User login
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Preoperative tramadol fails to improve function after knee surgery
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
FROM THE JOURNAL OF ARTHROPLASTY
Endoscopic duodenal mucosal resection found effective for some patients with T2D
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
Among patients with suboptimally controlled type 2 diabetes who use oral glucose-lowering medication, endoscopic duodenal mucosal resection (DMR) can be implemented safely and effectively, results from a multicenter, international, phase 2 study demonstrated.
“DMR elicited a substantial improvement in parameters of glycemia as well as a decrease in liver transaminase levels at 24 weeks, which was sustained at 12 months post procedure,” researchers led by Annieke C.G. van Baar, MD, wrote in a study published online in Gut. “These findings were also associated with an improvement in patients’ diabetes treatment satisfaction.”
For the study, Dr. van Baar, of the department of gastroenterology and hepatology at Amsterdam University Medical Center, and colleagues at seven clinical sites enrolled 46 patients with type 2 diabetes who were on stable glucose-lowering medication to undergo DMR. The procedure “involves circumferential hydrothermal ablation of the duodenal mucosa resulting in subsequent regeneration of the mucosa,” they wrote. “Before ablation, the mucosa is lifted with saline to protect the outer layers of the duodenum.” DMR was performed under either general anesthesia or deep sedation with propofol by a single endoscopist at each site with extensive experience in therapeutic upper GI endoscopy and guidewire management.
The mean age of the study participants was 55 years and 63% were male. Of the 46 patients, 37 (80%) underwent complete DMR and results were reported for 36 of them. A total of 24 patients had at least one adverse event related to DMR (52%), mostly GI symptoms such as diarrhea, abdominal pain, nausea, and oropharyngeal pain. Of these, 81% were mild. One serious adverse event was considered to be related to the procedure. “This concerned a patient with general malaise, mild fever, and increased C-reactive protein level on the first day after DMR,” the researchers wrote. “The mild fever resolved within 24 hours and [C-reactive protein] level normalized within 3 days.” No unanticipated adverse events were reported.
