User login
Immune checkpoint inhibitors and locally ablative therapy in NSCLC
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two phase 2 trials in stage IV non–small cell lung cancer (NSCLC) patients that appeared recently in JAMA Oncology. One summarizes a trial in stage IV NSCLC with four or fewer sites of metastasis (oligometastatic disease or OM), in which pembrolizumab is added to locally ablative therapy (LAT). The other examines whether LAT potentiates the response to immuno-oncology (I/O) in distant sites that were unexposed to LAT.
I/O added to LAT in OM-NSCLC
Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues, published findings from a nonrandomized phase 2 trial in OM-NSCLC in which patients could receive LAT by any technique (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449). Patients could have synchronous or metachronous OM-NSCLC, any histology, and any PD-L1 tumor proportion score. Patients with more than four sites of metastatic disease that regressed to OM-NSCLC after prior therapy (i.e., “oligoremnant NSCLC”) were excluded.
They reported on 51 patients who received conventional-dose pembrolizumab for eight cycles after LAT. Patients without toxicity or progression were allowed to receive up to eight additional cycles of pembrolizumab. The median progression-free survival (PFS) was 19.1 months (95% confidence interval, 9.4-28.7 months), significantly longer than the historical comparison group (median PFS, 6.6 months; P = .005). Additionally, the 24-month overall survival (OS) was 77.5%. With respect to safety, no quality of life decrement or new safety signals were seen.
What this means in practice
As Dr. Bauml and colleagues suggest, there is strong theoretical rationale for believing that OM-NSCLC represents a special, potentially curable, population of stage IV NSCLC patients. Like the recently published work of Daniel R. Gomez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues (J Clin Oncol. 2019 Jun 20;37[18]:1558-65), who studied LAT in comparison with consolidative/maintenance chemotherapy in a slightly different population of OM-NSCLC patients, the current trial moves clinical research forward.
Practically, this study has limitations that should temper a clinician’s enthusiasm for adopting the strategy of LAT, followed by I/O, as standard practice: small patient numbers, most with only one site of OM-NSCLC; comparison with historical controls; and no meaningful information about patient subsets who benefit from I/O and who do not. As the authors suggest, this study provides a strong rationale for a phase 3 trial with stratification for variables that could influence outcome. It does not inform clinical practice at the present time.
LAT added to I/O in stage IV NSCLC
We have limited ability to identify (the majority of) patients with metastatic NSCLC who will not benefit from I/O and no proven interventions to augment benefit in (the majority of) patients with low PD-L1 tumor proportion scores and/or low tumor mutation burden. However, the PEMBRO-RT study was designed to investigate whether LAT with stereotactic body radiation therapy (SBRT) could exploit the hypothesized increase in tumor antigen release and antigen presentation that could lead to better responses to I/O in untreated sites of disease among all patients with stage IV NSCLC.
As reported by Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues, the PEMBRO-RT study randomized 76 patients with stage IV NSCLC to pembro following SBRT to a single metastatic site (the experimental arm of the trial) or pembrolizumab alone. Pembrolizumab was given in a conventional dose and schedule in both arms of the trial and was administered within 7 days after SBRT on the experimental arm (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478).
The primary outcome was the overall response rate (ORR) at 12 weeks. Among patients on the experimental versus control arms, the ORR was 36% and 18%, respectively (P = .07). This did not meet the prespecified endpoint of improving ORR from 20% to 50% at 12 weeks. Additionally, although improved on the pembro plus SBRT arm of the trial, the median PFS and OS did not meet statistical criteria for improvement over the control arm, except among the 47 patients in the PD-L1 negative subset.
What this means in practice
There are a lot of potentially relevant variables in this small, randomized phase 2 study. As the authors discuss, if there is a dose and schedule of RT that facilitates antigen release and presentation and or an ideal latent period after radiotherapy that promotes an “abscopal effect” from I/O, it is unclear whether the ideal schema was used in the PEMBRO-RT trial.
At present, if a patient with stage IV NSCLC requires LAT for clinical reasons during I/O treatment, the patient can receive it safely, but without the expectation that the LAT will augment overall benefit from I/O. Additional preclinical work will need to help guide us about a rational way to design the next trial to test the concept of supra-additive benefit from these modalities. Not only is this combination “not ready for prime time” in clinical care, but it’s not ready for the large numbers of patients in a phase 3 clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Illusion of options
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
Mr. M wanted a second opinion. He was almost 80 years old and had been healthy his entire life. But recent abdominal discomfort prompted a CT scan, which prompted a biopsy. It appeared the tumor had started in his pancreas and then spread to the lymph nodes and the wall of his abdomen.
He asked his doctor to “give it to him straight,” and she did. She told him that it was incurable, but that chemotherapy might slow it down. He asked how long he had, and she said less than a year.
He wanted a straight answer, but that wasn’t the answer he wanted. Who would? So he did some reading and decided to come to a large academic hospital an hour away for a second opinion.
I interviewed him and then scrolled through his CT scans outside the room. There were a few things we could do, the attending and I discussed. We would send his tumor for genetic testing to see if there were any cancer mutations that could be targeted with drugs more specific than standard chemotherapy. We would also refer him to our cancer genetics clinic to get his blood tested for inherited mutations.
But mostly, all of that would likely turn up negative. Mostly, we agreed with his local oncologist.
We went back in the room. Explaining the genetic testing took the length of the visit because this is not a straightforward concept. We explained the difference between tumor mutations and inherited mutations. We wrote down a list of genetic variations we could discover. We discussed treatment options that could go along with each.
Do you have any questions?
He broke down. He reached for the tissue box sitting on the exam room table. “I feel so much better,” he said. “This is why I came here.” He felt safe, reassured, and hopeful.
I was happy to be helpful, but later, as I wrote my clinic note about him, I felt uneasy about the visit.
Everything we said was true. But somehow, it still felt as though we left him with an overly optimistic view of his illness. Did our emphasis on what could be done overshadow that it was unlikely to change the big picture? Did our in-depth discussion of slim possibilities mask that his prognosis was, in fact, still grim?
Working at a large academic medical center, I see many patients who come for a second opinion. I’m incredibly fortunate to learn at a place that is not just up to date in the most cutting-edge treatments but often leading in innovation.
And so we offer patients these options. They sound novel and exciting. They fill patients with hope because they fill the field with hope. I, too, get enraptured with the possibilities – circulating tumor DNA and clinical trials and targeted therapies.
At big cancer meetings every year, oncologists come together and speak about cancer therapies with enthusiasm and hope. Advances have exploded; it’s an exciting time to be learning and practicing.
And yet, the reality for many patients is very different. We are still discussing hospice after one line of chemotherapy has failed. We are still gently holding hands and saying that we have no more options to treat their aggressive cancers.
How can both of these worlds coexist? How can both be true?
A few years ago, a friend was diagnosed with a devastating neurologic condition. I went to a clinical trials website and typed in her disease. Immediately, hundreds of options popped up. I felt hopeful. The field is moving forward, I thought. There are options.
But in the exam room, there were none. When I asked about what I had read, the neurologist explained how many of these possibilities were being investigated. But in the end, my friend really had no good options.
After my visit with Mr. M, I thought about how commonly this story plays out in my field of hematology and oncology. Yes, there are instances in which we find a mutation that drastically changes management. It’s wonderful to witness: patients handed an ominous diagnosis and then living their normal lives, in remission or with stable disease, years later.
We all hope for that. But we rarely get it. The challenge comes when we spend 95% of a visit talking about something with a 1% chance of working. The numbers don’t add up – it’s an equation that easily results in false understanding. Cancer can be glossed with a veneer of innovative options, obscuring the reality that none are likely to work.
Weaving both truths into the conversation is a difficult skill, but one I decided to be more cognizant of after my encounter with Mr. M.
At our next visit, we were still waiting on the test results. But I decided to speak with him candidly. It’s important to have a plan B, I said, and asked what would be important to him if his time were limited. He nodded, thinking about this. “I’ve just been holding out hope for the mutation,” he admitted.
The next week his genetic testing came back negative, and he decided to get palliative chemotherapy closer to home. He had no reason to come to a large academic hospital anymore. With nothing special to offer him, I never saw him again.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
VHA Practice Guideline Recommendations for Diffuse Gliomas (FULL)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
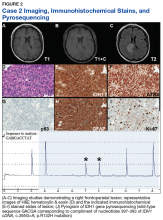
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
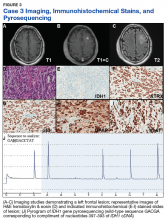
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
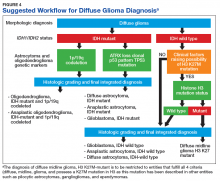
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
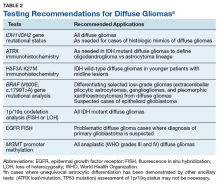
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.
Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
Over the past few decades, our understanding of the molecular underpinning of primary neoplasms of the central nervous system (CNS) has progressed substantially. Thanks in large part to this expansion in our knowledge base, the World Health Organization (WHO) has recently updated its classification of tumors of the CNS.1 One of the key elements of this update was the inclusion of molecular diagnostic criteria for the classification of infiltrating gliomas. While the previous classification system was based upon histologic subtypes of the tumor (astrocytoma, oligodendroglioma, and oligoastrocytoma), the revised classification system incorporates molecular testing to establish the genetic characteristics of the tumor to reach a final integrated diagnosis.
In this article, we present 3 cases to highlight some of these recent changes in the WHO diagnostic categories of primary CNS tumors and to illustrate the role of specific molecular tests in reaching a final integrated diagnosis. We then propose a clinical practice guideline for the Veterans Health Administration (VHA) that recommends use of molecular testing for veterans as part of the diagnostic workup of primary CNS neoplasms.
Purpose
In 2013 the VHA National Director of Pathology & Laboratory Medicine Services (P&LMS) chartered a national molecular genetics pathology workgroup (MGPW) that was charged with 4 specific tasks: (1) Provide recommendations about the effective use of molecular genetic testing for veterans; (2) Promote increased quality and availability of molecular testing within the VHA; (3) Encourage internal referral testing; and (4) Create an organizational structure and policies for molecular genetic testing and laboratory developed tests. The workgroup is currently composed of 4 subcommittees: genetic medicine, hematopathology, pharmacogenomics, and molecular oncology. The molecular oncology subcommittee is focused upon molecular genetic testing for solid tumors.
