User login
Early pregnancy loss and abortion: Medical management is safe, effective
NASHVILLE, TENN. – Medical management of abortion and early pregnancy loss is best achieved with both mifepristone and misoprostol, according to Sarah W. Prager, MD.
First-trimester procedures account for about 90% of elective abortions, with about two-thirds of those occurring before 8 weeks of gestation and 80% occurring in the first 10 weeks – and therefore considered eligible for medical management, Dr. Prager, director of the Family Planning Division and Family Planning Fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“We estimate that it’s approximately 31% of all abortions that are done using medication, but it’s about 45% of those eligible by gestational age,” she noted.
The alternative is uterine aspiration, and in the absence of a clear contraindication, patient preference should determine management choice, she said.
The same is true for early pregnancy loss (spontaneous abortion), which is the most common complication of early pregnancy, occurring in about 20% of clinically recognized pregnancies.
“That means that there are about 1 million spontaneous abortions happening annually in the United States, and about 80% of those are in the first trimester,” Dr. Prager said.
Expectant management is an additional option for managing early pregnancy loss, she noted.
Candidates
Medical management is appropriate for patients who are undergoing elective abortion at up to about 70 days of gestation or with pregnancy loss in the first trimester.
“They should have stable vital signs, no evidence of infection, no allergies to the medications being used, no serious social or medical problems,” Dr. Prager said, explaining that “a shared decision making process” is important for patients with extreme anxiety or homelessness/lack of stable housing, for example, in order to make sure that medical management is a good option.
“While she definitely gets to have the final say, unless there is a real medical contraindication, it definitely should be part of that decision making,” Dr. Prager said, adding that adequate counseling and acceptance by the patient of the risks and side effects also are imperative.
Protocol
The most effective evidence-based treatment protocol for elective abortion through day 70 of gestation includes a 200-mg oral dose of mifepristone, followed 24-72 hours later with at-home buccal or vaginal administration of an 800-mcg dose of misoprostol, with follow up within 1-2 weeks, Dr. Prager said, citing a 2010 Cochrane review.
The Food and Drug Administration–approved protocol, which was updated in April 2016, adheres closely to those findings, except that it calls for misoprostol within 48 hours of mifepristone dosing. Optional repeat dosing of misoprostol is allowed, as well, she noted.
Buccal or vaginal administration of misoprostol is preferable to oral and sublingual administration because while the latter approaches provide more rapid onset, the former approaches provide significantly better sustained action over a 5-hour period of time.
“And by not having that big peak at the beginning, it actually decreases the side effects that women experience with the misoprostol medication,” she said.
Misoprostol can also be given alone for early pregnancy loss management – also at a dose of 800 mcg buccally or vaginally – with repeat dosing at 12-24 hours for incomplete abortion. However, new data suggest that, before about 63 days of gestation, giving two doses 3 hours apart is slightly more effective. That approach can also be repeated if necessary, Dr. Prager said.
Pain management is an important part of treatment, as both miscarriage and medication abortion can range from uncomfortable to extremely painful, depending on the patient, her prior obstetric experience, and her life experiences.
“I recommend talking to all your patients about pain management. For most people, just using some type of NSAID is probably going to be sufficient,” she said, noting that some women will require a narcotic.
Antiemetic medication may also be necessary, as some women will experience nausea and vomiting.
Complications and intervention
Major complications are rare with medical management of first-trimester abortion and early pregnancy loss, but can include ongoing pregnancy, which is infrequent but possible; incomplete abortion, which is easily managed; and allergic reactions, which are “extremely rare,” Dr. Prager said.
Hemorrhage can occur, but isn’t common and usually is at a level that doesn’t require blood transfusion. “But it does require somebody to come in, potentially needing uterine aspiration or sometimes just a second dose of misoprostol,” she said.
Serious infections are “extraordinarily uncommon,” with an actual risk of infectious death of 0.5 per 100,000, and therefore antibiotic prophylaxis is not recommended.
“This is not to say that there can’t be serious infectious problems with medication abortion, and actually also with spontaneous abortion ... but it’s extremely rare,” Dr. Prager said, adding that “there are also consequences to giving everybody antibiotics if they are not necessary. I, personally, am way more afraid of antibiotic resistance these days than I am about preventing an infection from an medication abortion.”
Intervention is necessary in certain situations, including when the gestational sac remains and when the patient continues to have clinical symptoms or has developed clinical symptoms, she said.
“Does she now show signs of infection? Is she bleeding very heavily or [is she] extremely uncomfortable with cramping? Those are all really great reasons to intervene,” she said.
Sometimes patients just prefer to switch to an alternative method of management, particularly in cases of early pregnancy loss when medical management has “not been successful after some period of time,” Dr. Prager added.
Outcomes
Studies have shown that the success rates with a single dose of 400-800 mcg of misoprostol range from 25% to 88%, and with repeat dosing for incomplete abortion at 24 hours, the success rate improves to between 80% and 88%. The success rate with placebo is 16%-60%; this indicates that “some miscarriages just happen expectantly,” Dr. Prager explained.
“We already knew that ... and that’s why expectant management is an option with early pregnancy loss,” she said, adding that expectant management works about 50% of the time – “if you wait long enough.”
However, success rates with medical management depend on the type of miscarriage; the rate is close to 100% with incomplete abortion, but for other types, such as anembryonic pregnancy or fetal demise, it is slightly less effective at about 87%, Dr. Prager noted.
When mifepristone and misoprostol are both used, success rates for early pregnancy loss range from 52% to 84% in observational trials and using nonstandard doses, and between 90% and 93% with standard dosing.
Other recent data, which led to a 2016 “reaffirmation” of an ACOG practice bulletin on medical management of first-trimester abortion, show an 83% success rate with the combination therapy in anembryonic pregnancies, and a 25% reduction in the need for further intervention (N Engl J Med. 2018;378:2161-70).
“So it really was significantly more effective to be using that addition of the mifepristone,” she said. “My take-home message about this is that, if mifepristone is something that you have easily available to you at your clinical site, absolutely use it, because it creates better outcomes for your patients. However, if it’s not available to you ... it is still perfectly reasonable for patients to choose medication management of their early pregnancy loss and use misoprostol only.
“It is effective enough, and that is just part of your informed consent.”
Postabortion care
Postmiscarriage care is important and involves several components, Dr. Prager said.
- RhoGAM treatment. The use of RhoGAM to prevent Rh immunization has been routine, but data increasingly suggest this is not necessary, and in some countries it is not given at all, particularly at 8 or fewer weeks of gestation and sometimes even during the whole first trimester for early pregnancy loss. “That is not common practice yet in the United States; I’m not recommending at this time that everybody change their practice ... but I will say that there are some really interesting studies going on right now in the United States that are looking specifically at this, and I think we may, within the next 10 years or so, change this practice of giving RhoGAM at all gestational ages,” she said.
- Counseling about bleeding. Light to moderate bleeding after abortion is common for about 2 weeks after abortion, with normal menses returning between 4 and 8 weeks, and typically around 6 weeks. “I usually ask patients to come back and see me if they have not had what seems to be a normal period to them 8 weeks following their completed process,” Dr. Prager said.
- Counseling about human chorionic gonadotropin levels. It is also helpful to inform patients that human chorionic gonadotropin may remain present for about 2-4 weeks after completed abortion, resulting in a positive pregnancy test during that time. A positive test at 4 weeks may still be normal, but warrants evaluation to determine why the patient is testing positive.
- Counseling about conception timing. Data do not support delaying repeat pregnancy after abortion. Studies show no difference in the ability to conceive or in pregnancy outcomes among women who conceive without delay after early pregnancy loss and in those who wait at least 3 months. “So what I now tell women is ‘when you’re emotionally ready to start trying to get pregnant again, it’s perfectly medically acceptable to do so. There’s no biologic reason why you have to wait,’ ” she said.
- Contraception initiation. Contraception, including IUDs, can be initiated right away after elective or spontaneous abortion. However, for IUD insertion after medical abortion, it is important to first use ultrasound to confirm complete abortion, Dr. Prager said.
- Grief counseling. This may be appropriate in cases of early pregnancy loss and for elective abortions. “Both groups of people may need some counseling, may be experiencing grief around this process – and they may not be,” she said. “I think we just need to be sensitive about asking our patients what their needs might be around this.”
Future directions
The future of medical management for first trimester abortion may involve “demedicalization,” Dr. Prager said.
“There are many papers coming out now about clinic versus home use of mifepristone,” she said, explaining that home use would require removing the FDA’s Risk Evaluation and Mitigation Strategy restriction that requires that the drug be dispensed in a clinic by a physician or physician extender.
Studies are also looking at prescriptions, pharmacist provision of mifepristone, and mailing of medications to women in rural areas.
Another area of research beyond these “really creative ways of using these medications” is whether medical management is effective beyond 10 weeks. A study that will soon be published is looking at mifepristone and two doses of misoprostol at 11 weeks, she noted.
“I think from pregnancy diagnosis through at least week 10 – soon we will see potentially week 11 – medical abortion techniques are safe, they’re effective, and they’re extremely well accepted by patients,” she said. “Also ... a diverse group of clinicians can be trained to offer medical abortion and provide back-up so that access can be improved.”
Dr. Prager reported having no financial disclosures.
NASHVILLE, TENN. – Medical management of abortion and early pregnancy loss is best achieved with both mifepristone and misoprostol, according to Sarah W. Prager, MD.
First-trimester procedures account for about 90% of elective abortions, with about two-thirds of those occurring before 8 weeks of gestation and 80% occurring in the first 10 weeks – and therefore considered eligible for medical management, Dr. Prager, director of the Family Planning Division and Family Planning Fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“We estimate that it’s approximately 31% of all abortions that are done using medication, but it’s about 45% of those eligible by gestational age,” she noted.
The alternative is uterine aspiration, and in the absence of a clear contraindication, patient preference should determine management choice, she said.
The same is true for early pregnancy loss (spontaneous abortion), which is the most common complication of early pregnancy, occurring in about 20% of clinically recognized pregnancies.
“That means that there are about 1 million spontaneous abortions happening annually in the United States, and about 80% of those are in the first trimester,” Dr. Prager said.
Expectant management is an additional option for managing early pregnancy loss, she noted.
Candidates
Medical management is appropriate for patients who are undergoing elective abortion at up to about 70 days of gestation or with pregnancy loss in the first trimester.
“They should have stable vital signs, no evidence of infection, no allergies to the medications being used, no serious social or medical problems,” Dr. Prager said, explaining that “a shared decision making process” is important for patients with extreme anxiety or homelessness/lack of stable housing, for example, in order to make sure that medical management is a good option.
