User login
Signals of gut microbiome interaction with experimental Alzheimer’s drug prompt new trial
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
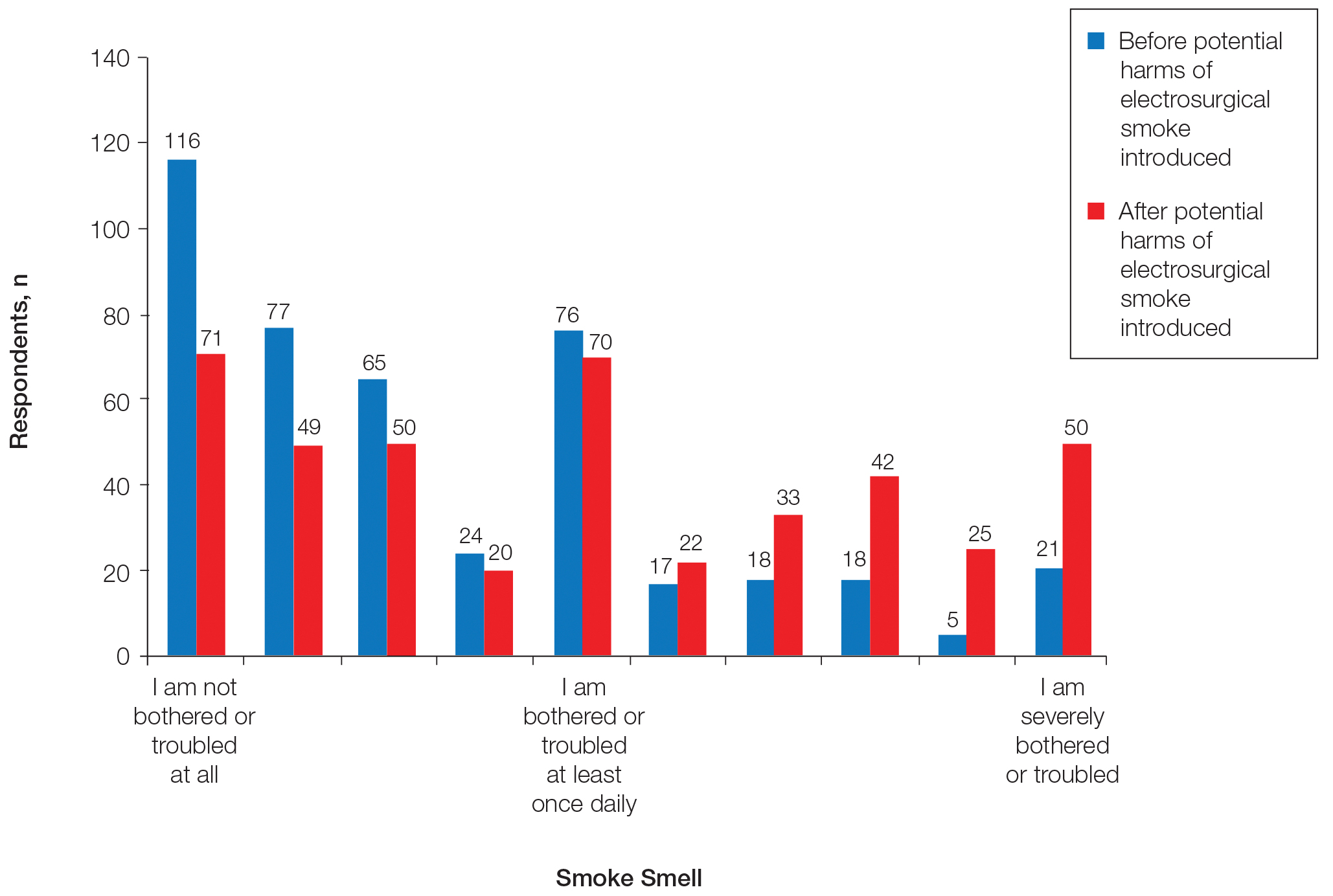
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
LOS ANGELES – A single look at the gut microbiome of patients with Alzheimer’s disease (AD) suggests an interaction between anti-inflammatory gut bacteria and long-term exposure to an investigational sigma 1 receptor agonist.