During follow-up measures taken 24 weeks after their DMR, hemoglobin A1c fell by a mean of 10 mmol/mol (P less than .001), fasting plasma glucose by 1.7 mmol/L (P less than .001), and the Homeostatic Model Assessment of Insulin Resistance improved significantly (P less than .001). In addition, the procedure conferred a moderate reduction in weight (a mean loss of 2.5 kg) and a decrease in hepatic transaminase levels. The effects were sustained at 12 months.
“While the majority of patients showed a durable glycemic response over 12 months, a minority exhibited less benefit from DMR and required additional glucose-lowering medication at 24 weeks,” the researchers wrote. “Of note, approximately two-thirds of the patients who required addition of antidiabetic medication in the latter phase of study had undergone insulin secretagogue medication withdrawal at screening. For future study, it may not be necessary to discontinue these medications before DMR, and this will allow an even more precise measure of DMR effect.”
Dr. van Baar and colleagues acknowledged certain limitations of the phase 2 study, including its open-label, uncontrolled design. “The results of this multicenter study need to be confirmed in a proper controlled study. Nevertheless, this study forms the requisite solid foundation for further research, and controlled studies are currently underway.”
The study was funded by Fractyl Laboratories. Dr. van Baar reported having no financial disclosures. Four of the study authors reported having financial relationships with numerous pharmaceutical and device companies.
SOURCE: van Baar ACG et al. Gut. 2019 Jul 22. doi: 10.1136/gutjnl-2019-318349.
FROM GUT
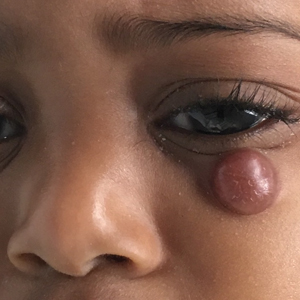
Rubbery Nodule on the Face of an Infant
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
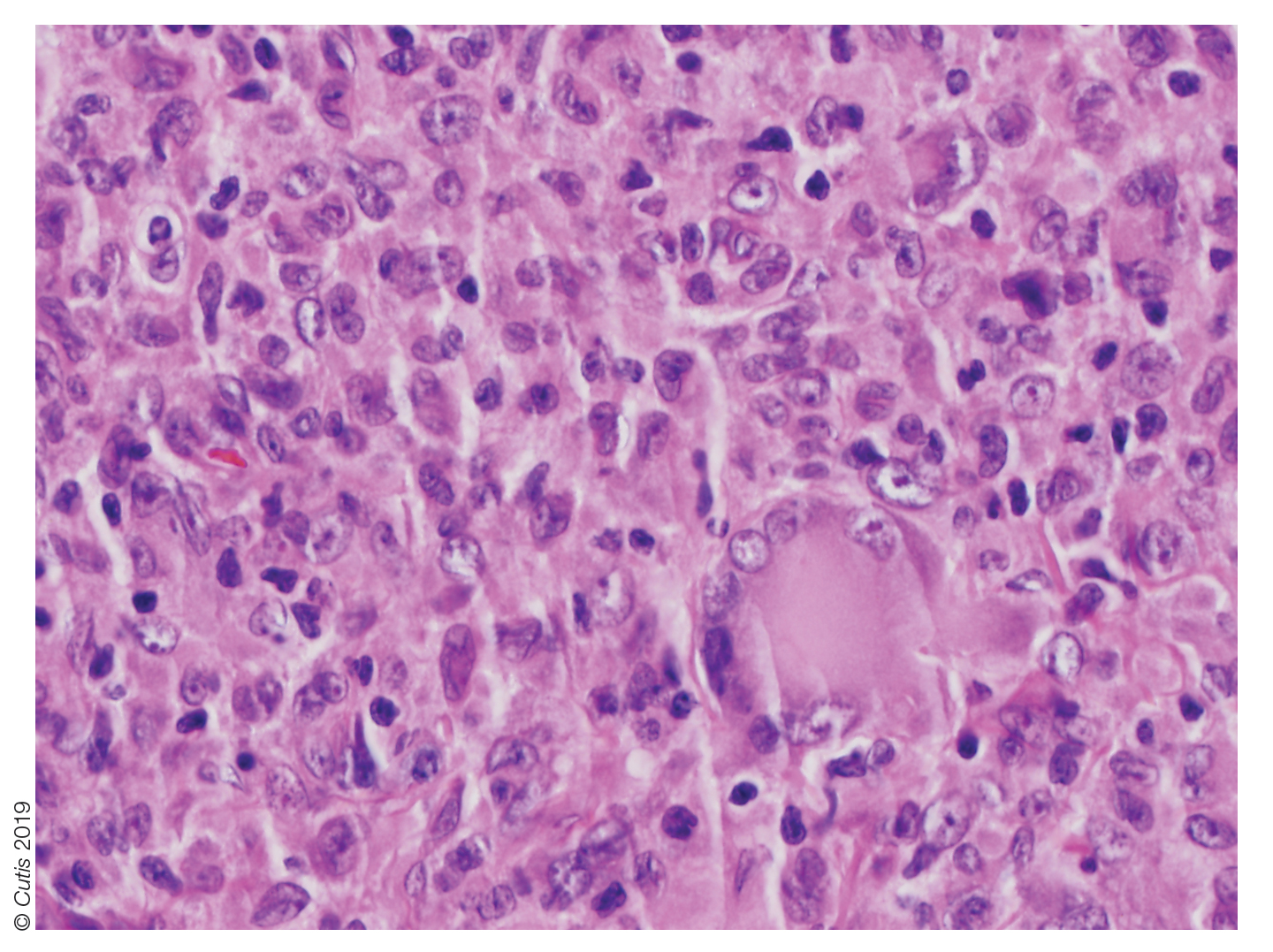
