User login
Recurrence of Linear Basal Cell Carcinoma
Case Report
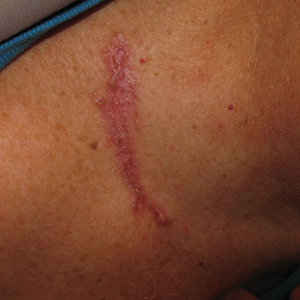
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
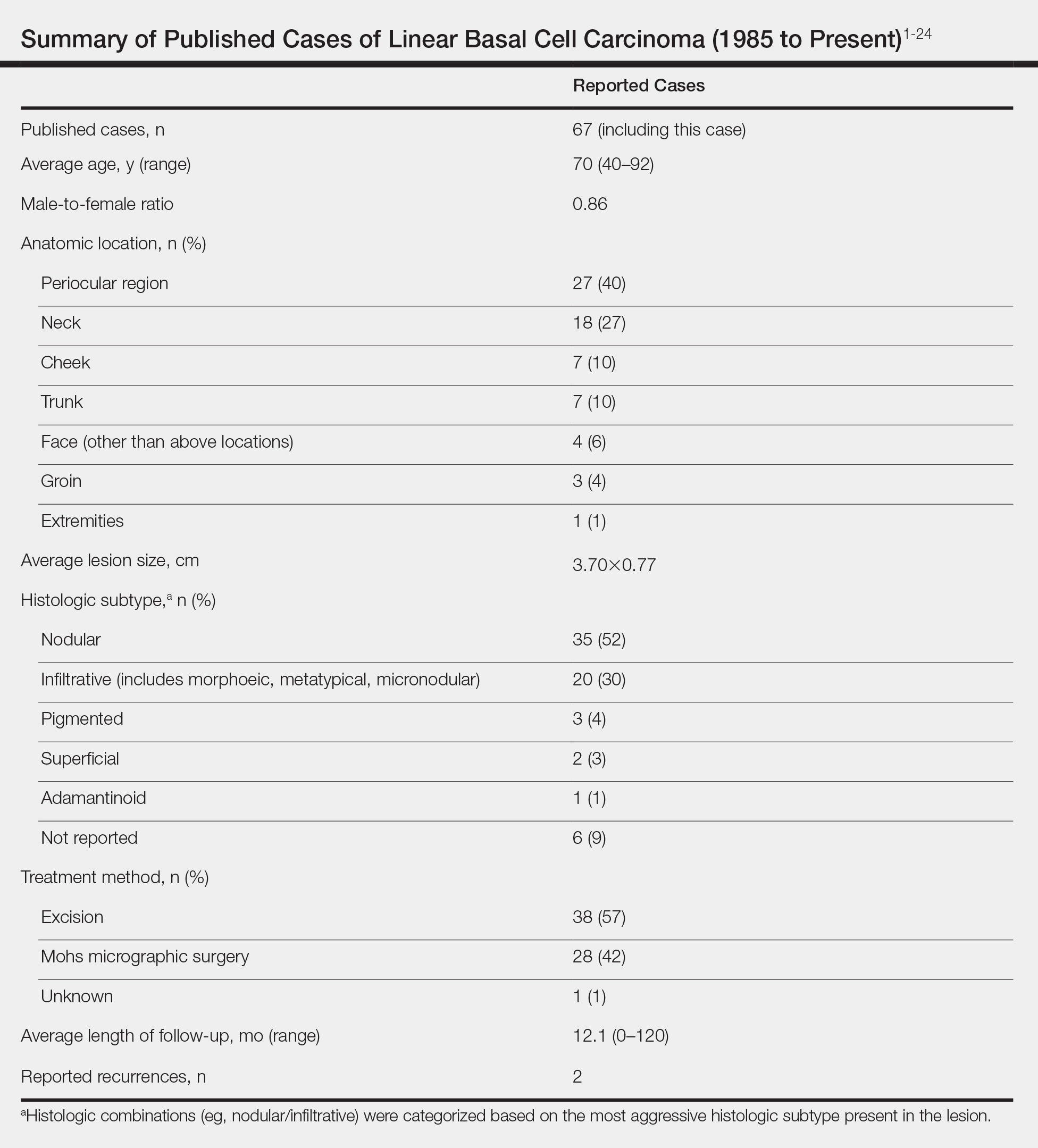
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
Case Report
A 63-year-old man was evaluated in the Mohs clinic for a lesion on the right supraclavicular neck, which he described as a linear asymptomatic “birthmark” that had been present since childhood and stable for many years. It began to enlarge approximately 5 years prior, became increasingly red, and had occasional crusting. The lesion also gradually became more irritated with repeated mild trauma when he carried a backpack while hiking. On physical examination, a 10×2-cm, linear, pink plaque with an irregular border, translucent rolled edges, and central smooth atrophic skin was seen on the right supraclavicular neck (Figure). There was no visible epidermal nevus or nevus sebaceous in the area. A shave biopsy of the lesion confirmed the pathologic diagnosis of basal cell carcinoma, nodular type, along with the morphologic diagnosis of linear basal cell carcinoma (LBCC). The tumor was completely removed with standard excision using 5-mm margins.
Approximately 10 months after the original excision, the patient developed an irritated erosion that occasionally bled when his backpack rubbed against it. He returned to the clinic after the erosion failed to heal. Physical examination revealed a 1.4×0.7-cm, eroded, pink papule with large telangiectases at the superior pole of the excision scar. A shave biopsy confirmed the diagnosis of a recurrent infiltrative basal cell carcinoma. The tumor was then completely excised using Mohs micrographic surgery.
Comment
Linear basal cell carcinoma, first described by Lewis1 in 1985, is a rare morphologic variant of basal cell carcinoma. In 2011, Al-Niaimi and Lyon2 performed a comprehensive literature search on LBCC (1985-2008) and found only 39 cases (including 2 of their own) had been published since the pioneer case in 1985. It was determined that the most common sites affected were the periorbital area and neck (n=13 each [67%]), and the majority were histologically nodular (n=27 [69%]). Mohs micrographic surgery was the most common treatment method (n=23 [59%]), followed by primary excision (n=17 [44%]). A history of trauma, radiotherapy, or prior operation in association with the site of the LBCC was discovered in only 7 cases (18%).2 Although Peschen et al3 proposed that trauma—both physical and surgical—and radiotherapy may play a role in the development of LBCCs, the low incidence reported suggests that other factors may be involved. To determine if genetic factors were contributing to the development of LBCCs, Yamaguchi et al4 investigated the expression of p27 and PCTAIRE1, both known to contribute to tumorigenesis when mutated, as well as somatic gene mutations using deep sequencing in a case of LBCC; they found no associated genetic mutation.
Reported Cases of LBCC
According to a PubMed search of articles indexed for MEDLINE using the terms linear and basal cell carcinoma, 67 cases (including the current case) of LBCC have been published since 1985. The patient demographics, anatomic location, histologic subtype, treatment methods, and frequency of recurrence for all reported cases of LBCC are summarized in the Table.1-24 There were 36 women and 31 men, with an average age of 70 years (range, 40–92 years). The most commonly affected sites were the periocular region (n=27) and neck (n=18). Histologically, most LBCCs were nodular (n=35), with the next most common histologic subtype being infiltrative (n=20), which included the morphoeic, metatypical, and micronodular subtypes under the overarching infiltrative subtype. The most frequently chosen treatment option was primary excision (n=38 [57%]), followed by Mohs micrographic surgery (n=28 [42%]). Risk factors previously identified by Al-Niaimi and Lyon,2 including trauma, radiotherapy, or prior operation, were reported in 12 of 67 cases. Recurrence was reported in only 2 of 67 cases, 1 being the current case; however, an accurate recurrence rate could not be calculated due to lack of follow-up or short length of follow-up in most of the reported cases.
Presentation and Treatment
Currently, there are no set criteria for the diagnosis of LBCC, but it has been shown to follow a characteristic morphologic pattern, favoring extension in one direction leading to a length-to-width ratio that typically is at least 3 to 1.5 With most lesions presenting in the periocular region along relaxed skin tension lines, it has been speculated that these tumors expand along wrinkles.2 Pierard and Lapiere25 proposed that the preferential parallel orientation and a straightening of thin collagen bundles and elastic fibers within the reticular dermis combined with relaxed skin tension lines and muscle contraction perpendicular to these stromal parts may influence the growth of tumors preferentially in one direction, contributing to linearity of the lesion. In addition, the clinical appearance is not a reliable indicator of subclinical extension.2 Therefore, Lim et al6 recommended Mohs micrographic surgery as the best initial treatment of LBCCs.
Conclusion
Linear basal cell carcinoma should be considered a distinct morphologic variant of basal cell carcinoma. Although likely underreported, this variant is uncommon. It presents most often in the periocular and neck regions. The most common histologic subtypes are nodular and infiltrative. Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype. As such, Mohs micrographic surgery or excision with complete circumferential peripheral and deep margin assessment is recommended as first-line treatment of LBCC.6
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1985;24:124-125.
- Al-Niaimi F, Lyon CC. Linear basal cell carcinoma: a distinct condition? Clin Exp Dermatol. 2011;36:231-234.
- Peschen M, Lo JS, Snow SN, et al. Linear basal cell carcinoma. Cutis. 1993;51:287-289.
- Yamaguchi Y, Yanagi T, Imafuku K, et al. A case of linear basal cell carcinoma: evaluation of proliferative activity by immunohistochemical staining of PCTAIRE1 and p27. J Eur Acad Dermatol Venereol. 2017;31:E359-E362.
- Mavirakis I, Malhotra R, Selva D, et al. Linear basal cell carcinoma: a distinct clinical entity. J Plast Reconstr Aesthet Surg. 2006;59:419-423.
- Lim KK, Randle HW, Roenigk RK, et al. Linear basal cell carcinoma: report of seventeen cases and review of the presentation and treatment. Dermatol Surg. 1999;25:63-67.
- Pardavila R, Rosón E, De la torre C, et al. Linear basal cell carcinoma. report of two cases [in Spanish]. Actas Dermosifiliogr. 2007;98:291.
- Shinsuke K, Hirohiko K, Yasuhiro T, et al. Linear basal cell carcinoma in an Asian patient. Open Ophthalmol J. 2007;1:20-22.
- Ning C, Chao S. Linear basal cell carcinoma of the scrotum. Dermatol Sinica. 2002;20:57-62.
- Chopra KF, Cohen PR. Linear basal cell carcinomas: report of multiple sequential tumors localized to a radiotherapy port and review of the literature. Tex Med. 1997;93:57-59.
- da Silva MO, Dadalt P, Santos OL, et al. Linear basal cell carcinoma. Int J Dermatol. 1995;34:488.
