User login
Managing dermatologic changes of targeted cancer therapy
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
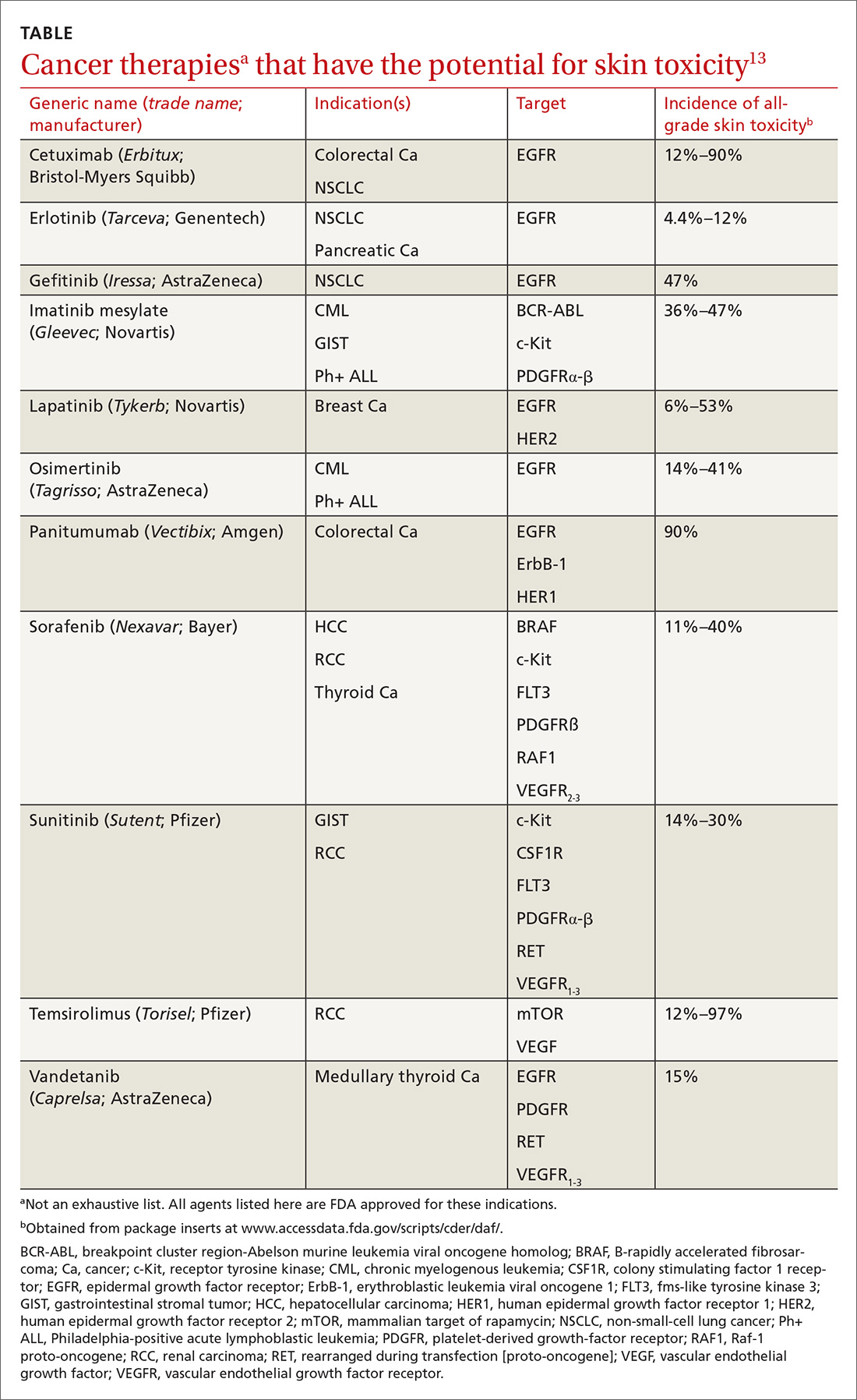
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13
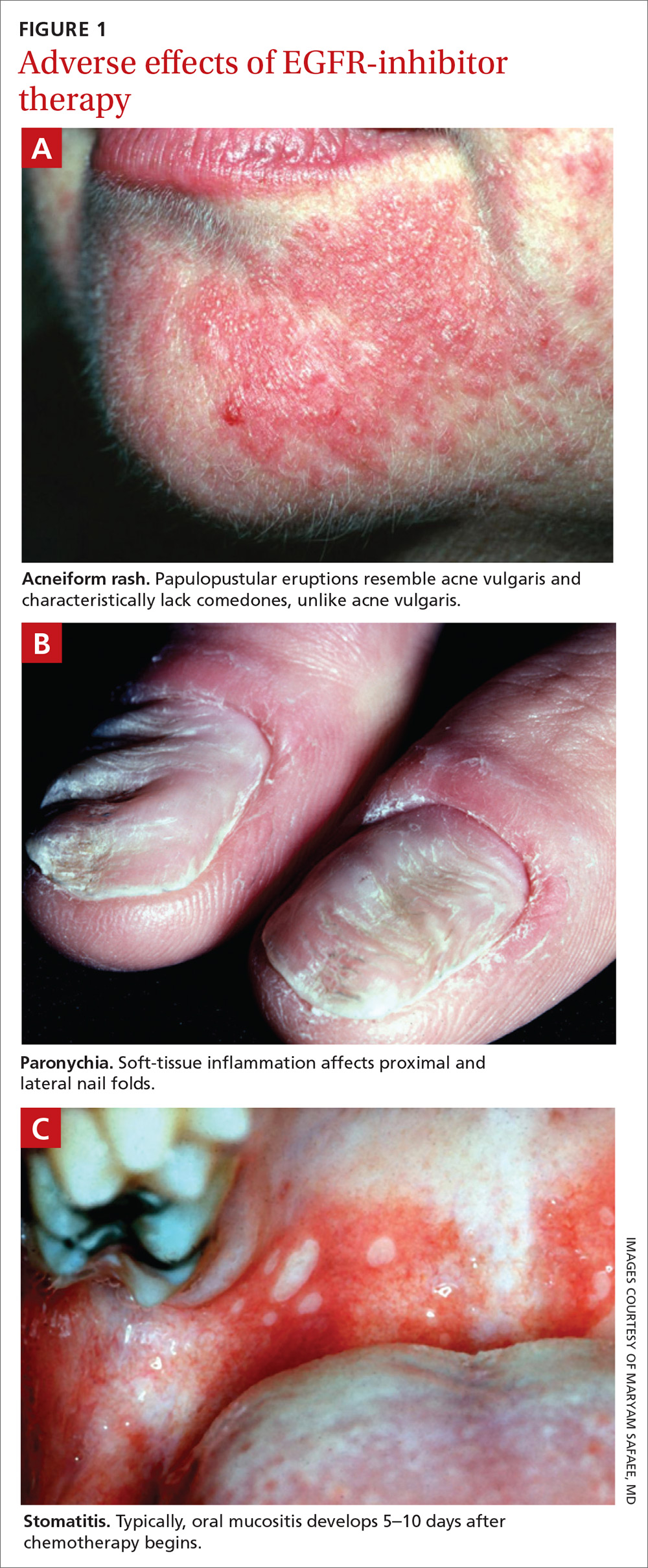
What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.
The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; Kayvan.zarrabi@gmail.com
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13
What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.
The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; Kayvan.zarrabi@gmail.com
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13
What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.
