User login
Predatory journals and HULLK’s prostate
The incredible HULLK
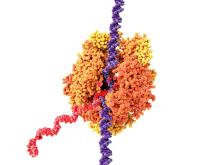
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.
The incredible HULLK
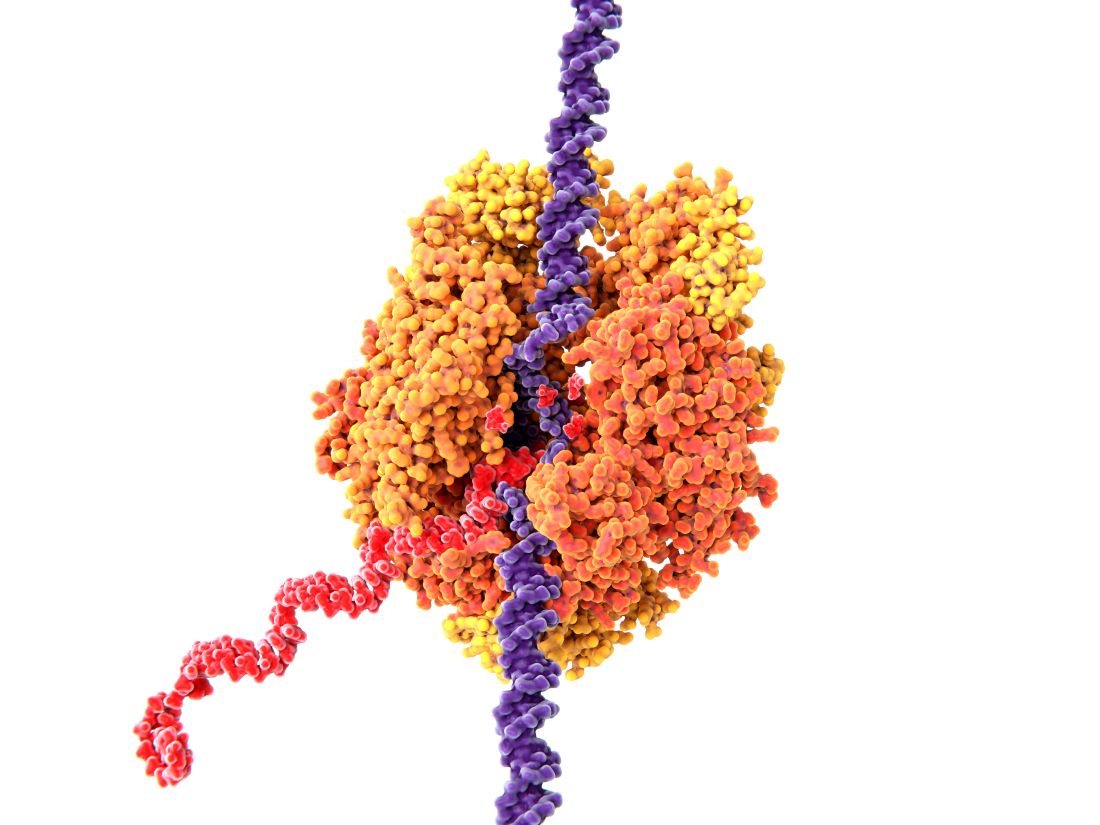
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.

The incredible HULLK
HULLK TYPE OF RNA THAT CONTROLS GROWTH OF PROSTATE CANCER CELLS. HULLK IS STRONGEST RNA THERE IS. HULLK SMASH CANCER!
Is everyone’s favorite not-so-jolly green giant the key to crushing prostate cancer? Not exactly: HULLK is a noncoding type of RNA. Which means, instead of coding a protein, it helps regulate cellular processes.
Cancer researchers from the University of Virginia found there was more HULLK in tumors from patients with advanced prostate cancer. They also found that decreasing the incredible RNA slows tumor cell growth.
In other words, we need a little more Bruce Banner, a little less HULLK.
The scientists who identified HULLK are hopeful that it can function as a biomarker and a therapeutic target in the future, and could be an integral discovery on the way to curing prostate cancer. HULLK LIKE.
Trading places with Sigmund Freud
Sometimes, it can be helpful to talk to someone about your problems. We’re not really going out on a limb here. That is, after all, pretty much the basis of psychotherapy.
But what if that someone else was really you, disguised as Sigmund Freud?
That was the premise for a recent study involving body swapping and immersive virtual reality. The investigators scanned each subject to create a 3D avatar that functioned as an online representation and moved as he or she moved during the experiment. In virtual reality, the subject’s avatar sat across a table from Sigmund Freud, who was the variable element in the study.
For the control group, Freud responded with prescripted questions and comments about the subject’s problem. The other group, however, was able to swap virtual bodies and respond to their own bodies as Freud. In other words, they could have a conversation with themselves, but it looked like they were talking to Freud.
A week after the virtual conversations, more than 80% of those in the body-swapping group experienced some sort of change with respect to their problem, compared with less than 50% of controls.
“We found that those in the body-swapping group got better knowledge, understanding, control, and new ideas about their problem, compared to the control group,” one of the investigators said.
We’re just wondering about their choice of Freud. It kind of makes sense, because that was his line of work; but would another person have been even more helpful?
How about Dr. Phil? Or maybe Oprah? Seems like Dwayne “the Rock” Johnson is everywhere else, so why not virtual reality? It would be hard to go wrong with some type of Kardashian, right?
Cue the ‘Jaws’ music
Doctors with freshly written studies beware, for you are not alone. Lurking in the undergrowth, stalking your every move, are the predatory journals. They’ve come for you. And they pose a danger not only to you, but to the entire field of medical literature.
However, there’s no need to fear, as you have a guide through the great Serengeti of the publication process: a new guideline on avoiding predatory journals from the American Medical Writers Association, European Medical Writers Association, and International Society for Medical Publication Professionals.
According to the guideline, some telltale characteristics of these journals include a lack of information, poorly made websites, a lack of indexing in a recognized system, promises of unrealistically quick peer review, and an insatiable thirst for your blood. We may have made that last one up.
The guideline authors call for all medical authors to conduct research while submitting to journals, and only submit to those journals that conduct a full peer review process and that genuinely seek to contribute to medical literature.
Failure to do so may result in a damaged reputation, being unwittingly appointed to an editorial board, losing your paper, or having your liver eaten by an eagle day after day until Hercules finally frees you from your torture.
We may have made that one up as well.

GLP-1 agonists, SGLT2 inhibitors offer more options in diabetes management
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Endocrine Society advises on diabetes care for older adults
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
FDA approves darolutamide for nonmetastatic CRPC
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
The Food and Drug Administration has approved darolutamide for nonmetastatic, castration-resistant prostate cancer.
The approval was based on improved metastasis-free survival (MFS) in the randomized ARAMIS trial of 1,509 patients with nonmetastatic, castration-resistant prostate cancer.
Median MFS was 40.4 months (95% confidence interval, 34.3 months to not reached) for patients treated with darolutamide, compared with 18.4 months (95% CI, 15.5-22.3 months) for those receiving placebo (hazard ratio, 0.41; 95% CI, 0.34-0.50; P less than .0001), according to the FDA.
MFS is defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first.
