User login
Machine-learning model predicts anti-TNF nonresponse in RA patients
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
A machine-learning model that uses clinical profiles and genetic information has shown promise in predicting which rheumatoid arthritis patients respond to anti–tumor necrosis factor drugs in a patient population of European descent.
The model can “help up to 40% of European-descent anti–tumor necrosis factor [TNF] nonresponders avoid ineffective treatments” when compared with the usual “trial-and-error practice,” according to the authors led by Yuanfang Guan, PhD, of the department of computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
The ability to accurately predict rheumatoid arthritis patients’ response to treatments would provide valuable information for optimal drug selection and would help potential nonresponders avoid drug expenses and side effects, such as an increased risk of infections, Dr. Guan and coauthors noted in Arthritis & Rheumatology.
The investigators used a modeling technique called Gaussian process regression (GPR) to predict anti-TNF drug responses. “GPR is designed to predict the unknown dependent variable for any given independent variables based on known but noisy observations of the dependent and independent variables,” they explained.
The model they used won first place in the Dialogue on Reverse Engineering Assessment and Methods: Rheumatoid Arthritis Responder Challenge, which used a crowd-based competition framework to develop a validated molecular predictor of anti-TNF response in RA.
The model was developed and cross-validated using 1,892 patients randomly selected from a training data set of 2,706 individuals of European ancestry compiled from 13 patient cohorts. All patients met 1987 American College of Rheumatology criteria for RA or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline, based on a 28-joint Disease Activity Score (DAS28) greater than 3.2.
The research team also evaluated the model using an independent dataset of 680 patients from the CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions) study.
The model combined demographic, clinical, and genetic markers to predict patients’ changes in DAS28 24 months after their baseline assessment, and identify nonresponders to anti-TNF treatments, the authors explained.
“Specifically, the [model] predicts the changes in [DAS28] of patients who have taken 12 months of anti-TNF treatments, and also classifies the patients’ responses based on the EULAR response metric,” they wrote.
Results showed that, in cross-validation tests, the model predicted changes in DAS28 with a correlation coefficient of 0.406, correctly classifying responses of 78% of subjects, with an area under the receiver operating characteristic curve (AUROC) of about 0.66.
In the independent test, the method achieved a Pearson correlation coefficient of 0.393 in predicting the change in DAS28.
Genetic SNP biomarkers provided a small additional contribution to the prediction on top of the clinical models, the authors noted.
“Compared to traditional trial-and-error practice, our model can help up to 40% of European-descent anti-TNF nonresponders avoid ineffective treatments. The model performance is even comparable to some published models utilizing additional biomarker data, whose AUROC ranges from 55% to 74% over various testing sets,” they wrote.
The GPR model has practical advantages in clinical application, unlike many sophisticated machine-learning algorithms, according to the authors. For example, GPR is a well-studied statistical model, its similarity-modeling approach is intuitive, and its results are easy to interpret.
“Our GPR model can predict subpopulations that do not respond to the treatment. This can help physicians tailor treatments for individual patients based on their conditions. ... The model can also estimate confidence intervals for its predictions, allowing physicians to judge how confident the predictions are,” the study authors wrote.
However, they cautioned that because the model was built using patients of European descent they did not expect it to achieve a similar performance in other populations. “Extension of the model over other populations requires new patient data and separate feature selection.”
The research was supported by the National Science Foundation and the National Natural Science Foundation of China. Several of the researchers reported financial relationships with pharmaceutical or technology companies.
SOURCE: Guan Y et al. Arthritis Rheumatol. 2019 Jul 24. doi: 10.1002/art.41056.
FROM ARTHRITIS & RHEUMATOLOGY
Did You Know? Psoriasis and cardiovascular disease



Infective endocarditis: Beyond the usual tests
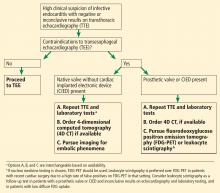
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
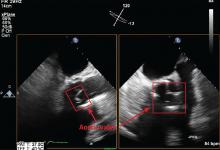
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
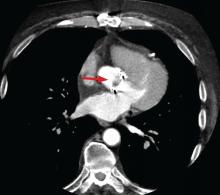
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
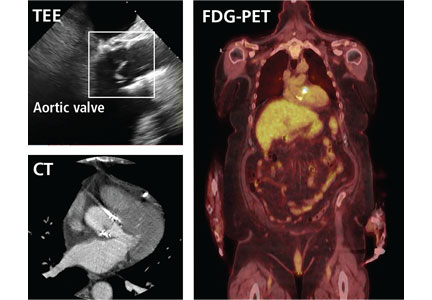
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
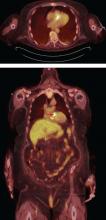
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
KEY POINTS
- Echocardiography can produce false-negative results in native-valve infective endocarditis and is even less sensitive in patients with a prosthetic valve or cardiac implanted electronic device.
- 4D CT is a reasonable alternative to transesophageal echocardiography. It can also be used as a second test if echocardiography is inconclusive. Coupled with angiography, it also provides a noninvasive method to evaluate coronary arteries perioperatively.
- Nuclear imaging tests—FDG-PET and leukocyte scintigraphy—increase the sensitivity of the Duke criteria for diagnosing infective endocarditis. They should be considered for evaluating suspected infective endocarditis in all patients who have a prosthetic valve or cardiac implanted electronic device, and whenever echocardiography is inconclusive and clinical suspicion remains high.
Adults with autism spectrum disorder: Updated considerations for healthcare providers
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
KEY POINTS
- Autism is becoming more common, with most recent statistics showing at least 1 in 59 children affected.
- Asperger syndrome is now included in the category of ASD, with possible implications for coverage of care.
- Some children with ASD get better as they get older, but many do not, and some do not receive a diagnosis until adulthood.
- Diagnosing ASD in adults can be difficult and involves specialists from multiple disciplines.
- Social support is important. Community programs and behavioral therapies can help. Drug therapy has not been rigorously tested and is not approved for use in adults with ASD. Caregivers may also need support.
Deciding when a picture is worth a thousand words and several thousand dollars
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
In a study from the University of Pennsylvania,2 Sedrak et al surveyed residents about their lab test ordering practices. Almost all responders recognized that they ordered “unnecessary tests.” The authors of the paper probed to understand why, and strikingly, the more common responses were the same that my resident peers and I would have given 4 decades ago: the culture of the system (“We don’t want to miss anything or be asked on rounds for data that hadn’t been checked”), the lack of transparency of cost of the tests, and the lack of role-modeling by teaching staff. There has been hope that the last of these would be resolved by increased visibility of subspecialists in hospital medicine, well-versed in the nuances of system-based practice. And the Society of Hospital Medicine, along with the American College of Physicians and others, has pushed hard to promote choosing wisely when ordering diagnostic studies. But we have a way to go.
Lab tests represent a small fraction of healthcare costs. Imaging tests, especially advanced and complex imaging studies, comprise a far greater fraction of healthcare costs. And here is the challenge: developers of new imaging modalities are now able to design and refine specific tests that are good enough to become the gold standard for diagnosis and staging of specific diseases—great for clinical care, bad for cost savings. One need only review a few new guidelines or clinical research protocols to appreciate the successful integration of these tests into clinical practice. Some tests are supplanting the need for aggressive biopsies, angiography, or a series of alternative imaging tests. This is potentially good for patients, but many of these tests are strikingly expensive and are being adopted for use prior to full vetting of their utility and limitations in large clinical studies; the cost of the tests can be an impediment to conducting a series of clinical studies that include appropriate patient subsets. The increasingly proposed use of positron emission tomography in patients with suspected malignancy, inflammation, or infection is a great example of a useful test that we are still learning how best to interpret in several conditions.
In this issue of the Journal, two testing scenarios are discussed. Lacy et al address the question of when patients with pyelonephritis should receive imaging studies. There are data to guide this decision process, but as noted in the study by Sedrak et al,2 there are forces at work that challenge the clinician to bypass the rational guidelines—not the least of which are the desire for efficiency (don’t take the chance that the test may be required later and delay discharge from the hospital or observation area) and greater surety in the clinical diagnosis. Although fear of litigation was not high on Sedrak’s list of reasons for ordering more “unnecessary” tests, I posit that a decrease in the confidence placed on clinical diagnosis drives a significant amount of imaging, in conjunction with the desire for shorter hospital stays.
The second paper, by Mgbojikwe et al, relates to the issue of which advanced technology should be ordered, and when. They review the limitations of traditional (echocardiographic) diagnosis and staging of infective endocarditis, and discuss the strengths and limitations of several advanced imaging tools in the setting of suspected or known infectious endocarditis. I suspect that in most medical centers the decisions to utilize these tests will rest with the infectious disease, cardiology, and cardiothoracic surgery consultants. But it is worth being aware of how the diagnostic and staging strategies are evolving, and of the limitations to these studies.