The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; Kayvan.zarrabi@gmail.com
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
PRACTICE RECOMMENDATIONS
› Counsel patients about their risk of rash before epidermal growth factor receptor–targeting treatment is initiated; early recognition of rash and intervention lead to milder symptoms. A
› Encourage daily skin care with an alcohol-free emollient cream. Instruct patients to avoid products that can cause skin drying, prolonged hot showers, perfumes, and soaps marketed for treating acne. B
› Instruct patients that oral hygiene to lower their risk of stomatitis should include a soft-bristle toothbrush and oral rinsing with normal saline—not with an alcohol-based commercial mouthwash. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What we know—and don’t—about non-nutritive sweeteners
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
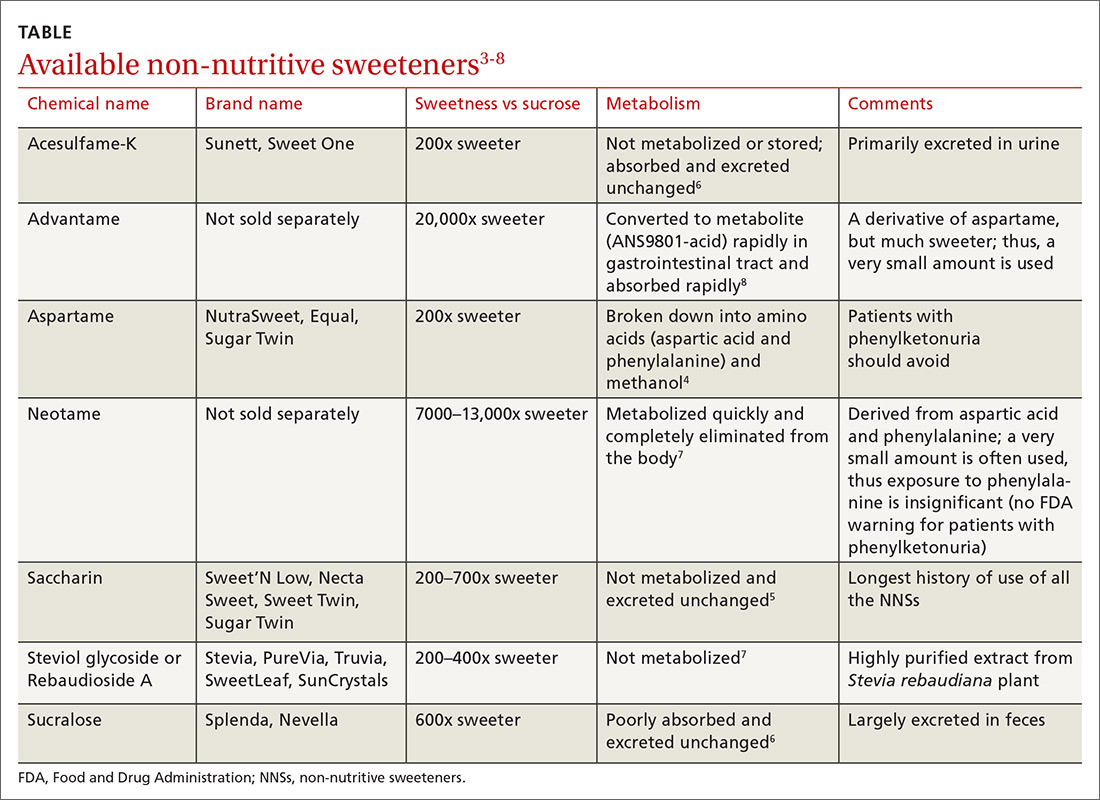
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.
Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; Clipper.young@tu.edu.
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.
Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; Clipper.young@tu.edu.
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.
Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; Clipper.young@tu.edu.
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
PRACTICE RECOMMENDATIONS
› Advise patients who are trying to lose weight that non-nutritive sweeteners (NNSs) are not beneficial for weight loss. A
› Reassure patients that NNSs do not appear to cause, or increase the risk of, developing type 2 diabetes mellitus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.
Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27
Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.
Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27
Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.
Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27
Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Pediatric luminal Crohn’s disease guideline issued
The new guideline provides evidence-based recommendations regarding optimal medical treatment strategies for achieving clinical remission based on a multi-item assessment of disease activity in pediatric patients with luminal CD. The guideline does not address surgical management, diagnosis, psychosocial therapies, preventative health considerations, or growth monitoring.
“The implications of inadequately treated CD are of particular importance in children because of the potentially serious and irreversible consequences,” wrote David R. Mack, MD, of the University of Ottawa and associates. Dr. Mack is the lead author of the pediatric practice guideline copublished in Gastroenterology and the Journal of the Canadian Association of Gastroenterology.
The consensus group reached its recommendations based on a systematic review of the literature for studies related to the medical treatment of pediatric CD. The majority of studies were randomized trials conducted in adults with CD.
“Evidence of efficacy of specific treatments in achieving mucosal healing is limited; therefore, “complete” or “deep” remission (clinical remission plus mucosal healing) was not the chosen primary outcome,” the guideline authors wrote.
The panel recommended that corticosteroids can be used as induction therapy in children with moderate to severe disease. Moreover, budesonide may be an appropriate alternative for induction therapy in patients with mild to moderate CD.
In contrast, the group recommended against the use of corticosteroids as maintenance therapy, largely because of adverse events reported with long-term use.
At diagnosis or initial stages of severe disease, as well as in patients who have failed with immunosuppressant and corticosteroid induction strategies, enteral nutrition should be used exclusively for induction therapy. In addition, anti–tumor necrosis factor biologics are an appropriate option for induction and maintenance therapy in these patients, according to the guideline.
“The group recommended against the use of oral 5-aminosalicylate for induction or maintenance therapy in patients with moderate disease, and recommended against thiopurines for induction therapy,” they wrote.
With respect to cannabis-based products, the panel made a strong recommendation against the use of these agents in all pediatric patients.
In terms of assessment, the team recommended that patients in clinical remission receiving methotrexate or a thiopurine agent as maintenance therapy should be evaluated for mucosal healing within 1 year of therapy initiation.
No consensus was reached on the adjuvant use of immunosuppressants during initiation therapy with a biologic drug, but the consensus panel recommended against the use of thiopurine combinations in male patients. Furthermore, no consensus was reached on the role of vedolizumab or antibiotics for induction or maintenance therapy, methotrexate for induction therapy, and the function of aminosalicylates in patients with mild CD.
The panel highlighted the importance of incorporating patient perspectives into treatment decision making.
“It is hoped that the available information will enhance the discussion between the clinician and the patient and enable the patient to make an evidence-based informed decision.”
The expert consensus was made up of 15 voting members that consisted of pediatric gastroenterologists throughout the United States and Canada, with expertise in several domains, including clinical epidemiology, nutrition, health services research, and patient engagement.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) criteria. The quality of evidence for each consensus statement was denoted as either high, moderate, low, or very low, based on the criteria.
The consensus statements were finalized at an in-person meeting conducted in Toronto in October 2017.
The guideline was supported through grant funding provided by AbbVie and Takeda. The authors reported financial affiliations with AbbVie and Takeda, as well as Janssen, Nestle Health Sciences, Shire, and several others.
SOURCE: Mack DR et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.022.
The new guideline provides evidence-based recommendations regarding optimal medical treatment strategies for achieving clinical remission based on a multi-item assessment of disease activity in pediatric patients with luminal CD. The guideline does not address surgical management, diagnosis, psychosocial therapies, preventative health considerations, or growth monitoring.
“The implications of inadequately treated CD are of particular importance in children because of the potentially serious and irreversible consequences,” wrote David R. Mack, MD, of the University of Ottawa and associates. Dr. Mack is the lead author of the pediatric practice guideline copublished in Gastroenterology and the Journal of the Canadian Association of Gastroenterology.
The consensus group reached its recommendations based on a systematic review of the literature for studies related to the medical treatment of pediatric CD. The majority of studies were randomized trials conducted in adults with CD.
“Evidence of efficacy of specific treatments in achieving mucosal healing is limited; therefore, “complete” or “deep” remission (clinical remission plus mucosal healing) was not the chosen primary outcome,” the guideline authors wrote.
The panel recommended that corticosteroids can be used as induction therapy in children with moderate to severe disease. Moreover, budesonide may be an appropriate alternative for induction therapy in patients with mild to moderate CD.
In contrast, the group recommended against the use of corticosteroids as maintenance therapy, largely because of adverse events reported with long-term use.
