User login
New RSV vaccine immunogenicity improved with protein engineering
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
FROM SCIENCE
Key clinical point:
Major finding: Epitope-neutralizing activity is 5-10 times greater 12 weeks after baseline with a 50 mcg or 150 mcg with and without alum adjuvant.
Study details: The findings are based on a prespecified interim analysis of 90 healthy adult participants in a phase 1, randomized, trial of DS-Cav1.
Disclosures: The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several authors are inventors on patents for stabilizing the RSV F protein.
Source: Crank MC et al. Science. 2019;365(6452):505-9.
Sepsis survivors’ persistent immunosuppression raises mortality risk
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
FROM JAMA NETWORK OPEN
Key clinical point: Markers of inflammation and immunosuppression persist in over two-thirds of patients hospitalized for sepsis, which could explain worsened outcomes and mortality up to 1 year after hospitalization.
Major finding: Patients with signs of hyperinflammation and immunosuppression had significantly increased mortality after 1 year and were significantly more likely to be readmitted or die because of cardiovascular disease or cancer.
Study details: A prospective cohort study of 483 patients who were hospitalized because of sepsis at 12 different centers between January 2012 and May 2017.
Disclosures: This study was funded by grants from National Institutes of Health and resources from the Veterans Affairs Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
Source: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
Smartphone mind control, wasp gyn remedy, and seagull stare downs
Don’t put THAT THERE!
As a doctor, you’ve probably thought, “I can’t believe I have to tell them this” more than once. Warnings that you think are common sense – please don’t try that home remedy, please don’t put that there, please don’t eat that anymore.
Well, here’s a new one for all doctors with female patients out there: Please don’t put a ground-up wasp’s nest into your vagina.
An intrepid ob.gyn. with a large Internet following found that someone has been selling oak galls online as vaginal medicine. Oak galls are little bumps that grow on a tree after the gall wasp lays its larvae. Fun insect-plant behavior! Very bad to put inside your body in any way!
One would think you don’t need to warn patients that tree/wasp paste is not the correct medicine to use on an episiotomy cut, as the Etsy seller suggested.
But with Gwyneth Paltrow out there trying to convince people that purposeful bee stings, healing stickers, and goat milk cleanses are all valid health tips, sometimes the obvious things just need to be spelled out.
Eye of the seagull
Rising up/Back on the beach/Did my time, made my sandwich
Went to the kitchen/Now I’m back on the street
Just a man and his will to surviiiiiiiiiive
It’s the eye of the seagull/It’s the thrill of the fight
Research shows you have to stare them down
When a seagull eyes your sandwich/Don’t let it have a bite
Don’t give in to the eye of the seagull.
That’s just a little ditty for you to sing this summer while enjoying your time on the sand, surrounded by those greedy flying sandwich thieves. Research out of the University of Exeter, England, suggests that staring down approaching seagulls can actually slow them down or even completely deter them from attempting to steal your snacks.
Perhaps the seagulls don’t like being watched while they commit their crimes? Maybe they can’t stand the shame of it all.
Whatever the reason, if you’re assaulted by a flock of seagulls this summer (the birds, not the band), try engaging in a staring contest to keep your food safe.
A gut microbe with a rye smile
Rye is not exactly a huge deal here in the good old U.S. of A., but rye bread happens to be the national food of Finland. So when the Finns say something about rye, it pays to listen.
Investigators at the University of Eastern Finland used metabolomics – which, we discovered the hard way, is the “analysis of metabolites in a biological specimen” and not the Finnish word for a “financial system based on rye bread” – to examine the effects of rye sourdough on the gut microbes of mice and an in vitro gastrointestinal model that mimicked the function of the human gut.
Many compounds found in rye sourdough, such as branched-chain amino acids and amino acid–containing small peptides known to have an impact on insulin metabolism, are processed by gut bacteria before getting absorbed into the body.
