User login
#MomsNeedToKnow mental health awareness campaign set to launch
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
One goal is to use social media to encourage women to let go of stigma
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Translucent particles found in Hemlibra
But toxicology and safety assessments conducted by the company found that the benefit-risk profile of the product remains unchanged.
Hemlibra is a bispecific factor IXa– and factor X–directed antibody that has been approved in the United States and other countries for routine prophylaxis in adult and pediatric patients with hemophilia A, with or without factor VIII inhibitors. It is administered as a subcutaneous injection.
The particles were first identified in March 2019 during a routine examination of drug product batches by Genentech. At the time, the company informed the U.S. Food and Drug Administration; the European Medicines Agency; Swissmedic; Health Canada; and the Ministry for Health, Labour, and Welfare in Japan. An initial company assessment found that the particles consisted of protein (Hemlibra drug substance) and silicone oil (polydimethylsiloxane), an organic polymer included in all parenteral medicines, according to Genentech.
Since the health authorities all agreed with the company’s initial conclusion that the product’s safety remained unchanged, no further action was taken.
The presence of the particles became more widely known in early October 2019, when Genentech notified the National Hemophilia Foundation, the NHF’s Medical and Scientific Advisory Council (MASAC), and the Hemophilia Federation of America. Genentech officials reached out to these groups after finishing a root cause investigation and issuing a final report to health authorities concluding that there was no change in the benefit-risk profile, according to a company spokesman.
MASAC issued its own statement recommending no change in prescribing or interruption in the use of Hemlibra. This is an “interim recommendation pending our assessment of the full review by Roche/Genentech of their manufacturing and quality control,” MASAC said. The council noted that it had been informed by representatives from Roche/Genentech that there have been no reports of adverse events linked to the particulate matter. Additionally, the problem had been present since the initial clinical trials of the product, but was only recently identified.
But at least one patient advocate is raising concerns about the timing of this notification. Jesse Clark, president and CEO of HemoAware, wants to know why patients were not informed of the particle issue for more than 6 months.
“The bleeding disorder community was never notified by either Genentech or any other agency,” Mr. Clark said in an interview. “The lack of transparency and communication is extremely concerning.”
A spokesman for Genentech said the company had not alerted patients earlier because there was no indication of an impact on the safety of the product. “We take inquiries from the community very seriously and are providing context and additional details in response to requests from patient organizations,” he said in an interview.
The company does not expect this issue to impact any patients in the United States and the availability of Hemlibra will not be affected, he added.
But toxicology and safety assessments conducted by the company found that the benefit-risk profile of the product remains unchanged.
Hemlibra is a bispecific factor IXa– and factor X–directed antibody that has been approved in the United States and other countries for routine prophylaxis in adult and pediatric patients with hemophilia A, with or without factor VIII inhibitors. It is administered as a subcutaneous injection.
The particles were first identified in March 2019 during a routine examination of drug product batches by Genentech. At the time, the company informed the U.S. Food and Drug Administration; the European Medicines Agency; Swissmedic; Health Canada; and the Ministry for Health, Labour, and Welfare in Japan. An initial company assessment found that the particles consisted of protein (Hemlibra drug substance) and silicone oil (polydimethylsiloxane), an organic polymer included in all parenteral medicines, according to Genentech.
Since the health authorities all agreed with the company’s initial conclusion that the product’s safety remained unchanged, no further action was taken.
The presence of the particles became more widely known in early October 2019, when Genentech notified the National Hemophilia Foundation, the NHF’s Medical and Scientific Advisory Council (MASAC), and the Hemophilia Federation of America. Genentech officials reached out to these groups after finishing a root cause investigation and issuing a final report to health authorities concluding that there was no change in the benefit-risk profile, according to a company spokesman.
MASAC issued its own statement recommending no change in prescribing or interruption in the use of Hemlibra. This is an “interim recommendation pending our assessment of the full review by Roche/Genentech of their manufacturing and quality control,” MASAC said. The council noted that it had been informed by representatives from Roche/Genentech that there have been no reports of adverse events linked to the particulate matter. Additionally, the problem had been present since the initial clinical trials of the product, but was only recently identified.
But at least one patient advocate is raising concerns about the timing of this notification. Jesse Clark, president and CEO of HemoAware, wants to know why patients were not informed of the particle issue for more than 6 months.
“The bleeding disorder community was never notified by either Genentech or any other agency,” Mr. Clark said in an interview. “The lack of transparency and communication is extremely concerning.”