In ARAMIS, patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n = 955) or matching placebo (n = 554). All patients received a gonadotropin-releasing hormone analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.
Overall survival data is not yet mature, the FDA said.
The most common adverse reactions in patients who received darolutamide were fatigue, extremity pain, and rash. Ischemic heart disease (4.3%) and heart failure (2.1%) were more common on the darolutamide arm, while seizure incidence was similar in the two arms (0.2%).
The recommended darolutamide dose is 600 mg (two 300-mg tablets) administered orally twice daily with food. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy, the FDA said.
Darolutamide is marketed as Nubeqa by Bayer HealthCare Pharmaceuticals.
Higher dietary vitamin A linked to lower SCC risk
in a large prospective study published in JAMA Dermatology.
There was also an inverse association between intake of carotenoids and risk of cutaneous SCC over the follow-up period of 26-28 years. The results of the study support “the protective role of vitamin A against SCC development,” wrote Jongwoo Kim, MD, of Brown University, Providence, R.I., and Inje University, Seoul, South Korea, and coauthors. “Our data further support the contention that supplemental and dietary vitamin A may be beneficial in preventing SCC,” they added.
The study evaluated intake of vitamin A and carotenoids and SCC risk with data from the Health Professionals Follow-Up Study (1986-2012) of 48,400 men, and the Nurses’ Health Study (1984-2012) of 75,170 women. Participants in those studies completed questionnaires based on lifestyle and medical history. Only white participants were included, because of the low number of SCC cases and low SCC risk in nonwhite participants, and participants who did not report diet and those who had a history of melanoma, SCC, or other cancer diagnoses at baseline were excluded.
Over the follow-up of 26-28 years, a total of 3,978 SCC cases were confirmed using pathological records. The investigators used different quintiles based on the median total amount of vitamin A intake. Using quintile 1 (lowest intake) as a reference, the pooled multivariate hazard ratios of vitamin A intake were 0.97, 0.97, 0.93, and 0.83 for quintiles 2, 3, 4, and 5, respectively (P less than .001 for the trend, in order of increasing quintiles).
In addition, they reported that greater intakes of retinol and several carotenoids were also significantly associated with a lower SCC risk.
The results were “generally consistent between men and women,” and “the inverse associations appeared to be more prominent among those with moles and those with burn or blistering sunburn reaction as children or adolescents,” they wrote.
The large sample size, prospective design, and confirmation of SCC cases by histology are among the strengths of the study, while a key limitation of the study was the homogeneous nature of the study population, which “may limit the generalizability of our findings,” the authors wrote.
The study was funded by the National Institutes of Health and Inje University (South Korea). One author reported serving as a consultant for AbbVie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, and Pfizer; and as a compensated investigator for Amgen, Regeneron, and Sanofi. Dr. Kim and the remaining three authors reported no disclosures.
SOURCE: Kim J et al. JAMA Dermatol. 2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937.
in a large prospective study published in JAMA Dermatology.
There was also an inverse association between intake of carotenoids and risk of cutaneous SCC over the follow-up period of 26-28 years. The results of the study support “the protective role of vitamin A against SCC development,” wrote Jongwoo Kim, MD, of Brown University, Providence, R.I., and Inje University, Seoul, South Korea, and coauthors. “Our data further support the contention that supplemental and dietary vitamin A may be beneficial in preventing SCC,” they added.
The study evaluated intake of vitamin A and carotenoids and SCC risk with data from the Health Professionals Follow-Up Study (1986-2012) of 48,400 men, and the Nurses’ Health Study (1984-2012) of 75,170 women. Participants in those studies completed questionnaires based on lifestyle and medical history. Only white participants were included, because of the low number of SCC cases and low SCC risk in nonwhite participants, and participants who did not report diet and those who had a history of melanoma, SCC, or other cancer diagnoses at baseline were excluded.
Over the follow-up of 26-28 years, a total of 3,978 SCC cases were confirmed using pathological records. The investigators used different quintiles based on the median total amount of vitamin A intake. Using quintile 1 (lowest intake) as a reference, the pooled multivariate hazard ratios of vitamin A intake were 0.97, 0.97, 0.93, and 0.83 for quintiles 2, 3, 4, and 5, respectively (P less than .001 for the trend, in order of increasing quintiles).
In addition, they reported that greater intakes of retinol and several carotenoids were also significantly associated with a lower SCC risk.
The results were “generally consistent between men and women,” and “the inverse associations appeared to be more prominent among those with moles and those with burn or blistering sunburn reaction as children or adolescents,” they wrote.
The large sample size, prospective design, and confirmation of SCC cases by histology are among the strengths of the study, while a key limitation of the study was the homogeneous nature of the study population, which “may limit the generalizability of our findings,” the authors wrote.
The study was funded by the National Institutes of Health and Inje University (South Korea). One author reported serving as a consultant for AbbVie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, and Pfizer; and as a compensated investigator for Amgen, Regeneron, and Sanofi. Dr. Kim and the remaining three authors reported no disclosures.
SOURCE: Kim J et al. JAMA Dermatol. 2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937.
in a large prospective study published in JAMA Dermatology.
There was also an inverse association between intake of carotenoids and risk of cutaneous SCC over the follow-up period of 26-28 years. The results of the study support “the protective role of vitamin A against SCC development,” wrote Jongwoo Kim, MD, of Brown University, Providence, R.I., and Inje University, Seoul, South Korea, and coauthors. “Our data further support the contention that supplemental and dietary vitamin A may be beneficial in preventing SCC,” they added.
The study evaluated intake of vitamin A and carotenoids and SCC risk with data from the Health Professionals Follow-Up Study (1986-2012) of 48,400 men, and the Nurses’ Health Study (1984-2012) of 75,170 women. Participants in those studies completed questionnaires based on lifestyle and medical history. Only white participants were included, because of the low number of SCC cases and low SCC risk in nonwhite participants, and participants who did not report diet and those who had a history of melanoma, SCC, or other cancer diagnoses at baseline were excluded.
Over the follow-up of 26-28 years, a total of 3,978 SCC cases were confirmed using pathological records. The investigators used different quintiles based on the median total amount of vitamin A intake. Using quintile 1 (lowest intake) as a reference, the pooled multivariate hazard ratios of vitamin A intake were 0.97, 0.97, 0.93, and 0.83 for quintiles 2, 3, 4, and 5, respectively (P less than .001 for the trend, in order of increasing quintiles).
In addition, they reported that greater intakes of retinol and several carotenoids were also significantly associated with a lower SCC risk.
The results were “generally consistent between men and women,” and “the inverse associations appeared to be more prominent among those with moles and those with burn or blistering sunburn reaction as children or adolescents,” they wrote.
The large sample size, prospective design, and confirmation of SCC cases by histology are among the strengths of the study, while a key limitation of the study was the homogeneous nature of the study population, which “may limit the generalizability of our findings,” the authors wrote.
The study was funded by the National Institutes of Health and Inje University (South Korea). One author reported serving as a consultant for AbbVie, Amgen, the Centers for Disease Control and Prevention, Janssen, Merck, Novartis, and Pfizer; and as a compensated investigator for Amgen, Regeneron, and Sanofi. Dr. Kim and the remaining three authors reported no disclosures.
SOURCE: Kim J et al. JAMA Dermatol. 2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937.