The gut microbes of mice fed rye sourdough also produce derivatives of trimethylglycine known as betaine, and at least one of these derivatives reduces the need for oxygen in heart muscle cells, which may protect the heart from ischemia or possibly even enhance its performance, the investigators said.
“The major role played by gut microbes in human health has become more and more evident over the past decades, and this is why gut microbes should be taken very good care of. It’s a good idea to avoid unnecessary antibiotics and feed gut microbes with optimal food, such as rye,” researcher Ville M. Koistinen said in a written statement.
The bottom line? A rye-filled gut microbe is a happy gut microbe. And this comes from Finland, the nation that gave the world “pantsdrunk,” so it must be true. On behalf of the Finns, you’re welcome, world.
This is your brain on smartphones
Hey. Hey, you. Do you want some scientists to install a small device in your brain that uses light and drugs to control your neurons and can be controlled externally by a smartphone? No?
Sounds like a terrible idea only fit for a cheesy science fiction dystopia, you say? Too bad, because a group of researchers from South Korea and the University of Colorado already have invented such a device.
To be fair, their study, published in Nature Biomedical Engineering, is relatively free of nefariousness. The device is meant to be used to search for brain diseases such as Alzheimer’s and Parkinson’s, as well as disorders such as depression, addiction, and pain.
The researchers say that their device is superior to current diagnostic technology available – which uses a similar drug/light combo but is bulkier, stationary, and causes long-term brain damage – because it can be used long term and outside the lab and – we’re sorry – but we’re straying back into dystopia here.
Okay, let’s try again. To test their device, the researchers conducted an animal study and found that, by manipulating the behavior of one animal using the drug/light combo from behind their smartphones, they could influence the behavior of the entire group.
Did we say that the study was relatively free of nefariousness? Sorry about that. At this point, we wouldn’t be surprised if the whole thing was bankrolled by a couple of genetically engineered lab mice from Acme Labs bent on world domination.
Don’t put THAT THERE!
As a doctor, you’ve probably thought, “I can’t believe I have to tell them this” more than once. Warnings that you think are common sense – please don’t try that home remedy, please don’t put that there, please don’t eat that anymore.
Well, here’s a new one for all doctors with female patients out there: Please don’t put a ground-up wasp’s nest into your vagina.
An intrepid ob.gyn. with a large Internet following found that someone has been selling oak galls online as vaginal medicine. Oak galls are little bumps that grow on a tree after the gall wasp lays its larvae. Fun insect-plant behavior! Very bad to put inside your body in any way!
One would think you don’t need to warn patients that tree/wasp paste is not the correct medicine to use on an episiotomy cut, as the Etsy seller suggested.
But with Gwyneth Paltrow out there trying to convince people that purposeful bee stings, healing stickers, and goat milk cleanses are all valid health tips, sometimes the obvious things just need to be spelled out.
Eye of the seagull
Rising up/Back on the beach/Did my time, made my sandwich
Went to the kitchen/Now I’m back on the street
Just a man and his will to surviiiiiiiiiive
It’s the eye of the seagull/It’s the thrill of the fight
Research shows you have to stare them down
When a seagull eyes your sandwich/Don’t let it have a bite
Don’t give in to the eye of the seagull.
That’s just a little ditty for you to sing this summer while enjoying your time on the sand, surrounded by those greedy flying sandwich thieves. Research out of the University of Exeter, England, suggests that staring down approaching seagulls can actually slow them down or even completely deter them from attempting to steal your snacks.
Perhaps the seagulls don’t like being watched while they commit their crimes? Maybe they can’t stand the shame of it all.
Whatever the reason, if you’re assaulted by a flock of seagulls this summer (the birds, not the band), try engaging in a staring contest to keep your food safe.
A gut microbe with a rye smile
Rye is not exactly a huge deal here in the good old U.S. of A., but rye bread happens to be the national food of Finland. So when the Finns say something about rye, it pays to listen.
