User login
Too many blood cultures ordered for pediatric SSTIs
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
REPORTING FROM PHM 2019
Poll: Medicare-for-all sees slight drop in support
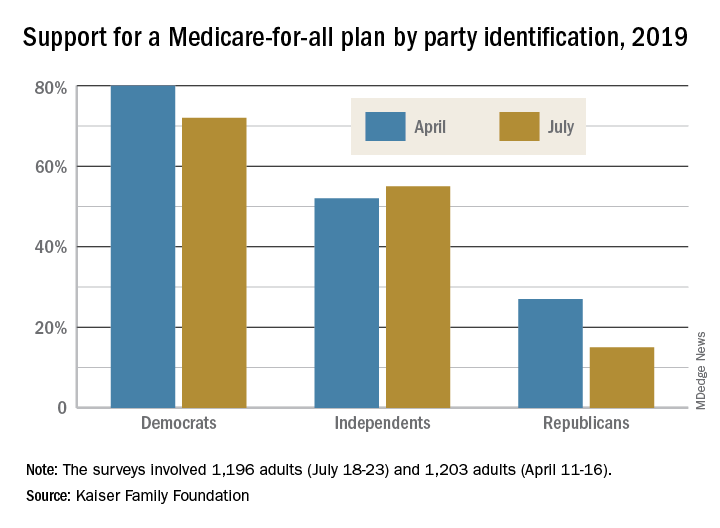
The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.
Dispatch from HM19: COPD updates
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
AI technology meets AFib detection
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
FROM THE LANCET
Preeclampsia doubles risk of postpartum transfusion reactions
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
Women with preeclampsia were found to be at the highest risk for transfusion reactions when receiving a blood transfusion post partum, according to results from a retrospective study.
Additionally, all women who received a transfusion postpartum were twice as likely to experience a procedure-related complication, compared with nonpregnant controls who received identical care.
“The objective of our study was to assess the incidence and risk factors for postpartum [transfusion reactions] in women transfused with red blood cells, plasma, or platelets post partum,” wrote Lars Thurn, PhD, of the Karolinska Institute in Stockholm and colleagues. The findings were reported in Blood Advances.
The researchers conducted a population-based cohort study that included a total of 517,854 women who gave birth in Stockholm County over a period of 21 years. Of those included, 12,183 (2.4%) received a blood transfusion postpartum.
Data was obtained from the Swedish National Birth Registry and was linked to the Stockholm Transfusion Database in order to evaluate the risk of transfusion reactions in pregnant women versus nonpregnant controls.
The researchers identified a total of 96 transfusion reactions postpartum for a prevalence of 79 per 10,000, compared with 40 per 10,000 among nonpregnant controls (odds ratio, 2.0; 95% confidence interval, 1.6-2.5).
The risk of transfusion-related reactions was more than double in pregnant women with preeclampsia versus pregnant women without the condition (OR, 2.1; 95% CI, 1.7-2.6).
“Preeclampsia, induced labor, and preterm delivery were significant risk factors for [transfusion reactions], but we found no differences due to parity, donor gender, or blood group,” the researchers wrote.
The large sample size was a major strength of the study, while a key limitation was the retrospective design.
“Our findings suggest heightened attention be paid when patients with preeclampsia are being evaluated for blood transfusions post partum,” the researchers concluded.
The study was partially funded by Södra Sjukvårdsregionen. The researchers reported having no conflicts of interest.
SOURCE: Thurn L et al. Blood Adv. 2019 Jul 31. doi: 10.1182/bloodadvances.2019000074.
FROM BLOOD ADVANCES
Enteral feeding is safe during bronchiolitis HFNC
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Large prospective trial offers reassurance for long-term PPI use
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Aside from a possible increased risk of enteric infections, long-term use of the proton pump inhibitor (PPI) pantoprazole appears safe in patients with stable atherosclerotic vascular disease, according to a prospective trial involving more than 17,000 participants.
In contrast with published observational studies, the present trial found no associations between long-term PPI use and previously reported risks such as pneumonia, fracture, or cerebrovascular events, according to lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Ont., and colleagues.
