User login
Exposure to patients with migraine increases likelihood of stigmatizing attitudes
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
REPORTING FROM AHS 2019
CMS plans to give MIPS an overhaul
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Key clinical point: The Centers for Medicare & Medicaid Services proposes an overhaul to the Merit-based Incentive Payment System track of the Quality Payment Program.
Major finding: The move is intended to make measures more meaningful to clinicians.
Study details: Measures would be more focused to specialties through Merit-based Incentive Payment System Value Pathways, with all those reporting on a specialty or condition reporting on more streamlined measures.
Disclosures: CMS, as the issuer of the rules, makes no disclosures.
Psoriasis Journal Scan: July 2019
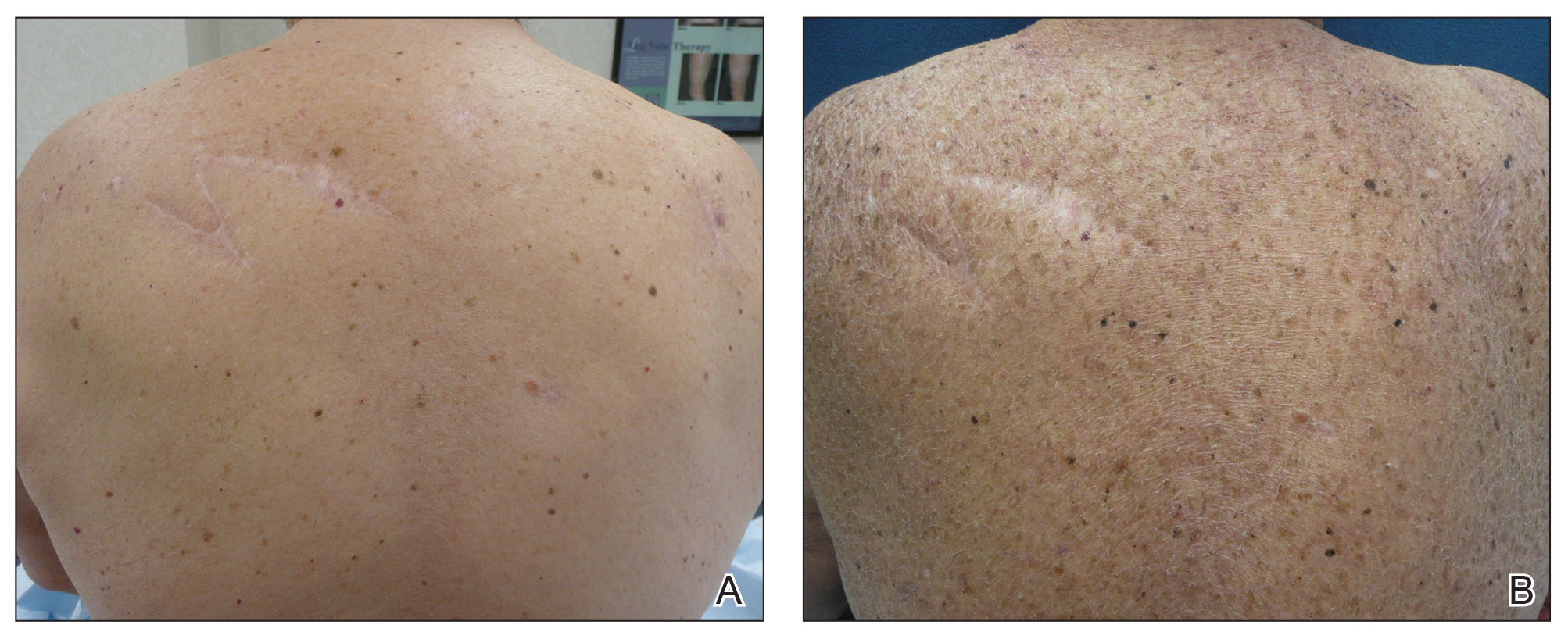
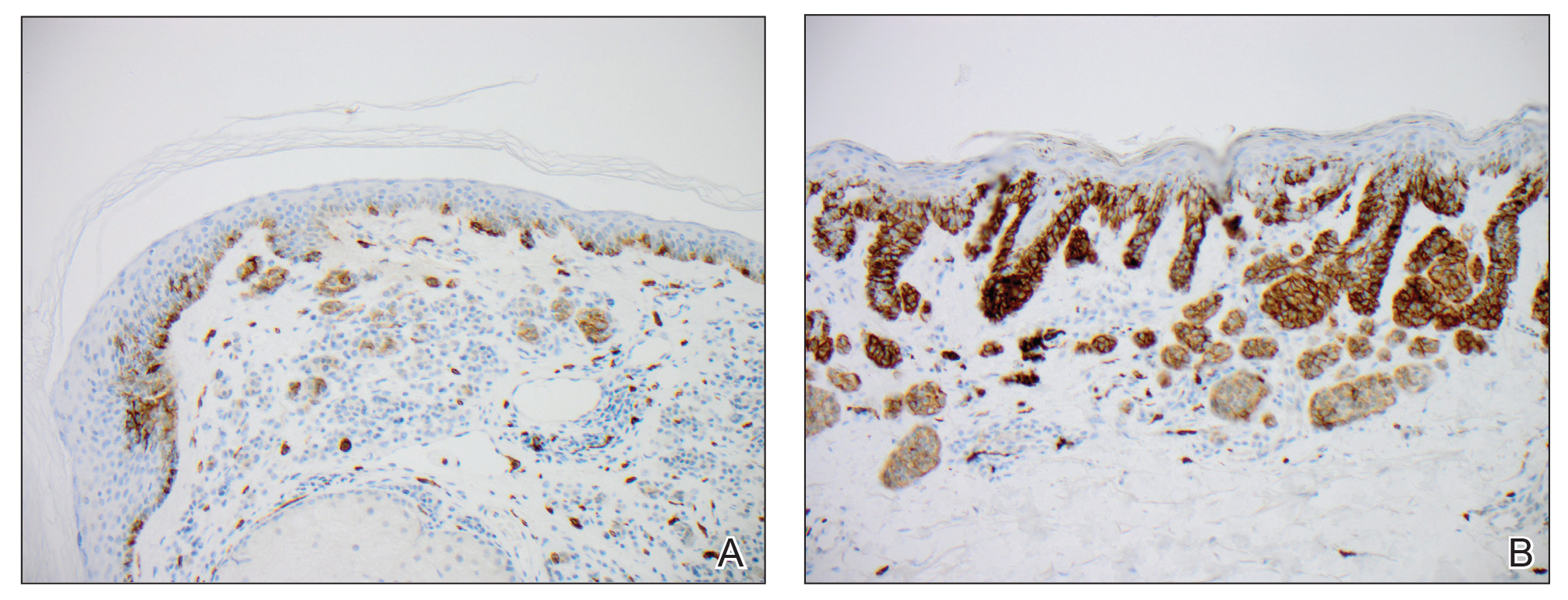
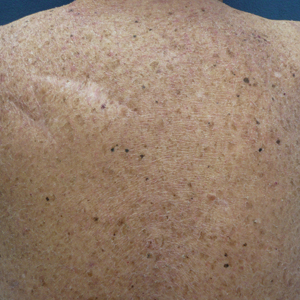
Facial involvement and the severity of psoriasis.
