User login
Dupilumab may reduce dysphagia in adults with eosinophilic esophagitis
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
FROM GASTROENTEROLOGY
Key clinical point: Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis.
Major finding: Change in the Straumann Dysphagia Instrument (SDI) score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab (least squares mean change of –3.0 from baseline, versus –1.3 for placebo; P = .0304).
Study details: A phase 2 trial including 47 adults with EoE randomized to dupilumab or placebo.
Disclosures: The research was sponsored by Sanofi and Regeneron Pharmaceuticals. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Regeneron, Adare, Allakos, Receptos/Celgene, Meritage, Shire, Alivio, Banner, Calypso, Enumeral, EsoCap, Glax-oSmithKline, and Robarts, among others.
Source: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
Prior maternal gastric bypass surgery tied to fewer birth defects
according to data from a cohort study of 2,921 women with a history of gastric bypass surgery and 30,573 matched controls.
“Obesity is associated with poor glucose control, which is teratogenic. Bariatric surgery results in weight loss and glucose normalization but is also associated with nutritional deficiencies and substance abuse, which could cause birth defects as hypothesized based on case series,” wrote Martin Neovius, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
To determine the risk of birth defects for infants born to women after gastric bypass surgery, the researchers used the Swedish Medical Birth Register to identify singleton infants born between 2007 and 2014 to women who underwent Roux-en-Y gastric bypass surgery and matched controls. The findings were published in a research letter in JAMA.
In the surgery group, the mean interval from surgery to conception was 1.6 years, and the mean weight loss was 40 kg for these women. In addition, the use of diabetes drugs decreased from 10% before surgery to 2% during the 6 months before conception.
Overall, major birth defects occurred in 3% of infants in the gastric bypass groups versus 5% of infants in the control group (risk ratio, 0.67). No neural tube defects occurred in the surgery group and 20 cases of neural tube defects were noted in the control group.
The study was limited by several factors including the lack of data on pregnancy termination, exclusion of stillbirths, and inability to analyze individual birth defects because of small numbers, the researchers noted.
Nonetheless, the results suggest that “a mechanism could be that surgery-induced improvements in glucose metabolism, and potentially other beneficial physiologic changes, led to a reduction of major birth defect risk to a level similar to that of the general population,” they said.
Dr. Neovius disclosed advisory board fees from Itrim and Ethicon Johnson & Johnson. Three coauthors reported grants or other fees from a variety of pharmaceutical companies. The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, by the Swedish Research Council, and by the Swedish Research Council for Health, Working Life, and Welfare.
SOURCE: Neovius M et al. JAMA. 2019 Oct 15; 322:1515-17.
according to data from a cohort study of 2,921 women with a history of gastric bypass surgery and 30,573 matched controls.
“Obesity is associated with poor glucose control, which is teratogenic. Bariatric surgery results in weight loss and glucose normalization but is also associated with nutritional deficiencies and substance abuse, which could cause birth defects as hypothesized based on case series,” wrote Martin Neovius, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
To determine the risk of birth defects for infants born to women after gastric bypass surgery, the researchers used the Swedish Medical Birth Register to identify singleton infants born between 2007 and 2014 to women who underwent Roux-en-Y gastric bypass surgery and matched controls. The findings were published in a research letter in JAMA.
In the surgery group, the mean interval from surgery to conception was 1.6 years, and the mean weight loss was 40 kg for these women. In addition, the use of diabetes drugs decreased from 10% before surgery to 2% during the 6 months before conception.
Overall, major birth defects occurred in 3% of infants in the gastric bypass groups versus 5% of infants in the control group (risk ratio, 0.67). No neural tube defects occurred in the surgery group and 20 cases of neural tube defects were noted in the control group.
The study was limited by several factors including the lack of data on pregnancy termination, exclusion of stillbirths, and inability to analyze individual birth defects because of small numbers, the researchers noted.
Nonetheless, the results suggest that “a mechanism could be that surgery-induced improvements in glucose metabolism, and potentially other beneficial physiologic changes, led to a reduction of major birth defect risk to a level similar to that of the general population,” they said.
Dr. Neovius disclosed advisory board fees from Itrim and Ethicon Johnson & Johnson. Three coauthors reported grants or other fees from a variety of pharmaceutical companies. The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, by the Swedish Research Council, and by the Swedish Research Council for Health, Working Life, and Welfare.
SOURCE: Neovius M et al. JAMA. 2019 Oct 15; 322:1515-17.
according to data from a cohort study of 2,921 women with a history of gastric bypass surgery and 30,573 matched controls.
“Obesity is associated with poor glucose control, which is teratogenic. Bariatric surgery results in weight loss and glucose normalization but is also associated with nutritional deficiencies and substance abuse, which could cause birth defects as hypothesized based on case series,” wrote Martin Neovius, PhD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.
To determine the risk of birth defects for infants born to women after gastric bypass surgery, the researchers used the Swedish Medical Birth Register to identify singleton infants born between 2007 and 2014 to women who underwent Roux-en-Y gastric bypass surgery and matched controls. The findings were published in a research letter in JAMA.
In the surgery group, the mean interval from surgery to conception was 1.6 years, and the mean weight loss was 40 kg for these women. In addition, the use of diabetes drugs decreased from 10% before surgery to 2% during the 6 months before conception.
Overall, major birth defects occurred in 3% of infants in the gastric bypass groups versus 5% of infants in the control group (risk ratio, 0.67). No neural tube defects occurred in the surgery group and 20 cases of neural tube defects were noted in the control group.
The study was limited by several factors including the lack of data on pregnancy termination, exclusion of stillbirths, and inability to analyze individual birth defects because of small numbers, the researchers noted.