“While she definitely gets to have the final say, unless there is a real medical contraindication, it definitely should be part of that decision making,” Dr. Prager said, adding that adequate counseling and acceptance by the patient of the risks and side effects also are imperative.
Protocol
The most effective evidence-based treatment protocol for elective abortion through day 70 of gestation includes a 200-mg oral dose of mifepristone, followed 24-72 hours later with at-home buccal or vaginal administration of an 800-mcg dose of misoprostol, with follow up within 1-2 weeks, Dr. Prager said, citing a 2010 Cochrane review.
The Food and Drug Administration–approved protocol, which was updated in April 2016, adheres closely to those findings, except that it calls for misoprostol within 48 hours of mifepristone dosing. Optional repeat dosing of misoprostol is allowed, as well, she noted.
Buccal or vaginal administration of misoprostol is preferable to oral and sublingual administration because while the latter approaches provide more rapid onset, the former approaches provide significantly better sustained action over a 5-hour period of time.
“And by not having that big peak at the beginning, it actually decreases the side effects that women experience with the misoprostol medication,” she said.
Misoprostol can also be given alone for early pregnancy loss management – also at a dose of 800 mcg buccally or vaginally – with repeat dosing at 12-24 hours for incomplete abortion. However, new data suggest that, before about 63 days of gestation, giving two doses 3 hours apart is slightly more effective. That approach can also be repeated if necessary, Dr. Prager said.
Pain management is an important part of treatment, as both miscarriage and medication abortion can range from uncomfortable to extremely painful, depending on the patient, her prior obstetric experience, and her life experiences.
“I recommend talking to all your patients about pain management. For most people, just using some type of NSAID is probably going to be sufficient,” she said, noting that some women will require a narcotic.
Antiemetic medication may also be necessary, as some women will experience nausea and vomiting.
Complications and intervention
Major complications are rare with medical management of first-trimester abortion and early pregnancy loss, but can include ongoing pregnancy, which is infrequent but possible; incomplete abortion, which is easily managed; and allergic reactions, which are “extremely rare,” Dr. Prager said.
Hemorrhage can occur, but isn’t common and usually is at a level that doesn’t require blood transfusion. “But it does require somebody to come in, potentially needing uterine aspiration or sometimes just a second dose of misoprostol,” she said.
Serious infections are “extraordinarily uncommon,” with an actual risk of infectious death of 0.5 per 100,000, and therefore antibiotic prophylaxis is not recommended.
“This is not to say that there can’t be serious infectious problems with medication abortion, and actually also with spontaneous abortion ... but it’s extremely rare,” Dr. Prager said, adding that “there are also consequences to giving everybody antibiotics if they are not necessary. I, personally, am way more afraid of antibiotic resistance these days than I am about preventing an infection from an medication abortion.”
Intervention is necessary in certain situations, including when the gestational sac remains and when the patient continues to have clinical symptoms or has developed clinical symptoms, she said.
“Does she now show signs of infection? Is she bleeding very heavily or [is she] extremely uncomfortable with cramping? Those are all really great reasons to intervene,” she said.
Sometimes patients just prefer to switch to an alternative method of management, particularly in cases of early pregnancy loss when medical management has “not been successful after some period of time,” Dr. Prager added.
Outcomes
Studies have shown that the success rates with a single dose of 400-800 mcg of misoprostol range from 25% to 88%, and with repeat dosing for incomplete abortion at 24 hours, the success rate improves to between 80% and 88%. The success rate with placebo is 16%-60%; this indicates that “some miscarriages just happen expectantly,” Dr. Prager explained.
“We already knew that ... and that’s why expectant management is an option with early pregnancy loss,” she said, adding that expectant management works about 50% of the time – “if you wait long enough.”
However, success rates with medical management depend on the type of miscarriage; the rate is close to 100% with incomplete abortion, but for other types, such as anembryonic pregnancy or fetal demise, it is slightly less effective at about 87%, Dr. Prager noted.
When mifepristone and misoprostol are both used, success rates for early pregnancy loss range from 52% to 84% in observational trials and using nonstandard doses, and between 90% and 93% with standard dosing.
Other recent data, which led to a 2016 “reaffirmation” of an ACOG practice bulletin on medical management of first-trimester abortion, show an 83% success rate with the combination therapy in anembryonic pregnancies, and a 25% reduction in the need for further intervention (N Engl J Med. 2018;378:2161-70).
“So it really was significantly more effective to be using that addition of the mifepristone,” she said. “My take-home message about this is that, if mifepristone is something that you have easily available to you at your clinical site, absolutely use it, because it creates better outcomes for your patients. However, if it’s not available to you ... it is still perfectly reasonable for patients to choose medication management of their early pregnancy loss and use misoprostol only.
“It is effective enough, and that is just part of your informed consent.”
Postabortion care
Postmiscarriage care is important and involves several components, Dr. Prager said.
- RhoGAM treatment. The use of RhoGAM to prevent Rh immunization has been routine, but data increasingly suggest this is not necessary, and in some countries it is not given at all, particularly at 8 or fewer weeks of gestation and sometimes even during the whole first trimester for early pregnancy loss. “That is not common practice yet in the United States; I’m not recommending at this time that everybody change their practice ... but I will say that there are some really interesting studies going on right now in the United States that are looking specifically at this, and I think we may, within the next 10 years or so, change this practice of giving RhoGAM at all gestational ages,” she said.
- Counseling about bleeding. Light to moderate bleeding after abortion is common for about 2 weeks after abortion, with normal menses returning between 4 and 8 weeks, and typically around 6 weeks. “I usually ask patients to come back and see me if they have not had what seems to be a normal period to them 8 weeks following their completed process,” Dr. Prager said.
- Counseling about human chorionic gonadotropin levels. It is also helpful to inform patients that human chorionic gonadotropin may remain present for about 2-4 weeks after completed abortion, resulting in a positive pregnancy test during that time. A positive test at 4 weeks may still be normal, but warrants evaluation to determine why the patient is testing positive.
- Counseling about conception timing. Data do not support delaying repeat pregnancy after abortion. Studies show no difference in the ability to conceive or in pregnancy outcomes among women who conceive without delay after early pregnancy loss and in those who wait at least 3 months. “So what I now tell women is ‘when you’re emotionally ready to start trying to get pregnant again, it’s perfectly medically acceptable to do so. There’s no biologic reason why you have to wait,’ ” she said.
- Contraception initiation. Contraception, including IUDs, can be initiated right away after elective or spontaneous abortion. However, for IUD insertion after medical abortion, it is important to first use ultrasound to confirm complete abortion, Dr. Prager said.
- Grief counseling. This may be appropriate in cases of early pregnancy loss and for elective abortions. “Both groups of people may need some counseling, may be experiencing grief around this process – and they may not be,” she said. “I think we just need to be sensitive about asking our patients what their needs might be around this.”
Future directions
The future of medical management for first trimester abortion may involve “demedicalization,” Dr. Prager said.
“There are many papers coming out now about clinic versus home use of mifepristone,” she said, explaining that home use would require removing the FDA’s Risk Evaluation and Mitigation Strategy restriction that requires that the drug be dispensed in a clinic by a physician or physician extender.
Studies are also looking at prescriptions, pharmacist provision of mifepristone, and mailing of medications to women in rural areas.
Another area of research beyond these “really creative ways of using these medications” is whether medical management is effective beyond 10 weeks. A study that will soon be published is looking at mifepristone and two doses of misoprostol at 11 weeks, she noted.
“I think from pregnancy diagnosis through at least week 10 – soon we will see potentially week 11 – medical abortion techniques are safe, they’re effective, and they’re extremely well accepted by patients,” she said. “Also ... a diverse group of clinicians can be trained to offer medical abortion and provide back-up so that access can be improved.”
Dr. Prager reported having no financial disclosures.
NASHVILLE, TENN. – Medical management of abortion and early pregnancy loss is best achieved with both mifepristone and misoprostol, according to Sarah W. Prager, MD.
First-trimester procedures account for about 90% of elective abortions, with about two-thirds of those occurring before 8 weeks of gestation and 80% occurring in the first 10 weeks – and therefore considered eligible for medical management, Dr. Prager, director of the Family Planning Division and Family Planning Fellowship at the University of Washington, Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“We estimate that it’s approximately 31% of all abortions that are done using medication, but it’s about 45% of those eligible by gestational age,” she noted.
The alternative is uterine aspiration, and in the absence of a clear contraindication, patient preference should determine management choice, she said.
The same is true for early pregnancy loss (spontaneous abortion), which is the most common complication of early pregnancy, occurring in about 20% of clinically recognized pregnancies.
“That means that there are about 1 million spontaneous abortions happening annually in the United States, and about 80% of those are in the first trimester,” Dr. Prager said.
Expectant management is an additional option for managing early pregnancy loss, she noted.
Candidates
Medical management is appropriate for patients who are undergoing elective abortion at up to about 70 days of gestation or with pregnancy loss in the first trimester.
“They should have stable vital signs, no evidence of infection, no allergies to the medications being used, no serious social or medical problems,” Dr. Prager said, explaining that “a shared decision making process” is important for patients with extreme anxiety or homelessness/lack of stable housing, for example, in order to make sure that medical management is a good option.
“While she definitely gets to have the final say, unless there is a real medical contraindication, it definitely should be part of that decision making,” Dr. Prager said, adding that adequate counseling and acceptance by the patient of the risks and side effects also are imperative.
Protocol
The most effective evidence-based treatment protocol for elective abortion through day 70 of gestation includes a 200-mg oral dose of mifepristone, followed 24-72 hours later with at-home buccal or vaginal administration of an 800-mcg dose of misoprostol, with follow up within 1-2 weeks, Dr. Prager said, citing a 2010 Cochrane review.
The Food and Drug Administration–approved protocol, which was updated in April 2016, adheres closely to those findings, except that it calls for misoprostol within 48 hours of mifepristone dosing. Optional repeat dosing of misoprostol is allowed, as well, she noted.
Buccal or vaginal administration of misoprostol is preferable to oral and sublingual administration because while the latter approaches provide more rapid onset, the former approaches provide significantly better sustained action over a 5-hour period of time.
“And by not having that big peak at the beginning, it actually decreases the side effects that women experience with the misoprostol medication,” she said.
Misoprostol can also be given alone for early pregnancy loss management – also at a dose of 800 mcg buccally or vaginally – with repeat dosing at 12-24 hours for incomplete abortion. However, new data suggest that, before about 63 days of gestation, giving two doses 3 hours apart is slightly more effective. That approach can also be repeated if necessary, Dr. Prager said.
Pain management is an important part of treatment, as both miscarriage and medication abortion can range from uncomfortable to extremely painful, depending on the patient, her prior obstetric experience, and her life experiences.