FROM JAMA DERMATOLOGY
Short-course azithromycin no benefit in pediatric asthma admissions
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
REPORTING FROM PHM 2019
Brain imaging could predict response to CBT in depression
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
Researchers have used functional MRI to identify key differences between individuals with depression who respond to cognitive-behavioral therapy and those who don’t.
In a paper published in Science Advances, Filippo Queirazza, MD, PhD, and coauthors reported the outcomes of a study of 37 individuals with untreated depression who took part in an online, self-guided cognitive-behavioral therapy (CBT) program.
The participants – 18 of whom were women – attended an appointment before and 2 months after completing the therapy program, at which they were clinically evaluated by a psychiatrist, and underwent fMRI scanning. Only 26 subjects completed the program and attended the posttreatment appointment, wrote Dr. Queirazza of the Institute of Neuroscience and Psychology at the University of Glasgow, Scotland.
To look for indication of treatment response in fMRI scanning, researchers got participants to do a series of reverse-learning tasks while they were being scanned. That activity involved learning which of two stimuli gave the highest payoff rate.
“The choice of a relevant generative model in MDD is dictated by a wealth of behavioral and neural findings, suggesting that learning from positive (reward) and negative (punishment) feedback [also known as reinforcement learning] is substantially impaired in depressed subjects,” the authors wrote.
The imaging suggested that individuals who responded showed more pretreatment neural activity in areas of the brain that deal with the acquisition and processing of feedback information, and that the level of activity was in proportion to the magnitude of their later treatment response.
They also found that activity in right striatum was best at discriminating responders from nonresponders. Based on their findings, they were able to predict responders as well as did pretreatment BDI-II scores.
“Our finding that neural activity in the right striatum is positively correlated with CBT response is consistent with a previous report that, in a group of adolescents with depression, greater pretreatment striatal responses to both anticipation and presentation of positive feedback during a monetary reward task were linked to posttreatment reduction in depression severity, particularly of anxiety symptoms,” they wrote.
The study was supported by several entities, including the Chief Scientist Office and the Dr Mortimer and Theresa Sackler Foundation. No competing interests were declared.
SOURCE: Queirazza F et al. Sci Adv. 2019 Jul 31. doi: 10.1126/sciadv.aav4962.
FROM SCIENCE ADVANCES
Placental bacteria not linked to adverse pregnancy outcomes
such as preeclampsia or spontaneous preterm birth, a study has found.
Dr. Marcus C. de Goffau, of Wellcome Sanger Institute, Cambridge, England, and coauthors wrote that placental dysfunction is linked to a number of common adverse pregnancy outcomes, but in many cases the cause of that dysfunction is unknown.
“Several studies have used sequencing-based methods for bacterial detection (metagenomics and 16S rRNA gene amplicon sequencing), and have concluded that the placenta is physiologically colonized by a diverse population of bacteria (the ‘placental microbiome’) and that the nature of this colonization may differ between healthy and complicated pregnancies,” they wrote.
In a paper published in the July 31 edition of Nature (doi: 10.1038/s41586-019-1451-5), researchers reported the outcomes of a study involving two cohorts. The first included 80 babies delivered by prelabor caesarean section, of whom 20 were small for gestational age, 20 were delivered to mothers with preeclampsia, and 40 were matched controls. The second cohort comprised 100 patients with preeclampsia, 100 small-for-gestational-age infants, 100 preterm births, and 198 matched controls, with two controls having been used twice.
The bacterial content of the placentas was analyzed via deep metagenomic sequencing of total DNA and 16S rRNA gene amplicon sequencing in cohort 1, and 16S rRNA gene amplicon from two different kits for cohort 2’s samples. In the first cohort, samples were also spiked with Salmonella bongori as a positive control.
For cohort 1, researchers were able to detect the S. bongori in all samples, but all other bacterial signals showed clear links to different batches, which the authors said showed they were the result of contamination. This was further supported by the discovery that the Escherichia coli signal seen in a number of batches was all from the same bacterial strain.
In the second cohort, researchers found Bradyrhizobium in nearly all samples, and Burkholderia – which has been thought to play a role in preterm birth – in some samples.
However in the case of the Burkholderia, significant variation between different runs of tests was found, and both Bradyrhizobium and Burkholderia were also found in negative controls.
Researchers also saw a high prevalence of four “ecologically unexpected” bacterial groups but were able to show that these were likely contaminants from a reagent used to wash the placental samples.
The study found that vaginal organisms such as lactobacilli and vaginosis-associated bacteria were more abundant in the second cohort, where babies were delivered by a mix of vaginal, intrapartum and prelabor caesarean section, compared with the first cohort, who were all prelabor caesarean section deliveries.
The only organism that met all the criteria for a genuine placenta-associated bacterial signal was Streptococcus agalactiae, but the authors found no association between it and pregnancy outcomes.
However, they did sound a warning that the perinatal transmission of S. agalactiae from the mother’s genital tract to the neonate could be a cause of fatal sepsis in the infant.
“Further studies will be required to determine the association between the presence of the organism in the placenta and fetal or neonatal disease,” they wrote.
The researchers also found significant associations between the delivery-associated vaginal bacteria Lactobacillus iners and preeclampsia, and between Streptococcus anginosus and the Ureaplasma genus – both of which are associated with vaginosis – and preterm birth.
“We conclude that bacterial placental infection is not a major cause of placentally related complications of human pregnancy and that the human placenta does not have a resident microbiome,” the authors wrote. “Although we see no evidence of a placental microbiome, the frequency of detection of vaginal bacteria in the placenta increased after intrapartum [cesarean] section, suggesting ascending or haematogenous spread.”
The Medical Research Council and the National Institute for Health Research Cambridge Biomedical Research Centre supported the study. Five authors declared grants and support from private industry outside the submitted work.
SOURCE: de Goffau M et al. Nature. 2019 Jul 31. doi: 10.1038/s41586-019-1451-5.
such as preeclampsia or spontaneous preterm birth, a study has found.
Dr. Marcus C. de Goffau, of Wellcome Sanger Institute, Cambridge, England, and coauthors wrote that placental dysfunction is linked to a number of common adverse pregnancy outcomes, but in many cases the cause of that dysfunction is unknown.
“Several studies have used sequencing-based methods for bacterial detection (metagenomics and 16S rRNA gene amplicon sequencing), and have concluded that the placenta is physiologically colonized by a diverse population of bacteria (the ‘placental microbiome’) and that the nature of this colonization may differ between healthy and complicated pregnancies,” they wrote.
In a paper published in the July 31 edition of Nature (doi: 10.1038/s41586-019-1451-5), researchers reported the outcomes of a study involving two cohorts. The first included 80 babies delivered by prelabor caesarean section, of whom 20 were small for gestational age, 20 were delivered to mothers with preeclampsia, and 40 were matched controls. The second cohort comprised 100 patients with preeclampsia, 100 small-for-gestational-age infants, 100 preterm births, and 198 matched controls, with two controls having been used twice.
The bacterial content of the placentas was analyzed via deep metagenomic sequencing of total DNA and 16S rRNA gene amplicon sequencing in cohort 1, and 16S rRNA gene amplicon from two different kits for cohort 2’s samples. In the first cohort, samples were also spiked with Salmonella bongori as a positive control.