This article is intended to be the first of several publications from the molecular oncology subcommittee of the MGPW that address some of the aforementioned tasks. Similar to the recent publication from the hematopathology subcommittee of the MGPW, this article focuses on CNS neoplasms.2
Scope of Problem
The incidence of tumors of the CNS in the US population varies among age groups. It is the most common solid tumor in children aged < 14 years and represents a significant cause of mortality across all age groups.3 Of CNS tumors, diffuse gliomas comprise about 20% of the tumors and more than 70% of the primary malignant CNS tumors.3 Analysis of the VA Central Cancer Registry data from 2010 to 2014 identified 1,186 veterans (about 237 veterans per year) who were diagnosed with diffuse gliomas. (Lynch, Kulich, Colman, unpublished data, February 2018). While the majority (nearly 80%) of these cases were glioblastomas (GBMs), unfortunately a majority of these cases did not undergo molecular testing (Lynch, Kulich, Colman, unpublished data, February 2018).
Although this low rate of testing may be in part reflective of the period from which these data were gleaned (ie, prior to the WHO release of their updated the classification of tumors of the CNS), it is important to raise VA practitioners’ awareness of these recent changes to ensure that veterans receive the proper diagnosis and treatment for their disease. Thus, while the number of veterans diagnosed with diffuse gliomas within the VHA is relatively small in comparison to other malignancies, such as prostatic adenocarcinomas and lung carcinomas, the majority of diffuse gliomas do not seem to be receiving the molecular testing that would be necessary for (1) appropriate classification under the recently revised WHO recommendations; and (2) making important treatment decisions.
Case Presentations
Case 1. A veteran of the Gulf War presented with a 3-month history of possible narcoleptic events associated with a motor vehicle accident. Magnetic resonance imaging (MRI) revealed a large left frontal mass lesion with minimal surrounding edema without appreciable contrast enhancement (Figures 1A, 1B, and 1C).
Neither mitotic figures nor endothelial proliferation were identified. Immunohistochemical stains revealed a lack of R132H mutant IDH1 protein expression, a loss of nuclear staining for ATRX protein within a substantial number of cells, and a clonal pattern of p53 protein overexpression (Figures 1E, 1F, and 1G). The lesion demonstrated diffuse glial fibrillary acidic protein (GFAP) immunoreactivity and a low proliferation index (as determined by Ki-67 staining; estimated at less than 5%) (Figures 1H and 1I).
Based upon these results, an initial morphologic diagnosis of diffuse glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. While fluorescence in situ hybridization (FISH) studies were negative for 1p/19q codeletion, pyrosequencing analysis revealed the presence of a c.394C>T (R132C) mutation of the IDH1 gene (Figure 1J). The University of Pittsburgh Medical Center’s GlioSeq targeted next-generation sequence (NGS) analysis confirmed the presence of the c.394C > T mutation in IDH1 gene.4 Based upon this additional information, a final integrated morphologic and molecular diagnosis of diffuse astrocytoma, IDH-mutant was rendered.
Case 2. A Vietnam War veteran presented with a 6-week history of new onset falls with associated left lower extremity weakness. A MRI revealed a right frontoparietal mass lesion with surrounding edema without appreciable contrast enhancement (Figures 2A, 2B, and 2C).
Immunohistochemical stains revealed R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, the lack of a clonal pattern of p53 protein overexpression, diffuse GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 20% (Figures 2E, 2F, 2G, 2H and 2I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were positive for 1p/19q codeletion, and pyrosequencing analysis confirmed the immunohistochemical findings of a c.395G>A (R132H) mutation of the IDH1 gene (Figure 2J). GlioSeq targeted NGS analysis confirmed the presence of the c.395G>A mutation in the IDH1 gene, a mutation in the telomerase reverse transcriptase (TERT) promoter, and possible decreased copy number of the CIC (chromosome 1p) and FUBP1 (chromosome 19q) genes.
A final integrated morphologic and molecular diagnosis of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted was rendered based on the additional information. With this final diagnosis, methylation analysis of the MGMT gene promoter, which was performed for prognostic and predictive purposes, was identified in this case.5,6
Case 3. A veteran of the Vietnam War presented with a new onset seizure. A MRI revealed a focally contrast-enhancing mass with surrounding edema within the left frontal lobe (Figures 3A, 3B, and 3C).
Hematoxylin and eosin (H&E) stained sections following formalin fixation and paraffin embedding demonstrated similar findings (Figure 3D), and while mitotic figures were readily identified, areas of necrosis were not identified and endothelial proliferation was not a prominent feature. Immunohistochemical stains revealed no evidence of R132H mutant IDH1 protein expression, retention of nuclear staining for ATRX protein, a clonal pattern of p53 protein overexpression, patchy GFAP immunoreactivity, and a proliferation index (as determined by Ki-67 staining) focally approaching 50% (Figures 3E, 3F, 3G, 3H, and 3I).
Based upon these results, an initial morphologic diagnosis of diffuse (high grade) glioma was issued, and the tissue was subjected to a variety of nucleic acid-based tests. The FISH studies were negative for EGFR gene amplification and 1p/19q codeletion, although a gain of the long arm of chromosome 1 was detected. Pyrosequencing analysis for mutations in codon 132 of the IDH1 gene revealed no mutations (Figure 3J). GlioSeq targeted NGS analysis identified mutations within the NF1, TP53, and PIK3CA genes without evidence of mutations in the IDH1, IDH2, ATRX, H3F3A, or EGFR genes or the TERT promoter. Based upon this additional information, a final integrated morphologic and molecular diagnosis of GBM, IDH wild-type was issued. The MGMT gene promoter was negative for methylation, a finding that has prognostic and predictive impact with regard to treatment with temazolamide.7-9
New Diffuse Glioma Classification
Since the issuance of the previous edition of the WHO classification of CNS tumors in 2007, several sentinel discoveries have been made that have advanced our understanding of the underlying biology of primary CNS neoplasms. Since a detailed review of these findings is beyond the scope and purpose of this manuscript and salient reviews on the topic can be found elsewhere, we will focus on the molecular findings that have been incorporated into the recently revised WHO classification.10 The importance of providing such information for proper patient management is illustrated by the recent acknowledgement by the American Academy of Neurology that molecular testing of brain tumors is a specific area in which there is a need for quality improvement.11 Therefore, it is critical that these underlying molecular abnormalities are identified to allow for proper classification and treatment of diffuse gliomas in the veteran population.
As noted previously, based on VA cancer registry data, diffuse gliomas are the most commonly encountered primary CNS cancers in the veteran population. Several of the aforementioned seminal discoveries have been incorporated into the updated classification of diffuse gliomas. While the recently updated WHO classification allows for the assignment of “not otherwise specified (NOS)” diagnostic designation, this category must be limited to cases where there is insufficient data to allow for a more precise classification due to sample limitations and not simply due to a failure of VA pathology laboratories to pursue the appropriate diagnostic testing.
Figure 4 presents the recommended diagnostic workflow for the workup of diffuse gliomas. As illustrated in the above cases, a variety of different methodologies, including immunohistochemical, FISH, loss of heterozygosity analysis, traditional and NGS may be applied when elucidating the status of molecular events at critical diagnostic branch points.
Diagnostic Uses of Molecular Testing
While the case studies in this article demonstrate the use of ancillary testing and provide a suggested strategy for properly subclassifying diffuse gliomas, inherent in this strategy is the assumption that, based upon the initial clinical and pathologic information available, one can accurately categorize the lesion as a diffuse glioma. In reality, such a distinction is not always a straightforward endeavor. It is well recognized that a proportion of low-grade, typically radiologically circumscribed, CNS neoplasms, such as pilocytic astrocytomas and glioneuronal tumors, may infiltrate the surrounding brain parenchyma. In addition, many of these low-grade CNS neoplasms also may have growth patterns that are shared with diffuse gliomas, a diagnostic challenge that often can be further hampered by the inherent limitations involved in obtaining adequate samples for diagnosis from the CNS.
Although there are limitations and caveats, molecular diagnostic testing may be invaluable in properly classifying CNS tumors in such situations. The finding of mutations in the IDH1 or IDH2 genes has been shown to be very valuable in distinguishing low-grade diffuse glioma from both nonneoplastic and low-grade circumscribed neuroepithelial neoplasms that may exhibit growth patterns that can mimic those of diffuse gliomas.15-17 Conversely, finding abnormalities in the BRAF gene in a brain neoplasm that has a low-grade morphology suggests that the lesion may represent one of these low-grade lesions such as a pleomorphic xanthoastrocytoma, pilocytic astrocytoma, or mixed neuronal-glial tumor as opposed to a diffuse glioma.18,19
Depending upon the environment in which one practices, small biopsy specimens may be prevalent, and unfortunately, it is not uncommon to obtain a biopsy that exhibits a histologic growth pattern that is discordant from what one would predict based on the clinical context and imaging findings. Molecular testing may be useful in resolving discordances in such situations. If a biopsy of a ring-enhancing lesion demonstrates a diffuse glioma that doesn’t meet WHO grade IV criteria, applying methodologies that look for genetic features commonly encountered in high-grade astrocytomas may identify genetic abnormalities that suggest a more aggressive lesion than is indicated by the histologic findings. The presence of genetic abnormalities such as homozygous deletion of the CDKN2A gene, TERT promoter mutation, loss of heterozygosity of chromosome 10q and/or phosphatase and tensin homolog (PTEN) mutations, EGFR gene amplification or the presence of the EGFR variant III are a few findings that would suggest the aforementioned sample may represent an undersampling of a higher grade diffuse astrocytoma, which would be important information to convey to the treating clinicians.20-26
Testing In the VA
The goals of the MPWG include promoting increased quality and availability of genetic testing within the VHA as well as encouraging internal referral testing. An informal survey of the chiefs of VA Pathology and Laboratory Medicine Services was conducted in November of 2017 in an attempt to identify internal VA pathology laboratories currently conducting testing that may be of use in the workup of diffuse gliomas (Table 1).
The VA currently offers NGS panels for patients with advanced-stage malignancies under the auspices of the Precision Oncology Program, whose reports provide both (1) mutational analyses for genes such as TP53, ATRX, NF1, BRAF, PTEN, TERT IDH1, and IDH2 that may be useful in the proper classifying of high-grade diffuse gliomas; and (2) information regarding clinical trials for which the veteran may be eligible for based on their glioma’s mutational profile. Interested VA providers should visit tinyurl.com/precisiononcology/ for more information about this program. Finally, although internal testing within VA laboratories is recommended to allow for the development of more cost-effective testing, testing may be performed through many nationally contracted reference laboratories.
Conclusion
In light of the recent progress made in our understanding of the molecular events of gliomagenesis, the way we diagnose diffuse gliomas within the CNS has undergone a major paradigm shift. While histology still plays a critical role in the process, we believe that additional ancillary testing is a requirement for all diffuse gliomas diagnosed within VA pathology laboratories. In the context of recently encountered cases, we have provided a recommended workflow highlighting the testing that can be performed to allow for the proper diagnosis of our veterans with diffuse gliomas (Figure 4).
Unless limited by the amount of tissue available for such tests, ancillary testing must be performed on all diffuse gliomas diagnosed within the VA system to ensure proper diagnosis and treatment of our veterans with diffuse gliomas.

Acknowledgments
The authors thank Dr. Craig M. Horbinski (Feinberg School of Medicine, Northwestern University) and Dr. Geoffrey H. Murdoch (University of Pittsburgh) for their constructive criticism of the manuscript. We also thank the following individuals for past service as members of the molecular oncology subcommittee of the MGPW: Dr. George Ansstas (Washington University School of Medicine), Dr. Osssama Hemadeh (Bay Pines VA Health Care System), Dr. James Herman (VA Pittsburgh Healthcare System), and Dr. Ryan Phan (formerly of the VA Greater Los Angeles Healthcare System) as well as the members of the Veterans Administration pathology and laboratory medicine service molecular genetics pathology workgroup.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies.
Dr. Kulich is the Acting Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System and member of the Division of Neuropathology at University of Pittsburgh Department of Pathology, Dr. Duvvuri is an Otolaryngologist at VA Pittsburgh Healthcare System, and Dr. Passero is the Section Chief of Hematology\Oncology at VA Pittsburgh Healthcare System in Pennsylvania. Dr. Becker is an Oncologist at VA-New York Harbor Healthcare System. Dr. Dacic is a Pathologist at University of Pittsburgh Department of Pathology in Pennsylvania. Dr. Ehsan is Chief of Pathology and Laboratory Medicine Services at the South Texas Veterans Healthcare System in San Antonio. Dr. Gutkin is the former Chief of Pathology and Laboratory Medicine Service at VA Pittsburgh Healthcare System. Dr. Hou is a Pathologist at St. Louis VA Medical Center in Missouri. Dr. Icardi is the VA National Director of Pathology and Laboratory Medicine Services. Dr. Lyle is a Pathologist at Bay Pine Health Care System in Florida. Dr. Lynch is an Investigator at VA Salt Lake Health Care System Informatics and Computing Infrastructure. Dr. Montgomery is an Oncologist at VA Puget Sound Health Care System, in Seattle, Washington. Dr. Przygodzki is the Director of Genomic Medicine Implementation and Associate Director of Genomic Medicine for the VA. Dr. Colman is a Neuro-Oncologist at George E. Wahlen VA Medical Center and the Director of Medical Neuro-Oncology at the Huntsman Cancer Institute, Salt Lake City, Utah.
Correspondence: Dr. Kulich (scott.kulich@va.gov)
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
2. Wang-Rodriguez J, Yunes A, Phan R, et al. The challenges of precision medicine and new advances in molecular diagnostic testing in hematolymphoid malignancies: impact on the VHA. Fed Pract. 2017;34(suppl 5):S38-S49.
3. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1-v88.
4. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18(3)379-387.
5. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473-1479.
6. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522.
7. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
8. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926.
9. van den Bent MJ, Kros JM. Predictive and prognostic markers in neuro-oncology. J Neuropathol Exp Neurol. 2007;66(12):1074-1081.
10. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284-297.
11. Jordan JT, Sanders AE, Armstrong T, et al. Quality improvement in neurology: neuro-oncology quality measurement set. Neurology. 2018;90(14):652-658.
12. Chen L, Voronovich Z, Clark K, et al. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro Oncol. 2014;16(11):1478-1483.
13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
14. Wick W, Platten M, Meisner C, et al; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715.
15. Horbinski C, Kofler J, Kelly LM, Murdoch GH, Nikiforova MN. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68(12):1319-1325.
16. Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509-511.
17. Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564-574.
18. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689-696.
19. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401-405.
20. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62(11):1118-1128.
21. Horbinski C. Practical molecular diagnostics in neuropathology: making a tough job a little easier. Semin Diagn Pathol. 2010;27(2):105-113.
22. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299-306.
23. Brennan CW, Verhaak RG, McKenna A, et al; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
24. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829-848.
25. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021-6026.
26. Nikiforova MN, Hamilton RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558-568.
Rheumatoid Arthritis: Therapeutic Strategies After Inadequate Response to Initial TNF Inhibitor Therapy
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
Association of Nausea and Length of Stay with Carbohydrate Loading Prior to Total Joint Arthroplasty
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
Decreasing Treatment of Asymptomatic Bacteriuria: An Interprofessional Approach to Antibiotic Stewardship
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
From the Mayo Clinic, Rochester, MN.
Abstract
- Objective: Asymptomatic bacteriuria (ASB) denotes asymptomatic carriage of bacteria within the urinary tract and does not require treatment in most patient populations. Unnecessary antimicrobial treatment has several consequences, including promotion of antimicrobial resistance, potential for medication adverse effects, and risk for Clostridiodes difficile infection. The aim of this quality improvement effort was to decrease both the unnecessary ordering of urine culture studies and unnecessary treatment of ASB.
- Methods: This is a single-center study of patients who received care on 3 internal medicine units at a large, academic medical center. We sought to determine the impact of information technology and educational interventions to decrease both inappropriate urine culture ordering and treatment of ASB. Data from included patients were collected over 3 1-month time periods: baseline, post-information technology intervention, and post-educational intervention.
- Results: There was a reduction in the percentage of patients who received antibiotics for ASB in the post-education intervention period as compared to baseline (35% vs 42%). The proportion of total urine cultures ordered by internal medicine clinicians did not change after an information technology intervention to redesign the computerized physician order entry screen for urine cultures.
- Conclusion: Educational interventions are effective ways to reduce rates of inappropriate treatment of ASB in patients admitted to internal medicine services.
Keywords: asymptomatic bacteriuria, UTI, information technology, education, quality.
Asymptomatic bacteriuria (ASB) is a common condition in which bacteria are recovered from a urine culture (UC) in patients without symptoms suggestive of urinary tract infection (UTI), with no pathologic consequences to most patients who are not treated.1,2 Patients with ASB do not exhibit symptoms of a UTI such as dysuria, increased frequency of urination, increased urgency, suprapubic tenderness, or costovertebral pain. Treatment with antibiotics is not indicated for most patients with ASB.1,3 According to the Infectious Diseases Society of America (IDSA), screening for bacteriuria and treatment for positive results is only indicated during pregnancy and prior to urologic procedures with anticipated breach of the mucosal lining.1
An estimated 20% to 52% of patients in hospital settings receive inappropriate treatment with antibiotics for ASB.4 Unnecessary prescribing of antibiotics has several negative consequences, including increased rates of antibiotic resistance, Clostridioides difficile infection, and medication adverse events, as well as increased health care costs.2,5 Antimicrobial stewardship programs to improve judicious use of antimicrobials are paramount to reducing these consequences, and their importance is heightened with recent requirements for antimicrobial stewardship put forth by The Joint Commission and the Centers for Medicare & Medicaid Services.6,7
A previous review of UC and antimicrobial use in patients for purposes of quality improvement at our institution over a 2-month period showed that of 59 patients with positive UCs, 47 patients (80%) did not have documented symptoms of a UTI. Of these 47 patients with ASB, 29 (61.7%) received antimicrobial treatment unnecessarily (unpublished data). We convened a group of clinicians and nonclinicians representing the areas of infectious disease, pharmacy, microbiology, statistics, and hospital internal medicine (IM) to examine the unnecessary treatment of ASB in our institution. Our objective was to address 2 antimicrobial stewardship issues: inappropriate UC ordering and unnecessary use of antibiotics to treat ASB. Our aim was to reduce the inappropriate ordering of UCs and to reduce treatment of ASB.
Methods
Setting
The study was conducted on 3 IM nursing units with a total of 83 beds at a large tertiary care academic medical center in the midwestern United States, and was approved by the organization’s Institutional Review Board.
Participants
We included all non-pregnant patients aged 18 years or older who received care from an IM primary service. These patients were admitted directly to an IM team through the emergency department (ED) or transferred to an IM team after an initial stay in the intensive care unit.
Data Source
Microbiology laboratory reports generated from the electronic health record were used to identify all patients with a collected UC sample who received care from an IM service prior to discharge. Urine samples were collected by midstream catch or catheterization. Data on urine Gram stain and urine dipstick were not included. Henceforth, the phrase “urine culture order” indicates that a UC was both ordered and performed. Data reports were generated for the month of August 2016 to determine the baseline number of UCs ordered. Charts of patients with positive UCs were reviewed to determine if antibiotics were started for the positive UC and whether the patient had signs or symptoms consistent with a UTI. If antibiotics were started in the absence of signs or symptoms to support a UTI, the patient was determined to have been unnecessarily treated for ASB. Reports were then generated for the month after each intervention was implemented, with the same chart review undertaken for positive UCs. Bacteriuria was defined in our study as the presence of microbial growth greater than 10,000 CFU/mL in UC.
Interventions
Initial analysis by our study group determined that lack of electronic clinical decision support (CDS) at the point of care and provider knowledge gaps in interpreting positive UCs were the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs, respectively. We reviewed the work of other groups who reported interventions to decrease treatment of ASB, ranging from educational presentations to pocket cards and treatment algorithms.8-13 We hypothesized that there would be a decrease in UC orders with CDS embedded in the computerized order entry screen, and that we would decrease unnecessary treatment of positive UCs by educating clinicians on indications for appropriate antibiotic prescribing in the setting of a positive UC.
Information technology intervention. The first intervention implemented involved redesign of the UC ordering screen in the computerized physician order entry (CPOE) system. This intervention went live hospital-wide, including the IM floors, intensive care units, and all other areas except the ED, on February 1, 2017 (Figure 1). The ordering screen required the prescriber to select from a list of appropriate indications for ordering a UC, including urine frequency, urgency, or dysuria; unexplained suprapubic or flank pain; fever in patients without another recognized cause; screening obtained prior to urologic procedure; or screening during pregnancy. An additional message advised prescribers to avoid ordering the culture if the patient had malodorous or cloudy urine, pyuria without urinary symptoms, or had an alternative cause of fever. Before we implemented the information technology (IT) intervention, there had been no specific point-of-care guidance on UC ordering.
Educational intervention. The second intervention, driven by clinical pharmacists, involved active and passive education of prescribers specifically designed to address unnecessary treatment of ASB. The IT intervention with CDS for UC ordering remained live. Presentations designed by the study group summarizing the appropriate indications for ordering a UC, distinguishing ASB from UTI, and discouraging treatment of ASB were delivered via a variety of routes by clinical pharmacists to nurses, nurse practitioners, physician assistants, pharmacists, medical residents, and staff physicians providing care to patients on the 3 IM units over a 1-month period in March 2017. The presentations contained the same basic content, but the information was delivered to target each specific audience group.
Medical residents received a 10-minute live presentation during a conference. Nurse practitioners, physician assistants, and staff physicians received a presentation via email, and highlights of the presentation were delivered by clinical pharmacists at their respective monthly group meetings. A handout was presented to nursing staff at nursing huddles, and presentation slides were distributed by email. Educational posters were posted in the medical resident workrooms, nursing breakrooms, and staff bathrooms on the units.
Outcome Measurements
The endpoints of interest were the percentage of patients with positive UCs unnecessarily treated for ASB before and after each intervention and the number of UCs ordered at baseline and after implementation of each intervention. Counterbalance measures assessed included the incidence of UTI, pyelonephritis, or urosepsis within 7 days of positive UC for patients who did not receive antibiotic treatment for ASB.
Results
Data from a total of 270 cultures were examined from IM nursing units. A total of 117 UCs were ordered during the baseline period before interventions were implemented. For a period of 1 month following activation of the IT intervention, 73 UCs were ordered. For a period of 1 month following the educational interventions, 80 UCs were ordered. Of these, 61 (52%) UCs were positive at baseline, 37 (51%) after the IT intervention, and 41 (51%) after the educational intervention. Patient characteristics were similar between the 3 groups (Table); 64.7% of patients were female in their early to mid-seventies. The majority of UCs were ordered by providers in the ED in all 3 periods examined (51%-70%). The percentage of patients who received antibiotics prior to UC for another indication (including bacteriuria) in the baseline, post-IT intervention, and post-education intervention groups were 30%, 27%, and 45%, respectively.
The study outcomes are summarized in Figure 2. Among patients with positive cultures, there was not a reduction in inappropriate treatment of ASB compared to baseline after the IT intervention (48% vs 42%). Following the education intervention, there was a reduction in unnecessary ASB treatment as compared both to baseline (35% vs 42%) and to post-IT intervention (35% vs 48%). There was no difference between the 3 study periods in the percentage of total UCs ordered by IM clinicians. The counterbalance measure showed that 1 patient who did not receive antibiotics within 7 days of a positive UC developed pyelonephritis, UTI, or sepsis due to a UTI in each intervention group.
Discussion
The results of this study demonstrate the role of multimodal interventions in antimicrobial stewardship and add to the growing body of evidence supporting the work of antimicrobial stewardship programs. Our multidisciplinary study group and multipronged intervention follow recent guideline recommendations for antimicrobial stewardship program interventions against unnecessary treatment of ASB.14 Initial analysis by our study group determined lack of CDS at the point of care and provider knowledge gaps in interpreting positive UCs as the 2 main contributors to unnecessary UC orders and unnecessary treatment of positive UCs in our local practice culture. The IT component of our intervention was intended to provide CDS for ordering UCs, and the education component focused on informing clinicians’ treatment decisions for positive UCs.
It has been suggested that the type of stewardship intervention that is most effective fits the specific needs and resources of an institution.14,15 And although the IDSA does not recommend education as a stand-alone intervention,16 we found it to be an effective intervention for our clinicians in our work environment. However, since the CPOE guidance was in place during the educational study periods, it is possible that the effect was due to a combination of these 2 approaches. Our pre-intervention ASB treatment rates were consistent with a recent meta-analysis in which the rate of inappropriate treatment of ASB was 45%.17 This meta-analysis found educational and organizational interventions led to a mean absolute risk reduction of 33%. After the education intervention, we saw a 7% decrease in unnecessary treatment of ASB compared to baseline, and a 13% decrease compared to the month just prior to the educational intervention.
Lessons learned from our work included how clear review of local processes can inform quality improvement interventions. For instance, we initially hypothesized that IM clinicians would benefit from point-of-care CDS guidance, but such guidance used alone without educational interventions was not supported by the results. We also determined that the majority of UCs from patients on general medicine units were ordered by ED providers. This revealed an opportunity to implement similar interventions in the ED, as this was the initial point of contact for many of these patients.
As with any clinical intervention, the anticipated benefits should be weighed against potential harm. Using counterbalance measures, we found there was minimal risk in the occurrence of UTI, pyelonephritis, or sepsis if clinicians avoided treating ASB. This finding is consistent with IDSA guideline recommendations and other studies that suggest that withholding treatment for asymptomatic bacteriuria does not lead to worse outcomes.1
This study has several limitations. Data were obtained through review of the electronic health record and therefore documentation may be incomplete. Also, antimicrobials for empiric coverage or treatment for other infections (eg, pneumonia, sepsis) may have confounded our results, as empirical antimicrobials were given to 27% to 45% of patients prior to UC. This was a quality improvement project carried out over defined time intervals, and thus our sample size was limited and not adequately powered to show statistical significance. Additionally, given the bundling of interventions, it is difficult to determine the impact of each intervention independently. Although CDS for UC ordering may not have influenced ordering, it is possible that the IT intervention raised awareness of ASB and influenced treatment practices.
Conclusion
Our work supports the principles of antibiotic stewardship as brought forth by IDSA.16 This work was the effort of a multidisciplinary team, which aligns with recommendations by Daniel and colleagues, published after our study had ended, for reducing overtreatment of ASB.14 Additionally, our study results provided valuable information for our institution. Although improvements in management of ASB were modest, the success of provider education and identification of other work areas and clinicians to target for future intervention were helpful in consideration of further studies. This work will also aid us in developing an expected effect size for future studies. We plan to provide ongoing education for IM providers as well as education in the ED to target providers who make first contact with patients admitted to inpatient services. In addition, the CPOE UC ordering screen message will continue to be used hospital-wide and will be expanded to the ED ordering system. Our interventions, experiences, and challenges may be used by other institutions to design effective antimicrobial stewardship interventions directed towards reducing rates of inappropriate ASB treatment.
Corresponding author: Prasanna P. Narayanan, PharmD, 200 First Street SW, Rochester, MN 55905; narayanan.prasanna@mayo.edu.
Financial disclosures: None.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
1. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:e83–75.
2. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175:1120-1127.
3. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332.
4. Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9:85-93.
5. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
6. The Joint Commission. Prepublication Requirements: New antimicrobial stewardship standard. Jun 22, 2016. www.jointcommission.org/assets/1/6/HAP-CAH_Antimicrobial_Prepub.pdf. Accessed January 24, 2019.
7. Federal Register. Medicare and Medicaid Programs; Hospital and Critical Access Hospital (CAH) Changes to Promote Innovation, Flexibility, and Improvement in Patient Care.Centers for Medicare & Medicaid Services. June 16, 2016. CMS-3295-P
8. Hartley SE, Kuhn L, Valley S, et al. Evaluating a hospitalist-based intervention to decrease unnecessary antimicrobial use in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2016;37:1044-1051.
9. Pavese P, Saurel N, Labarere J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? A controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30:596-599.
10. Kelley D, Aaronson P, Poon E, et al. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35:193-195.
11. Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2.
12. Bonnal C, Baune B, Mion M, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9:605-609.
13. Linares LA, Thornton DJ, Strymish J, et al. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32:644-648.
14. Daniel M, Keller S, Mozafarihashjin M, et al. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178:271-276.
15. Redwood R, Knobloch MJ, Pellegrini DC, et al. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4.
16. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e7.
17. Flokas ME, Andreatos N, Alevizakos M, et al. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:1-10.
Elevating Critical Care Pharmacy Services in a Resource-Limited Environment Through Establishment of a Pharmacist Team
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
From Robert Wood Johnson University Hospital Hamilton, Hamilton, NJ.
Abstract
- Background: Critical care pharmacy services are often provided by clinical specialists during limited hours and, otherwise, by general practice pharmacists, leading to varied level, expertise, and multidisciplinary expectations of these services.
- Objective: Since no published descriptions of successful models sustaining routine, high-quality critical care pharmacy services in a community-based, resource-limited environment exist, a critical care pharmacist team (CCPT) was created to meet this goal. After successful launch, the initiative’s primary goal was to assess whether team formation indeed standardized and increased the level of pharmacy services routinely provided. The secondary goal was to demonstrate cultural acceptance, and thus sustainability, of the model.
- Methods: A CCPT was formed from existing pharmacist resources. A longitudinal educational plan, including classroom, bedside, and practice modeling, assured consistent skills, knowledge, and confidence. Interventions performed by pharmacists before and after implementation were assessed to determine whether the model standardized type and level of service. Surveys of the CCPT and multidisciplinary teams assessed perceptions of expertise, confidence, and value as surrogates for model success and sustainability.
- Results: Interventions after CCPT formation reflected elevated and standardized critical care pharmacy services that advanced the multidisciplinary team’s perception of the pharmacist as an integral, essential team member. CCPT members felt empowered, as reflected by self-directed enrollment in PharmD programs and/or obtaining board certification. This success subsequently served to improve the culture of cooperation and spark similar evolution of other disciplines.
- Conclusion: The standardization and optimization of pharmacy services through a dedicated CCPT improved continuity of care and standardized multidisciplinary team expectations.
Keywords: critical care; clinical pharmacist; pharmaceutical care; standards of practice.
There has been significant evolution in the role, training, and overall understanding of the impact of critical care pharmacists over the past 2 decades. The specialized knowledge and role of pharmacists make them essential links in the provision of quality critical care services.1 The Society of Critical Care Medicine (SCCM) and the American College of Clinical Pharmacy (ACCP) have defined the level of clinical practice and specialized skills that characterize the critical care pharmacist and have made recommendations regarding both the personnel requirements for the provision of pharmaceutical care to critically ill patients and the fundamental, desirable, and optimal pharmacy services that should be provided to these patients (Table 1).2 Despite this, only two-thirds of US intensive care units (ICUs) have clinical pharmacists/specialists (defined as spending at least 50% of their time providing clinical services), resulting in fundamental activities dominating routine pharmacist services.3 The clinical nature of most desirable and optimal activities, such as code response and pharmacist-driven protocol management, is limited, but these activities correlate with decreases in mortality across hospitalized populations.4
Despite their demonstrated benefit and recognized role, critical care pharmacists remain a limited resource with limited physical presence in ICUs.5 This presents hospital pharmacies with a real dilemma: given that clinical pharmacy specialists are often a limited resource, what services (fundamental, desirable, or optimal) should be provided by which pharmacists over what hours and on which days? For many hospitals, personnel resources allow for a clinical pharmacy specialist (either trained or with significant experience in critical care) to participate in multidisciplinary rounds, but do not allow a specialist to be present 7 days per week across all times of the day. As a result, routine services may be inconsistent and limited to activities that are fundamental-to-desirable, due to the varied educational and training backgrounds of pharmacists providing nonrounding services. Where gaps have been identified, remote (tele-health) provision of targeted ICU pharmacist services are beneficial.5
In our organization, we recognized the significant variation created by this resource-defined model and sought to develop a process to move closer to published best practice standards for quality services2 through the creation of a formalized critical care pharmacist team (CCPT). This change was spurred by the transition of our organization’s clinical pharmacist to a board-certified, faculty-based specialist, which in turn spurred new focus on standardizing both the type and quality of services provided by the entire pharmacy team, targeting a higher, more consistent level of pharmacist care which better aligned with SCCM/ACCP-defined activities associated with quality services. The specialist proposed the formation of a CCPT, a process that involved targeted, intensive education and clinical skills development of a narrow pharmacist audience; administration approved this plan, provided that the CCPT arose from existing resources. This realignment focused on ensuring continuity of services across pharmacist roles (ie, rounding vs satellite) as well as across times (both days of the week and shifts). This report describes the methods used to recruit, train, and sustain a CCPT; the resulting changes observed in levels of pharmacy services after CCPT implementation; and the impressions of the CCPT members and the multidisciplinary team (physicians, nurses, dieticians, respiratory therapists, chaplains, and social workers in addition to the pharmacist), as cultural integration and perceived value are essential for sustainability and growth of the model.
Methods
Setting
Robert Wood Johnson University Hospital Hamilton is a 248-bed suburban community hospital in New Jersey with a 20-bed ICU that provides level II6 critical care services as part of an 11-hospital system. Critical care pharmacy services spanned from fundamental (eg, order review) to optimal (eg, independent pharmacotherapy evaluation) activities, with tremendous variability associated with who was engaged in care. In this original model, weekday ICU pharmacy services were provided by satellite-based general practice staff pharmacists (satellite pharmacy located in the ICU provides services for ICU, telemetry, and the emergency department) across 2 shifts (0700-2300; 9 pharmacists during the day shift and 2 on the evening shift). Satellite pharmacists largely focused on traditional/fundamental pharmacy practice, including order review, drug therapy evaluation, and adverse drug event identification. Additionally, a hospital-based, residency-trained clinical pharmacist rounded 3 days per week. General practice staff pharmacists provided weekend and overnight services. Very limited, prospective, independent clinical evaluation or individualized pharmacotherapy optimization occurred routinely. No established clinical assessment priorities or strategies existed, and thus expectations of pharmacy services were associated with the individual pharmacist present.
Team Structure and Recruitment
The staff pharmacists were well-established, with each having 25 to 41 years of practice experience. All 11 full-time staff pharmacists graduated with Bachelor of Science degrees in pharmacy, and 5 of them had returned to acquire Doctor of Pharmacy degrees prior to the initiative. None had completed post-doctoral training residencies, as residencies were not the standard when these pharmacists entered practice. The staffing model necessitated that pharmacists maintain Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) competency as members of inpatient emergency response teams.
Three volunteers were recruited to the initial transformational process. These volunteer pharmacists were preferentially assigned to the ICU, with a clinically focused weekend rotation, to provide 7-day/week rounding continuity, but maintained general competencies and cross-functionality. Weekend responsibilities included critical care assessments and multidisciplinary rounding, inpatient emergency response, patient education/medication histories, and inpatient warfarin management consultations.
Team Training and Development
Longitudinal education of the CCPT included classroom, bedside, and practice-modeling training strategies to complement routine exposure and integration into the pharmacist’s practice in providing direct patient care. Concentrated learning occurred over a 3-month period, with extended bedside and patient-case-based learning continuing for another 3 months. Expectations of the critical care pharmacist as an independent consultant to the interdisciplinary team targeting holistic pharmacotherapy optimization were established, instilling independence and accountability within the role. Next, lecture and bedside training targeted the development of crucial assessment skills, including an understanding of device and equipment implications on pharmacotherapy decisions, pharmacokinetic and pharmacodynamic variations in critically ill patients, and supportive care. A minimum of 5 hours of group lectures were included for all members of the CCPT, with additional instruction provided based on individual needs. Lectures explored the evidence and practice associated with common diagnoses, including review of related literature, core guidelines, and institutional order sets. Fundamental topics included pain, agitation, and delirium (PAD) during mechanical ventilation, infectious diseases, and hemodynamic management.
To reinforce knowledge, build bedside assessment skills, and increase confidence, pharmacists routinely partnered with the specialist during independent morning bedside evaluations and rounds. Over time, the specialist role became increasingly supportive as the critical care pharmacist grew into the primary role. On weekends the specialist was not present but remained on call to discuss cases with the rounding critical care pharmacist. This served to reinforce clinical decision-making and expand knowledge; these patient-specific lessons were communicated with the team to support continued development and standardization.
In addition to these internal efforts, the specialist simultaneously recalibrated expectations among key ICU stakeholders, establishing uniform quality and scope of service from the CCPT. Historically, physicians and nurses sought input from specific pharmacists, and thus a cultural change regarding the perceived value of the team was required. To reinforce this, those demanding a specific pharmacist were referred to the CCPT member present.
The initial training process involved a significant proportion of the specialist’s time. Initially focused on classroom lecture and core skills development, time increasingly focused on individual learner’s needs and learning styles. Mentoring and partnering were key during this period. In the first 6 months, weekend calls were routine, but these quickly tapered as the team gained experience and confidence in their knowledge and skills.
Tools and Team Support
Beyond standardizing knowledge and skills, team effectiveness depended on establishing routine assessment criteria (Table 2), communication tools, and references. Rounding and sign-out processes were standardized to support continuity of care. A patient census report generated by the clinical computer system was used as the daily worksheet and was stored on a sign-out clipboard to readily communicate clinically pertinent history, assessments, recommendations, and pending follow-up. The report included patient demographics, admitting diagnosis, and a list of consulting physicians. The pharmacist routinely recorded daily bedside observations, his/her independent assessments (topics outlined in Table 2), pertinent history, events, and goals established on rounds. Verbal sign-out occurred twice daily (during weekdays)—from the rounding to satellite pharmacist after rounds (unless 1 person fulfilled both roles) and between day and evening shifts. Additionally, a resource binder provided rapid accessibility to key information (eg, published evidence, tools, institutional protocols), with select references residing on the sign-out clipboard for immediate access during rounding.
Monthly meetings were established to promote full engagement of the team, demonstrate ownership, and provide opportunity for discussion and information sharing. Meetings covered operational updates, strategic development of the service, educational topics, and discussions of difficult cases.
Assessment
While not directly studied, existing evidence suggests that appropriately trained critical care pharmacists should be able to perform a broad range of services, from fundamental to optimal.7 To evaluate if CCPT training elevated and standardized the type of interventions routinely made, services provided prior to the team’s formation were compared to those provided after formation through interrogation of the institution’s surveillance system. As a baseline, a comparison of the types of ICU interventions documented by the specialist during a 2-month period prior to the team’s formation were compared to the interventions documented by the staff pharmacists who became part of the CCPT. Since standardization of skills and practice were goals of the CCPT formation, the same comparison was conducted after team formation to assess whether the intervention types normalized across roles, reflecting a consistent level of service.
As assignment to the CCPT is voluntary, with no additional compensation or tangible benefits, the success of the CCPT relies on active pharmacist engagement and ongoing commitment. Thus, a personal belief that their commitment was valuable and increased professional satisfaction was key to sustain change. An online, voluntary, anonymous survey was conducted to assess the CCPT member’s perceptions of their preparedness, development of skills and comfort level, and acceptance by the multidisciplinary team, as these elements would influence members’ beliefs regarding the impact and value of the team and their justification for commitment to continuous, uncompensated learning and training. Their thoughts on professional satisfaction and development were collected as a surrogate for the model’s sustainability.
Success and sustainability also depend on the multidisciplinary team’s acceptance and perceived value of the CCPT, especially given its evolution from a model in which clinical feedback was sought and accepted exclusively from the specialist. To evaluate these components, an online, voluntary, anonymous survey of the multidisciplinary members was conducted.
Results
CCPT Interventions and Level of Service
Prior to CCPT formation, intervention categories documented by the specialist differed from those of the staff (Figure 1). The staff’s baseline interventions represented those arising from the established, routine assessments performed by all pharmacists for all inpatients, such as renal dose assessments. The specialist’s interventions largely focused on independent pharmacotherapy assessments and optimization strategies. After team formation, intervention type became increasingly consistent across the CCPT, with all members aligning with the specialist’s interventions. Intervention categories reflected the clinically focused, independent assessments targeted during training (eg, supportive care and pain/sedation assessment), expanding beyond the routine assessments performed across the general hospitalized population.
When compared to SCCM/ACCP ideals, these interventions corresponded with an expansion from routinely fundamental to routinely broad (ie, fundamental, desirable, and optimal) critical care pharmacist activities, thus elevating the overall quality of services provided by the team while assuring continuity. Desirable activities adopted by the CCPT included multidisciplinary rounding on all ICU patients; drug history review for appropriate management during acute illness; and training of students and providing educational in-services. Optimal activities routinely integrated included independent and/or collaborative investigation of ICU guidelines/protocol impact and scholarship in peer-reviewed publications. Prior to CCPT formation, staff involvement of desirable activities was limited to resuscitation event response and clarification of effective dosage regimens, with no involvement in optimal activities.
CCPT Impressions
The online, voluntary, anonymous survey was completed by 5 of the 6 staff members (the 3 original members plus 3 staff members who were added several months into the program to enhance continuity and cross-shift coverage) comprising the team. Using a 5-point Likert scale, members ranked their comfort level with their critical care knowledge, bedside skills, ability to actively participate in rounds, and ability to address controversial clinical issues in their staffing role prior to team formation (ie, baseline) compared to their current CCPT practice. Overall, self-assessments reflected perceived increases across all categories. Prior to CCPT training and implementation, all team members were “not at all,” “slightly comfortable,” or “somewhat comfortable” with these points, while after training and implementation all reported being “comfortable” or “very comfortable” with the same points. All members reported feeling better prepared and confident in caring for critically ill patients and felt that the team and its standardized approach enhanced medication safety. When asked about their impressions of the perceived value of the CCPT by interdisciplinary peers, pharmacists felt it was perceived as bringing “a lot” or “a great deal” of value. Additionally, all members uniformly felt that the team supported their professional growth and enhanced their professional satisfaction.
Multidisciplinary Impressions of Service and Value
A total of 29 (90%) multidisciplinary team members completed the online, voluntary, anonymous survey of their impressions of the CCPT’s service and impact. Surveys represented the impressions of critical care physicians, the unit’s nursing leadership (administrative and clinical), nursing education, staff nurses, social work, and pastoral care. Using a 5-point Likert scale, all respondents reported that they “agreed” or “entirely agreed” that the CCPT enhanced care. Specifically, they reported that pharmacists were more visible and engaged, and provided more consistent and reliable care regardless of which member was present. Services were seen as more robust and seamless, meeting interdisciplinary needs. The CCPT was viewed as a cohesive, efficient group. Respondents felt that the CCPT’s presence and engagement on weekends enhanced continuity of pharmaceutical care. As a result, the CCPT was seen as enhancing interdisciplinary understanding of the pharmacist’s value in critical care.
Discussion
Realignment and development of existing personnel resources allowed our organization to assure greater continuity, consistency, and quality of pharmacy care in the critical care setting (Figure 2). By standardizing expectations and broadening multidisciplinary understanding of the CCPT’s unique value, the pharmacist’s role was solidified and became an integral, active part of routine patient bedside care.
Prior to forming the CCPT, the physical presence of the pharmacist, as well as the services provided, were inconsistent. While a general practice pharmacist was in the satellite pharmacy within the ICU for up to 2 shifts on weekdays, pharmacists largely focused on traditional functions associated with order review and drug dispensing or established hospital-wide programs such as renal dosing or intravenous-to-oral formulation switches. The pharmacist remained in the satellite, not visible on rounds or at the bedside. In fact, there was a clear lack of comfort, frequently articulated by the pharmacists, with clinical questions that required bedside assessment, leading to routine escalation to the clinical specialist, who was not always readily available. This dynamic set an expectation for the multidisciplinary team that there were segregated pharmacy services—the satellite provided order review and product and the clinical specialist, in the limited hours present, provided clinical consultation and education. The formation of the CCPT abolished this tiered level of expectations, establishing a physical and clinical presence of a critical care pharmacist with equal capability and comfort. Both the pharmacist and multidisciplinary members perceived enhancements and value associated with the standardization and consistency provided by implementing the CCPT. Intervention data from before and after team formation support that routine interventions in critical care normalized the care provided and increased the robustness of critical care pharmacy services, with a strong shift to both clinical and academic activities considered desirable to optimal by SCCM/ACCP standards.
The benefit of pharmacist presence in the ICU is well described, with studies showing that the presence of a pharmacist is associated with medication error prevention and adverse drug event identification.8-10 However, this body of evidence applies no standardized definition regarding critical care pharmacist qualifications, with many studies pre-dating the wider availability of post-doctoral training programs and national board certification for critical care pharmacists.11 Training and certification structures have evolved with increased recognition of the specialization required to optimize the pharmacist’s role in providing quality care, albeit at a slower pace than published standards.1,2 In 2018, 136 organizations offered America Society of Health-System Pharmacists–accredited critical care pharmacy residencies.12 National recognition of expertise as a critical care pharmacist was established by the Board of Pharmacy Specialists in 2015, with more than 1600 pharmacists currently recognized.12 Our project is the only known description of a pharmacist practice model that increases critical care pharmacist availability through the application of standardized criteria incorporating these updated qualifications, thus ensuring expertise and experience that correlates with practice quality and consistency.
Despite the advancements achieved through this project, several limitations exist. First, while this model largely normalized services over the day and evening shifts, our night shift continues to be covered by 1 general practice pharmacist. More recently, resource reallocation mandated reduction in satellite hours, although that CCPT member remains available from the main pharmacy. The specialist remains on call to support the general practice pharmacists, but in-house expertise cannot be made available in the absence of additional resources. To optimize existing staffing, the specialist begins clinical evaluations during the early morning, overlapping with the night-shift prior to the satellite pharmacist’s arrival. This both provides some pharmacist presence at the bedside for night shift nurses and extends the hours during which a critical care pharmacist is physically available. Second, while all efforts are made to stagger time off, unavoidable gaps in critical care pharmacist coverage occur; expansion of the original team from 3 to 6 members has greatly reduced the likelihood of such gaps. Last, the program was designed to achieve routine integration of activities shown in the literature as being associated with quality, and those activities were assessed as a surrogate for quality.
Informal input, confirmed through survey data, from various disciplines on our team has consistently supported that the establishment of the CCPT has met a need by both standardizing critical care pharmacy practice and optimizing the pharmacist role within the team. While we recognize the limitations associated with the size of these surveys, they represent large proportions of our team and reflect key elements known to be important in sustaining long-term cultural change—a belief that what one is doing is both justified and valuable. This success has been a catalyst for several ongoing projects, fostering the development and adoption of critical care pharmacist protocols to allow more autonomous practice within our scope. Team development and movement toward robust protocol management has sparked a cultural evolution across disciplines as we strive to achieve the SCCM description of a highly effective team2,13 that emphasizes each discipline practicing fully within its scope in a horizontal team structure. Thus, the ICU medical director has used the success of the CCPT structure as an example to support optimization and development of the practice by other disciplines within the team. This has led to a significant revision in our rounding structure and interdisciplinary care model.14
The survey of CCPT members revealed that the model both engaged and stimulated the pharmacists involved, reflective of the autonomy and accountability required for sustainable, transformational cultural change. Within a year of entering the CCPT, 2 of the 3 pharmacists initially engaged had earned their board certification in pharmacotherapy (ie, BCPS) and the other, who had not acquired her Doctor of Pharmacy degree prior to the CCPT initiative, enrolled in a program to do so. The pharmacists expressed that they obtained BCPS over the newly available critical care certification because of the expectation that they maintain expertise across patient populations. This level of self-driven motivation in the absence of compensation reflects the value and professional satisfaction gained from being voluntary members of the CCPT.
Conclusion
Critical care pharmacy practice has continued to evolve to include increasingly specialized training for newer graduates and, more recently, the availability of critical care pharmacist board certification. While it is optimal to apply these standards when filling open critical care pharmacist positions, many hospitals require existing staff to fulfill multiple roles across various patient populations, leading to a variation in educational, training, and practice backgrounds for pharmacists currently practicing in the ICU. To minimize the variation associated with this resource-limited structure in a manner that standardized and elevated the type and level of service provided, we created a CCPT with existing pharmacists who were willing to accept intensive training and demonstrate an ongoing commitment to maintain defined competencies and skills. Our goal was to solidify the essential role of the critical care pharmacist in providing quality critical care services as described in the literature. The CCPT was well-received by the multidisciplinary team and served as an example for other disciplines that had similar struggles. The team’s success expanded into several other ongoing initiatives, including critical care pharmacist–driven protocols.
Acknowledgment: The authors thank Nina Roberts, MSN, RN, CCRN, NEA-BC, and Carol Ash, DO, MBA, MHCDS, the ICU Nursing and Medical Directors, respectively, at the time of this program’s initiation, for supporting the development of the critical care pharmacist team initiative and review of this manuscript.
Corresponding author: Liza Barbarello Andrews, PharmD, BCCCP, BCPS, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, NJ 08854; lbarbarello@pharmacy.rutgers.edu.
Financial disclosures: None.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
1. Brilli RJ, Spevetz A, Branson RD, et al. American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007-2019.
2. Rudis MI, Brandl KM; Society of Critical Care Medicine and American College of Clinical Pharmacy Task Force on Critical Care Pharmacy Services. Position paper on critical care pharmacy services. Crit Care Med. 2000;28:3746-3750.
3. MacLaren R, Devlin JW, Martin SJ, et al. Critical care pharmacy services in United States hospitals. Ann Pharmacother. 2006;40:612-618.
4. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27:481-493.
5. Forni A, Skahan N, Hartman CA, et al. Evaluation of the impact of a tele-ICU pharmacist on the management of sedation in critically ill mechanically ventilated patients. Ann Pharmacother. 2010;44:432-438.
6. Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization on three levels of care. Crit Care Med. 2003;31:2677-2683.
7. Board of Pharmacy Specialties. Critical Care Pharmacy. www.bpsweb.org/bps-specialties/critical-care-pharmacy/.
8. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048.
9. Leape L, Cullen D, Clapp M, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267-270.
10. Horn E, Jacobi J. The critical care pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(suppl):S46-S51.
11. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30:1127-1128.
12. American Society of HealthSystem Pharmacists. Online residency directory. https://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx. Accessed June 26, 2019.
13. Weled BJ, Adzhigirey LA, Hodgman TM, et al. Critical care delivery: the importance of process of care and ICU structure to improved outcomes: an update from the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43:1520-1525.
14. Andrews LB, Roberts N, Ash C, et al. The LOTUS: a journey to value-based, patient-centered care. Creat Nurs. 2019;25:17-24.
Long-Term Exercise Training in Older Adults Is Associated with Reduced Injurious Falls and Fractures
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
Study Overview
Objective. To evaluate the association between long-term exercise interventions (duration ≥ 1 year) and risks of falls, injurious falls, multiple falls, fractures, hospitalization, and mortality in older adults.
Design. A systematic review of randomized controlled trials (RCTs) with preplanned meta-analysis was conducted to investigate the association between long-term exercise interventions and falls and fall-related adverse outcomes in adults older than 60 years. A literature search using electronic databases, including PubMed, Cochrane Central Register of Controlled Trials, SportDiscus, PsychInfo, and Ageline, was performed between February 20 and March 5, 2018. Studies selected were RCTs with exercise duration of 1 year or longer, where effects of exercise intervention were compared with a comparator group of participants aged 60 years or older. Articles were independently screened, abstracted, and assessed for risk of bias by 2 raters, who resolved divergences in data extraction and synthesis via in-person meetings.
Setting and participants. A total of 46 studies (22,709 participants; median of 203 participants per study) were included in the review and 40 studies (21,868 participants) were included in the meta-analysis. The participants’ mean age was 73.1 ± 7.1 years, and 66.3% (15,054 participants) were women. Studies were mostly conducted in Europe (n = 15), North America (n = 13), and Oceania (n = 10). Multicomponent training involving multiple exercises (eg, aerobic, strength and balance; 29 RCTs) was the most common intervention modality, followed by aerobic (8 RCTs) and strength (5 RCTs) training. Exercise interventions had a mean frequency of 3 times/week, with each session lasting approximately 50 minutes, and were administered at a moderate intensity. The average compliance rate with exercise training was 65%. Comparator groups were often active controls that ranged from attention controls to more intensive interventions.
Main outcome measures. The 6 binary outcomes investigated were fallers who fell at least once, multiple times, or at least twice; fractures; hospitalization; and mortality. Estimates of outcomes were combined using risk ratios (RRs) using DerSimonian and Laird’s random-effects model (Mantel-Haenszel method). Heterogeneity was evaluated using I2 statistics, and trials with low rates of compliance (< 30%) with exercise intervention or high attrition (> 40%) were excluded in primary analyses.
Main results. Exercise training significantly reduced the risk of falls by 12% (n = 20 RCTs; 4420 participants; RR, 0.88; 95% confidence interval [CI], 0.79-0.98) and injurious falls by 26% (9 RCTs; 4481 participants; RR, 0.74; 95% CI, 0.62-0.88), and reduced the risk of fractures by 16% (19 RCTs; 8410 participants; RR, 0.84; 95% CI, 0.71-1.00; P = 0.05). Exercise training did not decrease the risk of multiple falls (13 RCTs; 3060 participants; RR, 0.86; 95% CI, 0.68-1.08), hospitalization (12 RCTs; 5639 participants; RR 0.94; 95% CI, 0.80-1.12), or mortality (29 RCTs; 11,441 participants; RR 0.96; 95% CI, 0.85-1.09). Sensitivity analyses yielded similar results, with the exception of the fixed-effect meta-analysis for the risk of fracture that showed a significant effect of long-term exercise training (RR, 0.84; 95% CI, 0.70-1.00; P = 0.047). Meta-regression analysis on mortality and falls suggested that exercise frequency between 2 and 3 times per week was optimal and beneficial.
Conclusion. Long-term exercise training of 1 year or longer in duration is associated with a reduction in falls, injurious falls, and fractures in older adults. Moreover, moderate intensity, multicomponent exercise training performed 2 to 3 times weekly is likely safe and effective in this vulnerable population.
Commentary
Falls are exceedingly common (1 in 3 older Americans fall each year) and are the leading cause of fatal and nonfatal injuries in persons over the age of 65 years.1,2 While fall prevention is a public health priority and a topic of interest in many research studies, there are important gaps in knowledge regarding optimal strategies to prevent falls and fall-related injuries in this high-risk population. The study reported by de Souto Barreto and colleagues provides new insights to address several of these gaps and may have a significant impact on the clinical practice of fall prevention in geriatric medicine.
Studies show that a single exercise intervention of short- to medium-term duration can prevent falls in community-dwelling older adults.3 However, the effects of long-term exercise training (ie, intervention lasting longer than a year) on fall prevention in this population is less well characterized. This study is the first meta-analysis that aimed to evaluate the potential beneficial impact of long-term exercise training on falls and adverse fall-related outcomes in adults ≥ 60 years of age who are prone to falls. The study’s findings indicate that long-term exercise training reduces the risk of falling by 12%, injurious falls by 26%, and factures by 16%. These results are important in that they add compelling evidence that exercise training of any duration can reduce falls and some fall-related adverse outcomes. Furthermore, the positive effects of long-term exercise training appear to mitigate some of the fatal and nonfatal injuries attributable to falls—the leading cause of such injuries in older adults.
The modality (type) and dose (frequency) of exercise training are important components of “exercise prescription” for older adults. However, there is a lack of research evidence to help clearly define these exercise parameters to better guide development of consensus exercise recommendations for older patients. This gap in knowledge limits the clinicians’ ability to recommend evidence-based treatment regimens to older adults who are at higher risk for falls. Moreover, although exercise programs are rarely associated with serious adverse events, recent findings from the Lifestyle Interventions and Independence for Elders (LIFE) study found a modest and nonstatistically significant association between long-term, moderate-intensity physical activity programs and an increase in hospitalizations and mortality in older adults.4,5 Taken together, these gaps in knowledge highlight the urgent need to better understand the optimal methods for administering exercise programs in older adults as well as the need for critical appraisals of the benefits and harms associated with long-term exercise training in this vulnerable population.
The results reported by de Souto Barreto and colleagues helped to address these questions. In this study, the authors found that long-term multicomponent training, particularly moderate intensity with balance exercises performed 2 to 3 times a week, appears to be a safe and effective intervention for reducing falls and injurious falls in older adults. Importantly, this type of long-term exercise regimen does not increase hospitalization and mortality, and thus supports the notion that exercise therapy is safe in older adults. Therefore, information gained from this meta-analysis should help to guide clinicians to devise a patient-centered exercise prescription for fall prevention.
The current study was well designed and has a number of strengths. The design of the systematic review and meta-analysis allowed aggregation of data from multiple trials, resulting in a more robust point estimate to evaluate the effects of long-term exercise training on falls and fall-related outcomes that otherwise cannot be achieved with individual trials. In addition, the emphasis on long-term exercise training in older adults in the setting of falls and adverse fall-related outcomes addresses a key area of research that currently lacks a sufficient evidence base. There are also several limitations in this study, primarily due to the nature of its meta-analysis design. For instance, the study populations included in the analysis are highly heterogeneous and range from those with dementia to healthy participants. In addition, long-term exercise training, defined as a duration ≥ 1 year, was arbitrarily established as the minimum period of intervention. Thus, potential important studies that include interventions of significant duration, but less than 1 year, may not have been captured in this analysis.
Applications for Clinical Practice
Falls in older adults are common and may lead to devastating health consequences. The implementation of a long-term, multicomponent, moderate-intensity exercise regimen performed 2 to 3 times weekly can reduce falls and injurious falls in older adults.
—Fred Ko, MD, MS
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
1. Schiller JS, Kramarow EA, Dey AN. Fall injury episodes among noninstitutionalized older adults: United States, 2001-2003. Adv Data. 2007(392);1-16.
2. Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116-119.
3. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750-1758.
4. Liu CJ, Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;CD002759.
5. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387-2396.
Receipt of Primary Care Linked to High-Value Care, Better Health Care Experience
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
Study Overview
Objective. To examine whether receiving primary care is associated with receipt of high-value services and low-value services and quality of patient experience.
Design. Secondary data analysis of the Medical Expenditure
To define whether a respondent received primary care, respondents were asked if they have a “usual source of care” and to provide the name of a physician they usually visit if they “are sick or need advice” about their health. Four additional questions asked respondents if they would visit their usual source of care for (1) “new health problems,” (2) “preventive health care such as general checkups, examinations, and immunizations,” (3) “ongoing health problems,” and (4) “referrals to other health professionals when needed.” These questions were intended to reflect the essential functions of primary care: providing first contact care that is comprehensive, continuous, and coordinated. Any respondents who indicated that they did not have a usual source of care or answered no to any of the 4 questions were considered to not have primary care. Among respondents who identified a usual source of care, 95% met criteria for having primary care.
Setting and participants. The study included 49,286 US adults with primary care and 21,133 US adults without primary care. The average age was 50 years (95% confidence interval [CI], 50-51) among those with primary care and 38 years (95% CI, 38-39) among those without primary care. Among those who had primary care, 55% were female, 50% were non-Hispanic white, 32% Hispanic, and 13% black; among those without primary care, 43% were female, 43% were non-Hispanic white, 35% Hispanic, and 13% black. Among respondents with primary care, 58% considered their health status to be excellent or very good, as compared with 66% of respondents without primary care. Lack of insurance was reported by 7% of respondents with primary care and 34% of respondents without primary care. Chronic disease was reported in 78% of respondents without primary care, as compared with 42% of respondents with primary care. The study uses propensity score matching methods to produce a matched cohort, taking into account potential confounders. The matching procedure resulted in a final sample of 43,766 respondents with primary care matched to 17,964 respondents without primary care.
Main outcome measures. Main study outcome measures included 39 quality measures aggregated into quality composites (6 high-value services and 4 low-value services), and 7 patient care experience measures aggregated into an overall patient experience rating and 2 experience composites. High-value services are defined as delivery of services that are likely of benefit, and include the use of recommended cancer screening such as colorectal cancer screening in appropriate age groups; recommended diagnostic and preventive testing such as cholesterol measurement and influenza vaccination; recommended diabetes care such as hemoglobin A1c measurement; recommended medical treatment for medical conditions such as heart failure, coronary artery disease, and chronic obstructive pulmonary disease; and recommended counseling such as smoking cessation. Low-value services are defined as delivery of services that are considered either inappropriate or of little to no benefit, and include cancer screening in older adults; inappropriate use of antibiotics such as for bronchitis; inappropriate medical treatment such as anxiolytic, sedative, or hypnotic prescriptions for older adults; and inappropriate imaging tests for certain conditions.
Composites of underuse (high-value care) and overuse (low-value care) were constructed from each measure of high- or low-value services by identifying respondents who were eligible for the measure and determining the proportion in which recommended care was delivered (for high-value measures) or avoided (for low-value measures). Patient care experience was measured by standardized CAHPS (Consumer Assessment of Healthcare Providers and Systems) measurement for global rating of health care, doctor communication, and access to care. The patient care experience measures were dichotomized into positive responses as a rating of 8, 9, or 10 on items scored from 0 to 10, and 4 for items scored from 1 to 4. The experience composite was constructed by computing the mean for each respondent and then the mean for all respondents.
Main results. The study found that respondents with primary care were more likely to receive high-value care in 4 of 5 composite measures—cancer screening, diagnostic and preventive testing, diabetes care, and recommended counseling such as smoking cessation—but not in the composite recommended treatment for specific medical conditions such as heart failure. Respondents with primary care were more likely to receive recommended cancer screening, as compared to those without primary care (78% vs 67%, respectively, with a difference of 10.8%; 95% CI, 8.5%-13.0%). Respondents with primary care were also more likely to receive recommended diagnostic and preventive testing (with a difference of 9.9%; 95% CI, 8.7%-11.2%), to receive high-value diabetes care (with a difference of 7.8%; 95% CI, 1.2%-14.4%), and to receive counselling (with a difference of 6.9%; 95% CI, 4.1%-9.7%) when compared to respondents without primary care. However the rates of receipt of high-value medical treatments were similar among respondents with or without primary care (with a difference of –4.6% (95% CI, –14.3% to 5.0%). In contrast, rates of low-value care were similar for those with or without primary care in 3 of 4 composites, including low-value cancer screening, medical treatment, and imaging, while those with primary care had higher rates of low-value antibiotic use (with a difference of 11.0%; 95% CI, 2.8%-19.3%). Respondents with primary care reported better patient care experience, including global rating of their health care, physician communication, and access to care, when compared to those without primary care.
Conclusion. Receipt of primary care is associated with a better patient care experience, more high-value care, and slightly more low-value care.
Commentary
Primary care has long been considered the bedrock of modern health care, and the delivery of comprehensive, continuous, high-quality primary care yields benefits to patients and the health care system.1 Primary care is associated with better outcomes, such as lower mortality and reduced rates of potentially avoidable hospitalizations, and people living in areas with higher concentrations of primary care are more likely to report better health.2 Primary care is also associated with reductions in health care cost and utilization while maintaining quality.2 The current study adds to what is known about the potential benefits of primary care by directly examining the association of the use of primary care versus no primary care with outcomes of high-value care, low-value care, and patient care experience. Because this study used nationally representative data, it was able to examine adults in all age groups, not only older adults in Medicare, which prior studies have relied on.3 The study’s findings—that adults seen in primary care receive more high-value care and report better care experiences—are not surprising. The study also found that slightly more low-value care is being delivered in primary care. These findings are consistent with prior studies. Also, although primary care overall may be associated with health care benefits, there is substantial variation in the rates of overuse (of low-value care) and underuse (of high-value care) in primary care, and this may represent opportunities for improvement.4
This study has several limitations. Because the study defined primary care using questions that identify essential elements of primary care—first contact, comprehensiveness, continuity, and coordinated care—the findings may not apply to all individuals who have identified a primary care provider, but only to those who experience comprehensive, continuous, and coordinated care. Inclusion of all individuals who identify a usual source of primary care as the sole criteria may attenuate the association of primary care with the outcome measures. It is, however, reassuring that among those who identified a usual source of care (primary care), 95% indicated that they have care that is consistent with the principles of first contact care, comprehensiveness, continuity, and coordinated care. Another limitation is that the use of the criteria to indicate high- or low-value care may not capture the nuances of patient-centered care, preferences, or individualized decision-making that occurs in clinical care. Nonetheless, definitions used in the study for high- and low-value care are consistent with prior literature, and offer a standardized measure to indicate quality of care.
Applications for Clinical Practice
A recent trend in health care is the shift of continuity of care from primary care providers or practices to facility-based care or no continuity of care at all, and this shift disproportionately affects patients with low income and is associated with more emergency room visits.5 The current study makes a strong case for the potential benefits of receiving primary care that is comprehensive, continuous, and coordinated, as patients in primary care are more likely to receive high-value over low-value care, and to have a better care experience. The ongoing debate on changes to the health care system and insurance options must take into account the impact of any changes on the population receiving primary care coverage, with the goal that more, rather than fewer, individuals realize the potential benefits of comprehensive primary care.
— William W. Hung, MD MPH
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
1. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
2. American College of Physicians. How is a shortage of primary care physicians affecting the quality and cost of medical care? www.acponline.org/acp_policy/policies/primary_care_shortage_affecting_hc_2008.pdf. Published 2008. Accessed June 11, 2019.
3. Bazemore A, Petterson S, Peterson LE, et al. Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018;16:492-497.
4. O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8:e018557.
5. Liaw W, Jetty A, Petterson S, et al. Trends in the types of usual sources of care: a shift from people to places or nothing at all. Health Serv Res. 2018;53:2346-2367.
Enzalutamide Improves Progression-Free and Overall Survival in Metastatic Hormone-Sensitive Prostate Cancer
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
Study Overview
Objective. To evaluate the efficacy of enzalutamide compared with standard first-line testosterone suppression in men with newly diagnosed metastatic, castrate-sensitive prostate cancer.
Design. Multinational, open-label, randomized phase 3 trial.
Setting and participants. 1125 men were randomly assigned to receive enzalutamide (563 patients) or standard care (562 patients) from March 2014 through March 2017. Eligible patients had a histologic diagnosis of prostate adenocarcinoma with metastases documented by conventional imaging with computed tomography (CT) and/or technetium-99 bone scan. Prior use of adjuvant testosterone suppression was allowed for up to 2 years, provided this had been completed at least 12 months prior to enrollment.
Intervention. Patients were randomized in a 1:1 fashion to receive enzalutamide 160 mg daily or nonsteroidal antiandrogen therapy with bicalutamide, nilutamide, or flutamide. All patients received testosterone suppression with goserelin, leuprolide, or degarelix. Therapy was continued until disease progression or intolerable adverse effects occurred. In November 2014 the protocol was amended to allow for early administration of docetaxel 75 mg/m2 every 3 weeks for 6 cycles and androgen suppression. Patients were stratified according to having received docetaxel prior to randomization. This amendment was based on evidence of improved survival noted with this combination, and the decision to add docetaxel was up to the treating physician. The randomization was further stratified by disease volume, the use of bone-modifying agents, and comorbidity scores. High-volume disease was defined as the presence of visceral metastases or at least 4 bone lesions, with at least 1 being in the appendicular skeleton.
Main outcome measures. The primary endpoint was overall survival (OS). The secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical PFS, death from any cause, or the last known follow-up PSA. PSA progression was defined as an increase in PSA level from the nadir value by ≥ 25% and by ≥ 2 ng/mL.
Main results. The baseline characteristics were well balanced between the treatment arms. High-volume disease was present in 52% of patients. Early docetaxel was planned in 45% of patients; however, 22 patients in whom docetaxel treatment was planned did not receive it. All 6 cycles of docetaxel were given to 159 patients in the enzalutamide group and 181 patients in the standard-care group. After a median follow-up of 34 months, there were 102 deaths in the enzalutamide group and 143 deaths in the standard-care group, with a hazard ratio (HR) for death of 0.67 (95% confidence interval [CI], 0.52-0.86; P = 0.002). Early docetaxel treatment, volume of disease, and use of bone-modifying agents did not affect this outcome. At 3 years, the OS was 80% in the enzalutamide group and 72% in the standard-care group. The rate of PSA-determined PFS was higher in the enzalutamide group compared with the standard group (3-year event-free survival, 67% and 37%, respectively), with a HR of 0.39 (95% CI, 0.33-0.47; P < 0.001). There were fewer clinical PFS events in the enzalutamide group (167 events vs 320 events), with a HR of 0.40 (95% CI, 0.33-0.49; P < 0.001). Analysis of the stratified subgroups showed the effect on OS was diminished in those with use of bone-modifying agents, those with high-volume disease, and those who received early docetaxel. The clinical PFS benefit was maintained across all subgroups, albeit with a smaller effect in those with high-volume disease and in those with early docetaxel treatment.
Treatment discontinuation for reasons other than progressive disease occurred in 12% of those in the enzalutamide group and 19% of those in the standard-care group. Overall, the adverse events were consistent with the known safety profiles of the treatment regimen. Seizures occurred in 7 patients on enzalutamide and no patients in the standard-care group. Fatigue was more common with enzalutamide.
Conclusion. Enzalutamide treatment was associated with significantly longer PFS and OS compared with standard care in men with metastatic, hormone-sensitive prostate cancer receiving testosterone suppression.
Commentary
The current study shows that the addition of enzalutamide to standard androgen deprivation therapy (ADT) improves OS and PFS in men with newly diagnosed metastatic, hormone-sensitive prostate cancer. Until recently, antiandrogen therapy had been the standard of care for these men; however, with the advent of novel antiandrogen agents, outcomes in men with metastatic prostate cancer in both the androgen-sensitive and castrate-resistant settings have steadily improved.1-5 In the castrate-resistant setting, enzalutamide has previously been shown to improve survival in chemotherapy-naïve patients and those previously exposed to docetaxel chemotherapy.5-7 Similarly, in the hormone-sensitive setting the combination of ADT with either abiraterone or chemotherapy has been shown to improve outcomes. In the phase 3 LATITUDE and STAMPEDE trials, the combination of abiraterone plus prednisone and ADT resulted in a 30% and 37% improvement in OS, respectively.1,2 Six cycles of docetaxel in combination with ADT also resulted in a 37% increase in OS in those with high-volume metastatic disease.3
The current study adds to the growing body of literature suggesting that combination therapy in the upfront, hormone-sensitive setting improves outcomes. In the CHAARTED trial, the combination of docetaxel and ADT improved survival in men with high-volume disease, but it did not seem to benefit those with lower-volume disease.3 However, the current data suggests a survival advantage with enzalutamide with low-volume disease as well. The use of docetaxel was similar between the 2 groups, and this suggests that the benefits of enzalutamide cannot be attributed to early integration of docetaxel. It is important to note that the subgroup analysis of those who received early docetaxel showed that these patients did not experience the same survival benefit as those who did not receive docetaxel. However, this trial was not powered for this analysis, and thus it should be interpreted with caution. PFS benefit was maintained across those who received and did not receive early docetaxel. Also worth noting is the increased docetaxel-related toxicity in the combination docetaxel and enzalutamide arm of this study. The neurological toxicity of enzalutamide was again noted, with 7 seizure events documented in this study.
Because this report on the ENZAMET study is an interim analysis, it will be important to follow these outcomes as the data set matures to ensure these effects are maintained over time. Additionally, it will be important to see what implications the addition of enzalutamide have on quality of life measures, as these data have not yet been published.
Applications for Clinical Practice
The ENZAMET study provides evidence that in men with metastatic, hormone-sensitive prostate cancer receiving ADT, the addition of enzalutamide improves PFS and OS. In men who received early docetaxel, enzalutamide was associated with increased toxicity. Additionally, while PFS was improved in men who received enzalutamide and docetaxel, OS was not improved. The neurologic toxicities of enzalutamide should be considered, particularly in those with a prior history of seizure disorders. Based on these data, enzalutamide in combination with ADT represents a reasonable treatment option in men with metastatic, hormone-sensitive prostate cancer.
—Daniel Isaac, DO, MS
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. Kytriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080-1087.
4. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
5. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151-154.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with non-metastatic castration resistant prostate cancer. N Engl J Med. 2018;378:2465-2474.