A spokesman for Genentech said the company had not alerted patients earlier because there was no indication of an impact on the safety of the product. “We take inquiries from the community very seriously and are providing context and additional details in response to requests from patient organizations,” he said in an interview.
The company does not expect this issue to impact any patients in the United States and the availability of Hemlibra will not be affected, he added.
But toxicology and safety assessments conducted by the company found that the benefit-risk profile of the product remains unchanged.
Hemlibra is a bispecific factor IXa– and factor X–directed antibody that has been approved in the United States and other countries for routine prophylaxis in adult and pediatric patients with hemophilia A, with or without factor VIII inhibitors. It is administered as a subcutaneous injection.
The particles were first identified in March 2019 during a routine examination of drug product batches by Genentech. At the time, the company informed the U.S. Food and Drug Administration; the European Medicines Agency; Swissmedic; Health Canada; and the Ministry for Health, Labour, and Welfare in Japan. An initial company assessment found that the particles consisted of protein (Hemlibra drug substance) and silicone oil (polydimethylsiloxane), an organic polymer included in all parenteral medicines, according to Genentech.
Since the health authorities all agreed with the company’s initial conclusion that the product’s safety remained unchanged, no further action was taken.
The presence of the particles became more widely known in early October 2019, when Genentech notified the National Hemophilia Foundation, the NHF’s Medical and Scientific Advisory Council (MASAC), and the Hemophilia Federation of America. Genentech officials reached out to these groups after finishing a root cause investigation and issuing a final report to health authorities concluding that there was no change in the benefit-risk profile, according to a company spokesman.
MASAC issued its own statement recommending no change in prescribing or interruption in the use of Hemlibra. This is an “interim recommendation pending our assessment of the full review by Roche/Genentech of their manufacturing and quality control,” MASAC said. The council noted that it had been informed by representatives from Roche/Genentech that there have been no reports of adverse events linked to the particulate matter. Additionally, the problem had been present since the initial clinical trials of the product, but was only recently identified.
But at least one patient advocate is raising concerns about the timing of this notification. Jesse Clark, president and CEO of HemoAware, wants to know why patients were not informed of the particle issue for more than 6 months.
“The bleeding disorder community was never notified by either Genentech or any other agency,” Mr. Clark said in an interview. “The lack of transparency and communication is extremely concerning.”
A spokesman for Genentech said the company had not alerted patients earlier because there was no indication of an impact on the safety of the product. “We take inquiries from the community very seriously and are providing context and additional details in response to requests from patient organizations,” he said in an interview.
The company does not expect this issue to impact any patients in the United States and the availability of Hemlibra will not be affected, he added.
Wasteful health care spending could reach $935 billion
Wasteful spending in health care could reach almost $1 trillion, according to new research published in JAMA.
Review “of the current literature of the cost of waste in the U.S. health care system and evidence about projected savings from interventions that reduce waste suggest that the estimated total costs of waste and potential savings from interventions that address waste are as high as $760 billion to $935 billion and $191 billion to $282 billion, respectively,” William Shrank, MD, chief medical and corporate affairs officer at Humana, and colleagues wrote in an article published Oct. 7, 2019, in JAMA.
“These estimates represent approximately 25% of total health care expenditures in the Unites States, which have been projected to be $3.82 trillion for 2019,” the authors noted, adding that it is a little lower than other estimates that have waste as high as 34% of spending.
Authors looked at waste across six domains, including failure of care delivery, failure of care coordination, overtreatment or low-value care, pricing failure, fraud and abuse, and administrative complexity.
adding that there are no studies that identified savings from interventions to alleviate administrative complexity.
“Some of that complexity results from fragmentation in the health care system,” they stated. “Recent proposals by CMS [the Centers for Medicare & Medicaid Services] and the Office of the National Coordinator of [sic] Health Information Technology to foster data interoperability and government initiatives such as Blue Button 2.0 will hopefully alleviate some burden as information flows more freely and billing and authorization processes become more automated.”
They also point to greater use of value-based payments as a possible avenue toward greater cost savings in this category.
The second largest contributor is pricing failure, which is estimated to be in the range from $230.7 billion to $240.5 billion, with interventions generating savings ranging from $81.4 billion to $91.2 billion.
And as the health care system evolves to a value-based paradigm, it is expected to have the least impact in this category “since pharmaceutical pricing represents a major component of this waste domain and would not be affected by new approaches to care delivery and reimbursement,” Dr. Shrank and colleagues wrote.
