User login
Acute Kidney Injury: Treatment Depends on the Cause
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
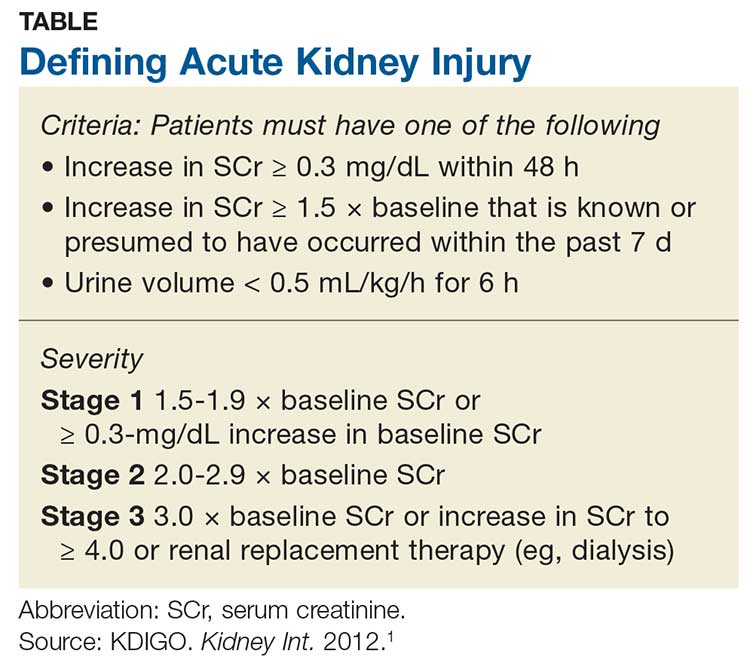
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1
AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1
AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1
AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
Chronic kidney disease may not be deterrent for B-FEVAR
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
CHICAGO – Although advanced renal dysfunction is a major contraindication for open repair of complex thoracoabdominal aneurysms (TAAA) and pararenal aneurysms (PRA), a single-center study of patients who had branched-fenestrated endovascular aneurysm repair (B-FEVAR) found that those with severe or moderate dysfunction and those with normal kidney function had similar results, according to a study reported at the annual meeting of the Midwestern Vascular Surgery Society.
“In our series of patients with stage 4 and 5 chronic kidney disease, branched-fenestration aneurysm repair for pararenal and thoracoabdominal aneurysm was associated with acceptable morbidity and mortality,” said Luis C. Cajas-Monson, MD, of the Mayo Clinic in Rochester, Minn. “Although often a contraindication for open repair, B-FEVAR could be a safe alternative for TAAA patients with poor renal function.”
The study evaluated 231 patients who had B-FEVAR for the following etiologies: 80 for PRA; 89 for Type I to III TAAA; and 62 for type IV TAAA. The patients had at least 1 year of follow-up. A small percentage of patients (4%; n = 9) had stage IV or V chronic kidney disease; the remainder had stage I to III CKD. The study compared results in the lower- and higher-stage CKD groups.
“The frequency of endovascular aortic aneurysm repair continues to increase, and it has advanced to treating more complex aortic pathology,” Dr. Cajas-Monson said. “There appears to be no significant decline in renal function with complex EVAR.” He noted that in open TAAA repair, the more severe the chronic kidney disease state, the worse the outcomes.
The Mayo researchers set out to evaluate the impact of renal function on survival after B-FEVAR for TAAA and PRA. “We hypothesized that renal function is not a significant factor in early and late survival after B-FEVAR,” Dr. Cajas-Monson said. TAAA patients represented 65% of the study population, with 59% having Extent I to III and 41% having Extent IV disease.
Dr. Cajas-Monson noted that demographics were comparable between the higher- and lower-stage CKD groups, with the exception of higher baseline creatinine levels in the CKD 4/5 patients: 3.14 vs. 1.13 (P less than .001). Operative outcomes and length of stay were also similar.
The higher-stage group had a higher overall rate of major adverse events, but given the small sample size this was not found to be significantly different (44% vs. 29%; P = .26). However, there were no events of perioperative death, stroke, paraplegia or estimated blood loss greater than 1 L in the higher-stage patients, while the lower-stage group had low percentages of these events.