Nonetheless, the results suggest that “a mechanism could be that surgery-induced improvements in glucose metabolism, and potentially other beneficial physiologic changes, led to a reduction of major birth defect risk to a level similar to that of the general population,” they said.
Dr. Neovius disclosed advisory board fees from Itrim and Ethicon Johnson & Johnson. Three coauthors reported grants or other fees from a variety of pharmaceutical companies. The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, by the Swedish Research Council, and by the Swedish Research Council for Health, Working Life, and Welfare.
SOURCE: Neovius M et al. JAMA. 2019 Oct 15; 322:1515-17.
FROM JAMA
Key clinical point: Infants whose mothers previously underwent gastric bypass surgery had a lower risk of birth defects than did the infants of matched controls.
Major finding: Major birth defects occurred in 3% of infants whose mothers had gastric bypass surgery, compared with 5% of infants born to control women.
Study details: The data come from a cohort study of 2,921 women with history of gastric bypass surgery and 30,573 matched controls.
Disclosures: Dr. Neovius disclosed advisory board fees from Itrim and Ethicon Johnson & Johnson. Three coauthors reported grants or other fees from a variety of pharmaceutical companies. The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, by the Swedish Research Council, and by the Swedish Research Council for Health, Working Life, and Welfare.
Source: Neovius M et al. JAMA. 2019 Oct 15; 322:1515-17.
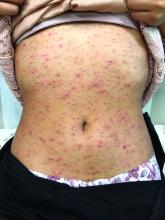
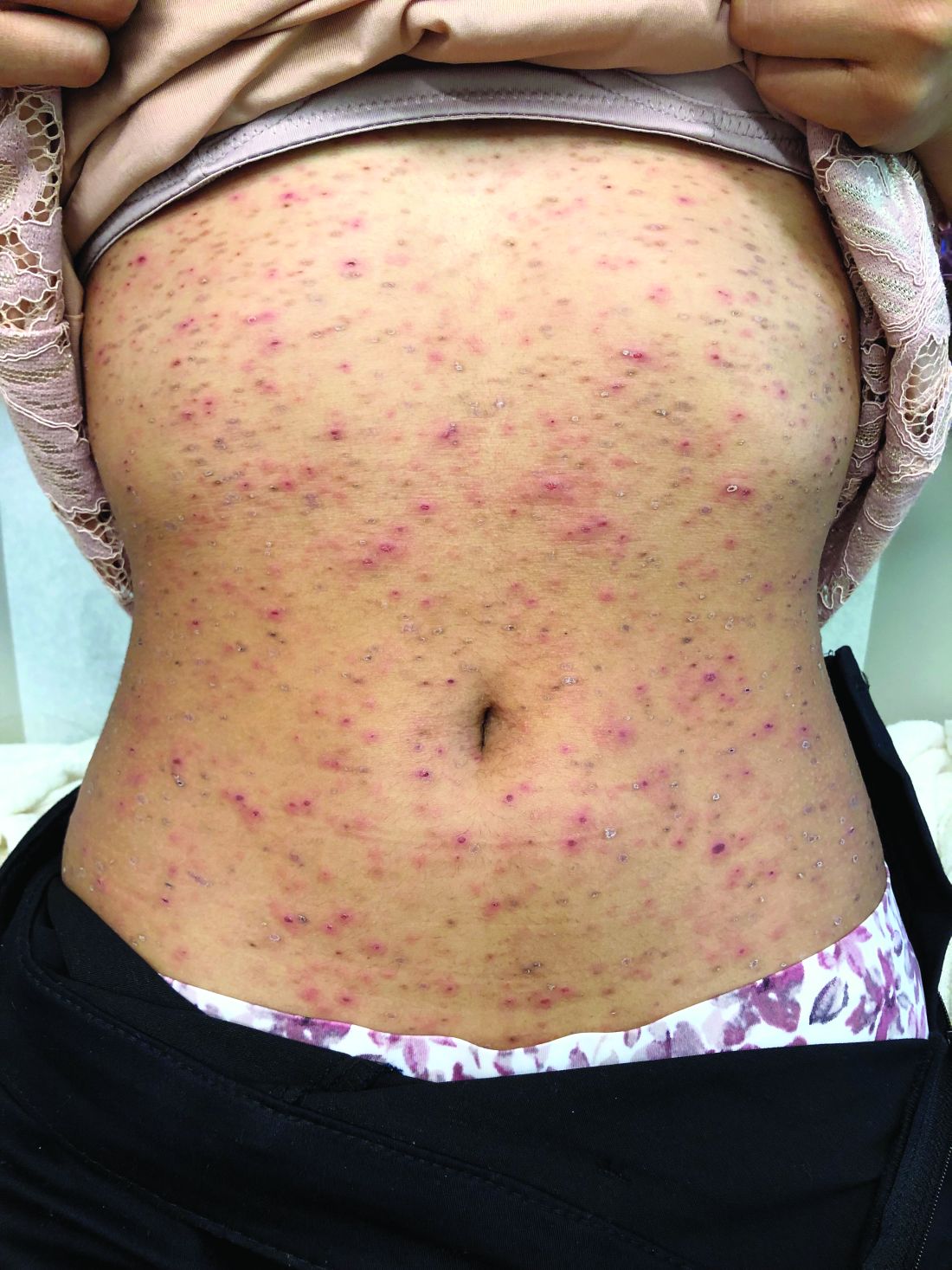
Pruritic, pink to violaceous, scaly papules
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at pdnews@mdedge.com.
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A healthy 14-year-old female was referred urgently by her pediatrician to our pediatric dermatology clinic for evaluation of a rash. The rash had been present for 4 weeks on her torso and proximal extremities, and had been spreading. She had been very itchy. She denied any fevers, chills, joint pain, oral or genital lesions.
She was visiting some family members in Washington State during the summer. The rash started 1 month after this visit.