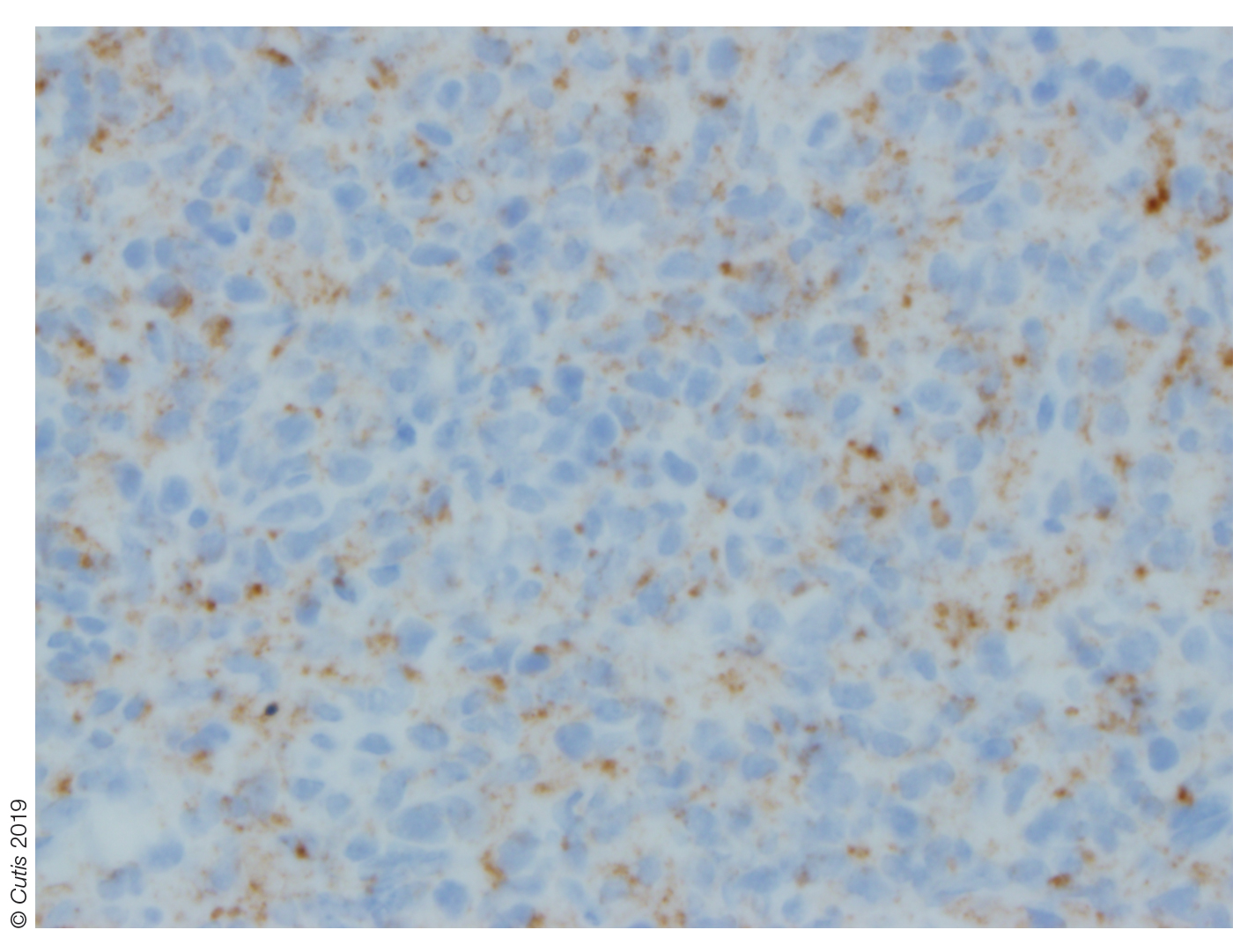
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 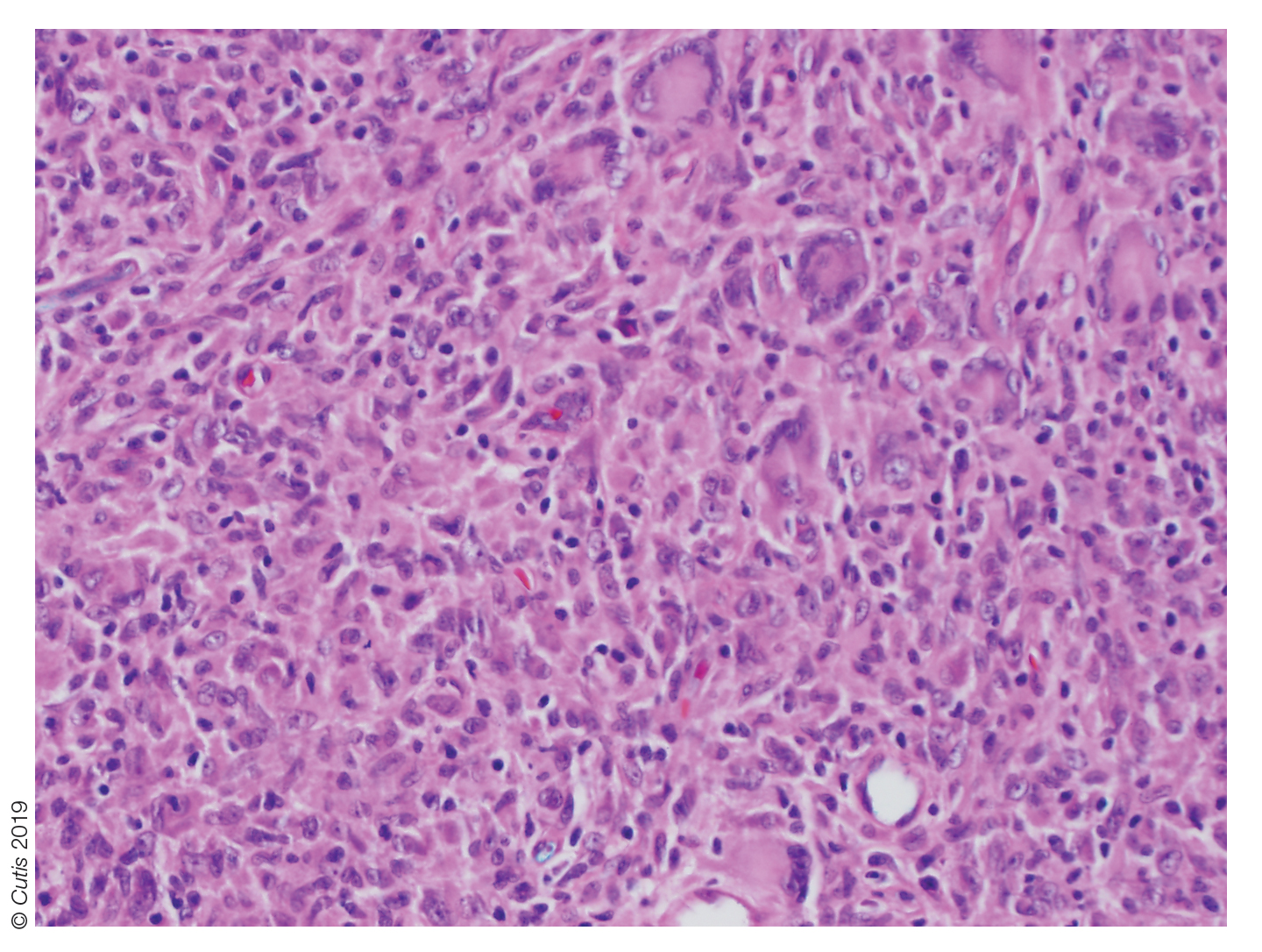
Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13
Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
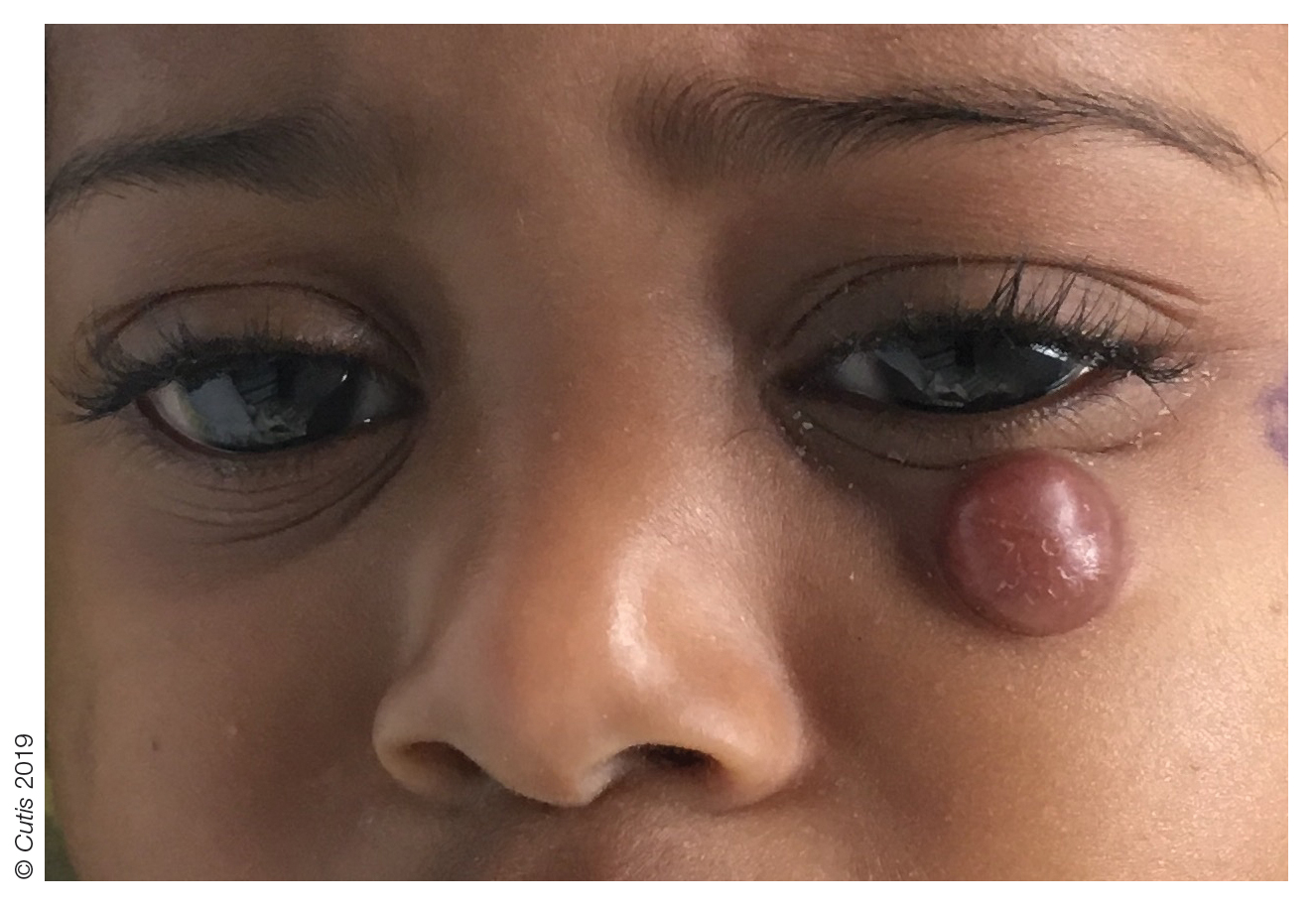
A 10-month-old girl presented with a facial nodule of 7 months’ duration that started as a small lesion. On physical examination, a single 10×10-mm, nontender, well-circumscribed, firm, freely mobile nodule was observed in the left infraorbital area. The patient was born full term at 37 weeks’ gestation via spontaneous vaginal delivery and had no other notable findings on physical examination. Excision was performed by an oculoplastic surgeon. Pathology revealed a relatively well-circumscribed, diffuse, dermal infiltrate of cells arranged in short fascicles and a storiform pattern. The cells had abundant clear to amphophilic cytoplasm, ovoid to reniform nuclei with vesicular chromatin and focal grooves, and diffuse CD68+ immunoreactivity, as well as scattered S-100–positive cells. The patient did well with the excision and no new lesions have developed.
IV fluid weaning unnecessary after gastroenteritis rehydration
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Are your patients ready for the transition to adult care?
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
AUSTIN, TEX. – All too often, children and adolescents stumble on their way to adult health care – if they make it at all.
In fact, data from an ongoing survey by the Department of Health & Human Services and the Health Resources and Services Administration indicate that only 40% of children with special health care needs aged 12-17 years receive the services necessary to make transitions to adult health care.
“Most fall off the proverbial cliff and do not engage with adult providers,” Cynthia Peacock, MD, said at the annual meeting of the Society for Pediatric Dermatology. She recalled meeting with one individual who, after being a patient at the children’s hospital for 13 years, was told over the phone to transition to an adult provider with not so much as a promised letter of introduction being sent on. “She did not know about the adult health care culture, which is fractured in care, relies on the patient to be their own advocate, and wants patients to be able to do their visit in 10-15 minutes.”