Passos AN, de A Rêgo VRP, Duarte GV, Santos E Miranda RC, de O Rocha B, de F S P de Oliveira M. Int J Dermatol. 2019 Jul 26.
The aim of this cross-sectional study is to compare the severity of psoriasis, measured by the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI), in patients with and without facial lesions.
Genital Psoriasis: Impact on Quality of Life and Treatment Options.
Kelly A, Ryan C. Am J Clin Dermatol. 2019 Jul 16
Psoriasis involving the genital skin occurs in up to two-thirds of psoriasis patients but is often overlooked by physicians. Furthermore, psoriasis objective and subjective severity indexes for common plaque psoriasis often neglect the impact this small area of psoriasis can have on a patient. It can have a significant impact on patients' psychosocial function due to intrusive physical symptoms such as genital itch and pain, and a detrimental impact on sexual health and impaired relationships.
Lifestyle changes for treating psoriasis.
Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Cochrane Database Syst Rev. 2019 Jul 16
The objective of this review is to assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions. Dietary intervention may reduce the severity of psoriasis (low-quality evidence) and probably improves quality of life and reduces BMI (moderate-quality evidence) in obese people when compared with usual care, while combined dietary intervention and exercise programme probably improves psoriasis severity and BMI when compared with information only (moderate-quality evidence).
The Incidence Rates and Risk Factors of Parkinson's Disease in Patients with Psoriasis: A Nationwide Population-based Cohort Study.
Lee JH, Han K, Gee HY. J Am Acad Dermatol. 2019 Jul 11.
This was a nationwide population-based cohort study to determine the incidence rates and risk factors of Parkinson's disease in patients with psoriasis. The psoriasis group showed a significantly increased risk of developing Parkinson's disease. The risk of Parkinson's disease was significantly high among the psoriasis patients who were not receiving systemic therapy and was low among the psoriasis patients on systemic therapy.
Psoriasis-associated itch: etiology, assessment, impact, and management.
Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. J Dermatolog Treat. 2019 Jul 5:1-9.
Pruritus, a very broad, subjective, and complex symptom, troubles the majority of patients with psoriasis. However, the subjective and multidimensional nature of the symptom renders it challenging for patients to appropriately communicate their experiences with itch to providers. This review explores current perspectives regarding the underlying mechanisms, assessment tools, burden, and treatment modalities for psoriatic pruritus. It emphasizes the significance of incorporating a standardized, thorough, and verified metric that incorporates severity, distribution, and character of pruritus as well as its effects on various aspects of quality of life. It also underscores the importance of continued research to fully understand the pathogenesis of psoriatic itch for establishment of novel, targeted therapeutics.
Facial involvement and the severity of psoriasis.
Passos AN, de A Rêgo VRP, Duarte GV, Santos E Miranda RC, de O Rocha B, de F S P de Oliveira M. Int J Dermatol. 2019 Jul 26.
The aim of this cross-sectional study is to compare the severity of psoriasis, measured by the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI), in patients with and without facial lesions.
Genital Psoriasis: Impact on Quality of Life and Treatment Options.
Kelly A, Ryan C. Am J Clin Dermatol. 2019 Jul 16
Psoriasis involving the genital skin occurs in up to two-thirds of psoriasis patients but is often overlooked by physicians. Furthermore, psoriasis objective and subjective severity indexes for common plaque psoriasis often neglect the impact this small area of psoriasis can have on a patient. It can have a significant impact on patients' psychosocial function due to intrusive physical symptoms such as genital itch and pain, and a detrimental impact on sexual health and impaired relationships.
Lifestyle changes for treating psoriasis.
Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Cochrane Database Syst Rev. 2019 Jul 16
The objective of this review is to assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions. Dietary intervention may reduce the severity of psoriasis (low-quality evidence) and probably improves quality of life and reduces BMI (moderate-quality evidence) in obese people when compared with usual care, while combined dietary intervention and exercise programme probably improves psoriasis severity and BMI when compared with information only (moderate-quality evidence).
The Incidence Rates and Risk Factors of Parkinson's Disease in Patients with Psoriasis: A Nationwide Population-based Cohort Study.
Lee JH, Han K, Gee HY. J Am Acad Dermatol. 2019 Jul 11.
This was a nationwide population-based cohort study to determine the incidence rates and risk factors of Parkinson's disease in patients with psoriasis. The psoriasis group showed a significantly increased risk of developing Parkinson's disease. The risk of Parkinson's disease was significantly high among the psoriasis patients who were not receiving systemic therapy and was low among the psoriasis patients on systemic therapy.
Psoriasis-associated itch: etiology, assessment, impact, and management.
Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. J Dermatolog Treat. 2019 Jul 5:1-9.
Pruritus, a very broad, subjective, and complex symptom, troubles the majority of patients with psoriasis. However, the subjective and multidimensional nature of the symptom renders it challenging for patients to appropriately communicate their experiences with itch to providers. This review explores current perspectives regarding the underlying mechanisms, assessment tools, burden, and treatment modalities for psoriatic pruritus. It emphasizes the significance of incorporating a standardized, thorough, and verified metric that incorporates severity, distribution, and character of pruritus as well as its effects on various aspects of quality of life. It also underscores the importance of continued research to fully understand the pathogenesis of psoriatic itch for establishment of novel, targeted therapeutics.