“I recommend talking to all your patients about pain management. For most people, just using some type of NSAID is probably going to be sufficient,” she said, noting that some women will require a narcotic.
Antiemetic medication may also be necessary, as some women will experience nausea and vomiting.
Complications and intervention
Major complications are rare with medical management of first-trimester abortion and early pregnancy loss, but can include ongoing pregnancy, which is infrequent but possible; incomplete abortion, which is easily managed; and allergic reactions, which are “extremely rare,” Dr. Prager said.
Hemorrhage can occur, but isn’t common and usually is at a level that doesn’t require blood transfusion. “But it does require somebody to come in, potentially needing uterine aspiration or sometimes just a second dose of misoprostol,” she said.
Serious infections are “extraordinarily uncommon,” with an actual risk of infectious death of 0.5 per 100,000, and therefore antibiotic prophylaxis is not recommended.
“This is not to say that there can’t be serious infectious problems with medication abortion, and actually also with spontaneous abortion ... but it’s extremely rare,” Dr. Prager said, adding that “there are also consequences to giving everybody antibiotics if they are not necessary. I, personally, am way more afraid of antibiotic resistance these days than I am about preventing an infection from an medication abortion.”
Intervention is necessary in certain situations, including when the gestational sac remains and when the patient continues to have clinical symptoms or has developed clinical symptoms, she said.
“Does she now show signs of infection? Is she bleeding very heavily or [is she] extremely uncomfortable with cramping? Those are all really great reasons to intervene,” she said.
Sometimes patients just prefer to switch to an alternative method of management, particularly in cases of early pregnancy loss when medical management has “not been successful after some period of time,” Dr. Prager added.
Outcomes
Studies have shown that the success rates with a single dose of 400-800 mcg of misoprostol range from 25% to 88%, and with repeat dosing for incomplete abortion at 24 hours, the success rate improves to between 80% and 88%. The success rate with placebo is 16%-60%; this indicates that “some miscarriages just happen expectantly,” Dr. Prager explained.
“We already knew that ... and that’s why expectant management is an option with early pregnancy loss,” she said, adding that expectant management works about 50% of the time – “if you wait long enough.”
However, success rates with medical management depend on the type of miscarriage; the rate is close to 100% with incomplete abortion, but for other types, such as anembryonic pregnancy or fetal demise, it is slightly less effective at about 87%, Dr. Prager noted.
When mifepristone and misoprostol are both used, success rates for early pregnancy loss range from 52% to 84% in observational trials and using nonstandard doses, and between 90% and 93% with standard dosing.
Other recent data, which led to a 2016 “reaffirmation” of an ACOG practice bulletin on medical management of first-trimester abortion, show an 83% success rate with the combination therapy in anembryonic pregnancies, and a 25% reduction in the need for further intervention (N Engl J Med. 2018;378:2161-70).
“So it really was significantly more effective to be using that addition of the mifepristone,” she said. “My take-home message about this is that, if mifepristone is something that you have easily available to you at your clinical site, absolutely use it, because it creates better outcomes for your patients. However, if it’s not available to you ... it is still perfectly reasonable for patients to choose medication management of their early pregnancy loss and use misoprostol only.
“It is effective enough, and that is just part of your informed consent.”
Postabortion care
Postmiscarriage care is important and involves several components, Dr. Prager said.
- RhoGAM treatment. The use of RhoGAM to prevent Rh immunization has been routine, but data increasingly suggest this is not necessary, and in some countries it is not given at all, particularly at 8 or fewer weeks of gestation and sometimes even during the whole first trimester for early pregnancy loss. “That is not common practice yet in the United States; I’m not recommending at this time that everybody change their practice ... but I will say that there are some really interesting studies going on right now in the United States that are looking specifically at this, and I think we may, within the next 10 years or so, change this practice of giving RhoGAM at all gestational ages,” she said.
- Counseling about bleeding. Light to moderate bleeding after abortion is common for about 2 weeks after abortion, with normal menses returning between 4 and 8 weeks, and typically around 6 weeks. “I usually ask patients to come back and see me if they have not had what seems to be a normal period to them 8 weeks following their completed process,” Dr. Prager said.
- Counseling about human chorionic gonadotropin levels. It is also helpful to inform patients that human chorionic gonadotropin may remain present for about 2-4 weeks after completed abortion, resulting in a positive pregnancy test during that time. A positive test at 4 weeks may still be normal, but warrants evaluation to determine why the patient is testing positive.
- Counseling about conception timing. Data do not support delaying repeat pregnancy after abortion. Studies show no difference in the ability to conceive or in pregnancy outcomes among women who conceive without delay after early pregnancy loss and in those who wait at least 3 months. “So what I now tell women is ‘when you’re emotionally ready to start trying to get pregnant again, it’s perfectly medically acceptable to do so. There’s no biologic reason why you have to wait,’ ” she said.
- Contraception initiation. Contraception, including IUDs, can be initiated right away after elective or spontaneous abortion. However, for IUD insertion after medical abortion, it is important to first use ultrasound to confirm complete abortion, Dr. Prager said.
- Grief counseling. This may be appropriate in cases of early pregnancy loss and for elective abortions. “Both groups of people may need some counseling, may be experiencing grief around this process – and they may not be,” she said. “I think we just need to be sensitive about asking our patients what their needs might be around this.”
Future directions
The future of medical management for first trimester abortion may involve “demedicalization,” Dr. Prager said.
“There are many papers coming out now about clinic versus home use of mifepristone,” she said, explaining that home use would require removing the FDA’s Risk Evaluation and Mitigation Strategy restriction that requires that the drug be dispensed in a clinic by a physician or physician extender.
Studies are also looking at prescriptions, pharmacist provision of mifepristone, and mailing of medications to women in rural areas.
Another area of research beyond these “really creative ways of using these medications” is whether medical management is effective beyond 10 weeks. A study that will soon be published is looking at mifepristone and two doses of misoprostol at 11 weeks, she noted.
“I think from pregnancy diagnosis through at least week 10 – soon we will see potentially week 11 – medical abortion techniques are safe, they’re effective, and they’re extremely well accepted by patients,” she said. “Also ... a diverse group of clinicians can be trained to offer medical abortion and provide back-up so that access can be improved.”
Dr. Prager reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Dr. Carl Bell always asked the hard questions
Carl C. Bell, MD, started his career by asking the hard questions that no one dared to ask. His curiosity, courage, and compassion for all communities would lead him to impact the world in ways that few psychiatrists could ever imagine.
His accomplishments were many and far-reaching, dating back over 4 decades of service and research. He was a prolific author and researcher, having written more than 400 books, chapters, and articles. His research covered a lot of ground, but four critical areas of focus were childhood trauma, violence prevention, criminal justice reform, and most recently, fetal alcohol spectrum disorders.
The word “visionary” is frequently overused. But when we apply it to Dr. Carl Bell, the word does not do him justice. If you take a closer look at all four of those areas, you can see a common thread: What are the elements of a society that can tear communities apart?
When I look at Dr. Bell’s research, I see a man with a dedicated vision to addressing each of those elements in systematic way, and a determination to bring the results of that research into his Southside Chicago community in numerous ways, including by serving as president and CEO of the Community Mental Health Council, and as director of the Institute for Juvenile Research at the University of Illinois at Chicago.
Dr. Bell was a leader for his patients as well as for black psychiatrists. He was a founding member of Black Psychiatrists of America and served as a mentor in some way to the vast majority of black psychiatrists currently practicing in the country. As a black male psychiatrist, I saw Dr. Bell as a source of inspiration in my career and the standard by which I measured myself. I’m not talking about awards or accomplishments, as Dr. Bell has countless accolades, including most recently, being presented this year with the American Psychiatric Association’s Adolph Meyer Award for Lifetime Achievement in Psychiatric Research and the National Medical Association’s Scroll of Merit. I am referring to Dr. Bell’s willingness to walk away from something he thought was wrong.
For every accolade he won or prestigious committee he served on, I would wager that he declined or stepped way from just as many. Dr. Bell’s character and vision for psychiatry in general, and the mental health of African American communities specifically, would not allow him to pay lip service to agendas that were self-serving, and did not push the field and communities forward.
During his service as an editorial advisory board member for Clinical Psychiatry News, Dr. Bell could always be relied upon to offer an insightful perspective to any discussion, ranging from violence prevention to the social determinants of health and their role in fetal alcohol spectrum disorders.
What I will remember most about Dr. Bell is his strong character. My guess is that he was never shy about stating the truth to a patient or a president of the United States. He possessed the intellect to back up any of his views while also having the humility of a dedicated community psychiatrist who worked for no other reason than to serve his patients.
When I first met Dr. Bell, he was giving a grand rounds at the George Washington University department of psychiatry. He was wearing a hat during the lecture and a belt with the Superman logo. I thought to myself, “Whoa, this is a different type of guy,” then I sat and listened to the talk, and was utterly astounded by his intellect, humor, honesty, and passion for his patients. I had never heard a psychiatrist speak with a combination of such command and approachability, and again, I thought to myself, “Whoa, this is a different type of guy.”
Little did I know that Dr. Bell and I would end up serving together on the editorial board of CPN. I loved seeing Dr. Bell, and catching up and gleaning from his wisdom. I was very humbled by how generous Dr. Bell was with his time and the extent to which he would make himself available as a mentor. During a CPN board meeting, we were trying to come up with a mantra that would capture the mission of the new MDedge Psychiatry website. Dr. Bell (in a hat, of course) let everyone else talk, and then, in a calm voice, said: “We ask the hard questions.” As editor in chief of MDedge Psychiatry, I knew this had to be the mantra. It was aspirational, gave us an identity, and held our feet to the fire – to always ask hard questions in the service of patients and readers. I looked forward to discussing the evolution of the site, seeing him at meetings and conferences, brainstorming the implications of new advances in the field, and simply walking down the street and laughing.
Those events will never occur again, because Carl left us on Thursday, Aug 1. Carl has left us with a legacy of work that we are still coming to appreciate. He left us with a mandate to pursue the truth and make an impact in our communities. He taught black psychiatrists what it meant to stand up unapologetically for your community and society. As I reflect on the scope of his life, I have one last hard question for Carl: “Why did you have to leave us so soon?”
Dr. Norris is assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Norris also is host of the MDedge Psychcast.
Carl C. Bell, MD, started his career by asking the hard questions that no one dared to ask. His curiosity, courage, and compassion for all communities would lead him to impact the world in ways that few psychiatrists could ever imagine.
His accomplishments were many and far-reaching, dating back over 4 decades of service and research. He was a prolific author and researcher, having written more than 400 books, chapters, and articles. His research covered a lot of ground, but four critical areas of focus were childhood trauma, violence prevention, criminal justice reform, and most recently, fetal alcohol spectrum disorders.
