User login
NAFLD unchecked is a ‘harbinger of deadly dysmetabolism’
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
EXPERT ANALYSIS FROM MEDS 2019
Treatment Facility: An Important Prognostic Factor for Dedifferentiated Liposarcoma Survival (FULL)
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
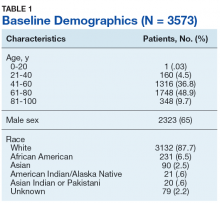
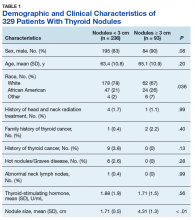
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
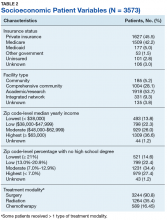
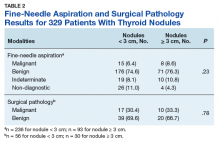
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
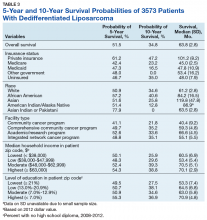
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
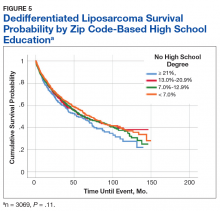
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
Approximately 17% to 25% of all softtissue sarcomas (STS) are liposarcomas, making liposarcoma the most common type of STS.1 The 2013 World Health Organization (WHO) classification separates liposarcoma into 4 histologic subtypes: atypical lipomatous tumor/well-differentiated (ALT/ WDLPS), dedifferentiated (DDLPS), myxoid, and pleomorphic.2 Each subtype has unique histology, morphology, and natural history. WDLPS and DDLPS are the most common histologic subtypes, comprising approximately 50% of all sarcomas that arise in the retroperitoneum.3 DDLPS represents 18% of all liposarcomas, making it the second most common subtype of liposarcoma.4
In 1979, DDLPS was first characterized.5 Most (90%) cases of DDLPS present de novo, whereas the other 10% transform from preexisting low-grade WDLPS.2 DDLPSs are formed by an amplification of 12q14-15 involving the MDM2 gene.4 These malignancies most commonly present in the retroperitoneum as a large painless mass, consisting of both fatty and nonfatty components.2 Primary site has been previously reported as a major prognostic factor for DDLPSs, with retroperitoneal DDLPSs demonstrating the worst prognosis.6 DDLPSs have a high risk of local recurrence, with some reports estimating recurrence rates approaching 40%.2 Overall mortality at 5 years for DDLPS is estimated to be between 30% and 40%.4
Previous literature has determined that median income, race, health insurance, and facility type are related to survival outcomes for patients with DDLPS.7-9 When comparing the most common types of cancers, residents of poorer US counties consistently had a higher risk of mortality than residents in affluent US counties, and all racial minorities showed worse survival outcomes when compared with white patients.7 Differences in survival outcomes have been reported in patients attending different treatment facilities for other cancers including pancreatic cancers, glioblastomas, and oral cancers, with multiple studies concluding that academic and research programs are associated with the longest survival outcomes.10-12 For many cancers, insurance status has been shown to be a significant prognostic factor, with private insurance typically resulting in the best prognosis.8,9
The goal of this retrospective study was to assess the prognostic effects of socioeconomic variables on the overall survival (OS) probabilities in a large cohort of DDLPS patients in order to inform clinicians about a potentially at-risk population.
Method
The National Cancer Database (NCDB) was created by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is the largest cancer database in the US and includes data on almost 70% of US patients with cancer. CoC-accredited cancer programs add data on patients with cancer to the NCDB. The authors accessed the NCDB data through the use of the NCDB Participant Use File program.
Patients’ data from 2004 through 2015 were abstracted. Only patients with the International Classification of Diseases for Oncology histology code 8858, corresponding to DDLPS, were analyzed. Patients with other comorbid malignant tumors were excluded to accurately capture the true survival rates for DDLPS. Variables analyzed included age, sex, race, insurance status, treatment facility type, median household income by zip code, and percentage of adults in the patient’s zip code with no high school (HS) education.
Median survival, 5- and 10-year OS probabilities, and Kaplan-Meier survival curves were calculated for multiple variables, specifically race, insurance status, treatment facility type, median family income, and percentage of adults without a HS degree. Both 5- and 10-year OS probabilities were determined by race with the patients separated into white, African American, Asian, American Indian/Alaska Native (AI/AN), and Asian Indian or Pakistani groups. Our study categorized Chinese, Japanese, Filipino, Hmong, Korean, Vietnamese, Thai, Guamanian, Asian not otherwise specified, and other Asian ethnicity patients together into one collective Asian group. Insurance status was classified into Medicare, Medicaid, other government insurance, and private insurance groups. Other government insurance consisted of US Department of Veterans Affairs, Indian Health Service, Public Health Service, and other government health care programs. Further analysis could not be performed into the distribution of the other government insurance variable.
Facility types were divided into 4 groups: community, comprehensive community, academic/ research, and integrated network cancer treatment facilities. Median income quartiles and the percentage of adults with no high school degree were estimated by comparison of the patient’s zip code with US Census Bureau data. Median household income was separated into 4 groups, including lowest level of household income (< $38,000), low level of household income ($38,000 to $47,999), moderate level of household income ($48,000 to $62,999), and highest level of household income (≥ $63,000). The percentages of adults with no high school degree were divided into 4 groups: lowest level of HS education (≥ 21% ), low level of HS education (13.0% to 20.9%), moderate level of HS education (7.0% to 12.9%), and highest level of HS education (≤ 7%). The 5- and 10-year survival probabilities were calculated using the number of months between the date of diagnosis and the date of death or last known contact.
Continuous variables are presented as median and interquartile range (IQR) whereas categorical variables are presented as frequencies and proportion. IBM SPSS version 25.0 was used to produce Kaplan-Meier survival curves and descriptive statistics. This study used Kaplan- Meier survival tables and log-rank tests to analyze both the 5- and 10-year OS rates for the 5 variables listed above. This study also used a multivariable Cox regression model that accommodated the correlative nature of outcomes within facilities to study the association of the treatment facility type and other socioeconomic factors, while controlling for age, race (which was collapsed into 3 categories), sex, primary site, tumor stage, and treatment approaches. The proportional hazards assumption was individually checked for all pertinent variables. Any patient records that were missing data were excluded from the multivariable Cox regression model, which was analyzed with SAS version 9.4 (Cary, NC). P < 0.05 was used to indicate statistical significance for all analyses.