For cohort 1, researchers were able to detect the S. bongori in all samples, but all other bacterial signals showed clear links to different batches, which the authors said showed they were the result of contamination. This was further supported by the discovery that the Escherichia coli signal seen in a number of batches was all from the same bacterial strain.
In the second cohort, researchers found Bradyrhizobium in nearly all samples, and Burkholderia – which has been thought to play a role in preterm birth – in some samples.
However in the case of the Burkholderia, significant variation between different runs of tests was found, and both Bradyrhizobium and Burkholderia were also found in negative controls.
Researchers also saw a high prevalence of four “ecologically unexpected” bacterial groups but were able to show that these were likely contaminants from a reagent used to wash the placental samples.
The study found that vaginal organisms such as lactobacilli and vaginosis-associated bacteria were more abundant in the second cohort, where babies were delivered by a mix of vaginal, intrapartum and prelabor caesarean section, compared with the first cohort, who were all prelabor caesarean section deliveries.
The only organism that met all the criteria for a genuine placenta-associated bacterial signal was Streptococcus agalactiae, but the authors found no association between it and pregnancy outcomes.
However, they did sound a warning that the perinatal transmission of S. agalactiae from the mother’s genital tract to the neonate could be a cause of fatal sepsis in the infant.
“Further studies will be required to determine the association between the presence of the organism in the placenta and fetal or neonatal disease,” they wrote.
The researchers also found significant associations between the delivery-associated vaginal bacteria Lactobacillus iners and preeclampsia, and between Streptococcus anginosus and the Ureaplasma genus – both of which are associated with vaginosis – and preterm birth.
“We conclude that bacterial placental infection is not a major cause of placentally related complications of human pregnancy and that the human placenta does not have a resident microbiome,” the authors wrote. “Although we see no evidence of a placental microbiome, the frequency of detection of vaginal bacteria in the placenta increased after intrapartum [cesarean] section, suggesting ascending or haematogenous spread.”
The Medical Research Council and the National Institute for Health Research Cambridge Biomedical Research Centre supported the study. Five authors declared grants and support from private industry outside the submitted work.
SOURCE: de Goffau M et al. Nature. 2019 Jul 31. doi: 10.1038/s41586-019-1451-5.
such as preeclampsia or spontaneous preterm birth, a study has found.
Dr. Marcus C. de Goffau, of Wellcome Sanger Institute, Cambridge, England, and coauthors wrote that placental dysfunction is linked to a number of common adverse pregnancy outcomes, but in many cases the cause of that dysfunction is unknown.
“Several studies have used sequencing-based methods for bacterial detection (metagenomics and 16S rRNA gene amplicon sequencing), and have concluded that the placenta is physiologically colonized by a diverse population of bacteria (the ‘placental microbiome’) and that the nature of this colonization may differ between healthy and complicated pregnancies,” they wrote.
In a paper published in the July 31 edition of Nature (doi: 10.1038/s41586-019-1451-5), researchers reported the outcomes of a study involving two cohorts. The first included 80 babies delivered by prelabor caesarean section, of whom 20 were small for gestational age, 20 were delivered to mothers with preeclampsia, and 40 were matched controls. The second cohort comprised 100 patients with preeclampsia, 100 small-for-gestational-age infants, 100 preterm births, and 198 matched controls, with two controls having been used twice.
The bacterial content of the placentas was analyzed via deep metagenomic sequencing of total DNA and 16S rRNA gene amplicon sequencing in cohort 1, and 16S rRNA gene amplicon from two different kits for cohort 2’s samples. In the first cohort, samples were also spiked with Salmonella bongori as a positive control.
For cohort 1, researchers were able to detect the S. bongori in all samples, but all other bacterial signals showed clear links to different batches, which the authors said showed they were the result of contamination. This was further supported by the discovery that the Escherichia coli signal seen in a number of batches was all from the same bacterial strain.
In the second cohort, researchers found Bradyrhizobium in nearly all samples, and Burkholderia – which has been thought to play a role in preterm birth – in some samples.
However in the case of the Burkholderia, significant variation between different runs of tests was found, and both Bradyrhizobium and Burkholderia were also found in negative controls.
Researchers also saw a high prevalence of four “ecologically unexpected” bacterial groups but were able to show that these were likely contaminants from a reagent used to wash the placental samples.
The study found that vaginal organisms such as lactobacilli and vaginosis-associated bacteria were more abundant in the second cohort, where babies were delivered by a mix of vaginal, intrapartum and prelabor caesarean section, compared with the first cohort, who were all prelabor caesarean section deliveries.
The only organism that met all the criteria for a genuine placenta-associated bacterial signal was Streptococcus agalactiae, but the authors found no association between it and pregnancy outcomes.
However, they did sound a warning that the perinatal transmission of S. agalactiae from the mother’s genital tract to the neonate could be a cause of fatal sepsis in the infant.
“Further studies will be required to determine the association between the presence of the organism in the placenta and fetal or neonatal disease,” they wrote.
The researchers also found significant associations between the delivery-associated vaginal bacteria Lactobacillus iners and preeclampsia, and between Streptococcus anginosus and the Ureaplasma genus – both of which are associated with vaginosis – and preterm birth.
“We conclude that bacterial placental infection is not a major cause of placentally related complications of human pregnancy and that the human placenta does not have a resident microbiome,” the authors wrote. “Although we see no evidence of a placental microbiome, the frequency of detection of vaginal bacteria in the placenta increased after intrapartum [cesarean] section, suggesting ascending or haematogenous spread.”
The Medical Research Council and the National Institute for Health Research Cambridge Biomedical Research Centre supported the study. Five authors declared grants and support from private industry outside the submitted work.
SOURCE: de Goffau M et al. Nature. 2019 Jul 31. doi: 10.1038/s41586-019-1451-5.
FROM NATURE
Key clinical point: Researchers found no evidence of a placental biome or a link between placental bacterial contamination and adverse pregnancy outcomes.
Major finding: No bacterial contamination of the placenta was associated with adverse pregnancy outcomes such as preeclampsia.
Study details: Cohort study of more than 500 placental samples.
Disclosures: The Medical Research Council and the National Institute for Health Research Cambridge Biomedical Research Centre supported the study. Five authors declared grants and support from private industry outside the submitted work.
Source: de Goffau M et al. Nature. 2019 Jul 31. doi: 10.1038/s41586-019-1451-5.
TP53 double hit predicts aggressive myeloma
Relapsed multiple myeloma becomes increasingly aggressive and difficult to treat with each additional TP53 alteration, according to investigators.
Findings from the study help illuminate the mechanics of myeloma disease progression and demonstrate the value of clonal competition assays, reported lead author Umair Munawar of the University Hospital Würzburg (Germany) and colleagues.
“The implications of mono-allelic TP53 lesions for the clinical outcome remain controversial, but clonal selection and evolution is a common feature of myeloma progression, and patients with TP53 wild-type or mono-allelic inactivation may present a double hit on relapse,” the investigators wrote in Blood. “Here, we addressed the hypothesis that sequential acquisition of TP53 hits lead to a gain of proliferative fitness of [multiple myeloma] cancer cells, inducing the expansion and domination of the affected clones within the patient’s bone marrow.”
