User login
Hadlima approved as fourth adalimumab biosimilar in U.S.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
Visit the AGA GI Patient Center for information to share with your patients about biologics and biosimilars at https://www.gastro.org/practice-guidance/gi-patient-center/topic/biosimilars.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
Visit the AGA GI Patient Center for information to share with your patients about biologics and biosimilars at https://www.gastro.org/practice-guidance/gi-patient-center/topic/biosimilars.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
Visit the AGA GI Patient Center for information to share with your patients about biologics and biosimilars at https://www.gastro.org/practice-guidance/gi-patient-center/topic/biosimilars.
Early phase trial shows durable responses to gene therapy for hemophilia A
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
REPORTING FROM 2019 ISTH CONGRESS
Probiotic protects against aspirin-related intestinal damage
Source: American Gastroenterological Association
Healthy volunteers given aspirin and Bif195 had significantly less damage and fewer ulcers in their small intestines than did control participants who received aspirin alone, reported lead author Brynjulf Mortensen, PhD, an employee of Chr. Hansen A/S, and colleagues in Gastroenterology. These findings may be relevant for millions of people, the investigators noted, because 30% of Americans older than 40 years take low-dose acetylsalicylic acid (ASA/aspirin) for cardiovascular disease (CVD).
NSAID-associated gastrointestinal issues are a long-standing and well-known problem, but the pathogenesis of this process in the small intestine appears more complex than in the stomach. The investigators pointed out that proton pump inhibitors, which limit gastropathy by suppressing acid, may actually worsen issues in the small intestine via disruption of microbiota.
“Whereas acid and pepsin are the principal luminal aggressors in NSAID-gastropathy, bile and indeed bacteria are the luminal factors in NSAID-enteropathy,” the investigators wrote.
“Given that deleterious compositional changes to the microbiota, in addition to direct effects on mucus and epithelial tissue, may increase the risk of NSAID-enteropathy, we hypothesized that an intervention targeting microbiome-host interactions may offer an attractive, preventative strategy,” the investigators wrote. They noted that previous human trials using probiotics for NSAID-enteropathy have been inconsistent; however, they suggested that Bifidobacteria remain worthy candidates because of their reported abilities to outcompete pathogenic bacteria, strengthen the intestinal epithelial layer, and modulate inflammation. “Our strain selection was based on the anti-inflammatory properties of certain Bifidobacteria and experimental preclinical evidence for a role of Bifidobacteria in NSAID-associated ulceration as well as unpublished preclinical screening data suggesting a particular potential of efficacy for the specific strain belonging to this genus.”
The double-blind, placebo-controlled trial involved 75 healthy volunteers aged 18-40 years who lived a sedentary lifestyle; during the study, they refrained from medications and bacterial products that might alter gastrointestinal function. Participants were randomized in a 1:1 ratio to received Bif195 or placebo for 8 weeks. Aspirin 300 mg was given daily to all participants for the first 6 weeks. At six points in time, video capsule endoscopy (VCE) was performed to determine the effect of treatment. The primary endpoint was intestinal damage, reported as area under the curve (AUC) for Lewis score, which incorporates stenosis, villous edema, and ulcers. The main secondary endpoint focused on ulcers, quantified by a separate AUC. Six other secondary endpoints evaluated symptoms, blood intestinal fatty acid binding protein (I-FABP), red spots visualized on VCE, and calprotectin.
After the 8-week period, 66 of 75 participants remained in the trial. Significantly less intestinal pathology was encountered among patients who received Bif195 than among those who did not. Specifically, for Lewis score, AUC in the Bif195 group was 3,040 plus or minus 1,340 arbitrary units (au), compared with 4,351 plus or minus 3,195 au in the placebo group (P = .0376). For ulcers alone, the Bif195 cohort had an AUC ulcer number of 50.4 plus or minus 53.1 au, versus 75.2 plus or minus 85.3 au for the placebo arm (P = .0258). Fecal calprotectin was also significantly lower in the Bif195 group than in the placebo group, whereas the remaining five secondary endpoints, which included symptom measurement, did not achieve statistical significance.
“Interestingly, fecal microbiome analysis revealed changes were limited to a marked increase in the total B. breve population in the Bif195 arm,” the investigators wrote. “These data provide further evidence that microbial intervention strategies targeting the microbiome can be clinically efficacious without inducing major alterations in the overall microbial population structure.”
Both aspirin and Bif195 were well tolerated during the trial, without statistically significant differences in adverse events between the treatment and placebo arms. No adverse events were considered related to Bif195.
“The trial results indicate that Bifidobacterium breve Bif195 confers significant and objectively verifiable protection against small-intestinal damage caused by a 6-week ASA challenge in healthy volunteers,” the investigators wrote.
“Further clinical trials are required to test whether the strain has clinical efficacy also in other settings and populations, i.e. in chronic users of ASA,” they concluded.
The study was funded by Chr. Hansen A/S. One author reported additional support from the Science Foundation Ireland.
SOURCE: Mortensen B et al. Gastroenterology. 2019 May 13. doi: 10.1053/j.gastro.2019.05.008.
Gastrointestinal bleeding related to nonsteroidal anti-inflammatory drug (NSAID) use is a significant cause of morbidity and mortality in patients taking these drugs. The risk of NSAID-related peptic ulcer can be reduced by proton pump inhibitor therapy, but no intervention has been proven to reduce ulceration beyond the duodenum in NSAID users. Animal models suggest the gut microbiota may be important in the development of NSAID-related small-bowel intestinal injury, but how this translates to patients is unclear.
Paul Moayyedi, MB ChB, MPH, PhD, is the Audrey Campbell Ulcerative Colitis Research Chair and assistant dean of research at McMaster University. He is also the principal investigator of the Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects network. He has no conflicts of interest.
Gastrointestinal bleeding related to nonsteroidal anti-inflammatory drug (NSAID) use is a significant cause of morbidity and mortality in patients taking these drugs. The risk of NSAID-related peptic ulcer can be reduced by proton pump inhibitor therapy, but no intervention has been proven to reduce ulceration beyond the duodenum in NSAID users. Animal models suggest the gut microbiota may be important in the development of NSAID-related small-bowel intestinal injury, but how this translates to patients is unclear.
Paul Moayyedi, MB ChB, MPH, PhD, is the Audrey Campbell Ulcerative Colitis Research Chair and assistant dean of research at McMaster University. He is also the principal investigator of the Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects network. He has no conflicts of interest.