The word “visionary” is frequently overused. But when we apply it to Dr. Carl Bell, the word does not do him justice. If you take a closer look at all four of those areas, you can see a common thread: What are the elements of a society that can tear communities apart?
When I look at Dr. Bell’s research, I see a man with a dedicated vision to addressing each of those elements in systematic way, and a determination to bring the results of that research into his Southside Chicago community in numerous ways, including by serving as president and CEO of the Community Mental Health Council, and as director of the Institute for Juvenile Research at the University of Illinois at Chicago.
Dr. Bell was a leader for his patients as well as for black psychiatrists. He was a founding member of Black Psychiatrists of America and served as a mentor in some way to the vast majority of black psychiatrists currently practicing in the country. As a black male psychiatrist, I saw Dr. Bell as a source of inspiration in my career and the standard by which I measured myself. I’m not talking about awards or accomplishments, as Dr. Bell has countless accolades, including most recently, being presented this year with the American Psychiatric Association’s Adolph Meyer Award for Lifetime Achievement in Psychiatric Research and the National Medical Association’s Scroll of Merit. I am referring to Dr. Bell’s willingness to walk away from something he thought was wrong.
For every accolade he won or prestigious committee he served on, I would wager that he declined or stepped way from just as many. Dr. Bell’s character and vision for psychiatry in general, and the mental health of African American communities specifically, would not allow him to pay lip service to agendas that were self-serving, and did not push the field and communities forward.
During his service as an editorial advisory board member for Clinical Psychiatry News, Dr. Bell could always be relied upon to offer an insightful perspective to any discussion, ranging from violence prevention to the social determinants of health and their role in fetal alcohol spectrum disorders.
What I will remember most about Dr. Bell is his strong character. My guess is that he was never shy about stating the truth to a patient or a president of the United States. He possessed the intellect to back up any of his views while also having the humility of a dedicated community psychiatrist who worked for no other reason than to serve his patients.
When I first met Dr. Bell, he was giving a grand rounds at the George Washington University department of psychiatry. He was wearing a hat during the lecture and a belt with the Superman logo. I thought to myself, “Whoa, this is a different type of guy,” then I sat and listened to the talk, and was utterly astounded by his intellect, humor, honesty, and passion for his patients. I had never heard a psychiatrist speak with a combination of such command and approachability, and again, I thought to myself, “Whoa, this is a different type of guy.”
Little did I know that Dr. Bell and I would end up serving together on the editorial board of CPN. I loved seeing Dr. Bell, and catching up and gleaning from his wisdom. I was very humbled by how generous Dr. Bell was with his time and the extent to which he would make himself available as a mentor. During a CPN board meeting, we were trying to come up with a mantra that would capture the mission of the new MDedge Psychiatry website. Dr. Bell (in a hat, of course) let everyone else talk, and then, in a calm voice, said: “We ask the hard questions.” As editor in chief of MDedge Psychiatry, I knew this had to be the mantra. It was aspirational, gave us an identity, and held our feet to the fire – to always ask hard questions in the service of patients and readers. I looked forward to discussing the evolution of the site, seeing him at meetings and conferences, brainstorming the implications of new advances in the field, and simply walking down the street and laughing.
Those events will never occur again, because Carl left us on Thursday, Aug 1. Carl has left us with a legacy of work that we are still coming to appreciate. He left us with a mandate to pursue the truth and make an impact in our communities. He taught black psychiatrists what it meant to stand up unapologetically for your community and society. As I reflect on the scope of his life, I have one last hard question for Carl: “Why did you have to leave us so soon?”
Dr. Norris is assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Norris also is host of the MDedge Psychcast.
Carl C. Bell, MD, started his career by asking the hard questions that no one dared to ask. His curiosity, courage, and compassion for all communities would lead him to impact the world in ways that few psychiatrists could ever imagine.
His accomplishments were many and far-reaching, dating back over 4 decades of service and research. He was a prolific author and researcher, having written more than 400 books, chapters, and articles. His research covered a lot of ground, but four critical areas of focus were childhood trauma, violence prevention, criminal justice reform, and most recently, fetal alcohol spectrum disorders.
The word “visionary” is frequently overused. But when we apply it to Dr. Carl Bell, the word does not do him justice. If you take a closer look at all four of those areas, you can see a common thread: What are the elements of a society that can tear communities apart?
When I look at Dr. Bell’s research, I see a man with a dedicated vision to addressing each of those elements in systematic way, and a determination to bring the results of that research into his Southside Chicago community in numerous ways, including by serving as president and CEO of the Community Mental Health Council, and as director of the Institute for Juvenile Research at the University of Illinois at Chicago.
Dr. Bell was a leader for his patients as well as for black psychiatrists. He was a founding member of Black Psychiatrists of America and served as a mentor in some way to the vast majority of black psychiatrists currently practicing in the country. As a black male psychiatrist, I saw Dr. Bell as a source of inspiration in my career and the standard by which I measured myself. I’m not talking about awards or accomplishments, as Dr. Bell has countless accolades, including most recently, being presented this year with the American Psychiatric Association’s Adolph Meyer Award for Lifetime Achievement in Psychiatric Research and the National Medical Association’s Scroll of Merit. I am referring to Dr. Bell’s willingness to walk away from something he thought was wrong.
For every accolade he won or prestigious committee he served on, I would wager that he declined or stepped way from just as many. Dr. Bell’s character and vision for psychiatry in general, and the mental health of African American communities specifically, would not allow him to pay lip service to agendas that were self-serving, and did not push the field and communities forward.
During his service as an editorial advisory board member for Clinical Psychiatry News, Dr. Bell could always be relied upon to offer an insightful perspective to any discussion, ranging from violence prevention to the social determinants of health and their role in fetal alcohol spectrum disorders.
What I will remember most about Dr. Bell is his strong character. My guess is that he was never shy about stating the truth to a patient or a president of the United States. He possessed the intellect to back up any of his views while also having the humility of a dedicated community psychiatrist who worked for no other reason than to serve his patients.
When I first met Dr. Bell, he was giving a grand rounds at the George Washington University department of psychiatry. He was wearing a hat during the lecture and a belt with the Superman logo. I thought to myself, “Whoa, this is a different type of guy,” then I sat and listened to the talk, and was utterly astounded by his intellect, humor, honesty, and passion for his patients. I had never heard a psychiatrist speak with a combination of such command and approachability, and again, I thought to myself, “Whoa, this is a different type of guy.”
Little did I know that Dr. Bell and I would end up serving together on the editorial board of CPN. I loved seeing Dr. Bell, and catching up and gleaning from his wisdom. I was very humbled by how generous Dr. Bell was with his time and the extent to which he would make himself available as a mentor. During a CPN board meeting, we were trying to come up with a mantra that would capture the mission of the new MDedge Psychiatry website. Dr. Bell (in a hat, of course) let everyone else talk, and then, in a calm voice, said: “We ask the hard questions.” As editor in chief of MDedge Psychiatry, I knew this had to be the mantra. It was aspirational, gave us an identity, and held our feet to the fire – to always ask hard questions in the service of patients and readers. I looked forward to discussing the evolution of the site, seeing him at meetings and conferences, brainstorming the implications of new advances in the field, and simply walking down the street and laughing.
Those events will never occur again, because Carl left us on Thursday, Aug 1. Carl has left us with a legacy of work that we are still coming to appreciate. He left us with a mandate to pursue the truth and make an impact in our communities. He taught black psychiatrists what it meant to stand up unapologetically for your community and society. As I reflect on the scope of his life, I have one last hard question for Carl: “Why did you have to leave us so soon?”
Dr. Norris is assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Norris also is host of the MDedge Psychcast.
2019 Update on female sexual dysfunction
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
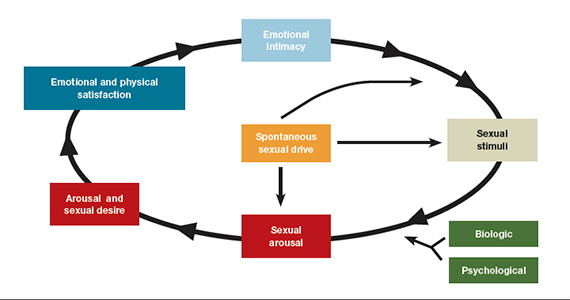
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4
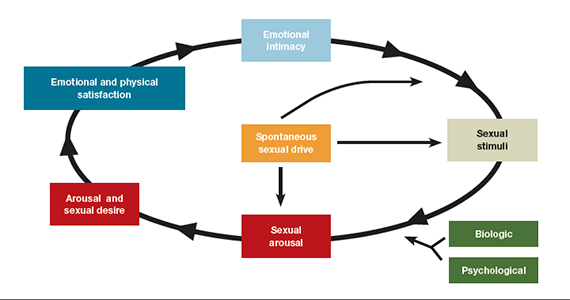
Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
Hypoactive sexual desire disorder (HSDD) is the most prevalent sexual health problem in women of all ages, with population-based studies showing that about 36% to 39% of women report low sexual desire, and 8% to 10% meet the diagnostic criteria of low sexual desire and associated distress.1,2 An expanded definition of HSDD may include3:
- lack of motivation for sexual activity (reduced or absent spontaneous desire or responsive desire to erotic cues and stimulation; inability to maintain desire or interest through sexual activity)
- loss of desire to initiate or participate in sexual activity (including avoiding situations that could lead to sexual activity) combined with significant personal distress (frustration, loss, sadness, worry) (FIGURE).4

Despite the high prevalence of HSDD, patients often are uncomfortable and reluctant to voice concerns about low sexual desire to their ObGyn. Further, clinicians may feel ill equipped to diagnose and treat patients with HSDD. ObGyns, however, are well positioned to initiate a general discussion about sexual concerns with patients and use screening tools, such as the Decreased Sexual Desire Screener (DSDS), to facilitate a discussion and clarify a diagnosis of generalized acquired HSDD (TABLES 1 and 2).5 Helpful guidance on HSDD is available from the American College of Obstetricians and Gynecologists and the International Society for the Study of Women’s Sexual Health.6-8
Importantly, clinicians have a new treatment option they can offer to patients with HSDD. Bremelanotide was approved by the US Food and Drug Administration (FDA) on June 21, 2019, to treat acquired, generalized HSDD in premenopausal women. Up until this approval, flibanserin (approved in 2015) was the only drug FDA approved for the treatment of HSDD.
Assessing and treating HSDD today can be likened to managing depression 30 years ago, before selective serotonin receptor inhibitors were available. ObGyns would refer patients with depression to other health care providers, or not even ask patients about depressive symptoms because we had so little to offer. Once safe and effective antidepressants became available, knowing we could provide pharmacologic options made inquiring about depressive symptoms and the use of screening tools more readily incorporated into standard clinical practice. Depression is now recognized as a medical condition with biologic underpinnings, just like HSDD, and treatment options are available for both disorders.