The investigators used sleeping beauty and CRISPR/Cas9 techniques to create double- and single-hit multiple myeloma cell lines that were stably transfected with fluorescent proteins. By observing coculture pairings of wild-type, single-hit, and double-hit cells, the investigators found a hierarchy of proliferation that depended on the number of TP53 alterations. For instance, when double-hit cells were cocultured with wild-type cells in a 1:3 ratio, it took 21 days for the double-hit cells to reach 50% of the total culture population. Similarly, single-hit cells outcompeted wild-type cells after 38 days, while double-hit cells took 35 days to overcome the single-hit population.
Further testing showed that comparatively smaller initial populations of TP53-aberrant cells required longer to outcompete larger wild-type populations, which could explain why deeper responses in the clinic are often followed by longer periods without disease progression, the investigators suggested.
A comparison of transcriptomes between wild-type cells and TP53 mutants revealed differences in about 900 genes, including 14 signaling pathways. Specifically, downregulation impacted antigen processing and presentation, chemokine signaling, and oxidative phosphorylation.
“These differences on the transcriptomic level well reflect the biology of ultra–high risk disease,” the investigators wrote, referring to increased glucose uptake on PET, resistance to immunotherapies, and extramedullary disease.
“[This study] underscores the power of clonal competition assays to decipher the effect of genomic lesions in tumors to better understand their impact on progression and disease relapse in [multiple myeloma],” the investigators concluded.
The study was funded by Deutsche Forschungsgemeinschaft, the CDW Stiftung, and the IZKF Würzburg. The investigators reported additional support from the CRIS foundation, the German Cancer Aid, and the University of Würzburg.
SOURCE: Munawar U et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019000080.
Relapsed multiple myeloma becomes increasingly aggressive and difficult to treat with each additional TP53 alteration, according to investigators.
Findings from the study help illuminate the mechanics of myeloma disease progression and demonstrate the value of clonal competition assays, reported lead author Umair Munawar of the University Hospital Würzburg (Germany) and colleagues.
“The implications of mono-allelic TP53 lesions for the clinical outcome remain controversial, but clonal selection and evolution is a common feature of myeloma progression, and patients with TP53 wild-type or mono-allelic inactivation may present a double hit on relapse,” the investigators wrote in Blood. “Here, we addressed the hypothesis that sequential acquisition of TP53 hits lead to a gain of proliferative fitness of [multiple myeloma] cancer cells, inducing the expansion and domination of the affected clones within the patient’s bone marrow.”
The investigators used sleeping beauty and CRISPR/Cas9 techniques to create double- and single-hit multiple myeloma cell lines that were stably transfected with fluorescent proteins. By observing coculture pairings of wild-type, single-hit, and double-hit cells, the investigators found a hierarchy of proliferation that depended on the number of TP53 alterations. For instance, when double-hit cells were cocultured with wild-type cells in a 1:3 ratio, it took 21 days for the double-hit cells to reach 50% of the total culture population. Similarly, single-hit cells outcompeted wild-type cells after 38 days, while double-hit cells took 35 days to overcome the single-hit population.
Further testing showed that comparatively smaller initial populations of TP53-aberrant cells required longer to outcompete larger wild-type populations, which could explain why deeper responses in the clinic are often followed by longer periods without disease progression, the investigators suggested.
A comparison of transcriptomes between wild-type cells and TP53 mutants revealed differences in about 900 genes, including 14 signaling pathways. Specifically, downregulation impacted antigen processing and presentation, chemokine signaling, and oxidative phosphorylation.
“These differences on the transcriptomic level well reflect the biology of ultra–high risk disease,” the investigators wrote, referring to increased glucose uptake on PET, resistance to immunotherapies, and extramedullary disease.
“[This study] underscores the power of clonal competition assays to decipher the effect of genomic lesions in tumors to better understand their impact on progression and disease relapse in [multiple myeloma],” the investigators concluded.
The study was funded by Deutsche Forschungsgemeinschaft, the CDW Stiftung, and the IZKF Würzburg. The investigators reported additional support from the CRIS foundation, the German Cancer Aid, and the University of Würzburg.
SOURCE: Munawar U et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019000080.
Relapsed multiple myeloma becomes increasingly aggressive and difficult to treat with each additional TP53 alteration, according to investigators.
Findings from the study help illuminate the mechanics of myeloma disease progression and demonstrate the value of clonal competition assays, reported lead author Umair Munawar of the University Hospital Würzburg (Germany) and colleagues.
“The implications of mono-allelic TP53 lesions for the clinical outcome remain controversial, but clonal selection and evolution is a common feature of myeloma progression, and patients with TP53 wild-type or mono-allelic inactivation may present a double hit on relapse,” the investigators wrote in Blood. “Here, we addressed the hypothesis that sequential acquisition of TP53 hits lead to a gain of proliferative fitness of [multiple myeloma] cancer cells, inducing the expansion and domination of the affected clones within the patient’s bone marrow.”
The investigators used sleeping beauty and CRISPR/Cas9 techniques to create double- and single-hit multiple myeloma cell lines that were stably transfected with fluorescent proteins. By observing coculture pairings of wild-type, single-hit, and double-hit cells, the investigators found a hierarchy of proliferation that depended on the number of TP53 alterations. For instance, when double-hit cells were cocultured with wild-type cells in a 1:3 ratio, it took 21 days for the double-hit cells to reach 50% of the total culture population. Similarly, single-hit cells outcompeted wild-type cells after 38 days, while double-hit cells took 35 days to overcome the single-hit population.
Further testing showed that comparatively smaller initial populations of TP53-aberrant cells required longer to outcompete larger wild-type populations, which could explain why deeper responses in the clinic are often followed by longer periods without disease progression, the investigators suggested.
A comparison of transcriptomes between wild-type cells and TP53 mutants revealed differences in about 900 genes, including 14 signaling pathways. Specifically, downregulation impacted antigen processing and presentation, chemokine signaling, and oxidative phosphorylation.
“These differences on the transcriptomic level well reflect the biology of ultra–high risk disease,” the investigators wrote, referring to increased glucose uptake on PET, resistance to immunotherapies, and extramedullary disease.
“[This study] underscores the power of clonal competition assays to decipher the effect of genomic lesions in tumors to better understand their impact on progression and disease relapse in [multiple myeloma],” the investigators concluded.
The study was funded by Deutsche Forschungsgemeinschaft, the CDW Stiftung, and the IZKF Würzburg. The investigators reported additional support from the CRIS foundation, the German Cancer Aid, and the University of Würzburg.
SOURCE: Munawar U et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019000080.
FROM BLOOD
Most authors of endoscopy practice guidelines have undisclosed financial conflicts
SAN DIEGO – Authors of endoscopy clinical practice guidelines (CPGs) received many more payments from industry than they disclosed, according to a new review of publicly available data.
Additionally, many of the payments came from pharmaceutical companies or medical device manufacturers whose products were directly related to the practice guideline topic at hand, said Amir Rumann, MD, and coauthors, who presented their findings in a poster session at the annual Digestive Disease Week®.
The researchers found that, of 581 authors listed in 38 CPGs, 127 had initially disclosed payments when the guidelines were published. A search of the federal Open Payments database, however, found that 452 of these authors had payments that they did not disclose in the CPG publication process.