- Warthan TL, Lewis JE. Giant linear basal cell epithelioma. Int J Dermatol. 1994;33:284.
- Lewis JE. Linear basal cell epithelioma. Int J Dermatol. 1989;28:682-684.
- Alcántara-Reifs CM, Salido-Vallejo R, González-Menchen A, et al. Linear basal cell carcinoma: report of three cases with dermoscopic findings. Indian J Dermatol Venereol Leprol. 2016;82:708-711.
- Lee MS, Cho E, Lee JH, et al. Linearly curved, blackish macule on the wrist. Cutis. 2016;97:384, 406-407.
- Bajaj S, Sharma PK, Kar HK. Linear adamantinoid basal cell carcinoma in the axilla. Dermatol Online J. 2015;21. pii:13030/qt8k0713nb.
- Iga N, Sakurai K, Fujii H, et al. Linear basal cell carcinoma at the external genitalia. J Dermatol. 2014;41:275-276.
- Ichinokawa Y, Ohtuki A, Hattori M, et al. Linear basal cell carcinoma: a case report. Case Rep Dermatol. 2011;3:142-146.
- Becher GL, Affleck A, Fleming C, et al. Linear basal cell carcinoma occurs most commonly on the lower eyelid. Clin Exp Dermatol. 2011;36:311-312.
- Jellouli A, Triki S, Zghal M, et al. Linear basal cell carcinoma. Actas Dermosifiliogr. 2010;101:648-650.
- Takiyoshi N, Nakano H, Kaneko T, et al. A linear basal cell carcinoma undergoing spontaneous regression. Clin Exp Dermatol. 2009;34:E411-E413.
- Yoleri L, Ozden S, Kandiloglu A. A 46-year-old male with an ulcerated linear lesion on his neck. Ann Saudi Med. 2008;28:57-58.
- Palleschi GM, Corradini D, Bruscino N, et al. Linear basal cell carcinoma: clinical significance and better surgical approach. G Ital Dermatol Venereol. 2016;151:119-121.
- Rodriguez-Garijo N, Redondo P. Linear basal cell carcinoma of the lower eyelid: reconstruction with a musculocutaneous transposition flap. JAAD Case Rep. 2018;4:633-635.
- Pierard GE, Lapiere CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987;9:219-224.
Practice Points
- Linear basal cell carcinoma (LBCC) follows a characteristic morphologic pattern of a length-to-width ratio that typically is at least 3 to 1.
- Linear basal cell carcinomas most commonly present in the periocular region and on the neck along relaxed skin tension lines.
- Because of the likelihood of subclinical spread, LBCC should be regarded as a high-risk subtype of basal cell carcinoma.
- Mohs micrographic surgery or excision with complete circumferential peripheral and deep-margin assessment is recommended as first-line treatment.
Noninvasive Imaging Tools in Dermatology
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
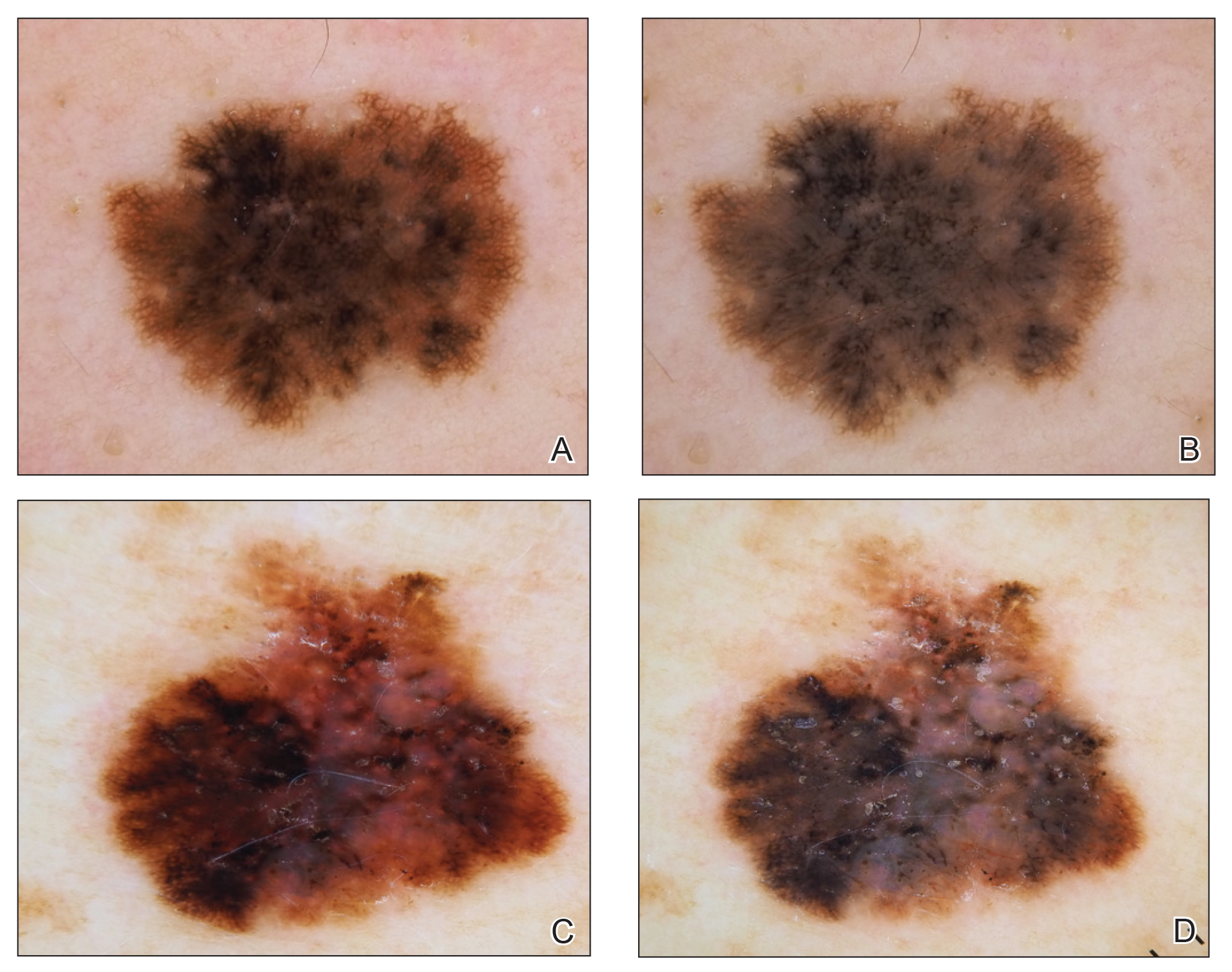
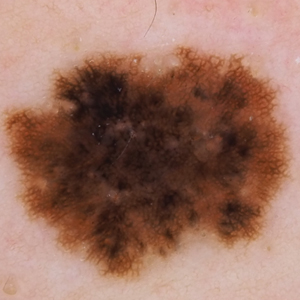
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
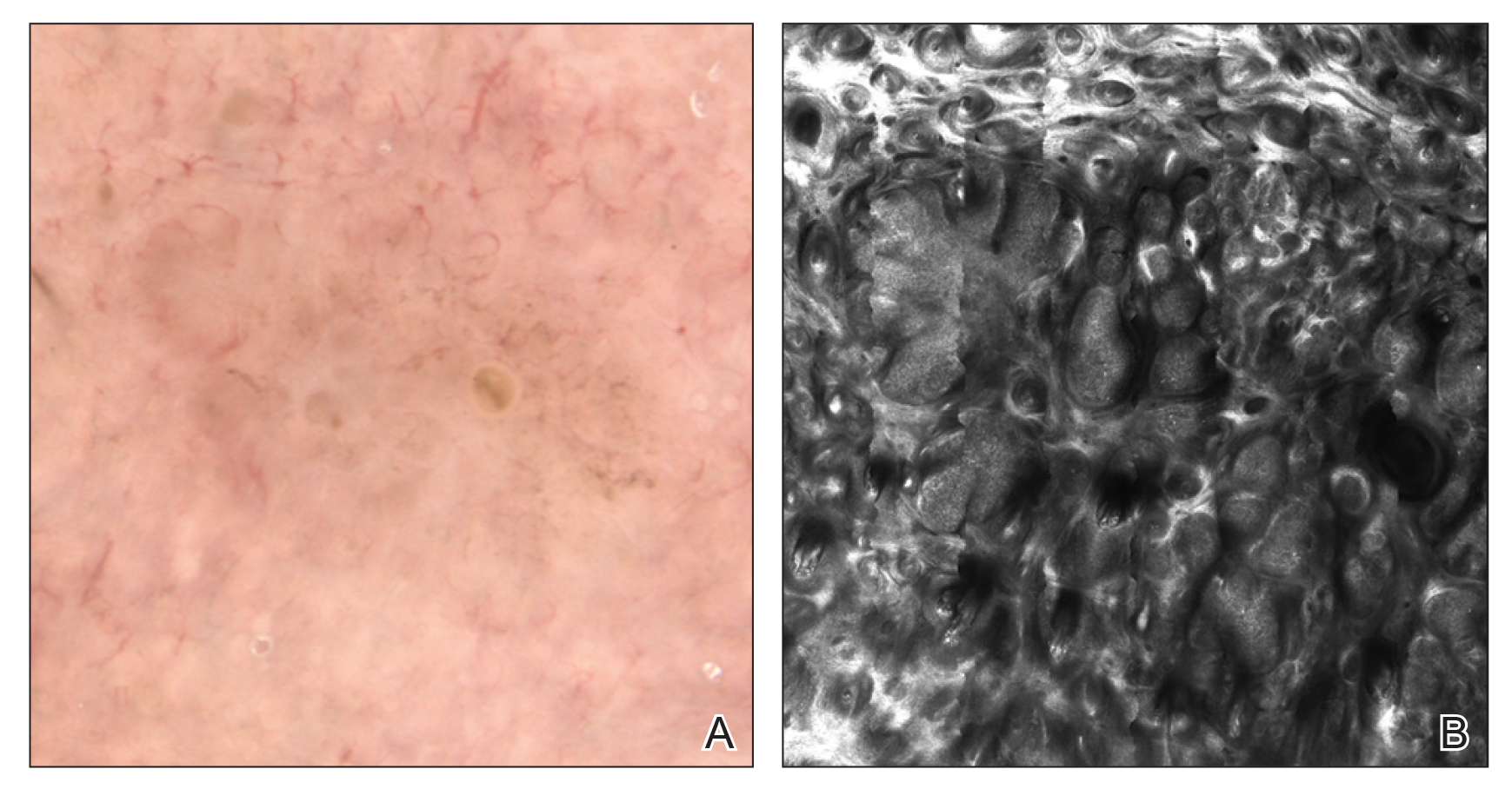
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
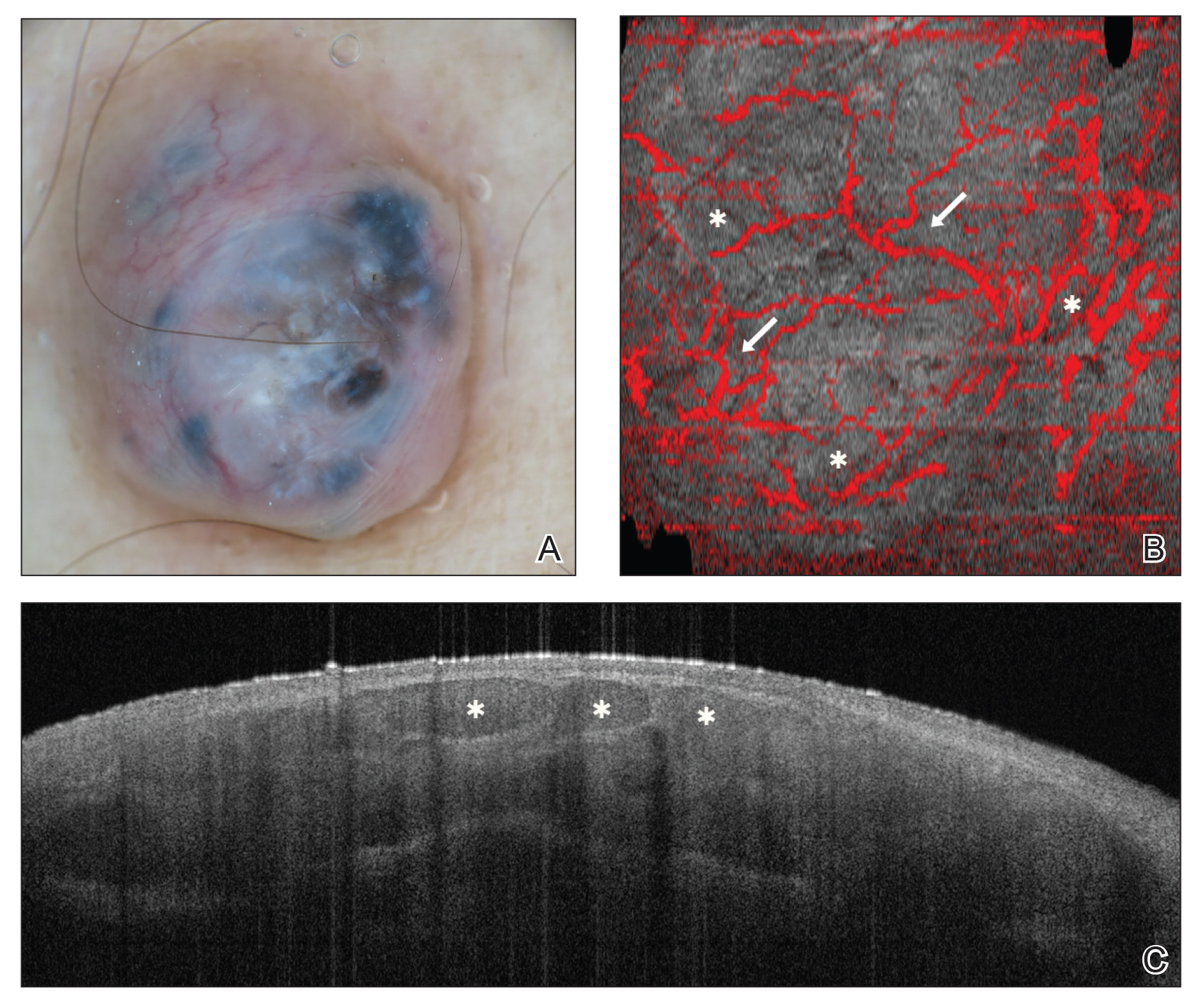
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Practice Points
- There are several new noninvasive imaging tools in dermatology that can be utilized to aid in the diagnosis and management of skin disease, including dermoscopy, reflectance confocal microscopy, and optical coherence tomography.
- Among these tools, there are several differences in cost, clinical integration, reimbursement, and accuracy.
Barriers and Job Satisfaction Among Dermatology Hospitalists
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Practice Points
• Dermatology hospitalists play a critical role in the specialized care of hospitalized patients with
skin conditions.
• Dermatology hospitalists have high job satisfaction, with opportunities to teach trainees and practice complex medical dermatology.
• Most dermatology hospitalists do not generate sufficient revenue providing inpatient dermatology consultations to fully support their salary for the time spent as consultants; alternate payment models are needed to maintain dermatology’s presence in the hospital.
Cardiovascular complications most common with carfilzomib in relapsed myeloma
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
Cardiovascular (CV) adverse events were common in patients receiving proteasome inhibitor therapy for relapsed multiple myeloma, especially with carfilzomib-based therapy, according to results from the PROTECT study.
While prior studies have shown an increased risk for CV toxicities with proteasome inhibitor therapy, detailed descriptions of the events and risk factors have been lacking. “Furthermore, there is no validated protocol to help determine which patients are at highest risk of CV toxicity during therapy, nor is there management guidance for patients who experience a [CV adverse event],” wrote Robert F. Cornell, MD, of Vanderbilt University, Nashville, Tenn., and colleagues in the Journal of Clinical Oncology.
The PROTECT (Prospective Observation of Cardiac Safety with Proteasome Inhibitor) study was conducted at Vanderbilt University Medical Center and the University of Pennsylvania Abramson Cancer Center, Philadelphia, between September 2015 and March 2018.
Researchers followed 95 patients with relapsed multiple myeloma who were treated with either bortezomib or carfilzomib for a total duration of 18 months. A total of 65 patients received a carfilzomib-based therapy and 30 patients received a bortezomib-based therapy.
Study patients received a CV assessment at baseline and at the beginning of each treatment cycle for the initial six cycles of proteasome inhibitor therapy. Subsequently, patients were monitored for the development of CV adverse events. CV assessments included ECG, echocardiography, and measurement of other cardiac biomarkers, such as NTproBNP and troponin I or T.