After up to 148 weeks treatment with Anavex 2-73, patients with stable or improved functional scores showed significantly higher levels of both Ruminococcaceae and Porphyromonadaceae, compared with patients who had declining function. Both bacterial families produce butyrate, an anti-inflammatory short-chain fatty acid.
Conversely, poor response was associated with a low level of Verrucomicrobia, a mucin-degrading phylum thought to be important in gut homeostasis. These bacteria live mainly in the intestinal mucosa – the physical interface between the microbiome and the rest of the body.
The data, presented at the Alzheimer’s Association International Conference, represent the first microbiome measurements reported in a clinical trial of an investigational Alzheimer’s therapy. Because they come from a single sample taken from a small group in an extension study, without a baseline comparator, it’s impossible to know what these associations mean. But the findings are enough to nudge Anavex Life Sciences into adding microbiome changes to its new study of Anavex 2-73, according to Christopher Missling, PhD, president and chief executive officer of the company.
The study, ramping up now, aims to recruit 450 patients with mild AD. They will be randomized to high-dose or mid-dose Anavex 2-73 for 48 weeks. The primary outcomes are measures of cognition and function. Stool sampling at baseline and at the end of the study will be included as well, Dr. Missling said in an interview.
Anavex 2-73 is a sigma-1 receptor agonist. A chaperone protein, sigma-1 is activated in response to acute and chronic cellular stressors, several which are important in neurodegeneration. The sigma-1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates several processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma-1 receptor activation can induce neuronal regrowth and functional recovery after stroke. It also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
Anavex 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients. This was followed by a 52-week, open-label extension trial of 10, 20, 30, and 50 mg/day orally, in which each patient was titrated to the maximum tolerated dose. The main endpoints were change on the Mini Mental State Exam and change on the Alzheimer’s Disease Cooperative Study-activities of daily living (ADCS-ADL) scale.
At 57 weeks, six patients had improved on the Mini Mental State Exam score: four with high plasma levels and two with low plasma levels, correlating to the dosage obtained. On the functional measure of activities of daily living, nine patients had improved, including five with high plasma levels, three with moderate levels, and one with a low level. One patient, with a moderate level, remained stable. The remaining 14 patients declined.
The company then enrolled 21 of the cohort in a 208-week extension trial, primarily because of patient request, Dr. Missling said. “They know they are doing better. Their families know they’re doing better. They did not want to give this up.”
Last fall, the company released 148-week functional and cognitive data confirming the initial findings: Patients with higher plasma levels (correlating with higher doses) declined about 2 points on the ADCS-ADL scale, compared with a mean decline of about 25 points among those with lower blood levels – an 88% difference in favor of treatment. Cognition scores showed a similar pattern, with the high-concentration group declining 64% less than the low-concentration group.
Sixteen patients consented to stool sampling. A sophisticated computer algorithm characterized the microbiome of each, measuring the relative abundance of phyla. Microbiome analysis wasn’t included as an endpoint in the original study design because, at that time, the idea of a connection between AD and the gut microbiome was barely on the research radar.
Things shifted dramatically in 2017, with a seminal paper finding that germ-free mice inoculated with stool from Parkinson’s patients developed Parkinson’s symptoms. This study was widely heralded as a breakthrough in the field – the first time any neurodegenerative disease had been conclusively linked to dysregulations in the human microbiome.
Last year, Vo Van Giau, PhD, of Gachon University, South Korea, and his colleagues published an extensive review of the data suggesting a similar link with Alzheimer’s disease.
Dr. Giau and his coauthors laid out a potential pathogenic pathway for this interaction.
“The microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of proinflammatory cytokines. Changes in the homeostatic state of the microbiota lead to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of proinflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders,” including Alzheimer’s, he wrote.
Dr. Missling said this paper, and smaller studies appearing at Alzheimer’s meetings, prompted the company to add the stool sampling as a follow-up measure.
“It’s something of great interest, we think, and deserves to be investigated.”
SOURCE: Missling C et al. AAIC 2019, Abstract 32260.
