User login
Uninsured population is big in Texas
There were just over 5 million uninsured people in the Lone Star State last year, representing an increase from 17.3% of the total population in 2017 to 17.7%, and that works out to an additional 186,000 residents with no health care coverage, the Census Bureau said in a recent report.
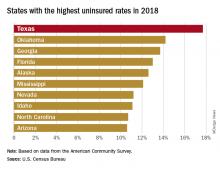
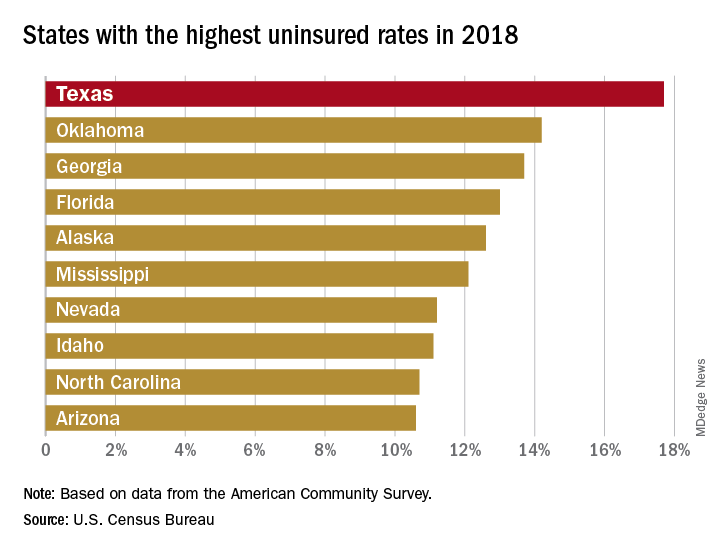
That 17.7% rate for 2018 gave Texas the highest proportion of uninsured population, putting it ahead of Oklahoma (14.2%), Georgia (13.7%), Florida (13.0%), Alaska (12.6%), and Mississippi (12.1%). Oklahoma had basically no change from 2017, while the other three each had a small but nonsignificant increase. Nationally, the rate of uninsured population went from 7.9% in 2017 to 8.5% in 2018, the Census Bureau investigators said.
On the other end of the coverage spectrum was Massachusetts, where only 2.8% of the population, or about 189,000 people, lacked health insurance in 2018. Washington, D.C., was next with an uninsured rate of 3.2%, followed by Vermont (4.0%), Hawaii (4.1%), Rhode Island (4.1%), and Minnesota (4.4%), they said, based on data from the American Community Survey.
A separate analysis of Census Bureau data by the personal finance website WalletHub showed that states that expanded Medicaid along with their Affordable Care Act implementation had an average uninsured rate of 7.0% in 2018, compared with 11.1% for states that did not expand eligibility.
All 50 states were in negative territory when changes in uninsured rates were calculated over a longer time period, 2010-2018, as the national rate fell by 6.6%. The largest drops among the states came in Nevada (–11.4%), California (–11.3%), Oregon (–10.1%), and New Mexico (–10.1%), while Massachusetts (–1.7%), Maine (–2.1%), and North Dakota (–2.5%) had the smallest declines, WalletHub reported.
There were just over 5 million uninsured people in the Lone Star State last year, representing an increase from 17.3% of the total population in 2017 to 17.7%, and that works out to an additional 186,000 residents with no health care coverage, the Census Bureau said in a recent report.
That 17.7% rate for 2018 gave Texas the highest proportion of uninsured population, putting it ahead of Oklahoma (14.2%), Georgia (13.7%), Florida (13.0%), Alaska (12.6%), and Mississippi (12.1%). Oklahoma had basically no change from 2017, while the other three each had a small but nonsignificant increase. Nationally, the rate of uninsured population went from 7.9% in 2017 to 8.5% in 2018, the Census Bureau investigators said.
On the other end of the coverage spectrum was Massachusetts, where only 2.8% of the population, or about 189,000 people, lacked health insurance in 2018. Washington, D.C., was next with an uninsured rate of 3.2%, followed by Vermont (4.0%), Hawaii (4.1%), Rhode Island (4.1%), and Minnesota (4.4%), they said, based on data from the American Community Survey.
A separate analysis of Census Bureau data by the personal finance website WalletHub showed that states that expanded Medicaid along with their Affordable Care Act implementation had an average uninsured rate of 7.0% in 2018, compared with 11.1% for states that did not expand eligibility.
All 50 states were in negative territory when changes in uninsured rates were calculated over a longer time period, 2010-2018, as the national rate fell by 6.6%. The largest drops among the states came in Nevada (–11.4%), California (–11.3%), Oregon (–10.1%), and New Mexico (–10.1%), while Massachusetts (–1.7%), Maine (–2.1%), and North Dakota (–2.5%) had the smallest declines, WalletHub reported.
There were just over 5 million uninsured people in the Lone Star State last year, representing an increase from 17.3% of the total population in 2017 to 17.7%, and that works out to an additional 186,000 residents with no health care coverage, the Census Bureau said in a recent report.
That 17.7% rate for 2018 gave Texas the highest proportion of uninsured population, putting it ahead of Oklahoma (14.2%), Georgia (13.7%), Florida (13.0%), Alaska (12.6%), and Mississippi (12.1%). Oklahoma had basically no change from 2017, while the other three each had a small but nonsignificant increase. Nationally, the rate of uninsured population went from 7.9% in 2017 to 8.5% in 2018, the Census Bureau investigators said.
On the other end of the coverage spectrum was Massachusetts, where only 2.8% of the population, or about 189,000 people, lacked health insurance in 2018. Washington, D.C., was next with an uninsured rate of 3.2%, followed by Vermont (4.0%), Hawaii (4.1%), Rhode Island (4.1%), and Minnesota (4.4%), they said, based on data from the American Community Survey.
A separate analysis of Census Bureau data by the personal finance website WalletHub showed that states that expanded Medicaid along with their Affordable Care Act implementation had an average uninsured rate of 7.0% in 2018, compared with 11.1% for states that did not expand eligibility.
All 50 states were in negative territory when changes in uninsured rates were calculated over a longer time period, 2010-2018, as the national rate fell by 6.6%. The largest drops among the states came in Nevada (–11.4%), California (–11.3%), Oregon (–10.1%), and New Mexico (–10.1%), while Massachusetts (–1.7%), Maine (–2.1%), and North Dakota (–2.5%) had the smallest declines, WalletHub reported.
What repair is best for juxtarenal aneurysm?
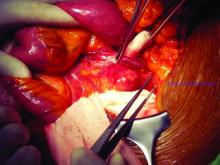
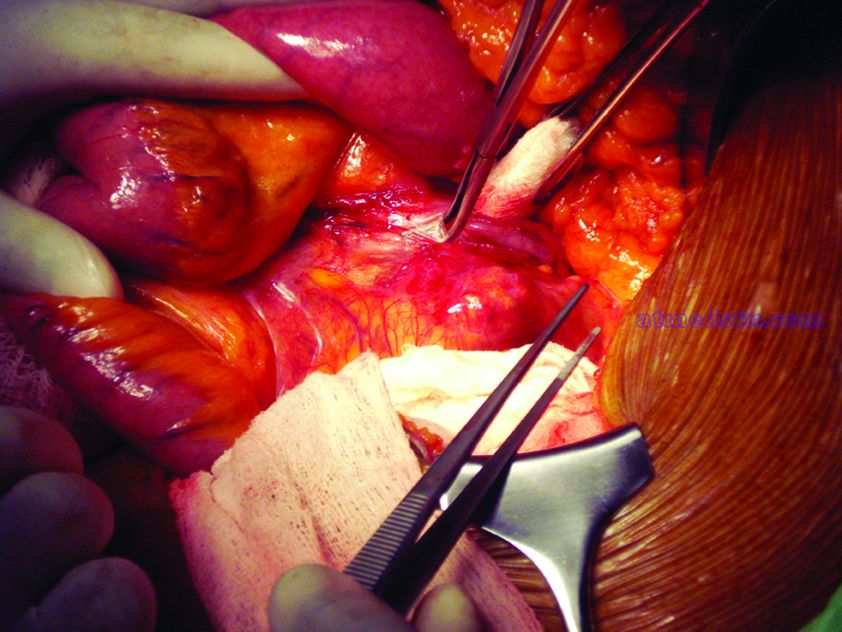
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
CHICAGO – Outcomes with fenestrated endografts and endograft anchors to repair abdominal aortic aneurysms (AAAs) in the region of the renal artery have improved as the techniques have gained popularity in recent years, but open repair may still achieve better overall results, vascular surgeons on opposite sides of the controversy contended during a debate at the annual meeting of the Midwestern Vascular Surgery Society.
Fenestrated endovascular aortic repair (FEVAR) “is as safe as open surgery to treat complex aneurysm,” said Carlos Bechara, MD, of Loyola University Medical Center in Chicago. “EndoAnchors [Medtronic] do provide an excellent off-the-shelf solution to treat short, hostile necks with promising short-term results.”
Arguing for open repair was Paul DiMusto, MD, of the University of Wisconsin–Madison. “Open repair has an equal perioperative mortality to FEVAR,” Dr. DiMusto said, adding that the open approach also has a higher long-term branch patency rate, lower secondary-intervention rate, a lower incidence of long-term renal failure, and higher long-term survival. “So putting that all together, open repair is best,” he said.
They staked out their positions by citing a host of published trials.
“The presence of a short neck can create a challenging clinical scenario for an endovascular repair of abdominal aortic aneurysm,” Dr. Bechara said. However, he noted he was discussing complex aneurysm in which the aortic clamp is placed above the renal arteries, differentiating it from infrarenal AAA in which the clamp is below the renal arteries with no renal ischemia time. He noted a 2011 study that determined a short neck was a predictor of Type 1A endoleak after AAA repair, but that compliance with best practices at the time was poor; more than 44% of EVARs did not follow the manufacturer’s instruction (Circulation. 2011;123:2848-55).
