User login
Peripheral nervous system events have lasting impact on SLE patients
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
REPORTING FROM ARTHRITIS & RHEUMATOLOGY
FDA approves Wakix for excessive daytime sleepiness
The Food and Drug Administration has approved pitolisant (Wakix) for excessive daytime sleepiness among patients with narcolepsy, according to a release from the drug’s developer.
Approval of this once-daily, selective histamine 3–receptor antagonist/inverse agonist was based on a pair of multicenter, randomized, double-blind, placebo-controlled studies that included a total of 261 patients. Patients in both studies experienced statistically significant improvements in excessive daytime sleepiness according to Epworth Sleepiness Scale scores.
Rates of adverse advents at or greater than 5% and more than double that of placebo included insomnia (6%), nausea (6%), and anxiety (5%). Patients with severe liver disease should not use pitolisant. Pitolisant has not been evaluated in patients under 18 years of age, and patients who are pregnant or planning to become pregnant are encouraged to enroll in a pregnancy exposure registry.
Full prescribing information, including contraindications and warnings, can be found on the FDA website.
The Food and Drug Administration has approved pitolisant (Wakix) for excessive daytime sleepiness among patients with narcolepsy, according to a release from the drug’s developer.
Approval of this once-daily, selective histamine 3–receptor antagonist/inverse agonist was based on a pair of multicenter, randomized, double-blind, placebo-controlled studies that included a total of 261 patients. Patients in both studies experienced statistically significant improvements in excessive daytime sleepiness according to Epworth Sleepiness Scale scores.
Rates of adverse advents at or greater than 5% and more than double that of placebo included insomnia (6%), nausea (6%), and anxiety (5%). Patients with severe liver disease should not use pitolisant. Pitolisant has not been evaluated in patients under 18 years of age, and patients who are pregnant or planning to become pregnant are encouraged to enroll in a pregnancy exposure registry.
Full prescribing information, including contraindications and warnings, can be found on the FDA website.
The Food and Drug Administration has approved pitolisant (Wakix) for excessive daytime sleepiness among patients with narcolepsy, according to a release from the drug’s developer.
Approval of this once-daily, selective histamine 3–receptor antagonist/inverse agonist was based on a pair of multicenter, randomized, double-blind, placebo-controlled studies that included a total of 261 patients. Patients in both studies experienced statistically significant improvements in excessive daytime sleepiness according to Epworth Sleepiness Scale scores.
Rates of adverse advents at or greater than 5% and more than double that of placebo included insomnia (6%), nausea (6%), and anxiety (5%). Patients with severe liver disease should not use pitolisant. Pitolisant has not been evaluated in patients under 18 years of age, and patients who are pregnant or planning to become pregnant are encouraged to enroll in a pregnancy exposure registry.
Full prescribing information, including contraindications and warnings, can be found on the FDA website.
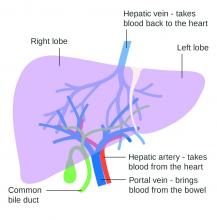
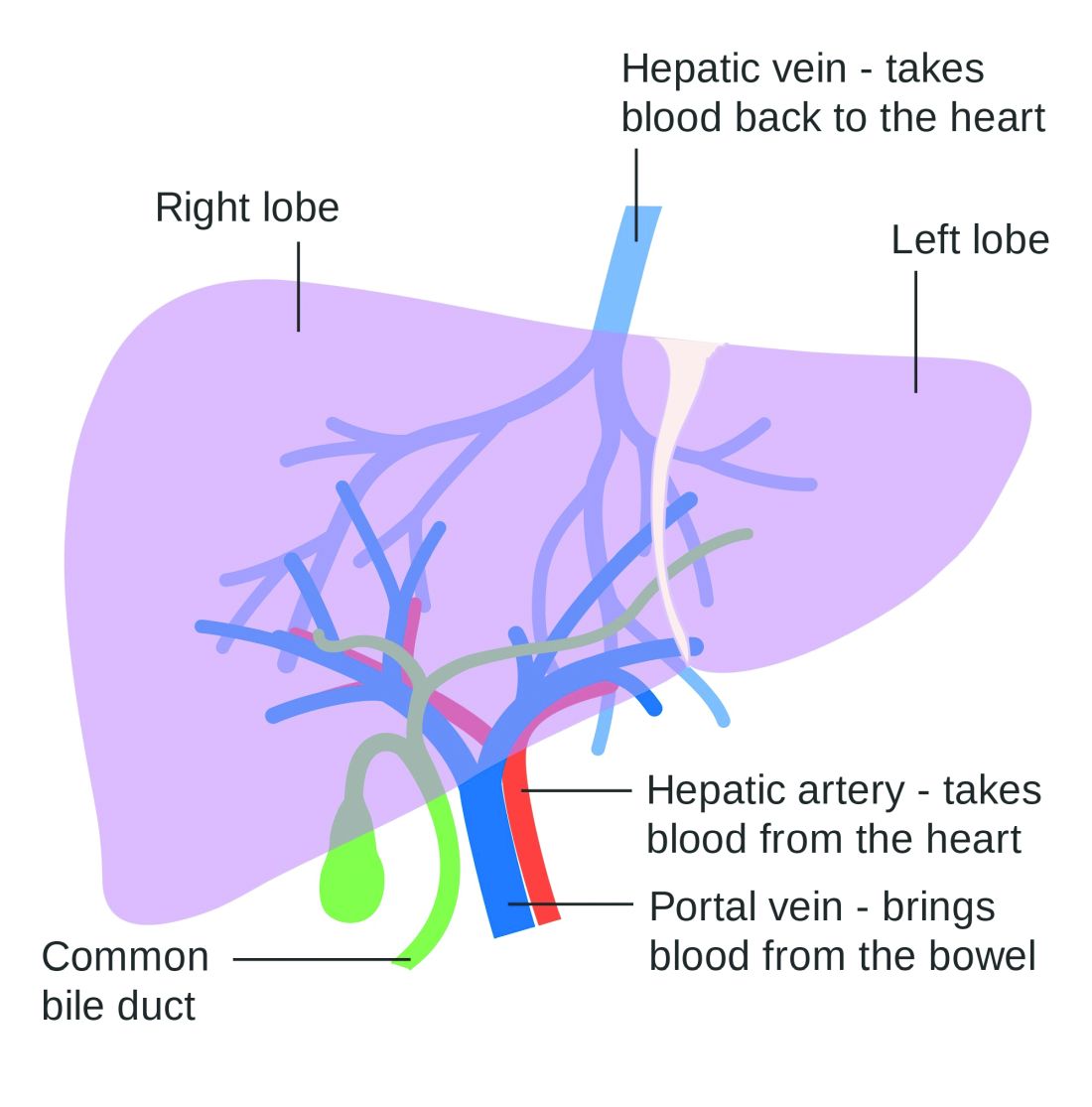
Lowering portal pressure boosts cirrhosis outcomes
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Monitoring Acne Patients on Oral Therapy: Survey of the Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
FDA approves lefamulin for community-acquired bacterial pneumonia treatment
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
Diet, exercise don’t improve breast cancer-related lymphedema
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
FROM JAMA ONCOLOGY
Recurrence score may help predict chemotherapy benefit in grade 3 breast cancers
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
FROM JCO PRECISION ONCOLOGY
Cardiovascular cost of smoking may last up to 25 years
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
FROM JAMA
Key clinical point:
Major finding: In the offspring cohort, heavy smokers showed elevated incidence of CVD for up to 25 years after quitting smoking.
Study details: A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study.
Disclosures: The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
Source: Duncan M et al. JAMA. 2019. doi: 10.1001/jama.2019.10298.