That being said, the authors stated that policy interventions “are needed to drive meaningful reductions in waste in this domain. Additionally, in the dynamic health care marketplace, where profit-motivated firms will respond to any new policy with strategies to protect their margins, no single policy is likely to suffice; a coordinated policy effort is likely needed to create long-standing change that will meaningfully reduce waste resulting from pricing failure.”
The three domains of failure of care delivery, failure of care coordination, and overtreatment or low-value care combined to account for $200 billion in waste, and the authors stated that there is “compelling empirical evidence in all three categories that interventions can produce meaningful savings and may reduce waste by as much as half.”
SOURCE: Shrank W et al. JAMA. 2019 Oct 7. doi: 10.1001/jama.2019.13978.
The biggest challenge in removing waste from the health care system is one of politics. People and organizations make huge profits from the current system and have a vested interest in maintaining the status quo and aren’t afraid to lobby both sides of the aisle to keep things as close to how they are.
Physicians hold power in this by championing more shared-risk payment structures that encourage everyone to be more conscious of waste. They also need to ensure their voices are heard politically to oppose greed and deception in pricing policies whenever they arise.
Donald M. Berwick, MD, president emeritus and senior fellow, the Institute for Health Care Improvement, Boston, and former CMS administrator, made his comments in an editorial published online in JAMA (2019 Oct 7. doi: 10.1001/jama.2019.14610 ).
The biggest challenge in removing waste from the health care system is one of politics. People and organizations make huge profits from the current system and have a vested interest in maintaining the status quo and aren’t afraid to lobby both sides of the aisle to keep things as close to how they are.
Physicians hold power in this by championing more shared-risk payment structures that encourage everyone to be more conscious of waste. They also need to ensure their voices are heard politically to oppose greed and deception in pricing policies whenever they arise.
Donald M. Berwick, MD, president emeritus and senior fellow, the Institute for Health Care Improvement, Boston, and former CMS administrator, made his comments in an editorial published online in JAMA (2019 Oct 7. doi: 10.1001/jama.2019.14610 ).
The biggest challenge in removing waste from the health care system is one of politics. People and organizations make huge profits from the current system and have a vested interest in maintaining the status quo and aren’t afraid to lobby both sides of the aisle to keep things as close to how they are.
Physicians hold power in this by championing more shared-risk payment structures that encourage everyone to be more conscious of waste. They also need to ensure their voices are heard politically to oppose greed and deception in pricing policies whenever they arise.
Donald M. Berwick, MD, president emeritus and senior fellow, the Institute for Health Care Improvement, Boston, and former CMS administrator, made his comments in an editorial published online in JAMA (2019 Oct 7. doi: 10.1001/jama.2019.14610 ).
Wasteful spending in health care could reach almost $1 trillion, according to new research published in JAMA.
Review “of the current literature of the cost of waste in the U.S. health care system and evidence about projected savings from interventions that reduce waste suggest that the estimated total costs of waste and potential savings from interventions that address waste are as high as $760 billion to $935 billion and $191 billion to $282 billion, respectively,” William Shrank, MD, chief medical and corporate affairs officer at Humana, and colleagues wrote in an article published Oct. 7, 2019, in JAMA.
“These estimates represent approximately 25% of total health care expenditures in the Unites States, which have been projected to be $3.82 trillion for 2019,” the authors noted, adding that it is a little lower than other estimates that have waste as high as 34% of spending.
Authors looked at waste across six domains, including failure of care delivery, failure of care coordination, overtreatment or low-value care, pricing failure, fraud and abuse, and administrative complexity.
adding that there are no studies that identified savings from interventions to alleviate administrative complexity.
“Some of that complexity results from fragmentation in the health care system,” they stated. “Recent proposals by CMS [the Centers for Medicare & Medicaid Services] and the Office of the National Coordinator of [sic] Health Information Technology to foster data interoperability and government initiatives such as Blue Button 2.0 will hopefully alleviate some burden as information flows more freely and billing and authorization processes become more automated.”
They also point to greater use of value-based payments as a possible avenue toward greater cost savings in this category.
The second largest contributor is pricing failure, which is estimated to be in the range from $230.7 billion to $240.5 billion, with interventions generating savings ranging from $81.4 billion to $91.2 billion.