Gastrointestinal bleeding related to nonsteroidal anti-inflammatory drug (NSAID) use is a significant cause of morbidity and mortality in patients taking these drugs. The risk of NSAID-related peptic ulcer can be reduced by proton pump inhibitor therapy, but no intervention has been proven to reduce ulceration beyond the duodenum in NSAID users. Animal models suggest the gut microbiota may be important in the development of NSAID-related small-bowel intestinal injury, but how this translates to patients is unclear.
Paul Moayyedi, MB ChB, MPH, PhD, is the Audrey Campbell Ulcerative Colitis Research Chair and assistant dean of research at McMaster University. He is also the principal investigator of the Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects network. He has no conflicts of interest.
Source: American Gastroenterological Association
Healthy volunteers given aspirin and Bif195 had significantly less damage and fewer ulcers in their small intestines than did control participants who received aspirin alone, reported lead author Brynjulf Mortensen, PhD, an employee of Chr. Hansen A/S, and colleagues in Gastroenterology. These findings may be relevant for millions of people, the investigators noted, because 30% of Americans older than 40 years take low-dose acetylsalicylic acid (ASA/aspirin) for cardiovascular disease (CVD).
NSAID-associated gastrointestinal issues are a long-standing and well-known problem, but the pathogenesis of this process in the small intestine appears more complex than in the stomach. The investigators pointed out that proton pump inhibitors, which limit gastropathy by suppressing acid, may actually worsen issues in the small intestine via disruption of microbiota.
“Whereas acid and pepsin are the principal luminal aggressors in NSAID-gastropathy, bile and indeed bacteria are the luminal factors in NSAID-enteropathy,” the investigators wrote.
“Given that deleterious compositional changes to the microbiota, in addition to direct effects on mucus and epithelial tissue, may increase the risk of NSAID-enteropathy, we hypothesized that an intervention targeting microbiome-host interactions may offer an attractive, preventative strategy,” the investigators wrote. They noted that previous human trials using probiotics for NSAID-enteropathy have been inconsistent; however, they suggested that Bifidobacteria remain worthy candidates because of their reported abilities to outcompete pathogenic bacteria, strengthen the intestinal epithelial layer, and modulate inflammation. “Our strain selection was based on the anti-inflammatory properties of certain Bifidobacteria and experimental preclinical evidence for a role of Bifidobacteria in NSAID-associated ulceration as well as unpublished preclinical screening data suggesting a particular potential of efficacy for the specific strain belonging to this genus.”
The double-blind, placebo-controlled trial involved 75 healthy volunteers aged 18-40 years who lived a sedentary lifestyle; during the study, they refrained from medications and bacterial products that might alter gastrointestinal function. Participants were randomized in a 1:1 ratio to received Bif195 or placebo for 8 weeks. Aspirin 300 mg was given daily to all participants for the first 6 weeks. At six points in time, video capsule endoscopy (VCE) was performed to determine the effect of treatment. The primary endpoint was intestinal damage, reported as area under the curve (AUC) for Lewis score, which incorporates stenosis, villous edema, and ulcers. The main secondary endpoint focused on ulcers, quantified by a separate AUC. Six other secondary endpoints evaluated symptoms, blood intestinal fatty acid binding protein (I-FABP), red spots visualized on VCE, and calprotectin.
After the 8-week period, 66 of 75 participants remained in the trial. Significantly less intestinal pathology was encountered among patients who received Bif195 than among those who did not. Specifically, for Lewis score, AUC in the Bif195 group was 3,040 plus or minus 1,340 arbitrary units (au), compared with 4,351 plus or minus 3,195 au in the placebo group (P = .0376). For ulcers alone, the Bif195 cohort had an AUC ulcer number of 50.4 plus or minus 53.1 au, versus 75.2 plus or minus 85.3 au for the placebo arm (P = .0258). Fecal calprotectin was also significantly lower in the Bif195 group than in the placebo group, whereas the remaining five secondary endpoints, which included symptom measurement, did not achieve statistical significance.
“Interestingly, fecal microbiome analysis revealed changes were limited to a marked increase in the total B. breve population in the Bif195 arm,” the investigators wrote. “These data provide further evidence that microbial intervention strategies targeting the microbiome can be clinically efficacious without inducing major alterations in the overall microbial population structure.”
Both aspirin and Bif195 were well tolerated during the trial, without statistically significant differences in adverse events between the treatment and placebo arms. No adverse events were considered related to Bif195.
“The trial results indicate that Bifidobacterium breve Bif195 confers significant and objectively verifiable protection against small-intestinal damage caused by a 6-week ASA challenge in healthy volunteers,” the investigators wrote.
“Further clinical trials are required to test whether the strain has clinical efficacy also in other settings and populations, i.e. in chronic users of ASA,” they concluded.
The study was funded by Chr. Hansen A/S. One author reported additional support from the Science Foundation Ireland.
SOURCE: Mortensen B et al. Gastroenterology. 2019 May 13. doi: 10.1053/j.gastro.2019.05.008.
Source: American Gastroenterological Association
Healthy volunteers given aspirin and Bif195 had significantly less damage and fewer ulcers in their small intestines than did control participants who received aspirin alone, reported lead author Brynjulf Mortensen, PhD, an employee of Chr. Hansen A/S, and colleagues in Gastroenterology. These findings may be relevant for millions of people, the investigators noted, because 30% of Americans older than 40 years take low-dose acetylsalicylic acid (ASA/aspirin) for cardiovascular disease (CVD).
NSAID-associated gastrointestinal issues are a long-standing and well-known problem, but the pathogenesis of this process in the small intestine appears more complex than in the stomach. The investigators pointed out that proton pump inhibitors, which limit gastropathy by suppressing acid, may actually worsen issues in the small intestine via disruption of microbiota.
“Whereas acid and pepsin are the principal luminal aggressors in NSAID-gastropathy, bile and indeed bacteria are the luminal factors in NSAID-enteropathy,” the investigators wrote.
“Given that deleterious compositional changes to the microbiota, in addition to direct effects on mucus and epithelial tissue, may increase the risk of NSAID-enteropathy, we hypothesized that an intervention targeting microbiome-host interactions may offer an attractive, preventative strategy,” the investigators wrote. They noted that previous human trials using probiotics for NSAID-enteropathy have been inconsistent; however, they suggested that Bifidobacteria remain worthy candidates because of their reported abilities to outcompete pathogenic bacteria, strengthen the intestinal epithelial layer, and modulate inflammation. “Our strain selection was based on the anti-inflammatory properties of certain Bifidobacteria and experimental preclinical evidence for a role of Bifidobacteria in NSAID-associated ulceration as well as unpublished preclinical screening data suggesting a particular potential of efficacy for the specific strain belonging to this genus.”