Results
Table 1 provides descriptive analysis for demographic characteristics of the 3573 patients including age, sex, and race. The median age at diagnosis was 64 years. There were 1073 more men (65%) than women (35%) in this analysis. Whites were the predominant racial category, comprising 87.7% of the patient population, followed by African Americans (6.5%) and Asians (2.5%).
Socioeconomic Variables
The largest proportion of the patient population (45.5%) had private insurance (Table 2). Medicare came in a close second covering almost 42.2% of the population, followed by Medicaid (5.0%), uninsured (2.8%), and other government insurance (1.5%). About half (53.7%) of the patients were treated at academic or research facilities, while the fewest number of patients (5.2%) underwent treatment at community cancer facilities. The largest percentage (36.6%) of patients lived in zip codes with the highest level of median household income, while 26.0% and 22.3% had moderate and low levels of income, respectively. About 14% of patients lived within an area of the lowest level of income. Similarly, almost 15% of patients lived in an area of lowest level of HS education. The greatest percentage of the patient population (34.5%) lived in a zip code with moderate level of HS education. Surgery was the most common treatment modality with 90.8% of the cohort undergoing surgery, while 35.4% and 16.5% were treated with radiation and chemotherapy, respectively (some patients received more than one type of treatment modality).
Survival Data
Survival data were available for 3112 patients. Kaplan-Meier survival curves were used to analyze OS according to insurance status, racial background, treatment facility type, median family income, and percentage of adults with no high school education. Overall 5- and 10- year OS probabilities were 51.5% and 34.8%, respectively, while the median OS (SD) was 63.57 (2.8) months (Table 3).
Private insurance showed significantly higher 5- and 10-year OS probabilities and median OS: 5-year OS was 61.2%, 10-year OS was 47.2%, and median survival (SD) was 101.2 (8.2) months compared with that of all other insurance groups (Medicare, Medicaid, other government insurance, and uninsured) (Figure 1). These other insurance types were fairly similar in their 5-year and median OS, but surprisingly, patients with no insurance had the second longest 10-year OS. The difference between the 5-year OS probabilities of private insurance compared with an average of the other insurances was 15.1%, which had almost doubled to 28.5% at 10 years, with a median OS difference of almost 5 years (56 months; data not shown).
Using the Kaplan-Meier survival curve, Asian Indians had the longest 5-year OS probability of 77.9% and African Americans had the longest 10-year OS probability of 40.6%. However, Asians as a group demonstrated the longest median (SD) OS outcome with 119.8 (47.8) months (Figure 2).
Overall, academic/research programs had the longest median OS and 5-year OS probability (SD) of 66.6 (4.5) months and 52.6%, respectively (Figure 3). Comprehensive community cancer programs and integrated network cancer programs had nearly identical 10-year OS rates (35.2% vs 35.1%, respectively). Community cancer programs had the worst 5- and 10-year OS probabilities (41.1% and 21.8%, respectively).
The top 2 income quartiles combined to demonstrate the longest median, 5-year, and 10-year OS probabilities and were very similar. Patients living in a zip code with the highest income level had the longest 5-year OS rates of 54.3%, while patients living in zip codes with a moderate income level had the longest 10-year OS at 39.3% and the longest median OS of about 71 months. Patients with the lowest level of median household income had the worst 5-year OS rates (48.3%) and a median (SD) OS of 53.4 (5.4) months (Figure 4).
A Kaplan-Meier curve for percentage of adults without a HS degree is displayed in Figure 5. Zip codes with the highest level of education had the longest 5-year OS rates and median (SD) OS of 55.3% and 70.9 (4.8) months, respectively. The longest 10-year OS outcomes at 38.1% were found in patients who lived in areas of low-education levels. The worst 5- and 10- year OS outcomes and median OS were found in the least educated zip codes.
Results from the Cox regression model of OS are displayed in Table 4. Race and ethnicity, zip code-level median household income, and zip code-level education were not associated with OS. Patients with no insurance had an increased risk of death (hazard ratio [HR], 1.84; 95% CI, 1.17-2.88; P < .01) when compared with patients with private insurance. Patients with other government insurance also had an increased risk of death (HR, 2.12; 95% CI, 1.27-3.54; P < .01) when compared with patients with private insurance while controlling for all other variables. Patients with Medicare had a decreased risk of death when compared with patients with other government insurance and no insurance (HR, 0.53; 95% CI, 0.31-0.92; P = .02 and HR, 0.62; 95% CI, 0.38-0.99; P = .05, respectively). Patients treated at academic centers had better OS when compared with patients treated at comprehensive treatment centers (HR, 0.77; 95% CI, 0.65-0.92;P < .01) and community treatment centers (HR, 0.62; 95% CI, 0.44-0.86; P < .01).
Discussion
This study is the largest study to date that specifically studies the type of treatment facilities and socioeconomic factors, including insurance status, race, income, and education, and how they affect survival of DDLPS. The overall 5- and 10-year OS probabilities for DDLPS in this study were 51.5% and 34.8%, respectively, with median OS of 63.6 months. These results were more encouraging than previous reports, which found a 5-year survival probability of 36.5% and a median OS of 45 months.13,14
The largest age grouping was aged 61 to 80 years (48.9% of the cohort), and the median age at diagnosis was 64 years. DDLPSs most typically present between the ages of 50 and 70 years.15 Our cohort was 65% male. Previous studies have indicated that DDLPSs affect the sexes equally; however, another study showed a similar male predominance (68.8%) at the MD Anderson Cancer Center in Houston, Texas.13,16
In our study, approximately 88% of patients were white, 6.5% were African American, and 2.5% were Asian, which differed from a previous study of 84 patients that had a 78.6% white, 4.8% Asian, and 1.2% African American patient population.14
Asian Indian or Pakistani patients had the best 5-year OS probability at 77.9%, followed by African American (57.2%), Asian (51.6%), AI/AN (51.4%), and white patients (50.9%). This trend had disappeared by 10 years and Asian, AI/AN, African American, and Asian Indian or Pakistani groups all demonstrated longer median OS than did white patients. In fact, Asian patients had the longest median OS at 119.8 months, which was almost double that of white patients with the lowest median OS of 61.2 months. This finding is contrary to previous studies, which reported that racial minorities typically had worse OS outcomes when compared with white patients in different types of cancer.7,17 Notably, these findings were not statistically significant in our current study in the log-rank or multivariable analyses.
Private insurance was the most common form of insurance followed in decreasing order by Medicare, Medicaid, uninsured, and other government insurance. About 42% of the cohort had Medicare, which is a federally funded US insurance program designated for patients aged ≥ 65 years and certain younger patients with disabilities.