Facial involvement and the severity of psoriasis.
Passos AN, de A Rêgo VRP, Duarte GV, Santos E Miranda RC, de O Rocha B, de F S P de Oliveira M. Int J Dermatol. 2019 Jul 26.
The aim of this cross-sectional study is to compare the severity of psoriasis, measured by the Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI), in patients with and without facial lesions.
Genital Psoriasis: Impact on Quality of Life and Treatment Options.
Kelly A, Ryan C. Am J Clin Dermatol. 2019 Jul 16
Psoriasis involving the genital skin occurs in up to two-thirds of psoriasis patients but is often overlooked by physicians. Furthermore, psoriasis objective and subjective severity indexes for common plaque psoriasis often neglect the impact this small area of psoriasis can have on a patient. It can have a significant impact on patients' psychosocial function due to intrusive physical symptoms such as genital itch and pain, and a detrimental impact on sexual health and impaired relationships.
Lifestyle changes for treating psoriasis.
Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Cochrane Database Syst Rev. 2019 Jul 16
The objective of this review is to assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions. Dietary intervention may reduce the severity of psoriasis (low-quality evidence) and probably improves quality of life and reduces BMI (moderate-quality evidence) in obese people when compared with usual care, while combined dietary intervention and exercise programme probably improves psoriasis severity and BMI when compared with information only (moderate-quality evidence).
The Incidence Rates and Risk Factors of Parkinson's Disease in Patients with Psoriasis: A Nationwide Population-based Cohort Study.
Lee JH, Han K, Gee HY. J Am Acad Dermatol. 2019 Jul 11.
This was a nationwide population-based cohort study to determine the incidence rates and risk factors of Parkinson's disease in patients with psoriasis. The psoriasis group showed a significantly increased risk of developing Parkinson's disease. The risk of Parkinson's disease was significantly high among the psoriasis patients who were not receiving systemic therapy and was low among the psoriasis patients on systemic therapy.
Psoriasis-associated itch: etiology, assessment, impact, and management.
Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. J Dermatolog Treat. 2019 Jul 5:1-9.
Pruritus, a very broad, subjective, and complex symptom, troubles the majority of patients with psoriasis. However, the subjective and multidimensional nature of the symptom renders it challenging for patients to appropriately communicate their experiences with itch to providers. This review explores current perspectives regarding the underlying mechanisms, assessment tools, burden, and treatment modalities for psoriatic pruritus. It emphasizes the significance of incorporating a standardized, thorough, and verified metric that incorporates severity, distribution, and character of pruritus as well as its effects on various aspects of quality of life. It also underscores the importance of continued research to fully understand the pathogenesis of psoriatic itch for establishment of novel, targeted therapeutics.
Researchers combine genetic and clinical factors in new VTE risk score
MELBOURNE – A venous thromboembolism risk score that combines clinical risk factors, such as lymphoma type and stage, along with genetic variables, could offer a better way to predict venous thromboembolism in patients with lymphoma, according to new findings presented at the International Society on Thrombosis and Haemostasis congress.
Cristina Pascual, MD, of the Hospital Universitario Gregorio Marañon in Madrid presented data from a development and validation study of a clinical-genetic risk model for thrombosis in lymphoma in 208 patients with lymphoma, 31 of whom experienced a venous thromboembolic event.
While the relationship between cancer and increased thrombosis risk is well recognized, lymphoma patients are at particularly high risk, with an estimated thrombosis incidence of 5%-10%, Dr. Pascual said.
Currently, the Khorana score is the most validated risk score for thrombosis in patients with solid tumors, using factors such as tumor site, platelet and leukocyte count, hemoglobin levels, and body mass index. However, Dr. Pascual pointed out that just 10% of the validation cohort for the Khorana score were lymphoma patients, and it had previously been found to be not as useful for that population.
More recently, researchers had developed the ThroLy score for predicting thromboembolic events specifically in patients with lymphoma, incorporating clinical variables such as mediastinal involvement and extranodal localization.
Another group took a different approach by incorporating genetic risk factors for thrombosis to create Thrombo inCode-Oncology (TiC-Onco) for solid tumors. This assessment included four genetic variants known to increase the risk of thromboembolic events in cancer patients, as well as the clinical risk factors of body mass index, family history of thrombosis, primary tumor site, and tumor stage.
Dr. Pascual and colleagues developed a unique risk factor model that combined both the ThroLy and TiC-Onco elements.
In 208 patients with lymphoma who were not receiving anticoagulant treatment, researchers identified five clinical factors that were most predictive of venous thrombosis: a history of thrombosis, immobilization for more than 3 days, lymphoma type, Ann Arbor score for lymphoma stage, and mediastinal extension.
They combined these clinical risk factors with the genetic risk factors from the TiC-Onco score to develop the TiC-Onco–associated lymphoma score (TiC-Lympho).
When validated in the same group of patients, the TiC-Lympho score had a sensitivity of 93.55%, a specificity of 54.49%, positive predictive value of 26.36%, and negative predictive value of 97.94%.
The researchers also compared TiC-Lympho’s performance with that of the ThroLy and TiC-Onco models, and found it performed better on sensitivity and negative predictive value. The area under the curve for TiC-Lympho (0.783) was significantly higher than that seen with the other two risk models.
Session chair Kate Burbury, MBBS, of the Peter MacCallum Cancer Centre in Melbourne, raised the question of how the score – and particularly the genetic risk factor assessment – might be applied in the real-world clinical setting.
In an interview, Dr. Pascual said the findings represented preliminary data only, so the model was not ready to be applied to clinical practice yet. She also stressed that this was based on retrospective data, and needed to be further validated in other cohorts of lymphoma patients.
No conflicts of interest were reported.
SOURCE: Pascual C et al. 2019 ISTH Congress, Abstract OC 41.3.
MELBOURNE – A venous thromboembolism risk score that combines clinical risk factors, such as lymphoma type and stage, along with genetic variables, could offer a better way to predict venous thromboembolism in patients with lymphoma, according to new findings presented at the International Society on Thrombosis and Haemostasis congress.