The double-blind, placebo-controlled trial involved 75 healthy volunteers aged 18-40 years who lived a sedentary lifestyle; during the study, they refrained from medications and bacterial products that might alter gastrointestinal function. Participants were randomized in a 1:1 ratio to received Bif195 or placebo for 8 weeks. Aspirin 300 mg was given daily to all participants for the first 6 weeks. At six points in time, video capsule endoscopy (VCE) was performed to determine the effect of treatment. The primary endpoint was intestinal damage, reported as area under the curve (AUC) for Lewis score, which incorporates stenosis, villous edema, and ulcers. The main secondary endpoint focused on ulcers, quantified by a separate AUC. Six other secondary endpoints evaluated symptoms, blood intestinal fatty acid binding protein (I-FABP), red spots visualized on VCE, and calprotectin.
After the 8-week period, 66 of 75 participants remained in the trial. Significantly less intestinal pathology was encountered among patients who received Bif195 than among those who did not. Specifically, for Lewis score, AUC in the Bif195 group was 3,040 plus or minus 1,340 arbitrary units (au), compared with 4,351 plus or minus 3,195 au in the placebo group (P = .0376). For ulcers alone, the Bif195 cohort had an AUC ulcer number of 50.4 plus or minus 53.1 au, versus 75.2 plus or minus 85.3 au for the placebo arm (P = .0258). Fecal calprotectin was also significantly lower in the Bif195 group than in the placebo group, whereas the remaining five secondary endpoints, which included symptom measurement, did not achieve statistical significance.
“Interestingly, fecal microbiome analysis revealed changes were limited to a marked increase in the total B. breve population in the Bif195 arm,” the investigators wrote. “These data provide further evidence that microbial intervention strategies targeting the microbiome can be clinically efficacious without inducing major alterations in the overall microbial population structure.”
Both aspirin and Bif195 were well tolerated during the trial, without statistically significant differences in adverse events between the treatment and placebo arms. No adverse events were considered related to Bif195.
“The trial results indicate that Bifidobacterium breve Bif195 confers significant and objectively verifiable protection against small-intestinal damage caused by a 6-week ASA challenge in healthy volunteers,” the investigators wrote.
“Further clinical trials are required to test whether the strain has clinical efficacy also in other settings and populations, i.e. in chronic users of ASA,” they concluded.
The study was funded by Chr. Hansen A/S. One author reported additional support from the Science Foundation Ireland.
SOURCE: Mortensen B et al. Gastroenterology. 2019 May 13. doi: 10.1053/j.gastro.2019.05.008.
FROM GASTROENTEROLOGY
Acupuncture cuts attacks in chronic angina
Adults with chronic stable angina who had acupuncture as an adjunct treatment had fewer angina attacks, compared with controls, based on data from a randomized trial of 398 patients in China.
“Acupuncture has been used as nonpharmacologic treatment for several decades, especially to relieve symptoms of myocardial ischemia, improve cardiac function, and prevent recurrence,” and several small studies have reported benefits in angina patients, wrote Ling Zhao, PhD, of Chengdu (China) University of Traditional Chinese Medicine and colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized patients aged 35-80 years with chronic stable angina into four groups: treatment on the disease-affected meridian (DAM), treatment on the nonaffected meridian (NAM), sham acupuncture (SA), and no acupuncture (wait list, or WL).
Chronic stable angina is defined by the American College of Cardiology and the American Heart Association as angina at least twice a week. Patients with other serious conditions including a history of MI, severe heart failure, valvular heart disease, and poorly controlled blood pressure or diabetes were excluded.
Each treatment group received three acupuncture sessions for 30 minutes each week for 4 weeks. Patients kept diaries of angina attacks and were assessed every 4 weeks for 16 weeks. After 16 weeks, the DAM patients had a significantly greater reduction in angina attacks, compared with the NAM group (4.1 fewer attacks), the SA group (5.2 fewer attacks), and the WL group (5.6 fewer attacks).
Overall, 16 patients reported adverse events related to acupuncture, including 5 cases of subcutaneous hemorrhage at the insertion point, 3 reports of tingling at the insertion point, and 8 reports of sleeplessness during the study period, but no patients discontinued the study because of these events. One patient in the WL group died of an acute MI and received no acupuncture treatment.
“We found that acupuncture on the DAM had superior and clinically relevant benefits in reducing angina frequency and pain intensity to a greater degree than acupuncture on a NAM, SA, or no acupuncture,” the researchers wrote. They found improvements in DAM patients on most metrics, including the Seattle Angina Questionnaire, compared with the other groups.
In addition, “compared with SA and no acupuncture, acupuncture on the DAM resulted in better regulation of anxiety and depression within the 12 weeks after treatment than at the end of the treatment period,” they wrote. “Acupuncture on the DAM causes autonomic remodeling by improving the balance between the vagus nerve and sympathetic nervous system during treatment.”
The findings were limited by several factors including the small sample size, potential performance bias based on variation in the acupuncturists’ experience, lack of analysis of doses of rescue medication, and lack of subgroup analysis, the researchers noted. However, the results are consistent with previous studies and support acupuncture as a potential adjunct treatment for patients with mild to moderate chronic angina.
The researchers had no financial conflicts to disclose. The study was funded by the National Natural Science Foundation of China and the State Key Program for Basic Research of China.
SOURCE: Zhao L et al. JAMA Intern Med. 2019 Jul 29. doi: 10.1001/jamainternmed.2019.2407.
Adults with chronic stable angina who had acupuncture as an adjunct treatment had fewer angina attacks, compared with controls, based on data from a randomized trial of 398 patients in China.
“Acupuncture has been used as nonpharmacologic treatment for several decades, especially to relieve symptoms of myocardial ischemia, improve cardiac function, and prevent recurrence,” and several small studies have reported benefits in angina patients, wrote Ling Zhao, PhD, of Chengdu (China) University of Traditional Chinese Medicine and colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized patients aged 35-80 years with chronic stable angina into four groups: treatment on the disease-affected meridian (DAM), treatment on the nonaffected meridian (NAM), sham acupuncture (SA), and no acupuncture (wait list, or WL).
Chronic stable angina is defined by the American College of Cardiology and the American Heart Association as angina at least twice a week. Patients with other serious conditions including a history of MI, severe heart failure, valvular heart disease, and poorly controlled blood pressure or diabetes were excluded.
Each treatment group received three acupuncture sessions for 30 minutes each week for 4 weeks. Patients kept diaries of angina attacks and were assessed every 4 weeks for 16 weeks. After 16 weeks, the DAM patients had a significantly greater reduction in angina attacks, compared with the NAM group (4.1 fewer attacks), the SA group (5.2 fewer attacks), and the WL group (5.6 fewer attacks).