Patients with private insurance demonstrated the longest OS, essentially twice the median OS of all other insured groups at 101 months. Medicare had the worst 5-year OS probability and median OS of all groups. A previous study of 77 patients with DDLPS reported that patients aged > 65 years had reduced OS.13 Medicare patients in this study were older, with a mean and median age at DDLPS diagnosis of 71 and 72 years, respectively, while private insurance had a mean and median age at diagnosis of 56 and 57 years, respectively. Medicare inherently covers older patients and this age difference could account for the decrease in overall survival.
Improved OS for privately insured patients was most notable compared with the uninsured or patients with other government insurance. Uninsured patients had an 83.7% increased risk of mortality when compared with patients with private insurance. When compared with patients with private insurance, patients with other government insurance had an 111.5% increased risk of mortality. Comparing patients with Medicare vs patients with no insurance or other government insurance, there was a decreased risk of mortality of 38.5% and 46.6%, respectively. This decreased OS in patients with other government insurance could be related to the choice of treatment facility, because only 31% of the patients with other government insurance went to academic or research centers when compared with the 58.4% and 50.8% of patients with private and Medicare insurance treated there (data not shown). Such centers often have access to more advanced technology and protocols that may not be available at other treatment facilities.
A little more than half of the patients in the cohort went to an academic or research center for treatment (53.7%); comprehensive community cancer programs were the second most common treatment facility at 28%. Patients treated at academic or research centers demonstrated the best outcomes with a 5-year OS of 52.6%, followed in decreasing order by comprehensive community cancer programs (49.7%), integrated network cancer programs (48.8%), and community cancer programs (41.1%). In our patient cocohort, patients treated at an academic/research center had slightly decreased 10-year OS rates compared with those patients treated at a comprehensive community cancer program, although the median OS for the academic/research centers were still the highest of all treatment facilities.
Treatment options varied significantly by facility, and the number of patients treated surgically followed a similar trend, with 92% undergoing surgery as the primary treatment at academic or research programs compared with 89% at comprehensive cancer programs and 82.7% at community cancer programs (data not shown). Another potential explaination for differing OS outcomes across facilities is the surgical margin outcome. Surgeries performed at community cancer programs or comprehensive cancer programs resulted with no residual tumor in 36% and 40% of cases, respectively, whereas cases performed at academic or research programs resulted with no residual tumor in 47% of cases (data not shown). Regardless, multivariate analysis demonstrated a marked decrease in the chance of mortality when comparing treatment received at academic facility centers with that received at comprehensive cancer centers (22.9%) and community cancer centers (38.3%) (data not shown).
A recent study demonstrated improved outcomes for patients with retroperitoneal or extremity STS treated at high-volume treatment centers.18 Patients treated at high-volume centers were found to have an 8% decreased risk of death compared with patients treated at low-volume centers. Notably, they found highvolume academic centers demonstrated the strongest improvement in survival, while highvolume community centers showed decreased survival.18 Similarly, we found that patients treated at academic/research institutions had improved 5-year OS and greater median OS than did patients treated at community cancer programs or comprehensive community cancer programs.
The top 2 income quartiles (≥ $48,000) combined to demonstrate the longest median, 5-year, and 10-year OS and were fairly similar between the quartiles. Patients living in zip codes with a median income of $38,000 to $47,999 had the worst 5-year OS and median OS. The log-rank analysis showed statistical evidence of differences in survival associated with income, but within the context of the multivariable analysis, there was no remaining evidence of a difference.
The longest 5-year OS outcomes were seen in patients living in zip codes with the highest level of education (55.3%). However, the difference in OS was not statistically significant using either the log-rank analysis or multivariate analysis.
Limitations
This study has certain inherent limitations in using a retrospective design and a large database such as the NCDB. Many different pathologists at CoC-accredited cancer programs perform the pathology that contributes to the data in the NCDB. There was no pathological review of these findings, which could potentially introduce error into the findings of this study. With the NCDB, potential selection bias is possible because patients in the database are added only from CoC-accredited cancer programs. This risk is minimized because NCDB contains data on most newly diagnosed cancer patients in the US. Further potential risks, which are unable to be controlled for, include potential interobserver error and data that may be incompletely, improperly, or inaccurately recorded from the patients’ charts. Without patient-specific information regarding income and education, it is challenging to utilize zip codes to estimate socioeconomic status and educational level. Even though a patient may live in a zip code identified with specific economic and educational characteristics, that patient may not share those characteristics. Furthermore, patients with Medicare tend to be older than patients with other forms of insurance, which limits the significance of comparisons across insurance groups. A future SEER (Surveillance, Epidemiology, and End Results) program study to confirm this study’s results and the effects of socioeconomic variables on DDLPS would be an excellent followup study.
Conclusion
This study used a large cohort of patients with DDLPS to study the effects of treatment facility, insurance status, and socioeconomic variables on survival outcomes. Although insurance status, median household income, and treatment facility were associated with differences in median OS and 5- and 10-year OS probabilities, evidence for a difference remained for only insurance status and facility type within the context of a multivariable analysis irrespective of age, race, sex, insurance status, education, and median income. Patients with private insurance and Medicaid had a decreased risk of mortality compared with other government insurance and no insurance. Patients receiving treatment at academic research programs had the highest median and 5-year OS of 66.6 months and 52.6%, respectively. Patients receiving treatment at academic centers had improved survival outcomes with a decrease in mortality of 23% and 38% compared to comprehensive or community cancer programs.
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550
1. Dodd LG. Update on liposarcoma: a review for cytopathologists. Diagn Cytopathol. 2012;40(12):1122-1131.
2. Mangham D. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Am. 2004;86(3):466.
3. Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381-391.
4. Coindre JM, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch. 2010;456(2):167-179.
5. Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3(6):507-523.
6. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271-281.
7. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93.
8. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231.
9. Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411.
10. Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103-110.
11. Chu Q, Medeiros K, Zhou M, et al. Effect of facility type on outcome following pancreatectomy for pancreatic adenocarcinoma: analysis of the National Cancer Data Base [Abstract FP26-02]. HPB (Oxford). 2016;18(suppl 1):E81-E82.
12. Rubin SJ, Cohen MB, Kirke DN, Qureshi MM, Truong MT, Jalisi S. Comparison of facility type outcomes for oral cavity cancer: analysis of the National Cancer Database. Laryngoscope. 2017;127(11):2551-2557.
13. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008;15(6):1585-1593.