For authors with undisclosed payments, the median value of the total undisclosed amounts was $2,445, from a median of 2.5 unique companies, according to records maintained by the Centers for Medicare & Medicaid Services. The interquartile range for payments was $167-$10,380.
“There is a high burden of undisclosed payments by pharmaceutical and medical device manufacturers to authors of endoscopy guidelines in the United States,” wrote Dr. Rumman and coauthors at the University of Toronto, where Dr. Rumann is a gastroenterology resident.
Of the authors who disclosed payments, 20 (16%) disclosed payments from sources directly related to the CPG in the publication. But the Open Payments database search yielded 314 (54%) disclosed payments that were judged directly related to the CPG topic at hand.
Of the 38 CPGs included in the analysis, 24 were from the American Society for Gastrointestinal Endoscopy (ASGE), and 4 were from the American Gastroenterological Association (AGA). According to the analysis performed by Dr. Rumman and his coauthors, 23 of these guidelines adhered to standards proposed by the National Academy of Medicine for development of “trustworthy” CPGs.
“The prevalence of conflicts of interest in the form of industry payments among physicians is well described in academic literature. A number of studies, including several from our group, have found there is a high burden of these payments among authors and chairs of CPGs in various therapeutic areas,” commented senior author Samir Grover, MD, a gastroenterologist at the University of Toronto. “Additionally, the existing literature has shown that many of these payments go undisclosed in the CPG themselves. This is concerning. Although the presence of these payments doesn’t necessarily indicate any obvious wrongdoing on the part of the recipients, it can undermine public and professional trust in guidelines.”
In its 2011 report, the National Academy of Medicine outlined three criteria for trustworthy guidelines. These include ensuring that committee chairs do not have financial conflicts of interest (COIs), limiting authors who do have financial COIs to less than half of the guideline drafting panel, and disclosing financially motivated relationships between individual health care providers and members of industry. “To our surprise, our findings demonstrated that none of the CPGs we identified met all of these criteria,” noted Dr. Grover, adding that “this finding highlights the importance of accountability at both the contributor level (to ensure conflicts are appropriately declared) and at the level of the sponsoring society (to ensure that guideline committee members and chairs are selected a priori for reasons that include impact of conflicts).”
Dr. Rumann and his colleagues conducted a cross-sectional study, including all endoscopy-related CPGs from AGA and ASGE published from 2014 through 2017. They performed a manual reconciliation between financial COIs disclosed in CPG documents and those reported in the Open Payments database maintained by CMS, which includes data from August 2013 to December 2017. “We decided to focus on CPGs of relevance to endoscopy, as the majority of the work to date has revolved around pharma and not devices, a potentially significant other source for industry payments to physicians,” explained Dr. Grover.
In the Open Payments database, payment types are recorded as general payments, research payments, or ownerships or investments. For each payment transaction, the type, amount, and date of payment are recorded, as is the payer.
In all, 91 individuals appeared as authors 581 times. The median number of CPGs per author was 2 with a range of 1-26.
About 80% of authors had payments reported in the Open Payments database. Though Dr. Grover acknowledged that reporting errors may exist in the Open Payments database, this fact, he said, “highlights the need for greater involvement of physicians in correct declaration of their payments.”
Among the guideline authors, 119 (20.5%) had originally disclosed general payments. However, more than three times that many authors had undisclosed records of general payments that showed up in the Open Payments database query. In all, 399 authors (68.6%) had received general payments, according to the database.
Though all 12 of the ownership or investment payments seen on Open Payments were disclosed, just 40 of 74 research payments were disclosed, said Dr. Rumann and coauthors.
“Given the potential impact of these undisclosed payments on CPGs, stricter policies on conflict of interest disclosures are needed,” they wrote.
When asked whether he would expect change to come from the findings, Dr. Grover replied, “First and foremost, we want to increase the awareness of the issue, among both the health professions and the public. While we believe that more research into COI in general is required, our study, along with similar papers in the field, show that CPGs authors need to be more cognizant of declaration of potential conflicts. In addition to being hopeful that readers of the article will be prompted to exert more diligence in tracking and declaring any payments they have received, we are hopeful that sponsoring societies self-regulate the membership of CPG committees and the choice of chairs in accordance with established criteria to mitigate conflicts.”
Dr. Rumann and other coauthors reported no conflicts of interest. Senior author Samir Grover, MD, reported financial relationships with several pharmaceutical companies.
SOURCE: Rumann A et al. DDW 2019, poster Sa1004.
The authors bring awareness to an important issue. Conflict of interest (COI) in guideline development has received a lot of attention over the past few years. COI, whether actual or perceived, can lead to mistrust of recommendations, limit uptake and dissemination of guidelines, and possibly lead to low-quality care.
As part of the AGA Institute’s mission, the Clinical Guidelines Committee (CGC) is charged with advancing the practice of gastroenterology through developing clinical practice guidelines that promote high-quality, evidence-based care. In accordance with the National Academies of Medicine (formerly Institute of Medicine) report for trustworthy guidelines, the CGC’s COI policy is in agreement with the following standards: more than 50% of guideline members have no financial COI, the methodologist cochair is completely free of COI, and the guideline cochair has no direct clincal practice guideline–relevant financial COI.
Guidelines developed by the AGA CGC are informed by a technical review or evidence synthesis. All panel members considered for the technical review and guideline panel must go through a stringent vetting process, which includes completion of a comprehensive COI disclosure form. All authors are asked to report any financial and intellectual interests from the past 3 years including stocks/stock options, speaking engagements, board and committee memberships, legal testimony, research grants, employment, patents, or intellectual property. A review of the Centers for Medicare & Medicaid Services Open Payments database is also performed. Open Payments is a national disclosure program and public database established in 2014 that reports financial relationships and lists any payment or gift to U.S. health care providers (physicians and teaching hospitals) valued at more than $10. While the AGA strives to form technical review and guideline panels that are free from financial and intellectual COI, technical review panels may, on occasion, include recognized clinical experts who have relationships deemed to be manageable because technical review authors serve as content experts and do not contribute to the actual recommendations in the guideline.
Furthermore, all official journals of the AGA Institute adhere to the standards set forth by the Committee on Publication Ethics and the International Committee of Medical Journal Editors. As such, at the time of publication, a uniform detailed self-report disclosure form is used to publicly disclose all relevant financial and nonfinancial COIs.
In light of the negative impact of perceived COI, substantial efforts have been made by professional organizations and medical editors to ensure proper disclosure of conflicts and the AGA CGC committee strengthened its COI policy further in 2018. The AGA CGC is committing to minimizing panel members with financial and nonfinancial COI and ensuring adequate disclosure and management of COI when present.
Shahnaz Sultan, MD, MHSc, AGAF, chair of the AGA Clinical Guideline Committee, and Yngve Falck-Ytter, MD, AGAF, chair emeritus of the AGA Clinical Guideline Committee.
The authors bring awareness to an important issue. Conflict of interest (COI) in guideline development has received a lot of attention over the past few years. COI, whether actual or perceived, can lead to mistrust of recommendations, limit uptake and dissemination of guidelines, and possibly lead to low-quality care.