At diagnosis or initial stages of severe disease, as well as in patients who have failed with immunosuppressant and corticosteroid induction strategies, enteral nutrition should be used exclusively for induction therapy. In addition, anti–tumor necrosis factor biologics are an appropriate option for induction and maintenance therapy in these patients, according to the guideline.
“The group recommended against the use of oral 5-aminosalicylate for induction or maintenance therapy in patients with moderate disease, and recommended against thiopurines for induction therapy,” they wrote.
With respect to cannabis-based products, the panel made a strong recommendation against the use of these agents in all pediatric patients.
In terms of assessment, the team recommended that patients in clinical remission receiving methotrexate or a thiopurine agent as maintenance therapy should be evaluated for mucosal healing within 1 year of therapy initiation.
No consensus was reached on the adjuvant use of immunosuppressants during initiation therapy with a biologic drug, but the consensus panel recommended against the use of thiopurine combinations in male patients. Furthermore, no consensus was reached on the role of vedolizumab or antibiotics for induction or maintenance therapy, methotrexate for induction therapy, and the function of aminosalicylates in patients with mild CD.
The panel highlighted the importance of incorporating patient perspectives into treatment decision making.
“It is hoped that the available information will enhance the discussion between the clinician and the patient and enable the patient to make an evidence-based informed decision.”
The expert consensus was made up of 15 voting members that consisted of pediatric gastroenterologists throughout the United States and Canada, with expertise in several domains, including clinical epidemiology, nutrition, health services research, and patient engagement.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) criteria. The quality of evidence for each consensus statement was denoted as either high, moderate, low, or very low, based on the criteria.
The consensus statements were finalized at an in-person meeting conducted in Toronto in October 2017.
The guideline was supported through grant funding provided by AbbVie and Takeda. The authors reported financial affiliations with AbbVie and Takeda, as well as Janssen, Nestle Health Sciences, Shire, and several others.
SOURCE: Mack DR et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.022.
The new guideline provides evidence-based recommendations regarding optimal medical treatment strategies for achieving clinical remission based on a multi-item assessment of disease activity in pediatric patients with luminal CD. The guideline does not address surgical management, diagnosis, psychosocial therapies, preventative health considerations, or growth monitoring.
“The implications of inadequately treated CD are of particular importance in children because of the potentially serious and irreversible consequences,” wrote David R. Mack, MD, of the University of Ottawa and associates. Dr. Mack is the lead author of the pediatric practice guideline copublished in Gastroenterology and the Journal of the Canadian Association of Gastroenterology.
The consensus group reached its recommendations based on a systematic review of the literature for studies related to the medical treatment of pediatric CD. The majority of studies were randomized trials conducted in adults with CD.
“Evidence of efficacy of specific treatments in achieving mucosal healing is limited; therefore, “complete” or “deep” remission (clinical remission plus mucosal healing) was not the chosen primary outcome,” the guideline authors wrote.
The panel recommended that corticosteroids can be used as induction therapy in children with moderate to severe disease. Moreover, budesonide may be an appropriate alternative for induction therapy in patients with mild to moderate CD.
In contrast, the group recommended against the use of corticosteroids as maintenance therapy, largely because of adverse events reported with long-term use.
At diagnosis or initial stages of severe disease, as well as in patients who have failed with immunosuppressant and corticosteroid induction strategies, enteral nutrition should be used exclusively for induction therapy. In addition, anti–tumor necrosis factor biologics are an appropriate option for induction and maintenance therapy in these patients, according to the guideline.
“The group recommended against the use of oral 5-aminosalicylate for induction or maintenance therapy in patients with moderate disease, and recommended against thiopurines for induction therapy,” they wrote.
With respect to cannabis-based products, the panel made a strong recommendation against the use of these agents in all pediatric patients.
In terms of assessment, the team recommended that patients in clinical remission receiving methotrexate or a thiopurine agent as maintenance therapy should be evaluated for mucosal healing within 1 year of therapy initiation.
No consensus was reached on the adjuvant use of immunosuppressants during initiation therapy with a biologic drug, but the consensus panel recommended against the use of thiopurine combinations in male patients. Furthermore, no consensus was reached on the role of vedolizumab or antibiotics for induction or maintenance therapy, methotrexate for induction therapy, and the function of aminosalicylates in patients with mild CD.
The panel highlighted the importance of incorporating patient perspectives into treatment decision making.
“It is hoped that the available information will enhance the discussion between the clinician and the patient and enable the patient to make an evidence-based informed decision.”
The expert consensus was made up of 15 voting members that consisted of pediatric gastroenterologists throughout the United States and Canada, with expertise in several domains, including clinical epidemiology, nutrition, health services research, and patient engagement.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) criteria. The quality of evidence for each consensus statement was denoted as either high, moderate, low, or very low, based on the criteria.
The consensus statements were finalized at an in-person meeting conducted in Toronto in October 2017.
The guideline was supported through grant funding provided by AbbVie and Takeda. The authors reported financial affiliations with AbbVie and Takeda, as well as Janssen, Nestle Health Sciences, Shire, and several others.
SOURCE: Mack DR et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.022.
FROM GASTROENTEROLOGY
CAG Clinical Practice Guideline: Luminal Crohn’s disease
The Canadian Association of Gastroenterology has released a new clinical practice guideline for the treatment of luminal Crohn’s disease (CD) in adults.
“In the last decade, treatment paradigms have changed, recognizing that certain clinical parameters carry an increased risk of progressive and disabling disease,” wrote Remo Panaccione, MD, of the University of Calgary (Canada) and collaborators. Dr. Panaccione is the lead author of this practice guideline copublished in Clinical Gastroenterology and Hepatology and the Journal of the Canadian Association of Gastroenterology.
The expert consensus panel consisted of 20 voting members, including both academic and community gastroenterologists, in addition to a specialist nurse practitioner. Other nonvoting members included two GRADE experts, lay observers, and a patient representative.
The panel systematically reviewed the body of literature for studies related to the management of CD in adults. After applying the search criteria, the team found that the majority of evidence was extracted from systematic reviews and meta-analyses of randomized trials.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) methodology. The quality of evidence for each consensus statement was classified as either high, moderate, low, or very low, based on the methodology’s criteria.
The consensus statements were finalized at a face-to-face meeting in Toronto held in September 2016. Prior to completion, a web-based system was used to allow for anonymous voting on level of agreement for each consensus statement.
The new guideline provides evidence-based recommendations about optimal treatment approaches for patients with mild to severe active luminal CD in an ambulatory setting, with particular focus on six major drug classes, including corticosteroids, biologic therapies, immunosuppressants, 5-aminosalicylate, antibiotics, and other therapies.
The consensus group recommended against the use of 5-aminosalicylate or antibiotics as induction or maintenance treatment strategies. Alternatively, they suggested that corticosteroids, including budesonide, could be used as induction therapy, but not as maintenance therapy.
“Parenteral methotrexate was proposed for induction and maintenance therapy in patients with corticosteroid-dependent CD,” they wrote.
With respect to immunosuppressive therapy, thiopurine agents could be an appropriate option for maintenance therapy in certain low-risk patients, but were not recommended as induction therapy, according to the guideline.
In patients who fail with conventional induction therapies, Dr. Panaccione and colleagues recommended that biological treatments, including ustekinumab, vedolizumab, and anti–tumor necrosis factor agents, could be used. No consensus was reached on the concomitant use of immunosuppressants and biologics.
In recent years, an increasing amount of evidence has emphasized the importance of mucosal healing as a key goal of therapy. In particular, the use of some therapies can result in mucosal healing and symptomatic improvement in certain patients with luminal CD.
In addition, the authors explained that mucosal healing has been linked to better clinical outcomes over the short and long term. As a result, the recommendations in the guideline target complete remission, defined as both endoscopic and symptomatic remission.
“The outcome assessed in most randomized controlled trials (RCTs) has been either symptomatic remission or symptomatic response, with only more contemporary clinical trials including endoscopic outcomes,” the guideline authors wrote.