14. Livingston JA, Bugano D, Barbo A, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep. 2017;7(1):11836.
15. Brennan MF, Antonescu CR, Alektiar KM, Maki RG. Management of Soft Tissue Sarcoma. 2nd ed. New York, NY: Springer; 2016.
16. Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia, PA: Saunders; 2014.
17. White A, Djenaba J, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001‐2009): findings from the CONCORD‐2 study. Cancer. 2017;123 (suppl 24):5014-5036.
18. Murphy JD, Padwal J, Guss ZD, Okamoto K, Sardar R. Impact of hospital volume on patterns of care and outcomes in soft tissue sarcoma [ASCO Abstract e23550]. J Clin Oncol. 2018;36(suppl 15):e23550
Undiagnosed COPD may change heart function before heart attacks occur
, data from a clinical trial suggest.
The finding could represent an opportunity for early intervention, Rita Pavasini, MD, wrote in COPD: Journal of Chronic Obstructive Pulmonary Disease.
“Early change of the right ventricular function, as detected by reduced right ventricular strain and reduced fractional area change, is present in [these] patients,” wrote Dr. Pavasini of Azienda Ospedaliero-Universitaria di Ferrara, Cona, Italy. “Further studies are warranted to determine whether these alterations can be reversed or modulated by an early identification and treatment and whether targeting early right ventricular strain reduction results in better clinical outcomes in these comorbid patients.”
Dr. Pavasini and colleagues reported a prespecified subanalysis of the Screening for COPD in ACS Patients (SCAP) trial, which showed that screening patients admitted for acute coronary syndromes with respiratory measures could identify those at risk for undiagnosed COPD. The substudy examined the echocardiographic characteristics of these patients.
SCAP comprised 137 patients who were current or past smokers. At baseline, 29% had a COPD diagnosis. However, most displayed airflow limitation (59% mild, 38% moderate). All underwent transthoracic echocardiogram at baseline and at 6 months.
At baseline, fractional area change (FAC) was already significantly lower in patients with occult COPD than in those without it (38% vs. 44%). Patients with undiagnosed COPD also had significantly reduced right ventricular strain (RVS) (–15 vs. –20).
“Interestingly, the inferior location of myocardial infarction did not influence the results,” the authors said. “Indeed, RVS and FAC did not differ between patients with inferior location of myocardial infarction vs. those without inferior location.”
At 6 months, these differences were unabated. FAC was still significantly lower among those with COPD (37% vs. 46%), and RVS still significantly less (–16 vs. –20). Systolic pulmonary artery pressure (sPAP) was also significantly higher in patients with concomitant COPD (35 vs. 27 mm Hg).
“The early impairment in RVS might reflect the impact of endothelial dysfunction, increased arterial stiffness and inflammation on right heart function, rather than only hypoxia, and even more in a population with mainly mild to moderate COPD,” the team said. “Thus, it is highly probable that sPAP in patients with undiagnosed COPD is mainly determined by concomitant pulmonary disease, which did not differ between acute and chronic phase.”
On the whole, the data suggest that the impairment of right ventricular strain was primarily related to the undiagnosed COPD, and not the cardiovascular event, they said,
“These data are consistent with the observed impairment of RVS and indirectly confirms that undiagnosed COPD determines early changes in the structure and function of the right heart.”
Dr. Pavasini had no relevant financial disclosures.
SOURCE: Pavasini R et al. COPD 2019 Jul 21. doi: 10.1080/15412555.2019.16451059.
, data from a clinical trial suggest.
The finding could represent an opportunity for early intervention, Rita Pavasini, MD, wrote in COPD: Journal of Chronic Obstructive Pulmonary Disease.
“Early change of the right ventricular function, as detected by reduced right ventricular strain and reduced fractional area change, is present in [these] patients,” wrote Dr. Pavasini of Azienda Ospedaliero-Universitaria di Ferrara, Cona, Italy. “Further studies are warranted to determine whether these alterations can be reversed or modulated by an early identification and treatment and whether targeting early right ventricular strain reduction results in better clinical outcomes in these comorbid patients.”
Dr. Pavasini and colleagues reported a prespecified subanalysis of the Screening for COPD in ACS Patients (SCAP) trial, which showed that screening patients admitted for acute coronary syndromes with respiratory measures could identify those at risk for undiagnosed COPD. The substudy examined the echocardiographic characteristics of these patients.
SCAP comprised 137 patients who were current or past smokers. At baseline, 29% had a COPD diagnosis. However, most displayed airflow limitation (59% mild, 38% moderate). All underwent transthoracic echocardiogram at baseline and at 6 months.
At baseline, fractional area change (FAC) was already significantly lower in patients with occult COPD than in those without it (38% vs. 44%). Patients with undiagnosed COPD also had significantly reduced right ventricular strain (RVS) (–15 vs. –20).
“Interestingly, the inferior location of myocardial infarction did not influence the results,” the authors said. “Indeed, RVS and FAC did not differ between patients with inferior location of myocardial infarction vs. those without inferior location.”
At 6 months, these differences were unabated. FAC was still significantly lower among those with COPD (37% vs. 46%), and RVS still significantly less (–16 vs. –20). Systolic pulmonary artery pressure (sPAP) was also significantly higher in patients with concomitant COPD (35 vs. 27 mm Hg).
“The early impairment in RVS might reflect the impact of endothelial dysfunction, increased arterial stiffness and inflammation on right heart function, rather than only hypoxia, and even more in a population with mainly mild to moderate COPD,” the team said. “Thus, it is highly probable that sPAP in patients with undiagnosed COPD is mainly determined by concomitant pulmonary disease, which did not differ between acute and chronic phase.”
On the whole, the data suggest that the impairment of right ventricular strain was primarily related to the undiagnosed COPD, and not the cardiovascular event, they said,
“These data are consistent with the observed impairment of RVS and indirectly confirms that undiagnosed COPD determines early changes in the structure and function of the right heart.”
Dr. Pavasini had no relevant financial disclosures.
SOURCE: Pavasini R et al. COPD 2019 Jul 21. doi: 10.1080/15412555.2019.16451059.
, data from a clinical trial suggest.
The finding could represent an opportunity for early intervention, Rita Pavasini, MD, wrote in COPD: Journal of Chronic Obstructive Pulmonary Disease.
“Early change of the right ventricular function, as detected by reduced right ventricular strain and reduced fractional area change, is present in [these] patients,” wrote Dr. Pavasini of Azienda Ospedaliero-Universitaria di Ferrara, Cona, Italy. “Further studies are warranted to determine whether these alterations can be reversed or modulated by an early identification and treatment and whether targeting early right ventricular strain reduction results in better clinical outcomes in these comorbid patients.”