Investigators at the University of Eastern Finland used metabolomics – which, we discovered the hard way, is the “analysis of metabolites in a biological specimen” and not the Finnish word for a “financial system based on rye bread” – to examine the effects of rye sourdough on the gut microbes of mice and an in vitro gastrointestinal model that mimicked the function of the human gut.
Many compounds found in rye sourdough, such as branched-chain amino acids and amino acid–containing small peptides known to have an impact on insulin metabolism, are processed by gut bacteria before getting absorbed into the body.
The gut microbes of mice fed rye sourdough also produce derivatives of trimethylglycine known as betaine, and at least one of these derivatives reduces the need for oxygen in heart muscle cells, which may protect the heart from ischemia or possibly even enhance its performance, the investigators said.
“The major role played by gut microbes in human health has become more and more evident over the past decades, and this is why gut microbes should be taken very good care of. It’s a good idea to avoid unnecessary antibiotics and feed gut microbes with optimal food, such as rye,” researcher Ville M. Koistinen said in a written statement.
The bottom line? A rye-filled gut microbe is a happy gut microbe. And this comes from Finland, the nation that gave the world “pantsdrunk,” so it must be true. On behalf of the Finns, you’re welcome, world.
This is your brain on smartphones
Hey. Hey, you. Do you want some scientists to install a small device in your brain that uses light and drugs to control your neurons and can be controlled externally by a smartphone? No?
Sounds like a terrible idea only fit for a cheesy science fiction dystopia, you say? Too bad, because a group of researchers from South Korea and the University of Colorado already have invented such a device.
To be fair, their study, published in Nature Biomedical Engineering, is relatively free of nefariousness. The device is meant to be used to search for brain diseases such as Alzheimer’s and Parkinson’s, as well as disorders such as depression, addiction, and pain.
The researchers say that their device is superior to current diagnostic technology available – which uses a similar drug/light combo but is bulkier, stationary, and causes long-term brain damage – because it can be used long term and outside the lab and – we’re sorry – but we’re straying back into dystopia here.
Okay, let’s try again. To test their device, the researchers conducted an animal study and found that, by manipulating the behavior of one animal using the drug/light combo from behind their smartphones, they could influence the behavior of the entire group.
Did we say that the study was relatively free of nefariousness? Sorry about that. At this point, we wouldn’t be surprised if the whole thing was bankrolled by a couple of genetically engineered lab mice from Acme Labs bent on world domination.

Don’t put THAT THERE!
As a doctor, you’ve probably thought, “I can’t believe I have to tell them this” more than once. Warnings that you think are common sense – please don’t try that home remedy, please don’t put that there, please don’t eat that anymore.
Well, here’s a new one for all doctors with female patients out there: Please don’t put a ground-up wasp’s nest into your vagina.
An intrepid ob.gyn. with a large Internet following found that someone has been selling oak galls online as vaginal medicine. Oak galls are little bumps that grow on a tree after the gall wasp lays its larvae. Fun insect-plant behavior! Very bad to put inside your body in any way!
One would think you don’t need to warn patients that tree/wasp paste is not the correct medicine to use on an episiotomy cut, as the Etsy seller suggested.
But with Gwyneth Paltrow out there trying to convince people that purposeful bee stings, healing stickers, and goat milk cleanses are all valid health tips, sometimes the obvious things just need to be spelled out.
Eye of the seagull
Rising up/Back on the beach/Did my time, made my sandwich
Went to the kitchen/Now I’m back on the street
Just a man and his will to surviiiiiiiiiive
It’s the eye of the seagull/It’s the thrill of the fight
Research shows you have to stare them down
When a seagull eyes your sandwich/Don’t let it have a bite
Don’t give in to the eye of the seagull.
That’s just a little ditty for you to sing this summer while enjoying your time on the sand, surrounded by those greedy flying sandwich thieves. Research out of the University of Exeter, England, suggests that staring down approaching seagulls can actually slow them down or even completely deter them from attempting to steal your snacks.