For this reason, the GRADE criteria–based quality of evidence for some of the consensus statements had to be lowered, they noted.
The panel acknowledged the importance of incorporating patient perspectives into treatment decision making; however, they reported that many gaps in clinical practice still remain.
“In many instances, factors that influence patient decisions relating to therapy choice and goals of therapy are not the same as those of the treating clinician,” they wrote. “[Current] surveys indicate a discrepancy between patient and physician treatment goals.”
In response, the guideline authors highlighted the importance of improved patient-physician collaboration and patient education.
The guideline was supported through grant funding provided by AbbVie, Janssen, Pfizer, and Takeda. The authors reported financial affiliations with AbbVie, Amgen, Baxter, Janssen, Shire, Takeda, and several others.
SOURCE: Panaccione R et al. Clin Gastroenterol Hepatol. 2019 Mar 7. doi: 10.1016/j.cgh.2019.02.043.
The Canadian Association of Gastroenterology has released a new clinical practice guideline for the treatment of luminal Crohn’s disease (CD) in adults.
“In the last decade, treatment paradigms have changed, recognizing that certain clinical parameters carry an increased risk of progressive and disabling disease,” wrote Remo Panaccione, MD, of the University of Calgary (Canada) and collaborators. Dr. Panaccione is the lead author of this practice guideline copublished in Clinical Gastroenterology and Hepatology and the Journal of the Canadian Association of Gastroenterology.
The expert consensus panel consisted of 20 voting members, including both academic and community gastroenterologists, in addition to a specialist nurse practitioner. Other nonvoting members included two GRADE experts, lay observers, and a patient representative.
The panel systematically reviewed the body of literature for studies related to the management of CD in adults. After applying the search criteria, the team found that the majority of evidence was extracted from systematic reviews and meta-analyses of randomized trials.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) methodology. The quality of evidence for each consensus statement was classified as either high, moderate, low, or very low, based on the methodology’s criteria.
The consensus statements were finalized at a face-to-face meeting in Toronto held in September 2016. Prior to completion, a web-based system was used to allow for anonymous voting on level of agreement for each consensus statement.
The new guideline provides evidence-based recommendations about optimal treatment approaches for patients with mild to severe active luminal CD in an ambulatory setting, with particular focus on six major drug classes, including corticosteroids, biologic therapies, immunosuppressants, 5-aminosalicylate, antibiotics, and other therapies.
The consensus group recommended against the use of 5-aminosalicylate or antibiotics as induction or maintenance treatment strategies. Alternatively, they suggested that corticosteroids, including budesonide, could be used as induction therapy, but not as maintenance therapy.
“Parenteral methotrexate was proposed for induction and maintenance therapy in patients with corticosteroid-dependent CD,” they wrote.
With respect to immunosuppressive therapy, thiopurine agents could be an appropriate option for maintenance therapy in certain low-risk patients, but were not recommended as induction therapy, according to the guideline.
In patients who fail with conventional induction therapies, Dr. Panaccione and colleagues recommended that biological treatments, including ustekinumab, vedolizumab, and anti–tumor necrosis factor agents, could be used. No consensus was reached on the concomitant use of immunosuppressants and biologics.
In recent years, an increasing amount of evidence has emphasized the importance of mucosal healing as a key goal of therapy. In particular, the use of some therapies can result in mucosal healing and symptomatic improvement in certain patients with luminal CD.
In addition, the authors explained that mucosal healing has been linked to better clinical outcomes over the short and long term. As a result, the recommendations in the guideline target complete remission, defined as both endoscopic and symptomatic remission.
“The outcome assessed in most randomized controlled trials (RCTs) has been either symptomatic remission or symptomatic response, with only more contemporary clinical trials including endoscopic outcomes,” the guideline authors wrote.
For this reason, the GRADE criteria–based quality of evidence for some of the consensus statements had to be lowered, they noted.
The panel acknowledged the importance of incorporating patient perspectives into treatment decision making; however, they reported that many gaps in clinical practice still remain.
“In many instances, factors that influence patient decisions relating to therapy choice and goals of therapy are not the same as those of the treating clinician,” they wrote. “[Current] surveys indicate a discrepancy between patient and physician treatment goals.”
In response, the guideline authors highlighted the importance of improved patient-physician collaboration and patient education.
The guideline was supported through grant funding provided by AbbVie, Janssen, Pfizer, and Takeda. The authors reported financial affiliations with AbbVie, Amgen, Baxter, Janssen, Shire, Takeda, and several others.
SOURCE: Panaccione R et al. Clin Gastroenterol Hepatol. 2019 Mar 7. doi: 10.1016/j.cgh.2019.02.043.
The Canadian Association of Gastroenterology has released a new clinical practice guideline for the treatment of luminal Crohn’s disease (CD) in adults.
“In the last decade, treatment paradigms have changed, recognizing that certain clinical parameters carry an increased risk of progressive and disabling disease,” wrote Remo Panaccione, MD, of the University of Calgary (Canada) and collaborators. Dr. Panaccione is the lead author of this practice guideline copublished in Clinical Gastroenterology and Hepatology and the Journal of the Canadian Association of Gastroenterology.
The expert consensus panel consisted of 20 voting members, including both academic and community gastroenterologists, in addition to a specialist nurse practitioner. Other nonvoting members included two GRADE experts, lay observers, and a patient representative.
The panel systematically reviewed the body of literature for studies related to the management of CD in adults. After applying the search criteria, the team found that the majority of evidence was extracted from systematic reviews and meta-analyses of randomized trials.
Quality of evidence and risk of bias was assessed using the GRADE (Grading of Recommendation Assessment, Development and Evaluation) methodology. The quality of evidence for each consensus statement was classified as either high, moderate, low, or very low, based on the methodology’s criteria.
The consensus statements were finalized at a face-to-face meeting in Toronto held in September 2016. Prior to completion, a web-based system was used to allow for anonymous voting on level of agreement for each consensus statement.
The new guideline provides evidence-based recommendations about optimal treatment approaches for patients with mild to severe active luminal CD in an ambulatory setting, with particular focus on six major drug classes, including corticosteroids, biologic therapies, immunosuppressants, 5-aminosalicylate, antibiotics, and other therapies.
The consensus group recommended against the use of 5-aminosalicylate or antibiotics as induction or maintenance treatment strategies. Alternatively, they suggested that corticosteroids, including budesonide, could be used as induction therapy, but not as maintenance therapy.
“Parenteral methotrexate was proposed for induction and maintenance therapy in patients with corticosteroid-dependent CD,” they wrote.
With respect to immunosuppressive therapy, thiopurine agents could be an appropriate option for maintenance therapy in certain low-risk patients, but were not recommended as induction therapy, according to the guideline.
In patients who fail with conventional induction therapies, Dr. Panaccione and colleagues recommended that biological treatments, including ustekinumab, vedolizumab, and anti–tumor necrosis factor agents, could be used. No consensus was reached on the concomitant use of immunosuppressants and biologics.
In recent years, an increasing amount of evidence has emphasized the importance of mucosal healing as a key goal of therapy. In particular, the use of some therapies can result in mucosal healing and symptomatic improvement in certain patients with luminal CD.
In addition, the authors explained that mucosal healing has been linked to better clinical outcomes over the short and long term. As a result, the recommendations in the guideline target complete remission, defined as both endoscopic and symptomatic remission.
“The outcome assessed in most randomized controlled trials (RCTs) has been either symptomatic remission or symptomatic response, with only more contemporary clinical trials including endoscopic outcomes,” the guideline authors wrote.
For this reason, the GRADE criteria–based quality of evidence for some of the consensus statements had to be lowered, they noted.
The panel acknowledged the importance of incorporating patient perspectives into treatment decision making; however, they reported that many gaps in clinical practice still remain.
“In many instances, factors that influence patient decisions relating to therapy choice and goals of therapy are not the same as those of the treating clinician,” they wrote. “[Current] surveys indicate a discrepancy between patient and physician treatment goals.”