REPORTING FROM AAIC 2019
HDAC/HMA combo shows ‘remarkable’ activity in PTCL
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
REPORTING FROM 15-ICML
FDA update: Higher late mortality with paclitaxel-coated devices
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Comment on “Analysis of Nail-Related Content of the Basic Dermatology Curriculum”
To the Editor:
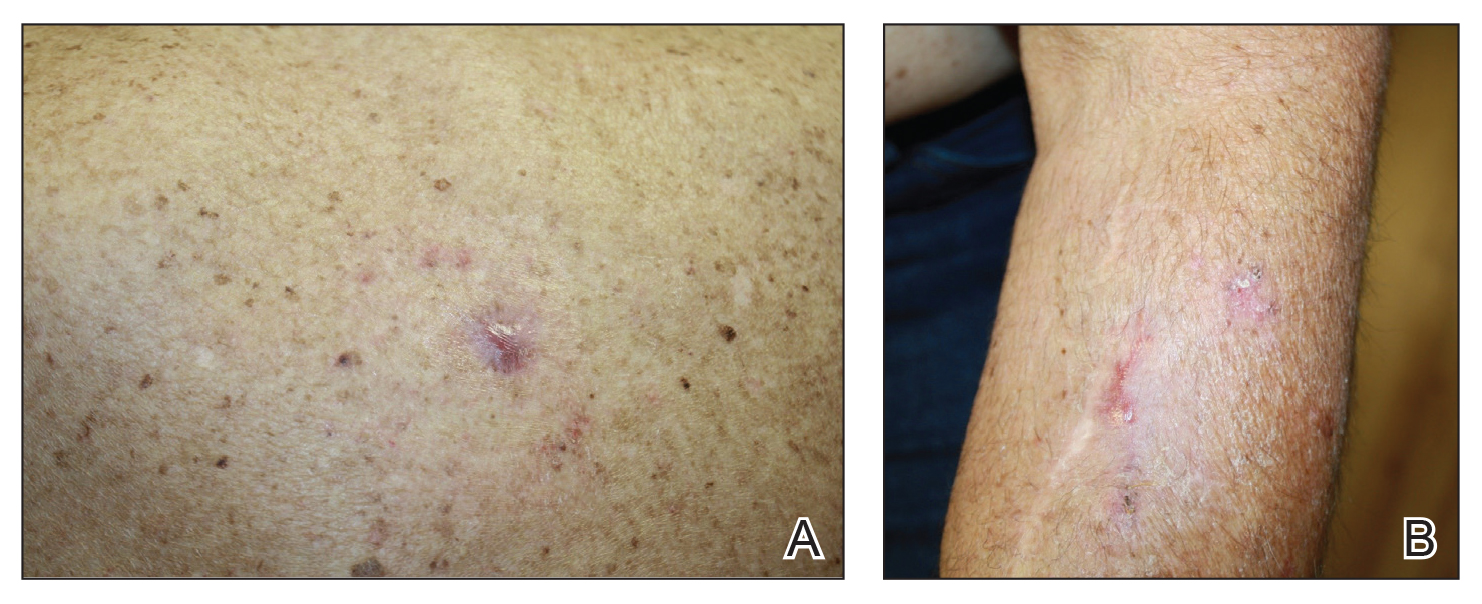
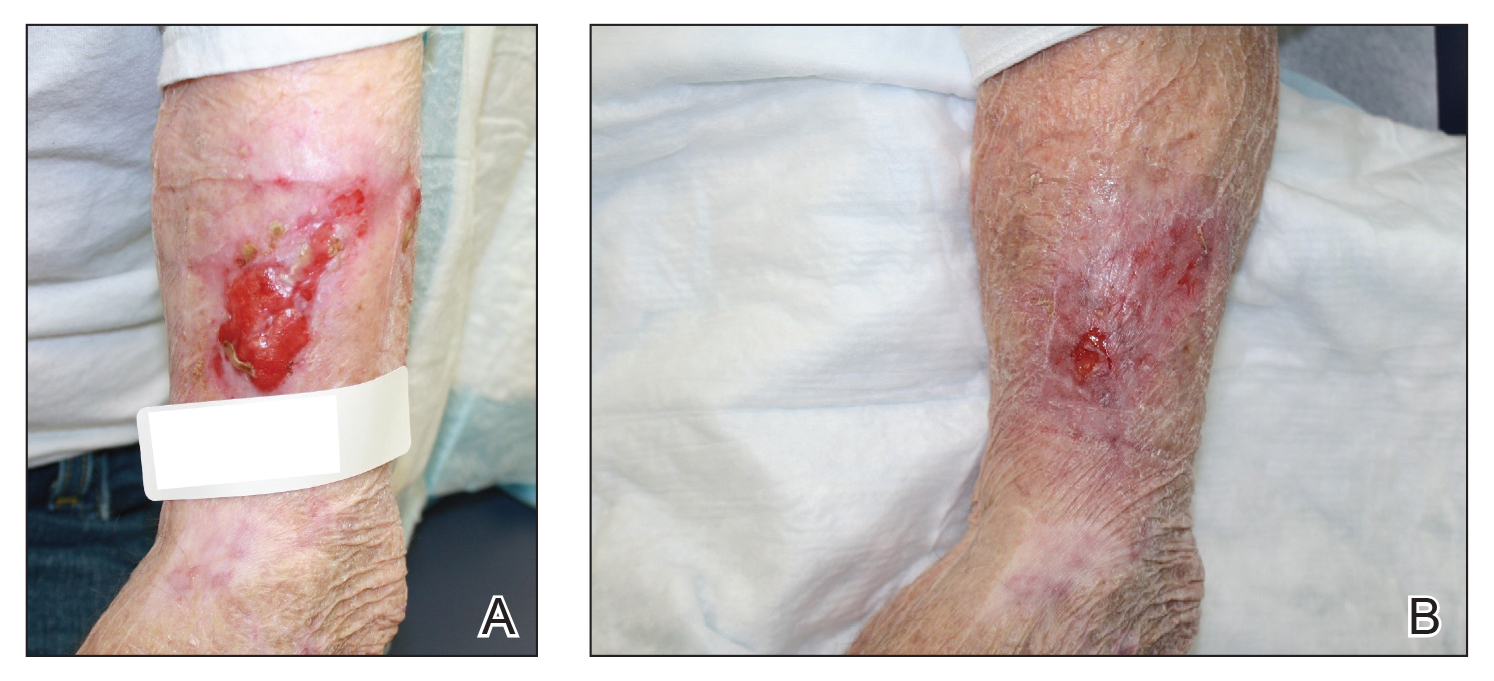
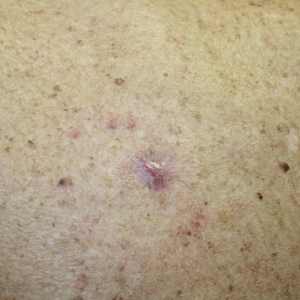
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
Practice Points
- Growing evidence suggests that the surgical smoke plume generated during electrosurgery may be harmful if inhaled.
- Our survey indicates that this information may affect clinician and staff perceptions about exposure to electrosurgical smoke and its remediation.
Diversity and Inclusivity Are Essential to the Future of Dermatology
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Methylisothiazolinone and Isothiazolinone Allergy
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Practice Points
- Methylisothiazolinone (MI) is a preservative found in water-based personal care products and is a common allergen in patch-tested populations.
- Methylisothiazolinone also has been identified in household products, industrial chemicals, paint, adhesives, and other unique sources.
- Benzisothiazolinone and octylisothiazolinone are structurally similar to MI, and a subset of MI-allergic patients may need to avoid them.
The changing landscape of medical education
A brave new world
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
A brave new world
A brave new world
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
It’s Monday morning, and your intern is presenting an overnight admission. Lost in the details of his disorganized introduction, your mind wanders. “Why doesn’t this intern know how to present? When I trained, all those admissions during long sleepless nights really taught me to do this right.” But can we equate hours worked with competency achieved? And if not, what is the alternative? This article introduces some major changes in medical education and their implications for hospitalists.
Most hospitalists trained in an educational system influenced by Sir William Osler. In the early 1900s, he introduced the natural method of teaching, positing that student exposure to patients and experience over time ensured that physicians in training would become competent doctors.1 His influence led to the current structure of medical education, which includes conventional third-year clerkships and time-limited rotations (such as a 2-week nephrology block).
While familiarity may be comforting, there are signs our current model of medical education is inefficient, inadequate, and obsolete.