But FEVAR was approved by the Food and Drug Administration in 2012, with an indication for an infrarenal neck length of 4-14 mm, Dr. Bechara noted. Since then, several studies have reported excellent outcomes with the technique. An early small study of 67 patients reported a 100% technical success rate with one patient having a Type 1 endoleak at 3 years (J Vasc Surg. 2014;60:1420-8).
This year, a larger study evaluated 6,825 patients in the American College of Surgeons National Surgical Quality Improvement Program who had FEVAR, open AAA repair or standard infrarenal endovascular repair during 2012-2016. “Actually, the fenestrated approach had fewer complications than open repair and the outcomes were comparable to standard EVAR,” Dr. Bechara noted. The trial reported FEVAR had lower rates of perioperative mortality (1.8% vs. 8.8%; P = .001), postoperative renal dysfunction (1.4% vs. 7.7%; P = .002), and overall complications (11% vs. 33%; P less than.001) than did open repair (J Vasc Surg. 2019;69:1670-78).
In regard to the use of endograft anchors for treatment of endoleaks, migrating grafts, and high-risk seal zones, Dr. Bechara noted they are a good “off-the-shelf” choice for complex AAA repair. He cited current results of a cohort of 70 patients with short-neck AAA (J Vasc Surg. 2019;70:732-40). “This study showed a procedural success rate at 97% and a technical success rate at 88.6%,” he said. “They had no stent migration, no increase in sac size or AAA rupture or open conversion.”
He also pointed to just-published results from a randomized trial of 881 patients with up to 14 years of follow-up that found comparable rates of death/secondary procedures, as well as durability, between patients who had endovascular and open repairs (77.7% and 75.5%, respectively, N Engl J Med. 2019;380:2126-35). Also, he noted that hospital volume is an important predictor of success with open repair, with high-volume centers reporting lower mortality (3.9%) than low-volume centers (9%; Ann Surg. 2018 Nov 29. doi: 10.1097/SLA.0000000000002873). “So not many centers are doing high-volume open aortic surgery,” he said.
To make his case that open surgery for juxtarenal AAAs is superior, Dr. DiMusto cited a number of recent studies, including a three-center trial of 200 patients who had open and FEVAR procedures (J Endovasc Ther. 2019;26:105-12). “There was no difference in perioperative mortality [2.2% for FEVAR, 1.9% in open repair], ” Dr. DiMusto said “There was a higher freedom from reintervention in the open group [96% vs. 78%], and there was higher long-term vessel patency in the open group” (97.5% having target patency for open vs. 93.3% for FEVAR).
He also pointed to a meta-analysis of 2,326 patients that found similar outcomes for mortality and postoperative renal insufficiency between FEVAR and open repair, around 4.1%, but showed significantly higher rates of renal failure in FEVAR, at 19.7% versus 7.7% (J Vasc Surg. 2015;61:242-55). This study also reported significantly more secondary interventions with FEVAR, 12.7% vs. 4.9%, Dr. DiMusto said.
Another study of 3,253 complex AAA repairs, including 887 FEVAR and 2,125 open procedures, showed that FEVAR had a technical success rate of 97%, with no appreciable difference in perioperative mortality between the two procedures (Ann Surg. 2019 Feb 1. doi: 10.1097/SLA.0000000000003094).
However, Dr. DiMusto said, adjusted 3-year mortality in this study was higher with FEVAR, and further analysis yielded outcomes that favored open repair. “After excluding perioperative deaths, differences remained, with 9% mortality for FEVAR and 5% for open repair [P = .02],” he said. “This corresponded to a 66% higher risk for overall mortality following FEVAR.”
What’s more, Dr. DiMusto said, draft guidelines from the National Institute for Health and Care Excellence in the United Kingdom advise against offering complex EVAR to people with an unruptured AAA under two scenarios: if open surgery is an option; and even if they’re unable to have surgery because of anesthetic or medical issues. The final guidelines have yet to be released.
Dr. Bechara disclosed financial relationships with Gore Medical and Cook Medical and equity interest in MOKITA Medical. Dr. DiMusto has no relevant financial disclosures.
EXPERT ANALYSIS FROM MIDWESTERN VASCULAR 2019
SSRIs may reduce fecundability, live birth rate in reproductive-age women
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
REPORTING FROM ASRM 2019
Bringing focus to the issue: Dr. Elizabeth Loder on gender in medicine
The recently published “Eleven Things Not to Say to Your Female Colleagues,” has sparked debate on medical Twitter. Senior author Elizabeth Loder, MD, developed the content collaboratively with members of the Migraine Mavens, a private Facebook group of North American headache practitioners and researchers.
In an interview, Dr. Loder, chief of the headache division in the neurology department at Brigham and Women’s Hospital, Boston, and professor of neurology at Harvard Medical School, Boston, shared the background and context for the article.
Q: Could you explain the impetus for putting this together? How did you arrive at the chart that is the center of the article?
A: In June, I gave the Seymour Solomon lecture at the American Headache Society annual scientific meeting. Because it was an award lecture, I was able to choose the topic. I decided to talk about gender-based problems faced by women in medicine, with a focus on the headache field.
These problems include sexual harassment, hurtful sex-based comments, gender-based barriers to career advancement, as well as the difficulties women face in getting institutions or professional societies to pay attention to these problems.
I wanted to provide real, recent examples of troubling behavior or comments, so I appealed to the Migraine Mavens group to describe their own experiences. I was not expecting the response I got. Not only did people post many examples of such behavior in the group, but I also received many private messages describing things that were so hurtful or private that the woman involved did not even feel comfortable posting them in our group.
I ended up with plenty of real-life vignettes. The title of my talk was “Time’s Up: Headache Medicine in the #MeToo Era.” Shortly after the talk, a member of the group posted this:
“Oh, Dr. Elizabeth Loder, how timely was your talk yesterday, and we have so much further to progress. ...
“Just now, I had this experience: I have been recently selected for a leadership position within AHS and I was talking to one of our male colleagues about it. ... He expressed his doubt in my ability to serve this role well.
“I thought it was because I am early in my career, and as I was reassuring him that I would reach out to him and others for mentorship, he then said ‘AND you have two small children. ... You don’t have time for this.’ ”
There was lively discussion in the group about how this poster could have responded and what bystanders could have said. One of the group members, Clarimar Borrero-Mejias, MD, a pediatric neurologist at Phoenix Children’s Hospital, pointed out that many men and women might benefit from knowing what kinds of things not to say to other colleagues. I suggested that we should take some of the problems we had discussed and write a paper, and that she should be the first author. We then crowdsourced the scenarios to be included.
The grid format came immediately to mind because I know that tables and charts and boxes are good ways to organize and present information. We also wanted to keep the article short and accessible, and thus the idea of “Ten Things” was born. At the end of our work, though, someone posted the vignette about the salary discussion. It was amazing to me how many women, even in this day and age, are still told that men deserve more money because of their family or other responsibilities. We thus decided that it had to be 11, not 10, things.
The article was possible only because of the supportive reaction of the editor of Headache, Thomas Ward, MD. He not only published the piece rapidly, but also agreed to make it free so that anyone who wanted to could access the entire article without hitting a paywall (Headache. 2019 Sep 26. doi: 10.1111/head.13647).
Q: Could you share some of the reactions you’ve gotten? I did see that Esther Choo, MD – an emergency medicine physician and prominent proponent for gender equity in medicine – highlighted the article on Twitter; are there other highlights, or surprising reactions, or pushback, that you’d like to share?
A: We were thrilled to be the subject of a “tweetorial” by Dr. Choo. It’s impossible to overestimate the boost this gave to the paper. She has over 75,000 Twitter followers, and it was quite impressive to watch the exponential increase in the article’s Altmetrics score after her tweetorial. This brought the article to the attention of people outside our own subspecialty. The experiences we described seem to be familiar to women doctors in every specialty and subspecialty, and also relevant outside medicine. I saw tweets from women lawyers, engineers, and others, many of whom said this sort of behavior is a problem in their own fields.
It’s probably not surprising that the vast majority of reactions came from women. A number of men tweeted the article, though, and recommended it to other men. This sort of #HeForShe support is gratifying. We did get some negative reactions, but there are Migraine Mavens on Twitter and we’ve taken them on.
Q: You offer suggestions for reframing many behaviors that reflect implicit bias. You also offer suggestions for bystanders to challenge these biases and support women who are on the receiving end of the behaviors you call out. Do you think exhibiting more of this kind of solidarity can help change the culture of medicine?
A: I believe many people who witness the behaviors are uncomfortable and would like to help but just don’t know what to say. Often, they are caught off guard. Some of our suggested responses are all-purpose lines that can be effective simply by calling attention to the behavior, for example, “What did you just say?” or “Why would you say something like that?” As Dr. Choo said, “Learn them, say them often.”
It’s critical to remember that problems like this are not in the past. This article gave real examples of things that have happened to real women recently. The sheer number of women who retweeted the article with statements such as, “How many of these have been said to you? Straw poll. I got 9,” demonstrates that behavior like this is common.
I recently received an email that forwarded a message written by a medical assistant. I’ve changed the names, but it otherwise read “Dr. Smith wants this patient to have a nerve block. ... You can schedule them with Abigail or Nancy.” Guess what? Abigail and Nancy are doctors. Not only that, they are Dr. Smith’s true peers in every way imaginable, having been hired at exactly the same time and having exactly the same titles and duties. There seems to be only one reason they are not addressed as doctor while their male colleague is, and that is their gender. So the struggle highlighted by #MyFirstNameIsDoctor is real. Women doctors live it every day.
koakes@mdedge.com
The recently published “Eleven Things Not to Say to Your Female Colleagues,” has sparked debate on medical Twitter. Senior author Elizabeth Loder, MD, developed the content collaboratively with members of the Migraine Mavens, a private Facebook group of North American headache practitioners and researchers.