In response, the guideline authors highlighted the importance of improved patient-physician collaboration and patient education.
The guideline was supported through grant funding provided by AbbVie, Janssen, Pfizer, and Takeda. The authors reported financial affiliations with AbbVie, Amgen, Baxter, Janssen, Shire, Takeda, and several others.
SOURCE: Panaccione R et al. Clin Gastroenterol Hepatol. 2019 Mar 7. doi: 10.1016/j.cgh.2019.02.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The Canadian Association of Gastroenterology has released a new clinical practice guideline for the treatment of mild to severe active luminal Crohn’s disease (CD).
Major finding: The new guideline includes 41 statements that focus on six major therapeutic classes.
Study details: The CAG Clinical Practice Guideline for Luminal CD.
Disclosures: The guideline was supported through grant funding provided by AbbVie, Janssen, Pfizer, and Takeda. The authors reported financial affiliations with AbbVie, Amgen, Baxter, Janssen, Shire, Takeda, and several others.
Source: Panaccione R et al. Clin Gastroenterol Hepatol. 2019 Mar 7. doi: 10.1016/j.cgh.2019.02.043.
Teens & tobacco use: USPSTF issues draft recs on prevention, cessation
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Reference
1. US Preventive Services Task Force. Draft Evidence Review for Prevention and Cessation of Tobacco Use in Children and Adolescents: Primary Care Interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 8, 2019.
Passing the torch
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me (bryson.katona@pennmedicine.upenn.edu), incoming editor in chief Vijaya Rao (vijayarao@medicine.bsd.uchicago.edu), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Not Salty Enough
We commend Gottenborg and Pierce on their well-written summary of the 2013 National Institutes of Care Excellence (NICE) guidelines on int
The recommendations for hypotonic solutions were largely developed from theoretical research in the 1950s before the first description of the syndrome of inappropriate secretion of antidiuretic hormone.5 Hospitalized patients are at significant risk for nonosmotic stimuli for antidiuretic hormone secretion, and hypotonic fluids increase the risk of hyponatremia, which can have catastrophic complications. We believe the pediatric evidence should be extrapolated and included with the supporting (albeit limited) adult evidence, and that when indicated, isotonic fluids should be the maintenance fluid for most hospitalized adults.3-4,6
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. https://doi.org/10.12788/jhm.3178
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/g174. Accessed April 6, 2019.
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6):170-171. https://doi.org/10.1542/peds.2018-3083.
4. Neilson J, O’Neill F, Dawoud D, Crean P, Guideline Development G. Intravenous fluids in children and young people: summary of NICE guidance. BMJ. 2015;351:h6388. https://doi.org/10.1136/bmj.h6388
5. Talbot NB, Crawford DJ, Butler AM. Medical progress; homeostatic limits to safe parenteral fluid therapy. N Engl J Med. 1953;248:1100-1108. https://doi.org/10.1056/NEJM195306252482605
6. Okada M, Egi M, Yokota Y, et al. Comparison of the incidences of hyponatremia in adult postoperative critically ill patients receiving intravenous maintenance fluids with 140 mmol/L or 35 mmol/L of sodium: retrospective before/after observational study. J Anesth. 2017;31(5):657-663 PubMed
We commend Gottenborg and Pierce on their well-written summary of the 2013 National Institutes of Care Excellence (NICE) guidelines on int
The recommendations for hypotonic solutions were largely developed from theoretical research in the 1950s before the first description of the syndrome of inappropriate secretion of antidiuretic hormone.5 Hospitalized patients are at significant risk for nonosmotic stimuli for antidiuretic hormone secretion, and hypotonic fluids increase the risk of hyponatremia, which can have catastrophic complications. We believe the pediatric evidence should be extrapolated and included with the supporting (albeit limited) adult evidence, and that when indicated, isotonic fluids should be the maintenance fluid for most hospitalized adults.3-4,6
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
We commend Gottenborg and Pierce on their well-written summary of the 2013 National Institutes of Care Excellence (NICE) guidelines on int
The recommendations for hypotonic solutions were largely developed from theoretical research in the 1950s before the first description of the syndrome of inappropriate secretion of antidiuretic hormone.5 Hospitalized patients are at significant risk for nonosmotic stimuli for antidiuretic hormone secretion, and hypotonic fluids increase the risk of hyponatremia, which can have catastrophic complications. We believe the pediatric evidence should be extrapolated and included with the supporting (albeit limited) adult evidence, and that when indicated, isotonic fluids should be the maintenance fluid for most hospitalized adults.3-4,6
Disclosures
We have no relevant conflicts of interest to report. No payment or services from a third party were received for any aspect of this submitted work. We have no financial relationships with entities in the bio-medical arena that could be perceived to influence, or that give the appearance of potentially influencing, what was written in this submitted work.
1. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. https://doi.org/10.12788/jhm.3178
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/g174. Accessed April 6, 2019.
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6):170-171. https://doi.org/10.1542/peds.2018-3083.
4. Neilson J, O’Neill F, Dawoud D, Crean P, Guideline Development G. Intravenous fluids in children and young people: summary of NICE guidance. BMJ. 2015;351:h6388. https://doi.org/10.1136/bmj.h6388
5. Talbot NB, Crawford DJ, Butler AM. Medical progress; homeostatic limits to safe parenteral fluid therapy. N Engl J Med. 1953;248:1100-1108. https://doi.org/10.1056/NEJM195306252482605
6. Okada M, Egi M, Yokota Y, et al. Comparison of the incidences of hyponatremia in adult postoperative critically ill patients receiving intravenous maintenance fluids with 140 mmol/L or 35 mmol/L of sodium: retrospective before/after observational study. J Anesth. 2017;31(5):657-663 PubMed
1. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. https://doi.org/10.12788/jhm.3178
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/g174. Accessed April 6, 2019.
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6):170-171. https://doi.org/10.1542/peds.2018-3083.
4. Neilson J, O’Neill F, Dawoud D, Crean P, Guideline Development G. Intravenous fluids in children and young people: summary of NICE guidance. BMJ. 2015;351:h6388. https://doi.org/10.1136/bmj.h6388
5. Talbot NB, Crawford DJ, Butler AM. Medical progress; homeostatic limits to safe parenteral fluid therapy. N Engl J Med. 1953;248:1100-1108. https://doi.org/10.1056/NEJM195306252482605
6. Okada M, Egi M, Yokota Y, et al. Comparison of the incidences of hyponatremia in adult postoperative critically ill patients receiving intravenous maintenance fluids with 140 mmol/L or 35 mmol/L of sodium: retrospective before/after observational study. J Anesth. 2017;31(5):657-663 PubMed
© 2019 Society of Hospital Medicine
Interhospital Transfers for Quality Assessment of Healthcare Systems
With the increasing percentage of our gross national product being allotted to healthcare and concerns about the care received by patients, the number of measures to assess the quality and efficiency of care delivered by healthcare professionals has increased. The paper by Mueller et al.1 adds to our understanding of an important yet relatively understudied group of patients: those that require transfer from one inpatient facility to another. In general, these patients are sicker and exhibit poor outcomes, especially with time-sensitive management conditions, such as cerebrovascular accidents, or conditions where the transfer itself may cause harm to the patient, such as the case of an infant born prematurely. However, transferring patients with less time-dependent conditions may not be associated with such negative results.1 The uniqueness of interhospital transfers is attributed to their ability to provide insights into the care practices of other actors within the healthcare system, namely, the transferring hospital and the larger healthcare system, and to describe how the care quality may change in hospitals during periods of stress, such as during overcrowding or high patient acuity.