As part of the AGA Institute’s mission, the Clinical Guidelines Committee (CGC) is charged with advancing the practice of gastroenterology through developing clinical practice guidelines that promote high-quality, evidence-based care. In accordance with the National Academies of Medicine (formerly Institute of Medicine) report for trustworthy guidelines, the CGC’s COI policy is in agreement with the following standards: more than 50% of guideline members have no financial COI, the methodologist cochair is completely free of COI, and the guideline cochair has no direct clincal practice guideline–relevant financial COI.
Guidelines developed by the AGA CGC are informed by a technical review or evidence synthesis. All panel members considered for the technical review and guideline panel must go through a stringent vetting process, which includes completion of a comprehensive COI disclosure form. All authors are asked to report any financial and intellectual interests from the past 3 years including stocks/stock options, speaking engagements, board and committee memberships, legal testimony, research grants, employment, patents, or intellectual property. A review of the Centers for Medicare & Medicaid Services Open Payments database is also performed. Open Payments is a national disclosure program and public database established in 2014 that reports financial relationships and lists any payment or gift to U.S. health care providers (physicians and teaching hospitals) valued at more than $10. While the AGA strives to form technical review and guideline panels that are free from financial and intellectual COI, technical review panels may, on occasion, include recognized clinical experts who have relationships deemed to be manageable because technical review authors serve as content experts and do not contribute to the actual recommendations in the guideline.
Furthermore, all official journals of the AGA Institute adhere to the standards set forth by the Committee on Publication Ethics and the International Committee of Medical Journal Editors. As such, at the time of publication, a uniform detailed self-report disclosure form is used to publicly disclose all relevant financial and nonfinancial COIs.
In light of the negative impact of perceived COI, substantial efforts have been made by professional organizations and medical editors to ensure proper disclosure of conflicts and the AGA CGC committee strengthened its COI policy further in 2018. The AGA CGC is committing to minimizing panel members with financial and nonfinancial COI and ensuring adequate disclosure and management of COI when present.
Shahnaz Sultan, MD, MHSc, AGAF, chair of the AGA Clinical Guideline Committee, and Yngve Falck-Ytter, MD, AGAF, chair emeritus of the AGA Clinical Guideline Committee.
The authors bring awareness to an important issue. Conflict of interest (COI) in guideline development has received a lot of attention over the past few years. COI, whether actual or perceived, can lead to mistrust of recommendations, limit uptake and dissemination of guidelines, and possibly lead to low-quality care.
As part of the AGA Institute’s mission, the Clinical Guidelines Committee (CGC) is charged with advancing the practice of gastroenterology through developing clinical practice guidelines that promote high-quality, evidence-based care. In accordance with the National Academies of Medicine (formerly Institute of Medicine) report for trustworthy guidelines, the CGC’s COI policy is in agreement with the following standards: more than 50% of guideline members have no financial COI, the methodologist cochair is completely free of COI, and the guideline cochair has no direct clincal practice guideline–relevant financial COI.
Guidelines developed by the AGA CGC are informed by a technical review or evidence synthesis. All panel members considered for the technical review and guideline panel must go through a stringent vetting process, which includes completion of a comprehensive COI disclosure form. All authors are asked to report any financial and intellectual interests from the past 3 years including stocks/stock options, speaking engagements, board and committee memberships, legal testimony, research grants, employment, patents, or intellectual property. A review of the Centers for Medicare & Medicaid Services Open Payments database is also performed. Open Payments is a national disclosure program and public database established in 2014 that reports financial relationships and lists any payment or gift to U.S. health care providers (physicians and teaching hospitals) valued at more than $10. While the AGA strives to form technical review and guideline panels that are free from financial and intellectual COI, technical review panels may, on occasion, include recognized clinical experts who have relationships deemed to be manageable because technical review authors serve as content experts and do not contribute to the actual recommendations in the guideline.
Furthermore, all official journals of the AGA Institute adhere to the standards set forth by the Committee on Publication Ethics and the International Committee of Medical Journal Editors. As such, at the time of publication, a uniform detailed self-report disclosure form is used to publicly disclose all relevant financial and nonfinancial COIs.
In light of the negative impact of perceived COI, substantial efforts have been made by professional organizations and medical editors to ensure proper disclosure of conflicts and the AGA CGC committee strengthened its COI policy further in 2018. The AGA CGC is committing to minimizing panel members with financial and nonfinancial COI and ensuring adequate disclosure and management of COI when present.
Shahnaz Sultan, MD, MHSc, AGAF, chair of the AGA Clinical Guideline Committee, and Yngve Falck-Ytter, MD, AGAF, chair emeritus of the AGA Clinical Guideline Committee.
SAN DIEGO – Authors of endoscopy clinical practice guidelines (CPGs) received many more payments from industry than they disclosed, according to a new review of publicly available data.
Additionally, many of the payments came from pharmaceutical companies or medical device manufacturers whose products were directly related to the practice guideline topic at hand, said Amir Rumann, MD, and coauthors, who presented their findings in a poster session at the annual Digestive Disease Week®.
The researchers found that, of 581 authors listed in 38 CPGs, 127 had initially disclosed payments when the guidelines were published. A search of the federal Open Payments database, however, found that 452 of these authors had payments that they did not disclose in the CPG publication process.
For authors with undisclosed payments, the median value of the total undisclosed amounts was $2,445, from a median of 2.5 unique companies, according to records maintained by the Centers for Medicare & Medicaid Services. The interquartile range for payments was $167-$10,380.
“There is a high burden of undisclosed payments by pharmaceutical and medical device manufacturers to authors of endoscopy guidelines in the United States,” wrote Dr. Rumman and coauthors at the University of Toronto, where Dr. Rumann is a gastroenterology resident.
Of the authors who disclosed payments, 20 (16%) disclosed payments from sources directly related to the CPG in the publication. But the Open Payments database search yielded 314 (54%) disclosed payments that were judged directly related to the CPG topic at hand.
Of the 38 CPGs included in the analysis, 24 were from the American Society for Gastrointestinal Endoscopy (ASGE), and 4 were from the American Gastroenterological Association (AGA). According to the analysis performed by Dr. Rumman and his coauthors, 23 of these guidelines adhered to standards proposed by the National Academy of Medicine for development of “trustworthy” CPGs.
“The prevalence of conflicts of interest in the form of industry payments among physicians is well described in academic literature. A number of studies, including several from our group, have found there is a high burden of these payments among authors and chairs of CPGs in various therapeutic areas,” commented senior author Samir Grover, MD, a gastroenterologist at the University of Toronto. “Additionally, the existing literature has shown that many of these payments go undisclosed in the CPG themselves. This is concerning. Although the presence of these payments doesn’t necessarily indicate any obvious wrongdoing on the part of the recipients, it can undermine public and professional trust in guidelines.”
In its 2011 report, the National Academy of Medicine outlined three criteria for trustworthy guidelines. These include ensuring that committee chairs do not have financial conflicts of interest (COIs), limiting authors who do have financial COIs to less than half of the guideline drafting panel, and disclosing financially motivated relationships between individual health care providers and members of industry. “To our surprise, our findings demonstrated that none of the CPGs we identified met all of these criteria,” noted Dr. Grover, adding that “this finding highlights the importance of accountability at both the contributor level (to ensure conflicts are appropriately declared) and at the level of the sponsoring society (to ensure that guideline committee members and chairs are selected a priori for reasons that include impact of conflicts).”