Three-year survival was 84% in the lower-stage group and 75% in the higher-stage group.
Dr. Cajas-Monson acknowledged that the small sample size was a limitation of the study. “Further evaluation of patients with renal dysfunction is needed to validate our initial findings,” he said.
This abstract of this study was published in the Journal of Vascular Surgery (2019. 70 [3]:e67).
Dr. Cajas-Monson had no financial relationships to disclose.
SOURCE: Cajas-Monson LC et al. Midwestern Vascular 2019, Abstract 19.
REPORTING FROM MIDWESTERN VASCULAR 2019
Nivolumab boosts overall survival in HCC
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
BARCELONA – Checkpoint inhibition with nivolumab led to a clinically meaningful, but not statistically significant, improvement in overall survival, compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma (HCC) in the phase 3 CheckMate 459 study.
Median overall survival (OS), the primary study endpoint, was 16.4 months in 371 patients randomized to receive the programmed death-1 (PD-1) inhibitor nivolumab, and 14.7 months in 372 patients who received the tyrosine kinase inhibitor sorafenib – the current standard for advanced HCC therapy (hazard ratio, 0.85; P = .0752), Thomas Yau, MD, reported at the European Society for Medical Oncology Congress.
The median OS seen with nivolumab is the longest ever reported in a first-line phase 3 HCC trial, but the difference between the arms did not meet the predefined threshold for statistical significance (HR, 0.84 and P = .419). However, clinical benefit was observed across predefined subgroups of patients, including those with hepatitis infection and those with vascular invasion and/or extrahepatic spread, said Dr. Yau of the University of Hong Kong.
The overall response rates (ORR) were 15% and 7% in the nivolumab and sorafenib arms, with 14 and 5 patients in each group experiencing a complete response (CR), respectively, he said.
At 12 and 24 months, the OS rates in the groups were 59.7% vs. 55.1%, and 36.5% vs. 33.1%, respectively. Median progression-free survival (PFS) was similar in the groups, at 3.7 and 3.8 months, respectively, and analysis by baseline tumor programmed death-ligand 1 (PD-L1) expression showed that ORR was 28% vs. 9% with PD-L1 expression of 1% or greater in the groups, respectively, and 12% vs. 7% among those with PD-L1 expression less than 1%.
Additionally, nivolumab had a more tolerable safety profile; grade 3/4 treatment-related adverse events were reported in 22% and 49% of patients in the groups, respectively, and led to discontinuation in 4% and 8%, respectively. No new safety signals were observed, Dr. Yau said.
Participants in the multicenter study were systemic therapy–naive adults with advanced disease. They were randomized 1:1 to receive intravenous nivolumab at a dose of 240 mg every 2 weeks or oral sorafenib at a dose of 400 mg twice daily, and were followed for at least 22.8 months.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” Dr. Yau said in an ESMO press release. “HCC is often diagnosed in the advanced stage, where effective treatment options are limited. The encouraging efficacy and favorable safety profile seen with nivolumab demonstrates the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
He further noted that the OS benefit seen in this study is “particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy, in the sorafenib arm,” and that the OS impact is bolstered by patient-reported outcomes suggesting improved quality of life in the nivolumab arm.
Nevertheless, the fact that CheckMate 459 did not meet its primary OS endpoint means the findings are unlikely to change the current standard of care, according to Angela Lamarca, MD, PhD, consultant medical oncologist and honorary senior lecturer at the Christie NHS Foundation Trust, University of Manchester (England).
She added, however, that the findings do underscore a potential role for immunotherapy in the first-line treatment of advanced HCC and noted that the clinically meaningful improvement in response rates with nivolumab, along with the checkpoint inhibitor’s favorable safety profile in this study, raise the possibility of its selection in this setting.
“In a hypothetical scenario in which both options ... were available and reimbursed, and if quality of life was shown to be better with nivolumab ... clinicians and patients may favor the option with a more tolerable safety profile,” she said in the press release.
She added, however, that at this point conclusions should be made cautiously and the high cost of immunotherapy should be considered.