Overall, 16 patients reported adverse events related to acupuncture, including 5 cases of subcutaneous hemorrhage at the insertion point, 3 reports of tingling at the insertion point, and 8 reports of sleeplessness during the study period, but no patients discontinued the study because of these events. One patient in the WL group died of an acute MI and received no acupuncture treatment.
“We found that acupuncture on the DAM had superior and clinically relevant benefits in reducing angina frequency and pain intensity to a greater degree than acupuncture on a NAM, SA, or no acupuncture,” the researchers wrote. They found improvements in DAM patients on most metrics, including the Seattle Angina Questionnaire, compared with the other groups.
In addition, “compared with SA and no acupuncture, acupuncture on the DAM resulted in better regulation of anxiety and depression within the 12 weeks after treatment than at the end of the treatment period,” they wrote. “Acupuncture on the DAM causes autonomic remodeling by improving the balance between the vagus nerve and sympathetic nervous system during treatment.”
The findings were limited by several factors including the small sample size, potential performance bias based on variation in the acupuncturists’ experience, lack of analysis of doses of rescue medication, and lack of subgroup analysis, the researchers noted. However, the results are consistent with previous studies and support acupuncture as a potential adjunct treatment for patients with mild to moderate chronic angina.
The researchers had no financial conflicts to disclose. The study was funded by the National Natural Science Foundation of China and the State Key Program for Basic Research of China.
SOURCE: Zhao L et al. JAMA Intern Med. 2019 Jul 29. doi: 10.1001/jamainternmed.2019.2407.
Adults with chronic stable angina who had acupuncture as an adjunct treatment had fewer angina attacks, compared with controls, based on data from a randomized trial of 398 patients in China.
“Acupuncture has been used as nonpharmacologic treatment for several decades, especially to relieve symptoms of myocardial ischemia, improve cardiac function, and prevent recurrence,” and several small studies have reported benefits in angina patients, wrote Ling Zhao, PhD, of Chengdu (China) University of Traditional Chinese Medicine and colleagues.
In a study published in JAMA Internal Medicine, the researchers randomized patients aged 35-80 years with chronic stable angina into four groups: treatment on the disease-affected meridian (DAM), treatment on the nonaffected meridian (NAM), sham acupuncture (SA), and no acupuncture (wait list, or WL).
Chronic stable angina is defined by the American College of Cardiology and the American Heart Association as angina at least twice a week. Patients with other serious conditions including a history of MI, severe heart failure, valvular heart disease, and poorly controlled blood pressure or diabetes were excluded.
Each treatment group received three acupuncture sessions for 30 minutes each week for 4 weeks. Patients kept diaries of angina attacks and were assessed every 4 weeks for 16 weeks. After 16 weeks, the DAM patients had a significantly greater reduction in angina attacks, compared with the NAM group (4.1 fewer attacks), the SA group (5.2 fewer attacks), and the WL group (5.6 fewer attacks).
Overall, 16 patients reported adverse events related to acupuncture, including 5 cases of subcutaneous hemorrhage at the insertion point, 3 reports of tingling at the insertion point, and 8 reports of sleeplessness during the study period, but no patients discontinued the study because of these events. One patient in the WL group died of an acute MI and received no acupuncture treatment.
“We found that acupuncture on the DAM had superior and clinically relevant benefits in reducing angina frequency and pain intensity to a greater degree than acupuncture on a NAM, SA, or no acupuncture,” the researchers wrote. They found improvements in DAM patients on most metrics, including the Seattle Angina Questionnaire, compared with the other groups.
In addition, “compared with SA and no acupuncture, acupuncture on the DAM resulted in better regulation of anxiety and depression within the 12 weeks after treatment than at the end of the treatment period,” they wrote. “Acupuncture on the DAM causes autonomic remodeling by improving the balance between the vagus nerve and sympathetic nervous system during treatment.”
The findings were limited by several factors including the small sample size, potential performance bias based on variation in the acupuncturists’ experience, lack of analysis of doses of rescue medication, and lack of subgroup analysis, the researchers noted. However, the results are consistent with previous studies and support acupuncture as a potential adjunct treatment for patients with mild to moderate chronic angina.
The researchers had no financial conflicts to disclose. The study was funded by the National Natural Science Foundation of China and the State Key Program for Basic Research of China.
SOURCE: Zhao L et al. JAMA Intern Med. 2019 Jul 29. doi: 10.1001/jamainternmed.2019.2407.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Adults with chronic stable angina who were treated with acupuncture had significantly fewer attacks than either those treated with sham acupuncture or controls who received no acupuncture.
Major finding: The reduction in angina attacks was 4.07 in disease-affected meridian versus an unaffected meridian, 5.18 versus a sham group, and 5.63 versus untreated controls.
Study details: The data come from a randomized trial of 398 patients at five clinical centers in China.
Disclosures: The researchers had no financial conflicts to disclose. The study was funded by the National Natural Science Foundation of China and the State Key Program for Basic Research of China.
Source: Zhao L et al. JAMA Intern Med. 2019 Jul 29. doi: 10.1001/jamainternmed.2019.2407.
Does endovascular thrombectomy benefit stroke patients with large infarcts?
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
FROM JAMA NEUROLOGY
Key clinical point: and who have a core volume less than 100 mL.
Major finding: At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27).
Study details: A prespecified secondary analysis of nonrandomized data from 105 patients in the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
Disclosures: The study was funded by an unrestricted grant from Stryker Neurovascular. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
Source: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Prescriptions for cough, cold medicine dropping for children
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.
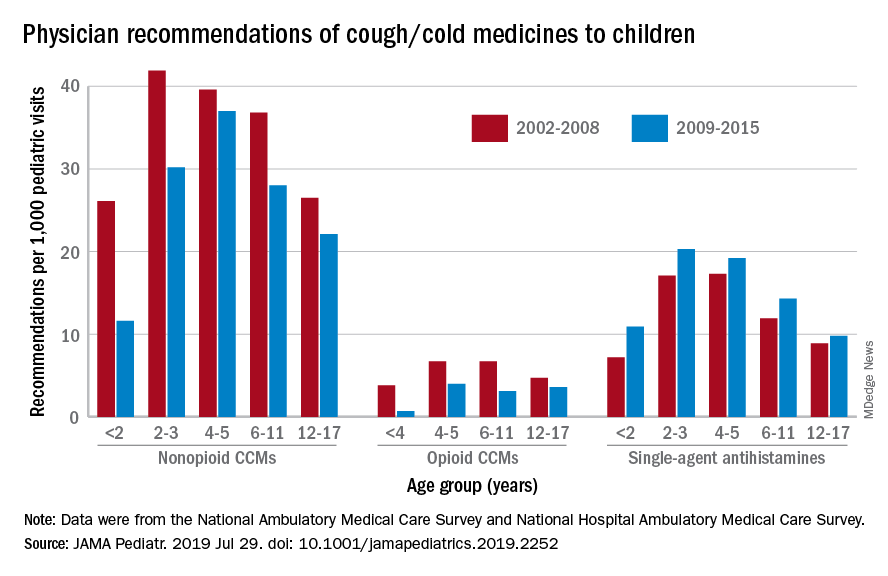
Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
with evidence suggesting replacement by off-label antihistamines, according to analysis of two national databases.