As described by Mueller et al. the care and outcomes of patients transferred to a hospital may provide information regarding the key aspects of care at the receiving hospital; these aspects include the capability for triage of potentially high-acuity patients and the management of such patients during periods of crowding and organizational stress. These measures of efficiency have rarely been studied in relation to the care provided to patients and their ultimate outcomes. The most studied efficiency measure is hospital crowding, which has been shown in numerous studies to be associated with lower efficiency as measured by the length of stay, lower quality of care, and higher mortality.2-3 This report by Mueller et al. is one of the first papers to highlight how other aspects of the care delivery system, including the triage practices and the response of a hospital system to stress, may influence care outcomes. The limitation of other studies in exploring the relationship between the measures of efficiency and quality of care, as noted by a systematic review of healthcare efficiency measures by Hussey et al.4 emphasizes the need to understand the drivers of low quality of care and to determine the specific times at which such care may be compromised by other factors, such as patient volumes.
Although interhospital transfers may offer certain insights into the efficiency of care delivered at the hospitals receiving these patients, they are generally rare and centered on a few quaternary hospitals within a region.3 In addition, the Mueller paper reveals that not all these transfers have high disease acuity, particularly for cardiac patients. Whether claims-based approaches to risk adjustment would sufficiently differentiate the reasons for the transfer/failure to transfer of patients is unclear and thus may be affected by the selection bias. With these issues, the outcome of transferred patients may be only of limited value when assessing the care quality of hospitals that generally receive transferred patients from other medical institutions within a given geographic area.5
Interhospital transfers may provide insights into the care of patients at the hospitals which transfer out such patients, focusing on the appropriateness of transfers, how these hospitals operate when such a sick patient arrives at that hospital, and the outcomes of patients with conditions that may require transfer. A few studies have explored the preventable transfer, particularly for trauma patients, where a preventable transfer was defined as a transfer that was was not admitted to the receiving hospital and did not receive any procedures or testing. Although not readily defined for numerous conditions, such a measure would provide insights into how hospitals decide whether a patient requires care at a higher-level hospital and assessing the processes needed to optimize this decision-making process, including where the patient ultimately is transferred. In a study of patients with acute myocardial infarction, 36.8% of cases that required transfer were not directed to hospitals with the best outcomes as measured by 30-day risk-adjusted mortality rates within a given geographic region.6 Such decisions would contribute to the potential worse outcomes observed in patients requiring interhospital transfer.
Finally, transfers provide insights into the functioning of the larger healthcare system. The measures assessing the functioning of the healthcare system are rare. In theory, interhospital transfers meet the goals of a functioning regional healthcare system by matching the patients to facilities with the suitable capabilities to manage the patient’s given type of illness or injury. Such a system, however, requires collaboration between hospitals who otherwise compete for patients. The literature suggests that such collaboration is widely variable and dependent on patient factors, such as the types of conditions and their insurance status,7 and the costs required by hospitals to add the services needed to care for increasingly ill patients. In addition, the growth of so-called narrow insurance networks, which limit the number of hospitals an insurance company will include on their preferred network, may place barriers on the appropriate location of such transfers based on the quality of the receiving hospital.8
The paper by Mueller et al. adds to the literature the unique aspects of the care needed by the patients requiring interhospital transfer. Unlike most other measures of care quality and efficiency, interhospital transfers potentially offer knowledge about the quality of the larger healthcare system, assessing the appropriateness and ultimate outcomes not only of patients who are transferred but similarly sick patients who could have potentially benefited from a transfer and how the actors within the system may respond to periods of high patient load and stress. By understanding the drivers of the appropriateness of where patients receive care, we can gain insights into the mechanisms needed to fulfill the goals of a functional regionalized healthcare system.
Disclosures
The author has no financial or other relevant conflicts of interest to disclose.
1. Mueller SK, Fiskio J, Schnipper J. Interhospital transfer: transfer processes and patient outcomes. J Hosp Med. 2019;(8):486-491. https://doi.org/10.12788/jhm.3192.
2. Lorch SA, Millman AM, Zhang X, Even-Shoshan O, Silber JH. Impact of admission-day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718-e730. https://doi.org/10.1542/peds.2007-1280.
3. Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605-611.e6. https://doi.org/10.1016/j.annemergmed.2012.10.026
4. Hussey PS, de Vries H, Romley J, et al. A systematic review of health care efficiency measures. Health Serv Res. 2009;44(3):784-805. https://doi.org/10.1111/j.1475-6773.2008.00942.x.
5. Lorch SA. National quality measures in perinatal medicine. Clin Perinatol. 2017;44(3):485-509. https://doi.org/10.1016/j.clp.2017.05.001
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. https://doi.org/10.1161/CIRCOUTCOMES.110.957993.
7. Green A, Showstack J, Rennie D, Goldman L. The relationship of insurance status, hospital ownership, and teaching status with interhospital transfers in California in 2000. Acad Med. 2005;80(8):774-779. https://doi.org/10.1097/00001888-200508000-00015
8. Colvin JD, Hall M, Thurm C, et al. Hypothetical network adequacy schemes for children fail to ensure patients’ access to in-network children’s hospital. Health Aff (Millwood). 2018;37(6):873-880. https://doi.org/10.1377/hlthaff.2017.1339.
With the increasing percentage of our gross national product being allotted to healthcare and concerns about the care received by patients, the number of measures to assess the quality and efficiency of care delivered by healthcare professionals has increased. The paper by Mueller et al.1 adds to our understanding of an important yet relatively understudied group of patients: those that require transfer from one inpatient facility to another. In general, these patients are sicker and exhibit poor outcomes, especially with time-sensitive management conditions, such as cerebrovascular accidents, or conditions where the transfer itself may cause harm to the patient, such as the case of an infant born prematurely. However, transferring patients with less time-dependent conditions may not be associated with such negative results.1 The uniqueness of interhospital transfers is attributed to their ability to provide insights into the care practices of other actors within the healthcare system, namely, the transferring hospital and the larger healthcare system, and to describe how the care quality may change in hospitals during periods of stress, such as during overcrowding or high patient acuity.
As described by Mueller et al. the care and outcomes of patients transferred to a hospital may provide information regarding the key aspects of care at the receiving hospital; these aspects include the capability for triage of potentially high-acuity patients and the management of such patients during periods of crowding and organizational stress. These measures of efficiency have rarely been studied in relation to the care provided to patients and their ultimate outcomes. The most studied efficiency measure is hospital crowding, which has been shown in numerous studies to be associated with lower efficiency as measured by the length of stay, lower quality of care, and higher mortality.2-3 This report by Mueller et al. is one of the first papers to highlight how other aspects of the care delivery system, including the triage practices and the response of a hospital system to stress, may influence care outcomes. The limitation of other studies in exploring the relationship between the measures of efficiency and quality of care, as noted by a systematic review of healthcare efficiency measures by Hussey et al.4 emphasizes the need to understand the drivers of low quality of care and to determine the specific times at which such care may be compromised by other factors, such as patient volumes.
Although interhospital transfers may offer certain insights into the efficiency of care delivered at the hospitals receiving these patients, they are generally rare and centered on a few quaternary hospitals within a region.3 In addition, the Mueller paper reveals that not all these transfers have high disease acuity, particularly for cardiac patients. Whether claims-based approaches to risk adjustment would sufficiently differentiate the reasons for the transfer/failure to transfer of patients is unclear and thus may be affected by the selection bias. With these issues, the outcome of transferred patients may be only of limited value when assessing the care quality of hospitals that generally receive transferred patients from other medical institutions within a given geographic area.5
Interhospital transfers may provide insights into the care of patients at the hospitals which transfer out such patients, focusing on the appropriateness of transfers, how these hospitals operate when such a sick patient arrives at that hospital, and the outcomes of patients with conditions that may require transfer. A few studies have explored the preventable transfer, particularly for trauma patients, where a preventable transfer was defined as a transfer that was was not admitted to the receiving hospital and did not receive any procedures or testing. Although not readily defined for numerous conditions, such a measure would provide insights into how hospitals decide whether a patient requires care at a higher-level hospital and assessing the processes needed to optimize this decision-making process, including where the patient ultimately is transferred. In a study of patients with acute myocardial infarction, 36.8% of cases that required transfer were not directed to hospitals with the best outcomes as measured by 30-day risk-adjusted mortality rates within a given geographic region.6 Such decisions would contribute to the potential worse outcomes observed in patients requiring interhospital transfer.