New biomarker model outmatches conventional risk factors for predicting mortality
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
A new model using 14 biomarkers may be more accurate at predicting longer-term mortality than a model comprising conventional risk factors, based on the largest metabolomics study to date.
The prognostic model was more accurate at predicting 5- and 10-year mortality across all ages, reported Joris Deelen, PhD, of Leiden (the Netherlands) University Medical Center and colleagues.
“These results suggest that metabolic biomarker profiling could potentially be used to guide patient care, if further validated in relevant clinical settings,” the investigators wrote in Nature Communications.
“There is no consensus on the ultimate set of predictors of longer-term [5-10 years] mortality risk, since the predictive power of the currently used risk factors is limited, especially at higher ages,” the investigators wrote. “However, it is especially this age group and follow-up time window for which a robust tool would aid clinicians in assessing whether treatment is still sensible.”
The current study was a survival meta-analysis of 44,168 individuals from 12 cohorts aged between 18 and 109 years at baseline. First, the investigators looked for associations between 226 metabolic biomarkers and all-cause mortality in the 5,512 people who died during follow-up. This revealed associations between mortality and 136 biomarkers, which increased to 159 biomarkers after adjusting for recently reported all-cause mortality associations with albumin, very low-density lipoprotein (VLDL) particle size, citrate, and glycoprotein acetyls. Because of strong correlations between many of the biomarkers evaluated, the investigators pared the field down to 63 biomarkers, then used a forward-backward procedure to ultimately identify 14 biomarkers independently associated with mortality. Of the four recently described biomarkers, citrate was excluded from the final model because of its minimal contribution to mortality estimates.
The 14 biomarkers were total lipids in chylomicrons and extremely large VLDL cholesterol, total lipids in small HDL cholesterol, mean diameter for VLDL cholesterol particles, ratio of polyunsaturated fatty acids to total fatty acids, glucose, lactate, histidine, isoleucine, leucine, valine, phenylalanine, acetoacetate, albumin, and glycoprotein acetyls.
“The 14 identified biomarkers are involved in various processes, such as lipoprotein and fatty acid metabolism, glycolysis, fluid balance, and inflammation. Although the majority of these biomarkers have been associated with mortality before, this is the first study that shows their independent effect when combined into one model,” the researchers wrote.
Implementation of the new biomarker model led to a score that typically ranged from –2 to 3. A 1-point increase was associated with a 173% increased risk of death (hazard ratio, 2.73; P less than 1 x 10–132). Analysis of cause-specific mortality revealed that most biomarkers were predictive of multiple causes of death. Some biomarkers were more focused; glucose, for example, was more predictive of cardiovascular-related death than of death because of cancer or nonlocalized infections. Compared with a model incorporating conventional risk factors, the biomarker model more accurately predicted 5- and 10-year mortality, with respective C-statistics of 0.837 versus 0.772 and 0.830 versus 0.790. This superiority was even more pronounced when only individuals aged older than 60 years were included.
The study was funded by Biobanking and BioMolecular resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
SOURCE: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
FROM NATURE COMMUNICATIONS
Key clinical point: A new model using 14 biomarkers may be more accurate at predicting 5- and 10-year mortality than a model comprising conventional risk factors.
Major finding: The biomarker model better predicted 5-year mortality than the conventional model (C-statistic, 0.837 vs. 0.772).
Study details: A retrospective metabolomics study involving 44,168 individuals.
Disclosures: The study was funded by Biobanking and BioMolecular Resources Research Initiative–Netherlands. The investigators reported additional relationships with Nightingale Health, Novo Nordisk, and Bayer.
Source: Deelen J et al. Nature Comm. 2019 Aug 20. doi: 10.1038/s41467-019-11311-9.
USPSTF expands BRCA1/2 testing recommendations
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
FROM JAMA