CV toxicities were reported among 5 patients (16.7%) of patients treated with bortezomib and 33 patients (50.7%) treated with carfilzomib (P = .005).
In total, there were 64 CV adverse events reported, most of which were grade 2 or 3, and 56 of which occurred while on carfilzomib-based therapy. For carfilzomib, the most common complications were heart failure (23 cases), followed by grade 3 or 4 hypertension (13 cases). Cardiac chest pain, atrial fibrillation, and acute coronary syndrome were reported in fewer cases.
The researchers also found that elevated natriuretic peptides that occurred before starting carfilzomib therapy or within the first 3 weeks of carfilzomib therapy were associated with a substantially higher risk of CV adverse events.
Patients who have multiple CV risk factors, and especially patients with a history of CV complications and elevated baseline natriuretic peptides, should be referred for a comprehensive cardiac evaluation, the researchers advised. “Such patients are at highest risk of CV [adverse events] with carfilzomib-based therapy, and optimization of CV therapy seems to improve overall care, allow continuation of potentially lifesaving cancer treatment, and affect severity or development of CV [adverse events],” they wrote.
A key limitation of the study was the lack of standardized treatment regimens. As a result, there was a broad dosing range for carfilzomib, in comparison to bortezomib.
Some authors reported financial relationships with carfilzomib maker Amgen and bortezomib maker Takeda, as well as with other companies.
SOURCE: Cornell RF et al. J Clin Oncol. 2019 Jun 12. doi: 10.1200/JCO.19.00231.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Laurence Wellikson, MD, MHM, announces retirement as CEO of Society of Hospital Medicine
Society recognizes Dr. Wellikson’s leadership, retains Spencer Stuart for successor search
Philadelphia – After serving as the first and only chief executive officer of the Society of Hospital Medicine since January of 2000, Laurence Wellikson, MD, MHM, has announced his retirement effective on Dec. 31, 2020. In parallel, the SHM Board of Directors have commenced a search for his successor.
“When I began as CEO 20 years ago, SHM – then known as the National Association of Inpatient Physicians – was a young national organization with approximately 500 members, and there was minimal understanding as to the value that hospitalists could add to their health communities,” Dr. Wellikson said. “I am proud to say that, nearly 20 years later, SHM boasts a growing membership of more than 17,000, and hospitalists are on the front line of innovation as a driving force in improving patient care.”
SHM has not only grown its membership but also its diverse portfolio of offerings for hospital medicine professionals under Dr. Wellikson’s leadership. Its first annual conference welcomed approximately 300 attendees; the most recent conference, Hospital Medicine 2019, saw that number increase more than tenfold to nearly 4,000. Its conferences, publications, online education, chapter program, advocacy efforts, quality improvement programs, and more have evolved significantly to ensure hospitalists at all stages of their careers – and those who support them – have access to resources to keep them up to date and demonstrate their value in America’s health care system.
During Dr. Wellikson’s tenure, SHM launched its peer-reviewed Journal of Hospital Medicine, the premier, ISI-indexed publication for the specialty, successfully advocated for a Focused Practice in Hospital Medicine certification option and C6 hospitalist specialty code, and earned the John M. Eisenberg Patient Safety and Quality Award for its quality improvement programs. These are just a few of the noteworthy accomplishments that have elevated SHM as a key partner for hospitalists and their institutions.
To assist with the search for SHM’s next CEO, the society has retained Spencer Stuart, a leading global executive and leadership advisory firm. The search process is being overseen by a diverse search committee led by the president-elect of SHM’s Board of Directors, Danielle Scheurer, MD, MSCR, SFHM.
“On behalf of the society and its members, I want to extend a sincere thank you to Larry for his years of dedication and service to SHM, its staff, and the hospital medicine professionals we serve,” said Christopher Frost, MD, SFHM, president of SHM’s Board of Directors. “His legacy will allow SHM to continue its growth trajectory through key programs and services supporting members’ needs for years to come. Larry has taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team can come for education, community, and betterment of the care we provide to our patients. We are indebted to him beyond words.”
Those who are interested in leading SHM into the future as its next CEO are encouraged to contact either Jennifer P. Heenan (jheenan@spencerstuart.com) or Mark Furman, MD (mfurman@spencerstuart.com).
Society recognizes Dr. Wellikson’s leadership, retains Spencer Stuart for successor search
Society recognizes Dr. Wellikson’s leadership, retains Spencer Stuart for successor search
Philadelphia – After serving as the first and only chief executive officer of the Society of Hospital Medicine since January of 2000, Laurence Wellikson, MD, MHM, has announced his retirement effective on Dec. 31, 2020. In parallel, the SHM Board of Directors have commenced a search for his successor.