Dr. Lamarca also highlighted the finding that patients with high PD-L1 expression had an increased response rate only in the nivolumab arm. This suggests a potential role for PD-L1 expression as a predictive biomarker in advanced HCC, but more research is needed to better understand how to select patients for immunotherapy, she said, adding that the lack of a reliable biomarker may have contributed to the study’s failure to show improved OS with nivolumab.
“In addition, the study design with a ‘high’ predefined threshold of statistical significance is generating confusion in the community, with potentially beneficial therapies generating statistically negative studies,” she noted.
CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau is an advisor and/or consultant to Bristol-Myers Squibb, and reported honoraria from the company to his institution. Dr. Lamarca reported honoraria, consultation fees, travel funding, and/or education funding from Eisai, Nutricia, Ipsen, Pfizer, Bayer, AAA, Sirtex, Delcath, Novartis, and Mylan, as well as participation in company-sponsored speaker bureaus for Pfizer, Ipsen, Merck, and Incyte.
SOURCE: Yau T et al. ESMO 2019, Abstract LBA38-PR
REPORTING FROM ESMO 2019
Retraining Working Memory to Reduce PTSD Symptoms
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.
Working memory (WM)—the function that allows us to temporarily store information and use it for cognitive tasks like learning, reasoning, and comprehension—is thought to play an important role in managing anxiety and intrusive symptoms of posttraumatic stress disorder (PTSD). Researchers have found inefficient filtering of threatening material from WM and increased storage of task-irrelevant threat distractors are associated with elevated anxiety. Thus, veterans with high levels of anxiety may disproportionately allocate cognitive resources toward threatening stimuli, and have trouble keeping threatening thoughts from entering WM and staying there. People with PTSD also have been shown to have problems using WM in emotional contexts, making it hard for them to deal with stressful situations in work and personal life. Some research has suggested that WM training that incorporates affective stimuli may increase the ability to use WM in emotional contexts. In a pilot study, researchers from Medical College of Wisconsin and University of Wisconsin-Milwaukee expanded on that possibility by testing 2 kinds of WM training in 21 veterans with elevated PTSD symptoms.
Participants completed a pretest, 15 sessions of at-home computerized training, a posttest, and a 1-month follow-up session. The computerized tests included tasks to measure WM capacity, such as solving math questions while trying to remember a set of unrelated numbers. The participants were then assigned to active emotional WM training (n-back) or control emotional WM training (1-back). The n-back training involved constant updating of information stored in WM and shifting between visual and auditory stimuli using 8 different faces and 8 different negative words. The training sessions started at the 1-back level and increased in difficulty level by level with performance with > 95% accuracy, or were scaled back with performance with < 75% accuracy. The control training was the same but consisted only of 1 level.
Overall, the researchers say, contrary to their hypothesis, they did not find a significant difference in results from the 2 groups. But they did find that both trainings had an overall “significant and sizable” impact on PTSD symptoms in both groups: 73% of the n-back group and 60% of the 1-back group had a reduction of ≥ 10 points on the PTSD Checklist total scores, which the researchers say is considered a clinically meaningful change. Moreover, both groups showed a clinically meaningful reduction in symptoms at follow-up: 55% and 40%, respectively.
It was interesting, the researchers say, that both groups showed improvement, especially with the 1-back intervention, which they had used as a “minimally effective” control condition. They also note that, anecdotally, the participants found both interventions “quite challenging.” It may be that in this population even the 1-back intervention is enough to detectably reduce symptoms, the researchers say. They were also encouraged to see that the n-back condition (marginally) outperformed the 1-back condition in improving reexperiencing symptoms, theoretically the most relevant training target of WM-focused intervention. The researchers believe that their study yields useful pilot data, suggesting that n-back training can have a clinical impact primarily by reducing reexperiencing symptoms, which are highly likely to indicate the presence of impaired WM functioning.
Psoriasis registry data provide evidence that adalimumab reduces mortality
MADRID – Psoriasis patients on adalimumab for up to 10 years in the prospective, observational, international, real-world ESPRIT registry had a sharply reduced likelihood of all-cause mortality, compared with the age- and sex-matched general population in participating countries, Diamant T. Thaci, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“If someone tells you that you as a dermatologist can save patient lives by controlling psoriasis, you may not believe it. But look at this standardized mortality ratio data,” said Dr. Thaci, professor of dermatology and head of the Comprehensive Center for Inflammation Medicine at the University of Luebeck (Germany).