And as the health care system evolves to a value-based paradigm, it is expected to have the least impact in this category “since pharmaceutical pricing represents a major component of this waste domain and would not be affected by new approaches to care delivery and reimbursement,” Dr. Shrank and colleagues wrote.
That being said, the authors stated that policy interventions “are needed to drive meaningful reductions in waste in this domain. Additionally, in the dynamic health care marketplace, where profit-motivated firms will respond to any new policy with strategies to protect their margins, no single policy is likely to suffice; a coordinated policy effort is likely needed to create long-standing change that will meaningfully reduce waste resulting from pricing failure.”
The three domains of failure of care delivery, failure of care coordination, and overtreatment or low-value care combined to account for $200 billion in waste, and the authors stated that there is “compelling empirical evidence in all three categories that interventions can produce meaningful savings and may reduce waste by as much as half.”
SOURCE: Shrank W et al. JAMA. 2019 Oct 7. doi: 10.1001/jama.2019.13978.
Wasteful spending in health care could reach almost $1 trillion, according to new research published in JAMA.
Review “of the current literature of the cost of waste in the U.S. health care system and evidence about projected savings from interventions that reduce waste suggest that the estimated total costs of waste and potential savings from interventions that address waste are as high as $760 billion to $935 billion and $191 billion to $282 billion, respectively,” William Shrank, MD, chief medical and corporate affairs officer at Humana, and colleagues wrote in an article published Oct. 7, 2019, in JAMA.
“These estimates represent approximately 25% of total health care expenditures in the Unites States, which have been projected to be $3.82 trillion for 2019,” the authors noted, adding that it is a little lower than other estimates that have waste as high as 34% of spending.
Authors looked at waste across six domains, including failure of care delivery, failure of care coordination, overtreatment or low-value care, pricing failure, fraud and abuse, and administrative complexity.
adding that there are no studies that identified savings from interventions to alleviate administrative complexity.
“Some of that complexity results from fragmentation in the health care system,” they stated. “Recent proposals by CMS [the Centers for Medicare & Medicaid Services] and the Office of the National Coordinator of [sic] Health Information Technology to foster data interoperability and government initiatives such as Blue Button 2.0 will hopefully alleviate some burden as information flows more freely and billing and authorization processes become more automated.”
They also point to greater use of value-based payments as a possible avenue toward greater cost savings in this category.
The second largest contributor is pricing failure, which is estimated to be in the range from $230.7 billion to $240.5 billion, with interventions generating savings ranging from $81.4 billion to $91.2 billion.
And as the health care system evolves to a value-based paradigm, it is expected to have the least impact in this category “since pharmaceutical pricing represents a major component of this waste domain and would not be affected by new approaches to care delivery and reimbursement,” Dr. Shrank and colleagues wrote.
That being said, the authors stated that policy interventions “are needed to drive meaningful reductions in waste in this domain. Additionally, in the dynamic health care marketplace, where profit-motivated firms will respond to any new policy with strategies to protect their margins, no single policy is likely to suffice; a coordinated policy effort is likely needed to create long-standing change that will meaningfully reduce waste resulting from pricing failure.”
The three domains of failure of care delivery, failure of care coordination, and overtreatment or low-value care combined to account for $200 billion in waste, and the authors stated that there is “compelling empirical evidence in all three categories that interventions can produce meaningful savings and may reduce waste by as much as half.”
SOURCE: Shrank W et al. JAMA. 2019 Oct 7. doi: 10.1001/jama.2019.13978.
FROM JAMA
CDC updates guidance on vaping-associated lung injury
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
REPORTING FROM A CDC TELEBRIEFING
Short-term statin use linked to risk of skin and soft tissue infections
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
according to a sequence symmetry analysis of prescription claims over a 10-year period reported in the British Journal of Clinical Pharmacology.
In the study, statin use for as little as 91 days was linked with elevated risks of SSTIs and diabetes. However, the increased risk of infection was seen in individuals who did and did not develop diabetes, wrote Humphrey Ko, of the school of pharmacy and biomedical sciences, Curtin University, Perth, Australia, and colleagues.
The current literature on the impact of statins on SSTIs is conflicted, they noted. Previous research shows that statins “may reduce the risk of community-acquired [Staphylococcus aureus] bacteremia and exert antibacterial effects against S. aureus,” and therefore may have potential for reducing SSTI risk “or evolve into promising novel treatments for SSTIs,” the researchers said; they noted, however, that other data show that statins may induce new-onset diabetes.