Cristina Pascual, MD, of the Hospital Universitario Gregorio Marañon in Madrid presented data from a development and validation study of a clinical-genetic risk model for thrombosis in lymphoma in 208 patients with lymphoma, 31 of whom experienced a venous thromboembolic event.
While the relationship between cancer and increased thrombosis risk is well recognized, lymphoma patients are at particularly high risk, with an estimated thrombosis incidence of 5%-10%, Dr. Pascual said.
Currently, the Khorana score is the most validated risk score for thrombosis in patients with solid tumors, using factors such as tumor site, platelet and leukocyte count, hemoglobin levels, and body mass index. However, Dr. Pascual pointed out that just 10% of the validation cohort for the Khorana score were lymphoma patients, and it had previously been found to be not as useful for that population.
More recently, researchers had developed the ThroLy score for predicting thromboembolic events specifically in patients with lymphoma, incorporating clinical variables such as mediastinal involvement and extranodal localization.
Another group took a different approach by incorporating genetic risk factors for thrombosis to create Thrombo inCode-Oncology (TiC-Onco) for solid tumors. This assessment included four genetic variants known to increase the risk of thromboembolic events in cancer patients, as well as the clinical risk factors of body mass index, family history of thrombosis, primary tumor site, and tumor stage.
Dr. Pascual and colleagues developed a unique risk factor model that combined both the ThroLy and TiC-Onco elements.
In 208 patients with lymphoma who were not receiving anticoagulant treatment, researchers identified five clinical factors that were most predictive of venous thrombosis: a history of thrombosis, immobilization for more than 3 days, lymphoma type, Ann Arbor score for lymphoma stage, and mediastinal extension.
They combined these clinical risk factors with the genetic risk factors from the TiC-Onco score to develop the TiC-Onco–associated lymphoma score (TiC-Lympho).
When validated in the same group of patients, the TiC-Lympho score had a sensitivity of 93.55%, a specificity of 54.49%, positive predictive value of 26.36%, and negative predictive value of 97.94%.
The researchers also compared TiC-Lympho’s performance with that of the ThroLy and TiC-Onco models, and found it performed better on sensitivity and negative predictive value. The area under the curve for TiC-Lympho (0.783) was significantly higher than that seen with the other two risk models.
Session chair Kate Burbury, MBBS, of the Peter MacCallum Cancer Centre in Melbourne, raised the question of how the score – and particularly the genetic risk factor assessment – might be applied in the real-world clinical setting.
In an interview, Dr. Pascual said the findings represented preliminary data only, so the model was not ready to be applied to clinical practice yet. She also stressed that this was based on retrospective data, and needed to be further validated in other cohorts of lymphoma patients.
No conflicts of interest were reported.
SOURCE: Pascual C et al. 2019 ISTH Congress, Abstract OC 41.3.
MELBOURNE – A venous thromboembolism risk score that combines clinical risk factors, such as lymphoma type and stage, along with genetic variables, could offer a better way to predict venous thromboembolism in patients with lymphoma, according to new findings presented at the International Society on Thrombosis and Haemostasis congress.
Cristina Pascual, MD, of the Hospital Universitario Gregorio Marañon in Madrid presented data from a development and validation study of a clinical-genetic risk model for thrombosis in lymphoma in 208 patients with lymphoma, 31 of whom experienced a venous thromboembolic event.
While the relationship between cancer and increased thrombosis risk is well recognized, lymphoma patients are at particularly high risk, with an estimated thrombosis incidence of 5%-10%, Dr. Pascual said.
Currently, the Khorana score is the most validated risk score for thrombosis in patients with solid tumors, using factors such as tumor site, platelet and leukocyte count, hemoglobin levels, and body mass index. However, Dr. Pascual pointed out that just 10% of the validation cohort for the Khorana score were lymphoma patients, and it had previously been found to be not as useful for that population.
More recently, researchers had developed the ThroLy score for predicting thromboembolic events specifically in patients with lymphoma, incorporating clinical variables such as mediastinal involvement and extranodal localization.
Another group took a different approach by incorporating genetic risk factors for thrombosis to create Thrombo inCode-Oncology (TiC-Onco) for solid tumors. This assessment included four genetic variants known to increase the risk of thromboembolic events in cancer patients, as well as the clinical risk factors of body mass index, family history of thrombosis, primary tumor site, and tumor stage.
Dr. Pascual and colleagues developed a unique risk factor model that combined both the ThroLy and TiC-Onco elements.
In 208 patients with lymphoma who were not receiving anticoagulant treatment, researchers identified five clinical factors that were most predictive of venous thrombosis: a history of thrombosis, immobilization for more than 3 days, lymphoma type, Ann Arbor score for lymphoma stage, and mediastinal extension.
They combined these clinical risk factors with the genetic risk factors from the TiC-Onco score to develop the TiC-Onco–associated lymphoma score (TiC-Lympho).
When validated in the same group of patients, the TiC-Lympho score had a sensitivity of 93.55%, a specificity of 54.49%, positive predictive value of 26.36%, and negative predictive value of 97.94%.
The researchers also compared TiC-Lympho’s performance with that of the ThroLy and TiC-Onco models, and found it performed better on sensitivity and negative predictive value. The area under the curve for TiC-Lympho (0.783) was significantly higher than that seen with the other two risk models.
Session chair Kate Burbury, MBBS, of the Peter MacCallum Cancer Centre in Melbourne, raised the question of how the score – and particularly the genetic risk factor assessment – might be applied in the real-world clinical setting.
In an interview, Dr. Pascual said the findings represented preliminary data only, so the model was not ready to be applied to clinical practice yet. She also stressed that this was based on retrospective data, and needed to be further validated in other cohorts of lymphoma patients.
No conflicts of interest were reported.
SOURCE: Pascual C et al. 2019 ISTH Congress, Abstract OC 41.3.
REPORTING FROM 2019 ISTH CONGRESS
Concizumab looks feasible in hemophilia A and B treatment
MELBOURNE – A once-daily subcutaneous treatment that inhibits the tissue factor 4 pathway inhibitor has shown significant reductions in bleeding rates in patients with hemophilia A and B, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Jan Astermark, MD, PhD, of the Centre for Thrombosis and Haemostasis at Lund University in Sweden, presented data from two phase 2, dose-escalation trials of the monoclonal antibody concizumab.