Dr. Peacock, medical director of the Transition Medicine Clinic at Baylor College of Medicine, Houston, described “Starting at age 12 is not too early,” she said. “It gives you time so that you can keep introducing the concept when that individual keeps coming back to see you, especially if it’s a chronic condition.”
She recommended that clinicians ask several questions to assess transition readiness of pediatric patients to adult care, including, Do you know your medications? Do you know how to take them? Do you know how to refill them? Do you know how to discuss them? Can you discuss your medical condition with the adult doctor? Can you call for a doctor’s appointment or get a prescription filled? “Adolescents are notorious for calling [the doctor’s office], and if they’re told they can’t make an appointment, that’s it; they stop right there,” Dr. Peacock said. “They don’t tend to problem solve. They don’t engage.”
Studies have suggested that the transfer of care is more likely to be successful if a formal transition program is in place to prepare the patient and to facilitate the change in health care providers. “There is a growing evidence base in the literature that skills training for young people with chronic illnesses can be associated with positive outcomes,” she said. “This can be as easy as telling the individual, ‘Do a book report about your condition. Talk to a friend. Tell a friend what your condition is. Or, do school science fair project and talk to your class about what you have.’ Get them past that uncomfortable feeling of having to talk about it.”
The earlier this happens, the better. “We know from research that if you get them to be their own [health care] advocate, that’s one less thing they have to do in the adult health care system,” she said. “They will move on to other things, such as getting a job or going to college.”