Indeed, the standardized in routine clinical practice, was 58% lower than expected, based upon published mortality rate data for the general population in the United States, Canada, and the 10 participating European countries. A total of 144 deaths were predicted in the matched general population, yet only 60 deaths occurred in adalimumab-treated registry participants.
This finding is all the more remarkable because ESPRIT participants had high rates of cardiovascular risk factors, as well as a substantial burden of comorbid conditions, as is typical for patients with chronic moderate to severe psoriasis encountered in real-world clinical practice. It’s a different population than enrollees in the long-term, open-label extensions of phase 3, double-blind, randomized, controlled clinical trials of biologics in psoriasis, which at the outset typically excluded patients with baseline substantial comorbidities, he noted.
Moreover, the incidence rates of serious infections, malignancies, and cardiovascular events in ESPRIT participants remained stable over time and well within the range of published rates in psoriasis patients not on biologic therapy.
“All of this underscores the importance of good control of psoriasis,” Dr. Thaci commented.
The ESPRIT registry began enrolling patients and evaluating them every 6 months in 2008, when the vast majority of dermatologists in clinical practice relied upon Physician Global Assessment (PGA) to evaluate treatment efficacy. For this reason, the PGA, rather than the Psoriasis Area and Severity Index, was the main efficacy measure utilized in the registry. The rate of PGA “clear” or “almost clear” was steady over time at 57% at 1 year, 62.1% at 5 years, and 61.5% at 10 years. It should be noted that this was reported in an “as-observed” analysis, which introduces bias favoring a rosier picture of efficacy since by 10 years slightly under half of patients remained on the tumor necrosis factor inhibitor.
However, the primary focus of this ESPRIT analysis was safety, not efficacy. The rate of serious infections was 1.0 event per 100 person-years on adalimumab, compared with published rates of 0.3-2.1 events/100 person-years in psoriasis patients not on a biologic. Malignancies occurred at a rate of 1.3 events/100 person-years in ESPRIT, compared with published rates of 0.5-2.0/100 person-years in psoriasis patients not on biologic therapy. Acute MI occurred at a rate of 0.1 cases/100 person-years in ESPRIT, stroke at 0.2 events/100 person-years, and heart failure at less than 0.1 event/100 person-years, versus a collective rate of 0.6-1.5 events/100 person-years in the comparator population.
Another view of the data is that, at 10 years, 99.4% of ESPRIT participants had not experienced an acute MI, 95.9% hadn’t had a malignancy, and 96.1% remained free of serious infection while on adalimumab, Dr. Thaci continued.
Injection-site reactions, a significant source of concern when adalimumab first reached the marketplace, occurred at a rate of 0.2 events/100 person-years over the course of 10 years. Oral candidiasis, active tuberculosis, demyelinating disorders, and lupus-like reactions were rare, each occurring at an incidence of less than 0.1 event/100 person-years.
Dr. Thaci’s 10-year update from the ongoing registry follows an earlier report of the 5-year results (J Am Acad Dermatol. 2015 Sep;73[3]:410-9.e6).
The ESPRIT registry is sponsored by AbbVie. Dr. Thaci reported serving as a consultant to and/or receiving research grants from that pharmaceutical company and nearly two dozen others.
MADRID – Psoriasis patients on adalimumab for up to 10 years in the prospective, observational, international, real-world ESPRIT registry had a sharply reduced likelihood of all-cause mortality, compared with the age- and sex-matched general population in participating countries, Diamant T. Thaci, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“If someone tells you that you as a dermatologist can save patient lives by controlling psoriasis, you may not believe it. But look at this standardized mortality ratio data,” said Dr. Thaci, professor of dermatology and head of the Comprehensive Center for Inflammation Medicine at the University of Luebeck (Germany).
Indeed, the standardized in routine clinical practice, was 58% lower than expected, based upon published mortality rate data for the general population in the United States, Canada, and the 10 participating European countries. A total of 144 deaths were predicted in the matched general population, yet only 60 deaths occurred in adalimumab-treated registry participants.