We have come a long way from diagnosing bacterial endocarditis with a valve abscess on the basis of finding changing murmurs, a Roth spot, a palpable spleen tip, new conduction abnormalities on the ECG, and documented daily afternoon fevers. Performing that physical examination is cheap but not highly reproducible. The new testing algorithms are not cheap but, hopefully, will offer superior sensitivity and specificity. Used correctly—and we likely have a way to go to learn what that means—these pictures may well be worth the cost.
Although someone still has to suspect the diagnosis of endocarditis.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150
- Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med 2016; 11(12):869–872. doi:10.1002/jhm.2645
Osteonecrosis of the femoral head with subchondral collapse
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
A 45-year-old woman with a history of multiple organ transplants presented with a 1-month history of anterior left hip pain with insidious onset. Although she was able to perform activities of daily living, she reported increasing difficulty with weight-bearing activities.
RISK FACTORS
Osteonecrosis of the hip is caused by prolonged interruption of blood flow to the femoral head.2 While idiopathic osteonecrosis is not uncommon, the condition is often associated with alcohol abuse or, as in our patient, long-term corticosteroid use after organ transplant.3 Corticosteroid use is also the most frequently reported risk factor for multifocal osteonecrosis.
Less common risk factors include systemic lupus erythematosus, antiphospholipid antibodies, coagulopathies, sickle cell disease, Gaucher disease, trauma, and external-beam therapy.
Young age is also associated with osteonecrosis, as nearly 75% of patients are between age 30 and 60.4
APPROACH TO DIAGNOSIS
Our patient had a typical clinical presentation of this disease: she was relatively young, was on long-term corticosteroids, and had acute anterior groin pain followed by progressive functional impairment.
The diagnostic evaluation consists of a detailed history, with attention to specific risk factors, and a thorough clinical examination followed by imaging, usually with plain radiography. However, plain radiographs are often unremarkable when the condition is in the early stages. In such cases, magnetic resonance imaging is recommended if clinical suspicion for osteonecrosis is high. It is far more sensitive (> 99%) and specific (> 99%) than plain radiography, and it detects early changes in the femoral head such as focal lesions and bone marrow edema.5
TREATMENT OPTIONS
Treatment of osteonecrosis is surgical and depends on the stage of disease.6
Joint preservation may be an option for small to medium-sized lesions before subchondral collapse has occurred; options include core decompression, bone grafting, and femoral osteotomy to preserve the native femoral head. These procedures have a higher success rate in young patients.
Subchondral collapse usually warrants hip replacement.
OUR PATIENT’S TREATMENT
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
- Pappas JN. The musculoskeletal crescent sign. Radiology 2000; 217(1):213–214. doi:10.1148/radiology.217.1.r00oc22213
- Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med 2015; 8(3):201–209. doi:10.1007/s12178-015-9277-8
- Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015; 6(8):590–601. doi:10.5312/wjo.v6.i8.590
- Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum 2002; 32(2):94–124. pmid:12430099
- Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med 2015; 8(3):221–227. doi:10.1007/s12178-015-9279-6
- Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017; 99-B(10):1267–1279. doi:10.1302/0301-620X.99B10.BJJ-2017-0233.R2
Should we stop aspirin before noncardiac surgery?
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
In patients with cardiac stents, do not stop aspirin. If the risk of bleeding outweighs the benefit (eg, with intracranial procedures), an informed discussion involving the surgeon, cardiologist, and patient is critical to ascertain risks vs benefits.
In patients using aspirin for secondary prevention, the decision depends on the patient’s cardiac status and an assessment of risk vs benefit. Aspirin has no role in patients undergoing noncardiac surgery who are at low risk of a major adverse cardiac event.1,2
Aspirin used for secondary prevention reduces rates of death from vascular causes,3 but data on the magnitude of benefit in the perioperative setting are still evolving. In patients with coronary stents, continuing aspirin is beneficial,4,5 whereas stopping it is associated with an increased risk of acute stent thrombosis, which causes significant morbidity and mortality.6
SURGERY AND THROMBOTIC RISK: WHY CONSIDER ASPIRIN?
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study7 prospectively screened 15,133 patients for myocardial injury with troponin T levels daily for the first 3 consecutive postoperative days; 1,263 (8%) of the patients had a troponin elevation of 0.03 ng/mL or higher. The 30-day mortality rate in this group was 9.8%, compared with 1.1% in patients with a troponin T level of less than 0.03 ng/mL (odds ratio 10.07; 95% confidence interval [CI] 7.84–12.94; P < .001).8 The higher the peak troponin T concentration, the higher the risk of death within 30 days:
- 0.01 ng/mL or less, risk 1.0%
- 0.02 ng/mL, risk 4.0%
- 0.03 to 0.29 ng/mL, risk 9.3%
- 0.30 ng/mL or greater, risk 16.9%.7
Myocardial injury is a common postoperative vascular complication.7 Myocardial infarction (MI) or injury perioperatively increases the risk of death: 1 in 10 patients dies within 30 days after surgery.8
Surgery creates substantial physiologic stress through factors such as fasting, anesthesia, intubation, surgical trauma, extubation, and pain. It promotes coagulation9 and inflammation with activation of platelets,10 potentially leading to thrombosis.11 Coronary thrombosis secondary to plaque rupture11,12 can result in perioperative MI. Perioperative hemodynamic variability, anemia, and hypoxia can lead to demand-supply mismatch and also cause cardiac ischemia.
Aspirin is an antiplatelet agent that irreversibly inhibits platelet aggregation by blocking the formation of cyclooxygenase. It has been used for several decades as an antithrombotic agent in primary and secondary prevention. However, its benefit in primary prevention is uncertain, and the magnitude of antithrombotic benefit must be balanced against the risk of bleeding.
The Antithrombotic Trialists’ Collaboration13 performed a systematic review of 6 primary prevention trials involving 95,000 patients and found that aspirin therapy was associated with a 12% reduction in serious vascular events, which occurred in 0.51% of patients taking aspirin per year vs 0.57% of controls (P = .0001). However, aspirin also increased the risk of major bleeding, at a rate of 0.10% vs 0.07% per year (P < .0001), with 2 bleeding events for every avoided vascular event.13
WILL ASPIRIN PROTECT PATIENTS AT CARDIAC RISK?
The second Perioperative Ischemic Evaluation trial (POISE 2),1 in patients with atherosclerotic disease or at risk for it, found that giving aspirin in the perioperative period did not reduce the rate of death or nonfatal MI, but increased the risk of a major bleeding event.
The trial included 10,010 patients undergoing noncardiac surgery who were randomly assigned to receive aspirin or placebo. The aspirin arm included 2 groups: patients who were not on aspirin (initiation arm), and patients on aspirin at the time of randomization (continuation arm).
Death or nonfatal MI (the primary outcome) occurred in 7.0% of patients on aspirin vs 7.1% of patients receiving placebo (hazard ratio [HR] 0.99, 95% CI 0.86–1.15, P = .92). The risk of major bleeding was 4.6% in the aspirin group vs 3.8% in the placebo group (HR 1.23, 95% CI 1.01–1.49, P = .04).1
George et al,14 in a prospective observational study in a single tertiary care center, found that fewer patients with myocardial injury in noncardiac surgery died if they took aspirin or clopidogrel postoperatively. Conversely, lack of antithrombotic therapy was an independent predictor of death (P < .001). The mortality rate in patients with myocardial injury who were on antithrombotic therapy postoperatively was 6.7%, compared with 12.1% in those without postoperative antithrombotic therapy (estimated number needed to treat, 19).14
PATIENTS WITH CORONARY STENTS UNDERGOING NONCARDIAC SURGERY
Percutaneous coronary intervention (PCI) accounts for 3.6% of all operating-room procedures in the United States,15 and 20% to 35% of patients who undergo PCI undergo noncardiac surgery within 2 years of stent implantation.16,17
Antiplatelet therapy is discontinued in about 20% of patients with previous PCI who undergo noncardiac surgery.18
Observational data have shown that stopping antiplatelet therapy in patients with previous PCI with stent placement who undergo noncardiac surgery is the single most important predictor of stent thrombosis and death.19–21 The risk increases if the interval between stent implantation and surgery is shorter, especially within 180 days.16,17 Patients who have stent thrombosis are at significantly higher risk of death.
Graham et al4 conducted a subgroup analysis of the POISE 2 trial comparing aspirin and placebo in 470 patients who had undergone PCI (427 had stent placement, and the rest had angioplasty or an unspecified type of PCI); 234 patients received aspirin and 236 placebo. The median time from stent implantation to surgery was 5.3 years.
Of the patients in the aspirin arm, 14 (6%) had the primary outcome of death or nonfatal MI compared with 27 patients (11.5%) in the placebo arm (absolute risk reduction 5.5%, 95% CI 0.4%–10.5%). The result, which differed from that in the primary trial,1 was due to reduction in MI in the PCI subgroup on aspirin. PCI patients who were on aspirin did not have increased bleeding risk. This subgroup analysis, albeit small and limited, suggests that continuing low-dose aspirin in patients with previous PCI, irrespective of the type of stent or the time from stent implantations, minimizes the risk of perioperative MI.
GUIDELINES AND RECOMMENDATIONS
Routine perioperative use of aspirin increases the risk of bleeding without a reduction in ischemic events.1 Patients with prior PCI are at increased risk of acute stent thrombosis when antiplatelet medications are discontinued.20,21 Available data, although limited, support continuing low-dose aspirin without interruption in the perioperative period in PCI patients,4 as do the guidelines from the American College of Cardiology.5
We propose a management algorithm for patients undergoing noncardiac surgery on antiplatelet therapy that takes into consideration whether the surgery is urgent, elective, or time-sensitive (Figure 1). It is imperative to involve the cardiologist, surgeon, anesthesiologist, and the patient in the decision-making process.
In the perioperative setting for patients undergoing noncardiac surgery:
- Discontinue aspirin in patients without coronary heart disease, as bleeding risk outweighs benefit.
- Consider aspirin in patients at high risk for a major adverse cardiac event if benefits outweigh risk.
- Continue low-dose aspirin without interruption in patients with a coronary stent, irrespective of the type of stent.
- If a patient has had PCI with stent placement but is not currently on aspirin, talk with the patient and the treating cardiologist to find out why, and initiate aspirin if no contraindications exist.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–e137. doi:10.1016/j.jacc.2014.07.944
- Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994; 308(6921):81–106. pmid:8298418
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
- Albaladejo P, Marret E, Samama CM, et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011; 97(19):1566–1572. doi:10.1136/hrt.2011.224519
- Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307(21):2295–2304. doi:10.1001/jama.2012.5502
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Gorka J, Polok K, Iwaniec T, et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br J Anaesth 2017; 118(5):713–719. doi:10.1093/bja/aex081
- Rajagopalan S, Ford I, Bachoo P, et al. Platelet activation, myocardial ischemic events and postoperative non-response to aspirin in patients undergoing major vascular surgery. J Thromb Haemost 2007; 5(10):2028–2035. doi:10.1111/j.1538-7836.2007.02694.x
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93(1):9–20. doi:10.1093/bja/aeh147
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173(6):627–634. doi:10.1503/cmaj.050011
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–1860. doi:10.1016/S0140-6736(09)60503-1
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Weiss AJ, Elixhauser A, Andrews RM; Healthcare Cost and Utilization Project (HCUP). Characteristics of operating room procedures in US hospitals, 2011: statistical brief #170. https://hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.jsp. Accessed May 3, 2019.
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310(14):1462–1472. doi:10.1001/jama.2013.278787
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126(11):1355–1362. doi:10.1161/CIRCULATIONAHA.112.102715
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011; 107(2):186–194. doi:10.1016/j.amjcard.2010.08.067
- Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation 2009; 119(12):1634–1642. doi:10.1161/CIRCULATIONAHA.108.813667
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293(17):2126–2130. doi:10.1001/jama.293.17.2126
- Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 2006; 98(3):352–356. doi:10.1016/j.amjcard.2006.02.039
An unusual cause of bruising
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
When does acute pyelonephritis require imaging?
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
A previously healthy 44-year-old woman presents to the emergency department with 1 day of fever, flank pain, dysuria, and persistent nausea and vomiting. Her temperature is 38.7°C (101.7°F), heart rate 102 beats per minute, and blood pressure 120/70 mm Hg. She has costovertebral angle tenderness. Laboratory testing reveals mild leukocytosis and a normal serum creatinine level; urinalysis shows leukocytes, as well as leukocyte esterase and nitrites. She has no personal or family history of nephrolithiasis. Urine cultures are obtained, and she is started on intravenous antibiotics and intravenous hydration to treat pyelonephritis.
Is imaging indicated at this point? And if so, which study is recommended?
KEY FEATURES
Acute pyelonephritis, infection of the renal parenchyma and collecting system, most often results from an ascending infection of the lower urinary tract. It is estimated to account for 250,000 office visits and 200,000 hospital admissions each year in the United States.1
Lower urinary tract symptoms such as urinary frequency, urgency, and dysuria accompanied by fever, nausea, vomiting, and flank pain raise suspicion for acute pyelonephritis. Flank pain is a key, nearly universal feature of upper urinary tract infection in patients without diabetes, though it may be absent in up to 50% of patients with diabetes.2
Additional findings include costovertebral angle tenderness on physical examination and leukocytosis, pyuria, and bacteriuria on laboratory studies.
PREDICTING THE NEED FOR EARLY IMAGING
Though guidelines state that imaging is inappropriate in most patients with pyelonephritis,2–4 it is nevertheless often done for diagnosis or identification of complications, which have been reported in more than two-thirds of patients.2–4
Acute pyelonephritis is generally classified as complicated or uncomplicated, though different definitions exist with regard to these classifications. The American College of Radiology’s Appropriateness Criteria2 consider patients with diabetes, immune compromise, a history of urolithiasis, or anatomic abnormality to be at highest risk for complications, and therefore recommend early imaging to assess for hydronephrosis, pyonephrosis, emphysematous pyelonephritis, and intrinsic or perinephric abscess.2
A clinical rule for predicting the need for imaging in acute pyelonephritis was developed and validated in an emergency department population in the Netherlands.3 The study suggested that restricting early imaging to patients with a history of urolithiasis, a urine pH of 7.0 or higher, or renal insufficiency—defined as a glomerular filtration rate (GFR) of 40 mL/min/1.73m2 or lower as estimated by the Modification of Diet in Renal Disease formula—would provide a negative predictive value of 94% to 100% for detection of an urgent urologic disorder (pyonephrosis, renal abscess, or urolithiasis). This high negative predictive value highlights that an absence of these signs and symptoms can safely identify patients who do not need renal imaging.
The positive predictive value was less useful, as only 5% to 23% of patients who had at least 1 risk factor went on to have urgent urologic risk factors.3
Implementation of this prediction rule would have resulted in a relative reduction in imaging of 40% and an absolute reduction of 28%. Of note, use of reduced GFR in this prediction rule is not clearly validated for patients with chronic kidney disease, as the previous GFR for most patients in this study was unknown.3
Based on these data, initial imaging is recommended in patients with diabetes, immune compromise, a history of urolithiasis, anatomic abnormality, a urine pH 7.0 or higher, or a GFR 40 mL/min or lower in a patient with no history of significant renal dysfunction. Early imaging would also be reasonable in patients with a complex clinical presentation, early recurrence of symptoms after treatment, clinical decompensation, or critical illness.
TREATMENT FAILURE
In a retrospective review of 62 patients hospitalized for acute renal infection, Soulen et al5 found that the most reliable indicator of complicated acute pyelonephritis was the persistence of fever and leukocytosis at 72 hours. And another small prospective study of patients with uncomplicated pyelonephritis reported a time to defervescence of no more than 4 days.6
In accordance with the Appropriateness Criteria2 and based on the best available evidence, imaging is recommended in all patients who remain febrile or have persistent leukocytosis after 72 hours of antibiotic therapy. In such cases, there should be high suspicion for a complication requiring treatment.
OPTIONS FOR IMAGING
Computed tomography
Computed tomography (CT) of the abdomen and pelvis with contrast is considered the study of choice in complicated acute pyelonephritis. CT can detect focal parenchymal abnormalities, emphysematous changes, and anatomic anomalies, and can also define the extent of disease. It can also detect perinephric fluid collections and abscesses that necessitate a change in management.2,5
A retrospective study in 2017 found that contrast-enhanced CT done without the usual noncontrast and excretory phases had an accuracy of 90% to 92% for pyelonephritis and 96% to 99% for urolithiasis, suggesting that reduction in radiation exposure through use of only the contrast-enhanced phase of CT imaging may be reasonable.7
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is increasingly acknowledged as effective in the evaluation of renal pathology, including the diagnosis of pyelonephritis; but it lacks the level of evidence that CT provides for detecting renal abscesses, calculi, and emphysematous pyelonephritis.2,8,9
Though it is more costly and time-consuming than CT with contrast enhancement, MRI is nevertheless the imaging study of choice if iodinated contrast or ionizing radiation must be avoided.
MRI typically involves a precontrast phase and a gadolinium contrast-enhanced phase, though there are data to support diffusion-weighted MRI when exposure to gadolinium poses a risk to the patient, such as in pregnancy or renal impairment (particularly when the estimated GFR is < 30 mL/min/1.73 m2).10
Ultrasonography
Conventional ultrasonography is appealing due to its relatively low cost, its availability and portability, and the lack of radiation and contrast exposure. It is most helpful in detecting hydronephrosis and pyonephrosis rather than intrarenal or perinephric abscess.2,9
Color and power Doppler ultrasonography may improve testing characteristics but not to the level of CT; in one study, sensitivity for detection of pyelonephritis was 33.3% with ultrasonography vs 81.0% with CT.11
Recent studies of ultrasonography with contrast enhancement show promising results,2 and it may ultimately prove to have a similar efficacy with lower risk for patients, but this has not been validated in large studies, and its availability remains limited.
Ultrasonography should be considered for patients in whom obstruction (with resulting hydronephrosis or pyonephrosis) is a primary concern, particularly when contrast exposure or radiation is contraindicated and MRI is unavailable.2
Abdominal radiography
While emphysematous pyelonephritis or a large staghorn calculus may be seen on abdominal radiography, it is not recommended for the assessment of complications in acute pyelonephritis because it lacks sensitivity.2
RETURN TO THE CASE SCENARIO
The patient in our case scenario meets the clinical criteria for uncomplicated pyelonephritis and is therefore not a candidate for imaging. Intravenous antibiotics should be started and should lead to rapid improvement in her condition.
Acknowledgment: The authors would like to thank Dr. Lisa Blacklock for her review of the radiology section of this paper.
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
- Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol 2003; 13(2):144–150. pmid:12559674
- Expert Panel on Urologic Imaging: Nikolaidis P, Dogra VS, Goldfarb S, et al. ACR appropriateness criteria acute pyelonephritis. J Am Coll Radiol 2018; 15(11S):S232–S239. doi:10.1016/j.jacr.2018.09.011
- van Nieuwkoop C, Hoppe BP, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis 2010; 51(11):1266–1272. doi:10.1086/657071
- Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49(1):22–30. doi:10.3947/ic.2017.49.1.22
- Soulen MC, Fishman EK, Goldman SM, Gatewood OM. Bacterial renal infection: role of CT. Radiology 1989; 171(3):703–707. doi:10.1148/radiology.171.3.2655002
- June CH, Browning MD, Smith LP, et al. Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 1985; 145(5):841–845. pmid:3888134
- Taniguchi LS, Torres US, Souza SM, Torres LR, D’Ippolito G. Are the unenhanced and excretory CT phases necessary for the evaluation of acute pyelonephritis? Acta Radiol 2017; 58(5):634–640. doi:10.1177/0284185116665424
- Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 2015; 56(2):244–249. doi:10.1177/0284185114520862
- Stunell H, Buckley O, Feeney J, Geoghegan T, Browne RF, Torreggiani WC. Imaging of acute pyelonephritis in the adult. Eur Radiol 2007; 17(7):1820–1828.
- American College of Radiology. ACR Manual on Contrast Media. www.acr.org/clinical-resources/contrast-manual. Accessed June 19, 2019.
- Yoo JM, Koh JS, Han CH, et al. Diagnosing acute pyelonephritis with CT, Tc-DMSA SPECT, and Doppler ultrasound: a comparative study. Korean J Urol 2010; 51(4):260–265. doi:10.4111/kju.2010.51.4.260
Primary care: Practice meets technology
Technology has infiltrated all parts of our everyday lives, including healthcare. Patients can make and cancel appointments, send e-mails directly to their physician, and request prescription refills—all through electronic portals. Physicians and healthcare providers must adjust to these changes in care-delivery models. Primary care providers must also adapt as younger generations seek access for their health needs outside of the doctor’s office.
And so it is with everyday life. Online banking and bill-paying is common. Groceries can be bought online and delivered within an hour. Connecting with family or friends around the world can be done with the touch of a button. In the United States, 90% of adults own a cell phone; many do not have a land line. More than 65% of adult Americans under age 75 own a smart phone, and 50% of the public owns a tablet computer.1
DRIVERS OF CHANGE: THE MILLENNIALS
The development and use of new technology is driven by the coming of age of the youngest adult population, ie, “Generation Y” or millennials, ie, persons born between 1981 and 1996.2 They now account for 28% of the US adult population, surpassing the baby boomers (born 1946 to 1964) by 8 million.3
Millennials have grown up with the World Wide Web at their fingertips. They are accustomed to an environment full of choices and unlimited, instantly available information.4
Millennials are cost-conscious shoppers who desire convenience and quick access. As patients, they often forgo traditional doctor’s office visits, turning instead to the Internet for quick answers to their questions in blogs and websites.5 A Kaiser Family Foundation survey in 2018 indicated that only a quarter of millennials see a primary care physician for healthcare needs.6
The shortage of primary care physicians
There are several reasons for this. Primary care physicians are in short supply, more Americans have insurance after the passage of the Affordable Care Act, and more physicians are working part-time or retiring earlier than in previous generations. There will be a continued shortfall of 15,000 to 49,000 full-time-equivalent primary care physicians by 2030.7 A survey of 15 large metropolitan markets found that the average wait time for a primary care new patient appointment increased to 24.1 days—a 30% increase from 2014. In some cities, the wait time can be 3 to 4 months.8
Older patients of the baby-boomer generation tend to discuss medical issues with their primary care physician, often relying on their feedback to improve their health lifestyle choices.9 Baby boomers who are Medicare subscribers tend to see their regular doctor at least once or twice a year10; trust is built with this continuity in care.
The rise of pharmacy clinics
But the shortage of primary care physicians and the desire of younger patients for immediate access to care have fueled the growth of new options for access, such as retail clinics in large pharmacies. These clinics are mostly found in the South and Midwest and are staffed by nurse practitioners,11 and 90% of their billing falls under 10 common diagnoses, including urinary tract and upper respiratory infections. More than 40% of patients seeking care at retail pharmacy clinics are 18 to 44 years of age, and less than 25% of this group have a primary care provider.11 These clinics have shorter wait times and limited out-of-pocket costs, and they are more convenient. In a study of adults visiting these clinics for vaccination, 30% did so during evening, weekend, and holiday hours, when traditional doctors’ offices are closed.12
Telemedicine’s foothold
Telemedicine has also taken a foothold in healthcare. Initially used for episodic illnesses, there is now growing acceptance of telemedicine for management of chronic physical and mental health problems. Accessibility to a doctor via a mobile device while at home has proven to be helpful to young, elderly, and minority patients living in rural areas,13 although reimbursement and legal issues continue to constrain its growth.14 Telemedicine is predicted to grow by nearly 15% from now to 2025, especially in North America and Europe, where technology has kept pace and government initiatives are encouraging its advancement.15
The American College of Physicians has published recommendations on how best to use telemedicine, especially when there is already an established patient-physician relationship. Telemedicine can bridge the divide for those who lack access to care because of geographic constraints or who cannot afford a regular doctor’s office appointment.16 It can also allow healthcare “extenders” like social workers, nutritionists, pharmacists, and nurses to work collaboratively with the primary care physician to improve patient education and outcomes.17
Wearable devices
The wearable device market continues to expand, in large part due to the increased availability and utilization of mobile technology. These gadgets can record steps, sleep, and heart rate. Consumer fitness trackers can give patients insight into their activity levels and encourage them to modify their behavior, ie, get up and move around more.17 The Deloitte Center for Health Solutions survey in 2018 showed that 62% of millennials use consumer fitness trackers to help meet their wellness goals, compared with 16% of seniors and 25% of baby boomers.18 There are few studies showing that these devices improve overall health promotion or decrease healthcare costs,17,19 but research is ongoing.
And the “generation gap” in technology’s uptake is slowly closing: 81% of American adults own a smartphone, and the rate in people over age 50 increased from 53% in 2015 to 67% in 2018.20 By comparison, 92% of millennials own a smartphone.1
Smartphone apps
A 2015 survey of more than 1,600 US adults found that 58% had downloaded an application to their smartphone to track their health needs, with 41% using more than 5 health-related apps; the most commonly downloaded apps tracked physical activity, food intake, exercise programs or weight loss progress.21
Users of mobile health apps are generally younger and more highly educated than nonusers.22 However, baby boomers are willing to try mobile health apps if the apps are intuitive, accessible, and effective; this is important, especially since this group accounts for more than 20% of US healthcare expenditures.23 Engaging and empowering baby boomers to use this technology may allow them to remain independent, live healthier, and avoid unnecessary office visits, thus decreasing strain on the limited healthcare workforce.23
ADAPTING TO THE GENERATIONAL SHIFT
Physicians and physician educators should be aware of this generational shift. Millennial-aged doctors will continue to embrace technology to achieve their work-life balance in order to avoid burnout and maintain robust primary care practices whether in the office or outside of it.
Medical school curricula
Medical schools need to adjust their curricula to prepare the next generation of physicians to engage with these new healthcare delivery models and technology. Practicing telemedicine, assessing mobile app safety and utility, and effectively integrating data from patient-specific devices represent a new skill set that is considerably different from the typical face-to-face encounters learners experience today.
Recognizing this, more than 50% of medical schools have added telemedicine and digital health to the curriculum,24 with suggestions to include telemedicine-related content in the Accreditation Council for Graduate Medical Education core competencies.25
Improving the electronic medical record
Maximizing the efficiency of electronic medical records will also be important because physicians currently spend more than 50% of their workday on documentation and administrative tasks; for every 1 hour of patient contact, physicians spend 2 hours in front of the electronic medical record.26 End-users (doctors, nurses, pharmacists, scribes) should interact or engage with developers of electronic medical record systems to promote platforms that enhance workflow, increase connectivity to mobile apps, foster team collaboration, and provide consistency in patient safety and privacy.27
Early and continuous education on use of the electronic medical record should be routine, as proficiency improves work-life balance, physician job satisfaction, and patient care by reducing after-hours note completion and in-box tasks leading to burnout.28
Technology-enabled primary care
Technology-enabled healthcare is here to stay and will continue to evolve, incorporating telehealth, smartphones, mobile apps, in-home and wearable devices, and online video communication.17 Clinicians will need to be adept at working with these technologies to advance quality care in population health. It will require clinician training and professional development, advances in technology, and revised reimbursement policies.17 But despite the increased use of mobile apps, there remain concerns about the possible dangers associated with their use, including breaches in confidentiality, conflicts of interest, and lack of professional medical involvement and evidence in their design.29
THE IMPORTANCE OF BEING SAVVY
There is a growing need for primary care providers to be technologically savvy and readily accessible via e-mail, healthcare portals, or in the office to keep up with the generational shifts and expectations occurring in this decade. Healthcare systems should have the right infrastructure in place, including efficient Web platforms to support telemedicine or to synchronize digital tracking devices, as well as a trained workforce to understand and implement these revolutionary changes into everyday practice. Educators will need to provide training in these changing platforms to medical students and residents. Primary care will evolve to redefine its role within the context of these emerging technologies17 and to adjust to these market demands in order to stay relevant.
- Jiang J. Millennials stand out for their technology use, but older generations also embrace digital life. Pew Research Center. www.pewresearch.org/fact-tank/2018/05/02/millennials-stand-out-for-their-technology-use-but-older-generations-also-embrace-digital-life. Accessed April 2, 2019.
- Dimock, M. Defining generations: Where Millennials end and post-Millennials begin. Pew Research Center. www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins. Accessed April 2, 2019.
- The Generation Gap in American Politics. Pew Research Center. www.people-press.org/2018/03/01/the-generation-gap-in-american-politics. Accessed April 2, 2019.
- Hopkins L, Hampton BS, Abbott, JF, et al. To the point: medical education, technology and the millennial learner. Am J Obstet Gynecol 2018; 218(2):188–192. doi:10.1016/j.ajog.2017.06.001
- DuPuis R. Courting the impatient patient: providers must embrace millennial’s health care expectations. Central Penn Business Journal. www.cpbj.com/courting-the-impatient-patient-providers-must-embrace-millennials-health-care-expectations. Accessed April 2, 2019.
- Boodman SG. Spurred by convenience, Millennials often spurn the “family doctor” model. Kaiser Health News. khn.org/news/spurred-by-convenience-millennials-often-spurn-the-family-doctor-model. Accessed April 2, 2019.
- Association of American Medical Colleges. 2018 update: the complexities of physician supply and demand: projections from 2016 to 2030. aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Accessed April 2, 2019.
- Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/uploadedFiles/MerrittHawkins/Content/Pdf/mha2017waittimesurveyPDF.pdf. Accessed April 2, 2019.
- SSRN. Employee Benefit Research Institute. Consumer engagement in health care among Millennials, Baby Boomers, and Generation X: findings from the 2017 Consumer Engagement in Health Care Survey. papers.ssrn.com/sol3/papers.cfm?abstract_id=3160059. Accessed April 2, 2019.
- Centers for Disease Control and Prevention (CDC). Summary health statistics: national health interview survey, 2016, Table A–18c. ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-18.pdf. Accessed April 2, 2019.
- Rand Corporation. The evolving role of retail clinics. www.rand.org/content/dam/rand/pubs/research_briefs/RB9400/RB9491-2/RAND_RB9491-2.pdf. Accessed April 2, 2019.
- Goad JA, Taitel MS, Fensterheim LE, Cannon, AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med 2013; 11(5):429–436. doi:10.1370/afm.1542
- Hansen MR, Okuda DT. Multiple sclerosis in the contemporary age: understanding the Millennial patient with multiple sclerosis to create next-generation care, Neurol Clin 2018; 36(1):219–230. doi:10.1016/j.ncl.2017.08.012
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016; 375(2):154–161. doi:10.1056/NEJMra1601705
- Landi, H. Report: telehealth market estimated to reach $19.5B by 2025. Healthcare Informatics. www.healthcare-informatics.com/news-item/telemedicine/report-telehealth-market-estimated-reach-195b-2025. Accessed April 2, 2019.
- Daniel H, Sulmasy LS; Health and Public Policy Committee of the American College of Physicians. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med 2015; 163(10):787–789. doi:10.7326/M15-0498
- Young HM, Nesbitt TS. Increasing the capacity of primary care through enabling technology. J Gen Intern Med 2017; 32(4):398–403. doi:10.1007/s11606-016-3952-3
- Abrams K, Korba C. Consumers are on board with virtual health options. Deloitte Insights, www2.deloitte.com/insights/us/en/industry/health-care/virtual-health-care-consumer-experience-survey.html. Accessed April 2, 2019.
- Coughlin SS, Stewart J. Use of consumer wearable devices to promote physical activity: a review of health intervention studies. J Environ Health Sci 2016; 2(6). doi:10.15436/2378-6841.16.1123
- Taylor K, Silver L. Smartphone ownership is growing rapidly around the world but not always equally. Pew Research Center. www.pewglobal.org/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally. Accessed April 2, 2019.
- Krebs P, Duncan DT. Health app use among us mobile phone owners: a national survey. JMIR Mhealth Uhealth 2015; 3(4):e101. doi:10.2196/mhealth.4924
- Carroll JK, Moorhead A, Bond R, LeBlanc WG, Petrella RJ, Fiscella K. Who uses mobile health apps and does use matter? A secondary data analytics approach. J Med Internet Res 2017; 19(4):e125. doi:10.2196/jmir.5604
- Kruse CS, Mileski M, Moreno J. Mobile health solutions for the aging population: a systematic narrative analysis. J Telemed Telecare 2017; 23(4):439–451. doi:10.1177/1357633X16649790
- Warshaw R. From bedside to webside: future doctors learn to practice remotely. AAMC News. news.aamc.org/medical-education/article/future-doctors-learn-practice-remotely. Accessed April 2, 2019.
- DeJong C, Lucey CR, Dudley RA. Incorporating a new technology while doing no harm, virtually. JAMA 2015; 314(22):2351–2352. doi:10.1001/jama.2015.13572
- Sinsky C, Colligan L, Li L, Prgomet M, Reynolds S, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- Kim MS. Improving electronic health records training through usability evaluation in primary care. J Health and Med Informat 2013; 4(5) e110. doi:10.4172/2157-7420.1000e110
- Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the i3 population collaborative. J Grad Med Educ 2017; 9(4):479–484. doi:10.4300/JGME-D-16-00123.1
- Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid Based Med 2013; 18(3):90–92. doi:10.1136/eb-2012-100885
Technology has infiltrated all parts of our everyday lives, including healthcare. Patients can make and cancel appointments, send e-mails directly to their physician, and request prescription refills—all through electronic portals. Physicians and healthcare providers must adjust to these changes in care-delivery models. Primary care providers must also adapt as younger generations seek access for their health needs outside of the doctor’s office.
And so it is with everyday life. Online banking and bill-paying is common. Groceries can be bought online and delivered within an hour. Connecting with family or friends around the world can be done with the touch of a button. In the United States, 90% of adults own a cell phone; many do not have a land line. More than 65% of adult Americans under age 75 own a smart phone, and 50% of the public owns a tablet computer.1
DRIVERS OF CHANGE: THE MILLENNIALS
The development and use of new technology is driven by the coming of age of the youngest adult population, ie, “Generation Y” or millennials, ie, persons born between 1981 and 1996.2 They now account for 28% of the US adult population, surpassing the baby boomers (born 1946 to 1964) by 8 million.3
Millennials have grown up with the World Wide Web at their fingertips. They are accustomed to an environment full of choices and unlimited, instantly available information.4
Millennials are cost-conscious shoppers who desire convenience and quick access. As patients, they often forgo traditional doctor’s office visits, turning instead to the Internet for quick answers to their questions in blogs and websites.5 A Kaiser Family Foundation survey in 2018 indicated that only a quarter of millennials see a primary care physician for healthcare needs.6
The shortage of primary care physicians
There are several reasons for this. Primary care physicians are in short supply, more Americans have insurance after the passage of the Affordable Care Act, and more physicians are working part-time or retiring earlier than in previous generations. There will be a continued shortfall of 15,000 to 49,000 full-time-equivalent primary care physicians by 2030.7 A survey of 15 large metropolitan markets found that the average wait time for a primary care new patient appointment increased to 24.1 days—a 30% increase from 2014. In some cities, the wait time can be 3 to 4 months.8
Older patients of the baby-boomer generation tend to discuss medical issues with their primary care physician, often relying on their feedback to improve their health lifestyle choices.9 Baby boomers who are Medicare subscribers tend to see their regular doctor at least once or twice a year10; trust is built with this continuity in care.
The rise of pharmacy clinics
But the shortage of primary care physicians and the desire of younger patients for immediate access to care have fueled the growth of new options for access, such as retail clinics in large pharmacies. These clinics are mostly found in the South and Midwest and are staffed by nurse practitioners,11 and 90% of their billing falls under 10 common diagnoses, including urinary tract and upper respiratory infections. More than 40% of patients seeking care at retail pharmacy clinics are 18 to 44 years of age, and less than 25% of this group have a primary care provider.11 These clinics have shorter wait times and limited out-of-pocket costs, and they are more convenient. In a study of adults visiting these clinics for vaccination, 30% did so during evening, weekend, and holiday hours, when traditional doctors’ offices are closed.12
Telemedicine’s foothold
Telemedicine has also taken a foothold in healthcare. Initially used for episodic illnesses, there is now growing acceptance of telemedicine for management of chronic physical and mental health problems. Accessibility to a doctor via a mobile device while at home has proven to be helpful to young, elderly, and minority patients living in rural areas,13 although reimbursement and legal issues continue to constrain its growth.14 Telemedicine is predicted to grow by nearly 15% from now to 2025, especially in North America and Europe, where technology has kept pace and government initiatives are encouraging its advancement.15
The American College of Physicians has published recommendations on how best to use telemedicine, especially when there is already an established patient-physician relationship. Telemedicine can bridge the divide for those who lack access to care because of geographic constraints or who cannot afford a regular doctor’s office appointment.16 It can also allow healthcare “extenders” like social workers, nutritionists, pharmacists, and nurses to work collaboratively with the primary care physician to improve patient education and outcomes.17
Wearable devices
The wearable device market continues to expand, in large part due to the increased availability and utilization of mobile technology. These gadgets can record steps, sleep, and heart rate. Consumer fitness trackers can give patients insight into their activity levels and encourage them to modify their behavior, ie, get up and move around more.17 The Deloitte Center for Health Solutions survey in 2018 showed that 62% of millennials use consumer fitness trackers to help meet their wellness goals, compared with 16% of seniors and 25% of baby boomers.18 There are few studies showing that these devices improve overall health promotion or decrease healthcare costs,17,19 but research is ongoing.
And the “generation gap” in technology’s uptake is slowly closing: 81% of American adults own a smartphone, and the rate in people over age 50 increased from 53% in 2015 to 67% in 2018.20 By comparison, 92% of millennials own a smartphone.1
Smartphone apps
A 2015 survey of more than 1,600 US adults found that 58% had downloaded an application to their smartphone to track their health needs, with 41% using more than 5 health-related apps; the most commonly downloaded apps tracked physical activity, food intake, exercise programs or weight loss progress.21
Users of mobile health apps are generally younger and more highly educated than nonusers.22 However, baby boomers are willing to try mobile health apps if the apps are intuitive, accessible, and effective; this is important, especially since this group accounts for more than 20% of US healthcare expenditures.23 Engaging and empowering baby boomers to use this technology may allow them to remain independent, live healthier, and avoid unnecessary office visits, thus decreasing strain on the limited healthcare workforce.23
ADAPTING TO THE GENERATIONAL SHIFT
Physicians and physician educators should be aware of this generational shift. Millennial-aged doctors will continue to embrace technology to achieve their work-life balance in order to avoid burnout and maintain robust primary care practices whether in the office or outside of it.
Medical school curricula
Medical schools need to adjust their curricula to prepare the next generation of physicians to engage with these new healthcare delivery models and technology. Practicing telemedicine, assessing mobile app safety and utility, and effectively integrating data from patient-specific devices represent a new skill set that is considerably different from the typical face-to-face encounters learners experience today.
Recognizing this, more than 50% of medical schools have added telemedicine and digital health to the curriculum,24 with suggestions to include telemedicine-related content in the Accreditation Council for Graduate Medical Education core competencies.25
Improving the electronic medical record
Maximizing the efficiency of electronic medical records will also be important because physicians currently spend more than 50% of their workday on documentation and administrative tasks; for every 1 hour of patient contact, physicians spend 2 hours in front of the electronic medical record.26 End-users (doctors, nurses, pharmacists, scribes) should interact or engage with developers of electronic medical record systems to promote platforms that enhance workflow, increase connectivity to mobile apps, foster team collaboration, and provide consistency in patient safety and privacy.27
Early and continuous education on use of the electronic medical record should be routine, as proficiency improves work-life balance, physician job satisfaction, and patient care by reducing after-hours note completion and in-box tasks leading to burnout.28
Technology-enabled primary care
Technology-enabled healthcare is here to stay and will continue to evolve, incorporating telehealth, smartphones, mobile apps, in-home and wearable devices, and online video communication.17 Clinicians will need to be adept at working with these technologies to advance quality care in population health. It will require clinician training and professional development, advances in technology, and revised reimbursement policies.17 But despite the increased use of mobile apps, there remain concerns about the possible dangers associated with their use, including breaches in confidentiality, conflicts of interest, and lack of professional medical involvement and evidence in their design.29
THE IMPORTANCE OF BEING SAVVY
There is a growing need for primary care providers to be technologically savvy and readily accessible via e-mail, healthcare portals, or in the office to keep up with the generational shifts and expectations occurring in this decade. Healthcare systems should have the right infrastructure in place, including efficient Web platforms to support telemedicine or to synchronize digital tracking devices, as well as a trained workforce to understand and implement these revolutionary changes into everyday practice. Educators will need to provide training in these changing platforms to medical students and residents. Primary care will evolve to redefine its role within the context of these emerging technologies17 and to adjust to these market demands in order to stay relevant.
Technology has infiltrated all parts of our everyday lives, including healthcare. Patients can make and cancel appointments, send e-mails directly to their physician, and request prescription refills—all through electronic portals. Physicians and healthcare providers must adjust to these changes in care-delivery models. Primary care providers must also adapt as younger generations seek access for their health needs outside of the doctor’s office.
And so it is with everyday life. Online banking and bill-paying is common. Groceries can be bought online and delivered within an hour. Connecting with family or friends around the world can be done with the touch of a button. In the United States, 90% of adults own a cell phone; many do not have a land line. More than 65% of adult Americans under age 75 own a smart phone, and 50% of the public owns a tablet computer.1
DRIVERS OF CHANGE: THE MILLENNIALS
The development and use of new technology is driven by the coming of age of the youngest adult population, ie, “Generation Y” or millennials, ie, persons born between 1981 and 1996.2 They now account for 28% of the US adult population, surpassing the baby boomers (born 1946 to 1964) by 8 million.3
Millennials have grown up with the World Wide Web at their fingertips. They are accustomed to an environment full of choices and unlimited, instantly available information.4
Millennials are cost-conscious shoppers who desire convenience and quick access. As patients, they often forgo traditional doctor’s office visits, turning instead to the Internet for quick answers to their questions in blogs and websites.5 A Kaiser Family Foundation survey in 2018 indicated that only a quarter of millennials see a primary care physician for healthcare needs.6
The shortage of primary care physicians
There are several reasons for this. Primary care physicians are in short supply, more Americans have insurance after the passage of the Affordable Care Act, and more physicians are working part-time or retiring earlier than in previous generations. There will be a continued shortfall of 15,000 to 49,000 full-time-equivalent primary care physicians by 2030.7 A survey of 15 large metropolitan markets found that the average wait time for a primary care new patient appointment increased to 24.1 days—a 30% increase from 2014. In some cities, the wait time can be 3 to 4 months.8
Older patients of the baby-boomer generation tend to discuss medical issues with their primary care physician, often relying on their feedback to improve their health lifestyle choices.9 Baby boomers who are Medicare subscribers tend to see their regular doctor at least once or twice a year10; trust is built with this continuity in care.
The rise of pharmacy clinics
But the shortage of primary care physicians and the desire of younger patients for immediate access to care have fueled the growth of new options for access, such as retail clinics in large pharmacies. These clinics are mostly found in the South and Midwest and are staffed by nurse practitioners,11 and 90% of their billing falls under 10 common diagnoses, including urinary tract and upper respiratory infections. More than 40% of patients seeking care at retail pharmacy clinics are 18 to 44 years of age, and less than 25% of this group have a primary care provider.11 These clinics have shorter wait times and limited out-of-pocket costs, and they are more convenient. In a study of adults visiting these clinics for vaccination, 30% did so during evening, weekend, and holiday hours, when traditional doctors’ offices are closed.12
Telemedicine’s foothold
Telemedicine has also taken a foothold in healthcare. Initially used for episodic illnesses, there is now growing acceptance of telemedicine for management of chronic physical and mental health problems. Accessibility to a doctor via a mobile device while at home has proven to be helpful to young, elderly, and minority patients living in rural areas,13 although reimbursement and legal issues continue to constrain its growth.14 Telemedicine is predicted to grow by nearly 15% from now to 2025, especially in North America and Europe, where technology has kept pace and government initiatives are encouraging its advancement.15
The American College of Physicians has published recommendations on how best to use telemedicine, especially when there is already an established patient-physician relationship. Telemedicine can bridge the divide for those who lack access to care because of geographic constraints or who cannot afford a regular doctor’s office appointment.16 It can also allow healthcare “extenders” like social workers, nutritionists, pharmacists, and nurses to work collaboratively with the primary care physician to improve patient education and outcomes.17
Wearable devices
The wearable device market continues to expand, in large part due to the increased availability and utilization of mobile technology. These gadgets can record steps, sleep, and heart rate. Consumer fitness trackers can give patients insight into their activity levels and encourage them to modify their behavior, ie, get up and move around more.17 The Deloitte Center for Health Solutions survey in 2018 showed that 62% of millennials use consumer fitness trackers to help meet their wellness goals, compared with 16% of seniors and 25% of baby boomers.18 There are few studies showing that these devices improve overall health promotion or decrease healthcare costs,17,19 but research is ongoing.
And the “generation gap” in technology’s uptake is slowly closing: 81% of American adults own a smartphone, and the rate in people over age 50 increased from 53% in 2015 to 67% in 2018.20 By comparison, 92% of millennials own a smartphone.1
Smartphone apps
A 2015 survey of more than 1,600 US adults found that 58% had downloaded an application to their smartphone to track their health needs, with 41% using more than 5 health-related apps; the most commonly downloaded apps tracked physical activity, food intake, exercise programs or weight loss progress.21
Users of mobile health apps are generally younger and more highly educated than nonusers.22 However, baby boomers are willing to try mobile health apps if the apps are intuitive, accessible, and effective; this is important, especially since this group accounts for more than 20% of US healthcare expenditures.23 Engaging and empowering baby boomers to use this technology may allow them to remain independent, live healthier, and avoid unnecessary office visits, thus decreasing strain on the limited healthcare workforce.23
ADAPTING TO THE GENERATIONAL SHIFT
Physicians and physician educators should be aware of this generational shift. Millennial-aged doctors will continue to embrace technology to achieve their work-life balance in order to avoid burnout and maintain robust primary care practices whether in the office or outside of it.
Medical school curricula
Medical schools need to adjust their curricula to prepare the next generation of physicians to engage with these new healthcare delivery models and technology. Practicing telemedicine, assessing mobile app safety and utility, and effectively integrating data from patient-specific devices represent a new skill set that is considerably different from the typical face-to-face encounters learners experience today.
Recognizing this, more than 50% of medical schools have added telemedicine and digital health to the curriculum,24 with suggestions to include telemedicine-related content in the Accreditation Council for Graduate Medical Education core competencies.25
Improving the electronic medical record
Maximizing the efficiency of electronic medical records will also be important because physicians currently spend more than 50% of their workday on documentation and administrative tasks; for every 1 hour of patient contact, physicians spend 2 hours in front of the electronic medical record.26 End-users (doctors, nurses, pharmacists, scribes) should interact or engage with developers of electronic medical record systems to promote platforms that enhance workflow, increase connectivity to mobile apps, foster team collaboration, and provide consistency in patient safety and privacy.27
Early and continuous education on use of the electronic medical record should be routine, as proficiency improves work-life balance, physician job satisfaction, and patient care by reducing after-hours note completion and in-box tasks leading to burnout.28
Technology-enabled primary care
Technology-enabled healthcare is here to stay and will continue to evolve, incorporating telehealth, smartphones, mobile apps, in-home and wearable devices, and online video communication.17 Clinicians will need to be adept at working with these technologies to advance quality care in population health. It will require clinician training and professional development, advances in technology, and revised reimbursement policies.17 But despite the increased use of mobile apps, there remain concerns about the possible dangers associated with their use, including breaches in confidentiality, conflicts of interest, and lack of professional medical involvement and evidence in their design.29
THE IMPORTANCE OF BEING SAVVY
There is a growing need for primary care providers to be technologically savvy and readily accessible via e-mail, healthcare portals, or in the office to keep up with the generational shifts and expectations occurring in this decade. Healthcare systems should have the right infrastructure in place, including efficient Web platforms to support telemedicine or to synchronize digital tracking devices, as well as a trained workforce to understand and implement these revolutionary changes into everyday practice. Educators will need to provide training in these changing platforms to medical students and residents. Primary care will evolve to redefine its role within the context of these emerging technologies17 and to adjust to these market demands in order to stay relevant.
- Jiang J. Millennials stand out for their technology use, but older generations also embrace digital life. Pew Research Center. www.pewresearch.org/fact-tank/2018/05/02/millennials-stand-out-for-their-technology-use-but-older-generations-also-embrace-digital-life. Accessed April 2, 2019.
- Dimock, M. Defining generations: Where Millennials end and post-Millennials begin. Pew Research Center. www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins. Accessed April 2, 2019.
- The Generation Gap in American Politics. Pew Research Center. www.people-press.org/2018/03/01/the-generation-gap-in-american-politics. Accessed April 2, 2019.
- Hopkins L, Hampton BS, Abbott, JF, et al. To the point: medical education, technology and the millennial learner. Am J Obstet Gynecol 2018; 218(2):188–192. doi:10.1016/j.ajog.2017.06.001
- DuPuis R. Courting the impatient patient: providers must embrace millennial’s health care expectations. Central Penn Business Journal. www.cpbj.com/courting-the-impatient-patient-providers-must-embrace-millennials-health-care-expectations. Accessed April 2, 2019.
- Boodman SG. Spurred by convenience, Millennials often spurn the “family doctor” model. Kaiser Health News. khn.org/news/spurred-by-convenience-millennials-often-spurn-the-family-doctor-model. Accessed April 2, 2019.
- Association of American Medical Colleges. 2018 update: the complexities of physician supply and demand: projections from 2016 to 2030. aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Accessed April 2, 2019.
- Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/uploadedFiles/MerrittHawkins/Content/Pdf/mha2017waittimesurveyPDF.pdf. Accessed April 2, 2019.
- SSRN. Employee Benefit Research Institute. Consumer engagement in health care among Millennials, Baby Boomers, and Generation X: findings from the 2017 Consumer Engagement in Health Care Survey. papers.ssrn.com/sol3/papers.cfm?abstract_id=3160059. Accessed April 2, 2019.
- Centers for Disease Control and Prevention (CDC). Summary health statistics: national health interview survey, 2016, Table A–18c. ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-18.pdf. Accessed April 2, 2019.
- Rand Corporation. The evolving role of retail clinics. www.rand.org/content/dam/rand/pubs/research_briefs/RB9400/RB9491-2/RAND_RB9491-2.pdf. Accessed April 2, 2019.
- Goad JA, Taitel MS, Fensterheim LE, Cannon, AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med 2013; 11(5):429–436. doi:10.1370/afm.1542
- Hansen MR, Okuda DT. Multiple sclerosis in the contemporary age: understanding the Millennial patient with multiple sclerosis to create next-generation care, Neurol Clin 2018; 36(1):219–230. doi:10.1016/j.ncl.2017.08.012
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016; 375(2):154–161. doi:10.1056/NEJMra1601705
- Landi, H. Report: telehealth market estimated to reach $19.5B by 2025. Healthcare Informatics. www.healthcare-informatics.com/news-item/telemedicine/report-telehealth-market-estimated-reach-195b-2025. Accessed April 2, 2019.
- Daniel H, Sulmasy LS; Health and Public Policy Committee of the American College of Physicians. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med 2015; 163(10):787–789. doi:10.7326/M15-0498
- Young HM, Nesbitt TS. Increasing the capacity of primary care through enabling technology. J Gen Intern Med 2017; 32(4):398–403. doi:10.1007/s11606-016-3952-3
- Abrams K, Korba C. Consumers are on board with virtual health options. Deloitte Insights, www2.deloitte.com/insights/us/en/industry/health-care/virtual-health-care-consumer-experience-survey.html. Accessed April 2, 2019.
- Coughlin SS, Stewart J. Use of consumer wearable devices to promote physical activity: a review of health intervention studies. J Environ Health Sci 2016; 2(6). doi:10.15436/2378-6841.16.1123
- Taylor K, Silver L. Smartphone ownership is growing rapidly around the world but not always equally. Pew Research Center. www.pewglobal.org/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally. Accessed April 2, 2019.
- Krebs P, Duncan DT. Health app use among us mobile phone owners: a national survey. JMIR Mhealth Uhealth 2015; 3(4):e101. doi:10.2196/mhealth.4924
- Carroll JK, Moorhead A, Bond R, LeBlanc WG, Petrella RJ, Fiscella K. Who uses mobile health apps and does use matter? A secondary data analytics approach. J Med Internet Res 2017; 19(4):e125. doi:10.2196/jmir.5604
- Kruse CS, Mileski M, Moreno J. Mobile health solutions for the aging population: a systematic narrative analysis. J Telemed Telecare 2017; 23(4):439–451. doi:10.1177/1357633X16649790
- Warshaw R. From bedside to webside: future doctors learn to practice remotely. AAMC News. news.aamc.org/medical-education/article/future-doctors-learn-practice-remotely. Accessed April 2, 2019.
- DeJong C, Lucey CR, Dudley RA. Incorporating a new technology while doing no harm, virtually. JAMA 2015; 314(22):2351–2352. doi:10.1001/jama.2015.13572
- Sinsky C, Colligan L, Li L, Prgomet M, Reynolds S, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- Kim MS. Improving electronic health records training through usability evaluation in primary care. J Health and Med Informat 2013; 4(5) e110. doi:10.4172/2157-7420.1000e110
- Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the i3 population collaborative. J Grad Med Educ 2017; 9(4):479–484. doi:10.4300/JGME-D-16-00123.1
- Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid Based Med 2013; 18(3):90–92. doi:10.1136/eb-2012-100885
- Jiang J. Millennials stand out for their technology use, but older generations also embrace digital life. Pew Research Center. www.pewresearch.org/fact-tank/2018/05/02/millennials-stand-out-for-their-technology-use-but-older-generations-also-embrace-digital-life. Accessed April 2, 2019.
- Dimock, M. Defining generations: Where Millennials end and post-Millennials begin. Pew Research Center. www.pewresearch.org/fact-tank/2019/01/17/where-millennials-end-and-generation-z-begins. Accessed April 2, 2019.
- The Generation Gap in American Politics. Pew Research Center. www.people-press.org/2018/03/01/the-generation-gap-in-american-politics. Accessed April 2, 2019.
- Hopkins L, Hampton BS, Abbott, JF, et al. To the point: medical education, technology and the millennial learner. Am J Obstet Gynecol 2018; 218(2):188–192. doi:10.1016/j.ajog.2017.06.001
- DuPuis R. Courting the impatient patient: providers must embrace millennial’s health care expectations. Central Penn Business Journal. www.cpbj.com/courting-the-impatient-patient-providers-must-embrace-millennials-health-care-expectations. Accessed April 2, 2019.
- Boodman SG. Spurred by convenience, Millennials often spurn the “family doctor” model. Kaiser Health News. khn.org/news/spurred-by-convenience-millennials-often-spurn-the-family-doctor-model. Accessed April 2, 2019.
- Association of American Medical Colleges. 2018 update: the complexities of physician supply and demand: projections from 2016 to 2030. aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Accessed April 2, 2019.
- Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. www.merritthawkins.com/uploadedFiles/MerrittHawkins/Content/Pdf/mha2017waittimesurveyPDF.pdf. Accessed April 2, 2019.
- SSRN. Employee Benefit Research Institute. Consumer engagement in health care among Millennials, Baby Boomers, and Generation X: findings from the 2017 Consumer Engagement in Health Care Survey. papers.ssrn.com/sol3/papers.cfm?abstract_id=3160059. Accessed April 2, 2019.
- Centers for Disease Control and Prevention (CDC). Summary health statistics: national health interview survey, 2016, Table A–18c. ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-18.pdf. Accessed April 2, 2019.
- Rand Corporation. The evolving role of retail clinics. www.rand.org/content/dam/rand/pubs/research_briefs/RB9400/RB9491-2/RAND_RB9491-2.pdf. Accessed April 2, 2019.
- Goad JA, Taitel MS, Fensterheim LE, Cannon, AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med 2013; 11(5):429–436. doi:10.1370/afm.1542
- Hansen MR, Okuda DT. Multiple sclerosis in the contemporary age: understanding the Millennial patient with multiple sclerosis to create next-generation care, Neurol Clin 2018; 36(1):219–230. doi:10.1016/j.ncl.2017.08.012
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016; 375(2):154–161. doi:10.1056/NEJMra1601705
- Landi, H. Report: telehealth market estimated to reach $19.5B by 2025. Healthcare Informatics. www.healthcare-informatics.com/news-item/telemedicine/report-telehealth-market-estimated-reach-195b-2025. Accessed April 2, 2019.
- Daniel H, Sulmasy LS; Health and Public Policy Committee of the American College of Physicians. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med 2015; 163(10):787–789. doi:10.7326/M15-0498
- Young HM, Nesbitt TS. Increasing the capacity of primary care through enabling technology. J Gen Intern Med 2017; 32(4):398–403. doi:10.1007/s11606-016-3952-3
- Abrams K, Korba C. Consumers are on board with virtual health options. Deloitte Insights, www2.deloitte.com/insights/us/en/industry/health-care/virtual-health-care-consumer-experience-survey.html. Accessed April 2, 2019.
- Coughlin SS, Stewart J. Use of consumer wearable devices to promote physical activity: a review of health intervention studies. J Environ Health Sci 2016; 2(6). doi:10.15436/2378-6841.16.1123
- Taylor K, Silver L. Smartphone ownership is growing rapidly around the world but not always equally. Pew Research Center. www.pewglobal.org/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally. Accessed April 2, 2019.
- Krebs P, Duncan DT. Health app use among us mobile phone owners: a national survey. JMIR Mhealth Uhealth 2015; 3(4):e101. doi:10.2196/mhealth.4924
- Carroll JK, Moorhead A, Bond R, LeBlanc WG, Petrella RJ, Fiscella K. Who uses mobile health apps and does use matter? A secondary data analytics approach. J Med Internet Res 2017; 19(4):e125. doi:10.2196/jmir.5604
- Kruse CS, Mileski M, Moreno J. Mobile health solutions for the aging population: a systematic narrative analysis. J Telemed Telecare 2017; 23(4):439–451. doi:10.1177/1357633X16649790
- Warshaw R. From bedside to webside: future doctors learn to practice remotely. AAMC News. news.aamc.org/medical-education/article/future-doctors-learn-practice-remotely. Accessed April 2, 2019.
- DeJong C, Lucey CR, Dudley RA. Incorporating a new technology while doing no harm, virtually. JAMA 2015; 314(22):2351–2352. doi:10.1001/jama.2015.13572
- Sinsky C, Colligan L, Li L, Prgomet M, Reynolds S, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med 2016; 165(11):753–760. doi:10.7326/M16-0961
- Kim MS. Improving electronic health records training through usability evaluation in primary care. J Health and Med Informat 2013; 4(5) e110. doi:10.4172/2157-7420.1000e110
- Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the i3 population collaborative. J Grad Med Educ 2017; 9(4):479–484. doi:10.4300/JGME-D-16-00123.1
- Buijink AW, Visser BJ, Marshall L. Medical apps for smartphones: lack of evidence undermines quality and safety. Evid Based Med 2013; 18(3):90–92. doi:10.1136/eb-2012-100885