Perhaps the seagulls don’t like being watched while they commit their crimes? Maybe they can’t stand the shame of it all.
Whatever the reason, if you’re assaulted by a flock of seagulls this summer (the birds, not the band), try engaging in a staring contest to keep your food safe.
A gut microbe with a rye smile
Rye is not exactly a huge deal here in the good old U.S. of A., but rye bread happens to be the national food of Finland. So when the Finns say something about rye, it pays to listen.
Investigators at the University of Eastern Finland used metabolomics – which, we discovered the hard way, is the “analysis of metabolites in a biological specimen” and not the Finnish word for a “financial system based on rye bread” – to examine the effects of rye sourdough on the gut microbes of mice and an in vitro gastrointestinal model that mimicked the function of the human gut.
Many compounds found in rye sourdough, such as branched-chain amino acids and amino acid–containing small peptides known to have an impact on insulin metabolism, are processed by gut bacteria before getting absorbed into the body.
The gut microbes of mice fed rye sourdough also produce derivatives of trimethylglycine known as betaine, and at least one of these derivatives reduces the need for oxygen in heart muscle cells, which may protect the heart from ischemia or possibly even enhance its performance, the investigators said.
“The major role played by gut microbes in human health has become more and more evident over the past decades, and this is why gut microbes should be taken very good care of. It’s a good idea to avoid unnecessary antibiotics and feed gut microbes with optimal food, such as rye,” researcher Ville M. Koistinen said in a written statement.
The bottom line? A rye-filled gut microbe is a happy gut microbe. And this comes from Finland, the nation that gave the world “pantsdrunk,” so it must be true. On behalf of the Finns, you’re welcome, world.
This is your brain on smartphones
Hey. Hey, you. Do you want some scientists to install a small device in your brain that uses light and drugs to control your neurons and can be controlled externally by a smartphone? No?
Sounds like a terrible idea only fit for a cheesy science fiction dystopia, you say? Too bad, because a group of researchers from South Korea and the University of Colorado already have invented such a device.
To be fair, their study, published in Nature Biomedical Engineering, is relatively free of nefariousness. The device is meant to be used to search for brain diseases such as Alzheimer’s and Parkinson’s, as well as disorders such as depression, addiction, and pain.
The researchers say that their device is superior to current diagnostic technology available – which uses a similar drug/light combo but is bulkier, stationary, and causes long-term brain damage – because it can be used long term and outside the lab and – we’re sorry – but we’re straying back into dystopia here.
Okay, let’s try again. To test their device, the researchers conducted an animal study and found that, by manipulating the behavior of one animal using the drug/light combo from behind their smartphones, they could influence the behavior of the entire group.
Did we say that the study was relatively free of nefariousness? Sorry about that. At this point, we wouldn’t be surprised if the whole thing was bankrolled by a couple of genetically engineered lab mice from Acme Labs bent on world domination.

Use of Mobile Messaging System for Self-Management of Chemotherapy Symptoms in Patients with Advanced Cancer (FULL)
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
Review of Radiologic Considerations in an Immunocompetent Patient With Primary Central Nervous System Lymphoma (FULL)
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
Central nervous system (CNS) lymphoma can be classified into 2 categories: primary CNS lymphoma (PCNSL), which includes disease limited to brain, eyes, spinal cord; and leptomeninges without coexisting or previous systemic lymphoma. Secondary CNS lymphoma (SCNSL) is essentially metastatic disease from a systemic primary site.1 The focus of this case presentation is PCNSL, with an emphasis on imaging characteristics and differential diagnosis.