This finding is all the more remarkable because ESPRIT participants had high rates of cardiovascular risk factors, as well as a substantial burden of comorbid conditions, as is typical for patients with chronic moderate to severe psoriasis encountered in real-world clinical practice. It’s a different population than enrollees in the long-term, open-label extensions of phase 3, double-blind, randomized, controlled clinical trials of biologics in psoriasis, which at the outset typically excluded patients with baseline substantial comorbidities, he noted.
Moreover, the incidence rates of serious infections, malignancies, and cardiovascular events in ESPRIT participants remained stable over time and well within the range of published rates in psoriasis patients not on biologic therapy.
“All of this underscores the importance of good control of psoriasis,” Dr. Thaci commented.
The ESPRIT registry began enrolling patients and evaluating them every 6 months in 2008, when the vast majority of dermatologists in clinical practice relied upon Physician Global Assessment (PGA) to evaluate treatment efficacy. For this reason, the PGA, rather than the Psoriasis Area and Severity Index, was the main efficacy measure utilized in the registry. The rate of PGA “clear” or “almost clear” was steady over time at 57% at 1 year, 62.1% at 5 years, and 61.5% at 10 years. It should be noted that this was reported in an “as-observed” analysis, which introduces bias favoring a rosier picture of efficacy since by 10 years slightly under half of patients remained on the tumor necrosis factor inhibitor.
However, the primary focus of this ESPRIT analysis was safety, not efficacy. The rate of serious infections was 1.0 event per 100 person-years on adalimumab, compared with published rates of 0.3-2.1 events/100 person-years in psoriasis patients not on a biologic. Malignancies occurred at a rate of 1.3 events/100 person-years in ESPRIT, compared with published rates of 0.5-2.0/100 person-years in psoriasis patients not on biologic therapy. Acute MI occurred at a rate of 0.1 cases/100 person-years in ESPRIT, stroke at 0.2 events/100 person-years, and heart failure at less than 0.1 event/100 person-years, versus a collective rate of 0.6-1.5 events/100 person-years in the comparator population.
Another view of the data is that, at 10 years, 99.4% of ESPRIT participants had not experienced an acute MI, 95.9% hadn’t had a malignancy, and 96.1% remained free of serious infection while on adalimumab, Dr. Thaci continued.
Injection-site reactions, a significant source of concern when adalimumab first reached the marketplace, occurred at a rate of 0.2 events/100 person-years over the course of 10 years. Oral candidiasis, active tuberculosis, demyelinating disorders, and lupus-like reactions were rare, each occurring at an incidence of less than 0.1 event/100 person-years.
Dr. Thaci’s 10-year update from the ongoing registry follows an earlier report of the 5-year results (J Am Acad Dermatol. 2015 Sep;73[3]:410-9.e6).
The ESPRIT registry is sponsored by AbbVie. Dr. Thaci reported serving as a consultant to and/or receiving research grants from that pharmaceutical company and nearly two dozen others.
MADRID – Psoriasis patients on adalimumab for up to 10 years in the prospective, observational, international, real-world ESPRIT registry had a sharply reduced likelihood of all-cause mortality, compared with the age- and sex-matched general population in participating countries, Diamant T. Thaci, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“If someone tells you that you as a dermatologist can save patient lives by controlling psoriasis, you may not believe it. But look at this standardized mortality ratio data,” said Dr. Thaci, professor of dermatology and head of the Comprehensive Center for Inflammation Medicine at the University of Luebeck (Germany).
Indeed, the standardized in routine clinical practice, was 58% lower than expected, based upon published mortality rate data for the general population in the United States, Canada, and the 10 participating European countries. A total of 144 deaths were predicted in the matched general population, yet only 60 deaths occurred in adalimumab-treated registry participants.
This finding is all the more remarkable because ESPRIT participants had high rates of cardiovascular risk factors, as well as a substantial burden of comorbid conditions, as is typical for patients with chronic moderate to severe psoriasis encountered in real-world clinical practice. It’s a different population than enrollees in the long-term, open-label extensions of phase 3, double-blind, randomized, controlled clinical trials of biologics in psoriasis, which at the outset typically excluded patients with baseline substantial comorbidities, he noted.