In an interview, Dr. Loder, chief of the headache division in the neurology department at Brigham and Women’s Hospital, Boston, and professor of neurology at Harvard Medical School, Boston, shared the background and context for the article.
Q: Could you explain the impetus for putting this together? How did you arrive at the chart that is the center of the article?
A: In June, I gave the Seymour Solomon lecture at the American Headache Society annual scientific meeting. Because it was an award lecture, I was able to choose the topic. I decided to talk about gender-based problems faced by women in medicine, with a focus on the headache field.
These problems include sexual harassment, hurtful sex-based comments, gender-based barriers to career advancement, as well as the difficulties women face in getting institutions or professional societies to pay attention to these problems.
I wanted to provide real, recent examples of troubling behavior or comments, so I appealed to the Migraine Mavens group to describe their own experiences. I was not expecting the response I got. Not only did people post many examples of such behavior in the group, but I also received many private messages describing things that were so hurtful or private that the woman involved did not even feel comfortable posting them in our group.
I ended up with plenty of real-life vignettes. The title of my talk was “Time’s Up: Headache Medicine in the #MeToo Era.” Shortly after the talk, a member of the group posted this:
“Oh, Dr. Elizabeth Loder, how timely was your talk yesterday, and we have so much further to progress. ...
“Just now, I had this experience: I have been recently selected for a leadership position within AHS and I was talking to one of our male colleagues about it. ... He expressed his doubt in my ability to serve this role well.
“I thought it was because I am early in my career, and as I was reassuring him that I would reach out to him and others for mentorship, he then said ‘AND you have two small children. ... You don’t have time for this.’ ”
There was lively discussion in the group about how this poster could have responded and what bystanders could have said. One of the group members, Clarimar Borrero-Mejias, MD, a pediatric neurologist at Phoenix Children’s Hospital, pointed out that many men and women might benefit from knowing what kinds of things not to say to other colleagues. I suggested that we should take some of the problems we had discussed and write a paper, and that she should be the first author. We then crowdsourced the scenarios to be included.
The grid format came immediately to mind because I know that tables and charts and boxes are good ways to organize and present information. We also wanted to keep the article short and accessible, and thus the idea of “Ten Things” was born. At the end of our work, though, someone posted the vignette about the salary discussion. It was amazing to me how many women, even in this day and age, are still told that men deserve more money because of their family or other responsibilities. We thus decided that it had to be 11, not 10, things.
The article was possible only because of the supportive reaction of the editor of Headache, Thomas Ward, MD. He not only published the piece rapidly, but also agreed to make it free so that anyone who wanted to could access the entire article without hitting a paywall (Headache. 2019 Sep 26. doi: 10.1111/head.13647).
Q: Could you share some of the reactions you’ve gotten? I did see that Esther Choo, MD – an emergency medicine physician and prominent proponent for gender equity in medicine – highlighted the article on Twitter; are there other highlights, or surprising reactions, or pushback, that you’d like to share?
A: We were thrilled to be the subject of a “tweetorial” by Dr. Choo. It’s impossible to overestimate the boost this gave to the paper. She has over 75,000 Twitter followers, and it was quite impressive to watch the exponential increase in the article’s Altmetrics score after her tweetorial. This brought the article to the attention of people outside our own subspecialty. The experiences we described seem to be familiar to women doctors in every specialty and subspecialty, and also relevant outside medicine. I saw tweets from women lawyers, engineers, and others, many of whom said this sort of behavior is a problem in their own fields.
It’s probably not surprising that the vast majority of reactions came from women. A number of men tweeted the article, though, and recommended it to other men. This sort of #HeForShe support is gratifying. We did get some negative reactions, but there are Migraine Mavens on Twitter and we’ve taken them on.
Q: You offer suggestions for reframing many behaviors that reflect implicit bias. You also offer suggestions for bystanders to challenge these biases and support women who are on the receiving end of the behaviors you call out. Do you think exhibiting more of this kind of solidarity can help change the culture of medicine?
A: I believe many people who witness the behaviors are uncomfortable and would like to help but just don’t know what to say. Often, they are caught off guard. Some of our suggested responses are all-purpose lines that can be effective simply by calling attention to the behavior, for example, “What did you just say?” or “Why would you say something like that?” As Dr. Choo said, “Learn them, say them often.”
It’s critical to remember that problems like this are not in the past. This article gave real examples of things that have happened to real women recently. The sheer number of women who retweeted the article with statements such as, “How many of these have been said to you? Straw poll. I got 9,” demonstrates that behavior like this is common.
I recently received an email that forwarded a message written by a medical assistant. I’ve changed the names, but it otherwise read “Dr. Smith wants this patient to have a nerve block. ... You can schedule them with Abigail or Nancy.” Guess what? Abigail and Nancy are doctors. Not only that, they are Dr. Smith’s true peers in every way imaginable, having been hired at exactly the same time and having exactly the same titles and duties. There seems to be only one reason they are not addressed as doctor while their male colleague is, and that is their gender. So the struggle highlighted by #MyFirstNameIsDoctor is real. Women doctors live it every day.
koakes@mdedge.com
The recently published “Eleven Things Not to Say to Your Female Colleagues,” has sparked debate on medical Twitter. Senior author Elizabeth Loder, MD, developed the content collaboratively with members of the Migraine Mavens, a private Facebook group of North American headache practitioners and researchers.
In an interview, Dr. Loder, chief of the headache division in the neurology department at Brigham and Women’s Hospital, Boston, and professor of neurology at Harvard Medical School, Boston, shared the background and context for the article.
Q: Could you explain the impetus for putting this together? How did you arrive at the chart that is the center of the article?
A: In June, I gave the Seymour Solomon lecture at the American Headache Society annual scientific meeting. Because it was an award lecture, I was able to choose the topic. I decided to talk about gender-based problems faced by women in medicine, with a focus on the headache field.
These problems include sexual harassment, hurtful sex-based comments, gender-based barriers to career advancement, as well as the difficulties women face in getting institutions or professional societies to pay attention to these problems.
I wanted to provide real, recent examples of troubling behavior or comments, so I appealed to the Migraine Mavens group to describe their own experiences. I was not expecting the response I got. Not only did people post many examples of such behavior in the group, but I also received many private messages describing things that were so hurtful or private that the woman involved did not even feel comfortable posting them in our group.
I ended up with plenty of real-life vignettes. The title of my talk was “Time’s Up: Headache Medicine in the #MeToo Era.” Shortly after the talk, a member of the group posted this:
“Oh, Dr. Elizabeth Loder, how timely was your talk yesterday, and we have so much further to progress. ...
“Just now, I had this experience: I have been recently selected for a leadership position within AHS and I was talking to one of our male colleagues about it. ... He expressed his doubt in my ability to serve this role well.
“I thought it was because I am early in my career, and as I was reassuring him that I would reach out to him and others for mentorship, he then said ‘AND you have two small children. ... You don’t have time for this.’ ”
There was lively discussion in the group about how this poster could have responded and what bystanders could have said. One of the group members, Clarimar Borrero-Mejias, MD, a pediatric neurologist at Phoenix Children’s Hospital, pointed out that many men and women might benefit from knowing what kinds of things not to say to other colleagues. I suggested that we should take some of the problems we had discussed and write a paper, and that she should be the first author. We then crowdsourced the scenarios to be included.
The grid format came immediately to mind because I know that tables and charts and boxes are good ways to organize and present information. We also wanted to keep the article short and accessible, and thus the idea of “Ten Things” was born. At the end of our work, though, someone posted the vignette about the salary discussion. It was amazing to me how many women, even in this day and age, are still told that men deserve more money because of their family or other responsibilities. We thus decided that it had to be 11, not 10, things.
The article was possible only because of the supportive reaction of the editor of Headache, Thomas Ward, MD. He not only published the piece rapidly, but also agreed to make it free so that anyone who wanted to could access the entire article without hitting a paywall (Headache. 2019 Sep 26. doi: 10.1111/head.13647).
Q: Could you share some of the reactions you’ve gotten? I did see that Esther Choo, MD – an emergency medicine physician and prominent proponent for gender equity in medicine – highlighted the article on Twitter; are there other highlights, or surprising reactions, or pushback, that you’d like to share?
A: We were thrilled to be the subject of a “tweetorial” by Dr. Choo. It’s impossible to overestimate the boost this gave to the paper. She has over 75,000 Twitter followers, and it was quite impressive to watch the exponential increase in the article’s Altmetrics score after her tweetorial. This brought the article to the attention of people outside our own subspecialty. The experiences we described seem to be familiar to women doctors in every specialty and subspecialty, and also relevant outside medicine. I saw tweets from women lawyers, engineers, and others, many of whom said this sort of behavior is a problem in their own fields.
It’s probably not surprising that the vast majority of reactions came from women. A number of men tweeted the article, though, and recommended it to other men. This sort of #HeForShe support is gratifying. We did get some negative reactions, but there are Migraine Mavens on Twitter and we’ve taken them on.
Q: You offer suggestions for reframing many behaviors that reflect implicit bias. You also offer suggestions for bystanders to challenge these biases and support women who are on the receiving end of the behaviors you call out. Do you think exhibiting more of this kind of solidarity can help change the culture of medicine?
A: I believe many people who witness the behaviors are uncomfortable and would like to help but just don’t know what to say. Often, they are caught off guard. Some of our suggested responses are all-purpose lines that can be effective simply by calling attention to the behavior, for example, “What did you just say?” or “Why would you say something like that?” As Dr. Choo said, “Learn them, say them often.”