Dr. Peacock, who is board certified in pediatrics and internal medicine, added that providing adolescents with the option of being seen by professionals without their parents is considered best practice. “You could start by introducing the concept at age 12, but say at age 13, ‘I want to spend a minute with you alone without your parent. I want you to bring in questions that you want to ask me that you may not want to ask in front of your parents,’” she said. “I guarantee you that on that third visit the adolescent will ask you a question.”
Optimistic messaging is another component of effective planning. “You may see someone in your office with a disease that you know has high mortality and high morbidity, and you’re trying to help the family cope,” Dr. Peacock said. “That young person needs to be asked, ‘What are your plans for your future?’ Think about it: In 10 or 20 years when you’re transitioning that individual out of your health care system, what medical miracles have happened?” She recalled visiting with the mother of a patient with Down syndrome who had significant congenital heart disease. He was in his 30s and struggled to keep his behavior in check. “We were trying to develop a behavior plan, but his past care team had never put one in place for him,” Dr. Peacock said. “The mother looked at me and said, accusingly, ‘It’s your fault. It’s all of your doctors’ faults because they never told me that this would happen. They told me to take him home and spoil him because he would not be around at this age.’”
Effective transition handoffs are collaborative, she continued, with care plans built around what is likely to happen with the patient over time. “At Baylor College of Medicine, pediatric dermatologists follow patients for life, but I take over everything else adult care related,” Dr. Peacock said. “The pediatric dermatologist has me come over to the hospital when we’re talking about quality-of-life issues – about advance care, advanced directives, those types of things.”
She recommends not transferring care to an adult provider during pregnancy, hospitalization, during active disease, or during changes to a patient’s medical therapy. “When pediatricians call me from the hospital and they want an urgent transfer, I tell them, ‘Your emergency is not my urgency. Get everything ready; get them discharged. Get them followed up and back on their chronic care management, and I’ll be happy to do that transition for you,’ ” she said. “It’s also good to leave that door open for the adult provider to call you. Give them your cell phone number because it may be just one question, like, ‘Can you tell me why his liver enzymes are elevated? We can’t figure it out.’ ”
Dr. Peacock advises pediatric providers to develop processes within their own practice that facilitates transfer, “even if it just means sharing information with the adult provider at the end of the time you’re seeing that young adult. Know your systems and your resources. Get that medical summary done, even if it’s making the patient do the medical summary.” More information for clinicians and for patients and their families can be found at www.gottransition.org.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM SPD 2019
Vaccination is not associated with increased risk of MS
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
FROM NEUROLOGY
Benzodiazepines, hypnotics don’t increase Alzheimer’s pathology
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
LOS ANGELES – Benzodiazepines and hypnotics, including the so-called “Z drugs,” don’t significantly increase the pathological features typical of Alzheimer’s disease but long-term users may experience some neuronal loss in the nucleus basalis, Chris Fox, MD, reported at the Alzheimer’s Association International Conference.
The nucleus basalis is rich in cholinergic neurons and associated with arousing stimuli, including positive and aversive appetite, sustained attention, and the interplay of reality and visual perception.
“Neuronal loss in the nucleus basalis offers mechanisms for the impact of benzodiazepine and anticholinergic drug use on the aging brain and highlights important areas for future research,” said Dr. Fox, professor of clinical psychiatry at the University of East Anglia, Norwich, England.
“The risk [for taking a Z drug] in the United Kingdom is high, with about 7.5 million older adults using potentially inappropriately prescribed anticholinergic and/or Z-drug medications. Despite well-documented cognitive impairment associated with these medicines, hypnotics are still used for long durations and exceed the recommended limits,” Dr. Fox said. “There’s no association with better cognition, quality of life, or improved behavior when they are given to people with dementia. In fact, we’ve seen a 60% increased risk of hip fractures – an increase from a 3% to a 15% yearly risk.”
Dr. Fox and colleagues studied the brains of 337 subjects who were included in the U.K. Medical Research Council’s Cognitive Function and Ageing Studies (CFAS). The study was intended to explore the incidence of dementia in the United Kingdom, examine incidence variation among regions, and explore factors increasing dementia risk and rate of progression.
The first study, which began in 1989 and lasted until 2015, followed subjects older than 65 years for up to 12 years. Each subject was regularly interviewed and underwent cognitive testing about every 1.5 years. Benzodiazepine use was considered an especially important aspect, because the medications are frequently used in the elderly and seem linked to injuries and cognitive status at last follow-up.
In CFAS, 21% of subjects reported at least one incidence of anticholinergic use, and 12% reported recurrent use. Another 17% reported any hypnotic use, and 11% reported recurrent use. The main indications were as an antidepressant (13%), for urological issues (4%), as antiparkinsonism drugs (1%), as antipsychotics (3%), and as antihistamines (3%). Overall, 18% reported concurrent use of benzodiazepines and hypnotics. At time of death, 46% had a diagnosis of dementia.
“Those reporting benzodiazepine use were more likely to be women and to have depression or sleep problems,” Dr. Fox noted, although he didn’t give specific hazard ratios. After adjustment for numerous factors, including age, sex, stroke, hypertension, depression, anxiety, asthma, Parkinson’s disease, duration of sleep problems, education, and smoking, he found no statistically increased risk of amyloid brain plaques or tau tangles, the pathologic hallmarks of Alzheimer’s disease.
Anticholinergic use was associated with a significant 60% reduction in cortical atrophy (odds ratio, 0.40) and recurrent use with a 61% reduction in amyloid angiopathy (OR, 0.39).