Moreover, the incidence rates of serious infections, malignancies, and cardiovascular events in ESPRIT participants remained stable over time and well within the range of published rates in psoriasis patients not on biologic therapy.
“All of this underscores the importance of good control of psoriasis,” Dr. Thaci commented.
The ESPRIT registry began enrolling patients and evaluating them every 6 months in 2008, when the vast majority of dermatologists in clinical practice relied upon Physician Global Assessment (PGA) to evaluate treatment efficacy. For this reason, the PGA, rather than the Psoriasis Area and Severity Index, was the main efficacy measure utilized in the registry. The rate of PGA “clear” or “almost clear” was steady over time at 57% at 1 year, 62.1% at 5 years, and 61.5% at 10 years. It should be noted that this was reported in an “as-observed” analysis, which introduces bias favoring a rosier picture of efficacy since by 10 years slightly under half of patients remained on the tumor necrosis factor inhibitor.
However, the primary focus of this ESPRIT analysis was safety, not efficacy. The rate of serious infections was 1.0 event per 100 person-years on adalimumab, compared with published rates of 0.3-2.1 events/100 person-years in psoriasis patients not on a biologic. Malignancies occurred at a rate of 1.3 events/100 person-years in ESPRIT, compared with published rates of 0.5-2.0/100 person-years in psoriasis patients not on biologic therapy. Acute MI occurred at a rate of 0.1 cases/100 person-years in ESPRIT, stroke at 0.2 events/100 person-years, and heart failure at less than 0.1 event/100 person-years, versus a collective rate of 0.6-1.5 events/100 person-years in the comparator population.
Another view of the data is that, at 10 years, 99.4% of ESPRIT participants had not experienced an acute MI, 95.9% hadn’t had a malignancy, and 96.1% remained free of serious infection while on adalimumab, Dr. Thaci continued.
Injection-site reactions, a significant source of concern when adalimumab first reached the marketplace, occurred at a rate of 0.2 events/100 person-years over the course of 10 years. Oral candidiasis, active tuberculosis, demyelinating disorders, and lupus-like reactions were rare, each occurring at an incidence of less than 0.1 event/100 person-years.
Dr. Thaci’s 10-year update from the ongoing registry follows an earlier report of the 5-year results (J Am Acad Dermatol. 2015 Sep;73[3]:410-9.e6).
The ESPRIT registry is sponsored by AbbVie. Dr. Thaci reported serving as a consultant to and/or receiving research grants from that pharmaceutical company and nearly two dozen others.
REPORTING FROM THE EADV CONGRESS
Wildfire smoke impact, part 2: Resources, advice for patients
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
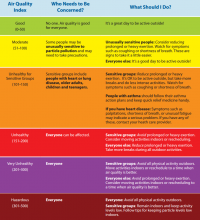
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Psoriasis & Psoriatic Arthritis: A Supplement
This Psoriasis and Psoriatic Arthritis supplement to Dermatology News includes commentary from Alan Menter, MD, and Joel M. Gelfand, MD. Highlights include: Dosage cuts of biologics; AAD/NPF guidelines; the impact of comorbidities; weight loss and outcomes; and treatment of elderly patients.
This Psoriasis and Psoriatic Arthritis supplement to Dermatology News includes commentary from Alan Menter, MD, and Joel M. Gelfand, MD. Highlights include: Dosage cuts of biologics; AAD/NPF guidelines; the impact of comorbidities; weight loss and outcomes; and treatment of elderly patients.
This Psoriasis and Psoriatic Arthritis supplement to Dermatology News includes commentary from Alan Menter, MD, and Joel M. Gelfand, MD. Highlights include: Dosage cuts of biologics; AAD/NPF guidelines; the impact of comorbidities; weight loss and outcomes; and treatment of elderly patients.
Multiple zoledronic acid doses may be needed after stopping denosumab
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
ORLANDO – A single infusion of zoledronic acid does not completely prevent bone mineral density (BMD) loss regardless of the timing of the infusion in patients with osteopenia who discontinued denosumab (Prolia), according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
“If you consider a treatment break in patients treated with [denosumab] for a long time, I suggest to aim for a higher BMD, allowing a smaller bone loss when transitioning via [zoledronic acid] to a treatment break, or maybe you need to give more than one infusion of [zoledronic acid],” said Bente Langdahl, MD, PhD, of Aarhus (Denmark) University Hospital during her presentation.