The adolescent had been treated with acyclovir, trimethoprim/sulfamethoxazole, and intramuscular triamcinolone without improvement. She had been taking children's multivitamins occasionally. Her vaccinations were up-to-date. She denied any history of varicella or herpes infection. Her mom has a history of cold sores. The teen is not sexually active.
On physical examination, the girl was not in acute distress. Her vital signs were stable. She was not febrile. She had pink, scaly, and hyperpigmented papules and plaques, some of which were crusted with violaceous centers on the trunk and proximal extremities. There were no lesions on the mouth, palms, or soles. She had no lymphadenopathy or hepatosplenomegaly.
Ankylosing spondylitis, axial PsA may be two different diseases
“Our study suggests that axial PsA and AS with psoriasis seem to be two different diseases with different genetics, demographics, and disease expression,” wrote Joy Feld, MD, of the University of Toronto and coauthors. Their findings were published in Rheumatology.
To investigate the similarities and differences between axPsA and AS patients, the researchers compared two adult cohorts recruited from Toronto clinics. The first was made up of AS patients and divided into two groups: with psoriasis (n = 91) and without psoriasis (n = 675). The second was made up of PsA patients and divided into two groups: axPsA (n = 477) and peripheral PsA (n = 826).
In comparing AS patients with and without psoriasis to axPsA patients, AS patients had a younger age at diagnosis (28.7 years and 30.4 years vs. 35.6 years; P less than .001), were more often male (76% and 72% vs. 64%; P less than .001), and were more likely to be HLA-B27 positive (82% and 75% vs. 19%; P less than .001).
At baseline, AS patients had more back pain (90% and 92% vs. 21%; P less than .001) and worse back metrology (Bath Ankylosing Spondylitis Metrology Index [BASMI] of 3.1 and 2.3 vs. 1.9; P less than .001).
The mean follow-up periods in the axial and peripheral PsA groups were 12.6 and 6.7 years, respectively, whereas in the AS groups with and without psoriasis the periods were 5.4 and 3.5 years. Over time and after longitudinal analysis, axPsA patients had more tender and swollen joints than AS patients with and without psoriasis (5.2 vs. 1.5 and 0.9; P less than .001) while AS patients with and without psoriasis had a higher BASMI (2.9 and 2.2 vs. 1.8; P less than .001) and worse axial disease activity scores (4.1 and 3.9 vs. 3.5; P = .02) as measured by the Bath Ankylosing Spondylitis Disease Activity Index.
After univariate analysis, AS with psoriasis was found to be more associated with HLA-B27 (odds ratio, 16.37; 95% confidence interval, 8.89-30.13; P less than .0001), a higher adjusted mean BASMI (OR, 1.41; 95% CI, 1.21-1.63; P less than .0001), worse sacroiliitis (OR, 7.58; 95% CI, 3.68-15.59; P less than .0001), and greater use of biologics (OR, 1.25; 95% CI, 0.77-1; P = .37), compared with axPsA. A multivariate analysis produced similar findings, including the lack of association between AS and active arthritis (OR, 0.75; 95% CI, 0.64-0.86; P less than .0001).
The authors acknowledged their study’s limitations, including the fact that symptoms often dictate which of the two clinics patients will be referred to, which can ultimately define the diagnosis. “Patients with significant back symptoms are more likely to be referred to the AS clinic,” they wrote, “while patients with more prominent peripheral symptoms are more likely to be referred to the PsA clinic.” Patients with AS in the study were also required to have back pain or limitations in spinal range of motion, while PsA patients were accepted even if they were asymptomatic.
Finally, they noted that some milder cases of the two diseases may have been missed in the cohort recruiting process, although they added that mild cases were, in fact, “present in the cohort, which might improve the generalizability of this study to primary rheumatology clinics.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation, but this study received no specific funding to carry out the research. Dr. Feld reported being supported by a grant from Novartis. The authors reported no conflicts of interest.
SOURCE: Feld J et al. Rheumatology. 2019 Oct 8. doi: 10.1093/rheumatology/kez457.
“Our study suggests that axial PsA and AS with psoriasis seem to be two different diseases with different genetics, demographics, and disease expression,” wrote Joy Feld, MD, of the University of Toronto and coauthors. Their findings were published in Rheumatology.
To investigate the similarities and differences between axPsA and AS patients, the researchers compared two adult cohorts recruited from Toronto clinics. The first was made up of AS patients and divided into two groups: with psoriasis (n = 91) and without psoriasis (n = 675). The second was made up of PsA patients and divided into two groups: axPsA (n = 477) and peripheral PsA (n = 826).
In comparing AS patients with and without psoriasis to axPsA patients, AS patients had a younger age at diagnosis (28.7 years and 30.4 years vs. 35.6 years; P less than .001), were more often male (76% and 72% vs. 64%; P less than .001), and were more likely to be HLA-B27 positive (82% and 75% vs. 19%; P less than .001).
At baseline, AS patients had more back pain (90% and 92% vs. 21%; P less than .001) and worse back metrology (Bath Ankylosing Spondylitis Metrology Index [BASMI] of 3.1 and 2.3 vs. 1.9; P less than .001).
The mean follow-up periods in the axial and peripheral PsA groups were 12.6 and 6.7 years, respectively, whereas in the AS groups with and without psoriasis the periods were 5.4 and 3.5 years. Over time and after longitudinal analysis, axPsA patients had more tender and swollen joints than AS patients with and without psoriasis (5.2 vs. 1.5 and 0.9; P less than .001) while AS patients with and without psoriasis had a higher BASMI (2.9 and 2.2 vs. 1.8; P less than .001) and worse axial disease activity scores (4.1 and 3.9 vs. 3.5; P = .02) as measured by the Bath Ankylosing Spondylitis Disease Activity Index.