The explorer 5 trial involved 36 adults with severe hemophilia A without inhibitors who were started on 0.15 mg/kg of concizumab for 24 weeks. If they experienced three or more bleeds during that time, they were escalated to 0.20 mg/kg, and then to 0.25mg/kg if they experienced an additional three bleeds. The initial 24-week treatment period was then extended by more than 52 weeks.
In the explorer 4 trial, 16 adults with hemophilia A and 10 adults with hemophilia B – all with inhibitors – were initially randomized 2:1 to either 24 weeks of 0.15mg/kg of concizumab, including a loading dose, or placebo, with similar dose escalation in response to breakthrough bleeds. After 24 weeks, the study continued with a 52-week extension, during which all patients were treated with concizumab.
Both studies saw reductions in bleeding rates associated with concizumab treatment.
In patients with hemophilia A and B with inhibitors, the mean annualized bleeding rate for all bleeds declined from 20.4 to 4.5 bleeds, spontaneous bleeds declined from 18.5 to 2.5, and joint bleeds declined from 15 to 3.2. All three of the reductions were statistically significant.
Almost all patients achieved a concizumab concentration of 100 ng/mL, which was the expected level based on data from the phase 1 trial. Some patients showed anticoncizumab antibodies, but these were transient and did not appear to have any effect on clinical outcomes, according to Dr. Astermark.
Most patients also reached a normal level of thrombin generation, although Dr. Astermark noted that there were some patients with hemophilia B with inhibitors who produced a lower amount of thrombin than normal.
Despite the increase in thrombin generation, there were no thromboembolic events, and no significant safety concerns emerged during the study, he reported.
“Importantly and interestingly, all patients completing the main phase went into the extension phase of this trial, indicating that it was something they think was a contribution to their treatment,” Dr. Astermark said.
Earlier in 2019, concizumab was granted breakthrough designation by the Food and Drug Administration for the treatment of patients with hemophilia B and inhibitors, allowing it to receive an accelerated review by the agency.
“What the FDA based their decision on was the B patients with inhibitors, because this is truly a group where we do not have so many options,” Dr. Astermark said in an interview. He also noted that the subcutaneous treatment, delivered via a pen-like device, was much more convenient for patients who, until now, had required repeated intravenous infusions.
Two phase 3 trials are now scheduled, in which patients will receive a higher loading dose than what was used in the phase 2 trials.
Novo Nordisk sponsored both studies. Dr. Astermark reported consultancies and research funding unrelated to the study.
SOURCE: Astermark J et al. 2019 ISTH Congress, Abstract LB 01.1.
MELBOURNE – A once-daily subcutaneous treatment that inhibits the tissue factor 4 pathway inhibitor has shown significant reductions in bleeding rates in patients with hemophilia A and B, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Jan Astermark, MD, PhD, of the Centre for Thrombosis and Haemostasis at Lund University in Sweden, presented data from two phase 2, dose-escalation trials of the monoclonal antibody concizumab.
The explorer 5 trial involved 36 adults with severe hemophilia A without inhibitors who were started on 0.15 mg/kg of concizumab for 24 weeks. If they experienced three or more bleeds during that time, they were escalated to 0.20 mg/kg, and then to 0.25mg/kg if they experienced an additional three bleeds. The initial 24-week treatment period was then extended by more than 52 weeks.
In the explorer 4 trial, 16 adults with hemophilia A and 10 adults with hemophilia B – all with inhibitors – were initially randomized 2:1 to either 24 weeks of 0.15mg/kg of concizumab, including a loading dose, or placebo, with similar dose escalation in response to breakthrough bleeds. After 24 weeks, the study continued with a 52-week extension, during which all patients were treated with concizumab.
Both studies saw reductions in bleeding rates associated with concizumab treatment.
In patients with hemophilia A and B with inhibitors, the mean annualized bleeding rate for all bleeds declined from 20.4 to 4.5 bleeds, spontaneous bleeds declined from 18.5 to 2.5, and joint bleeds declined from 15 to 3.2. All three of the reductions were statistically significant.
Almost all patients achieved a concizumab concentration of 100 ng/mL, which was the expected level based on data from the phase 1 trial. Some patients showed anticoncizumab antibodies, but these were transient and did not appear to have any effect on clinical outcomes, according to Dr. Astermark.
Most patients also reached a normal level of thrombin generation, although Dr. Astermark noted that there were some patients with hemophilia B with inhibitors who produced a lower amount of thrombin than normal.
Despite the increase in thrombin generation, there were no thromboembolic events, and no significant safety concerns emerged during the study, he reported.
“Importantly and interestingly, all patients completing the main phase went into the extension phase of this trial, indicating that it was something they think was a contribution to their treatment,” Dr. Astermark said.
Earlier in 2019, concizumab was granted breakthrough designation by the Food and Drug Administration for the treatment of patients with hemophilia B and inhibitors, allowing it to receive an accelerated review by the agency.
“What the FDA based their decision on was the B patients with inhibitors, because this is truly a group where we do not have so many options,” Dr. Astermark said in an interview. He also noted that the subcutaneous treatment, delivered via a pen-like device, was much more convenient for patients who, until now, had required repeated intravenous infusions.
Two phase 3 trials are now scheduled, in which patients will receive a higher loading dose than what was used in the phase 2 trials.
Novo Nordisk sponsored both studies. Dr. Astermark reported consultancies and research funding unrelated to the study.
SOURCE: Astermark J et al. 2019 ISTH Congress, Abstract LB 01.1.
MELBOURNE – A once-daily subcutaneous treatment that inhibits the tissue factor 4 pathway inhibitor has shown significant reductions in bleeding rates in patients with hemophilia A and B, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Jan Astermark, MD, PhD, of the Centre for Thrombosis and Haemostasis at Lund University in Sweden, presented data from two phase 2, dose-escalation trials of the monoclonal antibody concizumab.
The explorer 5 trial involved 36 adults with severe hemophilia A without inhibitors who were started on 0.15 mg/kg of concizumab for 24 weeks. If they experienced three or more bleeds during that time, they were escalated to 0.20 mg/kg, and then to 0.25mg/kg if they experienced an additional three bleeds. The initial 24-week treatment period was then extended by more than 52 weeks.