Dr. Pavasini and colleagues reported a prespecified subanalysis of the Screening for COPD in ACS Patients (SCAP) trial, which showed that screening patients admitted for acute coronary syndromes with respiratory measures could identify those at risk for undiagnosed COPD. The substudy examined the echocardiographic characteristics of these patients.
SCAP comprised 137 patients who were current or past smokers. At baseline, 29% had a COPD diagnosis. However, most displayed airflow limitation (59% mild, 38% moderate). All underwent transthoracic echocardiogram at baseline and at 6 months.
At baseline, fractional area change (FAC) was already significantly lower in patients with occult COPD than in those without it (38% vs. 44%). Patients with undiagnosed COPD also had significantly reduced right ventricular strain (RVS) (–15 vs. –20).
“Interestingly, the inferior location of myocardial infarction did not influence the results,” the authors said. “Indeed, RVS and FAC did not differ between patients with inferior location of myocardial infarction vs. those without inferior location.”
At 6 months, these differences were unabated. FAC was still significantly lower among those with COPD (37% vs. 46%), and RVS still significantly less (–16 vs. –20). Systolic pulmonary artery pressure (sPAP) was also significantly higher in patients with concomitant COPD (35 vs. 27 mm Hg).
“The early impairment in RVS might reflect the impact of endothelial dysfunction, increased arterial stiffness and inflammation on right heart function, rather than only hypoxia, and even more in a population with mainly mild to moderate COPD,” the team said. “Thus, it is highly probable that sPAP in patients with undiagnosed COPD is mainly determined by concomitant pulmonary disease, which did not differ between acute and chronic phase.”
On the whole, the data suggest that the impairment of right ventricular strain was primarily related to the undiagnosed COPD, and not the cardiovascular event, they said,
“These data are consistent with the observed impairment of RVS and indirectly confirms that undiagnosed COPD determines early changes in the structure and function of the right heart.”
Dr. Pavasini had no relevant financial disclosures.
SOURCE: Pavasini R et al. COPD 2019 Jul 21. doi: 10.1080/15412555.2019.16451059.
FROM COPD: JOURNAL OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Beyond sunscreen: Skin cancer preventive agents finding a role
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
NEW YORK – (KCs) that deserves to be considered for selective use in at-risk patients, according to an update at the American Academy of Dermatology summer meeting.
In providing her perspective on the available options, Rebecca Hartman, MD, MPH, director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, emphasized that the therapies are not interchangeable but deserve to be used selectively according to their relative protection and relative risks.
Of oral agents, she characterized two, nicotinamide and acitretin, as “clinic-ready.” Acitretin is “an oldie but goodie,” but there is an important issue of tolerability. In the published studies, 15%-39% of patients withdrew because of adverse events, according to Dr. Hartman, which suggests the need for a motivated patient.
In addition, acitretin can be esterified into etretinate, a teratogen that can persist as long as 3 years after the drug is discontinued, making this drug contraindicated in women of childbearing potential, she noted.
However, most patients in need of prophylaxis for KCs are older, so teratogenicity is not an issue. In her practice, she offers acitretin to patients who are developing three or more KCs per year, as well as in situations of extensive skin damage in which a course of acitretin might provide some degree of clearing.
“When you are faced with the potential of a large number of biopsies, you could start acitretin to see if lesions can be reduced,” Dr. Hartman said .
Prevention of KCs became somewhat more attractive as a routine practice following publication of a phase 3 trial with nicotinamide. In this study, nicotinamide, an over-the-counter water-soluble form of vitamin B3, was associated with significantly reduced nonmelanoma skin cancers, including KCs and actinic keratoses, relative to placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26). Importantly, there was no greater risk of adverse events relative to placebo.
When assessed individually, the relative reduction in squamous cell carcinomas (SCCs; P = .05) and basal cell carcinomas (P = .12) fell short of statistical significance, but there was a highly significant 13% reduction in actinic keratoses after 12 months (P less than .001). An increase in SCCs was observed after therapy was stopped, which led Dr. Hartman to conclude that nicotinamide must be used on a “use-it-or-lose-it” basis. However, she does routinely offer this option.
“When do I recommend nicotinamide? Any patient with multiple actinic keratoses who wants to get ahead of the game and wants something that is relative safe,” Dr. Hartman explained. She uses the same dosing employed in the study, which was 500 mg twice daily.
There are other options for chemoprevention of KCs, but they are less attractive.
For example, capecitabine is effective, but tolerability is an even greater issue with this agent than it is for acitretin. According to Dr. Hartman, “we use this therapy very rarely and only in select cases.” As an alternative to the 14 days on and 7 days off schedule used for treatment of cancer, capecitabine is sometimes better tolerated in a 7 day on and 7 day off schedule, she said.
Topical 5-fluorouracil with or without calcipotriol is another chemoprevention option for those who can tolerate a skin reaction that lasts several days, Dr. Hartman said. She cited one study that associated this therapy with a nearly 80% reduction in face and scalp SCC.
Ultimately, she offers 5-fluorouracil with or without calcipotriol to “patients who want an evidence-based chemoprevention,” but she indicated that patients must be motivated to endure the adverse effects.
Many remain unaware of the array of options for chemoprevention of KCs, but Dr. Hartman emphasized that this is an area of active research with new options expected.
“I am really excited about the future direction of chemoprevention in skin cancer,” said Dr. Hartman, citing ongoing work to develop vitamin A, polypodium leucotomas extract, and human papillomavirus vaccine as options.
“If we can stop skin cancer in the first place, avoiding the morbidity and mortality of treatment, we will also hopefully save costs as well,” she commented. So far, essentially all of the strategies for chemoprevention, other than sunscreen, involve KCs, which leaves a large unmet need for better ways to prevent melanoma. However, Dr. Hartman noted that KCs represent the most common type of cancer of any type.
Just days after Dr. Hartman spoke at the meeting, a prospective study of vitamin A that found an inverse association between vitamin A intake and cutaneous SCC risk was, in fact, published in JAMA Dermatology (2019 Jul 31. doi: 10.1001/jamadermatol.2019.1937).
Dr. Hartman reported no financial relationships relevant to her presentation.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Medical societies urge action to reduce gun violence
“With nearly 40,000 firearm-related deaths in 2017, the United States has reached a 20-year high, according to the Centers for Disease Control and Prevention,” the authors noted in the call to action (Ann Intern Med. 2019 Aug 7. doi: 10.7326/M19-2441).