Finally, transfers provide insights into the functioning of the larger healthcare system. The measures assessing the functioning of the healthcare system are rare. In theory, interhospital transfers meet the goals of a functioning regional healthcare system by matching the patients to facilities with the suitable capabilities to manage the patient’s given type of illness or injury. Such a system, however, requires collaboration between hospitals who otherwise compete for patients. The literature suggests that such collaboration is widely variable and dependent on patient factors, such as the types of conditions and their insurance status,7 and the costs required by hospitals to add the services needed to care for increasingly ill patients. In addition, the growth of so-called narrow insurance networks, which limit the number of hospitals an insurance company will include on their preferred network, may place barriers on the appropriate location of such transfers based on the quality of the receiving hospital.8
The paper by Mueller et al. adds to the literature the unique aspects of the care needed by the patients requiring interhospital transfer. Unlike most other measures of care quality and efficiency, interhospital transfers potentially offer knowledge about the quality of the larger healthcare system, assessing the appropriateness and ultimate outcomes not only of patients who are transferred but similarly sick patients who could have potentially benefited from a transfer and how the actors within the system may respond to periods of high patient load and stress. By understanding the drivers of the appropriateness of where patients receive care, we can gain insights into the mechanisms needed to fulfill the goals of a functional regionalized healthcare system.
Disclosures
The author has no financial or other relevant conflicts of interest to disclose.
With the increasing percentage of our gross national product being allotted to healthcare and concerns about the care received by patients, the number of measures to assess the quality and efficiency of care delivered by healthcare professionals has increased. The paper by Mueller et al.1 adds to our understanding of an important yet relatively understudied group of patients: those that require transfer from one inpatient facility to another. In general, these patients are sicker and exhibit poor outcomes, especially with time-sensitive management conditions, such as cerebrovascular accidents, or conditions where the transfer itself may cause harm to the patient, such as the case of an infant born prematurely. However, transferring patients with less time-dependent conditions may not be associated with such negative results.1 The uniqueness of interhospital transfers is attributed to their ability to provide insights into the care practices of other actors within the healthcare system, namely, the transferring hospital and the larger healthcare system, and to describe how the care quality may change in hospitals during periods of stress, such as during overcrowding or high patient acuity.
As described by Mueller et al. the care and outcomes of patients transferred to a hospital may provide information regarding the key aspects of care at the receiving hospital; these aspects include the capability for triage of potentially high-acuity patients and the management of such patients during periods of crowding and organizational stress. These measures of efficiency have rarely been studied in relation to the care provided to patients and their ultimate outcomes. The most studied efficiency measure is hospital crowding, which has been shown in numerous studies to be associated with lower efficiency as measured by the length of stay, lower quality of care, and higher mortality.2-3 This report by Mueller et al. is one of the first papers to highlight how other aspects of the care delivery system, including the triage practices and the response of a hospital system to stress, may influence care outcomes. The limitation of other studies in exploring the relationship between the measures of efficiency and quality of care, as noted by a systematic review of healthcare efficiency measures by Hussey et al.4 emphasizes the need to understand the drivers of low quality of care and to determine the specific times at which such care may be compromised by other factors, such as patient volumes.
Although interhospital transfers may offer certain insights into the efficiency of care delivered at the hospitals receiving these patients, they are generally rare and centered on a few quaternary hospitals within a region.3 In addition, the Mueller paper reveals that not all these transfers have high disease acuity, particularly for cardiac patients. Whether claims-based approaches to risk adjustment would sufficiently differentiate the reasons for the transfer/failure to transfer of patients is unclear and thus may be affected by the selection bias. With these issues, the outcome of transferred patients may be only of limited value when assessing the care quality of hospitals that generally receive transferred patients from other medical institutions within a given geographic area.5
Interhospital transfers may provide insights into the care of patients at the hospitals which transfer out such patients, focusing on the appropriateness of transfers, how these hospitals operate when such a sick patient arrives at that hospital, and the outcomes of patients with conditions that may require transfer. A few studies have explored the preventable transfer, particularly for trauma patients, where a preventable transfer was defined as a transfer that was was not admitted to the receiving hospital and did not receive any procedures or testing. Although not readily defined for numerous conditions, such a measure would provide insights into how hospitals decide whether a patient requires care at a higher-level hospital and assessing the processes needed to optimize this decision-making process, including where the patient ultimately is transferred. In a study of patients with acute myocardial infarction, 36.8% of cases that required transfer were not directed to hospitals with the best outcomes as measured by 30-day risk-adjusted mortality rates within a given geographic region.6 Such decisions would contribute to the potential worse outcomes observed in patients requiring interhospital transfer.
Finally, transfers provide insights into the functioning of the larger healthcare system. The measures assessing the functioning of the healthcare system are rare. In theory, interhospital transfers meet the goals of a functioning regional healthcare system by matching the patients to facilities with the suitable capabilities to manage the patient’s given type of illness or injury. Such a system, however, requires collaboration between hospitals who otherwise compete for patients. The literature suggests that such collaboration is widely variable and dependent on patient factors, such as the types of conditions and their insurance status,7 and the costs required by hospitals to add the services needed to care for increasingly ill patients. In addition, the growth of so-called narrow insurance networks, which limit the number of hospitals an insurance company will include on their preferred network, may place barriers on the appropriate location of such transfers based on the quality of the receiving hospital.8
The paper by Mueller et al. adds to the literature the unique aspects of the care needed by the patients requiring interhospital transfer. Unlike most other measures of care quality and efficiency, interhospital transfers potentially offer knowledge about the quality of the larger healthcare system, assessing the appropriateness and ultimate outcomes not only of patients who are transferred but similarly sick patients who could have potentially benefited from a transfer and how the actors within the system may respond to periods of high patient load and stress. By understanding the drivers of the appropriateness of where patients receive care, we can gain insights into the mechanisms needed to fulfill the goals of a functional regionalized healthcare system.
Disclosures
The author has no financial or other relevant conflicts of interest to disclose.
1. Mueller SK, Fiskio J, Schnipper J. Interhospital transfer: transfer processes and patient outcomes. J Hosp Med. 2019;(8):486-491. https://doi.org/10.12788/jhm.3192.
2. Lorch SA, Millman AM, Zhang X, Even-Shoshan O, Silber JH. Impact of admission-day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718-e730. https://doi.org/10.1542/peds.2007-1280.
3. Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605-611.e6. https://doi.org/10.1016/j.annemergmed.2012.10.026
4. Hussey PS, de Vries H, Romley J, et al. A systematic review of health care efficiency measures. Health Serv Res. 2009;44(3):784-805. https://doi.org/10.1111/j.1475-6773.2008.00942.x.
5. Lorch SA. National quality measures in perinatal medicine. Clin Perinatol. 2017;44(3):485-509. https://doi.org/10.1016/j.clp.2017.05.001
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. https://doi.org/10.1161/CIRCOUTCOMES.110.957993.
7. Green A, Showstack J, Rennie D, Goldman L. The relationship of insurance status, hospital ownership, and teaching status with interhospital transfers in California in 2000. Acad Med. 2005;80(8):774-779. https://doi.org/10.1097/00001888-200508000-00015
8. Colvin JD, Hall M, Thurm C, et al. Hypothetical network adequacy schemes for children fail to ensure patients’ access to in-network children’s hospital. Health Aff (Millwood). 2018;37(6):873-880. https://doi.org/10.1377/hlthaff.2017.1339.
1. Mueller SK, Fiskio J, Schnipper J. Interhospital transfer: transfer processes and patient outcomes. J Hosp Med. 2019;(8):486-491. https://doi.org/10.12788/jhm.3192.
2. Lorch SA, Millman AM, Zhang X, Even-Shoshan O, Silber JH. Impact of admission-day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718-e730. https://doi.org/10.1542/peds.2007-1280.
3. Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605-611.e6. https://doi.org/10.1016/j.annemergmed.2012.10.026
4. Hussey PS, de Vries H, Romley J, et al. A systematic review of health care efficiency measures. Health Serv Res. 2009;44(3):784-805. https://doi.org/10.1111/j.1475-6773.2008.00942.x.
5. Lorch SA. National quality measures in perinatal medicine. Clin Perinatol. 2017;44(3):485-509. https://doi.org/10.1016/j.clp.2017.05.001
6. Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468-475. https://doi.org/10.1161/CIRCOUTCOMES.110.957993.
7. Green A, Showstack J, Rennie D, Goldman L. The relationship of insurance status, hospital ownership, and teaching status with interhospital transfers in California in 2000. Acad Med. 2005;80(8):774-779. https://doi.org/10.1097/00001888-200508000-00015
8. Colvin JD, Hall M, Thurm C, et al. Hypothetical network adequacy schemes for children fail to ensure patients’ access to in-network children’s hospital. Health Aff (Millwood). 2018;37(6):873-880. https://doi.org/10.1377/hlthaff.2017.1339.
© 2019 Society of Hospital Medicine
Quantity, Quality, or Neither–Measuring the Effectiveness of Rounds
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
Medicine has a rich history of attending-led rounds, with some iteration of this ritual occurring as far back as the 1600s.1 In the early 1900s, the concept of “bedside rounds” was popularized by William Osler, who widely espoused their importance as a clinical and educational tool. Despite our best intentions, however, rounds today may be little reminiscent of the rounds of Osler’s day. Recent investigations into the characteristics of rounds have specifically revealed a “shift in the format from the beside to conference rooms and hallways.”2 Most of our practices for rounding in the modern era are built on tradition and belief rather than evidence. The ecosystem of modern hospital care is dramatically different than that of Osler’s day, and fundamental questions about the format, content, stakeholders, and processes of rounds remain. Perhaps the greatest and most needed change in rounding in recent years is the shift of rounds from a physician-centric activity to an activity that values the modern interprofessional hospital team. Ultimately, the very definition of “rounds” and the purpose they are meant to serve in the context of a dynamic and complicated hospital ecosystem has become increasingly complex and thus, difficult to assess and improve.
In this month’s Journal of Hospital Medicine, Sang et al.3 address this complexity by returning to basics and utilizing a novel approach to precisely measure the frequency and duration of a necessary (albeit insufficient) condition for interdisciplinary bedside rounding to occur: colocation of physician, nurse, and patient. Ultimately, their results provide a springboard to ask more complex and meaningful questions. Why, despite a recent culture shift prioritizing a return to bedside, is substantive physician and nurse colocation so persistently difficult to attain? How can we study outcomes of interdisciplinary bedside rounds if we cannot reliably facilitate their occurrence? What does “effective” rounding even mean? That is, what variables would be both meaningful and sensitive to changes in rounds?
After centuries of rounding, the medical community would be presumed to have perfected this art; however, we are instead left with more questions than answers. Prior research efforts have demonstrated the shifting of rounds away from the bedside, with bedside rounds occurring only 10%-40% of the time based on bias-prone survey data.2,4 Interestingly, a study by Huang et al., designed specifically to increase implementation of interdisciplinary bedside rounds, showed a frequency of only 64%.5 These studies are focused primarily on parameters such as patient and nursing satisfaction and did not include other important outcomes such as length of stay, readmission rates, diagnostic quality, patient engagement, or mortality.2,4,6
In Sang et al.,3 the authors utilized a real-time locator system, namely, radiofrequency identification, to precisely track the physical workflow of both attending hospitalists and bedside nurses and then subsequently used the data obtained to measure the frequency and duration of colocation at the patient bedside. The authors defined a physician “rounding event” as the physician’s presence in a single bed patient room for at least 10 seconds. The study revealed that colocation of physician and nurse (for at least 10 seconds) occurred in only 30% of all physician rounding events recorded. The duration of a physician rounding event was 5.68 minutes without nurse colocation and 9.56 minutes if a nurse was present. No difference in the frequency of physician-nurse overlap was observed between weekdays and weekends. Interestingly and not surprisingly, patient rooms located farther from the nursing station had a decreased likelihood of physician-nurse overlap.
A greater understanding of the medical community’s inability to reliably implement interdisciplinary bedside rounding may be found by examining the ecosystem of inpatient medicine. Physicians and nurses function in an environment with increasingly complex patients, more stringent (and non- evidence-based) documentation requirements, the physical decoupling of patients and their clinical information, and, as Sang et al.3 illuminate, complex geographical ward structures. As the rapidity with which patients are diagnosed and treated continues to escalate, physicians and nurses are also asked to attempt to squeeze an Oslerian-type rounding system into an ecosystem that is in overdrive. That the puzzle pieces do not fit should not be a surprise.
There is a risk that systems may implement interventions to “check the box” for interdisciplinary bedside rounding instead of seeking to change outcomes. How much time is time enough together at the bedside? Sang et al., among others, ponder whether a rounding duration of just under 10 minutes is enough.3,6 However, Rothberg et al. demonstrated that increased duration of communication alone is not necessarily associated with increased patient satisfaction or nurse–physician agreement on plan of care,7 suggesting that colocation and communication are necessary but not sufficient for true interdisciplinary patient care. The discordance between communication and understanding can potentially be explained by the varying agendas of the members of the interdisciplinary team during the same interaction.8
Ultimately, the future of interdisciplinary bedside rounding, and rounding in general, remains uncertain. Potential areas for improvement and further study include patient regionalization,3,5 tools to align agendas among stakeholders,8 integrating recommendations for interdisciplinary communication,9 and utilizing a common definition and taxonomy for study design.10 These interventions may improve future study designs and outcomes. However, these interventions are small tweaks in a complex ecosystem, and the return on these interventions may eventually reach an asymptote. Perhaps the concept of rounding as we know it is broken beyond repair, and a more radical approach is needed: either the creation of a completely innovative shared mental model of acute care that acknowledges the complex environment of inpatient medicine, or a complete restructuring of the ecosystem itself. Nonetheless, the findings of Sang et al.3 with respect to the ongoing difficulty of implementing interdisciplinary bedside rounding elucidate the need for innovation in study design and rounding implementation strategies; they also prompt us to ask—and answer—the complicated questions related to this integral component of our practice.
Disclosures
The authors have nothing to disclose.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
1. Linfors EW, Neelon FA. The case for bedside rounds. N Engl J Med. 1980;303(21):1230-1233. https://doi.org/10.1056/NEJM198011203032110.
2. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era. JAMA Intern Med. 2013;173(12):1084. https://doi.org/10.1001/jamainternmed.2013.6041.
3. Sang AX, Tisdale RL, Nielson D, et al. How much time are physicians and nurses spending together at the patient bedside? J Hosp Med. 2019;14(8):468-473. https://doi.org/10.12788/jhm.3204.
4. O’leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2015-005035.
5. Huang KTL, Minahan J, Brita-Rossi P, et al. All together now: impact of a regionalization and bedside rounding initiative on the efficiency and inclusiveness of clinical rounds OR. J Hosp Med. 2017;12(3):150-156. https://doi.org/10.12788/jhm.2696.
6. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
7. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Intern Med. 2012;27(2):185-189. https://doi.org/10.1007/s11606-011-1857-8.
8. Holton R, Patel R, Eggebrecht M, et al. Rounding on rounds. Am J Med Qual. 2015;30(5):493-493. https://doi.org/10.1177/1062860615596388.
9. Kassutto S , Seam N, Carlos WG, et al. Twelve tips for conducting successful interprofessional teaching rounds [published online ahead of print February 1, 2019]. Med Teach. https://doi.org/10.1080/0142159X.2018.1545086.
10. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. https://doi.org/10.1002/jhm.2575.
© 2019 Society of Hospital Medicine