Compared with older children, declines in both opioid and nonopioid cold and cough medicine (CCM) use “appeared to accelerate in children younger than 2 years … and among children younger than 6 years for opioid-containing CCM” after the Food and Drug Administration’s 2008 public health advisory on use of OTC forms of CCM, Daniel B. Horton, MD, of the Robert Wood Johnson Medical School, New Brunswick, N.J., and his associates wrote in JAMA Pediatrics.
Meanwhile, recommendations for single-agent antihistamines rose – for some age groups significantly – over the 14-year study period, which was divided into two eras: 2002-2008 and 2009-2015.
When the two eras were compared, trends for decreased use of CCM in children under 2 years of age (nonopioid) and under 4 years (opioid) approached – both were P = .05 – but did not quite reach the less than .05 considered statistically significant. Adjusted odds ratios for the other age groups were further off the mark. For antihistamines, the upward trend between the two eras was significant for children aged under 2 years, 2-3 years, and 6-11 years, Dr. Horton and associates reported.
The two youngest groups, under 2 years and 2-3, were combined for the opioid CCM analyses to avoid a population under 30, which would have yielded unreliable estimates. The investigators used data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, with the sample representing 3.1 billion pediatric visits from 2002 to 2015.
Dr. Horton is supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators reported no disclosures relevant to this study.
SOURCE: Horton DB et al. JAMA Pediatr. 2019 Jul 29. doi: 10.1001/jamapediatrics.2019.2252.
FROM JAMA PEDIATRICS
Pertussis: Comparison studies show DTwP more durable
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
FROM VACCINE
Second trimester fetal loss: Shared decision-making, patient preference are key
NASHVILLE, TENN. – , according to Sarah W. Prager, MD.
Therefore, in the absence of clear contraindications in settings where both options are available, patient preference should prevail, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, shared decision-making is imperative, she said.
Shared decision-making “can be extremely important for satisfaction with this process,” she said, explaining that provider-driven decisions can be paternalistic and often are based on what the provider might do in the same situation.
“But that may not be what the patient wants,” she added.
Conversely, patient-led decision-making can lead to information overload.
“She’s coming to you because you’re the expert. She wants your opinion on this,” Dr. Prager said, noting that sharing the process through “information transfer” allows for the “best, most appropriate decision” to be made.
“Patient engagement is the practice of actively involving and supporting the patient in health care and treatment decision-making activities, and this is really what I’m talking about,” she said, adding that patient engagement is “critically important in many situations, and especially in the setting of pregnancy loss.”
That’s because patients feel powerless in this situation, she explained. Engaging them in the decision-making process can “give them a little bit of that power back” by respecting autonomy, enhancing agency, improving health status, reducing decisional conflict, and limiting test use, thereby improving overall satisfaction.
Two randomized control trials, each designed to compare surgical management and medical management for terminations at up to 20 weeks of gestation, highlight the role and importance of patient preference, Dr. Prager said.
The first – a 2004 study – was stopped early because of slow enrollment, with 29 of 47 eligible subjects declining randomization. Among 93% of those who declined, there was a preference for surgical management. The second, a 2010 study, enrolled 122 patients after 107 of 229 eligible subjects (47%) declined randomization, again because most (67%) preferred surgery (BJOG. 2004;111[2]:148-53; BJOG. 2010;117[12]:1512-20).
Reasons given for preferring surgical management included less psychological trauma and deeper anesthesia, whereas reasons given for induction preference included less wait time and a desire to avoid general anesthesia.
Helping patients make the best decision requires a discussion about potential complications for each approach, Dr. Prager said.
Surgical management, which involves dilation and evacuation (D&E), is used for about 95% of second-trimester abortions overall, but medical management may be underreported, particularly for management of pregnancy loss, Dr. Prager said. “We don’t have clear statistics” in that setting.
The overall rate of complications is low for surgical management, with data suggesting a rate of up to 4%. Uterine perforation occurs in 0.2%-0.3% of procedures, cervical laceration occurs in up to 1%, and retained placenta occurs in less than 1%, she said.
The complication rate for medical management – induction with either misoprostol or mifepristone + misoprostol (the latter is the recommended approach) – is much higher at up to 29%, but that includes retained placenta, which happens in up to 10% of procedures. Uterine rupture occurs in 0.04%-0.28% of procedures, she said.
“With either surgical management or medication management of pregnancy loss, we need to keep in mind the possibility of disseminated intravascular coagulation, which is rare, but certainly possible,” she said.
Other factors that may be important to patients deciding between surgical and medical management for second-trimester fetal loss include:
- Anesthesia, which is local plus intravenous sedation for surgery, compared with IV narcotics and potentially an epidural or other type of regional anesthesia for medical management.
- Duration, which is 5-20 minutes for surgery, compared with 6-11 hours with mifepristone + misoprostol, and up to 20 hours with misoprostol alone.
- Location, which is done on an outpatient basis for surgery, compared with inpatient care for medical management.
- Cost, which is $1,000-$5,000 for surgery vs. $3,000-$9,000 for medical management.
- Contact with the fetus, which typically involves the possibility of partial viewing and an opportunity to obtain footprints as a memento if an intact procedure is attempted during surgery vs. full viewing and possibly holding the baby after delivery following medical management. This is often the key deciding factor for patients.
- Provider factors, in terms of training and skills. Surgery involves a need for specialized training, whereas medical management requires no extra training, she said, adding that “not all ob.gyns. across the country are competent or comfortable providing a D&E, particularly in the later second-trimester time period.” However, the availability of family planing fellowships will increase the number of centers across the country where both options will be available, she noted.
- The possibility of fetal autopsy, which surgery often (but not always) allows, but medical management always allows.
- Involvement level, which is provider heavy for surgery vs. patient heavy for medical management.
“Moving toward an evidence-based, patient-centered care model requires a lot of us, as providers, to really work at dropping our assumptions. We often have strong opinions about what we think we would do in that setting, and it can be tricky for us to set that aside and allow patients to really ask questions and discuss their values so that we can then advocate best for our patients after they know exactly what their options are,” she said.