They examined prescription claims (for statins, antidiabetic medications, and antistaphylococcal antibiotics) from 2001 to 2011 from the Australian Department of Veterans’ Affairs that included more than 228,000 veterans, war widows, and widowers. Prescriptions for antistaphylococcal antibiotics were used as a marker of SSTIs.
Overall, statins were significantly associated with an increased risk of SSTIs at 91 days (adjusted sequence ratio, 1.40). The risk of SSTIs from statin use was similar at 182 (ASR, 1.41) and 365 days (ASR, 1.40). In this case, the ASRs represent the incidence rate ratios of prescribing antibiotics in statin-exposed versus statin-nonexposed person-time.
Statins were associated with a significantly increased risk of new onset diabetes, but the SSTI risk was not significantly different between statin users with and without diabetes. Statin users who did not have diabetes had significant SSTI risks at 91, 182, and 365 days (ASR, 1.39, 1.41, and 1.37, respectively) and statin users with diabetes had similarly significant risks of SSTIs (ASR,1.43, 1.42, and 1.49, respectively).
In addition, socioeconomic status appeared to have no significant effect on the relationship between statin use, SSTIs, and diabetes, the researchers noted.
The findings were limited by several factors including the inability to account for patient compliance in taking the medications, a lack of dosage data to determine the impact of dosage on outcomes, and potential confounding by the presence of diabetes, they said. However, the results suggest that “it would seem prudent for clinicians to monitor blood glucose levels of statin users who are predisposed to diabetes, and be mindful of possible increased SSTI risks in such patients,” they concluded. Statins, they added, “may increase SSTI risk via direct or indirect mechanisms.”
More clinical trials are needed to confirm the mechanisms, and “to ascertain the effect of statins on gut dysbiosis, impaired bile acid metabolism, vitamin D levels, and cholesterol inhibition on skin function,” they wrote.
The study was supported in part by the Australian Government Research Training Program Scholarship, the Curtin Health Innovation Research Institute Biosciences Research Precinct Core Facility, and the School of Pharmacy and Biomedical Sciences (Curtin University). The researchers had no financial conflicts to disclose.
SOURCE: Ko H et al. Br J Clin Pharmacol. 2019 Oct 9. doi: 10.1111/bcp.14077.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Mesh nebulizer worked faster to control acute asthma
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
REPORTING FROM ERS 2019
Accounting for sex may improve diagnosis of amnestic MCI
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
FROM NEUROLOGY
Regional brain activation lower in bipolar disorder patients after multiple manic episodes
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
FROM BIPOLAR DISORDERS
Readmission burden high for those with sickle cell disease
, according to the Agency for Healthcare Research and Quality.
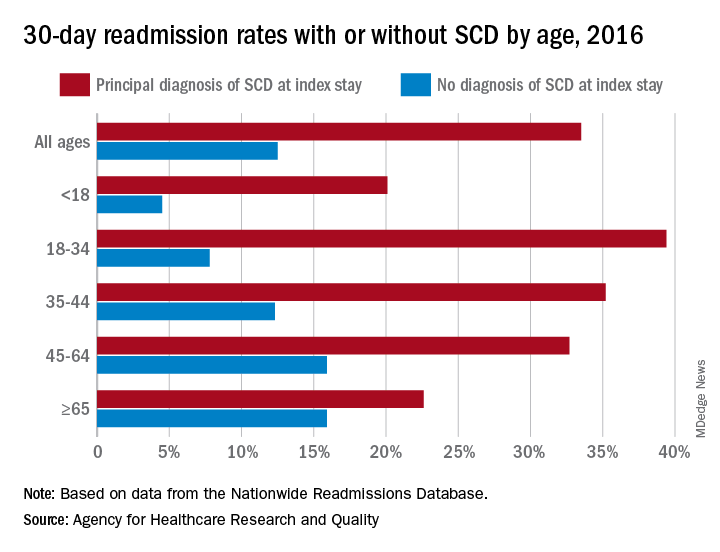
The 30-day all-cause readmission rate for index stays with a principal diagnosis of SCD was 33.5% in 2016, compared with 12.5% for non-SCD hospital stays. Patients with a secondary diagnosis of SCD had readmission rates of 32.9% with a pain crisis and 21.0% without one, and the overall readmission rate for an index stay with any SCD diagnosis was 31.1%, Kathryn R. Fingar, PhD, MPH, of IBM Watson Health, Sacramento, Calif., and associates wrote in an AHRQ statistical brief.