The median age at diagnosis for PCNSL is 65 years, and the overall incidence has been decreasing since the mid-1990s, likely related to the increased use of highly-active antiretroviral therapy (HAART) in patients with AIDS.2,3 Although overall incidence has decreased, incidence in the elderly population has increased.4 Historically, PCNSL has been considered an AIDS-defining illness.5 These patients, among other immunocompromised patients, such as those on chronic immunosuppressive therapy, are at a higher risk for developing the malignancy.6
Clinical presentation varies because of the location of CNS involvement and may present with headache, mood or personality disturbances, or focal neurologic deficits. Seizures are less likely due to the tendency of PCNSL to spare gray matter. Initial workup generally includes a head computed tomography (CT) scan, as well as a contrast-enhanced magnetic resonance image (MRI), which may help direct clinicians to the appropriate diagnosis. However, there is significant overlap between the imaging characteristics of PCNSL and numerous other disease processes, including glioblastoma and demyelination. The imaging characteristics of PCNSL are considerably different depending on the patient’s immune status.7
This case illustrates a rare presentation of PCNSL in an immunocompetent patient whose MRI characteristics were seemingly more consistent with those seen in patients with immunodeficiency. The main differential diagnoses and key imaging characteristics, which may help obtain accurate diagnosis, will be discussed.
Case Presentation
A 72-year-old male veteran presented with a 2-month history of subjective weakness in his upper and lower extremities progressing to multiple falls at home. He had no significant medical history other than a thymectomy at age 15 for an enlarged thymus, which per patient report, was benign. An initial laboratory test that included vitamin B12, folate, thyroid-stimulating hormone, complete blood cell count, and comprehensive metabolic panel, were unremarkable, with a white blood cell count of 8.5 K/uL. The initial neurologic evaluation did not show any focal neurologic deficits; however, during the initial hospital stay, the patient developed increasing lower extremity weakness on examination. A noncontrast CT head scan showed extensive nonspecific hypodensities within the periventricular white matter (Figure 1). A contrast-enhanced MRI showed enhancing lesions involving the corpus callosum, left cerebral peduncle, and right temporal lobe (Figures 2, 3, and 4). These lesions also exhibited significant restricted diffusion and a mild amount of surrounding vasogenic edema. The working diagnosis after the MRI included primary CNS lymphoma, multifocal glioblastoma, and tumefactive demyelinating disease. The patient was started on IV steroids and transferred for neurosurgical evaluation and biopsy at an outside hospital. The frontal lesion was biopsied, and the initial frozen section was consistent with lymphoma; a bone marrow biopsy was negative. The workup for immunodeficiency was unremarkable. Pathology revealed high-grade B-cell lymphoma, and the patient began a chemotherapy regimen.
Discussion
The workup of altered mental status, focal neurologic deficits, headaches, or other neurologic conditions often begins with a noncontrast CT scan. On CT, PCNSL generally appears isodense to hyperdense to gray matter, but appearance is variable. The often hyperdense appearance is attributable to the hypercellular nature of lymphoma. Many times, as in this case, CT may show only vague hypodensities, some of which may be associated with surrounding edema. This presentation is nonspecific and may be seen with advancing age due to changes of chronic microvascular ischemia as well as demyelination, other malignancies, and several other disease processes, both benign and malignant. After the initial CT scan, further workup requires evaluation with MRI. PCNSL exhibits restricted diffusion and variable signal intensity on T2-weighted imaging.
PCNSL is frequently centrally located within the periventricular white matter, often within the frontal lobe but can involve other lobes, the basal ganglia, brainstem, cerebellum, or less likely, the spinal canal.7 Contrary to primary CNS disease, secondary lymphoma within the CNS has been described classically as affecting a leptomeningeal (pia and arachnoid mater) distribution two-thirds of the time, with parenchymal involvement occurring in the other one-third of patients. A recent study by Malikova and colleagues found parenchymal involvement may be much more common than previously thought.1 Leptomeningeal spread of disease often involves the cranial nerves, subependymal regions, spinal cord, or spinal nerve roots. Dural involvement in primary or secondary lymphoma is rare.