The recommendations “stem largely from the individual positions previously approved by our organizations and ongoing collaborative discussion among leaders,” wrote the authors, who included leaders of the American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American College of Surgeons, American Medical Association, and American Psychiatric Association, as well as the American Public Health Association.
“Our organizations support a multifaceted public health approach to prevention of firearm injury and death similar to approaches that have successfully reduced the ill effects of tobacco use, motor vehicle accidents, and unintentional poisoning,” wrote Robert McLean, MD, president of the American College of Physicians, and colleagues. “While we recognize the significant political and philosophical differences about firearm ownership and regulation in the United States, we are committed to reaching out to bridge these differences to improve the health and safety of our patients, their families, and communities, while respecting the U.S. Constitution.”
The organizations specifically call for the following:
- Comprehensive criminal background checks for all firearm purchases and transfers between individuals, with limited exceptions.
- Research into the causes and consequences of firearm-related injury and death and the development and implementation of strategies to reduce these events.
- Extension of federal laws prohibiting access to firearms for domestic abusers to dating partners.
- The passage of child access prevention laws that hold accountable firearm owners who negligently store firearms under circumstances where minors could or do gain access to them.
- Improving access to mental health care for all individuals, while not broadly including all individuals with a mental health or substance use disorder in a category of individuals prohibited from purchasing firearms.
- Enactment of extreme risk protection order (ERPO) laws, which allow a judge to temporarily remove firearms from those who might be at imminent risk for using them to harm themselves or others. ERPO laws should be enacted in a manner consistent with due process.
- Physicians can and must be able to counsel at-risk patients about mitigating firearms-associated risks in the home.
- High-capacity magazines and firearms with features designed to increase their rapid and extended killing capacity should be the subject of special scrutiny and regulation.
For more than 2 decades, the American College of Physicians “has advocated for the urgent need for impactful legislation that would reduce firearms-related injuries and deaths,” ACP officials said in a statement.
“We need to protect our patients, their families, and our communities across the country from needless injuries and deaths; it’s time for the [United States] to put firearms violence prevention at the forefront of the health care conversation,” Dr. McLean said in a statement.“We are committed to working with all stakeholders, and continuing to speak out, to address this public health threat.”
Leaders at the American Academy of Family Physicians concurred.
“We need to come together as a nation on this issue,” AAFP President John Cullen, MD, said in a statement. “Treating firearm injuries as a public health issue is an important first step. We did this for motor vehicle accidents and have seen a significant decrease in injuries. We didn’t try to remove cars, we made them safer ... Both sides of the debate should come together now and work on solutions—including safe storage laws, expanded background checks, research and improved access to mental health services—we can all agree on.”
Additional firearms-related health policy content that has been published in Annals of Internal Medicine is available at: http://annals.org/aim/pages/firearm-related-content.
“With nearly 40,000 firearm-related deaths in 2017, the United States has reached a 20-year high, according to the Centers for Disease Control and Prevention,” the authors noted in the call to action (Ann Intern Med. 2019 Aug 7. doi: 10.7326/M19-2441).
The recommendations “stem largely from the individual positions previously approved by our organizations and ongoing collaborative discussion among leaders,” wrote the authors, who included leaders of the American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American College of Surgeons, American Medical Association, and American Psychiatric Association, as well as the American Public Health Association.
“Our organizations support a multifaceted public health approach to prevention of firearm injury and death similar to approaches that have successfully reduced the ill effects of tobacco use, motor vehicle accidents, and unintentional poisoning,” wrote Robert McLean, MD, president of the American College of Physicians, and colleagues. “While we recognize the significant political and philosophical differences about firearm ownership and regulation in the United States, we are committed to reaching out to bridge these differences to improve the health and safety of our patients, their families, and communities, while respecting the U.S. Constitution.”
The organizations specifically call for the following:
- Comprehensive criminal background checks for all firearm purchases and transfers between individuals, with limited exceptions.
- Research into the causes and consequences of firearm-related injury and death and the development and implementation of strategies to reduce these events.
- Extension of federal laws prohibiting access to firearms for domestic abusers to dating partners.
- The passage of child access prevention laws that hold accountable firearm owners who negligently store firearms under circumstances where minors could or do gain access to them.
- Improving access to mental health care for all individuals, while not broadly including all individuals with a mental health or substance use disorder in a category of individuals prohibited from purchasing firearms.
- Enactment of extreme risk protection order (ERPO) laws, which allow a judge to temporarily remove firearms from those who might be at imminent risk for using them to harm themselves or others. ERPO laws should be enacted in a manner consistent with due process.
- Physicians can and must be able to counsel at-risk patients about mitigating firearms-associated risks in the home.
- High-capacity magazines and firearms with features designed to increase their rapid and extended killing capacity should be the subject of special scrutiny and regulation.
For more than 2 decades, the American College of Physicians “has advocated for the urgent need for impactful legislation that would reduce firearms-related injuries and deaths,” ACP officials said in a statement.
“We need to protect our patients, their families, and our communities across the country from needless injuries and deaths; it’s time for the [United States] to put firearms violence prevention at the forefront of the health care conversation,” Dr. McLean said in a statement.“We are committed to working with all stakeholders, and continuing to speak out, to address this public health threat.”
Leaders at the American Academy of Family Physicians concurred.
“We need to come together as a nation on this issue,” AAFP President John Cullen, MD, said in a statement. “Treating firearm injuries as a public health issue is an important first step. We did this for motor vehicle accidents and have seen a significant decrease in injuries. We didn’t try to remove cars, we made them safer ... Both sides of the debate should come together now and work on solutions—including safe storage laws, expanded background checks, research and improved access to mental health services—we can all agree on.”
Additional firearms-related health policy content that has been published in Annals of Internal Medicine is available at: http://annals.org/aim/pages/firearm-related-content.
“With nearly 40,000 firearm-related deaths in 2017, the United States has reached a 20-year high, according to the Centers for Disease Control and Prevention,” the authors noted in the call to action (Ann Intern Med. 2019 Aug 7. doi: 10.7326/M19-2441).
The recommendations “stem largely from the individual positions previously approved by our organizations and ongoing collaborative discussion among leaders,” wrote the authors, who included leaders of the American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American College of Surgeons, American Medical Association, and American Psychiatric Association, as well as the American Public Health Association.
“Our organizations support a multifaceted public health approach to prevention of firearm injury and death similar to approaches that have successfully reduced the ill effects of tobacco use, motor vehicle accidents, and unintentional poisoning,” wrote Robert McLean, MD, president of the American College of Physicians, and colleagues. “While we recognize the significant political and philosophical differences about firearm ownership and regulation in the United States, we are committed to reaching out to bridge these differences to improve the health and safety of our patients, their families, and communities, while respecting the U.S. Constitution.”