“When I began as CEO 20 years ago, SHM – then known as the National Association of Inpatient Physicians – was a young national organization with approximately 500 members, and there was minimal understanding as to the value that hospitalists could add to their health communities,” Dr. Wellikson said. “I am proud to say that, nearly 20 years later, SHM boasts a growing membership of more than 17,000, and hospitalists are on the front line of innovation as a driving force in improving patient care.”
SHM has not only grown its membership but also its diverse portfolio of offerings for hospital medicine professionals under Dr. Wellikson’s leadership. Its first annual conference welcomed approximately 300 attendees; the most recent conference, Hospital Medicine 2019, saw that number increase more than tenfold to nearly 4,000. Its conferences, publications, online education, chapter program, advocacy efforts, quality improvement programs, and more have evolved significantly to ensure hospitalists at all stages of their careers – and those who support them – have access to resources to keep them up to date and demonstrate their value in America’s health care system.
During Dr. Wellikson’s tenure, SHM launched its peer-reviewed Journal of Hospital Medicine, the premier, ISI-indexed publication for the specialty, successfully advocated for a Focused Practice in Hospital Medicine certification option and C6 hospitalist specialty code, and earned the John M. Eisenberg Patient Safety and Quality Award for its quality improvement programs. These are just a few of the noteworthy accomplishments that have elevated SHM as a key partner for hospitalists and their institutions.
To assist with the search for SHM’s next CEO, the society has retained Spencer Stuart, a leading global executive and leadership advisory firm. The search process is being overseen by a diverse search committee led by the president-elect of SHM’s Board of Directors, Danielle Scheurer, MD, MSCR, SFHM.
“On behalf of the society and its members, I want to extend a sincere thank you to Larry for his years of dedication and service to SHM, its staff, and the hospital medicine professionals we serve,” said Christopher Frost, MD, SFHM, president of SHM’s Board of Directors. “His legacy will allow SHM to continue its growth trajectory through key programs and services supporting members’ needs for years to come. Larry has taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team can come for education, community, and betterment of the care we provide to our patients. We are indebted to him beyond words.”
Those who are interested in leading SHM into the future as its next CEO are encouraged to contact either Jennifer P. Heenan (jheenan@spencerstuart.com) or Mark Furman, MD (mfurman@spencerstuart.com).
Philadelphia – After serving as the first and only chief executive officer of the Society of Hospital Medicine since January of 2000, Laurence Wellikson, MD, MHM, has announced his retirement effective on Dec. 31, 2020. In parallel, the SHM Board of Directors have commenced a search for his successor.
“When I began as CEO 20 years ago, SHM – then known as the National Association of Inpatient Physicians – was a young national organization with approximately 500 members, and there was minimal understanding as to the value that hospitalists could add to their health communities,” Dr. Wellikson said. “I am proud to say that, nearly 20 years later, SHM boasts a growing membership of more than 17,000, and hospitalists are on the front line of innovation as a driving force in improving patient care.”
SHM has not only grown its membership but also its diverse portfolio of offerings for hospital medicine professionals under Dr. Wellikson’s leadership. Its first annual conference welcomed approximately 300 attendees; the most recent conference, Hospital Medicine 2019, saw that number increase more than tenfold to nearly 4,000. Its conferences, publications, online education, chapter program, advocacy efforts, quality improvement programs, and more have evolved significantly to ensure hospitalists at all stages of their careers – and those who support them – have access to resources to keep them up to date and demonstrate their value in America’s health care system.
During Dr. Wellikson’s tenure, SHM launched its peer-reviewed Journal of Hospital Medicine, the premier, ISI-indexed publication for the specialty, successfully advocated for a Focused Practice in Hospital Medicine certification option and C6 hospitalist specialty code, and earned the John M. Eisenberg Patient Safety and Quality Award for its quality improvement programs. These are just a few of the noteworthy accomplishments that have elevated SHM as a key partner for hospitalists and their institutions.
To assist with the search for SHM’s next CEO, the society has retained Spencer Stuart, a leading global executive and leadership advisory firm. The search process is being overseen by a diverse search committee led by the president-elect of SHM’s Board of Directors, Danielle Scheurer, MD, MSCR, SFHM.
“On behalf of the society and its members, I want to extend a sincere thank you to Larry for his years of dedication and service to SHM, its staff, and the hospital medicine professionals we serve,” said Christopher Frost, MD, SFHM, president of SHM’s Board of Directors. “His legacy will allow SHM to continue its growth trajectory through key programs and services supporting members’ needs for years to come. Larry has taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team can come for education, community, and betterment of the care we provide to our patients. We are indebted to him beyond words.”
Those who are interested in leading SHM into the future as its next CEO are encouraged to contact either Jennifer P. Heenan (jheenan@spencerstuart.com) or Mark Furman, MD (mfurman@spencerstuart.com).
Changing the VA’s OC Pill Dispensing Could Save Money—and Avoid Unwanted Pregnancy
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
2019 journal CHEST® impact factor update
June 27, 2019
The journal CHEST® has just been awarded an impact factor of 9.657, the highest in its history, which equates to a 26% increase over last year’s record-breaking score. CHEST is ranked 4th out of 33 journals in the Critical Care category and 5th out of 63 journals in the Respiratory System category.
Congratulations to all who contributed to this outstanding achievement.
June 27, 2019
The journal CHEST® has just been awarded an impact factor of 9.657, the highest in its history, which equates to a 26% increase over last year’s record-breaking score. CHEST is ranked 4th out of 33 journals in the Critical Care category and 5th out of 63 journals in the Respiratory System category.
Congratulations to all who contributed to this outstanding achievement.
June 27, 2019
The journal CHEST® has just been awarded an impact factor of 9.657, the highest in its history, which equates to a 26% increase over last year’s record-breaking score. CHEST is ranked 4th out of 33 journals in the Critical Care category and 5th out of 63 journals in the Respiratory System category.
Congratulations to all who contributed to this outstanding achievement.
Bronchoscopy coding and billing tips. HCV+ donors. Women and COPD. Treating penetrating trauma
Practice operations
Basic bronchoscopy coding and billing: Rules of the road
Although complex, reimbursement for bronchoscopy is based on appropriate billing, coding, and precise documentation. It is of utmost importance to have a detailed understanding of the various codes to optimize reimbursement. We understand this is a moving target and beyond the scope of this article to discuss all the specific details, so we will try to focus on “the road less travelled.”
Tip#1: When multiple techniques are performed during a bronchoscopy only one CPT® code is considered primary and fully paid while the rest are partially paid. However, there are certain CPT codes that are considered “add-ons” and, therefore, do not fall under the multiple bronchoscopy rules and are paid in full on top of the other codes.
Tip#2: When separate biopsies are performed on different sites or lesions during the same procedure, be sure to attach the Modifier 59 (distinct procedural service) code.
Tip#3: If the procedure performed was time consuming and/or difficult, attach the Modifier 22 (unusual procedural services) code as it increases the reimbursement by 20% to 25%.
Tip#4: The CPT codes for bronchoscopy with therapeutic aspiration are 31645 (initial) and 31646 (subsequent). These were revised in 2018. They are valued greater than 31622 (airway inspection).
Tip#5: Previously moderate sedation provided by the bronchoscopist was bundled in the CPT codes, but in 2017, CMS reduced the wRVUs of these codes by 0.25. This change was adapted due to the trend of billing for moderate sedation by separate providers and reflects the increased use of anesthetists in the endoscopy suite.
Different insurance companies have varying requirements regarding a lot of codes, particularly the modifiers. Therefore, physicians, hospitals, and the coders need to be aware of all the rules. Please do not hesitate to contact the Practice Operations NetWork for more information.
Salim Surani, MD, MPH, FCCP
Chair
Humayun Anjum, MD, FCCP
Vice-Chair
Additional reading:
Centers for Medicare & Medicaid Services (CMS). Fed Regist. 2017;82:52976.
Liu H, et al. JAMA. 2012;307:1178.
Nelson, ME. Chest. 2017;152:893.
Ninan N, et al. https://doi.org/10.1016/j.chest.2019.02.009
Transplant
Hepatitis C-positive donor organs and lung transplantation: Are we there yet?
The field of lung transplantation continues to be encumbered by the mismatch between organ supply and demand. Only approximately 15% of potential donor lungs are currently being used for transplantation, resulting in unacceptably high wait list mortality (17.2 deaths per 100 wait list years).
To counter this, the transplant community continues to invest in innovations such as ex vivo lung perfusion (EVLP) to increase the availability of suitable lungs for transplantation. At the same time, efforts to modify some of the existing practices are also underway. One area of interest has been the potential use of hepatitis C virus antibody positive (HCV +) donors in solid organ transplantation. Traditionally, the use of HCV + organs, especially when the donor is nucleic acid test (NAT)-positive, which indicates presence of HCV RNA, has been considered a contra-indication for solid organ transplantation. However, this has resulted in the exclusion of a significant number of potential HCV + donors (including young and otherwise healthy donor organs), the increased availability of which has been fueled by the opioid epidemic in the United States.
While kidney transplantation programs have been relatively more liberal with utilizing this subset of donors (due to requiring lesser degree of immunosuppression), heart and lung transplantation programs have shied away from this practice due to concerns for disease transmission and unfavorable outcomes, including reduced survival of the recipient (Englum BR, et al. J Heart Lung Transplant. 2016 Feb;35[2]:228).
Hepatitis C infection is one of the medical conditions for which the treatment of disease has changed substantially in the last decade. The advent of new classes of medications, direct acting antiviral agents (DAA), has ensured that a sustained virologic response (SVR), across all genotypes, is now possible in up to 98% of those who undergo treatment. Further, DAAs have a comparatively favorable pharmacokinetic profile and are well tolerated. Since the initial reports of success in the use of HCV + donor organs for lung transplantation, the results of a recently published trial lend further support to the continued use of these organs (Khan B, et al. Am J Transplant. 2017 Apr;17[4]:1129). One hundred percent of patients (n=35, 28 lung and 7 heart) who received organs from HCV + donors (NAT +) and were treated with DAA for 4 weeks (started immediately after transplantation) had an undetectable viral load and excellent graft function at 6 months posttransplantation (Woolley AE, et al. N Engl J Med. 2019 Apr 25;380[17]:1606). Similar studies with greater power and longer follow-up need to be conducted to instill greater confidence in the use of HCV + organs in potential lung recipients. In addition, ethical issues surrounding the use of HCV + organs should be carefully vetted, as the long-term outcomes regarding use of DAAs are not yet known. It is imperative that transplant centers ensure that patients who consent to receipt of HCV + organs fully comprehend the implications of doing so and have systematic posttransplant surveillance. It is also critical that ready access to the entire planned course of DAA is secured for recipients, since these agents could be cost-prohibitive in nonresearch settings. Willingness to comply with intense surveillance and therapy should also be assessed. While the notion of using HCV + donors has gained ground as a promising strategy, transplant centers have been rightfully cautious in its liberal use, until long-term outcomes are better characterized.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH, FCCP
Steering Committee
Women’s health
Women and COPD
While age-adjusted death rates from COPD declined for men in the US between 1999 and 2014, they did not change significantly for women. There have been increasing numbers of studies that have focused on differences in COPD risk factors and outcomes between men and women.