When age is factored in, the readmission gap between a principal SCD diagnosis and non-SCD becomes even greater – and smaller. The difference was greatest for patients aged 18-34 years – 39.4% with a principal diagnosis of SCD versus 7.8% without any SCD – and then narrowed as patients got older. For those aged 65 years and older, the rates were 22.6% with a principal diagnosis of SCD and 15.9% without, the investigators reported.
The approximately 100,000 Americans with SCD accounted for 134,000 admissions in 2016, and more than three-quarters of those stays involved a pain crisis. A principal diagnosis of SCD was recorded for almost 96,000 of those visits, and nearly all (96%) of those stays involved a pain crisis. For those with a secondary diagnosis of SCD, the most common reasons for hospitalization were diseases of the respiratory system (14.3% of those stays) and infectious and parasitic diseases (13.2%).
Patients with SCD were more likely than non-SCD patients to be admitted from the ED (79.6% vs. 51.3%), and they were more likely to discharged against medical advice (4.1% vs. 1.2%). Among those who left the hospital against medical advice, patients with SCD were much more likely to be readmitted than those without SCD (46.6% vs. 26.5%), based on data from the AHRQ’s Nationwide Readmissions Database.
Improved treatment of complications has “reduced mortality rates so that nearly 95% of individuals born with SCD in the United States reach 18 years of age [but] limited knowledge of SCD treatment guidelines among healthcare professionals continues to pose a barrier to effective patient-provider relationships, and this barrier contributes to lower quality of life,” Dr. Fingar and associates wrote.
, according to the Agency for Healthcare Research and Quality.

The 30-day all-cause readmission rate for index stays with a principal diagnosis of SCD was 33.5% in 2016, compared with 12.5% for non-SCD hospital stays. Patients with a secondary diagnosis of SCD had readmission rates of 32.9% with a pain crisis and 21.0% without one, and the overall readmission rate for an index stay with any SCD diagnosis was 31.1%, Kathryn R. Fingar, PhD, MPH, of IBM Watson Health, Sacramento, Calif., and associates wrote in an AHRQ statistical brief.
When age is factored in, the readmission gap between a principal SCD diagnosis and non-SCD becomes even greater – and smaller. The difference was greatest for patients aged 18-34 years – 39.4% with a principal diagnosis of SCD versus 7.8% without any SCD – and then narrowed as patients got older. For those aged 65 years and older, the rates were 22.6% with a principal diagnosis of SCD and 15.9% without, the investigators reported.
The approximately 100,000 Americans with SCD accounted for 134,000 admissions in 2016, and more than three-quarters of those stays involved a pain crisis. A principal diagnosis of SCD was recorded for almost 96,000 of those visits, and nearly all (96%) of those stays involved a pain crisis. For those with a secondary diagnosis of SCD, the most common reasons for hospitalization were diseases of the respiratory system (14.3% of those stays) and infectious and parasitic diseases (13.2%).
Patients with SCD were more likely than non-SCD patients to be admitted from the ED (79.6% vs. 51.3%), and they were more likely to discharged against medical advice (4.1% vs. 1.2%). Among those who left the hospital against medical advice, patients with SCD were much more likely to be readmitted than those without SCD (46.6% vs. 26.5%), based on data from the AHRQ’s Nationwide Readmissions Database.
Improved treatment of complications has “reduced mortality rates so that nearly 95% of individuals born with SCD in the United States reach 18 years of age [but] limited knowledge of SCD treatment guidelines among healthcare professionals continues to pose a barrier to effective patient-provider relationships, and this barrier contributes to lower quality of life,” Dr. Fingar and associates wrote.
, according to the Agency for Healthcare Research and Quality.

The 30-day all-cause readmission rate for index stays with a principal diagnosis of SCD was 33.5% in 2016, compared with 12.5% for non-SCD hospital stays. Patients with a secondary diagnosis of SCD had readmission rates of 32.9% with a pain crisis and 21.0% without one, and the overall readmission rate for an index stay with any SCD diagnosis was 31.1%, Kathryn R. Fingar, PhD, MPH, of IBM Watson Health, Sacramento, Calif., and associates wrote in an AHRQ statistical brief.
When age is factored in, the readmission gap between a principal SCD diagnosis and non-SCD becomes even greater – and smaller. The difference was greatest for patients aged 18-34 years – 39.4% with a principal diagnosis of SCD versus 7.8% without any SCD – and then narrowed as patients got older. For those aged 65 years and older, the rates were 22.6% with a principal diagnosis of SCD and 15.9% without, the investigators reported.