For one, the traditional system is failing to adequately prepare physicians to provide safe and complex care. Reports, such as the Institute of Medicine’s (IOM) “To Err is Human,”2 describe a high rate of preventable errors, highlighting considerable room for improvement in training the next generation of physicians.3,4
Meanwhile, trainees are still largely being deemed ready for the workforce by length of training completed (for example, completion of four-year medical school) rather than a skill set distinctly achieved. Our system leaves little flexibility to individualize learner goals, which is significant given some students and residents take shorter or longer periods of time to achieve proficiency. In addition, learner outcomes can be quite variable, as we have all experienced.
Even our methods of assessment may not adequately evaluate trainees’ skill sets. For example, most clerkships still rely heavily on the shelf exam5 as a surrogate for medical knowledge. As such, learners may conclude that testing performance trumps development of other professional skills.6 Efforts are being made to revamp evaluation systems to reflect mastery (such as Entrustable Professional Activities, or EPAs) toward competencies.7 Still, many institutions continue to rely on faculty evaluations that often reflect interpersonal dynamics rather than true critical thinking skills.6
Recognizing the above limitations, many educators have called for changing to outcome-based, or competency-based, training (CBME). CBME targets attainment of skills in performing concrete critical clinical activities,8 such as identifying unstable patients, providing initial management, and obtaining help. To be successful, supervisors must directly observe trainees, assess demonstrated skills, and provide feedback about progress.
Unfortunately, this considerable investment of time and effort is often poorly compensated. Additionally, unanswered questions remain. For example, how will residency programs continue to challenge physicians deemed “competent” in a required skill? What happens when a trainee is deficient and not appropriately progressing in a required skill? Is flexible training time part of the future of medical education? While CBME appears to be a more effective method of education, questions like these must be addressed during implementation.
Beyond the fact that hours worked cannot be used as a surrogate for competency, excessive unregulated work hours can be detrimental to learners, their supervisors, and patients. In 2003, the Accreditation Council for Graduate Medical Education (ACGME) implemented a major change in medical education: duty hour limitations. The premise that sleep-deprived providers are more prone to error is well established. However, controversy remains as to whether these regulations translate into improved patient care and provider well-being. Studies published following the ACGME change demonstrate increasing burnout among physicians,9-11 which has led some educators to explore the potential relationship between burnout and duty hour restrictions.
The recent “iCOMPARE” trial, which compared internal medicine (IM) residencies with “standard duty-hour” policies to those with “flexible” policies (that is, they did not specify limits on shift length or mandatory time off between shifts), supported a lack of correlation between hours worked and burnout.12 Researchers administered the Maslach Burnout Inventory to all participants.13 While those in the “flexible hours” arm reported greater dissatisfaction with the effect of the program on their personal lives, both groups reported significant burnout, with interns recording high scores in emotional exhaustion (79% in flexible programs vs. 72% in standard), depersonalization (75% vs. 72%), and lack of personal accomplishment (71% vs. 69%).
Disturbingly, these scores were not restricted to interns but were present in all residents. The good news? Limiting duty hours does not cause burnout. On the other hand, it does not protect from burnout. Trainee burnout appears to transcend the issue of hours worked. Clearly, we need to address the systemic flaws in our work environments that contribute to this epidemic. Nationwide, educators and organizations are continuing to define causes of burnout and test interventions to improve wellness.
A final front of change in medical education worth mentioning is the use of the electronic medical record (EMR). While the EMR has improved many aspects of patient care, its implementation is associated with decreased time spent with patients and parallels the rise in burnout. Another unforeseen consequence has been its disruptive impact on medical student documentation. A national survey of clerkship directors found that, while 64% of programs allowed students to use the EMR, only two-thirds of those programs permitted students to document electronically.14
Many institutions limit student access because of either liability concerns or the fact that student notes cannot be used to support medical billing. Concerning workarounds among preceptors, such as logging in students under their own credentials to write notes, have been identified.15 Yet medical students need to learn how to document a clinical encounter and maintain medical records.7,16 Authoring notes engages students, promotes a sense of patient ownership, and empowers them to feel like essential team members. Participating in the EMR also allows for critical feedback and skill development.
In 2016, the Society of Hospital Medicine joined several major internal medicine organizations in asking the federal government to reconsider guidelines prohibiting attendings from referring to medical student notes. In February 2018, the Centers for Medicare & Medicaid Services (CMS) revised its student documentation guidelines (see Box A), allowing teaching physicians to use all student documentation (not just Review of Systems, Family History, and Social History) for billable services.