Health and disease are impacted by both sex and gender. Sex refers to biological differences, including chromosomal differences, sex organs, and endogenous hormone profiles. Gender refers to social and cultural differences and includes socially constructed roles and behaviors that vary across cultures and over time.
The prevalence of COPD is increasing more rapidly in women. Women are more likely to be misdiagnosed or have a delay in diagnosis (Chapman, et al. Chest. 2001;119[6]:1691). Evidence suggests that women with COPD have more exacerbations, worse health status, and greater dyspnea (Roche, et al. Respir Res. 2014;15:20; Celli, et al. Am J Respir Crit Care Med. 2011;183[3]:317). Women diagnosed with COPD are more likely to be nonsmokers, and those who smoke are more susceptible to the harmful effects of tobacco (Vestbo, et al. Am J Respir Crit Care Med. 2013;187[4]:347).
In examining differences in exacerbation risk/severity between men and women, 48% of patients with incident COPD were women. Women were 17% more likely to have a moderate/severe first disease exacerbation and shorter time from diagnosis to exacerbation. During three years of follow-up, women had higher annual rates of moderate to severe exacerbations, most pronounced in ages > 40 years to < 65 years (Stolz et al. Submitted for publication. Chest 2019).
NHLBI convened a workshop of experts to review the current understanding of sex and gender on lung disease. They concluded that sex-specific susceptibility to COPD is poorly understood, and gender-specific approaches to COPD are imperative (Han et al. Am J Respir Crit Care Med. 2018;198[7]:850).
Margaret Pisani, MD, MS, FCCP
Vice-Chair
Disaster response and global health
Treating penetrating trauma
The management of penetrating trauma is an unfortunate but all too common facet of critical care practice. A recent emphasis has been placed on the use of extremity tourniquets for hemorrhage control.
It has been embraced by organizations such as the Hartford Consensus Joint Committee, in which hemorrhage control is viewed as the critical step in eliminating preventable prehospital death, secondary only to neutralizing the threat posed by the shooter (Brinsfield et al. Bull Am Coll Surg. 2015;100(1 Suppl):24). Interestingly, a recent retrospective review of mass shootings incorporating 12 events and 139 fatalities indicated that only 20% of victims sustained an injury to an extremity, while 58% were shot in the head or chest.
Only 7% of deaths occurred in victims with potentially survivable wounds, while the vast majority of fatalities followed wounds to the chest (89%), and there were no reported events of potential survivors exsanguinating from extremity wounds (Smith et al. J Trauma Acute Care Surg. 2016; 81:86). This differs from recent military data, where the use of extremity tourniquets has been widely lauded for improving survival. The majority of military combat injuries has been due to blast injury (62%-74%), with a minority (22%-23%) due to gunshots (Eastridge et al. J Trauma Acute Care Surg. 2012;73:S431; Champion et al. J Trauma. 2003;54:S13). These data suggest that widespread use of pre-hospital extremity tourniquets for hemorrhage control in the treatment of gunshot wounds may not result in the anticipated survival improvement that has led to its widespread advocacy. Basic tenets of trauma care, such as rapid control of the airway and treatment of penetrating trauma to the thorax and abdomen, will continue to be of paramount importance.
Michael Powers, MD
Ryan Maves, MD, FCCP
Michael Tripp, MD, FCCP
Steering Committee Members
Dr. Powers is a United States military service member. This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, the Department of Defense, nor the U.S. Government.
Practice operations
Basic bronchoscopy coding and billing: Rules of the road
Although complex, reimbursement for bronchoscopy is based on appropriate billing, coding, and precise documentation. It is of utmost importance to have a detailed understanding of the various codes to optimize reimbursement. We understand this is a moving target and beyond the scope of this article to discuss all the specific details, so we will try to focus on “the road less travelled.”
Tip#1: When multiple techniques are performed during a bronchoscopy only one CPT® code is considered primary and fully paid while the rest are partially paid. However, there are certain CPT codes that are considered “add-ons” and, therefore, do not fall under the multiple bronchoscopy rules and are paid in full on top of the other codes.
Tip#2: When separate biopsies are performed on different sites or lesions during the same procedure, be sure to attach the Modifier 59 (distinct procedural service) code.
Tip#3: If the procedure performed was time consuming and/or difficult, attach the Modifier 22 (unusual procedural services) code as it increases the reimbursement by 20% to 25%.
Tip#4: The CPT codes for bronchoscopy with therapeutic aspiration are 31645 (initial) and 31646 (subsequent). These were revised in 2018. They are valued greater than 31622 (airway inspection).
Tip#5: Previously moderate sedation provided by the bronchoscopist was bundled in the CPT codes, but in 2017, CMS reduced the wRVUs of these codes by 0.25. This change was adapted due to the trend of billing for moderate sedation by separate providers and reflects the increased use of anesthetists in the endoscopy suite.
Different insurance companies have varying requirements regarding a lot of codes, particularly the modifiers. Therefore, physicians, hospitals, and the coders need to be aware of all the rules. Please do not hesitate to contact the Practice Operations NetWork for more information.
Salim Surani, MD, MPH, FCCP
Chair
Humayun Anjum, MD, FCCP
Vice-Chair
Additional reading:
Centers for Medicare & Medicaid Services (CMS). Fed Regist. 2017;82:52976.
Liu H, et al. JAMA. 2012;307:1178.
Nelson, ME. Chest. 2017;152:893.
Ninan N, et al. https://doi.org/10.1016/j.chest.2019.02.009
Transplant
Hepatitis C-positive donor organs and lung transplantation: Are we there yet?
The field of lung transplantation continues to be encumbered by the mismatch between organ supply and demand. Only approximately 15% of potential donor lungs are currently being used for transplantation, resulting in unacceptably high wait list mortality (17.2 deaths per 100 wait list years).
To counter this, the transplant community continues to invest in innovations such as ex vivo lung perfusion (EVLP) to increase the availability of suitable lungs for transplantation. At the same time, efforts to modify some of the existing practices are also underway. One area of interest has been the potential use of hepatitis C virus antibody positive (HCV +) donors in solid organ transplantation. Traditionally, the use of HCV + organs, especially when the donor is nucleic acid test (NAT)-positive, which indicates presence of HCV RNA, has been considered a contra-indication for solid organ transplantation. However, this has resulted in the exclusion of a significant number of potential HCV + donors (including young and otherwise healthy donor organs), the increased availability of which has been fueled by the opioid epidemic in the United States.
While kidney transplantation programs have been relatively more liberal with utilizing this subset of donors (due to requiring lesser degree of immunosuppression), heart and lung transplantation programs have shied away from this practice due to concerns for disease transmission and unfavorable outcomes, including reduced survival of the recipient (Englum BR, et al. J Heart Lung Transplant. 2016 Feb;35[2]:228).
Hepatitis C infection is one of the medical conditions for which the treatment of disease has changed substantially in the last decade. The advent of new classes of medications, direct acting antiviral agents (DAA), has ensured that a sustained virologic response (SVR), across all genotypes, is now possible in up to 98% of those who undergo treatment. Further, DAAs have a comparatively favorable pharmacokinetic profile and are well tolerated. Since the initial reports of success in the use of HCV + donor organs for lung transplantation, the results of a recently published trial lend further support to the continued use of these organs (Khan B, et al. Am J Transplant. 2017 Apr;17[4]:1129). One hundred percent of patients (n=35, 28 lung and 7 heart) who received organs from HCV + donors (NAT +) and were treated with DAA for 4 weeks (started immediately after transplantation) had an undetectable viral load and excellent graft function at 6 months posttransplantation (Woolley AE, et al. N Engl J Med. 2019 Apr 25;380[17]:1606). Similar studies with greater power and longer follow-up need to be conducted to instill greater confidence in the use of HCV + organs in potential lung recipients. In addition, ethical issues surrounding the use of HCV + organs should be carefully vetted, as the long-term outcomes regarding use of DAAs are not yet known. It is imperative that transplant centers ensure that patients who consent to receipt of HCV + organs fully comprehend the implications of doing so and have systematic posttransplant surveillance. It is also critical that ready access to the entire planned course of DAA is secured for recipients, since these agents could be cost-prohibitive in nonresearch settings. Willingness to comply with intense surveillance and therapy should also be assessed. While the notion of using HCV + donors has gained ground as a promising strategy, transplant centers have been rightfully cautious in its liberal use, until long-term outcomes are better characterized.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH, FCCP
Steering Committee
Women’s health
Women and COPD
While age-adjusted death rates from COPD declined for men in the US between 1999 and 2014, they did not change significantly for women. There have been increasing numbers of studies that have focused on differences in COPD risk factors and outcomes between men and women.
Health and disease are impacted by both sex and gender. Sex refers to biological differences, including chromosomal differences, sex organs, and endogenous hormone profiles. Gender refers to social and cultural differences and includes socially constructed roles and behaviors that vary across cultures and over time.
The prevalence of COPD is increasing more rapidly in women. Women are more likely to be misdiagnosed or have a delay in diagnosis (Chapman, et al. Chest. 2001;119[6]:1691). Evidence suggests that women with COPD have more exacerbations, worse health status, and greater dyspnea (Roche, et al. Respir Res. 2014;15:20; Celli, et al. Am J Respir Crit Care Med. 2011;183[3]:317). Women diagnosed with COPD are more likely to be nonsmokers, and those who smoke are more susceptible to the harmful effects of tobacco (Vestbo, et al. Am J Respir Crit Care Med. 2013;187[4]:347).
In examining differences in exacerbation risk/severity between men and women, 48% of patients with incident COPD were women. Women were 17% more likely to have a moderate/severe first disease exacerbation and shorter time from diagnosis to exacerbation. During three years of follow-up, women had higher annual rates of moderate to severe exacerbations, most pronounced in ages > 40 years to < 65 years (Stolz et al. Submitted for publication. Chest 2019).
NHLBI convened a workshop of experts to review the current understanding of sex and gender on lung disease. They concluded that sex-specific susceptibility to COPD is poorly understood, and gender-specific approaches to COPD are imperative (Han et al. Am J Respir Crit Care Med. 2018;198[7]:850).
Margaret Pisani, MD, MS, FCCP
Vice-Chair
Disaster response and global health
Treating penetrating trauma
The management of penetrating trauma is an unfortunate but all too common facet of critical care practice. A recent emphasis has been placed on the use of extremity tourniquets for hemorrhage control.