Dr. Rumann and his colleagues conducted a cross-sectional study, including all endoscopy-related CPGs from AGA and ASGE published from 2014 through 2017. They performed a manual reconciliation between financial COIs disclosed in CPG documents and those reported in the Open Payments database maintained by CMS, which includes data from August 2013 to December 2017. “We decided to focus on CPGs of relevance to endoscopy, as the majority of the work to date has revolved around pharma and not devices, a potentially significant other source for industry payments to physicians,” explained Dr. Grover.
In the Open Payments database, payment types are recorded as general payments, research payments, or ownerships or investments. For each payment transaction, the type, amount, and date of payment are recorded, as is the payer.
In all, 91 individuals appeared as authors 581 times. The median number of CPGs per author was 2 with a range of 1-26.
About 80% of authors had payments reported in the Open Payments database. Though Dr. Grover acknowledged that reporting errors may exist in the Open Payments database, this fact, he said, “highlights the need for greater involvement of physicians in correct declaration of their payments.”
Among the guideline authors, 119 (20.5%) had originally disclosed general payments. However, more than three times that many authors had undisclosed records of general payments that showed up in the Open Payments database query. In all, 399 authors (68.6%) had received general payments, according to the database.
Though all 12 of the ownership or investment payments seen on Open Payments were disclosed, just 40 of 74 research payments were disclosed, said Dr. Rumann and coauthors.
“Given the potential impact of these undisclosed payments on CPGs, stricter policies on conflict of interest disclosures are needed,” they wrote.
When asked whether he would expect change to come from the findings, Dr. Grover replied, “First and foremost, we want to increase the awareness of the issue, among both the health professions and the public. While we believe that more research into COI in general is required, our study, along with similar papers in the field, show that CPGs authors need to be more cognizant of declaration of potential conflicts. In addition to being hopeful that readers of the article will be prompted to exert more diligence in tracking and declaring any payments they have received, we are hopeful that sponsoring societies self-regulate the membership of CPG committees and the choice of chairs in accordance with established criteria to mitigate conflicts.”
Dr. Rumann and other coauthors reported no conflicts of interest. Senior author Samir Grover, MD, reported financial relationships with several pharmaceutical companies.
SOURCE: Rumann A et al. DDW 2019, poster Sa1004.
SAN DIEGO – Authors of endoscopy clinical practice guidelines (CPGs) received many more payments from industry than they disclosed, according to a new review of publicly available data.
Additionally, many of the payments came from pharmaceutical companies or medical device manufacturers whose products were directly related to the practice guideline topic at hand, said Amir Rumann, MD, and coauthors, who presented their findings in a poster session at the annual Digestive Disease Week®.
The researchers found that, of 581 authors listed in 38 CPGs, 127 had initially disclosed payments when the guidelines were published. A search of the federal Open Payments database, however, found that 452 of these authors had payments that they did not disclose in the CPG publication process.
For authors with undisclosed payments, the median value of the total undisclosed amounts was $2,445, from a median of 2.5 unique companies, according to records maintained by the Centers for Medicare & Medicaid Services. The interquartile range for payments was $167-$10,380.
“There is a high burden of undisclosed payments by pharmaceutical and medical device manufacturers to authors of endoscopy guidelines in the United States,” wrote Dr. Rumman and coauthors at the University of Toronto, where Dr. Rumann is a gastroenterology resident.
Of the authors who disclosed payments, 20 (16%) disclosed payments from sources directly related to the CPG in the publication. But the Open Payments database search yielded 314 (54%) disclosed payments that were judged directly related to the CPG topic at hand.
Of the 38 CPGs included in the analysis, 24 were from the American Society for Gastrointestinal Endoscopy (ASGE), and 4 were from the American Gastroenterological Association (AGA). According to the analysis performed by Dr. Rumman and his coauthors, 23 of these guidelines adhered to standards proposed by the National Academy of Medicine for development of “trustworthy” CPGs.
“The prevalence of conflicts of interest in the form of industry payments among physicians is well described in academic literature. A number of studies, including several from our group, have found there is a high burden of these payments among authors and chairs of CPGs in various therapeutic areas,” commented senior author Samir Grover, MD, a gastroenterologist at the University of Toronto. “Additionally, the existing literature has shown that many of these payments go undisclosed in the CPG themselves. This is concerning. Although the presence of these payments doesn’t necessarily indicate any obvious wrongdoing on the part of the recipients, it can undermine public and professional trust in guidelines.”
In its 2011 report, the National Academy of Medicine outlined three criteria for trustworthy guidelines. These include ensuring that committee chairs do not have financial conflicts of interest (COIs), limiting authors who do have financial COIs to less than half of the guideline drafting panel, and disclosing financially motivated relationships between individual health care providers and members of industry. “To our surprise, our findings demonstrated that none of the CPGs we identified met all of these criteria,” noted Dr. Grover, adding that “this finding highlights the importance of accountability at both the contributor level (to ensure conflicts are appropriately declared) and at the level of the sponsoring society (to ensure that guideline committee members and chairs are selected a priori for reasons that include impact of conflicts).”
Dr. Rumann and his colleagues conducted a cross-sectional study, including all endoscopy-related CPGs from AGA and ASGE published from 2014 through 2017. They performed a manual reconciliation between financial COIs disclosed in CPG documents and those reported in the Open Payments database maintained by CMS, which includes data from August 2013 to December 2017. “We decided to focus on CPGs of relevance to endoscopy, as the majority of the work to date has revolved around pharma and not devices, a potentially significant other source for industry payments to physicians,” explained Dr. Grover.
In the Open Payments database, payment types are recorded as general payments, research payments, or ownerships or investments. For each payment transaction, the type, amount, and date of payment are recorded, as is the payer.
In all, 91 individuals appeared as authors 581 times. The median number of CPGs per author was 2 with a range of 1-26.
About 80% of authors had payments reported in the Open Payments database. Though Dr. Grover acknowledged that reporting errors may exist in the Open Payments database, this fact, he said, “highlights the need for greater involvement of physicians in correct declaration of their payments.”
Among the guideline authors, 119 (20.5%) had originally disclosed general payments. However, more than three times that many authors had undisclosed records of general payments that showed up in the Open Payments database query. In all, 399 authors (68.6%) had received general payments, according to the database.
Though all 12 of the ownership or investment payments seen on Open Payments were disclosed, just 40 of 74 research payments were disclosed, said Dr. Rumann and coauthors.
“Given the potential impact of these undisclosed payments on CPGs, stricter policies on conflict of interest disclosures are needed,” they wrote.
When asked whether he would expect change to come from the findings, Dr. Grover replied, “First and foremost, we want to increase the awareness of the issue, among both the health professions and the public. While we believe that more research into COI in general is required, our study, along with similar papers in the field, show that CPGs authors need to be more cognizant of declaration of potential conflicts. In addition to being hopeful that readers of the article will be prompted to exert more diligence in tracking and declaring any payments they have received, we are hopeful that sponsoring societies self-regulate the membership of CPG committees and the choice of chairs in accordance with established criteria to mitigate conflicts.”
Dr. Rumann and other coauthors reported no conflicts of interest. Senior author Samir Grover, MD, reported financial relationships with several pharmaceutical companies.
SOURCE: Rumann A et al. DDW 2019, poster Sa1004.
REPORTING FROM DDW 2019