It’s critical to remember that problems like this are not in the past. This article gave real examples of things that have happened to real women recently. The sheer number of women who retweeted the article with statements such as, “How many of these have been said to you? Straw poll. I got 9,” demonstrates that behavior like this is common.
I recently received an email that forwarded a message written by a medical assistant. I’ve changed the names, but it otherwise read “Dr. Smith wants this patient to have a nerve block. ... You can schedule them with Abigail or Nancy.” Guess what? Abigail and Nancy are doctors. Not only that, they are Dr. Smith’s true peers in every way imaginable, having been hired at exactly the same time and having exactly the same titles and duties. There seems to be only one reason they are not addressed as doctor while their male colleague is, and that is their gender. So the struggle highlighted by #MyFirstNameIsDoctor is real. Women doctors live it every day.
koakes@mdedge.com
Court of Appeals to decide fate of Medicaid work requirements
The debate over whether states can impose work requirements on Medicaid recipients is now in the hands of a federal appeals court.
The U.S. Court of Appeals for the District of Columbia heard oral arguments Oct. 11, 2019, in two cases that challenge state waivers that require work as part of Medicaid eligibility.
In Stewart v. Azar, 16 patients from Kentucky are suing the Department of Health & Human Services over its approval of changes to Kentucky’s Medicaid program that include work requirements, premiums, and lockouts. In Gresham v. Azar, several Arkansas residents are challenging HHS over the approval of modifications to Arkansas’ Medicaid program that require work requirements and eliminate retroactive coverage.
The restrictive conditions in the Medicaid waivers would cause thousands of Medicaid enrollees to lose coverage, according to Jane Perkins, legal director for the National Health Law Program, an advocacy firm representing the plaintiffs.
“Section 1115 of the Social Security Act only allows the [HHS] Secretary to approve experimental projects that further Medicaid’s purpose of furnishing medical assistance to low-income people,” Ms. Perkins said in a statement. “These waiver projects do not further this objective. By the government’s own framing, they are intended to transform Medicaid and explode Medicaid expansion. Only Congress can rewrite a statute – not this administration. We hope the appellate court will uphold the well-reasoned opinions of the district court.”
HHS argues that it has the authority to allow any experimental, pilot, or demonstration project likely to promote the objectives of Medicaid, which in addition to medical assistance include rehabilitation services that help patients attain or retain independence or self-care. The waivers from Kentucky and Arkansas are consistent with these objectives, attorneys for HHS argued in court documents.
Kentucky’s waiver project promotes beneficiary health and financial independence, while Arkansas’ demonstration is likely to assist in improving health outcomes through strategies that promote community engagement and address health determinants, according to letters from the Centers for Medicare & Medicaid Service approving the projects.
Arkansas’ demonstration project, approved in March 2018, includes a requirement that adults aged 19-49 years complete 80 hours per month of community engagement activities, such as employment, education, job-skills training, or community service, as a condition of continued Medicaid eligibility. Kentucky’s proposal, approved in November 2018, requires Medicaid patients to spend at least 80 hours per month on qualified activities, including employment, job skills training, education, community services and/or participation in substance use disorder treatment.
Medicaid patients in both states sued HHS shortly after the waivers were approved, arguing that the work requirements were arbitrary and capricious and that the agency exceeded its statutory authority in approving the projects. The U.S. District Court for the District of Columbia ruled in favor of the patients in March 2019, finding that HHS failed to fully consider the impact of the Kentucky and Arkansas changes on current and future Medicaid beneficiaries. In a decision for Kentucky and a separate ruling for Arkansas, the court vacated HHS’ approval of the projects and remanded both waivers back to HHS for reconsideration. In the interim, officials in both Kentucky and Arkansas halted implementation of the work requirements. The Department of Justice appealed in both cases.
According to court documents, 18,000 Arkansans lost coverage for failure to comply with the work requirements before the regulations were halted. In Kentucky, the state estimates that 95,000 Kentuckians could lose coverage if the project goes into effect.
A decision by the appeals court is expected by December 2019.
The debate over whether states can impose work requirements on Medicaid recipients is now in the hands of a federal appeals court.
The U.S. Court of Appeals for the District of Columbia heard oral arguments Oct. 11, 2019, in two cases that challenge state waivers that require work as part of Medicaid eligibility.
In Stewart v. Azar, 16 patients from Kentucky are suing the Department of Health & Human Services over its approval of changes to Kentucky’s Medicaid program that include work requirements, premiums, and lockouts. In Gresham v. Azar, several Arkansas residents are challenging HHS over the approval of modifications to Arkansas’ Medicaid program that require work requirements and eliminate retroactive coverage.
The restrictive conditions in the Medicaid waivers would cause thousands of Medicaid enrollees to lose coverage, according to Jane Perkins, legal director for the National Health Law Program, an advocacy firm representing the plaintiffs.
“Section 1115 of the Social Security Act only allows the [HHS] Secretary to approve experimental projects that further Medicaid’s purpose of furnishing medical assistance to low-income people,” Ms. Perkins said in a statement. “These waiver projects do not further this objective. By the government’s own framing, they are intended to transform Medicaid and explode Medicaid expansion. Only Congress can rewrite a statute – not this administration. We hope the appellate court will uphold the well-reasoned opinions of the district court.”
HHS argues that it has the authority to allow any experimental, pilot, or demonstration project likely to promote the objectives of Medicaid, which in addition to medical assistance include rehabilitation services that help patients attain or retain independence or self-care. The waivers from Kentucky and Arkansas are consistent with these objectives, attorneys for HHS argued in court documents.
Kentucky’s waiver project promotes beneficiary health and financial independence, while Arkansas’ demonstration is likely to assist in improving health outcomes through strategies that promote community engagement and address health determinants, according to letters from the Centers for Medicare & Medicaid Service approving the projects.
Arkansas’ demonstration project, approved in March 2018, includes a requirement that adults aged 19-49 years complete 80 hours per month of community engagement activities, such as employment, education, job-skills training, or community service, as a condition of continued Medicaid eligibility. Kentucky’s proposal, approved in November 2018, requires Medicaid patients to spend at least 80 hours per month on qualified activities, including employment, job skills training, education, community services and/or participation in substance use disorder treatment.
Medicaid patients in both states sued HHS shortly after the waivers were approved, arguing that the work requirements were arbitrary and capricious and that the agency exceeded its statutory authority in approving the projects. The U.S. District Court for the District of Columbia ruled in favor of the patients in March 2019, finding that HHS failed to fully consider the impact of the Kentucky and Arkansas changes on current and future Medicaid beneficiaries. In a decision for Kentucky and a separate ruling for Arkansas, the court vacated HHS’ approval of the projects and remanded both waivers back to HHS for reconsideration. In the interim, officials in both Kentucky and Arkansas halted implementation of the work requirements. The Department of Justice appealed in both cases.
According to court documents, 18,000 Arkansans lost coverage for failure to comply with the work requirements before the regulations were halted. In Kentucky, the state estimates that 95,000 Kentuckians could lose coverage if the project goes into effect.
A decision by the appeals court is expected by December 2019.
The debate over whether states can impose work requirements on Medicaid recipients is now in the hands of a federal appeals court.
The U.S. Court of Appeals for the District of Columbia heard oral arguments Oct. 11, 2019, in two cases that challenge state waivers that require work as part of Medicaid eligibility.
In Stewart v. Azar, 16 patients from Kentucky are suing the Department of Health & Human Services over its approval of changes to Kentucky’s Medicaid program that include work requirements, premiums, and lockouts. In Gresham v. Azar, several Arkansas residents are challenging HHS over the approval of modifications to Arkansas’ Medicaid program that require work requirements and eliminate retroactive coverage.
The restrictive conditions in the Medicaid waivers would cause thousands of Medicaid enrollees to lose coverage, according to Jane Perkins, legal director for the National Health Law Program, an advocacy firm representing the plaintiffs.
“Section 1115 of the Social Security Act only allows the [HHS] Secretary to approve experimental projects that further Medicaid’s purpose of furnishing medical assistance to low-income people,” Ms. Perkins said in a statement. “These waiver projects do not further this objective. By the government’s own framing, they are intended to transform Medicaid and explode Medicaid expansion. Only Congress can rewrite a statute – not this administration. We hope the appellate court will uphold the well-reasoned opinions of the district court.”
HHS argues that it has the authority to allow any experimental, pilot, or demonstration project likely to promote the objectives of Medicaid, which in addition to medical assistance include rehabilitation services that help patients attain or retain independence or self-care. The waivers from Kentucky and Arkansas are consistent with these objectives, attorneys for HHS argued in court documents.
Kentucky’s waiver project promotes beneficiary health and financial independence, while Arkansas’ demonstration is likely to assist in improving health outcomes through strategies that promote community engagement and address health determinants, according to letters from the Centers for Medicare & Medicaid Service approving the projects.
Arkansas’ demonstration project, approved in March 2018, includes a requirement that adults aged 19-49 years complete 80 hours per month of community engagement activities, such as employment, education, job-skills training, or community service, as a condition of continued Medicaid eligibility. Kentucky’s proposal, approved in November 2018, requires Medicaid patients to spend at least 80 hours per month on qualified activities, including employment, job skills training, education, community services and/or participation in substance use disorder treatment.
Medicaid patients in both states sued HHS shortly after the waivers were approved, arguing that the work requirements were arbitrary and capricious and that the agency exceeded its statutory authority in approving the projects. The U.S. District Court for the District of Columbia ruled in favor of the patients in March 2019, finding that HHS failed to fully consider the impact of the Kentucky and Arkansas changes on current and future Medicaid beneficiaries. In a decision for Kentucky and a separate ruling for Arkansas, the court vacated HHS’ approval of the projects and remanded both waivers back to HHS for reconsideration. In the interim, officials in both Kentucky and Arkansas halted implementation of the work requirements. The Department of Justice appealed in both cases.