For this Update, I had the opportunity to discuss the clinical trial experience with bremelanotide for HSDD with Dr. Sheryl Kingsberg, including efficacy and safety, dosage and administration, contraindications, and adverse events. She also details an ideal patient for treatment with bremelanotide, and we review pertinent aspects of flibanserin for comparative purposes.

Bremelanotide: A new therapeutic option
According to the product labeling for bremelanotide, the drug is indicated for the treatment of premenopausal women with acquired, generalized HSDD (low sexual desire that causes marked distress or interpersonal difficulty).9 This means that the HSDD developed in a woman who previously did not have problems with sexual desire, and that it occurred regardless of the type of stimulation, situation, or partner. In addition, the HSDD should not result from a coexisting medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug substance.
Flibanserin also is indicated for the treatment of premenopausal women with HSDD. While both bremelanotide and flibanserin have indications only for premenopausal women, 2 studies of flibanserin in postmenopausal women have been published.10,11 Results from these studies in naturally menopausal women suggest that flibanserin may be efficacious in this population, with improvement in sexual desire, reduced distress associated with low desire, and improvement in the number of satisfying sexual events (SSEs).
No trials of bremelanotide in postmenopausal women have been published, but since this drug acts on central nervous system receptors, as does flibanserin, it may have similar effectiveness in postmenopausal women as well.

Continue to: Clinical trials show bremelanotide improves desire, reduces distress...
Clinical trials show bremelanotide improves desire, reduces distress
Two phase 3 clinical trials, dubbed the Reconnect studies, demonstrated that, compared with placebo, bremelanotide was associated with statistically significant improvements in sexual desire and levels of distress regarding sexual desire.
The 2 identical, randomized, placebo-controlled multicenter trials included 1,247 premenopausal women with HSDD of at least 6 months' duration.9,12 Bremelanotide 1.75 mg (or placebo) was self-administered subcutaneously with an autoinjector on an as-desired basis. The 24-week double-blind treatment period was followed by a 52-week open-label extension study.
The co-primary efficacy end points were the change from baseline to end-of-study (week 24 of the double-blind treatment period) in the 1) Female Sexual Function Index (FSFI) desire domain score and 2) feeling bothered by low sexual desire as measured by Question 13 on the Female Sexual Distress Scale (FSDS). An increase in the FSFI desire domain score over time denotes improvement in sexual desire, while a decrease in the FSDS Question 13 score over time indicates improvement in the level of distress associated with low sexual desire.
In the 2 clinical studies, the mean change from baseline (SD) in the FSFI desire domain score, which ranged from 1.2 to 6.0 at study outset (higher scores indicate greater desire), was:
- study 1: 0.5 (1.1) in the bremelanotide-treated women and 0.2 (1.0) in the placebo-treated women (P = .0002)
- study 2: 0.6 (1.0) in the bremelanotide group versus 0.2 (0.9) in the placebo group (P<.0001).
For FSDS Question 13, for which the score range was 0 to 4 (higher scores indicate greater bother), the mean change from baseline score was:
- study 1: -0.7 (1.2) in the bremelanotide-treated group compared with -0.4 (1.1) in the placebo-treated group (P<.0001)
- study 2: -0.7 (1.1) in the bremelanotide group and -0.4 (1.1) in the placebo group (P = .0053).
It should be noted that, in the past, SSEs were used as a primary end point in clinical studies. However, we have shifted from SSEs to desire and distress as an end point because SSEs have little to do with desire. Women worry about and are distressed by the fact that they no longer have sexual appetite. They no longer "want to want" even though their body will be responsive and they can have an orgasm. That is exemplified by the woman in our case scenario (see box, page 18), who very much wants the experience of being able to anticipate with pleasure the idea of having an enjoyable connection with her partner.
Continue to: Physiologic target: The melanocortin receptor...
Physiologic target: The melanocortin receptor
Bremelanotide's theorized mechanism of action is that it works to rebalance neurotransmitters that are implicated in causing HSDD, acting as an agonist on the melanocortin receptor to promote dopamine release and allow women to perceive sexual cues as rewarding. They can then respond to those cues the way they used to and therefore experience desire. Flibanserin has affinity for serotonin (5-hydroxytryptamine [5-HT]) receptors, with agonist and antagonist activity, as well as moderate antagonist activity on some dopamine receptors.
The bottom line is that we now have treatments to address the underlying biologic aspect of HSDD, which is a biopsychosocial disorder. Again, this has parallels to depression and its biologic mechanism, for which we have effective treatments.
Dosing is an as-needed injection
Unlike the daily nighttime oral dose required with flibanserin, bremelanotide is a 1.75-mg dose administered as a subcutaneous injection (in either the thigh or the abdomen) with a pen-like autoinjector, on an as-needed basis. It should be administered at least 45 minutes before anticipated sexual activity. That is a benefit for many women who do not want to take a daily pill when they know that their "desire to desire" may be once per week or once every other week.
Regarding the drug delivery mode, nobody dropped out of the bremelanotide clinical trials because of having to take an injection with an autoinjector, which employs a very thin needle and is virtually painless. A small number of bremelanotide-treated women, about 13%, had injection site reactions (compared with 8% in the placebo group), which is common with subcutaneous injection. Even in the phase 2 clinical trial, in which a syringe was used to administer the drug, no participants discontinued the study because of the injection mode.
There are no clear pharmacokinetic data on how long bremelanotide's effects last, but it may be anywhere from 8 to 16 hours. Patients should not take more than 1 dose within 24 hours--but since the effect may last up to 16 hours that should not be a problem--and use of more than 8 doses per month is not recommended.
While bremelanotide improves desire, certainly better than placebo, there is also some peripheral improvement in arousal, although women in the trials had only HSDD. We do not know whether bremelanotide would treat arousal disorder, but it will help women with or without arousal difficulties associated with their HSDD, as shown in a subgroup analysis in the trials.13
Counsel patients on treatment potentialities
Clinicians should be aware of several precautions with bremelanotide use.
Blood pressure increases. After each dose of bremelanotide, transient increases in blood pressure (6 mm Hg in systolic and 3 mm Hg in diastolic blood pressure) and reductions in heart rate (up to 5 beats per minute) occur; these measurements return to baseline usually within 12 hours postdose.9 When you think about whether having sexual desire will increase blood pressure, this may be physiologic. It is similar to walking up a flight of stairs.
The drug is not recommended, however, for use in patients at high risk for cardiovascular disease, and it is contraindicated in women with uncontrolled hypertension or known cardiovascular disease. Blood pressure should be well controlled before bremelanotide is initiated--use of antihypertensive agents is not contraindicated with bremelanotide as the drugs do not interact.
Clinicians are not required to participate in a Risk Evaluation and Mitigation Strategy (REMS) program to prescribe bremelanotide as they are with flibanserin (because of the increased risk of severe hypotension and syncope due to flibanserin's interaction with alcohol).
Drug interactions. Bremelanotide is a melanocortin receptor agonist--a unique compound. Antidepressants, other psychoactive medications, and oral contraceptives are not contraindicated with bremelanotide as there are no known interactions. Alcohol use also is not a contraindication or caution, in contrast to flibanserin. (In April, the FDA issued a labeling change order for flibanserin, specifying that alcohol does not have to be avoided completely when taking flibanserin, but that women should discontinue drinking alcohol at least 2 hours before taking the drug at bedtime, or skip the flibanserin dose that evening.14) Bremelanotide may slow gastric emptying, though, so when a patient is taking oral drugs that require threshold concentrations for efficacy, such as antibiotics, they should avoid bremelanotide. In addition, some drugs, such as indomethacin, may have a delayed onset of action with concomitant bremelanotide use.9
Importantly, patients should avoid using bremelanotide if they are taking an oral naltrexone product for treatment of alcohol or opioid addiction, because bremelanotide may decrease systemic exposure of oral naltrexone. That would potentially lead to naltrexone treatment failure and its consequences.9
Skin pigmentation changes. Hyperpigmentation occurred with bremelanotide use on the face, gingiva, and breasts, as reported in the clinical trials, in 1% of treated patients who received up to 8 doses per month, compared with no such occurrences in placebo-treated patients. In addition, 38% of patients who received bremelanotide daily for 8 days developed focal hyperpigmentation. It was not confirmed in all patients whether the hyperpigmentation resolved. Women with dark skin were more likely to develop hyperpigmentation.9
Common adverse reactions. The most common adverse reactions with bremelanotide treatment are nausea, flushing, injection site reactions, and headache, with most events being mild to moderate in intensity. In the clinical trials, 40% of the bremelanotide-treated women experienced nausea (compared with 1% of placebo-treated women), with most occurrences being mild; for most participants nausea improved with the second dose. Women had nausea that either went away or was intermittent, or it was mild enough that the drug benefits outweighed the tolerability costs--of women who experienced nausea, 92% continued in the trial, and 8% dropped out because of nausea.9
The following scenario describes the experience of HSDD in one of Dr. Kingsberg's patients.
CASE Woman avoids sex because of low desire; marriage is suffering
A 40-year-old woman, Sandra, who has been married for 19 years and has fraternal twins aged 8, presented to the behavioral medicine clinic with distressing symptoms of low sexual desire. For several years into the marriage the patient experienced excellent sex drive. After 6 to 7 years, she noticed that her desire had declined and that she was starting to avoid sex. She was irritated when her husband initiated sex, and she would make excuses as to why it was not the right time.
Her husband felt hurt, frustrated, and rejected. The couple was close to divorce because he was angry and resentful. Sandra recognized there was a problem but did not know how to fix it. She could not understand why her interest had waned since she still loved her husband and considered him objectively very attractive.
Sandra came to see Dr. Kingsberg at the behavioral medicine clinic. Using the 5-item validated diagnostic tool called the Decreased Sexual Desire Screener, Dr. Kingsberg diagnosed hypoactive sexual desire disorder (HSDD), a term Sandra had never heard of and did not know was a condition. The patient was relieved to know that she was one of several million women affected by HSDD and that the problem was not just that she was a "bad wife" or that she had some kind of psychological block. She emphasized how much she loved her husband and how she wanted desperately to "want to want desire," as she recalled feeling previously.
Sandra was treated with counseling and psychotherapy in which we addressed the relationship issues, the avoidance of sex, the comfort with being sexual, and the recognition that responsive desire can be helpful (as she was able to have arousal and orgasm and have a satisfying sexual event). The issue was that she had no motivation to seek out sex and had no interest in experiencing that pleasure. In subsequent couple's therapy, the husband recognized that his wife was not intentionally rejecting him, but that she had a real medical condition.