“To our knowledge, this is the largest PPI trial for any indication and the first prospective randomized trial to evaluate the many long-term safety concerns related to PPI therapy,” the investigators wrote in Gastroenterology. “It is reassuring that there was no evidence for harm for most of these events other than an excess of enteric infections.”
“Given how commonly acid suppressive medications are used, it is important to ensure that this class of drugs is safe,” the investigators wrote. They noted that patients are often alarmed by “sensational headlines” about PPI safety. “There are balancing articles that more carefully discuss the risks and benefits of taking PPI therapy but these receive less media attention,” the investigators added.
The present, prospective trial, COMPASS, involved 17,598 participants from 33 countries with stable peripheral artery disease and cardiovascular disease. “We use the term participants, rather than patients, as not all of those taking part in this research would have been patients throughout the trial but all participated in the randomized controlled trial,” the investigators wrote.
In addition to evaluating the safety of pantoprazole, the study was initially designed to measure the efficacy of pantoprazole for preventing upper gastrointestinal events in participants taking rivaroxaban and/or aspirin, which, in combination, were recently shown to reduce cardiovascular outcomes among patients with stable cardiovascular conditions. As such, participants in the trial were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or 2.5-mg rivaroxaban twice daily combined with 100-mg aspirin once daily. The primary efficacy outcomes for these three groups were stroke, myocardial infarction, and cardiovascular death. This portion of the trial was discontinued early because of evidence that showed the superiority of combination therapy over aspirin alone; however, the pantoprazole component of the trial continued, as planned, for 3 years.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. Pantoprazole safety outcomes centered on those previously reported by observational studies, including dementia, chronic kidney disease, gastric atrophy, fracture, cancer, pneumonia, diabetes mellitus, chronic obstructive lung disease, Clostrididoides difficile infection, and other enteric infections. Hospitalization rates for noncardiovascular and cardiovascular events were also reported. Data were gathered via questionnaires, which were conducted every 6 months.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking an NSAID (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis of cardiovascular outcomes revealed no significant differences between placebo and pantoprazole groups. Of all the evaluated safety measures, only enteric infections differed significantly between groups, occurring at a higher rate in the pantoprazole group than in the placebo group (1.4% vs. 1.0%; odds ratio, 1.33; 95% confidence interval, 1.01-1.75). Although C. difficile infection was more common among pantoprazole users, only 13 such events occurred, precluding statistical significance.
According to the investigators, these findings should offer reassurance to PPI prescribers and users; they noted that previous findings from observational studies warrant skepticism. “A significant proportion of patients are prescribed PPI therapy inappropriately, and in these cases, it is reasonable to advocate strategies to discontinue acid suppression. However, when there is a clinical need for PPI therapy, these data suggest that the benefits are likely to outweigh any putative risks.”
In regard to the possible increased risk of enteric infection, the investigators again urged a conservative interpretation, as the increased rate of enteric infection among PPI users was still lower than rates reported by systematic reviews. “The data in the current randomized trial were not adjusted for multiple testing so this result should be interpreted with caution,” the investigators wrote. Although acid suppression may allow for increased ingestion of pathogenic organisms, which could theoretically increase the risk of enteric infection, the investigators stated that the benefits of PPIs likely outweigh their risks.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Bayer, Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
AGA patient education on GERD can help your patients better understand and manage the disorder. Post this education or your practice website or share you’re your patients at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
FROM GASTROENTEROLOGY
Key clinical point: Aside from a possible increased risk of enteric infections, long-term use of pantoprazole is safe in patients with stable peripheral artery and cardiovascular disease.
Major finding: Enteric infections were 33% more common in the pantoprazole group than in the placebo group.
Study details: A placebo-controlled, double-blind, randomized trial involving 17,598 patients with stable peripheral artery disease and cardiovascular disease.
Disclosures: The COMPASS trial was funded by Bayer AG. The investigators disclosed relationships with Bayer, Allergan, Takeda, Janssen, and others.
Source: Moayyedi P et al. Gastroenterology. 2019 May 29. doi: 10.1053/j.gastro.2019.05.056.
Cost a factor in breast cancer treatment decisions
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
Treatment costs are a significant factor in women’s decision making around breast cancer surgery, investigators reported.
With the health care costs of breast cancer estimated to reach $20 billion by 2020 in the United States, many of those costs are being shifted onto patients themselves, wrote Rachel A. Greenup, MD, from Duke University, Durham, N.C., and coauthors in the Journal of Oncology Practice.
“This financial hardship is now recognized as a major adverse effect of cancer care and has been associated with reduced quality of life, nonadherence, and an increased risk of early mortality,” they wrote.
Researchers surveyed 607 women with a history of breast cancer to examine the impact that cost had on their decisions about surgery and what financial harm they had experienced after breast cancer surgery.
Overall, 43% of women said they considered costs when making decisions about breast cancer treatment, 28% said cost influenced their decision making around breast cancer surgery, and 14% said costs were extremely important in that decision.
Women in the lowest income bracket – earning at or below $45,000 per year – identified cost as the most influential factor in their decision about breast cancer surgery, above loss of sensation, breast preservation or appearance, the need for long-term surveillance, or avoiding radiation.
However, more than three-quarters of women said they never discussed costs with their medical team.
Bilateral mastectomy, with and without reconstruction, was associated with higher patient-reported out-of-pocket costs, higher debt, higher rates of cancer-induced financial hardship, and higher rates of altered or reduced employment, compared with breast-conserving surgery.
More than one-third of participants reported significant to catastrophic financial burden because of their breast cancer care.
Even in the highest income brackets, two-thirds of women were financially unprepared for the cost of treatment, and 26% said their treatment costs were higher than expected.
The authors commented that “cost transparency” was uncommon between oncologically equivalent surgical treatments, “thus, patients with breast cancer may unknowingly be guiding therapeutic decisions that increase the risk of financial harm.”
“To date, patient out-of-pocket costs and subsequent risk of financial harm have not been routinely incorporated into shared decisions for breast cancer surgery, a process that has otherwise highly revered patient values,” they wrote.
The investigators suggested that revealing the greater risk for financial burden associated with treatments like bilateral mastectomy could help inform surgical treatment decisions.
The study was supported by the National Institutes of Health and the Duke Cancer Institute. Six authors reported honoraria, research funding, prior employment, and other support from the pharmaceutical sector.
SOURCE: Greenup RA et al. J Oncol Pract. 2019 Jul 29. doi: 10.1200/JOP.18.00796.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
DRC Ebola epidemic continues unabated despite international response
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
, “currently the outbreak continues at the same pace, so we don’t see evidence of slowing,” according to Henry Walke, MD, director of the Division of Preparedness and Emerging Infections and Incident Manager, 2018 CDC Ebola Response, Centers for Disease Control and Prevention.
He added that new cases of Ebola have been seen in Goma, which is outside the initial outbreak area. Goma is the largest city in the eastern part of the DRC and a major trading port.
Dr. Walke made his remarks in a telephone media briefing Aug. 1 by the U. S. Department of Health and Human Services outlining the current state of the U.S. response to the outbreak.
He described the efforts of the CDC to provide support to the DRC both from Atlanta and in the field. These efforts included support for vaccination activities in DRC’s North Kivu and Ituri provinces for the population and for at-risk health-care workers in the DRC and neighboring countries. In addition, the United States is involved in the testing of experimental therapeutics and vaccines in the DRC in an effort to aid in this and future outbreaks.
“There are no cases of Ebola in the United States,” said Dr. Walke, and the CDC believes the risk to the United States from the outbreak is low. He cited the limited number of travelers from DRC. “There [are] about 16,000 from the DRC to the U.S. on an annual basis, and only about 100 from Goma itself. There aren’t direct flights and we have at the Goma airport both entry and exit screening.”
According to a World Health Organization report, this Ebola outbreak is the second deadliest on record and has killed 1,750 people out of around 2,518 confirmed cases as of July 23.
Efforts to control the epidemic are severely hampered by civil unrest in the area, public mistrust of the government and health care workers, and a comparative lack of international aid compared to previous Ebola outbreaks.
REPORTING FROM A MEDIA BRIEFING BY HHS
Conflicts of interest common among authors of ASCO guidelines
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.