After univariate analysis, AS with psoriasis was found to be more associated with HLA-B27 (odds ratio, 16.37; 95% confidence interval, 8.89-30.13; P less than .0001), a higher adjusted mean BASMI (OR, 1.41; 95% CI, 1.21-1.63; P less than .0001), worse sacroiliitis (OR, 7.58; 95% CI, 3.68-15.59; P less than .0001), and greater use of biologics (OR, 1.25; 95% CI, 0.77-1; P = .37), compared with axPsA. A multivariate analysis produced similar findings, including the lack of association between AS and active arthritis (OR, 0.75; 95% CI, 0.64-0.86; P less than .0001).
The authors acknowledged their study’s limitations, including the fact that symptoms often dictate which of the two clinics patients will be referred to, which can ultimately define the diagnosis. “Patients with significant back symptoms are more likely to be referred to the AS clinic,” they wrote, “while patients with more prominent peripheral symptoms are more likely to be referred to the PsA clinic.” Patients with AS in the study were also required to have back pain or limitations in spinal range of motion, while PsA patients were accepted even if they were asymptomatic.
Finally, they noted that some milder cases of the two diseases may have been missed in the cohort recruiting process, although they added that mild cases were, in fact, “present in the cohort, which might improve the generalizability of this study to primary rheumatology clinics.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation, but this study received no specific funding to carry out the research. Dr. Feld reported being supported by a grant from Novartis. The authors reported no conflicts of interest.
SOURCE: Feld J et al. Rheumatology. 2019 Oct 8. doi: 10.1093/rheumatology/kez457.
“Our study suggests that axial PsA and AS with psoriasis seem to be two different diseases with different genetics, demographics, and disease expression,” wrote Joy Feld, MD, of the University of Toronto and coauthors. Their findings were published in Rheumatology.
To investigate the similarities and differences between axPsA and AS patients, the researchers compared two adult cohorts recruited from Toronto clinics. The first was made up of AS patients and divided into two groups: with psoriasis (n = 91) and without psoriasis (n = 675). The second was made up of PsA patients and divided into two groups: axPsA (n = 477) and peripheral PsA (n = 826).
In comparing AS patients with and without psoriasis to axPsA patients, AS patients had a younger age at diagnosis (28.7 years and 30.4 years vs. 35.6 years; P less than .001), were more often male (76% and 72% vs. 64%; P less than .001), and were more likely to be HLA-B27 positive (82% and 75% vs. 19%; P less than .001).
At baseline, AS patients had more back pain (90% and 92% vs. 21%; P less than .001) and worse back metrology (Bath Ankylosing Spondylitis Metrology Index [BASMI] of 3.1 and 2.3 vs. 1.9; P less than .001).
The mean follow-up periods in the axial and peripheral PsA groups were 12.6 and 6.7 years, respectively, whereas in the AS groups with and without psoriasis the periods were 5.4 and 3.5 years. Over time and after longitudinal analysis, axPsA patients had more tender and swollen joints than AS patients with and without psoriasis (5.2 vs. 1.5 and 0.9; P less than .001) while AS patients with and without psoriasis had a higher BASMI (2.9 and 2.2 vs. 1.8; P less than .001) and worse axial disease activity scores (4.1 and 3.9 vs. 3.5; P = .02) as measured by the Bath Ankylosing Spondylitis Disease Activity Index.
After univariate analysis, AS with psoriasis was found to be more associated with HLA-B27 (odds ratio, 16.37; 95% confidence interval, 8.89-30.13; P less than .0001), a higher adjusted mean BASMI (OR, 1.41; 95% CI, 1.21-1.63; P less than .0001), worse sacroiliitis (OR, 7.58; 95% CI, 3.68-15.59; P less than .0001), and greater use of biologics (OR, 1.25; 95% CI, 0.77-1; P = .37), compared with axPsA. A multivariate analysis produced similar findings, including the lack of association between AS and active arthritis (OR, 0.75; 95% CI, 0.64-0.86; P less than .0001).
The authors acknowledged their study’s limitations, including the fact that symptoms often dictate which of the two clinics patients will be referred to, which can ultimately define the diagnosis. “Patients with significant back symptoms are more likely to be referred to the AS clinic,” they wrote, “while patients with more prominent peripheral symptoms are more likely to be referred to the PsA clinic.” Patients with AS in the study were also required to have back pain or limitations in spinal range of motion, while PsA patients were accepted even if they were asymptomatic.
Finally, they noted that some milder cases of the two diseases may have been missed in the cohort recruiting process, although they added that mild cases were, in fact, “present in the cohort, which might improve the generalizability of this study to primary rheumatology clinics.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation, but this study received no specific funding to carry out the research. Dr. Feld reported being supported by a grant from Novartis. The authors reported no conflicts of interest.
SOURCE: Feld J et al. Rheumatology. 2019 Oct 8. doi: 10.1093/rheumatology/kez457.
FROM RHEUMATOLOGY
Changes to public charge rule blocked by courts
In three separate decisions, the U.S. District Court for the Southern District of New York, the U.S. District Court for the Northern District of California, and the U.S. District Court for the Eastern District of Washington temporarily barred the administration’s changes to the public charge rule from moving forward. The judges said lawsuits challenging the rule are likely to prevail in court.
The injunctions are much needed to protect patients and families, said R. Shawn Martin, senior vice president of advocacy, practice advancement, and policy for the American Academy of Family Physicians.
“The court decisions are important,” Mr. Martin said in an interview. “The AAFP believes that the public charge rule, as proposed by the administration, would have an immediate and negative impact on the health of thousands of people, including children. There is evidence that policies such as this create a culture where patients forgo interactions with the health care system out of fear. We should never be a country that compromises the health and well-being of individuals, especially those that are most vulnerable.”
