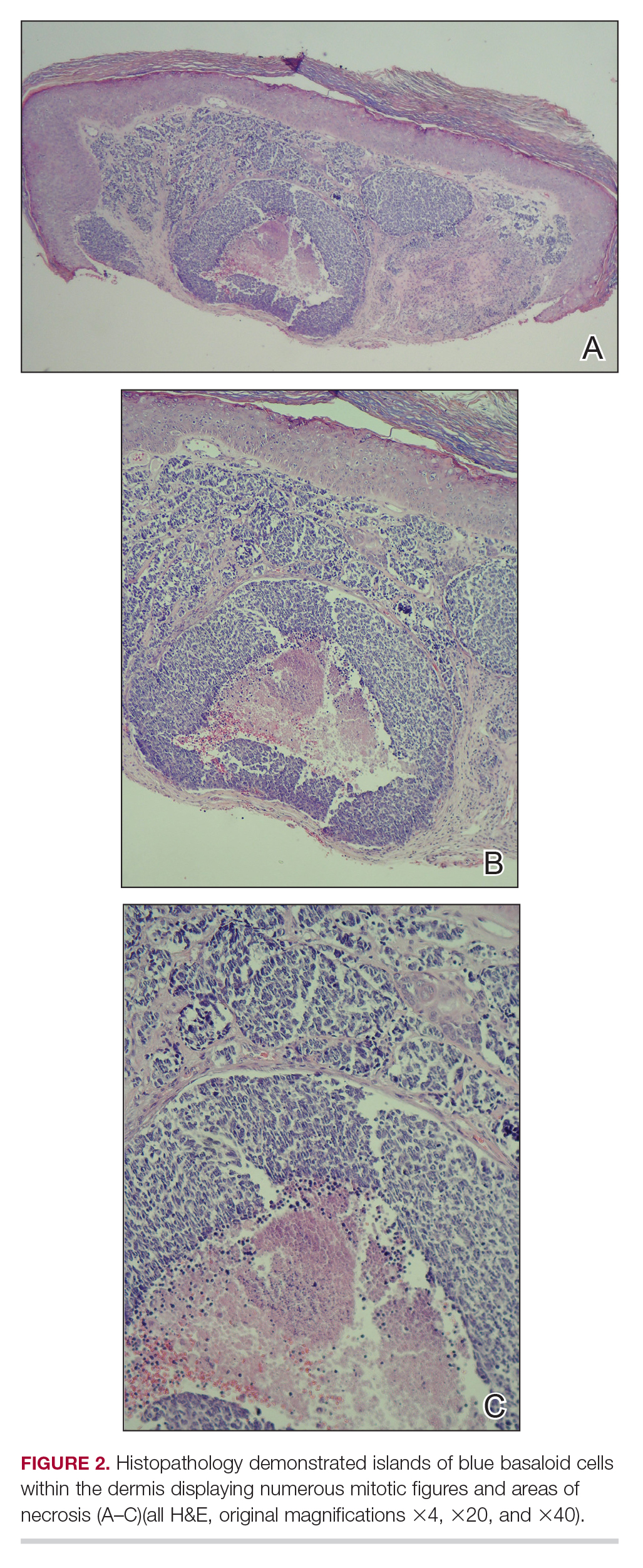
Treatment
He subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression. The patient underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Patient Outcome
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted, including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed, and the findings were consistent with metastatic MCC, indicating progression to stage IV disease. The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period and tolerated the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
This case was adapted from Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
Adjunctive therapy is among the roles for topical agents in psoriasis
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – is not dead,” Linda Stein Gold, MD, said at the annual Coastal Dermatology Symposium.
“We have to remember when we think back to our practice, how many topical prescriptions do we write, compared to preventive prescriptions? Probably most are topical,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Hospital Center, Detroit.
Topical agents have a place when a patient is doing well on treatment with a biologic but is not responding completely, she noted. One open-label, single-arm study looked at adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% (Enstilar) foam, applied once daily for 4 week, then twice a week on consecutive days for 12 weeks in 25 patients with psoriasis who had a mean body surface area (BSA) of less than 5% but significant remaining disease despite treatment with biologics.
At week 4, 76% achieved a BSA of 1% or less and Physician’s Global Assessment score of 1 or less at week 4, as did 68% at week 16. This was compared with 12% and 4%, respectively (J Drugs Dermatol. 2018 Aug 1;17[8]:845-50). “They found that a good potent topical on top of a biologic does really well. That can really kick up the last part of the efficacy to get the patients almost to clear,” she observed.
At the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education, Dr. Stein Gold also discussed tazarotene, a topical retinoid approved by the Food and Drug Administration for treating psoriasis and is available as a 0.1% and 0.05% cream and gel. About 10%-30% of patients experience side effects with tazarotene, such as pruritus, stinging, and burning. Topical corticosteroids can help, which prompted development of a combined product, she noted.
She referred to a phase 2 study of patients with moderate to severe plaque psoriasis, which compared the fixed combination lotion formulation of tazarotene plus halobetasol propionate to the two components alone. The investigators found almost a 9% rate of treatment success with tazarotene alone, versus about 23% with halobetasol propionate alone and about 43% with the combined product. The combined individual effect of the two drugs was about 32%, so the 43% efficacy of the combined product had an absolute synergistic effect of about 11%, Dr. Stein Gold pointed out.
Two phase 3 trials of adults with moderate to severe psoriasis supported the phase 2 results of the combined lotion formulation (halobetasol 0.01% with tazarotene 0.045%), said Dr. Stein Gold, the first author (J Am Acad Dermatol. 2018 Aug;79[2]:287-93). Treatment success was defined as at least a 2-grade Investigator’s Global Assessment score and improvement from baseline and a score of “clear” or “almost clear.” In one of the studies, 36% of those on the combination versus 7% of those on the vehicle met this endpoint at week 8, as did 45% versus 13%, respectively, in the second study (P less than .001 for both studies).
Patients also had less itching, drying, and stinging than typically seen with tazarotene alone, Dr. Stein Gold said. In the studies, contact dermatitis was the most common side effect associated with treatment, reported in 6.3%
Dr. Stein Gold has received research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, and Foamix. She has been a consultant for Sol-gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promis, Anacor, and Medimetriks. She has been on the speakers bureau of Galderma, Leo, Valeant, Novartis, Celgene, and Allergan. She has been a member of scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Guidelines updated for treating community-acquired pneumonia
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE