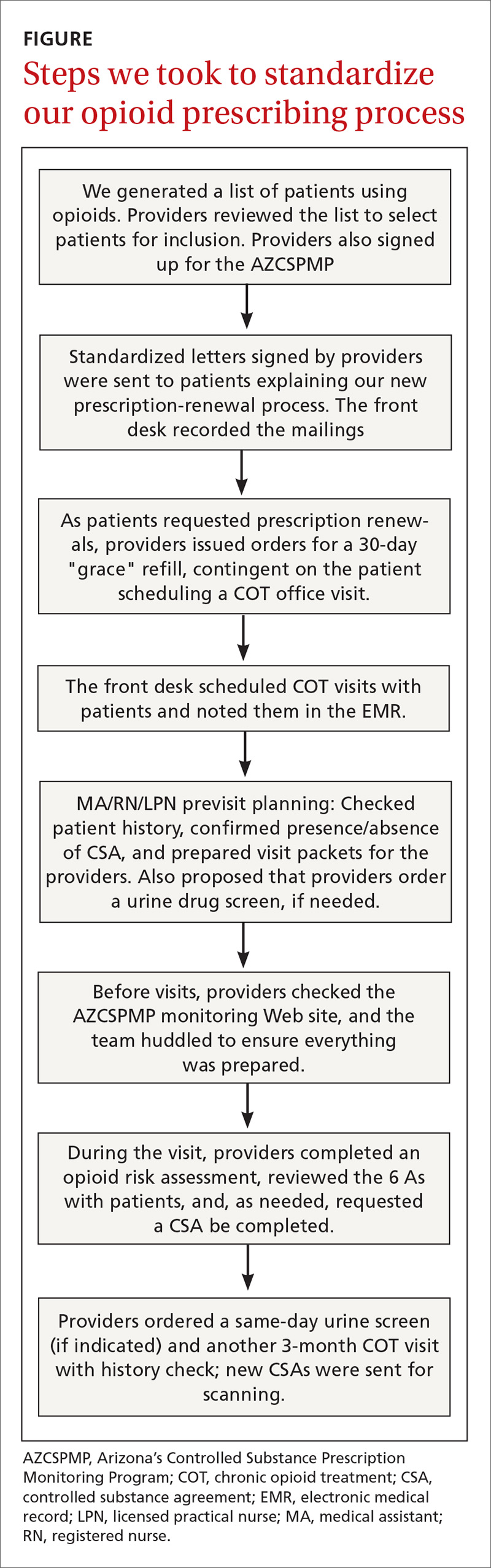
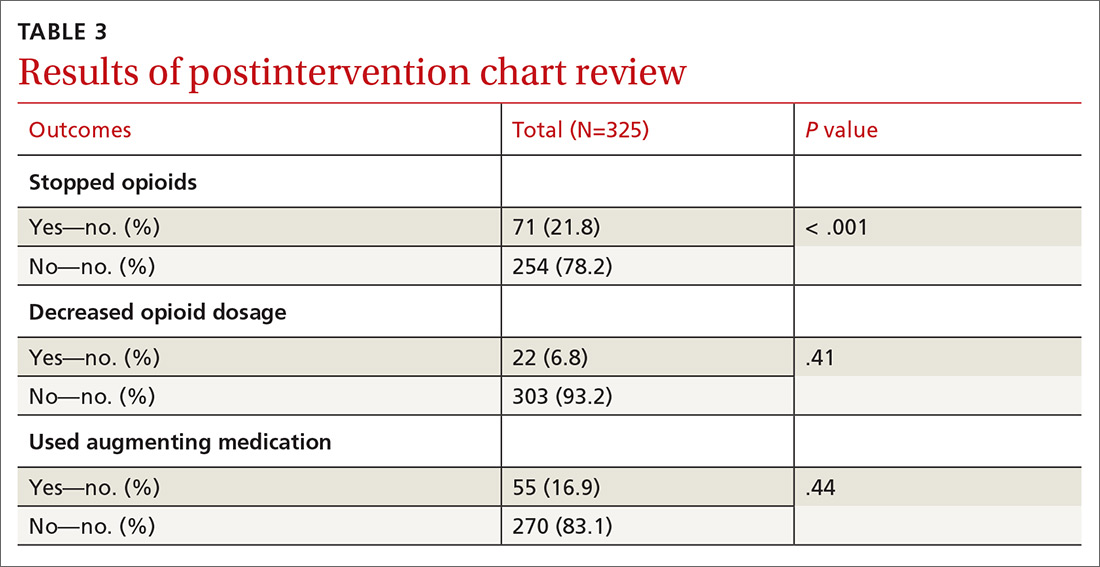
User login
A solution for reducing referrals (and malpractice suits)
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
I agree with Dr. Hickner’s editorial “To refer—or not?” (J Fam Pract. 2019;68:8) that family physicians could manage about 30% of the patients they refer to specialists. Still, it’s worth noting that many referrals are motivated by the threat of unmerited malpractice suits. Until the medical liability system becomes less adversarial and unmerited suits are eliminated, all primary care doctors—not just family physicians—will continue to send patients to specialists—even when these physicians are themselves capable of treating such patients.
What might help mitigate malpractice suits? There could be benefit from oversight of health courts, which would be presided over by judges with special training in medical malpractice. Being nonadversarial, health courts would cut down on legal wrangling, settle suits, and get awards to patients quicker. They would also cut down on attorney and court fees, which account for almost half of the total amount spent on litigation. These courts wouldn’t completely eliminate unnecessary referrals to specialists, but they could help make a difference.
Edward Volpintesta, MD
Bethel, Conn
Acute hearing loss, tinnitus, and fullness in the left ear • Weber test lateralized to the right ear • Positive Rinne test and normal tympanometry • Dx?
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
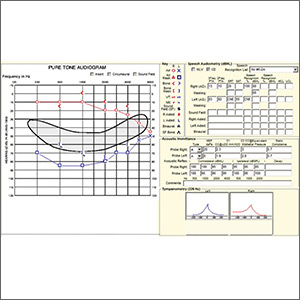
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; Sergio.Huerta@UTSouthwestern.edu
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; Sergio.Huerta@UTSouthwestern.edu
THE CASE
A healthy 48-year-old man presented to our otolaryngology clinic with a 2-hour history of hearing loss, tinnitus, and fullness in the left ear. He denied any vertigo, nausea, vomiting, otalgia, or otorrhea. He had noticed signs of a possible upper respiratory infection, including a sore throat and headache, the day before his symptoms started. His medical history was unremarkable. He denied any history of otologic surgery, trauma, or vision problems, and he was not taking any medications.
The patient was afebrile on physical examination with a heart rate of 48 beats/min and blood pressure of 117/68 mm Hg. A Weber test performed using a 512-Hz tuning fork lateralized to the right ear. A Rinne test showed air conduction was louder than bone conduction in the affected left ear—a normal finding. Tympanometry and otoscopic examination showed the bilateral tympanic membranes were normal.
THE DIAGNOSIS
Pure tone audiometry showed severe sensorineural hearing loss in the left ear and a poor speech discrimination score. The Weber test confirmed the hearing loss was sensorineural and not conductive, ruling out a middle ear effusion. Additionally, the normal tympanogram made conductive hearing loss from a middle ear effusion or tympanic membrane perforation unlikely. The positive Rinne test was consistent with a diagnosis of idiopathic sudden sensorineural hearing loss (SSNHL).
DISCUSSION
SSNHL is defined by hearing loss of more than 30 dB in at least 3 consecutive frequencies with acute onset of less than 72 hours.1,2 The most common symptoms include acute hearing loss, tinnitus, and fullness in the affected ear.1 The majority of cases of SSNHL are unilateral. The typical age of onset is in the fourth and fifth decades, occurring with equal distribution in both sexes.
Etiology.
Diagnosis. The initial evaluation should include an otoscopic examination, tuning fork tests, and pure tone audiometry.1-3 Weber and Rinne tests are essential when evaluating patients for unilateral hearing loss and determining the type of loss (ie, sensorineural vs conductive). The Weber test (ideally using a 512-Hz tuning fork) can detect either conductive or sensorineural hearing loss. In a normal Weber test, the patient should hear the vibration of the tuning fork equally in both ears. The tuning fork will be heard in both ears in conductive hearing loss but will only be heard in the unaffected hear if sensorineural hearing loss is present. So, for instance, if a patient has a perforation in the right tympanic membrane causing conductive hearing loss in the right hear, the tuning fork would be heard in both ears. If the patient has sensorineural hearing loss in the right ear, the tuning fork would only be heard in the left ear.
The Rinne test compares the perception of sound waves transmitted by air conduction vs bone conduction and serves as a rapid screen for conductive hearing loss.
Continue to: Magnetic resonance imaging...
Magnetic resonance imaging (MRI) of the brain and brainstem with gadolinium contrast can reveal vascular events (thrombotic or hemorrhagic), demyelinating disorders, or retrocochlear lesions such as vestibular schwannoma and is indicated in all cases of suspected SSNHL.4,5
Treatment and management. The current standard of care for treatment of idiopathic SSNHL is systemic steroids.1,2 Although the gold standard currently is oral prednisolone or methylprednisolone (1 mg/kg/d for 10 to 14 days with a taper,1,2 the evidence for this regimen stems from a single placebo-controlled trial (N = 67) that demonstrated greater improvement in the steroid group compared with the placebo group (61% vs 32%).6 A Cochrane review and other systematic analyses have not demonstrated clear efficacy of corticosteroid treatment for the management of idiopathic SSNHL.7,8
Because of the potential systemic adverse effects associated with oral corticosteroids, intratympanic (IT) corticosteroids have been advocated as an alternative treatment option. A prospective, randomized, noninferiority trial comparing the efficacy of oral vs IT corticosteroids for idiopathic SSNHL found IT corticosteroids to be noninferior to systemic treatment.9 IT treatment also has been advocated as a rescue therapy for patients who do not respond to systemic treatment.10
A combination of oral and IT corticosteroids was investigated in a retrospective study analyzing multiple treatment modalities.10 Researchers first compared 122 patients receiving one of 3 treatments: (1) IT corticosteroids, (2) oral corticosteroids, and (3) combination treatment (IT + oral corticosteroids). There was no difference in hearing recovery among any of the treatments. Fifty-eight patients who were refractory to initial treatment were then included in a second analysis in which they were divided into those who received additional IT corticosteroids (salvage treatment) vs no treatment (control). There was no difference in hearing recovery between the 2 groups. The authors concluded that IT corticosteroids were as effective as oral treatment and that salvage IT treatment did not add any benefit.10
The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recently published guidelines on the diagnosis and management of SSNHL.11 The guidelines state that IT steroids should be considered in patients who cannot tolerate oral steroids, such as patients with diabetes. It is important to note, however, that the high cost of IT treatment (~$2000 for dexamethasone or methylprednisolone vs < $10 for oral prednisolone) is an issue that needs to be considered as health care costs continue to rise.
Continue to: Antivirals
Antivirals. Because an underlying viral etiology has been speculated as a potential cause of idiopathic SSNHL, antiviral agents such as valacyclovir or famciclovir also are potential treatment agents.12 Antiviral medications have minimal adverse effects and are relatively inexpensive, but the benefits have not yet been proven in randomized controlled trials,and they currently are not endorsed by the AAO-HNS in their guidelines for the management of SSNHL.11
Spontaneous recovery occurs in up to 40% of patients with idiopathic SSNHL. As many as 65% of those who experience recovery do so within 2 weeks of the onset of symptoms, regardless of treatment.1,2 Treatment beyond 2 weeks after onset of symptoms is unlikely to be of any benefit, although some otolaryngologists will treat for up to 6 weeks after the onset of hearing loss.
A substantial number of patients with SSNHL may not recover. Management of these patients begins with referral to an appropriate specialist to initiate counseling and lifestyle changes. Depending on the degree of hearing loss, audiologic rehabilitation may include use of a traditional or bone-anchored hearing aid or a frequency-modulation system.1,2,11 Tinnitus retraining therapy might be of benefit for patients with persistent tinnitus.11
Our patient. After a discussion of his treatment options, our patient decided on a combination of oral prednisolone (60 mg once daily for 9 days followed by a taper for 5 days) and intratympanic dexamethasone injections (1 mL [10 mg/mL] once weekly for 3 weeks).
The rationale for this approach was the minimal adverse effects associated with short-term (ie, days to 1–2 weeks) use of high-dose (ie, > 30 mg/d) corticosteroids. Although steroid therapy has been associated with adverse effects such as aseptic necrosis of the hip, these complications usually arise after longer periods (ie, months to years) of high-dose steroid therapy with a mean cumulative dose much higher than what was used in our patient.13
Continue to: Our patient...
Our patient noticed slight improvement within 48 hours of the initial onset of symptoms that continued for the next several weeks until full recovery was attained. An MRI performed 5 days after the onset of symptoms was negative for retrocochlear pathology.
THE TAKEAWAY
SSNHL is a medical emergency that requires prompt recognition and diagnosis. The steps in evaluating sudden hearing loss include: (1) appropriate history and physical examination (eg, otoscopic examination, tuning fork tests), (2) urgent audiometry to confirm hearing loss, (3) immediate referral to an otolaryngologist for further testing (eg, tympanometry, blood tests, MRI), and (4) initiation of treatment.
If a specific etiology is identified (eg, vestibular schwannoma), the patient should be referred to a specialist for appropriate treatment. If there is no identifiable cause (idiopathic SSNHL), the patient should be treated with oral and/or intratympanic steroids. Patients who do not recover following treatment should be offered audiologic rehabilitation.
CORRESPONDENCE
Sergio Huerta, MD, UT Southwestern Medical Center, 4500 S Lancaster Road #112L, Dallas, TX 75216; Sergio.Huerta@UTSouthwestern.edu
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
1. Schreiber BE, Agrup C, Haskard DO, et al. Sudden sensorineural hearing loss. Lancet. 2010;375:1203-1211.
2. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833-840.
3. Paul BC, Roland JT Jr. An abnormal audiogram. JAMA. 2015;313:85-86.
4. Aarnisalo AA, Suoranta H, Ylikoski J. Magnetic resonance imaging findings in the auditory pathway of patients with sudden deafness. Otol Neurotol. 2004;25:245-249.
5. Cadoni G, Cianfoni A, Agostino S, et al. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J Otolaryngol. 2006;35:310-316.
6. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106:772-776.
7. Wei BPC, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013. doi:10.1002/14651858.CD003998.pub3.
8. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. a meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582-586.
9. Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305:2071-2079.
10. Lee KH, Ryu SH, Lee HM, et al. Is intratympanic dexamethasone injection effective for the treatment of idiopathic sudden sensorineural hearing loss? J Audiol Otol. 2015;19:154-158.
11. Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1-S35.
12. Westerlaken BO, Stokroos RJ, Dhooge IJ, et al. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. 2003;112:993-1000.
13. Nowak DA, Yeung J. Steroid-induced osteonecrosis in dermatology: a review [published online March 30, 2015]. J Cutan Med Surg. 2015;19:358-360.
Can unintended pregnancies be reduced by dispensing a year’s worth of hormonal contraception?
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
EVIDENCE SUMMARY
A 2013 systematic review studied the effect of dispensing a larger amount of pills on pregnancy rate, abortion rate, and overall cost to the health care system.1 Three of the 4 studies analyzed found lower rates of pregnancy and abortion, as well as lower cost despite increased pill wastage, in the groups that received more medication. The 1 study that didn’t show a significant difference between groups compared only short durations (1 vs 4 months).
The systematic review included a large retrospective cohort study from 2011 that examined public insurance data from more than 84,000 patients to compare pregnancy rates in women who were given a 1-year supply of oral contraceptives (12 or 13 packs) vs those given 1 or 3 packs at a time.2 The study found pregnancy rates of 2.9%, 3.3%, and 1.2% for 1, 3, and 12 or 13 months, respectively (P < .05; absolute risk reduction [ARR] = 1.7%; number needed to treat [NNT] = 59; relative risk reduction = 41%).
More pills lead to longer use of contraception
The systematic review also included a 2011 trial of 700 women starting oral contraceptives.3 It randomized them to receive a 7- or 3-month supply at their initial visit, then evaluated use of oral contraception at 6 months. All women were invited back for a 3-month follow-up visit, at which time the 3-month supply group would receive additional medication.
Fifty-one percent of the 7-month group were still using oral contraceptives at 6 months compared with 35% of the 3-month group (P < .001; NNT = 7). The contrast was starker for women younger than 18 years (49% vs 12%; NNT = 3). Notably, of the women who stopped using contraception, more in the 3-month group stopped because they ran out of medication (P = .02). Subjects in the 7-month group were more likely to have given birth and more likely to have 2 or more children.
A 2017 case study examined proposed legislation in California that required health plans to cover a 12-month supply of combined hormonal contraceptives.4 The California Health Benefits Review Program surveyed health insurers and reviewed contraception usage patterns. They found that, if the legislation passed, the state could expect a 30% reduction in unintended pregnancy (ARR = 2%; NNT = 50), resulting in 6000 fewer live births and 7000 fewer abortions per year.
RECOMMENDATIONS
The Centers for Disease Control and Prevention (CDC)’s Selected Practice Recommendations for Contraceptive Use recommend prescribing or providing as much as a 1-year supply of combined hormonal contraceptives at the initial visit and each return visit.5
The American College of Obstetricians and Gynecologists (ACOG) supports over-the-counter access to oral contraceptives, effectively allowing an unlimited supply.6
EDITOR’S TAKEAWAY
Adequate evidence of benefits and strong support from the CDC and ACOG should encourage us to offer 1-year supplies of combined oral contraceptives. Even though the higher-quality studies reviewed also showed a cost savings, up-front patient expense may remain a challenge.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
1. Steenland MW, Rodriguez MI, Marchbanks PA, et al. How does the number of oral contraceptive pill packs dispensed or prescribed affect continuation and other measures of consistent and correct use? A systematic review. Contraception. 2013;87:605-610.
2. Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
3. White KO, Westhoff C. The effect of pack supply on oral contraceptive pill continuation: a randomized controlled trial. Obstet Gynecol. 2011;118:615-622.
4. McMenamin SB, Charles SA, Tabatabaeepour N, et al. Implications of dispensing self-administered hormonal contraceptives in a 1-year supply: a California case study. Contraception. 2017;95:449-451.
5. Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
6. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 544: Over-the-counter access to oral contraceptives. Obstet Gynecol. 2012;120:1527-1531.
EVIDENCE-BASED ANSWER:
Probably, although studies that looked directly at this outcome are limited. A systematic review showed that women who received a larger number of pills at one time were more likely to continue using combined hormonal contraception 7 to 15 months later (strength of recommendation [SOR]: A, consistent evidence from 2 cohort studies and 1 randomized, controlled trial), which might be extrapolated to indicate lower unintended pregnancy rates.
One of the large retrospective cohort studies included in the review demonstrated a significantly lower rate of pregnancy among women who received 12 or 13 packs of oral contraceptives at an office visit compared with 1 or 3 packs (SOR: B, large retrospective cohort study).
Do A-fib patients continue to benefit from vitamin K antagonists with advancing age?
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE-BASED ANSWER:
Yes, patients with atrial fibrilla- tion who are between the ages of 50 and 90 years continue to benefit from vitamin K antagonist therapy (warfarin) (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and large cohorts). Regardless of age, warfarin produces a reduction in risk of thrombotic events that is 2- to 4-fold greater than the risk of hemorrhagic events.
Facial swelling in an adolescent
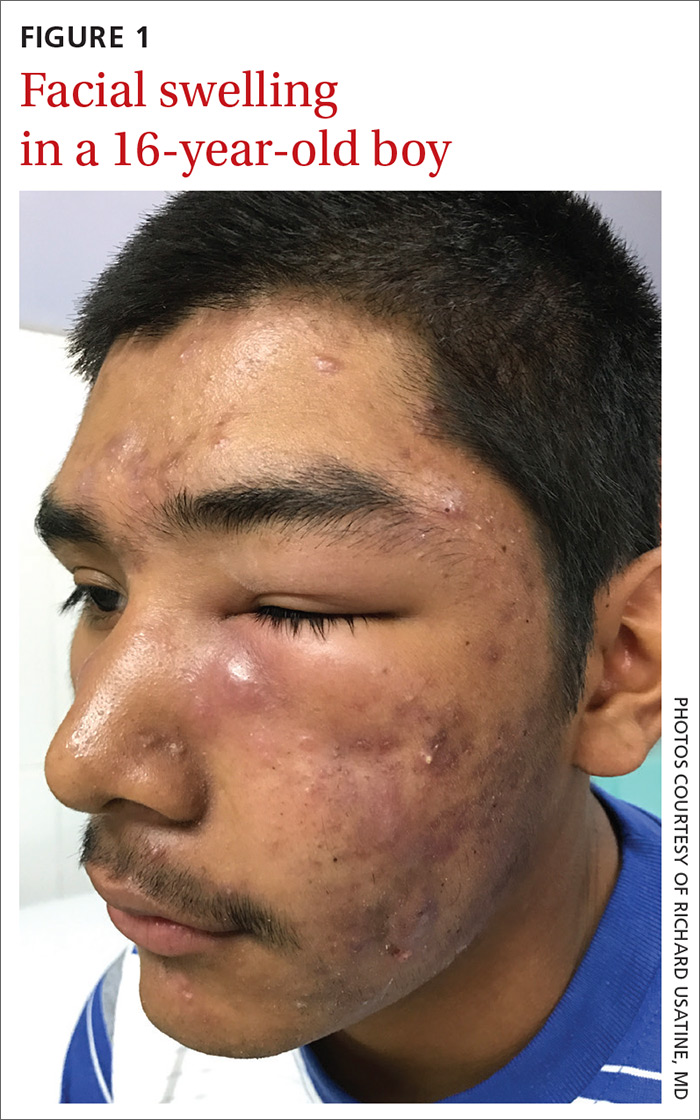
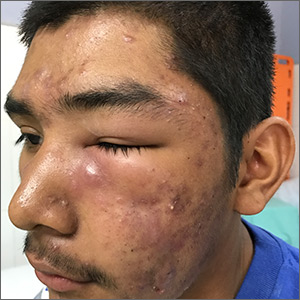
A 16-year-old boy sought care at a rural hospital in Panama for facial swelling that began 3 months earlier. He was seen by a family physician (RU) and a team of medical students who were there as part of a volunteer effort. The patient had difficulty opening his left eye. He denied fever and chills, and said he felt well—other than his inability to see out of his left eye. He denied any changes to his vision when he held the swollen eyelids open. The patient lived on a ranch far outside of town, and he walked down a mountain road alone for 6 hours with one eye swollen shut to present for treatment. The patient was not taking any medications and had not received any health care since his last vaccine several years ago. On physical exam, his vital signs were normal, and the swelling under his left eye was somewhat tender and slightly warm to the touch. There were no lesions on his trunk and the remainder of the exam was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nodulocystic acne
The family physician (FP) diagnosed severe inflammatory nodulocystic acne in this patient. He initially was concerned about possible cellulitis or an abscess, but his clinical experience suggested the swelling was secondary to severe inflammation and not a bacterial infection. The FP noted that the patient was afebrile and lacked systemic symptoms. In addition, the presence of open and closed comedones on the face, as well as the patient’s age and sex, supported the diagnosis of acne. No tests were performed; the diagnosis was made clinically.
A case of acne, or a bacterial infection?
The FP considered acne conglobata, acne fulminans, and a bacterial infection as other possible causes of the patient’s facial swelling.
Acne conglobata is a form of severe inflammatory cystic acne that affects the face, chest, and back. It is characterized by nodules, cysts, large open comedones, and interconnecting sinuses.1,2 Although this case of acne was severe, the young man did not have large open comedones or interconnecting sinus tracts. In addition, his trunk was unaffected.
Acne fulminans is a type of severe cystic acne with systemic symptoms, which is mainly seen in adolescent males. It may have a sudden onset and is characterized by ulcerated, nodular, and painful acne that bleeds, crusts, and results in severe scarring. Patients may present with fever, joint pain, and weight loss.1,2 Our patient did not have systemic symptoms despite the severe facial swelling.
Bacterial infections of the skin usually are caused by Staphylococcus aureus (S aureus) or Streptococcus pyogenes and can lead to cellulitis and/or abscess formation.3 This process was considered as a complication of the severe acne, but the clinical picture was consistent with severe inflammation rather than a bacterial superinfection.
Continue to: Treatment of choice includes prednisone and doxycycline
Treatment of choice includes prednisone and doxycycline
The FP knew that the severe inflammation and swelling needed to be treated with a systemic steroid, so he started the patient on prednisone 60 mg orally once daily at the time of presentation. Additionally, the FP prescribed doxycycline 100 mg bid to treat the inflammation and to cover a possible superinfection.
Doxycycline is the oral antibiotic of choice for inflammatory acne.2 It also is a good antibiotic for cutaneous methicillin-resistant S aureus infection.3 Although it is not the treatment of choice for a nonpurulent cellulitis, it is a good option for cellulitis with purulence.3
With the working diagnosis of severe inflammatory acne, it was expected that the prednisone and doxycycline would be effective. Treating with antibiotics alone (for fear of causing immunosuppression with steroids) would have likely been less effective. Since the patient lived 6 hours from the hospital by foot and was alone, he was admitted overnight for observation (with parental permission obtained over the phone).
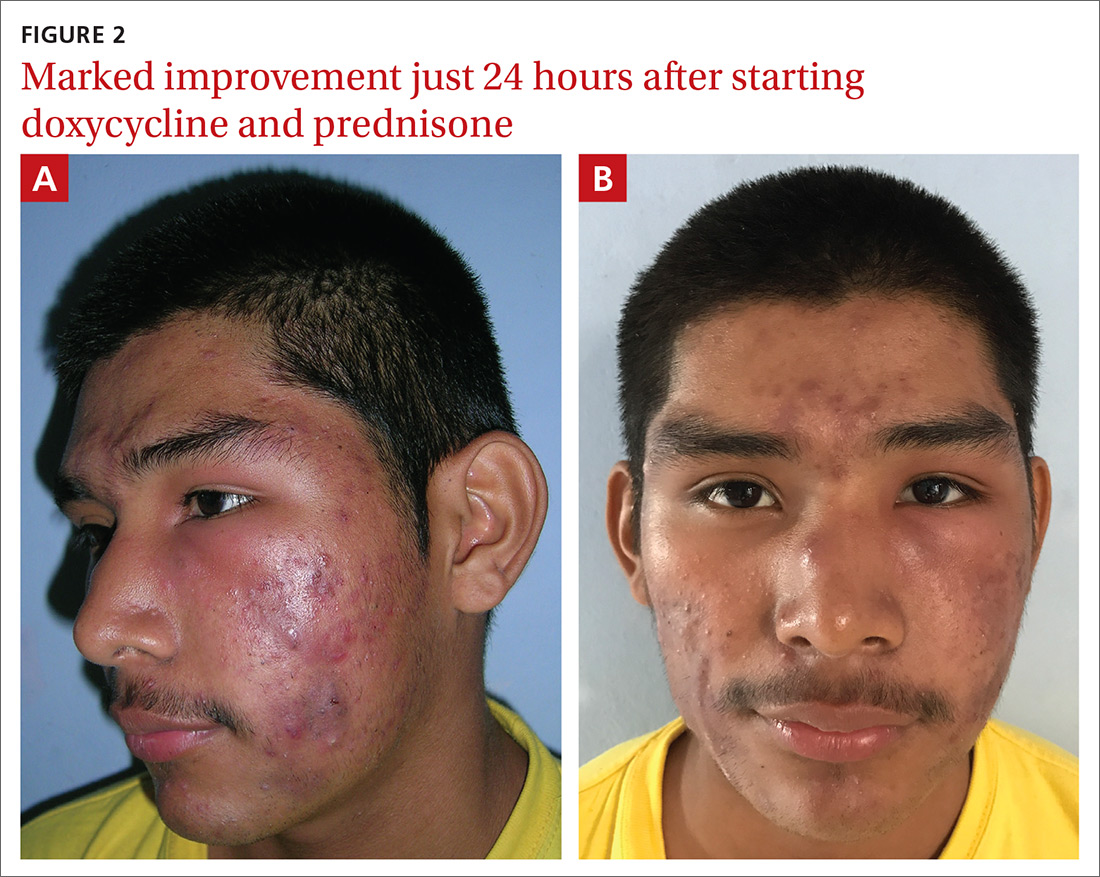
The patient’s condition improved overnight. Marked improvement in the swelling and inflammation was noted the following morning (FIGURES 2A and 2B). The patient was pleased with the results and was discharged to return home (transportation provided by the hospital) with directions on how to continue the oral prednisone and doxycycline. He was given 1 month of doxycycline to continue (100 mg bid) and enough oral prednisone to take 40 mg/d for 1 week and 20 mg/d for another week. He was given a follow-up appointment for 2 weeks to assess his acne and his ability to tolerate the medications.
He was warned to avoid the sun as much as possible, as doxycycline is photosensitizing, and to use a large hat and sunscreen when the sun could not be avoided. (Another option would have been to prescribe minocycline 100 mg bid because it is equally effective for acne with a lower risk for photosensitization.2)
Continue to: Access to medical care was limited
Access to medical care was limited. Although this patient was a good candidate for oral isotretinoin treatment, he did not have access to this medication in rural Panama. Managing his acne was challenging because of the severity of the case and the patient’s sun exposure in this tropical country. Access to the full range of topical anti-acne treatments also is limited in rural Panama, but fortunately his response to the initial oral medications was good.
The future plan at the follow-up visit consisted of continuing the doxycycline, stopping the prednisone, and adding topical benzoyl peroxide. The purpose of the benzoyl peroxide was to prevent bacterial resistance to the antibiotic.2
CORRESPONDENCE
Richard Usatine, MD, Skin Clinic, 903 W Martin Ave, Historic Building, San Antonio, TX 78207; usatine@uthscsa.edu
1. Usatine R, Bambekova P, Shiu V. Acne vulgaris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:717-724.
2. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
3. Stevens DL, Bisno AL, Chambers HF; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:E10-E52.
A 16-year-old boy sought care at a rural hospital in Panama for facial swelling that began 3 months earlier. He was seen by a family physician (RU) and a team of medical students who were there as part of a volunteer effort. The patient had difficulty opening his left eye. He denied fever and chills, and said he felt well—other than his inability to see out of his left eye. He denied any changes to his vision when he held the swollen eyelids open. The patient lived on a ranch far outside of town, and he walked down a mountain road alone for 6 hours with one eye swollen shut to present for treatment. The patient was not taking any medications and had not received any health care since his last vaccine several years ago. On physical exam, his vital signs were normal, and the swelling under his left eye was somewhat tender and slightly warm to the touch. There were no lesions on his trunk and the remainder of the exam was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nodulocystic acne
The family physician (FP) diagnosed severe inflammatory nodulocystic acne in this patient. He initially was concerned about possible cellulitis or an abscess, but his clinical experience suggested the swelling was secondary to severe inflammation and not a bacterial infection. The FP noted that the patient was afebrile and lacked systemic symptoms. In addition, the presence of open and closed comedones on the face, as well as the patient’s age and sex, supported the diagnosis of acne. No tests were performed; the diagnosis was made clinically.
A case of acne, or a bacterial infection?
The FP considered acne conglobata, acne fulminans, and a bacterial infection as other possible causes of the patient’s facial swelling.
Acne conglobata is a form of severe inflammatory cystic acne that affects the face, chest, and back. It is characterized by nodules, cysts, large open comedones, and interconnecting sinuses.1,2 Although this case of acne was severe, the young man did not have large open comedones or interconnecting sinus tracts. In addition, his trunk was unaffected.
Acne fulminans is a type of severe cystic acne with systemic symptoms, which is mainly seen in adolescent males. It may have a sudden onset and is characterized by ulcerated, nodular, and painful acne that bleeds, crusts, and results in severe scarring. Patients may present with fever, joint pain, and weight loss.1,2 Our patient did not have systemic symptoms despite the severe facial swelling.
Bacterial infections of the skin usually are caused by Staphylococcus aureus (S aureus) or Streptococcus pyogenes and can lead to cellulitis and/or abscess formation.3 This process was considered as a complication of the severe acne, but the clinical picture was consistent with severe inflammation rather than a bacterial superinfection.
Continue to: Treatment of choice includes prednisone and doxycycline
Treatment of choice includes prednisone and doxycycline
The FP knew that the severe inflammation and swelling needed to be treated with a systemic steroid, so he started the patient on prednisone 60 mg orally once daily at the time of presentation. Additionally, the FP prescribed doxycycline 100 mg bid to treat the inflammation and to cover a possible superinfection.
Doxycycline is the oral antibiotic of choice for inflammatory acne.2 It also is a good antibiotic for cutaneous methicillin-resistant S aureus infection.3 Although it is not the treatment of choice for a nonpurulent cellulitis, it is a good option for cellulitis with purulence.3
With the working diagnosis of severe inflammatory acne, it was expected that the prednisone and doxycycline would be effective. Treating with antibiotics alone (for fear of causing immunosuppression with steroids) would have likely been less effective. Since the patient lived 6 hours from the hospital by foot and was alone, he was admitted overnight for observation (with parental permission obtained over the phone).
The patient’s condition improved overnight. Marked improvement in the swelling and inflammation was noted the following morning (FIGURES 2A and 2B). The patient was pleased with the results and was discharged to return home (transportation provided by the hospital) with directions on how to continue the oral prednisone and doxycycline. He was given 1 month of doxycycline to continue (100 mg bid) and enough oral prednisone to take 40 mg/d for 1 week and 20 mg/d for another week. He was given a follow-up appointment for 2 weeks to assess his acne and his ability to tolerate the medications.
He was warned to avoid the sun as much as possible, as doxycycline is photosensitizing, and to use a large hat and sunscreen when the sun could not be avoided. (Another option would have been to prescribe minocycline 100 mg bid because it is equally effective for acne with a lower risk for photosensitization.2)
Continue to: Access to medical care was limited
Access to medical care was limited. Although this patient was a good candidate for oral isotretinoin treatment, he did not have access to this medication in rural Panama. Managing his acne was challenging because of the severity of the case and the patient’s sun exposure in this tropical country. Access to the full range of topical anti-acne treatments also is limited in rural Panama, but fortunately his response to the initial oral medications was good.
The future plan at the follow-up visit consisted of continuing the doxycycline, stopping the prednisone, and adding topical benzoyl peroxide. The purpose of the benzoyl peroxide was to prevent bacterial resistance to the antibiotic.2
CORRESPONDENCE
Richard Usatine, MD, Skin Clinic, 903 W Martin Ave, Historic Building, San Antonio, TX 78207; usatine@uthscsa.edu
A 16-year-old boy sought care at a rural hospital in Panama for facial swelling that began 3 months earlier. He was seen by a family physician (RU) and a team of medical students who were there as part of a volunteer effort. The patient had difficulty opening his left eye. He denied fever and chills, and said he felt well—other than his inability to see out of his left eye. He denied any changes to his vision when he held the swollen eyelids open. The patient lived on a ranch far outside of town, and he walked down a mountain road alone for 6 hours with one eye swollen shut to present for treatment. The patient was not taking any medications and had not received any health care since his last vaccine several years ago. On physical exam, his vital signs were normal, and the swelling under his left eye was somewhat tender and slightly warm to the touch. There were no lesions on his trunk and the remainder of the exam was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nodulocystic acne
The family physician (FP) diagnosed severe inflammatory nodulocystic acne in this patient. He initially was concerned about possible cellulitis or an abscess, but his clinical experience suggested the swelling was secondary to severe inflammation and not a bacterial infection. The FP noted that the patient was afebrile and lacked systemic symptoms. In addition, the presence of open and closed comedones on the face, as well as the patient’s age and sex, supported the diagnosis of acne. No tests were performed; the diagnosis was made clinically.
A case of acne, or a bacterial infection?
The FP considered acne conglobata, acne fulminans, and a bacterial infection as other possible causes of the patient’s facial swelling.
Acne conglobata is a form of severe inflammatory cystic acne that affects the face, chest, and back. It is characterized by nodules, cysts, large open comedones, and interconnecting sinuses.1,2 Although this case of acne was severe, the young man did not have large open comedones or interconnecting sinus tracts. In addition, his trunk was unaffected.
Acne fulminans is a type of severe cystic acne with systemic symptoms, which is mainly seen in adolescent males. It may have a sudden onset and is characterized by ulcerated, nodular, and painful acne that bleeds, crusts, and results in severe scarring. Patients may present with fever, joint pain, and weight loss.1,2 Our patient did not have systemic symptoms despite the severe facial swelling.
Bacterial infections of the skin usually are caused by Staphylococcus aureus (S aureus) or Streptococcus pyogenes and can lead to cellulitis and/or abscess formation.3 This process was considered as a complication of the severe acne, but the clinical picture was consistent with severe inflammation rather than a bacterial superinfection.
Continue to: Treatment of choice includes prednisone and doxycycline
Treatment of choice includes prednisone and doxycycline
The FP knew that the severe inflammation and swelling needed to be treated with a systemic steroid, so he started the patient on prednisone 60 mg orally once daily at the time of presentation. Additionally, the FP prescribed doxycycline 100 mg bid to treat the inflammation and to cover a possible superinfection.
Doxycycline is the oral antibiotic of choice for inflammatory acne.2 It also is a good antibiotic for cutaneous methicillin-resistant S aureus infection.3 Although it is not the treatment of choice for a nonpurulent cellulitis, it is a good option for cellulitis with purulence.3
With the working diagnosis of severe inflammatory acne, it was expected that the prednisone and doxycycline would be effective. Treating with antibiotics alone (for fear of causing immunosuppression with steroids) would have likely been less effective. Since the patient lived 6 hours from the hospital by foot and was alone, he was admitted overnight for observation (with parental permission obtained over the phone).
The patient’s condition improved overnight. Marked improvement in the swelling and inflammation was noted the following morning (FIGURES 2A and 2B). The patient was pleased with the results and was discharged to return home (transportation provided by the hospital) with directions on how to continue the oral prednisone and doxycycline. He was given 1 month of doxycycline to continue (100 mg bid) and enough oral prednisone to take 40 mg/d for 1 week and 20 mg/d for another week. He was given a follow-up appointment for 2 weeks to assess his acne and his ability to tolerate the medications.
He was warned to avoid the sun as much as possible, as doxycycline is photosensitizing, and to use a large hat and sunscreen when the sun could not be avoided. (Another option would have been to prescribe minocycline 100 mg bid because it is equally effective for acne with a lower risk for photosensitization.2)
Continue to: Access to medical care was limited
Access to medical care was limited. Although this patient was a good candidate for oral isotretinoin treatment, he did not have access to this medication in rural Panama. Managing his acne was challenging because of the severity of the case and the patient’s sun exposure in this tropical country. Access to the full range of topical anti-acne treatments also is limited in rural Panama, but fortunately his response to the initial oral medications was good.
The future plan at the follow-up visit consisted of continuing the doxycycline, stopping the prednisone, and adding topical benzoyl peroxide. The purpose of the benzoyl peroxide was to prevent bacterial resistance to the antibiotic.2
CORRESPONDENCE
Richard Usatine, MD, Skin Clinic, 903 W Martin Ave, Historic Building, San Antonio, TX 78207; usatine@uthscsa.edu
1. Usatine R, Bambekova P, Shiu V. Acne vulgaris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:717-724.
2. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
3. Stevens DL, Bisno AL, Chambers HF; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:E10-E52.
1. Usatine R, Bambekova P, Shiu V. Acne vulgaris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:717-724.
2. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
3. Stevens DL, Bisno AL, Chambers HF; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:E10-E52.
The benefits of a standardized approach to opioid prescribing
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.
RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.
Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; patchett.david@mayo.edu
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
Doing our part to dismantle the opioid crisis
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
Multiple hyperpigmented papules and plaques
A 2-year-old girl with Fitzpatrick skin type VI who had recently emigrated from Djibouti presented to our dermatology clinic for a rash that appeared when she was 2 months old. The lesions occasionally were pruritic after sun exposure or after crawling outside. There was no associated abdominal pain, diarrhea, flushing, or hypotension. Topical moisturizers were not helpful.
The patient had no known allergies or other notable medical history. Physical examination revealed multiple hyperpigmented, oval-shaped, slightly raised papules and plaques on her torso (FIGURE 1), neck, and arms, and fewer lesions on her face and legs. Darier sign was not appreciated on the initial consult. A 4-mm punch biopsy of the patient’s right middle back was performed at this visit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mastocytosis
The presence of a hyperpigmented skin lesion that becomes pruritic, raised, and erythematous when rubbed (Darier sign) is the major criterion for the diagnosis of cutaneous mastocytosis.1 Minor criteria include a skin biopsy demonstrating a 4- to 8-fold increase in the number of mast cells within the papillary dermis and the presence of cKIT mutations.1 In this case, the patient’s punch biopsy results came back positive for clonal mast cell infiltration of the papillary dermis (FIGURES 2A and 2B), and her physician elicited a slightly positive Darier sign during a follow-up visit.
Mastocytosis is characterized by pathognomonic proliferation of clonal mast cells with either cutaneous or systemic involvement.1 Mast cell expansion most commonly is found in the skin and bone marrow; however, involvement of the gastrointestinal tract, liver, spleen, and lymph nodes also has been documented.1 Nonhereditary somatic mutations of the cKIT gene have been observed in many studies and may be associated with increased proliferation of clonal mast cells.2
A review of the English-language literature on cutaneous mastocytosis reveals a paucity of reports in darker skin types. However, it is important for clinicians to be able to recognize this disease in all skin types.
Three subcategories. Cutaneous mastocytosis is divided into 3 subcategories: mastocytoma of the skin (1–3 solitary skin lesions), maculopapular cutaneous mastocytosis, also known as urticaria pigmentosa (as seen in our patient; usually ≥ 4 lesions); and diffuse cutaneous mastocytosis (confluent lesions; leathery appearance of skin).1,3,4 Maculopapular cutaneous mastocytosis, which our patient had, classically develops within the first 6 months of life and presents as hyperpigmented macules and papules.
Keep in mind that systemic involvement in cutaneous mastocytosis occasionally occurs and can be associated with anaphylaxis, flushing, headache, dyspnea, nausea, emesis, diarrhea, abdominal pain, and hepatosplenomegaly.2
Continue to: Narrowing down a broad differential diagnosis
Narrowing down a broad differential diagnosis
Our cas a ase was made somewhat challenging by the patient’s darkly pigmented skin color, which made any erythema and other cutaneous signs less visible. The differential was broad and included postinflammatory hyperpigmentation from eczema, multiple congenital nevi, sarcoidosis, leprosy, and cutaneous tuberculosis, all of which can be eliminated by performing a biopsy of the lesion.
Diagnostic criteria include biopsy and laboratory findings
In addition to a skin biopsy, initial assessment in cases of suspected cutaneous mastocytosis should include a complete blood cell count with differential, liver function tests (+/- liver ultrasound), a serum tryptase level, and a peripheral smear. Serum tryptase levels > 20 ng/mL have been shown to correlate with a higher probability of systemic mastocytosis. Higher tryptase levels also correlate with severity of disease in children.5
Treatment focuses on minimizing mast cell degranulation
Due to the relatively benign course of cutaneous mastocytosis, the mainstay of treatment is focused on minimizing mast cell degranulation to control subsequent symptoms. Avoiding precipitating factors such as temperature extremes, external stimulation of lesions, dry skin, infection, and certain medications (eg, nonsteroidal anti-inflammatory drugs, aspirin, morphine, polymyxin B sulphate, anticholinergics, some systemic anesthetics) that can stimulate mast cell degranulation is encouraged.2 H1 and H2 antihistamines are the first-line treatment for mild to moderate symptoms. In refractory cases, leukotriene receptor antagonists or oral cromolyn sodium may be considered.2
Regular follow-up every 6 to 12 months should be established after diagnosis.1 If symptoms persist into adulthood or concern for disease progression is high, a bone marrow biopsy is recommended.1
Our patient
Our patient’s laboratory results revealed a normal serum tryptase level (3.4 ng/mL; reference range < 11.5 ng/mL) and complete blood cell count. A complete metabolic panel demonstrated elevated and high-normal liver function tests with alanine aminotransferase (ALT) at 111 u/L (reference range, 10–40 u/L) and aspartate aminotransferase (AST) at 55 u/L (reference range, 7–56 u/L).
Continue to: As a precaution...
As a precaution and for consideration of further work-up, such as the need for a liver ultrasound, the patient was referred to Pediatric Gastroenterology. The specialist repeated the liver function tests, the results of which were lower (ALT, 42 u/L; AST, 24 u/L); a liver ultrasound was thought to be unnecessary.
Our patient’s otherwise negative review of systems led the specialist to feel confident that no systemic disease was present at the time. These results were discussed with the patient’s parents and counseling about trigger avoidance was provided. The patient was given pediatric-dosed oral and topical H1 antihistamines for symptom relief.
CORRESPONDENCE
Joshua D. Sparling, MD, 6 E Chestnut Street, Ste 340, Augusta, ME 04330; Joshua.Sparling@MaineGeneral.org
1. Hartmann K, Escribano L, Grattan C, et al . Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
2. Abid A, Malone MA, Curci K. Mastocytosis. Prim Care. 2016;43:505-518.
3. Forster A, Hartmann K, Horny HP, et al. Large maculopapular cutaneous lesions are associated with favourable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol. 2015;136:1581-1590.
4. Tharp MD, Sofen BD. Mastocytosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2103-2104.
5. Carter MC, Clayton ST, Komarow MD, et al. Assessment of clinical findings, tryptase levels and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin
A 2-year-old girl with Fitzpatrick skin type VI who had recently emigrated from Djibouti presented to our dermatology clinic for a rash that appeared when she was 2 months old. The lesions occasionally were pruritic after sun exposure or after crawling outside. There was no associated abdominal pain, diarrhea, flushing, or hypotension. Topical moisturizers were not helpful.
The patient had no known allergies or other notable medical history. Physical examination revealed multiple hyperpigmented, oval-shaped, slightly raised papules and plaques on her torso (FIGURE 1), neck, and arms, and fewer lesions on her face and legs. Darier sign was not appreciated on the initial consult. A 4-mm punch biopsy of the patient’s right middle back was performed at this visit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mastocytosis
The presence of a hyperpigmented skin lesion that becomes pruritic, raised, and erythematous when rubbed (Darier sign) is the major criterion for the diagnosis of cutaneous mastocytosis.1 Minor criteria include a skin biopsy demonstrating a 4- to 8-fold increase in the number of mast cells within the papillary dermis and the presence of cKIT mutations.1 In this case, the patient’s punch biopsy results came back positive for clonal mast cell infiltration of the papillary dermis (FIGURES 2A and 2B), and her physician elicited a slightly positive Darier sign during a follow-up visit.
Mastocytosis is characterized by pathognomonic proliferation of clonal mast cells with either cutaneous or systemic involvement.1 Mast cell expansion most commonly is found in the skin and bone marrow; however, involvement of the gastrointestinal tract, liver, spleen, and lymph nodes also has been documented.1 Nonhereditary somatic mutations of the cKIT gene have been observed in many studies and may be associated with increased proliferation of clonal mast cells.2
A review of the English-language literature on cutaneous mastocytosis reveals a paucity of reports in darker skin types. However, it is important for clinicians to be able to recognize this disease in all skin types.
Three subcategories. Cutaneous mastocytosis is divided into 3 subcategories: mastocytoma of the skin (1–3 solitary skin lesions), maculopapular cutaneous mastocytosis, also known as urticaria pigmentosa (as seen in our patient; usually ≥ 4 lesions); and diffuse cutaneous mastocytosis (confluent lesions; leathery appearance of skin).1,3,4 Maculopapular cutaneous mastocytosis, which our patient had, classically develops within the first 6 months of life and presents as hyperpigmented macules and papules.
Keep in mind that systemic involvement in cutaneous mastocytosis occasionally occurs and can be associated with anaphylaxis, flushing, headache, dyspnea, nausea, emesis, diarrhea, abdominal pain, and hepatosplenomegaly.2
Continue to: Narrowing down a broad differential diagnosis
Narrowing down a broad differential diagnosis
Our cas a ase was made somewhat challenging by the patient’s darkly pigmented skin color, which made any erythema and other cutaneous signs less visible. The differential was broad and included postinflammatory hyperpigmentation from eczema, multiple congenital nevi, sarcoidosis, leprosy, and cutaneous tuberculosis, all of which can be eliminated by performing a biopsy of the lesion.
Diagnostic criteria include biopsy and laboratory findings
In addition to a skin biopsy, initial assessment in cases of suspected cutaneous mastocytosis should include a complete blood cell count with differential, liver function tests (+/- liver ultrasound), a serum tryptase level, and a peripheral smear. Serum tryptase levels > 20 ng/mL have been shown to correlate with a higher probability of systemic mastocytosis. Higher tryptase levels also correlate with severity of disease in children.5
Treatment focuses on minimizing mast cell degranulation
Due to the relatively benign course of cutaneous mastocytosis, the mainstay of treatment is focused on minimizing mast cell degranulation to control subsequent symptoms. Avoiding precipitating factors such as temperature extremes, external stimulation of lesions, dry skin, infection, and certain medications (eg, nonsteroidal anti-inflammatory drugs, aspirin, morphine, polymyxin B sulphate, anticholinergics, some systemic anesthetics) that can stimulate mast cell degranulation is encouraged.2 H1 and H2 antihistamines are the first-line treatment for mild to moderate symptoms. In refractory cases, leukotriene receptor antagonists or oral cromolyn sodium may be considered.2
Regular follow-up every 6 to 12 months should be established after diagnosis.1 If symptoms persist into adulthood or concern for disease progression is high, a bone marrow biopsy is recommended.1
Our patient
Our patient’s laboratory results revealed a normal serum tryptase level (3.4 ng/mL; reference range < 11.5 ng/mL) and complete blood cell count. A complete metabolic panel demonstrated elevated and high-normal liver function tests with alanine aminotransferase (ALT) at 111 u/L (reference range, 10–40 u/L) and aspartate aminotransferase (AST) at 55 u/L (reference range, 7–56 u/L).
Continue to: As a precaution...
As a precaution and for consideration of further work-up, such as the need for a liver ultrasound, the patient was referred to Pediatric Gastroenterology. The specialist repeated the liver function tests, the results of which were lower (ALT, 42 u/L; AST, 24 u/L); a liver ultrasound was thought to be unnecessary.
Our patient’s otherwise negative review of systems led the specialist to feel confident that no systemic disease was present at the time. These results were discussed with the patient’s parents and counseling about trigger avoidance was provided. The patient was given pediatric-dosed oral and topical H1 antihistamines for symptom relief.
CORRESPONDENCE
Joshua D. Sparling, MD, 6 E Chestnut Street, Ste 340, Augusta, ME 04330; Joshua.Sparling@MaineGeneral.org
A 2-year-old girl with Fitzpatrick skin type VI who had recently emigrated from Djibouti presented to our dermatology clinic for a rash that appeared when she was 2 months old. The lesions occasionally were pruritic after sun exposure or after crawling outside. There was no associated abdominal pain, diarrhea, flushing, or hypotension. Topical moisturizers were not helpful.
The patient had no known allergies or other notable medical history. Physical examination revealed multiple hyperpigmented, oval-shaped, slightly raised papules and plaques on her torso (FIGURE 1), neck, and arms, and fewer lesions on her face and legs. Darier sign was not appreciated on the initial consult. A 4-mm punch biopsy of the patient’s right middle back was performed at this visit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mastocytosis
The presence of a hyperpigmented skin lesion that becomes pruritic, raised, and erythematous when rubbed (Darier sign) is the major criterion for the diagnosis of cutaneous mastocytosis.1 Minor criteria include a skin biopsy demonstrating a 4- to 8-fold increase in the number of mast cells within the papillary dermis and the presence of cKIT mutations.1 In this case, the patient’s punch biopsy results came back positive for clonal mast cell infiltration of the papillary dermis (FIGURES 2A and 2B), and her physician elicited a slightly positive Darier sign during a follow-up visit.
Mastocytosis is characterized by pathognomonic proliferation of clonal mast cells with either cutaneous or systemic involvement.1 Mast cell expansion most commonly is found in the skin and bone marrow; however, involvement of the gastrointestinal tract, liver, spleen, and lymph nodes also has been documented.1 Nonhereditary somatic mutations of the cKIT gene have been observed in many studies and may be associated with increased proliferation of clonal mast cells.2
A review of the English-language literature on cutaneous mastocytosis reveals a paucity of reports in darker skin types. However, it is important for clinicians to be able to recognize this disease in all skin types.
Three subcategories. Cutaneous mastocytosis is divided into 3 subcategories: mastocytoma of the skin (1–3 solitary skin lesions), maculopapular cutaneous mastocytosis, also known as urticaria pigmentosa (as seen in our patient; usually ≥ 4 lesions); and diffuse cutaneous mastocytosis (confluent lesions; leathery appearance of skin).1,3,4 Maculopapular cutaneous mastocytosis, which our patient had, classically develops within the first 6 months of life and presents as hyperpigmented macules and papules.
Keep in mind that systemic involvement in cutaneous mastocytosis occasionally occurs and can be associated with anaphylaxis, flushing, headache, dyspnea, nausea, emesis, diarrhea, abdominal pain, and hepatosplenomegaly.2
Continue to: Narrowing down a broad differential diagnosis
Narrowing down a broad differential diagnosis
Our cas a ase was made somewhat challenging by the patient’s darkly pigmented skin color, which made any erythema and other cutaneous signs less visible. The differential was broad and included postinflammatory hyperpigmentation from eczema, multiple congenital nevi, sarcoidosis, leprosy, and cutaneous tuberculosis, all of which can be eliminated by performing a biopsy of the lesion.
Diagnostic criteria include biopsy and laboratory findings
In addition to a skin biopsy, initial assessment in cases of suspected cutaneous mastocytosis should include a complete blood cell count with differential, liver function tests (+/- liver ultrasound), a serum tryptase level, and a peripheral smear. Serum tryptase levels > 20 ng/mL have been shown to correlate with a higher probability of systemic mastocytosis. Higher tryptase levels also correlate with severity of disease in children.5
Treatment focuses on minimizing mast cell degranulation
Due to the relatively benign course of cutaneous mastocytosis, the mainstay of treatment is focused on minimizing mast cell degranulation to control subsequent symptoms. Avoiding precipitating factors such as temperature extremes, external stimulation of lesions, dry skin, infection, and certain medications (eg, nonsteroidal anti-inflammatory drugs, aspirin, morphine, polymyxin B sulphate, anticholinergics, some systemic anesthetics) that can stimulate mast cell degranulation is encouraged.2 H1 and H2 antihistamines are the first-line treatment for mild to moderate symptoms. In refractory cases, leukotriene receptor antagonists or oral cromolyn sodium may be considered.2
Regular follow-up every 6 to 12 months should be established after diagnosis.1 If symptoms persist into adulthood or concern for disease progression is high, a bone marrow biopsy is recommended.1
Our patient
Our patient’s laboratory results revealed a normal serum tryptase level (3.4 ng/mL; reference range < 11.5 ng/mL) and complete blood cell count. A complete metabolic panel demonstrated elevated and high-normal liver function tests with alanine aminotransferase (ALT) at 111 u/L (reference range, 10–40 u/L) and aspartate aminotransferase (AST) at 55 u/L (reference range, 7–56 u/L).
Continue to: As a precaution...
As a precaution and for consideration of further work-up, such as the need for a liver ultrasound, the patient was referred to Pediatric Gastroenterology. The specialist repeated the liver function tests, the results of which were lower (ALT, 42 u/L; AST, 24 u/L); a liver ultrasound was thought to be unnecessary.
Our patient’s otherwise negative review of systems led the specialist to feel confident that no systemic disease was present at the time. These results were discussed with the patient’s parents and counseling about trigger avoidance was provided. The patient was given pediatric-dosed oral and topical H1 antihistamines for symptom relief.
CORRESPONDENCE
Joshua D. Sparling, MD, 6 E Chestnut Street, Ste 340, Augusta, ME 04330; Joshua.Sparling@MaineGeneral.org
1. Hartmann K, Escribano L, Grattan C, et al . Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
2. Abid A, Malone MA, Curci K. Mastocytosis. Prim Care. 2016;43:505-518.
3. Forster A, Hartmann K, Horny HP, et al. Large maculopapular cutaneous lesions are associated with favourable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol. 2015;136:1581-1590.
4. Tharp MD, Sofen BD. Mastocytosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2103-2104.
5. Carter MC, Clayton ST, Komarow MD, et al. Assessment of clinical findings, tryptase levels and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin
1. Hartmann K, Escribano L, Grattan C, et al . Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
2. Abid A, Malone MA, Curci K. Mastocytosis. Prim Care. 2016;43:505-518.
3. Forster A, Hartmann K, Horny HP, et al. Large maculopapular cutaneous lesions are associated with favourable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol. 2015;136:1581-1590.
4. Tharp MD, Sofen BD. Mastocytosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2103-2104.
5. Carter MC, Clayton ST, Komarow MD, et al. Assessment of clinical findings, tryptase levels and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin
Do probiotics reduce C diff risk in hospitalized patients?
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
PRACTICE CHANGER
Start probiotics within 1 to 2 days of starting antibiotics in hospitalized patients to reduce the risk of Clostridium difficile infection.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900. e9.
Facts to help you keep pace with the vaccine conversation
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.