Although Sandra's relationship was now more stable and she and her husband were both working toward finding a solution to Sandra's loss of desire, she was still very distressed by her lack of desire. Sandra tried flibanserin for 3 months but unfortunately did not respond. Sandra heard about the recent approval of bremelanotide and is looking forward to the drug being available so that she can try it.
Final considerations
Asking patients about sexual function and using sexual function screening tools can help clinicians identify patients with the decreased sexual desire and associated distress characteristic of HSDD. ObGyns are the appropriate clinicians to treat these women and soon will have 2 pharmacologic options--bremelanotide (anticipated to be available in Fall 2019) and flibanserin--to offer patients with this biopsychosocial disorder that can adversely impact well-being and quality of life. Clinicians should individualize treatment, which may include psychotherapeutic counseling, and counsel patients on appropriate drug use and potential adverse effects.
AMAG Pharmaceuticals, Inc. has announced that they will have a copay assistance program for bremelanotide, where the first prescription of four autoinjectors will be a $0 copay, followed by a $99 copay or less for refills.15
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
- Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441-1449.
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions: part II. J Sex Med. 2016;13:1888-1906.
- Basson R. Using a different model for female sexual response to address women's problematic low sexual desire. J Sex Marital Ther. 2001;27:395-403.
- Clayton AH, Goldfischer ER, Goldstein I, et al. Validation of the Decreased Sexual Desire Screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J Sex Med. 2009;6:730-738.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction. Obstet Gynecol. 2019;134:e1-e18.
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women's Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc. 2018;93:467-487.
- Vyleesi [package insert]. Waltham, MA: AMAG Pharmaceuticals; 2019.
- Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21;633-640.
- Portman DJ, Brown L, Yuan, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834-842.
- Kingsberg SA, Clayton AH, Portman D, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol. Forthcoming.
- Clayton AH, Lucas J, Jordon R, et al. Efficacy of the Investigational drug bremelanotide in the Reconnect studies. Poster presented at: 30th ECNP Congress of Applied and Translational Neuroscience; September 2-5, 2017, Paris, France.
- US Food and Drug Administration. FDA orders important safety labeling changes for Addyi [press release]. April 11, 2019. https://www.fda.gov/news-events/press-announcements/fda-orders-important-safety-labeling-changes-addyi. Accessed July 17, 2019.
- Vyleesi website (https://vyleesipro.com). Accessed August 5, 2019.
Morcellation use in gynecologic surgery: Current clinical recommendations and cautions
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7
Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
In memory of Dr. Carl Compton Bell
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
It was a simple message in the body of an email: “A strong voice in and for psychiatry is now silent.”
That is how I shared the news of the passing of Carl Compton Bell, MD, with the leadership of the American Psychiatric Association and what I think Carl would have approved be shared. Although he was a member of APA, he was never interested in the trappings of leadership there or any other organizations of which he was a longtime member, really. He preferred to “do the work” and was known to not suffer fools who were in it to promote themselves. He was always ready, willing, and able to offer guidance or assistance in your work and never failed to have an opinion on what else you needed to do. Some of my favorite memories of Carl are the talks he initiated at the drop of a hat where he “dropped some knowledge” about what he was doing or what you should be doing.
Upon hearing of his death, I described him to someone as fearless, unapologetic, smart, and ready to advocate for black people at the drop of one of the many hats he wore over the years. In fact, his decades of wardrobes is one the other things many of us will remember – the CMHC baseball cap with the “Stop Black on Black” crime T-shirt, the Obama cap paired with an assortment of message T-shirts (depending on what issue he was focused on at the time), and, most recently, the longer hair sticking out from under the wide brim leather cowboy hat with the highway patrol polarized sunglasses.
And whether it was the Surgeon General or an audience at the Carter Center, the message was consistent and powerful. An international researcher, clinician, teacher, and author of more than 500 books, chapters, and articles, he spent most of his career directly addressing issues of violence and HIV prevention, misdiagnosis of psychiatric disorders in African Americans, and the psychological effects on children exposed to violence.
Honoring the legacy of Carl Bell is about more than how we can all follow in his footsteps and more about being like him – unapologetically fearless and focused on improving the health, mental health, and overall well-being of black people. He was very clear that his talents, his skills, his focus – whether it was clinical care, training, or research – would be on black people, and he was often amused at the response, mostly from white people, when he stated clearly that this was his focus. He was often challenged by them, and his response as I frequently heard him say was: “I care about black people; I want to help black people.” I think he basically felt that, if it was good for black people, it would also benefit everyone else.
So, there’s a lesson for us as we heap on the well-deserved accolades on him and his life’s work, and reminisce about our personal encounters and experiences with him over the decades. As we reflect on what he meant to each of us as a friend, a colleague, and a history maker, I think the lesson is that if Carl were here today, he’d say: “OK, that’s all good, thank you for the nice words but what are you doing for black people today? What are you doing to improve their health and life condition today?” I think if we really want to honor his legacy and continue his work, we must be as fearless and focused as he was as we follow his lead and carry on with the work that promotes mental health in the black community. And when we are challenged for wanting to do this work, we must be just as unapologetic and thoughtful as he was, even channeling our own “Carl Bell” moment if needed. As a lifelong martial arts practitioner, I will end with this: “The bamboo which bends in the wind is stronger than the mighty oak which breaks in a storm.” Carl was the bamboo, and he’s with the Ancestors now, encouraging us to do the work and bend not break. Rest, my brother; job well done!
Dr. Stewart is immediate past president of the American Psychiatric Association.
Hormone therapy and cognition: What is best for the midlife brain?
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).
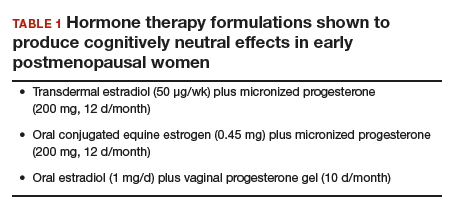
What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).
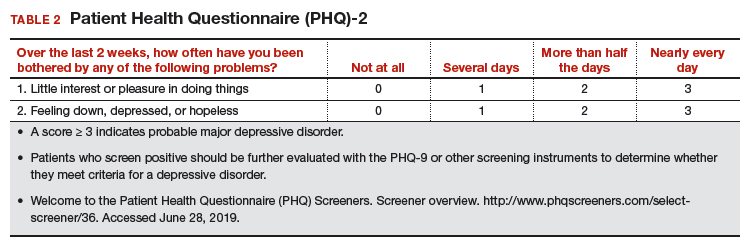
Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
CASE HT for vasomotor symptoms in perimenopausal woman with cognitive concerns
Jackie is a 49-year-old woman. Her body mass index is 33 kg/m2, and she has mild hypertension that is effectively controlled with antihypertensive medications. Otherwise, she is in good health.During her annual gynecologic exam, she reports that for the past 9 months her menstrual cycles have not been as regular as they used to be and that 3 months ago she skipped a cycle. She is having bothersome vasomotor symptoms (VMS) and is concerned about her memory. She says she is forgetful at work and in social situations. During a recent presentation, she could not remember the name of one of her former clients. At a work happy hour, she forgot the name of her coworker’s husband, although she did remember it later after returning home.
Her mother has Alzheimer disease (AD), and Jackie worries about whether she, too, might be developing dementia and whether her memory will fail her in social situations.
She is concerned about using hormone therapy (HT) for her vasomotor symptoms because she has heard that it can lead to breast cancer and/or AD.
How would you advise her?
HT remains the most effective treatment for bothersome VMS, but concerns about its cognitive safety persist. Such concerns, and indeed a black-box warning about the risk of dementia with HT use, initially arose following the 2003 publication of the Women’s Health Initiative Memory Study (WHIMS), a randomized, placebo-controlled trial of HT for the primary prevention of dementia in women aged 65 years and older at baseline.1 The study found that combination estrogen/progestin therapy was associated with a 2-fold increase in dementia when compared with placebo.
One of the critical questions arising even before WHIMS was whether the cognitive risks associated with HT that were seen in WHIMS apply to younger women. Attempting to answer the question and adding fuel to the fire are the results of a recent case-control study from Finland.2 This study compared HT use in Finnish women with and without AD and found that HT use was higher among Finnish women with AD compared with those without AD, regardless of age. The authors concluded, “Our data must be implemented into information for the present and future users of HT, even though the absolute risk increase is small.”
However, given the limitations inherent to observational and registry studies, and the contrasting findings of 3 high-quality, randomized controlled trials (RCTs; more details below), providers actually can reassure younger peri- and postmenopausal women about the cognitive safety of HT.3 They also can explain to patients that cognitive symptoms like the ones described in the case example are normal and provide general guidance to midlife women on how to optimize brain health.

Continue to: Closer look at WHI and RCT research pinpoints cognitively neutral HT...
Closer look at WHI and RCT research pinpoints cognitively neutral HT
In WHIMS, the combination of conjugated equine estrogen (CEE; 0.625 mg/d) plus medroxyprogesterone acetate (MPA; 2.5 mg/d) led to a doubling of the risk of all-cause dementia compared with placebo in a sample of 4,532 women aged 65 years and older at baseline.1 CEE alone (0.625 mg) did not lead to an increased risk of all-cause dementia.4
Whether those formulations led to cognitive impairment in younger postmenopausal women was the focus of WHIMS-Younger (WHIMS-Y), which involved WHI participants aged 50 to 55 years at baseline.5 Results revealed neutral cognitive effects (ie, no differences in cognitive performance in women randomly assigned to HT or placebo) in women tested 7.2 years after the end of the WHI trial. WHIMS-Y findings indicated that there were no sustained cognitive risks of CEE or CEE/MPA therapy. Two randomized, placebo-controlled trials involving younger postmenopausal women yielded similar findings.6,7 HT shown to produce cognitively neutral effects during active treatment included transdermal estradiol plus micronized progesterone,6 CEE plus progesterone,6 and oral estradiol plus vaginal progesterone gel.7 The findings of these randomized trials are critical for guiding decisions regarding the cognitive risks of HT in early postmenopausal women (TABLE 1).

What about women with VMS?
A key gap in knowledge about the cognitive effects of HT is whether HT confers cognitive advantages to women with bothersome VMS. This is a striking absence given that the key indication for HT is the treatment of VMS. While some symptomatic women were included in the trials of HT in younger postmenopausal women described above, no large trial to date has selectively enrolled women with moderate-to-severe VMS to determine if HT is cognitively neutral, beneficial, or detrimental in that group. Some studies involving midlife women have found associations between VMS (as measured with ambulatory skin conductance monitors) and multiple measures of brain health, including memory performance,8 small ischemic lesions on structural brain scans,9 and altered brain function.10 In a small trial of a nonhormonal intervention for VMS, improvement in VMS following the intervention was directly related to improvement in memory performance.11 The reliability of these findings continues to be evaluated but raises the hypothesis that VMS treatments might improve memory in midlife women.