White House Press Secretary Stephanie Grisham called the court rulings extremely disappointing and said the recent changes to the public charge rule restore integrity to the immigration system.
“The rulings today prevent our nation’s immigration officers from ensuring that immigrants seeking entry to the United States will be self-sufficient and instead allow noncitizens to continue taking advantage of our generous but limited public resources reserved for vulnerable Americans,” Ms. Graham said in a statement. “These injunctions are the latest inexplicable example of the administration being ordered to comply with the flawed or lawless guidance of a previous administration instead of the actual laws passed by Congress.”
Under the longstanding public charge rule, officials can refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation, which was scheduled to take effect on Oct. 15, 2019, would allow officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs. The revised regulation would continue to allow immigrants to access emergency medical care and disaster relief without public charge repercussions.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued in Washington state by 14 states. On Oct. 11, Judge Rosanna Malouf Peterson, U.S. district judge for the Eastern District of Washington, issued a nationwide ban of the revised public charge regulation, ruling that the plaintiff states will suffer irreparable harm if the rule moves forward.
“The plaintiff states have shown a significant threat of irreparable injury as a result of the impending enactment of the public charge rule by numerous individuals disenrolling from benefits for which they or their relatives were qualified, out of fear or confusion, that accepting those noncash public benefits will deprive them of an opportunity for legal permanent residency,” Judge Peterson wrote in her decision. “The plaintiff states have further demonstrated how that chilling effect predictably would cause irreparable injury by creating long-term costs to the plaintiff states from providing ongoing triage for residents who have missed opportunities for timely diagnoses, vaccinations, or building a strong foundation in childhood that will allow U.S. citizen children and future U.S. citizens to flourish and contribute to their communities as taxpaying adults.”
Judge Phyllis J. Hamilton, U.S. district judge for the Northern District of California, ruled similarly on Oct. 11, as did George Benjamin Daniels of the U.S. District Court for the Southern District of New York.
Physician associations previously voiced opposition to the administration’s changes to the public charge rule. In a joint statement, the AAFP, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, the American Osteopathic Association, the American College of Physicians, and the American Psychiatric Association expressed concern that the new regulation will discourage immigrants from seeking needed health care since such assistance may be used to deny green cards and visas, or even lead to deportations.
“Rather than face that threat, impacted patients currently served by our members almost certainly will avoid needed care from their trusted physicians, jeopardizing their own health and that of their communities,” the medical societies stated. “Many of our members have already witnessed this chilling effect among their own patient populations, with patients avoiding health services and programs out of fear.”
In three separate decisions, the U.S. District Court for the Southern District of New York, the U.S. District Court for the Northern District of California, and the U.S. District Court for the Eastern District of Washington temporarily barred the administration’s changes to the public charge rule from moving forward. The judges said lawsuits challenging the rule are likely to prevail in court.
The injunctions are much needed to protect patients and families, said R. Shawn Martin, senior vice president of advocacy, practice advancement, and policy for the American Academy of Family Physicians.
“The court decisions are important,” Mr. Martin said in an interview. “The AAFP believes that the public charge rule, as proposed by the administration, would have an immediate and negative impact on the health of thousands of people, including children. There is evidence that policies such as this create a culture where patients forgo interactions with the health care system out of fear. We should never be a country that compromises the health and well-being of individuals, especially those that are most vulnerable.”
White House Press Secretary Stephanie Grisham called the court rulings extremely disappointing and said the recent changes to the public charge rule restore integrity to the immigration system.
“The rulings today prevent our nation’s immigration officers from ensuring that immigrants seeking entry to the United States will be self-sufficient and instead allow noncitizens to continue taking advantage of our generous but limited public resources reserved for vulnerable Americans,” Ms. Graham said in a statement. “These injunctions are the latest inexplicable example of the administration being ordered to comply with the flawed or lawless guidance of a previous administration instead of the actual laws passed by Congress.”
Under the longstanding public charge rule, officials can refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation, which was scheduled to take effect on Oct. 15, 2019, would allow officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs. The revised regulation would continue to allow immigrants to access emergency medical care and disaster relief without public charge repercussions.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued in Washington state by 14 states. On Oct. 11, Judge Rosanna Malouf Peterson, U.S. district judge for the Eastern District of Washington, issued a nationwide ban of the revised public charge regulation, ruling that the plaintiff states will suffer irreparable harm if the rule moves forward.
“The plaintiff states have shown a significant threat of irreparable injury as a result of the impending enactment of the public charge rule by numerous individuals disenrolling from benefits for which they or their relatives were qualified, out of fear or confusion, that accepting those noncash public benefits will deprive them of an opportunity for legal permanent residency,” Judge Peterson wrote in her decision. “The plaintiff states have further demonstrated how that chilling effect predictably would cause irreparable injury by creating long-term costs to the plaintiff states from providing ongoing triage for residents who have missed opportunities for timely diagnoses, vaccinations, or building a strong foundation in childhood that will allow U.S. citizen children and future U.S. citizens to flourish and contribute to their communities as taxpaying adults.”
Judge Phyllis J. Hamilton, U.S. district judge for the Northern District of California, ruled similarly on Oct. 11, as did George Benjamin Daniels of the U.S. District Court for the Southern District of New York.
Physician associations previously voiced opposition to the administration’s changes to the public charge rule. In a joint statement, the AAFP, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, the American Osteopathic Association, the American College of Physicians, and the American Psychiatric Association expressed concern that the new regulation will discourage immigrants from seeking needed health care since such assistance may be used to deny green cards and visas, or even lead to deportations.
“Rather than face that threat, impacted patients currently served by our members almost certainly will avoid needed care from their trusted physicians, jeopardizing their own health and that of their communities,” the medical societies stated. “Many of our members have already witnessed this chilling effect among their own patient populations, with patients avoiding health services and programs out of fear.”