Dr. Langdahl and her colleagues enrolled 60 patients with osteopenia in a 2-year, randomized, open-label interventional study of patients who discontinued denosumab after more than 2 years of use (average treatment, 4.6 years). One group of 20 patients received 5 mg of zoledronic acid at 6 months after their last denosumab injection. Another group of 20 patients underwent monthly monitoring starting at 6 months after their last denosumab injection and received zoledronic acid if their s-carboxy-terminal collagen crosslinks (s-CTX) increased more than 1.26 mcg/L or if they reached 9 months after their last denosumab injection. A third group underwent observation with monthly monitoring starting at 6 months after their last denosumab injection, and they received zoledronic acid if s-CTX increased more than 1.26 mcg/L, BMD loss was 5% or more at any site, they experienced a fragility vertebral or hip fracture, or they reached 12 months after their last denosumab injection. All patients received a readministration of zoledronic acid if their BMD decreased by greater than 5% or if their s-CTX increased more than 1.26 mcg/L after 6 months, 12 months, or 24 months.
The researchers included postmenopausal women and men older than 50 years (mean, 67.7 years), but the majority of patients were women (n = 53) distributed evenly between the 6-month, 9-month, and observation groups. Patients were excluded if they had a low-energy vertebral fracture, had any hip fracture within 12 months, had a T score of less than –2.5 at any site, had received alendronate more than 3 years prior to taking denosumab, used glucocorticoids, had metabolic bone disease, had received hormone replacement therapy, or had cancer.
The observational group patients who met criteria to receive zoledronic acid because of increased s-CTX included one at 1 month after stopping denosumab, two at 2 months, six at 3 months, and one at 4 months. Of six patients who met BMD criteria for treatment at 3 months, one received retreatment 6 months after their first administration of zoledronic acid, and four patients received retreatment at 12 months.
At 2 months, two patients in the 9-month group met s-CTX criteria for treatment, four patients underwent retreatment under BMD criteria 6 months after the first administration of zoledronic acid, and one patient underwent retreatment at 12 months under BMD criteria. In the 6-month group, one patient met s-CTX criteria for retreatment at 6 months, and one patient at 12 months, with five patients meeting BMD criteria for retreatment at 6 months and at 12 months.
Overall, the average bone loss was 4.6% at the lumbar spine and 3.2% for total hip with no clinically significant between-group differences for either site. At 12 months, lumbar spine bone loss was 4.8% in the 6-month group, 4.2% in the 9-month group, and 4.9% in the observational group (P less than or equal to .006), and total hip bone loss was 2.6% in the 6-month group, 3.3% in the 9-month group, and 3.8% in the observational group (P less than or equal to .001).
Although the study followed patients for 2 years after the first zoledronic acid injection, data were available for the first year only, and the study is ongoing, Dr. Langdahl said.
This study was funded in part by Amgen, the Foundation of Vilhelm Pedersen and wife, Aarhus University, the Danish Osteoporosis Society Research Foundation, the P. Carl Petersens Foundation, and the Torkil Steenbeck Foundation. Dr. Langdahl reported receiving research funding from Amgen and Novo Nordisk and is on the advisory board for Amgen, Eli Lilly, and UCB.
SOURCE: Sølling A. ASBMR 2019. Abstract LB-1169
REPORTING FROM ASBMR 2019
Retinal artery blockage doesn’t necessarily portend stroke
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
CHICAGO – Occlusion of the retinal artery has been thought to be a predictor of stroke, but an analysis of patients with diagnosed retinal artery occlusion at Cleveland Clinic has found that their risk of stroke is about the same as the general population, a researcher reported at the annual meeting of the Midwestern Vascular Surgery Society.
“Subsequent hemispheric stroke is rare with or following retinal artery occlusion (RAO),” said David Laczynski, MD, a vascular surgeon at the Cleveland Clinic. “We do caution that large database studies may be overestimating the risk of stroke after RAO.” Studies have reported a stroke rate of up to 20% at 1 year, he said (Am J Ophthalmol. 2012;154:645-52).