The organizations specifically call for the following:
- Comprehensive criminal background checks for all firearm purchases and transfers between individuals, with limited exceptions.
- Research into the causes and consequences of firearm-related injury and death and the development and implementation of strategies to reduce these events.
- Extension of federal laws prohibiting access to firearms for domestic abusers to dating partners.
- The passage of child access prevention laws that hold accountable firearm owners who negligently store firearms under circumstances where minors could or do gain access to them.
- Improving access to mental health care for all individuals, while not broadly including all individuals with a mental health or substance use disorder in a category of individuals prohibited from purchasing firearms.
- Enactment of extreme risk protection order (ERPO) laws, which allow a judge to temporarily remove firearms from those who might be at imminent risk for using them to harm themselves or others. ERPO laws should be enacted in a manner consistent with due process.
- Physicians can and must be able to counsel at-risk patients about mitigating firearms-associated risks in the home.
- High-capacity magazines and firearms with features designed to increase their rapid and extended killing capacity should be the subject of special scrutiny and regulation.
For more than 2 decades, the American College of Physicians “has advocated for the urgent need for impactful legislation that would reduce firearms-related injuries and deaths,” ACP officials said in a statement.
“We need to protect our patients, their families, and our communities across the country from needless injuries and deaths; it’s time for the [United States] to put firearms violence prevention at the forefront of the health care conversation,” Dr. McLean said in a statement.“We are committed to working with all stakeholders, and continuing to speak out, to address this public health threat.”
Leaders at the American Academy of Family Physicians concurred.
“We need to come together as a nation on this issue,” AAFP President John Cullen, MD, said in a statement. “Treating firearm injuries as a public health issue is an important first step. We did this for motor vehicle accidents and have seen a significant decrease in injuries. We didn’t try to remove cars, we made them safer ... Both sides of the debate should come together now and work on solutions—including safe storage laws, expanded background checks, research and improved access to mental health services—we can all agree on.”
Additional firearms-related health policy content that has been published in Annals of Internal Medicine is available at: http://annals.org/aim/pages/firearm-related-content.
FROM ANNALS OF INTERNAL MEDICINE
Product Update: Osphena’s NDA, new hysteroscope, TempSure RF technology, Resilient stirrup covers
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
Genomic Medicine and Genetic Counseling in the Department of Veterans Affairs and Department of Defense (FULL)
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Prevalence of Cancer in Thyroid Nodules In the Veteran Population (FULL)
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
Peeling skin with chills
The physician thought that this was most likely toxic epidermal necrolysis (TEN). He was in a small hospital without a burn unit, so he drew baseline labs and blood cultures, and started to give intravenous fluids to treat the dehydration.
TEN is on the most severe side of a spectrum of disorders that includes erythema multiforme and Stevens-Johnson syndrome (SJS). Erythema multiforme is diagnosed when < 10% of the body surface area is involved, SJS/TEN when between 10% and 30% is involved, and TEN when >30% is involved. Drugs that are most commonly known to cause SJS and TEN include sulfonamide antibiotics, allopurinol, nonsteroidal anti-inflammatory agents, amine antiepileptic drugs (phenytoin and carbamazepine), and lamotrigine. In this case, the amoxicillin was the likely culprit.
The physician waited until the patient was hemodynamically stable before transferring her to the closest city hospital where a dermatologist could manage her care. The dermatologist agreed with the diagnosis of TEN and continued supportive care. While the hospital did not have intravenous immunoglobulin or cyclosporine on hand, the health care team was able to provide the necessary supportive care. The patient survived, and she was warned to never take any type of penicillin again.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The physician thought that this was most likely toxic epidermal necrolysis (TEN). He was in a small hospital without a burn unit, so he drew baseline labs and blood cultures, and started to give intravenous fluids to treat the dehydration.
TEN is on the most severe side of a spectrum of disorders that includes erythema multiforme and Stevens-Johnson syndrome (SJS). Erythema multiforme is diagnosed when < 10% of the body surface area is involved, SJS/TEN when between 10% and 30% is involved, and TEN when >30% is involved. Drugs that are most commonly known to cause SJS and TEN include sulfonamide antibiotics, allopurinol, nonsteroidal anti-inflammatory agents, amine antiepileptic drugs (phenytoin and carbamazepine), and lamotrigine. In this case, the amoxicillin was the likely culprit.
The physician waited until the patient was hemodynamically stable before transferring her to the closest city hospital where a dermatologist could manage her care. The dermatologist agreed with the diagnosis of TEN and continued supportive care. While the hospital did not have intravenous immunoglobulin or cyclosporine on hand, the health care team was able to provide the necessary supportive care. The patient survived, and she was warned to never take any type of penicillin again.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The physician thought that this was most likely toxic epidermal necrolysis (TEN). He was in a small hospital without a burn unit, so he drew baseline labs and blood cultures, and started to give intravenous fluids to treat the dehydration.
TEN is on the most severe side of a spectrum of disorders that includes erythema multiforme and Stevens-Johnson syndrome (SJS). Erythema multiforme is diagnosed when < 10% of the body surface area is involved, SJS/TEN when between 10% and 30% is involved, and TEN when >30% is involved. Drugs that are most commonly known to cause SJS and TEN include sulfonamide antibiotics, allopurinol, nonsteroidal anti-inflammatory agents, amine antiepileptic drugs (phenytoin and carbamazepine), and lamotrigine. In this case, the amoxicillin was the likely culprit.
The physician waited until the patient was hemodynamically stable before transferring her to the closest city hospital where a dermatologist could manage her care. The dermatologist agreed with the diagnosis of TEN and continued supportive care. While the hospital did not have intravenous immunoglobulin or cyclosporine on hand, the health care team was able to provide the necessary supportive care. The patient survived, and she was warned to never take any type of penicillin again.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
FDA panel backs Descovy as HIV PrEP for men and transgender women who have sex with men
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
The Food and Drug Administration’s Antimicrobial Drugs Advisory Committee backed the fixed dose combination of emtricitabine and tenofovir alafenamide (TAF; Descovy, Gilead) for pre-exposure prophylaxis (PrEP) against HIV for men and transgender women who have sex with men.
In a discussion after a 16-2 vote, committee members cited analysis by the study’s sponsor and the FDA showing efficacy and a generally good safety profile in the DISCOVER trial, the single new clinical trial conducted to support TAF’s use for pre-exposure prophylaxis (PrEP).