In three separate decisions, the U.S. District Court for the Southern District of New York, the U.S. District Court for the Northern District of California, and the U.S. District Court for the Eastern District of Washington temporarily barred the administration’s changes to the public charge rule from moving forward. The judges said lawsuits challenging the rule are likely to prevail in court.
The injunctions are much needed to protect patients and families, said R. Shawn Martin, senior vice president of advocacy, practice advancement, and policy for the American Academy of Family Physicians.
“The court decisions are important,” Mr. Martin said in an interview. “The AAFP believes that the public charge rule, as proposed by the administration, would have an immediate and negative impact on the health of thousands of people, including children. There is evidence that policies such as this create a culture where patients forgo interactions with the health care system out of fear. We should never be a country that compromises the health and well-being of individuals, especially those that are most vulnerable.”
White House Press Secretary Stephanie Grisham called the court rulings extremely disappointing and said the recent changes to the public charge rule restore integrity to the immigration system.
“The rulings today prevent our nation’s immigration officers from ensuring that immigrants seeking entry to the United States will be self-sufficient and instead allow noncitizens to continue taking advantage of our generous but limited public resources reserved for vulnerable Americans,” Ms. Graham said in a statement. “These injunctions are the latest inexplicable example of the administration being ordered to comply with the flawed or lawless guidance of a previous administration instead of the actual laws passed by Congress.”
Under the longstanding public charge rule, officials can refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation, which was scheduled to take effect on Oct. 15, 2019, would allow officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs. The revised regulation would continue to allow immigrants to access emergency medical care and disaster relief without public charge repercussions.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued in Washington state by 14 states. On Oct. 11, Judge Rosanna Malouf Peterson, U.S. district judge for the Eastern District of Washington, issued a nationwide ban of the revised public charge regulation, ruling that the plaintiff states will suffer irreparable harm if the rule moves forward.
“The plaintiff states have shown a significant threat of irreparable injury as a result of the impending enactment of the public charge rule by numerous individuals disenrolling from benefits for which they or their relatives were qualified, out of fear or confusion, that accepting those noncash public benefits will deprive them of an opportunity for legal permanent residency,” Judge Peterson wrote in her decision. “The plaintiff states have further demonstrated how that chilling effect predictably would cause irreparable injury by creating long-term costs to the plaintiff states from providing ongoing triage for residents who have missed opportunities for timely diagnoses, vaccinations, or building a strong foundation in childhood that will allow U.S. citizen children and future U.S. citizens to flourish and contribute to their communities as taxpaying adults.”
Judge Phyllis J. Hamilton, U.S. district judge for the Northern District of California, ruled similarly on Oct. 11, as did George Benjamin Daniels of the U.S. District Court for the Southern District of New York.
Physician associations previously voiced opposition to the administration’s changes to the public charge rule. In a joint statement, the AAFP, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, the American Osteopathic Association, the American College of Physicians, and the American Psychiatric Association expressed concern that the new regulation will discourage immigrants from seeking needed health care since such assistance may be used to deny green cards and visas, or even lead to deportations.
“Rather than face that threat, impacted patients currently served by our members almost certainly will avoid needed care from their trusted physicians, jeopardizing their own health and that of their communities,” the medical societies stated. “Many of our members have already witnessed this chilling effect among their own patient populations, with patients avoiding health services and programs out of fear.”
Neurologists publish consensus statement on stridor in MSA
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
FROM NEUROLOGY
Rivaroxaban trends toward higher thrombotic risk than vitamin K antagonists in APS
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
The recent trial by Ordi-Ros et al. revealed similar findings to a previous trial, TRAPS, by Pengo et al., which compared rivaroxaban with warfarin among patients with thrombotic antiphospholipid syndrome and triple positivity for antiphospholipid antibodies. Despite the caveat that TRAPS was prematurely terminated, in both studies, a higher proportion of patients in the rivaroxaban group than the vitamin K antagonist group had thrombotic events, most of which were arterial, whether considering MI or stroke. Furthermore, both studies did not show noninferiority of rivaroxaban versus dose-adjusted vitamin K antagonists.
The reasons for this failure of noninferiority remain unclear.
Denis Wahl, MD, PhD, and Virginie Dufrost, MD, are with the University of Lorraine, Nancy, France, and the Centre Hospitalier Universitaire de Nancy. No conflicts of interest were reported. His remarks are adapted from an accompanying editorial (Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-2815).
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
suggests a recent trial conducted in Spain.
Stroke was also more common among those taking rivaroxaban, while major bleeding was slightly less common, reported lead author Josep Ordi-Ros, MD, PhD, of Vall d’Hebrón University Hospital Research Institute in Barcelona, and colleagues in Annals of Internal Medicine.
“Two randomized, controlled trials comparing rivaroxaban with warfarin suggested that rivaroxaban may be efficacious in patients with previous venous thromboembolism who are receiving standard-intensity anticoagulation but showed an increased thrombotic risk in those with triple-positive antiphospholipid antibodies,” the investigators wrote. However, they also noted that these findings required a cautious interpretation because of study limitations, such as premature termination caused by an excess of study events and the use of a laboratory surrogate marker as a primary outcome.
To learn more, the investigators performed an open-label, phase 3 trial involving 190 patients with thrombotic APS. Patients were randomized in a 1:1 ratio to receive either rivaroxaban (20 mg per day, or 15 mg per day for patients with a creatinine clearance of 30-49 mL/min per 1.73 m2) or an adjusted dosage of vitamin K antagonists (target international normalized ratio of 2.0-3.0, or 3.1-4.0 for those with a history of recurrent thrombosis).