PCNSL nearly always shows enhancement. Linear enhancement along perivascular spaces is highly characteristic of PCNSL. The typical appearance of PCNSL associated with immunodeficiency varies from that seen in an otherwise immunocompetent patient. Patients with immunodeficiency usually have multifocal involvement, central necrosis leading to a ring enhancement appearance, and have more propensity for spontaneous hemorrhage.7 Immunocompetent patients are less likely to present with multifocal disease and rarely show ring enhancement. Also, spontaneous hemorrhage is rare in immunocompetent patients. In our case, extensive multifocal involvement was present, whereas typically immunocompetent patients will present with a solitary homogeneously enhancing parenchymal mass.
The primary differential for PCNSL includes malignant glioma, tumefactive multiple sclerosis, metastatic disease, and in an immunocompromised patient, toxoplasmosis. The degree of associated vasogenic edema and mass effect is generally lower in PCNSL than that of malignant gliomas and metastasis. Also, PCNSL tends to spare the cerebral cortex.8
Classically, PCNSL, malignant gliomas, and demyelinating disease have been considered the main differential for lesions that cross midline and involve both cerebral hemispheres. Lymphoma generally exhibits more restricted diffusion than malignant gliomas and metastasis, attributable to the highly cellular nature of lymphoma.7 Tumefactive multiple sclerosis is associated with relatively minimal mass effect for lesion size and exhibits less restricted diffusion values when compared to high grade gliomas and PCNSL. One fairly specific finding for tumefactive demyelinating lesions is incomplete rim enhancement.9 Unfortunately, an MRI is not reliable in differentiating these entities, and biopsy is required for definitive diagnosis. Many advancing imaging modalities may help provide the correct diagnosis of PCNSL, including diffusion-weighted and apparent diffusion coefficient imaging, diffusion tensor imaging, MR spectroscopy and PET imaging.7
Conclusion
With the increasing use of HAART, the paradigm of PCNSL is shifting toward one predominantly affecting immunocompetent patients. PCNSL should be considered in any patient with multiple enhancing CNS lesions, regardless of immune status. Several key imaging characteristics may help differentiate PCNSL and other disease processes; however, at this time, biopsy is recommended for definitive diagnosis.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
1. Malikova H, Burghardtova M, Koubska E, Mandys V, Kozak T, Weichet J. Secondary central nervous system lymphoma: spectrum of morphological MRI appearances. Neuropsychiatr Dis Treat. 2018;4:733-740.
2. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro-Oncol. 2012;14(suppl 5):v1-v49.
3. Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106(1):128-135.
4. O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkins lymphoma. Am J Hematol. 2013;88(12):997-1000.
5. [no authors listed]. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(rr-17):1-19.
6. Maiuri F. Central nervous system lymphomas and immunodeficiency. Neurological Research. 1989;11(1):2-5.
7. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2010;32(6):984-992.
8. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: spectrum of findings and differential characteristics. Radiología. 2018;60(4):280-289.
9. Mabray MC, Cohen BA, Villanueva-Meyer JE, et al. Performance of Apparent Diffusion Coefficient Values and Conventional MRI Features in Differentiating Tumefactive Demyelinating Lesions From Primary Brain Neoplasms. American Journal of Roentgenology. 2015;205(5):1075-1085.
Which birth defects are associated with childhood cancer risk?