ROA is a thromboembolic disorder of the vessels that provide blood to the back of the eye. American Academy of Ophthalmology preferred practice patterns recommend that patients with central RAO should be referred to the emergency department or a stroke center.
“As the vascular surgeon who’s on the receiving end of these consults, we have little data to provide to our patients as far as what their prognosis is,” Dr. Laczynski said. He noted the pathogenesis varies and that the diagnosis is difficult to arrive at. Fluorescein angiography imaging of the retina is essential to confirm diagnosis of ROA, but Dr. Laczynski said that many institutions do not have access to this level of imaging.
The study evaluated 221 patients whose RAO was confirmed with fluorescein angiography from 2004 to 2018 at the Cleveland Clinic Cole Eye Institute. The impetus of the study was to use the eye center to evaluate the institution’s experience with RAO, Dr. Laczynski said. “We were specifically concerned with looking at confirmed, symptomatic RAO with the risk of subsequent stroke,” he said. The study’s hypothesis was that RAO is not associated with an increased risk of stroke. The study population is the largest series in ROA ever reported, Dr. Laczynski said.
The average age of patients was 66 years. With a median follow-up of 2.2 years, the stroke rate was 2.3% (n = 5), with four of the strokes occurring at the time of RAO and one at 1.2 years later. Only one stroke patient had greater than 50% stenosis of the carotid artery. The rate of stroke, death, or MI was 10% (n = 22), Dr. Laczynski said. When concurrent ischemic events were excluded, the stroke rate was less than 1%.
“Sixty-three percent of patients (n = 141) had carotid imaging, but only 14.2% (n = 20) had more than 50% stenosis of the carotid artery,” Dr. Laczynski said. “Ten patients had carotid intervention.”
Among study limitations Dr. Laczynski pointed out were its single-center, retrospective nature and that not all patients had carotid artery imaging. “We cannot make any conclusion in regard to RAO and carotid artery disease,” Dr. Laczynski said.
This study was also published in the Journal of Vascular Surgery (2019 Sep;70[3]:e59-60).
Dr. Laczynski has no financial relationships to disclose.
SOURCE: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
REPORTING FROM MIDWESTERN VASCULAR 2019
Key clinical point: Retinal artery occlusion may not necessarily increase one’s stroke risk.
Major finding: The risk of stroke in patients with RAO was 2.3%.
Study details: A retrospective, single-institution review of 221 patients from 2004 to 2018.
Disclosures: Dr. Laczynski has no financial relationships to disclose.
Source: Laczynski DJ et al. Midwestern Vascular 2019. J Vasc Surg. 2019 Sep;70[3]:e59-60.
Continuation of Natalizumab Treatment Reduces Risk of MS Relapses During Pregnancy
Key clinical point: Continuing natalizumab treatment during pregnancy reduces the risk of relapse and does not increase risks to newborns.
Major finding: The annualized relapse rate among women who continued treatment after the first trimester was 0.09.
Study details: A comparison of two cohorts of women with multiple sclerosis who received treatment with natalizumab and became pregnant.
Disclosures: The authors received funding from companies such as Biogen, Merck Serono, and Teva.
Citation: Landi D et al. ECTRIMS 2019, Abstract 338.
Key clinical point: Continuing natalizumab treatment during pregnancy reduces the risk of relapse and does not increase risks to newborns.
Major finding: The annualized relapse rate among women who continued treatment after the first trimester was 0.09.
Study details: A comparison of two cohorts of women with multiple sclerosis who received treatment with natalizumab and became pregnant.
Disclosures: The authors received funding from companies such as Biogen, Merck Serono, and Teva.
Citation: Landi D et al. ECTRIMS 2019, Abstract 338.
Key clinical point: Continuing natalizumab treatment during pregnancy reduces the risk of relapse and does not increase risks to newborns.
Major finding: The annualized relapse rate among women who continued treatment after the first trimester was 0.09.
Study details: A comparison of two cohorts of women with multiple sclerosis who received treatment with natalizumab and became pregnant.
Disclosures: The authors received funding from companies such as Biogen, Merck Serono, and Teva.
Citation: Landi D et al. ECTRIMS 2019, Abstract 338.