Patients underwent evaluations every month for the first 3 months and then every 3 months thereafter, each of which involved a variety of laboratory diagnostics such as checks for antinuclear antibodies and lupus anticoagulant, among others. Statistical analyses aimed to determine if rivaroxaban was noninferior to therapy with vitamin K antagonists based on parameters drawn from previous meta-analyses, as no studies had compared the two types of treatment when the present study was designed.
After 3 years of follow-up, almost twice as many patients in the rivaroxaban group had experienced recurrent thrombosis (11.6% vs. 6.3%), although this finding lacked statistical significance for both noninferiority of rivaroxaban (P = .29) and superiority of vitamin K antagonists (P = .20). Still, supporting a similar trend toward differences in efficacy, stroke was more common in the rivaroxaban group, in which nine events occurred, compared with none in the vitamin K antagonist group. In contrast, major bleeding was slightly less common with rivaroxaban than vitamin K antagonists (6.3% vs. 7.4%).
“In conclusion, rivaroxaban did not demonstrate noninferiority to dose-adjusted vitamin K antagonists for secondary thromboprophylaxis in patients with thrombotic APS,” the investigators wrote. “Instead, our results indicate a recurrent thrombotic rate that is nearly double, albeit without statistical significance.”
The study was funded by Bayer Hispania. One coauthor reported additional relationships with Pfizer, Lilly, Janssen, and others.
SOURCE: Ordi-Ros J et al. Ann Intern Med. 2019 Oct 15. doi: 10.7326/M19-0291.
FROM ANNALS OF INTERNAL MEDICINE
No clear benefit from conservative oxygen in mechanical ventilation
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
FROM NEJM
Key clinical point: Conservative oxygen therapy during mechanical ventilation does not increase ventilation-free days.
Major finding: The number of ventilation-free days was similar in adults on conservative oxygen therapy and those on usual care.
Study details: Parallel-group randomized controlled trial in 965 adults undergoing mechanical ventilation.
Disclosures: The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
Source: Mackle D et al. NJEM 2019, October 14. DOI: 10.1056/NEJMoa1903297.
MRI saves money, better than CT in acute stroke
ST. LOUIS – , according to a review from Johns Hopkins University, Baltimore.
MRI as the first scan leads to “a definitive diagnoses sooner and helps you manage the person more rapidly and appropriately, without negatively affecting outcomes even in stroke patients who receive endovascular therapy,” said neurologist and senior investigator Argye Hillis, MD, director of the Center of Excellence in Stroke Detection and Diagnosis at Hopkins. “Consider skipping the CT and getting an MRI, and get the MRI while they are still in the emergency room.”
Almost all emergency departments in the United States are set up to get a CT first, but MRI is known to be the better study, according to the researchers. MRI is much more sensitive to stroke, especially in the first 24 hours, and pinpoints the location and extent of the damage. It can detect causes of stroke invisible to CT, with no radiation, and rule out stroke entirely, whereas CT can rule out only intracranial bleeding. Increasingly in Europe, MRI is the first study in suspected stroke, and new EDs in the United States are being designed with an in-house MRI, or one nearby.
The ED at Hopkins’ main campus in downtown Baltimore already has an MRI, and uses it first whenever possible. The problem has been that MRI techs are available only during weekdays, so physicians have to default back to CT at night and on weekends. The impetus for the review, presented at the annual meeting of the American Neurological Association, was to see if savings from unnecessary admissions prevented by MRI would be enough to offset the cost of around-the-clock staffing for the MRI scanner.
Dr. Hillis and her team reviewed 320 patients with suspected ischemic stroke who were seen at the main campus in 2018 and had CT in the ED, and then definitive diagnosis by MRI, which is the usual approach in most U.S. hospitals.
A total of 134 patients had a final diagnosis on MRI that did not justify admission; techs were available to give 75 of them MRIs in the ED after the CT, and those patients were sent home. Techs were not available, however, for 59 patients and since the CT was not able to rule out stroke, those patients were admitted. The cost of those 59 admissions was $814,016.
The cost of the noncontrast CTs for the 75 patients who were sent home after definitive MRI imaging was $28,050, plus an additional $46,072 for those who had CT neck/head angiograms. Altogether, skipping the CT and going straight to the MRI would have saved Hopkins $888,138 in 2018, enough to cover round-the-clock MRI staffing in the ED, which is now the plan at the main campus.
Once the facility moves to 24-and-7 MRI coverage, the next step in the project is to compare stroke outcomes with Johns Hopkins Bayview Medical Center, also in Baltimore, which will continue to do CT first. “We know MRI first is cheaper. We want to see if we have better outcomes. If we find they’re much better, I think many hospitals will say it’s worth the 5 minutes longer it takes to get to the MRI scanner,” Dr. Hillis said.
Stroke mimics among the 134 patients included peripheral nerve palsy and migraine, but also people simply faking it for a hot meal and a warm bed. “Its pretty common, unfortunately,” she said.
The average age for stroke admissions at Hopkins is 55 years, with as many men as women.
There was no industry funding, and Dr. Hillis didn’t have any relevant disclosures.
ST. LOUIS – , according to a review from Johns Hopkins University, Baltimore.
MRI as the first scan leads to “a definitive diagnoses sooner and helps you manage the person more rapidly and appropriately, without negatively affecting outcomes even in stroke patients who receive endovascular therapy,” said neurologist and senior investigator Argye Hillis, MD, director of the Center of Excellence in Stroke Detection and Diagnosis at Hopkins. “Consider skipping the CT and getting an MRI, and get the MRI while they are still in the emergency room.”
Almost all emergency departments in the United States are set up to get a CT first, but MRI is known to be the better study, according to the researchers. MRI is much more sensitive to stroke, especially in the first 24 hours, and pinpoints the location and extent of the damage. It can detect causes of stroke invisible to CT, with no radiation, and rule out stroke entirely, whereas CT can rule out only intracranial bleeding. Increasingly in Europe, MRI is the first study in suspected stroke, and new EDs in the United States are being designed with an in-house MRI, or one nearby.