Dr. Prager reported having no relevant disclosures.
NASHVILLE, TENN. – , according to Sarah W. Prager, MD.
Therefore, in the absence of clear contraindications in settings where both options are available, patient preference should prevail, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, shared decision-making is imperative, she said.
Shared decision-making “can be extremely important for satisfaction with this process,” she said, explaining that provider-driven decisions can be paternalistic and often are based on what the provider might do in the same situation.
“But that may not be what the patient wants,” she added.
Conversely, patient-led decision-making can lead to information overload.
“She’s coming to you because you’re the expert. She wants your opinion on this,” Dr. Prager said, noting that sharing the process through “information transfer” allows for the “best, most appropriate decision” to be made.
“Patient engagement is the practice of actively involving and supporting the patient in health care and treatment decision-making activities, and this is really what I’m talking about,” she said, adding that patient engagement is “critically important in many situations, and especially in the setting of pregnancy loss.”
That’s because patients feel powerless in this situation, she explained. Engaging them in the decision-making process can “give them a little bit of that power back” by respecting autonomy, enhancing agency, improving health status, reducing decisional conflict, and limiting test use, thereby improving overall satisfaction.
Two randomized control trials, each designed to compare surgical management and medical management for terminations at up to 20 weeks of gestation, highlight the role and importance of patient preference, Dr. Prager said.
The first – a 2004 study – was stopped early because of slow enrollment, with 29 of 47 eligible subjects declining randomization. Among 93% of those who declined, there was a preference for surgical management. The second, a 2010 study, enrolled 122 patients after 107 of 229 eligible subjects (47%) declined randomization, again because most (67%) preferred surgery (BJOG. 2004;111[2]:148-53; BJOG. 2010;117[12]:1512-20).
Reasons given for preferring surgical management included less psychological trauma and deeper anesthesia, whereas reasons given for induction preference included less wait time and a desire to avoid general anesthesia.
Helping patients make the best decision requires a discussion about potential complications for each approach, Dr. Prager said.
Surgical management, which involves dilation and evacuation (D&E), is used for about 95% of second-trimester abortions overall, but medical management may be underreported, particularly for management of pregnancy loss, Dr. Prager said. “We don’t have clear statistics” in that setting.
The overall rate of complications is low for surgical management, with data suggesting a rate of up to 4%. Uterine perforation occurs in 0.2%-0.3% of procedures, cervical laceration occurs in up to 1%, and retained placenta occurs in less than 1%, she said.
The complication rate for medical management – induction with either misoprostol or mifepristone + misoprostol (the latter is the recommended approach) – is much higher at up to 29%, but that includes retained placenta, which happens in up to 10% of procedures. Uterine rupture occurs in 0.04%-0.28% of procedures, she said.
“With either surgical management or medication management of pregnancy loss, we need to keep in mind the possibility of disseminated intravascular coagulation, which is rare, but certainly possible,” she said.
Other factors that may be important to patients deciding between surgical and medical management for second-trimester fetal loss include:
- Anesthesia, which is local plus intravenous sedation for surgery, compared with IV narcotics and potentially an epidural or other type of regional anesthesia for medical management.
- Duration, which is 5-20 minutes for surgery, compared with 6-11 hours with mifepristone + misoprostol, and up to 20 hours with misoprostol alone.
- Location, which is done on an outpatient basis for surgery, compared with inpatient care for medical management.
- Cost, which is $1,000-$5,000 for surgery vs. $3,000-$9,000 for medical management.
- Contact with the fetus, which typically involves the possibility of partial viewing and an opportunity to obtain footprints as a memento if an intact procedure is attempted during surgery vs. full viewing and possibly holding the baby after delivery following medical management. This is often the key deciding factor for patients.
- Provider factors, in terms of training and skills. Surgery involves a need for specialized training, whereas medical management requires no extra training, she said, adding that “not all ob.gyns. across the country are competent or comfortable providing a D&E, particularly in the later second-trimester time period.” However, the availability of family planing fellowships will increase the number of centers across the country where both options will be available, she noted.
- The possibility of fetal autopsy, which surgery often (but not always) allows, but medical management always allows.
- Involvement level, which is provider heavy for surgery vs. patient heavy for medical management.
“Moving toward an evidence-based, patient-centered care model requires a lot of us, as providers, to really work at dropping our assumptions. We often have strong opinions about what we think we would do in that setting, and it can be tricky for us to set that aside and allow patients to really ask questions and discuss their values so that we can then advocate best for our patients after they know exactly what their options are,” she said.
Dr. Prager reported having no relevant disclosures.
NASHVILLE, TENN. – , according to Sarah W. Prager, MD.
Therefore, in the absence of clear contraindications in settings where both options are available, patient preference should prevail, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, shared decision-making is imperative, she said.
Shared decision-making “can be extremely important for satisfaction with this process,” she said, explaining that provider-driven decisions can be paternalistic and often are based on what the provider might do in the same situation.
“But that may not be what the patient wants,” she added.
Conversely, patient-led decision-making can lead to information overload.
“She’s coming to you because you’re the expert. She wants your opinion on this,” Dr. Prager said, noting that sharing the process through “information transfer” allows for the “best, most appropriate decision” to be made.
“Patient engagement is the practice of actively involving and supporting the patient in health care and treatment decision-making activities, and this is really what I’m talking about,” she said, adding that patient engagement is “critically important in many situations, and especially in the setting of pregnancy loss.”
That’s because patients feel powerless in this situation, she explained. Engaging them in the decision-making process can “give them a little bit of that power back” by respecting autonomy, enhancing agency, improving health status, reducing decisional conflict, and limiting test use, thereby improving overall satisfaction.
Two randomized control trials, each designed to compare surgical management and medical management for terminations at up to 20 weeks of gestation, highlight the role and importance of patient preference, Dr. Prager said.
The first – a 2004 study – was stopped early because of slow enrollment, with 29 of 47 eligible subjects declining randomization. Among 93% of those who declined, there was a preference for surgical management. The second, a 2010 study, enrolled 122 patients after 107 of 229 eligible subjects (47%) declined randomization, again because most (67%) preferred surgery (BJOG. 2004;111[2]:148-53; BJOG. 2010;117[12]:1512-20).
Reasons given for preferring surgical management included less psychological trauma and deeper anesthesia, whereas reasons given for induction preference included less wait time and a desire to avoid general anesthesia.
Helping patients make the best decision requires a discussion about potential complications for each approach, Dr. Prager said.