In the explorer 4 trial, 16 adults with hemophilia A and 10 adults with hemophilia B – all with inhibitors – were initially randomized 2:1 to either 24 weeks of 0.15mg/kg of concizumab, including a loading dose, or placebo, with similar dose escalation in response to breakthrough bleeds. After 24 weeks, the study continued with a 52-week extension, during which all patients were treated with concizumab.
Both studies saw reductions in bleeding rates associated with concizumab treatment.
In patients with hemophilia A and B with inhibitors, the mean annualized bleeding rate for all bleeds declined from 20.4 to 4.5 bleeds, spontaneous bleeds declined from 18.5 to 2.5, and joint bleeds declined from 15 to 3.2. All three of the reductions were statistically significant.
Almost all patients achieved a concizumab concentration of 100 ng/mL, which was the expected level based on data from the phase 1 trial. Some patients showed anticoncizumab antibodies, but these were transient and did not appear to have any effect on clinical outcomes, according to Dr. Astermark.
Most patients also reached a normal level of thrombin generation, although Dr. Astermark noted that there were some patients with hemophilia B with inhibitors who produced a lower amount of thrombin than normal.
Despite the increase in thrombin generation, there were no thromboembolic events, and no significant safety concerns emerged during the study, he reported.
“Importantly and interestingly, all patients completing the main phase went into the extension phase of this trial, indicating that it was something they think was a contribution to their treatment,” Dr. Astermark said.
Earlier in 2019, concizumab was granted breakthrough designation by the Food and Drug Administration for the treatment of patients with hemophilia B and inhibitors, allowing it to receive an accelerated review by the agency.
“What the FDA based their decision on was the B patients with inhibitors, because this is truly a group where we do not have so many options,” Dr. Astermark said in an interview. He also noted that the subcutaneous treatment, delivered via a pen-like device, was much more convenient for patients who, until now, had required repeated intravenous infusions.
Two phase 3 trials are now scheduled, in which patients will receive a higher loading dose than what was used in the phase 2 trials.
Novo Nordisk sponsored both studies. Dr. Astermark reported consultancies and research funding unrelated to the study.
SOURCE: Astermark J et al. 2019 ISTH Congress, Abstract LB 01.1.
REPORTING FROM 2019 ISTH CONGRESS
CMS is proposing higher payments for E/M visits
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
AAD, NPF update use of phototherapy for psoriasis
, according to updated guidelines issued jointly by the American Academy of Dermatology and the National Psoriasis Foundation.
“Phototherapy serves as a reasonable and effective treatment option for patients requiring more than topical medications and/or those wishing to avoid systemic medications or simply seeking an adjunct to a failing regimen,” wrote working group cochair Craig A. Elmets, MD, professor of dermatology at the University of Alabama at Birmingham, and coauthors.
The guidelines, which focus on phototherapy for adults with psoriasis, join a multipart series on psoriasis being published this year in the Journal of the American Academy of Dermatology.
The working group used an evidence-based model to examine efficacy, effectiveness, and adverse effects of the following modalities: narrow-band ultraviolet B (NB-UVB); broadband ultraviolet B (BB-UVB); targeted phototherapy using excimer laser and excimer lamp; psoralen plus ultraviolet A (PUVA) therapy, including topical, oral, and bath PUVA; photodynamic therapy (PDT), grenz ray therapy, climatotherapy; visible light therapy; Goeckerman therapy; and pulsed dye laser/intense pulsed light.
NB-UVB, which can be used to treat generalized plaque psoriasis, refers to wavelengths of 311-313 nm. The recommended treatment is two or three times a week, with a starting dose based on skin phenotype or minimal erythema dose. Although oral PUVA has shown higher clearance rates, compared with NB-UVB, NB-UVB has demonstrated fewer side effects. NB-UVB also has shown effectiveness for psoriasis in combination with medications including oral retinoids, “particularly useful in patients at increased risk for skin cancer,” the working group wrote. Genital shielding and eye protection are recommended during all phototherapy sessions to reduce the risk of cancer and cataracts, they emphasized.
BB-UVB, an older version of NB-UVB, is still effective for generalized plaque psoriasis as monotherapy, but evidence does not support additional benefit in combination with other treatments, and overall BB-UVB is less effective than either NB-UVB or oral PUVA, the working group said.
For treatment of localized psoriatic lesions, some evidence supports the ability of targeted UVB therapy to improve lesions in fewer treatments and at a lower cumulative dose, compared with nontargeted phototherapy, for palmoplantar plaque psoriasis and palmoplantar pustulosis. Excimer lasers also have shown effectiveness against scalp psoriasis, the working group noted. However, “there is insufficient evidence to recommend the excimer laser rather than topical PUVA for treatment of localized plaque psoriasis,” they said.
PUVA treatments are available as topical creams, or they can be taken orally, or mixed with bath water. All forms of PUVA include psoralens, photosensitizing agents that prepare target cells for the effects of UVA light. Topical PUVA has demonstrated particular effectiveness for palmoplantar psoriasis, the working group noted, but there is a risk of phototoxicity, so it has become less popular, they added. Similarly, evidence supports effectiveness of oral and bath PUVA, but all forms are used less frequently because of the increased availability of NB-UVB phototherapy, they said.
PDT is primarily used to destroy premalignant or malignant cells, and in theory “PDT-induced apoptosis of T lymphocytes could lead to reductions in inflammatory cytokines and, in turn, to improvement of psoriasis,” the working group noted. However, “clinical studies have failed to find significant benefit” of PDT using either 5-aminolevulinic acid (ALA) or methyl aminolevulinic acid (MAL) for psoriasis, or any significant benefits of MAL-PDT for nail psoriasis.
The grenz ray is an effective, but rarely used treatment in which 75% of long-wavelength ionizing radiation is absorbed by the first 1 mm of skin and 95% is absorbed within the first 3 mm of skin to protect the deeper tissues from radiation. Although more alternatives are available, grenz rays can be used for psoriasis patients unable to tolerate UV therapy, according to the working group.
Climatotherapy involves temporary or permanent relocation of the patient to a part of the world with a climate that could be favorable for psoriasis because of the unique effects of environmental factors in those areas. The evidence to support climatotherapy is both subjective and objective, but considered safe.