It has been embraced by organizations such as the Hartford Consensus Joint Committee, in which hemorrhage control is viewed as the critical step in eliminating preventable prehospital death, secondary only to neutralizing the threat posed by the shooter (Brinsfield et al. Bull Am Coll Surg. 2015;100(1 Suppl):24). Interestingly, a recent retrospective review of mass shootings incorporating 12 events and 139 fatalities indicated that only 20% of victims sustained an injury to an extremity, while 58% were shot in the head or chest.
Only 7% of deaths occurred in victims with potentially survivable wounds, while the vast majority of fatalities followed wounds to the chest (89%), and there were no reported events of potential survivors exsanguinating from extremity wounds (Smith et al. J Trauma Acute Care Surg. 2016; 81:86). This differs from recent military data, where the use of extremity tourniquets has been widely lauded for improving survival. The majority of military combat injuries has been due to blast injury (62%-74%), with a minority (22%-23%) due to gunshots (Eastridge et al. J Trauma Acute Care Surg. 2012;73:S431; Champion et al. J Trauma. 2003;54:S13). These data suggest that widespread use of pre-hospital extremity tourniquets for hemorrhage control in the treatment of gunshot wounds may not result in the anticipated survival improvement that has led to its widespread advocacy. Basic tenets of trauma care, such as rapid control of the airway and treatment of penetrating trauma to the thorax and abdomen, will continue to be of paramount importance.
Michael Powers, MD
Ryan Maves, MD, FCCP
Michael Tripp, MD, FCCP
Steering Committee Members
Dr. Powers is a United States military service member. This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, the Department of Defense, nor the U.S. Government.
Practice operations
Basic bronchoscopy coding and billing: Rules of the road
Although complex, reimbursement for bronchoscopy is based on appropriate billing, coding, and precise documentation. It is of utmost importance to have a detailed understanding of the various codes to optimize reimbursement. We understand this is a moving target and beyond the scope of this article to discuss all the specific details, so we will try to focus on “the road less travelled.”
Tip#1: When multiple techniques are performed during a bronchoscopy only one CPT® code is considered primary and fully paid while the rest are partially paid. However, there are certain CPT codes that are considered “add-ons” and, therefore, do not fall under the multiple bronchoscopy rules and are paid in full on top of the other codes.
Tip#2: When separate biopsies are performed on different sites or lesions during the same procedure, be sure to attach the Modifier 59 (distinct procedural service) code.
Tip#3: If the procedure performed was time consuming and/or difficult, attach the Modifier 22 (unusual procedural services) code as it increases the reimbursement by 20% to 25%.
Tip#4: The CPT codes for bronchoscopy with therapeutic aspiration are 31645 (initial) and 31646 (subsequent). These were revised in 2018. They are valued greater than 31622 (airway inspection).
Tip#5: Previously moderate sedation provided by the bronchoscopist was bundled in the CPT codes, but in 2017, CMS reduced the wRVUs of these codes by 0.25. This change was adapted due to the trend of billing for moderate sedation by separate providers and reflects the increased use of anesthetists in the endoscopy suite.
Different insurance companies have varying requirements regarding a lot of codes, particularly the modifiers. Therefore, physicians, hospitals, and the coders need to be aware of all the rules. Please do not hesitate to contact the Practice Operations NetWork for more information.
Salim Surani, MD, MPH, FCCP
Chair
Humayun Anjum, MD, FCCP
Vice-Chair
Additional reading:
Centers for Medicare & Medicaid Services (CMS). Fed Regist. 2017;82:52976.
Liu H, et al. JAMA. 2012;307:1178.
Nelson, ME. Chest. 2017;152:893.
Ninan N, et al. https://doi.org/10.1016/j.chest.2019.02.009
Transplant
Hepatitis C-positive donor organs and lung transplantation: Are we there yet?
The field of lung transplantation continues to be encumbered by the mismatch between organ supply and demand. Only approximately 15% of potential donor lungs are currently being used for transplantation, resulting in unacceptably high wait list mortality (17.2 deaths per 100 wait list years).
To counter this, the transplant community continues to invest in innovations such as ex vivo lung perfusion (EVLP) to increase the availability of suitable lungs for transplantation. At the same time, efforts to modify some of the existing practices are also underway. One area of interest has been the potential use of hepatitis C virus antibody positive (HCV +) donors in solid organ transplantation. Traditionally, the use of HCV + organs, especially when the donor is nucleic acid test (NAT)-positive, which indicates presence of HCV RNA, has been considered a contra-indication for solid organ transplantation. However, this has resulted in the exclusion of a significant number of potential HCV + donors (including young and otherwise healthy donor organs), the increased availability of which has been fueled by the opioid epidemic in the United States.
While kidney transplantation programs have been relatively more liberal with utilizing this subset of donors (due to requiring lesser degree of immunosuppression), heart and lung transplantation programs have shied away from this practice due to concerns for disease transmission and unfavorable outcomes, including reduced survival of the recipient (Englum BR, et al. J Heart Lung Transplant. 2016 Feb;35[2]:228).
Hepatitis C infection is one of the medical conditions for which the treatment of disease has changed substantially in the last decade. The advent of new classes of medications, direct acting antiviral agents (DAA), has ensured that a sustained virologic response (SVR), across all genotypes, is now possible in up to 98% of those who undergo treatment. Further, DAAs have a comparatively favorable pharmacokinetic profile and are well tolerated. Since the initial reports of success in the use of HCV + donor organs for lung transplantation, the results of a recently published trial lend further support to the continued use of these organs (Khan B, et al. Am J Transplant. 2017 Apr;17[4]:1129). One hundred percent of patients (n=35, 28 lung and 7 heart) who received organs from HCV + donors (NAT +) and were treated with DAA for 4 weeks (started immediately after transplantation) had an undetectable viral load and excellent graft function at 6 months posttransplantation (Woolley AE, et al. N Engl J Med. 2019 Apr 25;380[17]:1606). Similar studies with greater power and longer follow-up need to be conducted to instill greater confidence in the use of HCV + organs in potential lung recipients. In addition, ethical issues surrounding the use of HCV + organs should be carefully vetted, as the long-term outcomes regarding use of DAAs are not yet known. It is imperative that transplant centers ensure that patients who consent to receipt of HCV + organs fully comprehend the implications of doing so and have systematic posttransplant surveillance. It is also critical that ready access to the entire planned course of DAA is secured for recipients, since these agents could be cost-prohibitive in nonresearch settings. Willingness to comply with intense surveillance and therapy should also be assessed. While the notion of using HCV + donors has gained ground as a promising strategy, transplant centers have been rightfully cautious in its liberal use, until long-term outcomes are better characterized.
Anupam Kumar, MD
Fellow-in-Training Member
J. W. Awori Hayanga, MD, MPH, FCCP
Steering Committee
Women’s health
Women and COPD
While age-adjusted death rates from COPD declined for men in the US between 1999 and 2014, they did not change significantly for women. There have been increasing numbers of studies that have focused on differences in COPD risk factors and outcomes between men and women.
Health and disease are impacted by both sex and gender. Sex refers to biological differences, including chromosomal differences, sex organs, and endogenous hormone profiles. Gender refers to social and cultural differences and includes socially constructed roles and behaviors that vary across cultures and over time.
The prevalence of COPD is increasing more rapidly in women. Women are more likely to be misdiagnosed or have a delay in diagnosis (Chapman, et al. Chest. 2001;119[6]:1691). Evidence suggests that women with COPD have more exacerbations, worse health status, and greater dyspnea (Roche, et al. Respir Res. 2014;15:20; Celli, et al. Am J Respir Crit Care Med. 2011;183[3]:317). Women diagnosed with COPD are more likely to be nonsmokers, and those who smoke are more susceptible to the harmful effects of tobacco (Vestbo, et al. Am J Respir Crit Care Med. 2013;187[4]:347).
In examining differences in exacerbation risk/severity between men and women, 48% of patients with incident COPD were women. Women were 17% more likely to have a moderate/severe first disease exacerbation and shorter time from diagnosis to exacerbation. During three years of follow-up, women had higher annual rates of moderate to severe exacerbations, most pronounced in ages > 40 years to < 65 years (Stolz et al. Submitted for publication. Chest 2019).
NHLBI convened a workshop of experts to review the current understanding of sex and gender on lung disease. They concluded that sex-specific susceptibility to COPD is poorly understood, and gender-specific approaches to COPD are imperative (Han et al. Am J Respir Crit Care Med. 2018;198[7]:850).
Margaret Pisani, MD, MS, FCCP
Vice-Chair
Disaster response and global health
Treating penetrating trauma
The management of penetrating trauma is an unfortunate but all too common facet of critical care practice. A recent emphasis has been placed on the use of extremity tourniquets for hemorrhage control.
It has been embraced by organizations such as the Hartford Consensus Joint Committee, in which hemorrhage control is viewed as the critical step in eliminating preventable prehospital death, secondary only to neutralizing the threat posed by the shooter (Brinsfield et al. Bull Am Coll Surg. 2015;100(1 Suppl):24). Interestingly, a recent retrospective review of mass shootings incorporating 12 events and 139 fatalities indicated that only 20% of victims sustained an injury to an extremity, while 58% were shot in the head or chest.
Only 7% of deaths occurred in victims with potentially survivable wounds, while the vast majority of fatalities followed wounds to the chest (89%), and there were no reported events of potential survivors exsanguinating from extremity wounds (Smith et al. J Trauma Acute Care Surg. 2016; 81:86). This differs from recent military data, where the use of extremity tourniquets has been widely lauded for improving survival. The majority of military combat injuries has been due to blast injury (62%-74%), with a minority (22%-23%) due to gunshots (Eastridge et al. J Trauma Acute Care Surg. 2012;73:S431; Champion et al. J Trauma. 2003;54:S13). These data suggest that widespread use of pre-hospital extremity tourniquets for hemorrhage control in the treatment of gunshot wounds may not result in the anticipated survival improvement that has led to its widespread advocacy. Basic tenets of trauma care, such as rapid control of the airway and treatment of penetrating trauma to the thorax and abdomen, will continue to be of paramount importance.
Michael Powers, MD
Ryan Maves, MD, FCCP
Michael Tripp, MD, FCCP
Steering Committee Members
Dr. Powers is a United States military service member. This work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, the Department of Defense, nor the U.S. Government.
From the President: IPF—Success or failure?
Last month, I attended the funeral of my uncle who died at age 88 from pulmonary fibrosis, a condition that he battled for more than a decade. Having the honor of delivering a tribute to his life during the ceremony, I included an explanation of the basics of the disease to the audience that had packed the church located in an upscale neighborhood in the Chicago suburbs.