According to court documents, 18,000 Arkansans lost coverage for failure to comply with the work requirements before the regulations were halted. In Kentucky, the state estimates that 95,000 Kentuckians could lose coverage if the project goes into effect.
A decision by the appeals court is expected by December 2019.
Proteinuria and Albuminuria: What’s the Difference?
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
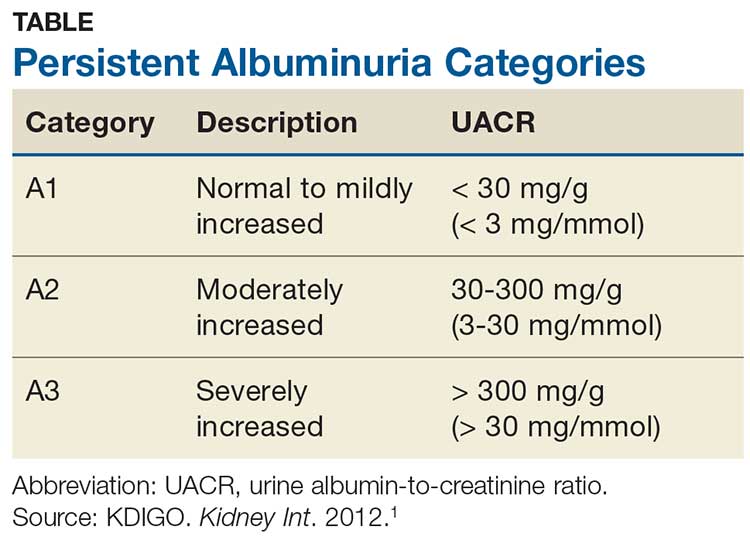
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.
While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.
While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.
While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
FDA approves rivaroxaban for VTE prevention in hospitalized, acutely ill patients
The Food and Drug Administration has approved rivaroxaban (Xarelto) for the prevention of venous thromboembolism (VTE) in hospitalized, acutely ill patients at risk for thromboembolic complications who do not have a high bleeding risk, according to a release from Janssen.
FDA approval for the new indication is based on results from the phase 3 MAGELLAN and MARINER trials, which included more than 20,000 hospitalized, acutely ill patients. In MAGELLAN, rivaroxaban demonstrated noninferiority to enoxaparin, a low-molecular-weight heparin, in short-term usage, and it was superior over the long term, compared with short-term enoxaparin followed by placebo.
While VTE and VTE-related deaths were not reduced in MARINER, compared with placebo, patients who received rivaroxaban did see a significantly reduction in symptomatic VTE with a favorable safety profile.
According to the indication, rivaroxaban can be administered to patients during hospitalization and can be continued after discharge for 31-39 days. The safety profile in MAGELLAN and MARINER was consistent with that already seen, with the most common adverse event being bleeding.
The new indication is the eighth for rivaroxaban, the most of any direct oral anticoagulant; six of these are specifically for the treatment, prevention, and reduction in the risk of VTE recurrence.
“With this new approval, Xarelto as an oral-only option now has the potential to change how acutely ill medical patients are managed for the prevention of blood clots, both in the hospital and for an extended period after discharge,” said Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital, New York, and a member of the steering committee of the MAGELLAN trial.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved rivaroxaban (Xarelto) for the prevention of venous thromboembolism (VTE) in hospitalized, acutely ill patients at risk for thromboembolic complications who do not have a high bleeding risk, according to a release from Janssen.
FDA approval for the new indication is based on results from the phase 3 MAGELLAN and MARINER trials, which included more than 20,000 hospitalized, acutely ill patients. In MAGELLAN, rivaroxaban demonstrated noninferiority to enoxaparin, a low-molecular-weight heparin, in short-term usage, and it was superior over the long term, compared with short-term enoxaparin followed by placebo.
While VTE and VTE-related deaths were not reduced in MARINER, compared with placebo, patients who received rivaroxaban did see a significantly reduction in symptomatic VTE with a favorable safety profile.
According to the indication, rivaroxaban can be administered to patients during hospitalization and can be continued after discharge for 31-39 days. The safety profile in MAGELLAN and MARINER was consistent with that already seen, with the most common adverse event being bleeding.
The new indication is the eighth for rivaroxaban, the most of any direct oral anticoagulant; six of these are specifically for the treatment, prevention, and reduction in the risk of VTE recurrence.
“With this new approval, Xarelto as an oral-only option now has the potential to change how acutely ill medical patients are managed for the prevention of blood clots, both in the hospital and for an extended period after discharge,” said Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital, New York, and a member of the steering committee of the MAGELLAN trial.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved rivaroxaban (Xarelto) for the prevention of venous thromboembolism (VTE) in hospitalized, acutely ill patients at risk for thromboembolic complications who do not have a high bleeding risk, according to a release from Janssen.
FDA approval for the new indication is based on results from the phase 3 MAGELLAN and MARINER trials, which included more than 20,000 hospitalized, acutely ill patients. In MAGELLAN, rivaroxaban demonstrated noninferiority to enoxaparin, a low-molecular-weight heparin, in short-term usage, and it was superior over the long term, compared with short-term enoxaparin followed by placebo.
While VTE and VTE-related deaths were not reduced in MARINER, compared with placebo, patients who received rivaroxaban did see a significantly reduction in symptomatic VTE with a favorable safety profile.
According to the indication, rivaroxaban can be administered to patients during hospitalization and can be continued after discharge for 31-39 days. The safety profile in MAGELLAN and MARINER was consistent with that already seen, with the most common adverse event being bleeding.
The new indication is the eighth for rivaroxaban, the most of any direct oral anticoagulant; six of these are specifically for the treatment, prevention, and reduction in the risk of VTE recurrence.
“With this new approval, Xarelto as an oral-only option now has the potential to change how acutely ill medical patients are managed for the prevention of blood clots, both in the hospital and for an extended period after discharge,” said Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital, New York, and a member of the steering committee of the MAGELLAN trial.
Find the full press release on the Janssen website.
#MyFirstNameIsDoctor: Why it matters, and what you can do
When Shawnté James, MD, picked up the phone at work recently, a male physician on the other end was calling for a peer-to-peer review of a patient’s insurance issue.
“Hi, this is Dr. Y, calling to speak with Shawnté about patient X. Is she available?” asked the physician. “No,” replied Dr. James, an assistant professor of pediatrics at MedStar Georgetown University Hospital, Washington.
She related the rest of the interaction in a recent tweet:
“I’m Dr. XY calling a peer-to-peer review of a denial. Is Shawnté available?”
— Shawnté James (@ShawnteJamesMD) October 10, 2019
Me: “No.”
Him: “Is she in today?”
Me: “There’s no Shawnté here.”
Him: “Oh this is the number I have for Dr. Shawnté James”
Me: “Oh, DR. JAMES. Yes, that’s me. How can I help?”#MyFirstNameIsDoctor
The tweet, along with many others that used the hashtag #MyFirstNameIsDoctor, struck a chord among female physicians on Twitter. In tweets of their own, they related instance after instance of peers, coworkers, and patients assuming first-name familiarity with them – but not their male colleagues.
“This time it’s a peer-to-peer review. Last time it was being introduced to new hospital leadership as, ‘Shawnté, one of our pediatricians,’ ” Dr. James said in an interview. “The truth is, for physician women – particularly women of color – this is a regular occurrence.”
Data show an ongoing problem
Objective evidence that female physicians and scientists are significantly less likely than their male peers to be addressed by their titles came in a just-published study of presentations at the annual meeting of the American Society for Clinical Oncology in 2017 and 2018.
Narjust Duma, MD, the study’s first author, described her growing awareness of the problem.
Dr. Duma recalled a session on the last day of the ASCO 2018 meeting. Five presenters were speaking – four men and a woman. “The woman is the one who knows the most about this subject. She’s the only one at the table who’s a full professor,” Dr. Duma, assistant professor of hematology/oncology at the University of Wisconsin–Madison, said in an interview. “And then everybody is introduced as ‘Dr. So-and-so,’ and when they come to her, they introduce her as ‘Julie.’ ”
“Is it just me?” Dr. Duma asked herself. The same day, she began a Twitter poll to ask whether her female peers were experiencing this phenomenon, and got an “overwhelming” response.
“We need data to learn the extent of the problem,” she said she realized.
The ASCO annual meeting afforded an ideal opportunity for data gathering, said Dr. Duma, because presentations are recorded and written transcripts generated. Dr. Duma assembled a research team that had a 50-50 gender balance and racial and ethnic diversity. The team combed ASCO transcripts to code introductions according to whether title and surname were used or whether speakers were addressed by first name only.
After excluding videos that did not capture speaker introductions, Dr. Duma and collaborators were left with 781 videos to watch and code.
Female speakers overall were less likely to be addressed by their professional title (62% vs. 81% for males, P less than .001). Male introducers used professional titles 53% of the time when introducing female speakers, and 80% of the time when introducing male speakers (P less than .01). No gender differences were seen when females were the introducers (J Clin Oncol. 2019 Oct 11. doi: 10.1200/JCO.19.01608).
Looking further, male introducers addressed female speakers by first name only in 24% of the cases. Female introducers used first names only with female speakers 7% of the time, a statistically significant difference. “This is the part that is really sad,” said Dr. Duma.
She and her coauthors also performed multivariable analysis to adjust for factors such as seniority and geographic location; after adjustment, males were still over 2.5 times as likely as females to be introduced with their professional title, and females were nearly six times as likely as males to be introduced by their first names only. When the introducer was male, a female speaker was over three times more likely to be introduced by her first name only.
Dr. Duma and colleagues are working with the ASCO 2020 planning team to develop a template that standardizes presenter introductions. They’re also planning for prospective data collection at that meeting, and will include self-reported race and ethnicity data for presenters and introducers who choose to provide it.
“We do not plan to create a ‘her versus him’ battle,” said Dr. Duma. “The goal is to use this hardcore data to bring attention to the problem.” She pointed out that, though fewer females introduced other females by first name only, the problem wasn’t limited only to male introducers at ASCO.
“The problem is unconscious bias. Nobody’s exempt,” said Dr. Duma. She related that she herself had just sent a work-related email to a female colleague that addressed her by her first name, and had copied many of their mutual colleagues. Realizing her gaffe, she held herself to her own standard by apologizing to her colleague and copying everyone who saw the first email. “The goal is to bring attention to the difference, so we can improve gender bias in medicine together.”
Patient interactions: Sometimes, a delicate balance
What’s the right approach when a patient, uninvited, addresses you by your first name? Natalie Strand, MD, had been thinking about the best way to handle this sticky situation for some time. Recently, she tried it out on a patient and shared her approach in a tweet:
So proud of myself!
— Natalie Strand (@DrNatStrand) October 11, 2019
After introducing myself as Dr. Strand to a patient, he looked at my name badge and said- oh, so Natalie.
Usually I’m stuck feeling afraid to rock the boat...
Not today!
“Yes, but I go by Dr. Strand at work! “
I finally said it!!!
There was an awkward moment with the patient, Dr. Strand acknowledged, “but we moved past it.”
Asserting one’s hard-earned status despite a societally ingrained desire to please or to avoid confrontation can be difficult, she acknowledged, but it’s worth it. Put simply, she said, “I want to be called Dr. Strand.”
The importance of this issue can sometimes be hard for male colleagues to understand, said Dr. Strand, who practices outpatient interventional pain medicine at the Mayo Clinic, Scottsdale, Ariz. “The people that have privilege – they don’t see it as privilege. And that’s not anybody’s fault. That’s just the reality of it, because that’s the norm. … That’s why putting a name to microaggression and microinsults is so powerful, because once you name it, then you can respond to it.”
Beginning from a point of mutual professionalism is a good place to start, Dr. Strand said in an interview. She always begins by addressing her patients by their surname and waits for patients to invite her to call them by their first names. “The most professional approach is the best first step,” she said. When she has a longstanding relationship with patients and she knows that trust and mutual respect have been established, she may also invite first-name familiarity.
“Patients don’t do this to be mean,” emphasized Dr. Strand, adding that, particularly with older patients, “they are trying to be sweet.” That’s part of the difficulty in finding a gentle but firm way to bring the relationship back to a professional footing.
Judging by the responses she’s gotten from other female physicians, this delicate situation, and the best way to ask for professionalism with patients, is a common struggle. Many of her female peers have said they’ll consider adopting her approach, she said.
“Male physicians are our allies,” said Dr. Strand. “The needs of the patients come first. This isn’t about power; it’s not about holding a power differential against the patient. It’s about having a culture of mutual respect, and being seen as a physician. Not as a female physician, not as a male physician. Just being seen as a physician, so you can act as a physician.”
Whether they come from patients or peers, said Dr. James, who adroitly called out the physician reviewer who asked for her by first name, “These microaggressions are uncomfortable to address at the time they occur – but they are teachable moments that we should all take advantage of. Usually, a gentle correction, such as, ‘I prefer to be addressed as Dr. James while at work,’ is sufficient.” However, she added, “sometimes, a firmer ‘I feel disrespected when you address me by my first name to colleagues and patients’ is needed.”
This article was updated 10/15/19.
When Shawnté James, MD, picked up the phone at work recently, a male physician on the other end was calling for a peer-to-peer review of a patient’s insurance issue.
“Hi, this is Dr. Y, calling to speak with Shawnté about patient X. Is she available?” asked the physician. “No,” replied Dr. James, an assistant professor of pediatrics at MedStar Georgetown University Hospital, Washington.
She related the rest of the interaction in a recent tweet:
“I’m Dr. XY calling a peer-to-peer review of a denial. Is Shawnté available?”
— Shawnté James (@ShawnteJamesMD) October 10, 2019
Me: “No.”
Him: “Is she in today?”
Me: “There’s no Shawnté here.”
Him: “Oh this is the number I have for Dr. Shawnté James”
Me: “Oh, DR. JAMES. Yes, that’s me. How can I help?”#MyFirstNameIsDoctor
The tweet, along with many others that used the hashtag #MyFirstNameIsDoctor, struck a chord among female physicians on Twitter. In tweets of their own, they related instance after instance of peers, coworkers, and patients assuming first-name familiarity with them – but not their male colleagues.
“This time it’s a peer-to-peer review. Last time it was being introduced to new hospital leadership as, ‘Shawnté, one of our pediatricians,’ ” Dr. James said in an interview. “The truth is, for physician women – particularly women of color – this is a regular occurrence.”
Data show an ongoing problem
Objective evidence that female physicians and scientists are significantly less likely than their male peers to be addressed by their titles came in a just-published study of presentations at the annual meeting of the American Society for Clinical Oncology in 2017 and 2018.
Narjust Duma, MD, the study’s first author, described her growing awareness of the problem.
Dr. Duma recalled a session on the last day of the ASCO 2018 meeting. Five presenters were speaking – four men and a woman. “The woman is the one who knows the most about this subject. She’s the only one at the table who’s a full professor,” Dr. Duma, assistant professor of hematology/oncology at the University of Wisconsin–Madison, said in an interview. “And then everybody is introduced as ‘Dr. So-and-so,’ and when they come to her, they introduce her as ‘Julie.’ ”
“Is it just me?” Dr. Duma asked herself. The same day, she began a Twitter poll to ask whether her female peers were experiencing this phenomenon, and got an “overwhelming” response.
“We need data to learn the extent of the problem,” she said she realized.
The ASCO annual meeting afforded an ideal opportunity for data gathering, said Dr. Duma, because presentations are recorded and written transcripts generated. Dr. Duma assembled a research team that had a 50-50 gender balance and racial and ethnic diversity. The team combed ASCO transcripts to code introductions according to whether title and surname were used or whether speakers were addressed by first name only.
After excluding videos that did not capture speaker introductions, Dr. Duma and collaborators were left with 781 videos to watch and code.
Female speakers overall were less likely to be addressed by their professional title (62% vs. 81% for males, P less than .001). Male introducers used professional titles 53% of the time when introducing female speakers, and 80% of the time when introducing male speakers (P less than .01). No gender differences were seen when females were the introducers (J Clin Oncol. 2019 Oct 11. doi: 10.1200/JCO.19.01608).
Looking further, male introducers addressed female speakers by first name only in 24% of the cases. Female introducers used first names only with female speakers 7% of the time, a statistically significant difference. “This is the part that is really sad,” said Dr. Duma.
She and her coauthors also performed multivariable analysis to adjust for factors such as seniority and geographic location; after adjustment, males were still over 2.5 times as likely as females to be introduced with their professional title, and females were nearly six times as likely as males to be introduced by their first names only. When the introducer was male, a female speaker was over three times more likely to be introduced by her first name only.
Dr. Duma and colleagues are working with the ASCO 2020 planning team to develop a template that standardizes presenter introductions. They’re also planning for prospective data collection at that meeting, and will include self-reported race and ethnicity data for presenters and introducers who choose to provide it.
“We do not plan to create a ‘her versus him’ battle,” said Dr. Duma. “The goal is to use this hardcore data to bring attention to the problem.” She pointed out that, though fewer females introduced other females by first name only, the problem wasn’t limited only to male introducers at ASCO.
“The problem is unconscious bias. Nobody’s exempt,” said Dr. Duma. She related that she herself had just sent a work-related email to a female colleague that addressed her by her first name, and had copied many of their mutual colleagues. Realizing her gaffe, she held herself to her own standard by apologizing to her colleague and copying everyone who saw the first email. “The goal is to bring attention to the difference, so we can improve gender bias in medicine together.”
Patient interactions: Sometimes, a delicate balance
What’s the right approach when a patient, uninvited, addresses you by your first name? Natalie Strand, MD, had been thinking about the best way to handle this sticky situation for some time. Recently, she tried it out on a patient and shared her approach in a tweet:
So proud of myself!
— Natalie Strand (@DrNatStrand) October 11, 2019
After introducing myself as Dr. Strand to a patient, he looked at my name badge and said- oh, so Natalie.
Usually I’m stuck feeling afraid to rock the boat...
Not today!
“Yes, but I go by Dr. Strand at work! “
I finally said it!!!
There was an awkward moment with the patient, Dr. Strand acknowledged, “but we moved past it.”
Asserting one’s hard-earned status despite a societally ingrained desire to please or to avoid confrontation can be difficult, she acknowledged, but it’s worth it. Put simply, she said, “I want to be called Dr. Strand.”
The importance of this issue can sometimes be hard for male colleagues to understand, said Dr. Strand, who practices outpatient interventional pain medicine at the Mayo Clinic, Scottsdale, Ariz. “The people that have privilege – they don’t see it as privilege. And that’s not anybody’s fault. That’s just the reality of it, because that’s the norm. … That’s why putting a name to microaggression and microinsults is so powerful, because once you name it, then you can respond to it.”
Beginning from a point of mutual professionalism is a good place to start, Dr. Strand said in an interview. She always begins by addressing her patients by their surname and waits for patients to invite her to call them by their first names. “The most professional approach is the best first step,” she said. When she has a longstanding relationship with patients and she knows that trust and mutual respect have been established, she may also invite first-name familiarity.
“Patients don’t do this to be mean,” emphasized Dr. Strand, adding that, particularly with older patients, “they are trying to be sweet.” That’s part of the difficulty in finding a gentle but firm way to bring the relationship back to a professional footing.
Judging by the responses she’s gotten from other female physicians, this delicate situation, and the best way to ask for professionalism with patients, is a common struggle. Many of her female peers have said they’ll consider adopting her approach, she said.
“Male physicians are our allies,” said Dr. Strand. “The needs of the patients come first. This isn’t about power; it’s not about holding a power differential against the patient. It’s about having a culture of mutual respect, and being seen as a physician. Not as a female physician, not as a male physician. Just being seen as a physician, so you can act as a physician.”
Whether they come from patients or peers, said Dr. James, who adroitly called out the physician reviewer who asked for her by first name, “These microaggressions are uncomfortable to address at the time they occur – but they are teachable moments that we should all take advantage of. Usually, a gentle correction, such as, ‘I prefer to be addressed as Dr. James while at work,’ is sufficient.” However, she added, “sometimes, a firmer ‘I feel disrespected when you address me by my first name to colleagues and patients’ is needed.”
This article was updated 10/15/19.
When Shawnté James, MD, picked up the phone at work recently, a male physician on the other end was calling for a peer-to-peer review of a patient’s insurance issue.
“Hi, this is Dr. Y, calling to speak with Shawnté about patient X. Is she available?” asked the physician. “No,” replied Dr. James, an assistant professor of pediatrics at MedStar Georgetown University Hospital, Washington.
She related the rest of the interaction in a recent tweet:
“I’m Dr. XY calling a peer-to-peer review of a denial. Is Shawnté available?”
— Shawnté James (@ShawnteJamesMD) October 10, 2019
Me: “No.”
Him: “Is she in today?”
Me: “There’s no Shawnté here.”
Him: “Oh this is the number I have for Dr. Shawnté James”
Me: “Oh, DR. JAMES. Yes, that’s me. How can I help?”#MyFirstNameIsDoctor
The tweet, along with many others that used the hashtag #MyFirstNameIsDoctor, struck a chord among female physicians on Twitter. In tweets of their own, they related instance after instance of peers, coworkers, and patients assuming first-name familiarity with them – but not their male colleagues.
“This time it’s a peer-to-peer review. Last time it was being introduced to new hospital leadership as, ‘Shawnté, one of our pediatricians,’ ” Dr. James said in an interview. “The truth is, for physician women – particularly women of color – this is a regular occurrence.”
Data show an ongoing problem
Objective evidence that female physicians and scientists are significantly less likely than their male peers to be addressed by their titles came in a just-published study of presentations at the annual meeting of the American Society for Clinical Oncology in 2017 and 2018.
Narjust Duma, MD, the study’s first author, described her growing awareness of the problem.
Dr. Duma recalled a session on the last day of the ASCO 2018 meeting. Five presenters were speaking – four men and a woman. “The woman is the one who knows the most about this subject. She’s the only one at the table who’s a full professor,” Dr. Duma, assistant professor of hematology/oncology at the University of Wisconsin–Madison, said in an interview. “And then everybody is introduced as ‘Dr. So-and-so,’ and when they come to her, they introduce her as ‘Julie.’ ”
“Is it just me?” Dr. Duma asked herself. The same day, she began a Twitter poll to ask whether her female peers were experiencing this phenomenon, and got an “overwhelming” response.
“We need data to learn the extent of the problem,” she said she realized.
The ASCO annual meeting afforded an ideal opportunity for data gathering, said Dr. Duma, because presentations are recorded and written transcripts generated. Dr. Duma assembled a research team that had a 50-50 gender balance and racial and ethnic diversity. The team combed ASCO transcripts to code introductions according to whether title and surname were used or whether speakers were addressed by first name only.
After excluding videos that did not capture speaker introductions, Dr. Duma and collaborators were left with 781 videos to watch and code.
Female speakers overall were less likely to be addressed by their professional title (62% vs. 81% for males, P less than .001). Male introducers used professional titles 53% of the time when introducing female speakers, and 80% of the time when introducing male speakers (P less than .01). No gender differences were seen when females were the introducers (J Clin Oncol. 2019 Oct 11. doi: 10.1200/JCO.19.01608).
Looking further, male introducers addressed female speakers by first name only in 24% of the cases. Female introducers used first names only with female speakers 7% of the time, a statistically significant difference. “This is the part that is really sad,” said Dr. Duma.
She and her coauthors also performed multivariable analysis to adjust for factors such as seniority and geographic location; after adjustment, males were still over 2.5 times as likely as females to be introduced with their professional title, and females were nearly six times as likely as males to be introduced by their first names only. When the introducer was male, a female speaker was over three times more likely to be introduced by her first name only.
Dr. Duma and colleagues are working with the ASCO 2020 planning team to develop a template that standardizes presenter introductions. They’re also planning for prospective data collection at that meeting, and will include self-reported race and ethnicity data for presenters and introducers who choose to provide it.
“We do not plan to create a ‘her versus him’ battle,” said Dr. Duma. “The goal is to use this hardcore data to bring attention to the problem.” She pointed out that, though fewer females introduced other females by first name only, the problem wasn’t limited only to male introducers at ASCO.
“The problem is unconscious bias. Nobody’s exempt,” said Dr. Duma. She related that she herself had just sent a work-related email to a female colleague that addressed her by her first name, and had copied many of their mutual colleagues. Realizing her gaffe, she held herself to her own standard by apologizing to her colleague and copying everyone who saw the first email. “The goal is to bring attention to the difference, so we can improve gender bias in medicine together.”
Patient interactions: Sometimes, a delicate balance
What’s the right approach when a patient, uninvited, addresses you by your first name? Natalie Strand, MD, had been thinking about the best way to handle this sticky situation for some time. Recently, she tried it out on a patient and shared her approach in a tweet:
So proud of myself!
— Natalie Strand (@DrNatStrand) October 11, 2019
After introducing myself as Dr. Strand to a patient, he looked at my name badge and said- oh, so Natalie.
Usually I’m stuck feeling afraid to rock the boat...
Not today!
“Yes, but I go by Dr. Strand at work! “
I finally said it!!!
There was an awkward moment with the patient, Dr. Strand acknowledged, “but we moved past it.”
Asserting one’s hard-earned status despite a societally ingrained desire to please or to avoid confrontation can be difficult, she acknowledged, but it’s worth it. Put simply, she said, “I want to be called Dr. Strand.”
The importance of this issue can sometimes be hard for male colleagues to understand, said Dr. Strand, who practices outpatient interventional pain medicine at the Mayo Clinic, Scottsdale, Ariz. “The people that have privilege – they don’t see it as privilege. And that’s not anybody’s fault. That’s just the reality of it, because that’s the norm. … That’s why putting a name to microaggression and microinsults is so powerful, because once you name it, then you can respond to it.”
Beginning from a point of mutual professionalism is a good place to start, Dr. Strand said in an interview. She always begins by addressing her patients by their surname and waits for patients to invite her to call them by their first names. “The most professional approach is the best first step,” she said. When she has a longstanding relationship with patients and she knows that trust and mutual respect have been established, she may also invite first-name familiarity.
“Patients don’t do this to be mean,” emphasized Dr. Strand, adding that, particularly with older patients, “they are trying to be sweet.” That’s part of the difficulty in finding a gentle but firm way to bring the relationship back to a professional footing.
Judging by the responses she’s gotten from other female physicians, this delicate situation, and the best way to ask for professionalism with patients, is a common struggle. Many of her female peers have said they’ll consider adopting her approach, she said.
“Male physicians are our allies,” said Dr. Strand. “The needs of the patients come first. This isn’t about power; it’s not about holding a power differential against the patient. It’s about having a culture of mutual respect, and being seen as a physician. Not as a female physician, not as a male physician. Just being seen as a physician, so you can act as a physician.”
Whether they come from patients or peers, said Dr. James, who adroitly called out the physician reviewer who asked for her by first name, “These microaggressions are uncomfortable to address at the time they occur – but they are teachable moments that we should all take advantage of. Usually, a gentle correction, such as, ‘I prefer to be addressed as Dr. James while at work,’ is sufficient.” However, she added, “sometimes, a firmer ‘I feel disrespected when you address me by my first name to colleagues and patients’ is needed.”
This article was updated 10/15/19.
In patients with CIS, combined omics predicts conversion to clinically definite MS
STOCKHOLM –
Further, “combined omics improves diagnostic accuracy” over oligoclonal band (OCB) status alone in differentiating patients with multiple sclerosis (MS) from a group of normal control patients, said Fay Probert, PhD, speaking at a poster session at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Not everyone with clinically isolated syndrome (CIS) converts to clinically definite MS, and there is large variability in the time to progression, explained Dr. Probert, a postdoctoral fellow in the department of pharmacology at Oxford (England) University, and colleagues. Though the revised McDonald criteria now allow earlier diagnosis of MS, individuals who will be early converters cannot be identified by these criteria, they noted, citing earlier work showing that just over half (52%) of CIS patients who are OCB positive will have clinically definite MS at the 3-year mark.
To see whether combining analysis of multiple proteins and metabolites improved diagnostic accuracy, Dr. Probert and colleagues examined cerebrospinal fluid (CSF) samples from 41 patients with clinically definite MS, 71 patients with CIS, and 64 control participants without MS. In their analysis, the investigators used nuclear MR metabolomics and a commercially available proteomics assay that identifies and quantifies more than 5,000 proteins.
The multivariate analysis strategy achieved 10-fold external cross-validation of the samples, repeating training and testing of the analysis model while shuffling data. This, explained Dr. Probert and colleagues, “ensures that any discrimination observed cannot have occurred by chance.” Further analysis “identifies the optimal combination of proteomics and metabolomics features which results in the highest diagnostic accuracy.”
Both the nuclear MR metabolomics and the proteomic analyses were able to discriminate between those with clinically definite MS and the control participants, with accuracy of 71% and 75%, respectively.