Visible light has been associated with improvement in erythema in psoriasis, with hyperpigmentation as the only notable side effect based on the evidence reviewed. However, the working group found the current evidence insufficient to recommend the use of intense pulsed light for treating psoriasis.
Goeckerman therapy, a method that combines coal tar and UVB phototherapy, has shown safety and effectiveness for patients with recalcitrant or severe psoriasis, and remains a recommended treatment, according to the working group research. However, this method is underused, especially in the United States, because of the messiness of the application, challenge of insurance reimbursement, and investment of time for outpatient care, the work group noted.
Pulsed dye laser treatment is effective for nail psoriasis, and reported adverse effects have been mild, according to the working group.
Overall, the guidelines emphasize that quality of life and disease severity should be considered and discussed with patients along with efficacy and safety information so they can make informed decisions about adding phototherapy to a current regimen or switching among modalities.
The guidelines have no funding sources. Several coauthors disclosed relationships with multiple companies, including manufacturers of psoriasis products; however, a minimum of 51% of the work group had no relevant financial conflicts to disclose, in accordance with AAD policy. Work group members with potential conflicts recused themselves from discussion and drafting of recommendations in the relevant topic areas. Alan Menter, MD, chairman of the division of dermatology, Baylor University Medical Center, Dallas, is the other cochair of the work group.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2019 Jul 18. doi: 10.1016/j.jaad.2019.04.042.
, according to updated guidelines issued jointly by the American Academy of Dermatology and the National Psoriasis Foundation.
“Phototherapy serves as a reasonable and effective treatment option for patients requiring more than topical medications and/or those wishing to avoid systemic medications or simply seeking an adjunct to a failing regimen,” wrote working group cochair Craig A. Elmets, MD, professor of dermatology at the University of Alabama at Birmingham, and coauthors.
The guidelines, which focus on phototherapy for adults with psoriasis, join a multipart series on psoriasis being published this year in the Journal of the American Academy of Dermatology.
The working group used an evidence-based model to examine efficacy, effectiveness, and adverse effects of the following modalities: narrow-band ultraviolet B (NB-UVB); broadband ultraviolet B (BB-UVB); targeted phototherapy using excimer laser and excimer lamp; psoralen plus ultraviolet A (PUVA) therapy, including topical, oral, and bath PUVA; photodynamic therapy (PDT), grenz ray therapy, climatotherapy; visible light therapy; Goeckerman therapy; and pulsed dye laser/intense pulsed light.
NB-UVB, which can be used to treat generalized plaque psoriasis, refers to wavelengths of 311-313 nm. The recommended treatment is two or three times a week, with a starting dose based on skin phenotype or minimal erythema dose. Although oral PUVA has shown higher clearance rates, compared with NB-UVB, NB-UVB has demonstrated fewer side effects. NB-UVB also has shown effectiveness for psoriasis in combination with medications including oral retinoids, “particularly useful in patients at increased risk for skin cancer,” the working group wrote. Genital shielding and eye protection are recommended during all phototherapy sessions to reduce the risk of cancer and cataracts, they emphasized.
BB-UVB, an older version of NB-UVB, is still effective for generalized plaque psoriasis as monotherapy, but evidence does not support additional benefit in combination with other treatments, and overall BB-UVB is less effective than either NB-UVB or oral PUVA, the working group said.
For treatment of localized psoriatic lesions, some evidence supports the ability of targeted UVB therapy to improve lesions in fewer treatments and at a lower cumulative dose, compared with nontargeted phototherapy, for palmoplantar plaque psoriasis and palmoplantar pustulosis. Excimer lasers also have shown effectiveness against scalp psoriasis, the working group noted. However, “there is insufficient evidence to recommend the excimer laser rather than topical PUVA for treatment of localized plaque psoriasis,” they said.
PUVA treatments are available as topical creams, or they can be taken orally, or mixed with bath water. All forms of PUVA include psoralens, photosensitizing agents that prepare target cells for the effects of UVA light. Topical PUVA has demonstrated particular effectiveness for palmoplantar psoriasis, the working group noted, but there is a risk of phototoxicity, so it has become less popular, they added. Similarly, evidence supports effectiveness of oral and bath PUVA, but all forms are used less frequently because of the increased availability of NB-UVB phototherapy, they said.
PDT is primarily used to destroy premalignant or malignant cells, and in theory “PDT-induced apoptosis of T lymphocytes could lead to reductions in inflammatory cytokines and, in turn, to improvement of psoriasis,” the working group noted. However, “clinical studies have failed to find significant benefit” of PDT using either 5-aminolevulinic acid (ALA) or methyl aminolevulinic acid (MAL) for psoriasis, or any significant benefits of MAL-PDT for nail psoriasis.
The grenz ray is an effective, but rarely used treatment in which 75% of long-wavelength ionizing radiation is absorbed by the first 1 mm of skin and 95% is absorbed within the first 3 mm of skin to protect the deeper tissues from radiation. Although more alternatives are available, grenz rays can be used for psoriasis patients unable to tolerate UV therapy, according to the working group.
Climatotherapy involves temporary or permanent relocation of the patient to a part of the world with a climate that could be favorable for psoriasis because of the unique effects of environmental factors in those areas. The evidence to support climatotherapy is both subjective and objective, but considered safe.
Visible light has been associated with improvement in erythema in psoriasis, with hyperpigmentation as the only notable side effect based on the evidence reviewed. However, the working group found the current evidence insufficient to recommend the use of intense pulsed light for treating psoriasis.
Goeckerman therapy, a method that combines coal tar and UVB phototherapy, has shown safety and effectiveness for patients with recalcitrant or severe psoriasis, and remains a recommended treatment, according to the working group research. However, this method is underused, especially in the United States, because of the messiness of the application, challenge of insurance reimbursement, and investment of time for outpatient care, the work group noted.
Pulsed dye laser treatment is effective for nail psoriasis, and reported adverse effects have been mild, according to the working group.
Overall, the guidelines emphasize that quality of life and disease severity should be considered and discussed with patients along with efficacy and safety information so they can make informed decisions about adding phototherapy to a current regimen or switching among modalities.