The approximately 100,000 Americans with SCD accounted for 134,000 admissions in 2016, and more than three-quarters of those stays involved a pain crisis. A principal diagnosis of SCD was recorded for almost 96,000 of those visits, and nearly all (96%) of those stays involved a pain crisis. For those with a secondary diagnosis of SCD, the most common reasons for hospitalization were diseases of the respiratory system (14.3% of those stays) and infectious and parasitic diseases (13.2%).
Patients with SCD were more likely than non-SCD patients to be admitted from the ED (79.6% vs. 51.3%), and they were more likely to discharged against medical advice (4.1% vs. 1.2%). Among those who left the hospital against medical advice, patients with SCD were much more likely to be readmitted than those without SCD (46.6% vs. 26.5%), based on data from the AHRQ’s Nationwide Readmissions Database.
Improved treatment of complications has “reduced mortality rates so that nearly 95% of individuals born with SCD in the United States reach 18 years of age [but] limited knowledge of SCD treatment guidelines among healthcare professionals continues to pose a barrier to effective patient-provider relationships, and this barrier contributes to lower quality of life,” Dr. Fingar and associates wrote.
Low-FODMAP diet eases gut symptoms in IBD
A diet low in fermentable carbohydrates can reduce gut symptoms related to inflammatory bowel disease (IBD), according to a study by U.K. researchers.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) occur in a number of common foods, including certain fruits, vegetables, and dairy products. They can draw increased water to the gut, and through microbial fermentation increase hydrogen in the colon.
While previous research has shown that a low-FODMAP diet can relieve gut symptoms such as swelling and flatulence in people with irritable bowel syndrome, the diet has been little studied in IBD patients, for whom gut symptoms often persist even in the absence of gastrointestinal inflammation. In a study published in Gastroenterology, Selina Cox, MD, of King’s College, London, and colleagues randomized 52 people with ulcerative colitis or Crohn’s disease with persistent gut symptoms but without active inflammation to 4 weeks on a low-FODMAP diet (n = 27) or a control diet comprising sham dietary advice (n = 25). Investigators were not blinded to treatment allocation.
At 4 weeks, Dr. Cox and her colleagues reported more patients on the low-FODMAP diet reported “adequate” relief of gut symptoms (52% vs. 16%, P = .007), and saw slight improvements in health-related quality of life scores, compared with the control group. Patient-reported flatulence and bloating were significantly lower in the treatment group, while few other symptom-specific differences were seen between groups.
Stool samples collected at baseline and at the study’s endpoint showed significantly reduced abundance of three types of gut bacteria thought to have a role in immune response – Bifidobacterium adolescentis, B longum, and Faecalibacterium prausnitzii – compared with control subjects. But there were no significant between-group differences in bacterial diversity or in biomarkers of inflammation.
“A major strength of this trial is that low-FODMAP dietary advice was compared to sham dietary advice, providing the first placebo-controlled evidence of effectiveness in IBD,” the researchers wrote in their analysis. Weaknesses of the study include its single-blinded design and inability to control for nutritional alterations related to the low-FODMAP diet.
Ms. Cox and her colleagues recommended a 4-week low-FODMAP diet along with “expert advice and intensive follow-up” for the management of gut symptoms in IBD, but cautioned that longer-term use may not be appropriate.
The study was funded by the U.S.-based Kenneth Rainin Foundation. Two of Dr. Cox’s coauthors declared financial conflicts of interest from a patent on a mobile application to support the low-FODMAP diet; the study’s corresponding author, Kevin Whelan, PhD, additionally reported receiving fees or research support from food and nutrition firms.
SOURCE: Cox S et al. Gastroenterology 2019. doi: 10.1053/j.gastro.2019.09.024.
A diet low in fermentable carbohydrates can reduce gut symptoms related to inflammatory bowel disease (IBD), according to a study by U.K. researchers.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) occur in a number of common foods, including certain fruits, vegetables, and dairy products. They can draw increased water to the gut, and through microbial fermentation increase hydrogen in the colon.
While previous research has shown that a low-FODMAP diet can relieve gut symptoms such as swelling and flatulence in people with irritable bowel syndrome, the diet has been little studied in IBD patients, for whom gut symptoms often persist even in the absence of gastrointestinal inflammation. In a study published in Gastroenterology, Selina Cox, MD, of King’s College, London, and colleagues randomized 52 people with ulcerative colitis or Crohn’s disease with persistent gut symptoms but without active inflammation to 4 weeks on a low-FODMAP diet (n = 27) or a control diet comprising sham dietary advice (n = 25). Investigators were not blinded to treatment allocation.