However, both medication classes were associated with greater neuronal loss in the nucleus basalis. Recurrent use of anticholinergic drugs increased neuronal loss by 300% (OR, 4.12), while any use nearly tripled it (OR, 2.87). Recurrent use of benzodiazepines was associated with increased neuronal loss in the region (OR, 3.76) as well. However, these associations did not reach statistical significance. But there was a statistically significant association with any use of benzodiazepines and neuronal loss in the nucleus basalis (OR, 6.84).
“We did find greater neuronal loss in the nucleus basalis associated with benzodiazepine and anticholinergic drugs use,” Dr. Fox said. “The nucleus basalis is rich in neurons that stimulate the cholinergic system of the neocortex. Neuronal loss in this region is thought to occur in the early stages of Alzheimer’s. Other studies have suggested that volume loss in the basal forebrain cholinergic site leads to widespread cortical atrophy in patients with mild cognitive impairment. We did not observe the widespread cortical atrophy, however.
“Given that the strongest associations were observed for benzodiazepines and neuronal loss in the nucleus basalis, it may be that the drugs were prescribed to treat the symptoms of ‘cholinergic deficiency syndrome,’ Our findings suggest that the symptoms of dementia lead to an increase of benzodiazepines as opposed to the medications actually causing Alzheimer’s disease,” he said.
Dr. Fox reported no financial disclosures.
SOURCE: Fox C et al. AAIC 2019, Abstract 34017.
REPORTING FROM AAIC 2019
Researchers examine potential causes of dementia in CTE
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
The study by Alosco et al. provides new insights into the pathogenesis of dementia in deceased former football players with chronic traumatic encephalopathy (CTE), Julie A. Schneider, MD, professor of neuropathology at Rush University, Chicago, wrote in an accompanying editorial (JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.1089).
Significant and widespread white matter injury is an established result of head trauma resulting from acceleration-deceleration injuries. In addition, studies of single and repetitive traumatic brain injury have shown disruption of axons and white matter. The findings of Alosco et al. “underscore the importance of studying the risk factors and mechanisms for the white matter rarefaction, in addition to the tauopathy, in individuals who have played U.S. football and have CTE,” Dr. Schneider wrote.
The comprehensive neuropathologic examinations, advanced statistical techniques, and multiple sensitivity analyses that the investigators performed are among the study’s strengths. An important limitation, however, is selection bias. “The frequency of pathologic characteristics in this group should not be generalized to estimate the prevalence of neuropathologic conditions in living individuals who have played or are playing U.S. football,” Dr. Schneider wrote. “Moreover, individuals who played football who were selected for autopsy and found to have CTE may differ in other important ways from those who did not undergo autopsy or did not have CTE.” Recall bias could alter associations between years of play and dementia diagnosis, and the study’s semiquantitative assessments could result in decreased power to observe relevant associations, she said.
“In spite of these limitations, the authors should be applauded for elegant work and compelling support for multiple pathologic pathways to dementia in football players with CTE,” Dr. Schneider concluded.
Dr. Schneider is with the Rush Alzheimer’s Disease Center at Rush University, Chicago. She has been an expert consultant for the National Football League and the National Hockey League.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
, according to a cross-sectional study published online Aug. 5 in JAMA Neurology.
The study of older, deceased former American football players with CTE showed that more years of play were associated with more severe white matter rarefaction and greater burden of neurofibrillary tau tangles in the dorsolateral frontal cortex, wrote Michael L. Alosco, PhD, assistant professor of neurology at Boston University’s CTE Center, and colleagues.
An analysis of donated brains
Repetitive head impacts are associated with CTE. The clinical presentation of CTE includes cognitive, behavioral, and mood changes that can progress to dementia. The contributions of pathologic changes in phosphorylated tau, white matter degeneration, and cerebrovascular disease to dementia in the context of CTE are poorly understood. Dr. Alosco and colleagues examined arteriosclerosis, infarcts, microinfarcts, microbleeds, and white matter rarefaction in donated brains to illuminate these contributions.
The researchers examined data from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank. The population included deceased men who had played football and had received a neuropathologic diagnosis of CTE. Eligible participants had a history of repetitive head impacts. Brains that had been donated after a prolonged time postmortem and those with poor tissue quality were excluded.
Neuropathologists blinded to clinical data analyzed patients’ CTE stage and severity of neurofibrillary tangle burden in the dorsolateral frontal cortex as semiquantitative scales of phosphorylated tau severity. Neurofibrillary tangle burden was dichotomized as none or mild versus moderate or severe. The neuropathologists also rated white matter rarefaction and arteriolosclerosis severity using a scale of 0 points (i.e., none) to 3 points (i.e., severe changes). The investigators obtained clinical data through online surveys and retrospective telephone interviews with informants. They adjudicated consensus diagnoses of dementia based on modified criteria from DSM-IV.
White matter rarefaction was common
Dr. Alosco and colleagues included 180 individuals in their analysis, excluding those aged younger than 40 years because of low pathologic burden and minimal presence of dementia. Mean age at death was nearly 68 years. Fifty patients had no or mild neurofibrillary tangle burden, and 130 had moderate to severe burden. Thirty-five patients had CTE at stage I or II, and 145 had CTE at stage III or IV. In all, 120 patients were determined to have had dementia. About 47% of the sample had moderate to severe white matter rarefaction, and about 47% had arteriolosclerosis. Infarcts, microinfarcts, and microbleeds were uncommon.
When the investigators created a simultaneous equations regression model and controlled for age and race, they found that more years of play was associated with more severe white matter rarefaction, greater phosphorylated tau accumulation, and high CTE stage. Furthermore, white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles were associated with dementia. The association of years of play with dementia was mediated by white matter rarefaction and neurofibrillary tangle burden. Arteriolosclerosis was not associated with years of play, but arteriolosclerosis was independently associated with dementia.