Memory complaints common among midlife women
About 60% of women report an undesirable change in memory performance at midlife as compared with earlier in their lives.12,13 Complaints of forgetfulness are higher in perimenopausal and postmenopausal women compared with premenopausal women, even when those women are similar in age.14 Two large prospective studies found that memory performance decreases during the perimenopause and then rebounds, suggesting a transient decrease in memory.15,16 Although cognitive complaints are common among women in their 40s and 50s, AD is rare in that age group. The risk is largely limited to those women with a parent who developed dementia before age 65, as such cases suggest a familial form of AD.
Continue to: What causes cognitive difficulties during midlife?
What causes cognitive difficulties during midlife?
First, some cognitive decline is expected at midlife based on increasing age. Second, above and beyond the role of chronologic aging (ie, getting one year older each year), ovarian aging plays a role. A role of estrogen was verified in clinical trials showing that memory decreased following oophorectomy in premenopausal women in their 40s but returned to presurgical levels following treatment with estrogen therapy (ET).17 Cohort studies indicate that women who undergo oophorectomy before the typical age of menopause are at increased risk for cognitive impairment or dementia, but those who take ET after oophorectomy until the typical age of menopause do not show that risk.18
Third, cognitive problems are linked not only to VMS but also to sleep disturbance, depressed mood, and increased anxiety—all of which are common in midlife women.15,19 Lastly, health factors play a role. Hypertension, obesity, insulin resistance, diabetes, and smoking are associated with adverse brain changes at midlife.20
Giving advice to your patients
First, normalize the cognitive complaints, noting that some cognitive changes are an expected part of aging for all people regardless of whether they are male or female. Advise that while the best studies indicate that these cognitive lapses are especially common in perimenopausal women, they appear to be temporary; women are likely to resume normal cognitive function once the hormonal changes associated with menopause subside.15,16 Note that the one unknown is the role that VMS play in memory problems and that some studies indicate a link between VMS and cognitive problems. Women may experience some cognitive improvement if VMS are effectively treated.
Advise patients that the Endocrine Society, the North American Menopause Society (NAMS), and the International Menopause Society all have published guidelines saying that the benefits of HT outweigh the risks for most women aged 50 to 60 years.21 For concerns about the cognitive adverse effects of HT, discuss the best quality evidence—that which comes from randomized trials—which shows no harmful effects of HT in midlife women.5-7 Especially reassuring is that one of these high-quality studies was conducted by the same researchers who found that HT can be risky in older women (ie, the WHI Investigators).5
Going one step further: Protecting brain health
As primary care providers to midlife women, ObGyns can go one step further and advise patients on how to proactively nurture their brain health. Great evidence-based resources for information on maintaining brain health include the Alzheimer’s Association (https://www.alz.org) and the Women’s Brain Health Initiative (https://womensbrainhealth.org). Primary prevention of AD begins decades before the typical age of an AD diagnosis, and many risk factors for AD are modifiable.22 Patients can keep their brains healthy through myriad approaches including treating hypertension, reducing body mass index, engaging in regular aerobic exercise (brisk walking is fine), eating a Mediterranean diet, maintaining an active social life, and engaging in novel challenging activities like learning a new language or a new skill like dancing.20
Also important is the overlap between cognitive issues, mood, and alcohol use. In the opening case, Jackie mentions alcohol use and social withdrawal. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), low-risk drinking for women is defined as no more than 3 drinks on any single day and no more than 7 drinks per week.23 Heavy alcohol use not only affects brain function but also mood, and depressed mood can lead women to drink excessively.24
In addition, Jackie’s mother has AD, and that stressor can contribute to depressed feelings, especially if Jackie is involved in caregiving. A quick screen for depression with an instrument like the Patient Health Questionnaire-2 (PHQ-2; TABLE 2)25 can rule out a more serious mood disorder—an approach that is particularly important for patients with a history of major depression, as 58% of those patients experience a major depressive episode during the menopausal transition.26 For this reason, it is important to ask patients like Jackie if they have a history of depression; if they do and were treated medically, consider prescribing the antidepressant that worked in the past. For information on menopause and mood-related issues, providers can access new guidelines from NAMS and the National Network of Depression Centers (NNDC).27 There is also a handy patient information sheet to accompany those guidelines on the NAMS website (https://www.menopause.org/).

Continue to: CASE Resolved...
CASE Resolved
When approaching Jackie, most importantly, I would normalize her experience and tell her that memory problems are common in the menopausal transition, especially for women with bothersome VMS. Research suggests that the memory problems she is experiencing are related to hormonal changes and not to AD, and that her memory will likely improve once she has transitioned through the menopause. I would tell her that AD is rare at midlife unless there is a family history of early onset of AD (before age 65), and I would verify the age at which her mother was diagnosed to confirm that it was late-onset AD.
For now, I would recommend that she be prescribed HT for her bothersome hot flashes using one of the “safe” formulations in the Table on page 24. I also would tell her that there is much she can do to lower her risk of AD and that it is best to start now as she enters her 50s because that is when AD changes typically start in the brain, and she can start to prevent those changes now.
I would tell her that experts in the field of AD agree that these lifestyle interventions are currently the best way to prevent AD and that the more of them she engages in, the more her brain will benefit. I would advise her to continue to manage her hypertension and to consider ways of lowering her BMI to enhance her brain health. Engaging in regular brisk walking or other aerobic exercise, as well as incorporating more of the Mediterranean diet into her daily food intake would also benefit her brain. As a working woman, she is exercising her brain, and she should consider other cognitively challenging activities to keep her brain in good shape.
I would follow up with her in a few months to see if her memory functioning is better. If it is not, and if her VMS continue to be bothersome, I would increase her dose of HT. Only if her VMS are treated but her memory problems are getting worse would I screen her with a Mini-Mental State Exam and refer her to a neurologist for an evaluation.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651-2662.
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
- Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429-1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 2015;12:e1001833.
- Henderson VW, St. John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87:699-708.
- Maki PM, Drogos LL, Rubin LH, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848-856.
- Thurston RC, Aizenstein HJ, Derby CA, et al. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23:27-32.
- Thurston RC, Maki PM, Derby CA, et al. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103:1572-1578.e1.
- Maki PM, Rubin LH, Savarese A, et al. Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129.
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7:257-265.