Afterward, I wondered if medical science and we as medical providers are making any real progress in fighting this relentless fibrotic interstitial lung disease. After all, I had watched him gradually decline, first plagued by an endless nonproductive cough, then exertional dyspnea, exertional hypoxia, and followed by hypoxia at rest. Eventually, he was short of breath with moving from his bed to the bathroom – even on high flow supplemental oxygen. It was a slow, gradual process of asphyxiation.
He had every resource available to him. He was seen by the best subspecialty providers in his community and saw experts in major medical centers across the country. He had been given the seemingly usual trial of corticosteroids initially, and then for unclear reasons, several inhalers. Maybe because his doctors didn’t have much else to offer. He was then treated with pirfenidone, and later switched to nintedanib when the side effects were too much for him to handle. Lung transplant was considered and then ruled out. With more respiratory symptoms, and despite my caution against it, he even tried experimental therapy with stem cells – an unproven treatment with additional potential for untoward consequences. At that point, he considered my suggestion to hold off, shrugged, and indicated that if he had the wherewithal and means to try it, why not?
Besides watching the desperation of finding that elusive therapy that will somehow erase the progressive symptoms of shortness of breath and cough, what I realized from being on the “other side of the gurney” with a family member is that sometimes obtaining basic treatments like supplemental oxygen can be more challenging than obtaining the more expensive pharmacologic therapies. And, that having to use supplemental oxygen is like being tethered, especially if your portable oxygen delivery device is unreliable or battery power is questionable. I’ve thought about folks with lung disease who do not have a “best practice” resource that includes unlimited medical direction and access to care. What about the people who can’t afford experimental therapies, who cannot easily navigate the medical maze, or who do not have someone to phone and call to help them when their portable oxygen concentrator develops a fatal hardware error?
I pondered the reality of his illness. Yes, at some point all of us will die. But in the end, were his treatments a success? What exactly does success look like when it comes to treatment of idiopathic pulmonary fibrosis? Did we as a medical community slow down his inevitable decline in lung function? Did we make a meaningful difference? Ultimately, does it matter if someone has a better FEV1, or trek 30 feet farther on a 6-minute walk for a few more months? Maybe. Then, as I traveled with my uncle on his medical journey, I realized that, yes, even the small things really do matter, retrospectively.
I think in my uncle’s case, his eventual demise came, but only after great successes by the medical community. The inevitable was delayed for several years at a minimum. He remained comfortable. He was able to do things on his “bucket list” that he would not likely have been able to enjoy without treatment. It allowed him to take an Honor Flight to Washington, DC, to be remembered for his heroic service in the Korean War. It allowed him to attend the 150th annual convention of the American Legion last summer, as he completed his service as the Commander of his local post. On a personal and maybe more selfish level, it allowed me to be able to enjoy dinner with him on two or three occasions while visiting Chicago during CHEST business meetings at the headquarters location. How much value do we place on being able to hear a few more family stories, to watch someone who you know is going to die smile and laugh, and share memories together for what could be the final time?
Through this experience, I have been reminded to recognize that there are many small, silent victories for our medical community in the war against devastating disease. We need to celebrate even the tiny advances. The medical community did not let him down. It rallied to give him the best we could offer with the tools we had at hand. And, keeping in mind those yet to develop this condition, it’s time to keep working hard to make a difference. Whether doing basic research, creating the medications of the future to treat respiratory illness, diagnosing conditions earlier and more accurately, or providing compassionate, patient-centered care, let’s keep our focus on crushing lung disease -- a little bit at a time.
Last month, I attended the funeral of my uncle who died at age 88 from pulmonary fibrosis, a condition that he battled for more than a decade. Having the honor of delivering a tribute to his life during the ceremony, I included an explanation of the basics of the disease to the audience that had packed the church located in an upscale neighborhood in the Chicago suburbs.
Afterward, I wondered if medical science and we as medical providers are making any real progress in fighting this relentless fibrotic interstitial lung disease. After all, I had watched him gradually decline, first plagued by an endless nonproductive cough, then exertional dyspnea, exertional hypoxia, and followed by hypoxia at rest. Eventually, he was short of breath with moving from his bed to the bathroom – even on high flow supplemental oxygen. It was a slow, gradual process of asphyxiation.
He had every resource available to him. He was seen by the best subspecialty providers in his community and saw experts in major medical centers across the country. He had been given the seemingly usual trial of corticosteroids initially, and then for unclear reasons, several inhalers. Maybe because his doctors didn’t have much else to offer. He was then treated with pirfenidone, and later switched to nintedanib when the side effects were too much for him to handle. Lung transplant was considered and then ruled out. With more respiratory symptoms, and despite my caution against it, he even tried experimental therapy with stem cells – an unproven treatment with additional potential for untoward consequences. At that point, he considered my suggestion to hold off, shrugged, and indicated that if he had the wherewithal and means to try it, why not?
Besides watching the desperation of finding that elusive therapy that will somehow erase the progressive symptoms of shortness of breath and cough, what I realized from being on the “other side of the gurney” with a family member is that sometimes obtaining basic treatments like supplemental oxygen can be more challenging than obtaining the more expensive pharmacologic therapies. And, that having to use supplemental oxygen is like being tethered, especially if your portable oxygen delivery device is unreliable or battery power is questionable. I’ve thought about folks with lung disease who do not have a “best practice” resource that includes unlimited medical direction and access to care. What about the people who can’t afford experimental therapies, who cannot easily navigate the medical maze, or who do not have someone to phone and call to help them when their portable oxygen concentrator develops a fatal hardware error?
I pondered the reality of his illness. Yes, at some point all of us will die. But in the end, were his treatments a success? What exactly does success look like when it comes to treatment of idiopathic pulmonary fibrosis? Did we as a medical community slow down his inevitable decline in lung function? Did we make a meaningful difference? Ultimately, does it matter if someone has a better FEV1, or trek 30 feet farther on a 6-minute walk for a few more months? Maybe. Then, as I traveled with my uncle on his medical journey, I realized that, yes, even the small things really do matter, retrospectively.
I think in my uncle’s case, his eventual demise came, but only after great successes by the medical community. The inevitable was delayed for several years at a minimum. He remained comfortable. He was able to do things on his “bucket list” that he would not likely have been able to enjoy without treatment. It allowed him to take an Honor Flight to Washington, DC, to be remembered for his heroic service in the Korean War. It allowed him to attend the 150th annual convention of the American Legion last summer, as he completed his service as the Commander of his local post. On a personal and maybe more selfish level, it allowed me to be able to enjoy dinner with him on two or three occasions while visiting Chicago during CHEST business meetings at the headquarters location. How much value do we place on being able to hear a few more family stories, to watch someone who you know is going to die smile and laugh, and share memories together for what could be the final time?
Through this experience, I have been reminded to recognize that there are many small, silent victories for our medical community in the war against devastating disease. We need to celebrate even the tiny advances. The medical community did not let him down. It rallied to give him the best we could offer with the tools we had at hand. And, keeping in mind those yet to develop this condition, it’s time to keep working hard to make a difference. Whether doing basic research, creating the medications of the future to treat respiratory illness, diagnosing conditions earlier and more accurately, or providing compassionate, patient-centered care, let’s keep our focus on crushing lung disease -- a little bit at a time.
Last month, I attended the funeral of my uncle who died at age 88 from pulmonary fibrosis, a condition that he battled for more than a decade. Having the honor of delivering a tribute to his life during the ceremony, I included an explanation of the basics of the disease to the audience that had packed the church located in an upscale neighborhood in the Chicago suburbs.
Afterward, I wondered if medical science and we as medical providers are making any real progress in fighting this relentless fibrotic interstitial lung disease. After all, I had watched him gradually decline, first plagued by an endless nonproductive cough, then exertional dyspnea, exertional hypoxia, and followed by hypoxia at rest. Eventually, he was short of breath with moving from his bed to the bathroom – even on high flow supplemental oxygen. It was a slow, gradual process of asphyxiation.
He had every resource available to him. He was seen by the best subspecialty providers in his community and saw experts in major medical centers across the country. He had been given the seemingly usual trial of corticosteroids initially, and then for unclear reasons, several inhalers. Maybe because his doctors didn’t have much else to offer. He was then treated with pirfenidone, and later switched to nintedanib when the side effects were too much for him to handle. Lung transplant was considered and then ruled out. With more respiratory symptoms, and despite my caution against it, he even tried experimental therapy with stem cells – an unproven treatment with additional potential for untoward consequences. At that point, he considered my suggestion to hold off, shrugged, and indicated that if he had the wherewithal and means to try it, why not?
Besides watching the desperation of finding that elusive therapy that will somehow erase the progressive symptoms of shortness of breath and cough, what I realized from being on the “other side of the gurney” with a family member is that sometimes obtaining basic treatments like supplemental oxygen can be more challenging than obtaining the more expensive pharmacologic therapies. And, that having to use supplemental oxygen is like being tethered, especially if your portable oxygen delivery device is unreliable or battery power is questionable. I’ve thought about folks with lung disease who do not have a “best practice” resource that includes unlimited medical direction and access to care. What about the people who can’t afford experimental therapies, who cannot easily navigate the medical maze, or who do not have someone to phone and call to help them when their portable oxygen concentrator develops a fatal hardware error?
I pondered the reality of his illness. Yes, at some point all of us will die. But in the end, were his treatments a success? What exactly does success look like when it comes to treatment of idiopathic pulmonary fibrosis? Did we as a medical community slow down his inevitable decline in lung function? Did we make a meaningful difference? Ultimately, does it matter if someone has a better FEV1, or trek 30 feet farther on a 6-minute walk for a few more months? Maybe. Then, as I traveled with my uncle on his medical journey, I realized that, yes, even the small things really do matter, retrospectively.
I think in my uncle’s case, his eventual demise came, but only after great successes by the medical community. The inevitable was delayed for several years at a minimum. He remained comfortable. He was able to do things on his “bucket list” that he would not likely have been able to enjoy without treatment. It allowed him to take an Honor Flight to Washington, DC, to be remembered for his heroic service in the Korean War. It allowed him to attend the 150th annual convention of the American Legion last summer, as he completed his service as the Commander of his local post. On a personal and maybe more selfish level, it allowed me to be able to enjoy dinner with him on two or three occasions while visiting Chicago during CHEST business meetings at the headquarters location. How much value do we place on being able to hear a few more family stories, to watch someone who you know is going to die smile and laugh, and share memories together for what could be the final time?
Through this experience, I have been reminded to recognize that there are many small, silent victories for our medical community in the war against devastating disease. We need to celebrate even the tiny advances. The medical community did not let him down. It rallied to give him the best we could offer with the tools we had at hand. And, keeping in mind those yet to develop this condition, it’s time to keep working hard to make a difference. Whether doing basic research, creating the medications of the future to treat respiratory illness, diagnosing conditions earlier and more accurately, or providing compassionate, patient-centered care, let’s keep our focus on crushing lung disease -- a little bit at a time.