The ED at Hopkins’ main campus in downtown Baltimore already has an MRI, and uses it first whenever possible. The problem has been that MRI techs are available only during weekdays, so physicians have to default back to CT at night and on weekends. The impetus for the review, presented at the annual meeting of the American Neurological Association, was to see if savings from unnecessary admissions prevented by MRI would be enough to offset the cost of around-the-clock staffing for the MRI scanner.
Dr. Hillis and her team reviewed 320 patients with suspected ischemic stroke who were seen at the main campus in 2018 and had CT in the ED, and then definitive diagnosis by MRI, which is the usual approach in most U.S. hospitals.
A total of 134 patients had a final diagnosis on MRI that did not justify admission; techs were available to give 75 of them MRIs in the ED after the CT, and those patients were sent home. Techs were not available, however, for 59 patients and since the CT was not able to rule out stroke, those patients were admitted. The cost of those 59 admissions was $814,016.
The cost of the noncontrast CTs for the 75 patients who were sent home after definitive MRI imaging was $28,050, plus an additional $46,072 for those who had CT neck/head angiograms. Altogether, skipping the CT and going straight to the MRI would have saved Hopkins $888,138 in 2018, enough to cover round-the-clock MRI staffing in the ED, which is now the plan at the main campus.
Once the facility moves to 24-and-7 MRI coverage, the next step in the project is to compare stroke outcomes with Johns Hopkins Bayview Medical Center, also in Baltimore, which will continue to do CT first. “We know MRI first is cheaper. We want to see if we have better outcomes. If we find they’re much better, I think many hospitals will say it’s worth the 5 minutes longer it takes to get to the MRI scanner,” Dr. Hillis said.
Stroke mimics among the 134 patients included peripheral nerve palsy and migraine, but also people simply faking it for a hot meal and a warm bed. “Its pretty common, unfortunately,” she said.
The average age for stroke admissions at Hopkins is 55 years, with as many men as women.
There was no industry funding, and Dr. Hillis didn’t have any relevant disclosures.
ST. LOUIS – , according to a review from Johns Hopkins University, Baltimore.
MRI as the first scan leads to “a definitive diagnoses sooner and helps you manage the person more rapidly and appropriately, without negatively affecting outcomes even in stroke patients who receive endovascular therapy,” said neurologist and senior investigator Argye Hillis, MD, director of the Center of Excellence in Stroke Detection and Diagnosis at Hopkins. “Consider skipping the CT and getting an MRI, and get the MRI while they are still in the emergency room.”
Almost all emergency departments in the United States are set up to get a CT first, but MRI is known to be the better study, according to the researchers. MRI is much more sensitive to stroke, especially in the first 24 hours, and pinpoints the location and extent of the damage. It can detect causes of stroke invisible to CT, with no radiation, and rule out stroke entirely, whereas CT can rule out only intracranial bleeding. Increasingly in Europe, MRI is the first study in suspected stroke, and new EDs in the United States are being designed with an in-house MRI, or one nearby.
The ED at Hopkins’ main campus in downtown Baltimore already has an MRI, and uses it first whenever possible. The problem has been that MRI techs are available only during weekdays, so physicians have to default back to CT at night and on weekends. The impetus for the review, presented at the annual meeting of the American Neurological Association, was to see if savings from unnecessary admissions prevented by MRI would be enough to offset the cost of around-the-clock staffing for the MRI scanner.
Dr. Hillis and her team reviewed 320 patients with suspected ischemic stroke who were seen at the main campus in 2018 and had CT in the ED, and then definitive diagnosis by MRI, which is the usual approach in most U.S. hospitals.
A total of 134 patients had a final diagnosis on MRI that did not justify admission; techs were available to give 75 of them MRIs in the ED after the CT, and those patients were sent home. Techs were not available, however, for 59 patients and since the CT was not able to rule out stroke, those patients were admitted. The cost of those 59 admissions was $814,016.
The cost of the noncontrast CTs for the 75 patients who were sent home after definitive MRI imaging was $28,050, plus an additional $46,072 for those who had CT neck/head angiograms. Altogether, skipping the CT and going straight to the MRI would have saved Hopkins $888,138 in 2018, enough to cover round-the-clock MRI staffing in the ED, which is now the plan at the main campus.
Once the facility moves to 24-and-7 MRI coverage, the next step in the project is to compare stroke outcomes with Johns Hopkins Bayview Medical Center, also in Baltimore, which will continue to do CT first. “We know MRI first is cheaper. We want to see if we have better outcomes. If we find they’re much better, I think many hospitals will say it’s worth the 5 minutes longer it takes to get to the MRI scanner,” Dr. Hillis said.
Stroke mimics among the 134 patients included peripheral nerve palsy and migraine, but also people simply faking it for a hot meal and a warm bed. “Its pretty common, unfortunately,” she said.
The average age for stroke admissions at Hopkins is 55 years, with as many men as women.
There was no industry funding, and Dr. Hillis didn’t have any relevant disclosures.
REPORTING FROM ANA 2019
Key clinical point: Getting an MRI first for suspected stroke, instead of a CT, saves money by avoiding unnecessary admissions and might lead to better outcomes.
Major finding: An MRI-first approach at a busy ED in downtown Baltimore would have saved $888,138 in 1 year.
Study details: Review of 320 patients with suspected ischemic strokes.
Disclosures: There was no industry funding, and the senior investigator did not have any relevant disclosures.
Source: Sherry E et al. ANA 2019. Abstract M123.
Did You Know? Psoriasis and inflammatory bowel disease