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351-362.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152:463-473.
Office hysteroscopic evaluation of postmenopausal bleeding
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
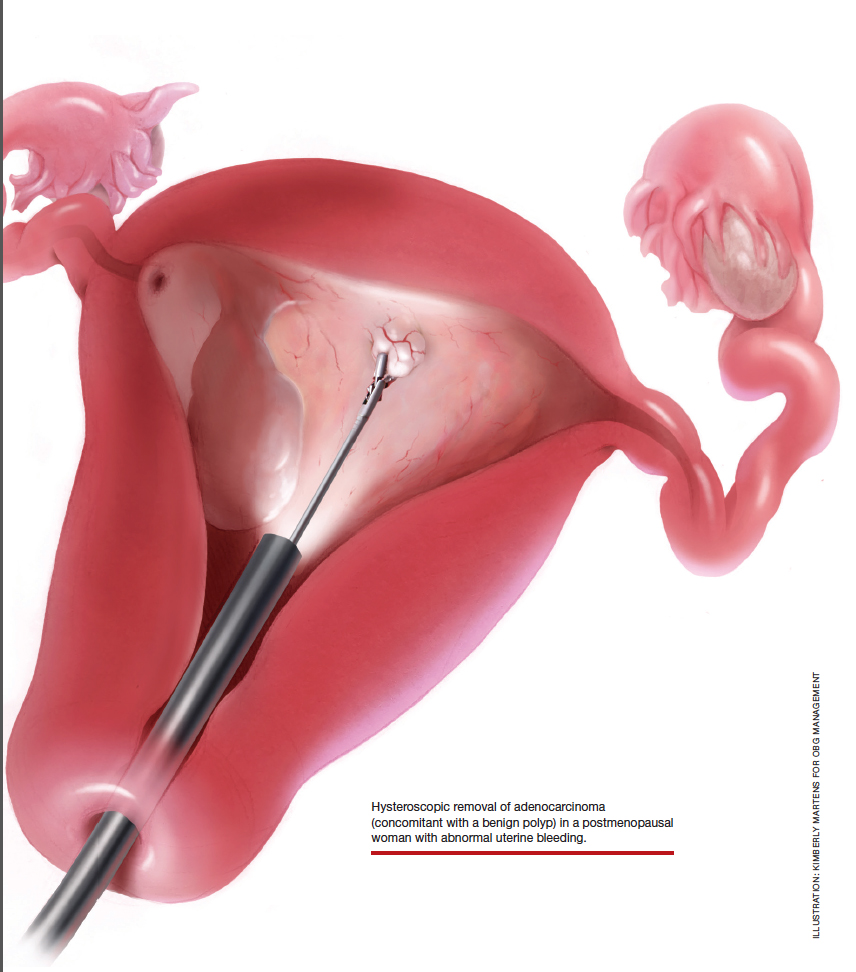
Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
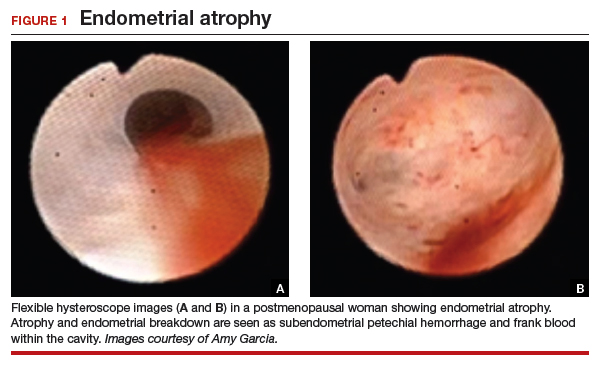
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
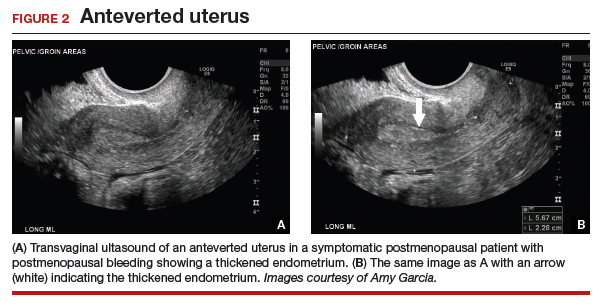
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
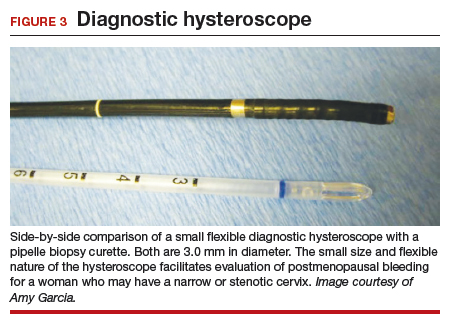
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
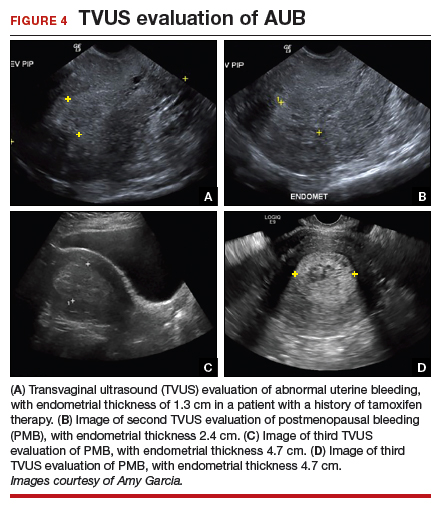
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

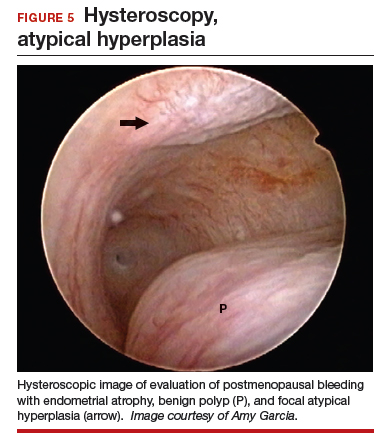
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

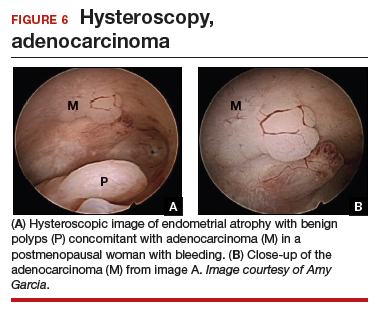
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
The states of health care: Ranking the best and worst
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.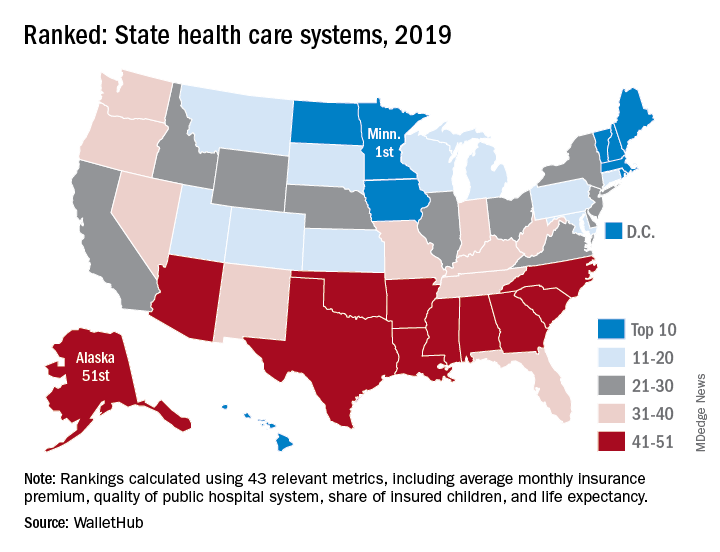
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
and the last frontier is also last in the nation in health care.
In the 2019 edition of its annual ranking of state health care systems, personal finance website WalletHub named Minnesota the best less than a week after U.S. News & World Report called the Mayo Clinic the top hospital in the country.
The WalletHub ranking, which included Washington, D.C., also put Alaska’s health care system at No. 51. Just one step above in the 50th spot was North Carolina, with Mississippi (49), South Carolina (48), and Arkansas (47) occupying the rest of the bottom five. Louisiana, which jumped from 51st in 2018 to 44th this year, was the only state to move out of the bottom five, WalletHub reported.
Joining Minnesota in the top five were Massachusetts (2), Rhode Island (3), D.C. (4), and Vermont (5), which was the home of last year’s best health care. North Dakota, which moved up 12 spots this year all the way to 9th, had the second-largest climb of any state: Montana catapulted 15 spots this year to finish 17th overall, WalletHub said.
For 2019, the company compared the states and D.C. using 43 measures of cost, accessibility, and outcome. The cost dimension’s six metrics included cost of a medical visit and share of adults with no doctor visits because of cost. The accessibility dimension consisted of 23 metrics, including hospital beds per capita, geriatricians per population aged 65 years and older, and Medicaid acceptance rate among physicians. The outcomes dimension included 14 metrics, among them share of patients readmitted to hospitals and share of nonimmunized children.
Minnesota was the only state to finish in the top 10 of all three dimensions. D.C. was ranked first in cost and Maine was the leader in access – as each was last year – and Massachusetts was ranked first in outcomes, the WalletHub data show.
The lowest-ranked states for each category in 2019 were the same as in 2018: Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from 21 sources, including the Bureau of Labor Statistics, the Centers for Medicare & Medicaid Services, and the Kaiser Family Foundation.
Burnout gets personal for 68% of physicians
by real-time market insights technology firm InCrowd.
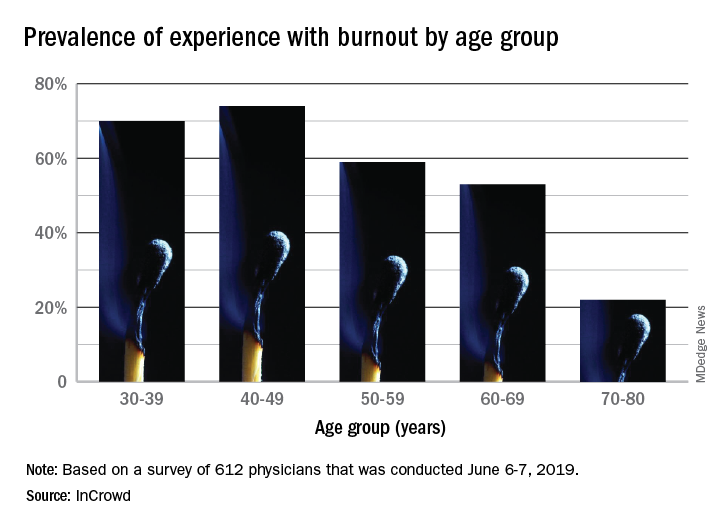
The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
by real-time market insights technology firm InCrowd.

The overall prevalence of personal burnout experience was 68% among respondents, and another 28% said that they had not felt burned out but knew other physicians who had, InCrowd reported Aug. 6.
Specialty appeared to play a part given that 79% of primary care physicians reported experiencing burnout versus 57% of specialists. In response to an open-ended question about ability to manage burnout, the most common answer (23%) was that specialty played a large role, with “no role/all specialties affected equally” next at 13%. Equal proportions of respondents, however, said that specialists (24%) and primary care physicians (24%) were the group most affected, InCrowd said.
There was also a disconnect regarding age. When answering another open-ended question about the effects of age, 23% of those surveyed said that older physicians are more affected, compared with 9% who put the greater burden on younger physicians. The self-reporting of burnout, however, showed that younger physicians were much more likely to experience its effects than their older counterparts: 70% of those aged 30-39 years and 74% of those 40-49 versus 22% of those aged 70-80, InCrowd reported.
InCrowd noted that its results fall within the range of other recent surveys involving burnout in physicians that have shown levels that were lower, at 44% (MedScape, 2019) or 43.9% (American Academy of Family Physicians, 2019), and those that were higher, at 77.8% (The Physicians Foundation/Merritt Hawkins, 2018).
“The alarming persistence of physician burnout over the years and across multiple studies unfortunately demonstrates that we have not yet turned the tide on this problematic issue,” Diane Hayes, PhD, president of InCrowd, said in a statement accompanying the survey results. “Since we last looked at this in 2016, there really haven’t been any notable improvements. The healthcare industry would benefit from refining and expanding current initiatives to assure adequate staffing levels needed to deliver the quality care patients deserve.”
The survey was conducted June 6-7, 2019, and involved responses from 612 physicians (51% primary care providers, 49% specialists).
“Hidden” HIV in Cerebrospinal Fluid Cells May Lead to Cognitive Problems
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.
Even when a standard HIV RNA test is negative, some patients may still have viral DNA in their cerebrospinal fluid (CSF)—and that could be a predictor of later memory and concentration problems, say researchers who conducted a National Institute of Health (NIH)-funded study of patients on long-term antiretroviral therapy (ART).
The 69 participants, enrolled in the AIDS Clinical Trials Group HIV Reservoirs Cohort Study, had infections controlled with ART for a median of 9 years. Calling it a “striking observation,” the researchers found nearly half of the patients had viral DNA in CSF cells, although the standard viral load tests of the cell-free CSF fluid were positive in only 4% of the patients.
Of the 30 patients with persistent HIV, 9 (30%) experienced neurocognitive difficulties in a 7-domain neuropsychological test battery. Among 35 participants with no detectable HIV DNA in CSF, 4 (11%) were clinically impaired.
The low rates of detectable HIV RNA in the cell-free CSF fraction and within CSF cell pellets suggest low levels of HIV transcription within cells and infrequent release into the extracellular space during systemically suppressive ART, the researchers say.
The brain is one of the first targets of the virus, and CNS manifestations are common. Neurocognitive impairment in HIV-positive patients is probably related to multiple factors, including HIV infection, age, neuroinflammation, and comorbid conditions, including substance abuse, the researchers say. There also may be a “legacy effect,” in which processes associated with long-term exposure to HIV before ART leads to irreversible neurologic injury and more extensive infection of CSF cells. Lack of an association with inflammatory biomarkers in this study suggested that current inflammation does not lead to present neurocognitive dysfunction, the researchers say, but does not rule out prior inflammation as the underlying cause of neuronal injury.
Still, given that brain tissue in living individuals is “inaccessible” (as the researchers put it), CSF offers a window into neuropathogenesis of HIV. Studies have found a range between 15% and 55% of participants develop an HIV-associated neurocognitive disorder (HAND). The new findings may support a role of persistent HIV-infected cells, but the researchers emphasize that the association does not confirm that HIV DNA causes HAND. However, they add, persistent HIV in “sanctuary sites” despite ART presents a barrier to curing the infection. Their study, they say, indicates that examination of CSF cells is important in assessing residual HIV in compartments during ART.