The odds ratio for dementia was 1.69 among participants with more severe white matter rarefaction and 1.81 among patients with arteriolosclerosis. After the researchers controlled for age and race, the odds ratio of dementia was 2.65 among participants with a high neurofibrillary tangle burden, compared with participants with a low burden.
“Studies that include direct cardiovascular disease and repetitive head impacts metrics and refined measures of white matter integrity are needed to improve understanding of the pathogenesis of white matter rarefaction and cerebral small vessel changes in CTE,” Dr. Alosco and colleagues wrote.
The study was funded by grants from the National Institute on Aging, National Institute of Neurological Disorders and Stroke, the Department of Veterans Affairs, the Nick and Lynn Buoniconti Foundation, and the National Center for Advancing Translational Sciences. Some of the authors reported financial ties to the pharmaceutical industry and serving on professional sports committees.
SOURCE: Alosco ML et al. JAMA Neurol. 2019 Aug 5. doi: 10.1001/jamaneurol.2019.2244.
FROM JAMA NEUROLOGY
Tales From VA Anesthesiology
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
The patient grabbed my attention as I glanced through our clinic schedule. It was his age: He was 99 years old and scheduled for eye surgery. The plastic surgery resident’s note read: “Patient understands that this would involve surgery under general anesthesia and is agreeable to moving forward...Extremely high risk of anesthesia emphasized.”
I reviewed the patient’s history. At baseline, he had severe pulmonary hypertension, severe aortic stenosis (AS), diastolic heart failure, chronic atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate of 26 mL/min [normal is > 60 mL/min]), anemia (hematocrit 26%), and a standing do not resuscitate (DNR) order. His maximal daily exercise was walking slowly across a room, primarily limited by joint pain. Recent geropsychiatry notes indicated mild cognitive impairment. The anesthesia record from an urgent hip fracture repair 7 months before under general anesthesia was unremarkable.
I phoned the attending plastic surgeon. Our conversation was as follows:
“Hi, I’m about to see a 99-year-old patient with a DNR who is scheduled for resection of an eyelid tumor. His medical history makes me nervous. Are you sure this is a good idea?”“Hmmm, 99-year-old…okay, that’s right,” he responded. “He has an invasive squamous that could become a big problem. The actual procedure is under 10 minutes. Waiting for the pathology report will be the longest part of the procedure.”
“Can it be done under local?” I asked.
“Yes,” he replied.
“Okay, I’ll talk to him and call you back.”
I found the patient in the waiting room, flanked by his 2 daughters and invited them into the clinic room. After introductions, I began asking whether they had any questions about the anesthesia. By midsentence a daughter was prompting him to discuss what happened “last time.” He described a history of posttraumatic stress disorder (PTSD) stemming from his hip surgery, which he blamed squarely on the anesthesia. His emotion was evident in the gathering pauses. “I hate that I am so emotional since they kept me awake during my surgery.”
Through the fog of multiple accounts, it became clear that he was traumatized by the loss of control during the administration of and emergence from the anesthesia.
“They told me it was only oxygen,” he said. “They lied. There was a taste to it…I was awake and skinned alive…They said I was a monster when I woke up thrashing.” He went on, explaining that in the recovery room “there were 2 people bothering me, man-handling me, asking me questions.”
One of his daughters showed me pictures of bruises on his face from ripping off the mask and pulling out the breathing tube. They were visibly upset by the memory of his postoperative combativeness and paranoia. The note written by the orthopedic surgery resident on the day after surgery stated succinctly, “Doing well, had some delirium from anesthesia overnight.” Subsequent geropsychiatry home visits attested to intrusive thoughts, flashbacks, and nightmares from his time as a combat soldier in World War II, 65 years in the past.
“It took me months…months to recover,” he said.
He was in the mood to reminisce, however, perhaps a willful distraction. He had the floor for at least 30 minutes, during which I spoke about 5 sentences. With every sad story he told there was a happy, humorous one, such as meeting his future wife while on leave in New Zealand during the war, recalled down to exact dates. And another story:
There we were in New Caledonia. All our supplies went out to replace what sank on [USS] Coolidge, including a lot of food. Well, there were deer on the island. So we took out a truck and a rifle and wouldn’t you know we came upon a roadblock in the form of a big steer. We figured it looked enough like a deer. My buddy shot it dead with one shot. We dressed it and loaded it into the jeep. Hardly before we even got back to the mess hall, the officers’ cook came sniffing around. He and our captain agreed it was easily the biggest deer they’d ever seen and appropriated it to the officers’ mess. Next day the CO [commanding officer] of the whole outfit came by and announced it was the best tasting ‘venison’ he’d ever had. I heard the farmer got paid a pretty penny for that steer. I didn’t get a damn bite.
He delivered this last bit with relish.
When the conversation returned to anesthesia, I read them the record of his hip fracture repair. I explained that on the face of it, the report seemed uneventful. One daughter asked astute questions about his awareness. I explained that although awareness during general anesthesia is possible, it seemed from the record, he’d had plenty of anesthesia during the case and that there is always less at the beginning and end, the periods that apparently had caused him distress. I also explained that most studies report the incidence of true awareness as at most 1 out of thousands of events and that he had none of the established risk factors for it, such as female gender, young age, chronic substance abuse, cardiac and obstetric surgery, and history of awareness.1
The other daughter wondered why he was so agitated afterward. I recited data on the frequency of postoperative delirium in elderly patients but explained that the range is wide, depending on the study and population, from about 1% in elderly patients undergoing ambulatory surgery to 65% for open aortic surgery.2,3 I added that their father had 2 of the strongest risk factors for delirium, advanced age and cognitive impairment.3 Only after airing each question about the hip surgery in detail were they ready to discuss the eye surgery.