Surgical management, which involves dilation and evacuation (D&E), is used for about 95% of second-trimester abortions overall, but medical management may be underreported, particularly for management of pregnancy loss, Dr. Prager said. “We don’t have clear statistics” in that setting.
The overall rate of complications is low for surgical management, with data suggesting a rate of up to 4%. Uterine perforation occurs in 0.2%-0.3% of procedures, cervical laceration occurs in up to 1%, and retained placenta occurs in less than 1%, she said.
The complication rate for medical management – induction with either misoprostol or mifepristone + misoprostol (the latter is the recommended approach) – is much higher at up to 29%, but that includes retained placenta, which happens in up to 10% of procedures. Uterine rupture occurs in 0.04%-0.28% of procedures, she said.
“With either surgical management or medication management of pregnancy loss, we need to keep in mind the possibility of disseminated intravascular coagulation, which is rare, but certainly possible,” she said.
Other factors that may be important to patients deciding between surgical and medical management for second-trimester fetal loss include:
- Anesthesia, which is local plus intravenous sedation for surgery, compared with IV narcotics and potentially an epidural or other type of regional anesthesia for medical management.
- Duration, which is 5-20 minutes for surgery, compared with 6-11 hours with mifepristone + misoprostol, and up to 20 hours with misoprostol alone.
- Location, which is done on an outpatient basis for surgery, compared with inpatient care for medical management.
- Cost, which is $1,000-$5,000 for surgery vs. $3,000-$9,000 for medical management.
- Contact with the fetus, which typically involves the possibility of partial viewing and an opportunity to obtain footprints as a memento if an intact procedure is attempted during surgery vs. full viewing and possibly holding the baby after delivery following medical management. This is often the key deciding factor for patients.
- Provider factors, in terms of training and skills. Surgery involves a need for specialized training, whereas medical management requires no extra training, she said, adding that “not all ob.gyns. across the country are competent or comfortable providing a D&E, particularly in the later second-trimester time period.” However, the availability of family planing fellowships will increase the number of centers across the country where both options will be available, she noted.
- The possibility of fetal autopsy, which surgery often (but not always) allows, but medical management always allows.
- Involvement level, which is provider heavy for surgery vs. patient heavy for medical management.
“Moving toward an evidence-based, patient-centered care model requires a lot of us, as providers, to really work at dropping our assumptions. We often have strong opinions about what we think we would do in that setting, and it can be tricky for us to set that aside and allow patients to really ask questions and discuss their values so that we can then advocate best for our patients after they know exactly what their options are,” she said.
Dr. Prager reported having no relevant disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Real-world data for immunotherapy-treated NSCLC found robust
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
Real-world outcome data from patients with advanced non–small cell lung cancer (NSCLC) treated with immunotherapy are robust enough to use for regulatory and payer decisions, suggests an analysis of six data sets and more than 13,000 patients.
“Study of routinely collected health care data is increasingly important for various stakeholders who are interested in better understanding particular patient populations, evaluating drug safety in the postmarketing setting, measuring health care use and clinical outcomes, performing comparative effectiveness research, and optimizing drug pricing models,” noted lead investigator Mark Stewart, PhD, Friends of Cancer Research, Washington, and coinvestigators. “However, before [real-world data] finds widespread use as an adjunct to – or in unique settings, an alternative for – [randomized clinical trials], the validity of readily extractable clinical outcomes measures – real-world endpoints – must be established.”
The investigators undertook a retrospective cohort study using administrative claims and electronic health records for patients with advanced NSCLC treated with inhibitors of programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) between January 2011 and October 2017 in real-world settings. They analyzed six data sets having 269 to 6,924 patients each (13,639 patients total).
Results reported in JCO Clinical Cancer Informatics showed that the real-world intermediate endpoints of time to treatment discontinuation and time to next treatment were moderately to highly correlated with real-world overall survival (Spearman’s rank correlation coefficient, 0.36 to 0.89, with most values 0.60 or higher).
In real-world settings, the 1-year rate of overall survival after starting immunotherapy ranged from 40% to 57%. Real-world data and trial data were similar with respect to median overall survival (8.6-13.5 months vs. 9.2-12.7 months).
The data sources used for the study have been extensively used for research and are curated on an ongoing basis to ensure the data are accurate and as complete as possible, Dr. Stewart and coinvestigators noted.
“These findings demonstrate that real-world endpoints are generally consistent with each other and with outcomes observed in randomized clinical trials, which substantiates the potential validity of real-world data to support regulatory and payer decision-making,” they maintained. “Differences observed likely reflect true differences between real-world and protocol-driven practices.
“Additional studies are needed to further support the use of [real-world evidence] and inform the development of regulatory guidance,” the investigators concluded. “Standardizing definitions for real-world endpoints and determining appropriate analytic methodologies for [real-world data] will be critical for broader adoption of real-world studies and will provide greater confidence in associated findings. As more refined and standardized approaches are developed that incorporate deep clinical and bioinformatics expertise, the greater the utility of [real-world data] will be for detecting even small, but important, differences in treatment effects.”
Dr. Stewart disclosed no conflicts of interest. The study was supported in part by the National Cancer Institute and the Patient Centered Outcomes Research Institute.
SOURCE: Stewart M et al. JCO Clin Cancer Inform. 2019 July 23. doi: 10.1200/CCI.18.00155.
FROM JCO CLINICAL CANCER INFORMATICS
Improving Comorbidities With Psoriasis Treatment
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
Psoriasis is a common immune-mediated inflammatory skin disorder that affects up to 3.2% of adults in the United States.1 It is a TH1, TH17, and TH22 inflammatory disease resulting in increased levels of cytokines in the skin, including IFN-γ, tumor necrosis factor (TNF), IL-17, and IL-22. Dendritic antigen-presenting cells also are increased in the skin of patients with psoriasis resulting in increased levels of IL-23.2 Although skin disease often is its most prominent and sometimes its only documented manifestation, an understanding of psoriasis as a chronic multisystem inflammatory disorder is essential to optimize outcomes.1,3 Multiple comorbidities that may affect treatment selection often are associated with psoriasis, including psoriatic arthritis, cardiovascular disease, depression, obesity, metabolic syndrome, cardiovascular disease (CVD), cerebrovascular disease, and peripheral vascular disease.