The levels of seven metabolites present in CSF were predictive of clinically definite MS, compared with non-MS status, independent of OCB status. In fact, noted Dr. Probert and colleagues, “the CSF myoinositol concentration alone diagnosed [clinically definite] MS in this cohort with a specificity of 74% but did not outperform OCB status overall.”
Using the combined omics approach, though, “significantly improved the discrimination” between the non-MS control CSF samples and those of patients with clinically definite MS, wrote Dr. Probert and colleagues. Using a combination of up to five CSF proteins and metabolites yielded accuracy of 85 plus or minus 2%, sensitivity of 85 plus or minus 3%, and specificity of 85 plus or minus 3%. For comparison, using just OCB status provides accuracy of 74%, sensitivity of 88% and specificity of 63%.
Then, Dr. Probert and colleagues turned to the CSF samples from patients with CIS to look for predictors of “fast” (4 years or less) or “slow” (greater than 4 years) conversion to clinically definite MS. “While important for diagnosis, OCB status was not predictive of early conversion,” the investigators noted. However, baseline CSF proteomics analysis alone did differentiate the fast from the slow converters among the CIS subgroup, with an accuracy of 77%.
For patients with CIS who were OCB positive, their baseline metabolite and proteomic profiles were “indistinguishable” from those with clinically definite MS, wrote Dr. Probert and colleagues. The omics analysis was also able to distinguish between OCB-positive CIS patients and the non-MS control patients.
“These results indicate that combined metabolomics and proteomics analysis could not only be used as an adjunct in diagnosis of [clinically definite] MS but could be used as a prognostic test to identify CIS patients at high risk of a second clinical attack within 4 years of onset,” wrote Dr. Probert and coauthors. They noted that the method reported in the poster is the first to offer this prognostic accuracy, but that more work is needed before routine clinical use.
Dr. Probert reported that she had no financial conflicts of interest. One coauthor reported being a consultant to Novartis. Two coauthors reported financial relationships with multiple pharmaceutical companies, including Merck, which partially funded the study. Numares Health, the U.K. Medical Research Council, and the Multiple Sclerosis Society also provided funding support.
SOURCE: Probert F et al. ECTRIMS 2019, Abstract P586.
STOCKHOLM –
Further, “combined omics improves diagnostic accuracy” over oligoclonal band (OCB) status alone in differentiating patients with multiple sclerosis (MS) from a group of normal control patients, said Fay Probert, PhD, speaking at a poster session at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Not everyone with clinically isolated syndrome (CIS) converts to clinically definite MS, and there is large variability in the time to progression, explained Dr. Probert, a postdoctoral fellow in the department of pharmacology at Oxford (England) University, and colleagues. Though the revised McDonald criteria now allow earlier diagnosis of MS, individuals who will be early converters cannot be identified by these criteria, they noted, citing earlier work showing that just over half (52%) of CIS patients who are OCB positive will have clinically definite MS at the 3-year mark.
To see whether combining analysis of multiple proteins and metabolites improved diagnostic accuracy, Dr. Probert and colleagues examined cerebrospinal fluid (CSF) samples from 41 patients with clinically definite MS, 71 patients with CIS, and 64 control participants without MS. In their analysis, the investigators used nuclear MR metabolomics and a commercially available proteomics assay that identifies and quantifies more than 5,000 proteins.
The multivariate analysis strategy achieved 10-fold external cross-validation of the samples, repeating training and testing of the analysis model while shuffling data. This, explained Dr. Probert and colleagues, “ensures that any discrimination observed cannot have occurred by chance.” Further analysis “identifies the optimal combination of proteomics and metabolomics features which results in the highest diagnostic accuracy.”
Both the nuclear MR metabolomics and the proteomic analyses were able to discriminate between those with clinically definite MS and the control participants, with accuracy of 71% and 75%, respectively.
The levels of seven metabolites present in CSF were predictive of clinically definite MS, compared with non-MS status, independent of OCB status. In fact, noted Dr. Probert and colleagues, “the CSF myoinositol concentration alone diagnosed [clinically definite] MS in this cohort with a specificity of 74% but did not outperform OCB status overall.”
Using the combined omics approach, though, “significantly improved the discrimination” between the non-MS control CSF samples and those of patients with clinically definite MS, wrote Dr. Probert and colleagues. Using a combination of up to five CSF proteins and metabolites yielded accuracy of 85 plus or minus 2%, sensitivity of 85 plus or minus 3%, and specificity of 85 plus or minus 3%. For comparison, using just OCB status provides accuracy of 74%, sensitivity of 88% and specificity of 63%.
Then, Dr. Probert and colleagues turned to the CSF samples from patients with CIS to look for predictors of “fast” (4 years or less) or “slow” (greater than 4 years) conversion to clinically definite MS. “While important for diagnosis, OCB status was not predictive of early conversion,” the investigators noted. However, baseline CSF proteomics analysis alone did differentiate the fast from the slow converters among the CIS subgroup, with an accuracy of 77%.
For patients with CIS who were OCB positive, their baseline metabolite and proteomic profiles were “indistinguishable” from those with clinically definite MS, wrote Dr. Probert and colleagues. The omics analysis was also able to distinguish between OCB-positive CIS patients and the non-MS control patients.
“These results indicate that combined metabolomics and proteomics analysis could not only be used as an adjunct in diagnosis of [clinically definite] MS but could be used as a prognostic test to identify CIS patients at high risk of a second clinical attack within 4 years of onset,” wrote Dr. Probert and coauthors. They noted that the method reported in the poster is the first to offer this prognostic accuracy, but that more work is needed before routine clinical use.
Dr. Probert reported that she had no financial conflicts of interest. One coauthor reported being a consultant to Novartis. Two coauthors reported financial relationships with multiple pharmaceutical companies, including Merck, which partially funded the study. Numares Health, the U.K. Medical Research Council, and the Multiple Sclerosis Society also provided funding support.
SOURCE: Probert F et al. ECTRIMS 2019, Abstract P586.
STOCKHOLM –
Further, “combined omics improves diagnostic accuracy” over oligoclonal band (OCB) status alone in differentiating patients with multiple sclerosis (MS) from a group of normal control patients, said Fay Probert, PhD, speaking at a poster session at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Not everyone with clinically isolated syndrome (CIS) converts to clinically definite MS, and there is large variability in the time to progression, explained Dr. Probert, a postdoctoral fellow in the department of pharmacology at Oxford (England) University, and colleagues. Though the revised McDonald criteria now allow earlier diagnosis of MS, individuals who will be early converters cannot be identified by these criteria, they noted, citing earlier work showing that just over half (52%) of CIS patients who are OCB positive will have clinically definite MS at the 3-year mark.
To see whether combining analysis of multiple proteins and metabolites improved diagnostic accuracy, Dr. Probert and colleagues examined cerebrospinal fluid (CSF) samples from 41 patients with clinically definite MS, 71 patients with CIS, and 64 control participants without MS. In their analysis, the investigators used nuclear MR metabolomics and a commercially available proteomics assay that identifies and quantifies more than 5,000 proteins.
The multivariate analysis strategy achieved 10-fold external cross-validation of the samples, repeating training and testing of the analysis model while shuffling data. This, explained Dr. Probert and colleagues, “ensures that any discrimination observed cannot have occurred by chance.” Further analysis “identifies the optimal combination of proteomics and metabolomics features which results in the highest diagnostic accuracy.”
Both the nuclear MR metabolomics and the proteomic analyses were able to discriminate between those with clinically definite MS and the control participants, with accuracy of 71% and 75%, respectively.
The levels of seven metabolites present in CSF were predictive of clinically definite MS, compared with non-MS status, independent of OCB status. In fact, noted Dr. Probert and colleagues, “the CSF myoinositol concentration alone diagnosed [clinically definite] MS in this cohort with a specificity of 74% but did not outperform OCB status overall.”
Using the combined omics approach, though, “significantly improved the discrimination” between the non-MS control CSF samples and those of patients with clinically definite MS, wrote Dr. Probert and colleagues. Using a combination of up to five CSF proteins and metabolites yielded accuracy of 85 plus or minus 2%, sensitivity of 85 plus or minus 3%, and specificity of 85 plus or minus 3%. For comparison, using just OCB status provides accuracy of 74%, sensitivity of 88% and specificity of 63%.
Then, Dr. Probert and colleagues turned to the CSF samples from patients with CIS to look for predictors of “fast” (4 years or less) or “slow” (greater than 4 years) conversion to clinically definite MS. “While important for diagnosis, OCB status was not predictive of early conversion,” the investigators noted. However, baseline CSF proteomics analysis alone did differentiate the fast from the slow converters among the CIS subgroup, with an accuracy of 77%.
For patients with CIS who were OCB positive, their baseline metabolite and proteomic profiles were “indistinguishable” from those with clinically definite MS, wrote Dr. Probert and colleagues. The omics analysis was also able to distinguish between OCB-positive CIS patients and the non-MS control patients.
“These results indicate that combined metabolomics and proteomics analysis could not only be used as an adjunct in diagnosis of [clinically definite] MS but could be used as a prognostic test to identify CIS patients at high risk of a second clinical attack within 4 years of onset,” wrote Dr. Probert and coauthors. They noted that the method reported in the poster is the first to offer this prognostic accuracy, but that more work is needed before routine clinical use.
Dr. Probert reported that she had no financial conflicts of interest. One coauthor reported being a consultant to Novartis. Two coauthors reported financial relationships with multiple pharmaceutical companies, including Merck, which partially funded the study. Numares Health, the U.K. Medical Research Council, and the Multiple Sclerosis Society also provided funding support.
SOURCE: Probert F et al. ECTRIMS 2019, Abstract P586.
REPORTING FROM ECTRIMS 2019
Holy basil: A member of the Ocimum family
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.