He started that conversation with the right question: “Do I really need it?”
I quoted my surgical colleague’s concern. I told him that, should he opt to undergo the surgery, I was confident that this time around his experience would be different from the last.
“If you’re okay with it, all you need is some numbing medicine from the surgeon; you won’t need any anesthesia from me.”
I walked step-by-step through what they could expect on the day of surgery. Maintaining control was of obvious importance to him. He felt comfortable going forward. His daughters intuited that less would be more for a quick recovery.
We then addressed the DNR directive. I acknowledged his absolute right to self-determination and explained that the need for resuscitation is, at times, a consequence of the surgery and anesthesia. I reassured them that our plan made resuscitation and intubation highly unlikely. They also asked to use any interventions necessary to restart his heart if it should stop beating. I documented their decision in my notes and communicated it to the surgical team. We had talked for 90 minutes.
I met the patient and his daughters on the day of surgery in the preoperative holding area. I inserted an IV, applied electrocardiography leads, and affixed a pulse oximeter and a noninvasive blood pressure cuff. In the operating room (OR) we took time to place his 99-year-old joints into, as he said, the “least worst” position. He tolerated the injection of the local by the surgeon perfectly well. We were in the OR for 3 hours, during which he taught me a fair amount about boating and outboard engines among other things. Pathology reported clean margins. He was discharged home soon after and had an uneventful recovery.
Patient-First Approach
A core competency of the Accreditation Council for Graduate Medical Education for an anesthesia residency is the Interpersonal and Communication Skills program. A comprehensive discussion of communication is far beyond the scope here. But not surprisingly, deficient communication between physicians and patients can cause emotional distress, significant dissatisfaction among family members, and negative patient judgment of how well we communicate.4-6 These observations are particularly true in our increasingly elderly surgical population, in which both surgeons and anesthesiologists often feel unequal to the task of discussing concepts such as code status.7,8
In our practice and in residency training, the preoperative clinic often is the location where patient/provider communication occurs. Here we consider the latest American College of Cardiology/American Heart Association guidelines, examine airways, review electrocardiograms, and formulate plans agreeable to and understood by our anxious patients and their families. The potent anxiolytic effect of a preoperative visit by an anesthesiologist is well established.9 Anxiety about surgery is a risk factor for impaired decision making before surgery.10 And surgery is traumatic—as many as 7.6% of postoperative patients experience symptoms consistent with PTSD attributable to the surgery, placing it on a par with being mugged (8.0%).11,12
The patient in this case presented several communication challenges even absent his revelation of prior traumatic experience with anesthesia. He was elderly, anxious, and had multiple comorbidities. He had mild cognitive impairment and required a code status discussion. There also were the clinical challenges—navigating a 99-year-old with severe aortic stenosis and a right ventricular systolic pressure > 90 mm Hg through a general anesthetic gave me a sinking feeling.
He was fortunate that the procedure could be done with local anesthesia, mitigating his risk of cognitive dysfunction, including delirium. He also was fortunate in that his anesthesiologist and surgeon had created a collaborative, patient-first approach and that his US Department of Veterans Affairs (VA) clinic had the time, space, and staffing to accommodate an unexpected 90-minute visit. A big investment in communication, mainly my keeping quiet, made the intraoperative management simple. Such is life in an integrated health care system without financial incentives for high-volume care—and another reminder that VA physicians are blessed to guide patients through some of the most vulnerable and distressing moments of their lives.
Postscript
During the preparation of this manuscript, the patient passed away at the age of 100. His obituary was consistent with what I had learned about him and his family during our 2 encounters: a long successful career in local industry; extensive involvement in his community; an avid sportsman; and nearly 30 grandchildren, great-grandchildren, and great-great grandchildren. But there was one more detail that never came up during my extensive discussion with him and his daughters: He was awarded the Purple Heart for his service in World War II.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
1. Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: risk factors, causes and sequelae: a review of reported cases in the literature. Anesth Analg. 2009;108(2):527-535.
2. Aya AGM, Pouchain PH, Thomas H, Ripart J, Cuvillon P. Incidence of postoperative delirium in elderly ambulatory patients: a prospective evaluation using the FAM-CAM instrument. J Clin Anesth. 2019;53:35-38.
3. Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: a systematic review. Int J Surg. 2016;35:1-6.
4. Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians’ interviewing skills and reducing patients’ emotional distress: a randomized clinical trial. Arch Intern Med. 1995;155(17):1877-1884.
5. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
6. Hall JA, Roter DL, Rand CS. Communication of affect between patient and physician. J Health Soc Behav. 1981;22(1):18-30.
7. Cooper Z, Meyers M, Keating NL, Gu X, Lipsitz SR, Rogers SO. Resident education and management of end-of-life care: the resident’s perspective. J Surg Educ. 2010;67(2):79-84.
8. Hickey TR, Cooper Z, Urman RD, Hepner DL, Bader AM. An agenda for improving perioperative code status discussion. A A Case Rep. 2016;6(12):411-415.
9. Egbert LD, Battit GE, Turndorf H, Beecher HK. The value of the preoperative visit by an anesthetist. JAMA. 1963;185(7):553-555.
10. Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns. 2014;94(3):328-333.
11. Whitlock EL, Rodebaugh TL, Hassett AL, et al. Psychological sequelae of surgery in a prospective cohort of patients from three intraoperative awareness prevention trials. Anesth Analg. 2015;120(1):87-95.
12. Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.