As with other immune-mediated inflammatory diseases, it has been hypothesized that psoriasis may influence comorbidities through shared genetic risks, environmental factors, and pathogenic factors or inflammatory pathways.2-4 For example, emerging evidence suggests that comorbidities such as metabolic syndrome may be related to the chronic inflammation that accompanies psoriasis, a finding that has important clinical implications.3
The interplay and dependence or interdependence of psoriasis and its comorbidities is complex, and it is an area deserving of vigorous research.1 At present, observational and epidemiological data such as the present case suggest that effective treatment of psoriasis could lead to benefits “beyond the skin” and potentially even prevent future disease-associated comorbidity.1-3
Metabolic Comorbidities and Psoriasis Treatment
Although the prevalence of CVD and CVD risk factors is increased in patients with psoriasis, studies suggest that the suppression of systemic inflammation that accompanies adequate psoriasis treatment, particularly in patients with moderate to severe disease, may decrease the risk for cardiovascular comorbidities.5 For example, a number of studies have found treatment of psoriasis with methotrexate may decrease the risk for cardiovascular events, including ischemic heart disease, stroke, and cardiovascular death.6-10 Low-dose methotrexate has been shown to be particularly advantageous for decreasing CVD in patients with psoriasis.5,8
Tumor necrosis factor α inhibitors, which are frequently used for moderate to severe plaque psoriasis, also may notably decrease cardiovascular risk.5 One study showed a significant decrease in the risk for myocardial infarction in patients with psoriasis who were treated with TNF-α inhibitors (hazard ratio, 0.50; 95% CI, 0.32-0.79)11; other studies have confirmed this benefit.12-17 Moreover, the reduction in cardiovascular events may be greater with TNF-α inhibitors than with methotrexate when the former is used for psoriasis treatment, with longer duration of TNF-α inhibition leading to greater risk reduction.18,19
In patients with severe psoriasis, treatment with TNF-α inhibitors has been associated with improvements in subclinical CVD (abnormalities in echocardiogram), improved coronary microvascular function (determined by transthoracic Doppler echocardiography), and reduced progression in coronary artery disease (assessed by coronary computed tomography).20-22 Improvement in endothelial function (brachial artery reactivity) and carotid arterial stiffness also has been reported following 6 months of treatment with adalimumab for moderate to severe psoriasis.21
Data concerning potential cardiovascular risk reduction with treatment of psoriasis utilizing newer agents are continuing to emerge. To date, no increase in the incidence of major adverse cardiovascular events has been shown in patients with psoriasis treated with anti–IL-17 agents, such as secukinumab; however, additional long-term studies are needed.18,23-25
Apremilast, an oral phosphodiesterase 4 inhibitor, is another addition to the psoriasis armamentarium.26 No increase in the risk for major cardiac events has been shown in randomized controlled trials of patients with psoriasis receiving apremilast for up to 156 weeks.27,28 As with secukinumab, additional long-term, large-scale studies are needed to determine the effects of apremilast on cardiovascular risk in patients with psoriasis.5
Other Comorbidities
Effective treatment of psoriasis also appears to benefit various other comorbidities. Numerous studies have shown an increased incidence of depression in patients with psoriasis vs controls and a concurrent improvement in psychiatric symptoms with psoriasis disease control.1 For instance, a multicenter, randomized, open-label study of 352 patients with psoriasis showed treatment with etanercept, a TNF inhibitor, significantly improved scores for concomitant depression and anxiety (P<.05).29 Similarly, a double-blind, randomized clinical trial of patients with psoriasis found significant improvement in depression at 12 weeks in patients treated with adalimumab vs placebo (P<.001).30 Likewise, a multicenter phase 3 trial of more than 600 psoriatic patients showed improved Beck depression inventory and Hamilton depression rating scale scores at 12 weeks in patients with psoriasis treated with etanercept compared to placebo.31
A much larger analysis of 7490 patients with psoriasis compared the rates of depression among patients receiving biologic therapy, phototherapy, and conventional systemic therapy. The greatest impact on depression symptoms was seen with biologic therapy (incidence rate, 3.01/100 patient-years), followed by conventional systemic therapy (5.70/100 patient-years), and phototherapy (5.85/100 patient-years).32
Uveitis, or inflammation of the middle layer of the eye (the uvea), frequently is seen in patients with psoriasis. In a cohort study of 60,000 patients with mild psoriasis and more than 7000 patients with severe psoriasis, the incidence of uveitis in patients was significantly increased in both patients with severe disease and those with mild disease (P<.001 for both).33 In a case series of 8 patients with concomitant psoriasis and uveitis, 4 patients were treated with infliximab and 4 with adalimumab; 7 patients treated achieved remission of their uveitis.34
Role of the TNF-α Blockade in Sickle Cell Disease
Presently, no reported human studies have shown TNF-α blockade as a possible treatment of sickle cell disease.35 However, increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and to the severity of sickle cell disease due to their integral role in the development of vascular wall dysfunction and ischemia.35,36 Studies have shown that TNF-α is released in homozygous sickle cell anemia (HbSS) disease and impedes blood flow during sickle cell crisis, resulting in worsening ischemia and painful infarction.35,36 Moreover, cytokine analysis has shown significantly (P<.05) elevated levels of TNF-α during sickle cell crises and at baseline in patients with HbSS vs healthy controls, suggesting a possible role of TNF-α in the pathogenesis of sickle cell crisis.36
The case patient reported a 50% reduction in pain level and the use of pain medications that overlapped with the initiation of adalimumab for treatment of her psoriasis. Moreover, although radiographs showed possible psoriatic changes of the distal metatarsal row, she described sickle cell pain and pain crises that were uncharacteristic of psoriatic arthralgia.35 Although these findings are observational in nature and limited to one patient, they do suggest an interesting hypothesis. If a common inflammatory mediator is the culprit, it is possible that TNF-α inhibitors could be the preferred treatment option for patients with psoriasis and comorbid HbSS or HbSC disease. Further studies are needed to analyze the role of TNF-α inhibition in sickle cell disease.
Bottom Line
Psoriasis may influence comorbidities through shared genetic risks, environmental factors, or inflammatory pathways. Improvement in metabolic and other comorbidities have been shown with the effective treatment of psoriasis. The case described here showed improvement in sickle cell crises and pain with treatment of psoriasis with adalimumab. Tumor necrosis factor inhibitors may be an optimal choice for patients with both psoriasis and sickle cell disease.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
- Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
- Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
- Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
- Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
- Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
- Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
- Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
- Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
- Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
- Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
- Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
- Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
- Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area). The patient’s medical history was positive for sickle cell disease, specifically hemoglobin SC disease (HbSC). She reported chronic dull arthralgia in the ankles that was worse at night. She was being treated by hematology with ibuprofen and ketorolac. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. At the current presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on its potential for improvement in HbSC symptoms as well as psoriasis.
Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and she reported a decrease in her HbSC pain from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. She also reported decreased use of pain medication with rare sickle cell pain crises following initiation of adalimumab.