Update from AMA Annual Meeting 2019
The American Medical Association (AMA) conducted the Annual Meeting of the AMA House of Delegates from June 8-12 in Chicago. The House of Delegates (HOD) is the principal policymaking body of the AMA, consisting of more than 600 delegates and accompanying alternate delegates who represent the medical specialty societies (including CHEST); the state and territorial medical associations; the uniformed services; and other stakeholder organizations. Leading policymakers including Centers for Medicare & Medicaid Services (CMS) Administrator Seema Verma and the Surgeon General of the United States, Vice Admiral Jerome M. Adams, MD, also participated in the meeting.
This year, the delegates (CHEST has three delegate positions) considered more than 200 policy proposals (resolutions and reports) in a multi-step process: caucuses, Reference Committees, and hearings before the full House of Delegates.
The caucuses are an important first step in the HOD process. The Chest/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met the day before the Reference Committee hearings to:
• Decide what resolutions and reports are most important to the chest diseases, critical care medicine, sleep medicine, and allergy communities;
• Determine (if possible) a unified position (support/oppose);
• Develop talking points; and
• Identify who will speak for the caucus (or as individuals if there were differing positions) at the various Reference Committee meetings.
Under the leadership of Tina Shah, MD, MPH, from the Society of Critical Care Medicine, the caucus decided to focus on 16 reports and resolutions that were slated for discussion at 7 different Reference Committees. The caucus used the GroupMe mobile, a group messaging app, to stay in touch during the meeting to ensure that someone from the caucus would be at all pertinent sessions and to communicate progress and results in real time.
The topics of the reports and resolutions selected by the Caucus for involvement included:
• Returning Liquid Oxygen to the Medicare Fee Schedule
• COPD National Action Plan
• Low Nicotine Product Standard
• Addressing the Vaping Crisis
• Regulating Liquid Nicotine and E-Cigarettes
• Put Over-the-Counter Inhaled Epinephrine Behind Pharmacy Counter
• Change in Marijuana Classification to Allow Research
• Promotion of Early Recognition and Treatment of Sepsis by Out-of-Hospital Healthcare Providers
• The Climate Change Lecture for US Medical Schools
• Physician-Assisted Suicide
• End-of-Life Care
The Reference Committees, where both AMA members and nonmembers (with permission) may testify, are organized by topic:
• Medical Service
• Legislation, Legal, and Regulatory Issues
• Medical Education
• Public Health
• Science and Technology
• AMA Governance and Finance
• Medical Practice
• Constitution and Bylaws
The Reference Committees hear testimony on each resolution, adjourn, and then meet privately (often into the wee hours) to develop recommendations to the full House. Their options include:
• Recommend Adoption
• Recommend Adoption With Amendment
• Recommend Referral (further study by one of several Councils)
• Recommend Referral for Decision (by the Board of Trustees after further study)
• Recommend for Non-Adoption
During the following 3 days, the full House of Delegates considers the Reference Committee recommendations. Any delegate may object to any recommendation and cause it to be debated and voted on by the full House of Delegates. Details about the outcomes of the 200+ resolutions are available at the AMA website (ama-assn.org).
The compendium of policies covers the entire range of topics impacting the practice of medicine – ethics, legislation, regulation, public health, individual health, and medical education among them. The full range of policies may be found in the AMA’s Policy Manual available on the AMA website (ama-assn.org).
CHEST members with an interest in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills with no cost at scores of educational sessions and will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at jnemkovich@chestnet.org for details.
Dr. Desai is with the Chicago Chest Center and Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates. Mr. Newman is the Senior Director of Strategy, Product, & Global Development at CHEST.
The American Medical Association (AMA) conducted the Annual Meeting of the AMA House of Delegates from June 8-12 in Chicago. The House of Delegates (HOD) is the principal policymaking body of the AMA, consisting of more than 600 delegates and accompanying alternate delegates who represent the medical specialty societies (including CHEST); the state and territorial medical associations; the uniformed services; and other stakeholder organizations. Leading policymakers including Centers for Medicare & Medicaid Services (CMS) Administrator Seema Verma and the Surgeon General of the United States, Vice Admiral Jerome M. Adams, MD, also participated in the meeting.
This year, the delegates (CHEST has three delegate positions) considered more than 200 policy proposals (resolutions and reports) in a multi-step process: caucuses, Reference Committees, and hearings before the full House of Delegates.
The caucuses are an important first step in the HOD process. The Chest/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met the day before the Reference Committee hearings to:
• Decide what resolutions and reports are most important to the chest diseases, critical care medicine, sleep medicine, and allergy communities;
• Determine (if possible) a unified position (support/oppose);
• Develop talking points; and
• Identify who will speak for the caucus (or as individuals if there were differing positions) at the various Reference Committee meetings.
Under the leadership of Tina Shah, MD, MPH, from the Society of Critical Care Medicine, the caucus decided to focus on 16 reports and resolutions that were slated for discussion at 7 different Reference Committees. The caucus used the GroupMe mobile, a group messaging app, to stay in touch during the meeting to ensure that someone from the caucus would be at all pertinent sessions and to communicate progress and results in real time.
The topics of the reports and resolutions selected by the Caucus for involvement included:
• Returning Liquid Oxygen to the Medicare Fee Schedule
• COPD National Action Plan
• Low Nicotine Product Standard
• Addressing the Vaping Crisis
• Regulating Liquid Nicotine and E-Cigarettes
• Put Over-the-Counter Inhaled Epinephrine Behind Pharmacy Counter
• Change in Marijuana Classification to Allow Research
• Promotion of Early Recognition and Treatment of Sepsis by Out-of-Hospital Healthcare Providers
• The Climate Change Lecture for US Medical Schools
• Physician-Assisted Suicide
• End-of-Life Care
The Reference Committees, where both AMA members and nonmembers (with permission) may testify, are organized by topic:
• Medical Service
• Legislation, Legal, and Regulatory Issues
• Medical Education
• Public Health
• Science and Technology
• AMA Governance and Finance
• Medical Practice
• Constitution and Bylaws
The Reference Committees hear testimony on each resolution, adjourn, and then meet privately (often into the wee hours) to develop recommendations to the full House. Their options include:
• Recommend Adoption
• Recommend Adoption With Amendment
• Recommend Referral (further study by one of several Councils)
• Recommend Referral for Decision (by the Board of Trustees after further study)
• Recommend for Non-Adoption
During the following 3 days, the full House of Delegates considers the Reference Committee recommendations. Any delegate may object to any recommendation and cause it to be debated and voted on by the full House of Delegates. Details about the outcomes of the 200+ resolutions are available at the AMA website (ama-assn.org).
The compendium of policies covers the entire range of topics impacting the practice of medicine – ethics, legislation, regulation, public health, individual health, and medical education among them. The full range of policies may be found in the AMA’s Policy Manual available on the AMA website (ama-assn.org).
CHEST members with an interest in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills with no cost at scores of educational sessions and will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at jnemkovich@chestnet.org for details.
Dr. Desai is with the Chicago Chest Center and Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates. Mr. Newman is the Senior Director of Strategy, Product, & Global Development at CHEST.
The American Medical Association (AMA) conducted the Annual Meeting of the AMA House of Delegates from June 8-12 in Chicago. The House of Delegates (HOD) is the principal policymaking body of the AMA, consisting of more than 600 delegates and accompanying alternate delegates who represent the medical specialty societies (including CHEST); the state and territorial medical associations; the uniformed services; and other stakeholder organizations. Leading policymakers including Centers for Medicare & Medicaid Services (CMS) Administrator Seema Verma and the Surgeon General of the United States, Vice Admiral Jerome M. Adams, MD, also participated in the meeting.
This year, the delegates (CHEST has three delegate positions) considered more than 200 policy proposals (resolutions and reports) in a multi-step process: caucuses, Reference Committees, and hearings before the full House of Delegates.
The caucuses are an important first step in the HOD process. The Chest/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met the day before the Reference Committee hearings to:
• Decide what resolutions and reports are most important to the chest diseases, critical care medicine, sleep medicine, and allergy communities;
• Determine (if possible) a unified position (support/oppose);
• Develop talking points; and
• Identify who will speak for the caucus (or as individuals if there were differing positions) at the various Reference Committee meetings.
Under the leadership of Tina Shah, MD, MPH, from the Society of Critical Care Medicine, the caucus decided to focus on 16 reports and resolutions that were slated for discussion at 7 different Reference Committees. The caucus used the GroupMe mobile, a group messaging app, to stay in touch during the meeting to ensure that someone from the caucus would be at all pertinent sessions and to communicate progress and results in real time.
The topics of the reports and resolutions selected by the Caucus for involvement included:
• Returning Liquid Oxygen to the Medicare Fee Schedule
• COPD National Action Plan
• Low Nicotine Product Standard
• Addressing the Vaping Crisis
• Regulating Liquid Nicotine and E-Cigarettes
• Put Over-the-Counter Inhaled Epinephrine Behind Pharmacy Counter
• Change in Marijuana Classification to Allow Research
• Promotion of Early Recognition and Treatment of Sepsis by Out-of-Hospital Healthcare Providers
• The Climate Change Lecture for US Medical Schools
• Physician-Assisted Suicide
• End-of-Life Care
The Reference Committees, where both AMA members and nonmembers (with permission) may testify, are organized by topic:
• Medical Service
• Legislation, Legal, and Regulatory Issues
• Medical Education
• Public Health
• Science and Technology
• AMA Governance and Finance
• Medical Practice
• Constitution and Bylaws
The Reference Committees hear testimony on each resolution, adjourn, and then meet privately (often into the wee hours) to develop recommendations to the full House. Their options include:
• Recommend Adoption
• Recommend Adoption With Amendment
• Recommend Referral (further study by one of several Councils)
• Recommend Referral for Decision (by the Board of Trustees after further study)
• Recommend for Non-Adoption
During the following 3 days, the full House of Delegates considers the Reference Committee recommendations. Any delegate may object to any recommendation and cause it to be debated and voted on by the full House of Delegates. Details about the outcomes of the 200+ resolutions are available at the AMA website (ama-assn.org).
The compendium of policies covers the entire range of topics impacting the practice of medicine – ethics, legislation, regulation, public health, individual health, and medical education among them. The full range of policies may be found in the AMA’s Policy Manual available on the AMA website (ama-assn.org).
CHEST members with an interest in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills with no cost at scores of educational sessions and will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at jnemkovich@chestnet.org for details.
Dr. Desai is with the Chicago Chest Center and Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates. Mr. Newman is the Senior Director of Strategy, Product, & Global Development at CHEST.