While the guidelines officially went into effect in March 2018, many institutions are still fine-tuning their implementation, in part because of nonspecific policy language. For instance, if a student composes a note and a resident edits and signs it, can the attending physician simply cosign the resident note? Also, once a student has presented a case, can the attending see the patient and verify findings without the student present?
Despite the above challenges, the revision to CMS guidelines is a significant “win” and can potentially reduce the documentation burden on teaching physicians. With more oversight of their notes, the next generation of students will be encouraged to produce accurate, high-quality documentation.
In summary, these changes in the way we define competency, in duty hours, and in the use of the EMR demonstrate that medical education is continuously improving via robust critique and educator engagement in outcomes. We are fortunate to train in a system that respects the scientific method and utilizes data and critical events to drive important changes in practice. Understanding these changes might help hospitalists relate to the backgrounds and needs of learners. And who knows – maybe next time that intern will do a better job presenting!
Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System (VASDHS) and an associate professor at the University of California, San Diego, in the division of hospital medicine. He is the chair of the SHM Physicians in Training committee. Dr. Sebasky is an associate clinical professor at UCSD in the division of hospital medicine. Dr. Muchmore is a hematologist/oncologist and professor of clinical medicine in the department of medicine at UCSD and associate chief of staff for education at VASDHS.
References
1. Osler W. “The Hospital as a College.” In Aequanimitas. Osler W, Ed. (Philadelphia: P. Blakiston’s Son & Co., 1932).
2. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health Care System. (Washington: National Academies Press, 1999).
3. Ten Cate O. Competency-based postgraduate medical education: Past, present and future. GMS J Med Educ. 2017 Nov 15. doi: 10.3205/zma001146.
4. Carraccio C, Englander R, Van Melle E, et al. Advancing competency-based medical education: A charter for clinician–educators. Acad Med. 2016;91(5):645-9.
5. 2016 NBME Clinical Clerkship Subject Examination Survey.
6. Mehta NB, Hull AL, Young JB, et al. Just imagine: New paradigms for medical education. Acad Med. 2013;88(10):1418-23.
7. Fazio SB, Ledford CH, Aronowitz PB, et al. Competency-based medical education in the internal medicine clerkship: A report from the Alliance for Academic Internal Medicine Undergraduate Medical Education Task Force. Acad Med. 2018;93(3):421-7.
8. Ten Cate O, Scheele F. Competency-based postgraduate training: Can we bridge the gap between theory and clinical practice? Acad Med. 2007 Jun;82(6):542-7.
9. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: A systematic review. BMJ Open. 2017. doi: 10.1136/bmjopen-2016-015141.
10. Hall LH, Johnson J, Watt I, et al. Healthcare Staff wellbeing, burnout, and patient safety: A systematic review. PLoS ONE. 2016. doi: 10.1371/journal.pone.0159015.
11. Salyers MP, Bonfils KA, Luther L, et al. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. Gen Intern Med. 2017 Apr; 32(4):475-82.
12. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty hour flexibility trial in internal medicine. N Engl J Med. 2018 378:1494-508.
13. Maslach C, Jackson SE, Leiter MP. Maslach burnout inventory manual. 3rd ed. (Palo Alto, CA: Consulting Psychologists Press, 1996).
14. Hammoud MM, Margo K, Christner JG, et al. Opportunities and challenges in integrating electronic health records into undergraduate medical education: A national survey of clerkship directors. Teach Learn Med. 2012;24(3):219-24.
15. White J, Anthony D, WinklerPrins V, et al. Electronic medical records, medical students, and ambulatory family physicians: A multi-institution study. Acad Med. 2017;92(10):1485-90.
16. Pageler NM, Friedman CP, Longhurst CA. Refocusing medical education in the EMR era. JAMA 2013;310(21):2249-50.
Box A
“Students may document services in the medical record. However, the teaching physician must verify in the medical record all student documentation or findings, including history, physical exam, and/or medical decision making. The teaching physician must personally perform (or re-perform) the physical exam and medical decision making activities of the E/M service being billed, but may verify any student documentation of them in the medical record, rather than re-documenting this work.”
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.