The guidelines have no funding sources. Several coauthors disclosed relationships with multiple companies, including manufacturers of psoriasis products; however, a minimum of 51% of the work group had no relevant financial conflicts to disclose, in accordance with AAD policy. Work group members with potential conflicts recused themselves from discussion and drafting of recommendations in the relevant topic areas. Alan Menter, MD, chairman of the division of dermatology, Baylor University Medical Center, Dallas, is the other cochair of the work group.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2019 Jul 18. doi: 10.1016/j.jaad.2019.04.042.
, according to updated guidelines issued jointly by the American Academy of Dermatology and the National Psoriasis Foundation.
“Phototherapy serves as a reasonable and effective treatment option for patients requiring more than topical medications and/or those wishing to avoid systemic medications or simply seeking an adjunct to a failing regimen,” wrote working group cochair Craig A. Elmets, MD, professor of dermatology at the University of Alabama at Birmingham, and coauthors.
The guidelines, which focus on phototherapy for adults with psoriasis, join a multipart series on psoriasis being published this year in the Journal of the American Academy of Dermatology.
The working group used an evidence-based model to examine efficacy, effectiveness, and adverse effects of the following modalities: narrow-band ultraviolet B (NB-UVB); broadband ultraviolet B (BB-UVB); targeted phototherapy using excimer laser and excimer lamp; psoralen plus ultraviolet A (PUVA) therapy, including topical, oral, and bath PUVA; photodynamic therapy (PDT), grenz ray therapy, climatotherapy; visible light therapy; Goeckerman therapy; and pulsed dye laser/intense pulsed light.
NB-UVB, which can be used to treat generalized plaque psoriasis, refers to wavelengths of 311-313 nm. The recommended treatment is two or three times a week, with a starting dose based on skin phenotype or minimal erythema dose. Although oral PUVA has shown higher clearance rates, compared with NB-UVB, NB-UVB has demonstrated fewer side effects. NB-UVB also has shown effectiveness for psoriasis in combination with medications including oral retinoids, “particularly useful in patients at increased risk for skin cancer,” the working group wrote. Genital shielding and eye protection are recommended during all phototherapy sessions to reduce the risk of cancer and cataracts, they emphasized.
BB-UVB, an older version of NB-UVB, is still effective for generalized plaque psoriasis as monotherapy, but evidence does not support additional benefit in combination with other treatments, and overall BB-UVB is less effective than either NB-UVB or oral PUVA, the working group said.
For treatment of localized psoriatic lesions, some evidence supports the ability of targeted UVB therapy to improve lesions in fewer treatments and at a lower cumulative dose, compared with nontargeted phototherapy, for palmoplantar plaque psoriasis and palmoplantar pustulosis. Excimer lasers also have shown effectiveness against scalp psoriasis, the working group noted. However, “there is insufficient evidence to recommend the excimer laser rather than topical PUVA for treatment of localized plaque psoriasis,” they said.
PUVA treatments are available as topical creams, or they can be taken orally, or mixed with bath water. All forms of PUVA include psoralens, photosensitizing agents that prepare target cells for the effects of UVA light. Topical PUVA has demonstrated particular effectiveness for palmoplantar psoriasis, the working group noted, but there is a risk of phototoxicity, so it has become less popular, they added. Similarly, evidence supports effectiveness of oral and bath PUVA, but all forms are used less frequently because of the increased availability of NB-UVB phototherapy, they said.
PDT is primarily used to destroy premalignant or malignant cells, and in theory “PDT-induced apoptosis of T lymphocytes could lead to reductions in inflammatory cytokines and, in turn, to improvement of psoriasis,” the working group noted. However, “clinical studies have failed to find significant benefit” of PDT using either 5-aminolevulinic acid (ALA) or methyl aminolevulinic acid (MAL) for psoriasis, or any significant benefits of MAL-PDT for nail psoriasis.
The grenz ray is an effective, but rarely used treatment in which 75% of long-wavelength ionizing radiation is absorbed by the first 1 mm of skin and 95% is absorbed within the first 3 mm of skin to protect the deeper tissues from radiation. Although more alternatives are available, grenz rays can be used for psoriasis patients unable to tolerate UV therapy, according to the working group.
Climatotherapy involves temporary or permanent relocation of the patient to a part of the world with a climate that could be favorable for psoriasis because of the unique effects of environmental factors in those areas. The evidence to support climatotherapy is both subjective and objective, but considered safe.
Visible light has been associated with improvement in erythema in psoriasis, with hyperpigmentation as the only notable side effect based on the evidence reviewed. However, the working group found the current evidence insufficient to recommend the use of intense pulsed light for treating psoriasis.
Goeckerman therapy, a method that combines coal tar and UVB phototherapy, has shown safety and effectiveness for patients with recalcitrant or severe psoriasis, and remains a recommended treatment, according to the working group research. However, this method is underused, especially in the United States, because of the messiness of the application, challenge of insurance reimbursement, and investment of time for outpatient care, the work group noted.
Pulsed dye laser treatment is effective for nail psoriasis, and reported adverse effects have been mild, according to the working group.
Overall, the guidelines emphasize that quality of life and disease severity should be considered and discussed with patients along with efficacy and safety information so they can make informed decisions about adding phototherapy to a current regimen or switching among modalities.
The guidelines have no funding sources. Several coauthors disclosed relationships with multiple companies, including manufacturers of psoriasis products; however, a minimum of 51% of the work group had no relevant financial conflicts to disclose, in accordance with AAD policy. Work group members with potential conflicts recused themselves from discussion and drafting of recommendations in the relevant topic areas. Alan Menter, MD, chairman of the division of dermatology, Baylor University Medical Center, Dallas, is the other cochair of the work group.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2019 Jul 18. doi: 10.1016/j.jaad.2019.04.042.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Darkening and Eruptive Nevi During Treatment With Erlotinib
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
Practice Points
- Cutaneous side effects of erlotinib include acneform eruption, xerosis, paronychia, and pruritus.
- Clinicians should monitor patients for darkening and/or eruptive nevi as well as melanoma during treatment with erlotinib.
Hypothyroidism may have more impact on cardiac health than hyperthyroidism
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
EXPERT ANALYSIS FROM CARPS 2019
Metastatic Adamantinoma Presenting as a Cutaneous Papule
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
Practice Points
- Metastatic adamantinoma of the skin is a rare clinical scenario.
- Dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.