However, this trial included no cisgender women; the sponsor asked for approval based primarily on extrapolation from the DISCOVER results and previous results with tenofovir disoproxil fumarate (TDF) in cisgender women. Both formulations of tenofovir are prodrugs and converted to tenofovir diphosphate intracellularly in peripheral blood mononuclear cells, though many aspects of their pharmacokinetics differ.
The committee voted 10-8 against the proposition that these data supported an indication of TAF for PrEP in cisgender women, in a narrowly worded question from the FDA.
Many members who voted on either side of the question had strongly worded reservations about the lack of data for cisgender women. Said committee chair Lindsey R. Baden, MD, director of the infectious disease service at Dana-Farber Cancer Institute, Boston, who voted against the indication for cisgender women, “We’ve failed women. To be at this point and not have the data to guide decision-making is a shame on all of us.”
Ighovwerha Ofotokun, MD, who voted yes, concurred: “I agree it is a terrible failure that the agency, as well as the sponsor, would come to this committee with a lack of data on women.” But for Dr. Ofotokun, a professor of infectious diseases at Emory University, Atlanta, not including cisgender women in the approval was a distasteful proposition. “Creating a two-tier prevention and treatment hierarchy would not be helpful. We should remind ourselves that there are more women living with HIV in the world than there are men, and the risk of new HIV infection is higher among women than among men, if you look at this globally,” he said.
“I find it disrespectful and an issue of research equity. Women deserve the same quality of data about the safety and efficacy of the drugs they are exposed to that men get and that is not the situation we find ourselves in at the moment,” said Dawn K. Smith, MD, MPH, a lead scientist at the Centers for Disease Control and Prevention (CDC), Atlanta, who voted against approval for cisgender women.
Michael Green, MD, MPH, professor of pediatrics, surgery and clinical and translational science at the University of Pittsburgh, echoed the frustration of many committee members when he said, “I voted yes, almost abstained, then almost voted no.” He, along with all who voted yes, emphasized the importance of mandatory postmarketing studies in cisgender women to ensure efficacy data are obtained.
Transgender women made up only about 1% of the DISCOVER population, a fact that also gave many committee members pause.
If TAF is approved, labeling and package materials should be clear that the data support only noninferiority, not superiority, compared with TDF, said several advisory committee members who voted for approval for men and transgender women who have sex with men. “My expectation of this approval is that it should be marketed responsibly from the perspective of not creating these disparities and having Truvada be a drug for poor people and Descovy be a drug for rich people,” said Demetre Dasklalakis, MD, assistant commissioner of the Bureau of HIV/AIDS Prevention and Control at the city of New York’s Department of Health and Hygiene, and of the Icahn School of Medicine at Mount Sinai, N.Y. Truvada is slated to be offered as a generic drug in 2020, according to a Securities and Exchange Commission filing by Gilead Sciences.
The CDC reported earlier in 2019 that rates of new HIV infections have plateaued in recent years. Uptake of PrEP has been particularly low among at-risk members of minority populations, in rural areas, and in the South, according to a CDC report.
The DISCOVER trial is a 96-week ongoing trial to test TAF’s noninferiority to a fixed-drug combination of emcitrabine and tenofovir dimethyl fumarate (TDF; Truvada, Gilead) for PrEP. Both drugs are already approved to treat HIV infection, and TDF is approved for PrEP. Non-inferiority was preestablished at a rate ratio of HIV incidence of 1.62 (TAF:TDF) between the two study arms.
DISCOVER has enrolled 5,387 men and transgender women who have sex with men and are deemed at high risk for HIV, and found an incidence rate ratio of 0.47, with the upper bound of the confidence interval at 1.15. Since this figure was less than the prespecified noninferiority margin, both Gilead presenters and the FDA agreed, TAF’s noninferiority for efficacy was established.
Characteristics were similar between patients in the TAF arm (N = 2,694) and the TDF arm (N = 2,693). About 60% of patients in each arm reported having receptive anal sex with at least two partners in the previous 12 weeks, and recent rectal gonorrhea, syphilis, and chlamydia rates were 9-13% at baseline. Two thirds of participants reported recreational drug use, and about one in four reported binge drinking.
Sexual behavior and sexually transmitted infection rates continued generally unchanged from baseline during the study period.
The median age was 34 years, and most participants (84%) were white. Black participants made up 9% of the study population, and about 25% were of Hispanic or Latin ethnic origin.
Known decreases in bone mineral density occur with TDF; these were not seen with TAF, and bone mineral density increased while on TAF for the DISCOVER population aged 19-25 years.
Renal biomarkers of concern with TDF included two proteins linked with proximal tubule dysfunction, as well as estimated glomerular filtration rate. According to the sponsor’s analysis, eGFR fell by 2.3 mL/min for the TAF group, compared with a 1.8 mL/min rise while on TDF (P less than .001). Changes of similar statistical significance were seen for proximal tubular proteinuria. Also, improvements were seen in renal measures for the subset of patients enrolled who were on TDF PrEP at baseline but switched to TAF, in a prespecified subgroup analysis.
However, patients who were on TDF had a significant decrease in total cholesterol and both low- and high-density lipoprotein cholesterol compared with those on TAF, who had minimal changes or slight increases in lipids (P less than .001 for all). Triglycerides rose for those on TAF and remained unchanged for those on TDF (P = .002).
The PrEP indication sought by Gilead includes adults and adolescents, defined as those who weigh more than 35 kg. A nonvoting question put before the committee asked whether the totality of tenofovir data supported an indication of TAF for cisgender men who have insertive vaginal sex; though this extrapolation didn’t give the committee as much pause as the request for approval in cisgender women, they cited similar concerns and noted that cervicovaginal mucosa are different in many ways from rectal mucosa.
The study included no cisgender women, for a host of reasons cited by the sponsor and the FDA. These included high nonadherence rates among this population, relatively lower HIV infection rates among cisgender women in the United States, and mixed efficacy results in previous tenofovir clinical trials; the latter point made establishing a noninferiority margin problematic, according to the FDA.
For Dr. Baden, “The optics of approval for population A but not for population B are problematic.” Speaking to both the sponsor and the FDA, he said, “Everyone agrees there needs to be actual data. Please do the study as quickly as possible.” What’s needed is the collective will to make it happen, he added: “I don’t accept that it’s too big, too hard, too difficult.”
The FDA usually follows the recommendations of its advisory committees.
This article was updated 8/8/19.
FROM AN FDA ADVISORY COMMITTEE MEETING