At 4 weeks, Dr. Cox and her colleagues reported more patients on the low-FODMAP diet reported “adequate” relief of gut symptoms (52% vs. 16%, P = .007), and saw slight improvements in health-related quality of life scores, compared with the control group. Patient-reported flatulence and bloating were significantly lower in the treatment group, while few other symptom-specific differences were seen between groups.
Stool samples collected at baseline and at the study’s endpoint showed significantly reduced abundance of three types of gut bacteria thought to have a role in immune response – Bifidobacterium adolescentis, B longum, and Faecalibacterium prausnitzii – compared with control subjects. But there were no significant between-group differences in bacterial diversity or in biomarkers of inflammation.
“A major strength of this trial is that low-FODMAP dietary advice was compared to sham dietary advice, providing the first placebo-controlled evidence of effectiveness in IBD,” the researchers wrote in their analysis. Weaknesses of the study include its single-blinded design and inability to control for nutritional alterations related to the low-FODMAP diet.
Ms. Cox and her colleagues recommended a 4-week low-FODMAP diet along with “expert advice and intensive follow-up” for the management of gut symptoms in IBD, but cautioned that longer-term use may not be appropriate.
The study was funded by the U.S.-based Kenneth Rainin Foundation. Two of Dr. Cox’s coauthors declared financial conflicts of interest from a patent on a mobile application to support the low-FODMAP diet; the study’s corresponding author, Kevin Whelan, PhD, additionally reported receiving fees or research support from food and nutrition firms.
SOURCE: Cox S et al. Gastroenterology 2019. doi: 10.1053/j.gastro.2019.09.024.
A diet low in fermentable carbohydrates can reduce gut symptoms related to inflammatory bowel disease (IBD), according to a study by U.K. researchers.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) occur in a number of common foods, including certain fruits, vegetables, and dairy products. They can draw increased water to the gut, and through microbial fermentation increase hydrogen in the colon.
While previous research has shown that a low-FODMAP diet can relieve gut symptoms such as swelling and flatulence in people with irritable bowel syndrome, the diet has been little studied in IBD patients, for whom gut symptoms often persist even in the absence of gastrointestinal inflammation. In a study published in Gastroenterology, Selina Cox, MD, of King’s College, London, and colleagues randomized 52 people with ulcerative colitis or Crohn’s disease with persistent gut symptoms but without active inflammation to 4 weeks on a low-FODMAP diet (n = 27) or a control diet comprising sham dietary advice (n = 25). Investigators were not blinded to treatment allocation.
At 4 weeks, Dr. Cox and her colleagues reported more patients on the low-FODMAP diet reported “adequate” relief of gut symptoms (52% vs. 16%, P = .007), and saw slight improvements in health-related quality of life scores, compared with the control group. Patient-reported flatulence and bloating were significantly lower in the treatment group, while few other symptom-specific differences were seen between groups.
Stool samples collected at baseline and at the study’s endpoint showed significantly reduced abundance of three types of gut bacteria thought to have a role in immune response – Bifidobacterium adolescentis, B longum, and Faecalibacterium prausnitzii – compared with control subjects. But there were no significant between-group differences in bacterial diversity or in biomarkers of inflammation.
“A major strength of this trial is that low-FODMAP dietary advice was compared to sham dietary advice, providing the first placebo-controlled evidence of effectiveness in IBD,” the researchers wrote in their analysis. Weaknesses of the study include its single-blinded design and inability to control for nutritional alterations related to the low-FODMAP diet.
Ms. Cox and her colleagues recommended a 4-week low-FODMAP diet along with “expert advice and intensive follow-up” for the management of gut symptoms in IBD, but cautioned that longer-term use may not be appropriate.
The study was funded by the U.S.-based Kenneth Rainin Foundation. Two of Dr. Cox’s coauthors declared financial conflicts of interest from a patent on a mobile application to support the low-FODMAP diet; the study’s corresponding author, Kevin Whelan, PhD, additionally reported receiving fees or research support from food and nutrition firms.
SOURCE: Cox S et al. Gastroenterology 2019. doi: 10.1053/j.gastro.2019.09.024.
FROM GASTROENTEROLOGY