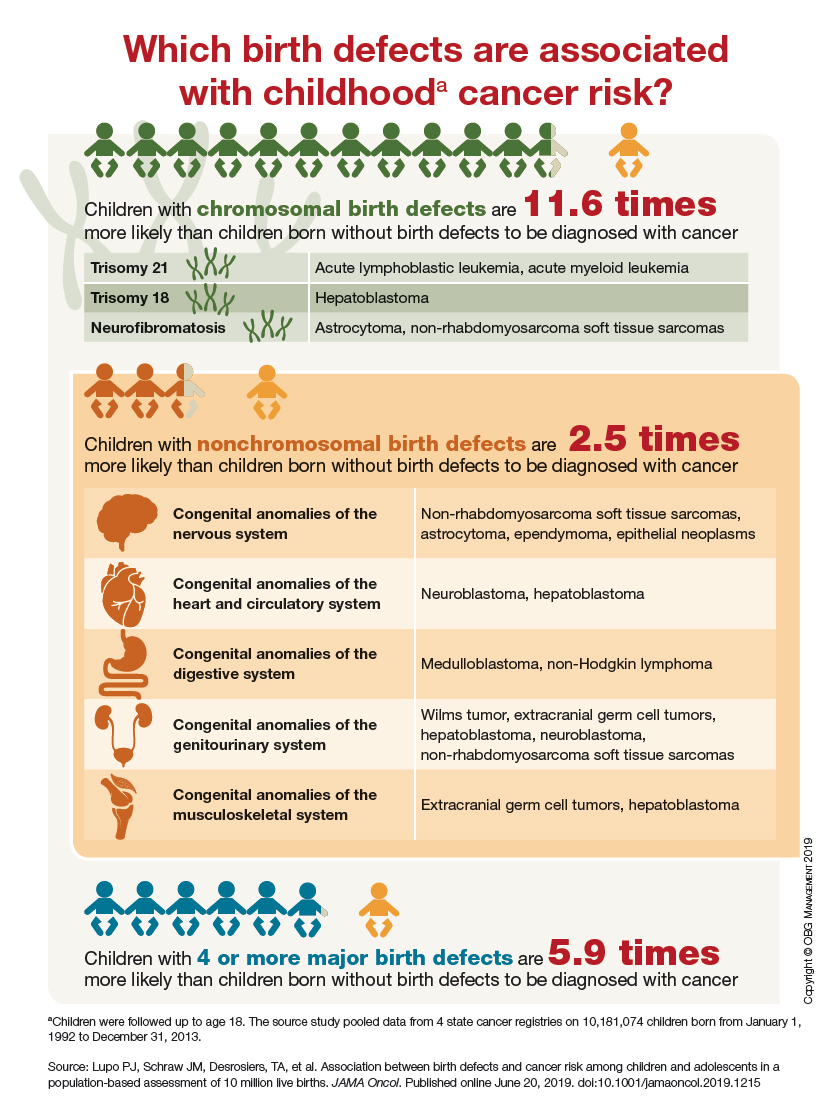


Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer (FULL)
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
T cells and IL-2 drive acute celiac symptoms
CD4+ T-cell reactivation and interleukin (IL)–2 release are responsible for acute gastrointestinal symptoms when patients with celiac disease are exposed to gluten, according to investigators.
Although T cells have been well studied in previous celiac disease research, clinical symptoms after acute gluten exposure have never been linked with specific cytokine changes, reported lead author Gautam Goel, PhD, of Massachusetts General Hospital in Boston, and colleagues.
“If treated [celiac disease] patients, i.e., those following a strict [gluten-free diet], are exposed to gluten-containing food, they typically suffer from gastrointestinal reactions occurring 1 to 2 hours after the gluten exposure,” the investigators wrote in Science Advances. “There is currently no explanation for the acute gluten-induced symptoms seen in treated [celiac disease] patients.”
The current study was prompted by two phase 1 trials involving the therapeutic vaccine Nexvax2, which uses peptide fragments of gluten proteins to desensitize celiac patients to gluten, the investigators explained. During those trials, intradermal injections of Nexvax2 above a certain dose threshold led to gastrointestinal symptoms within 2-5 hours, but not injection site reactions, which would have been indicative of a cutaneous response to recall antigen.
“Our observations from these phase 1 studies led us to hypothesize that cytokine release occurs following natural gluten exposure and could be used to implicate which arms of the immune system drive early symptoms.”
Of the 28 patients in the two trials, all underwent intradermal testing, while 19 also participated in an oral gluten challenge. Following intradermal injection of gluten peptides, patients exhibited gastrointestinal symptoms, along with coordinated elevations of least 15 plasma cytokines; most significantly IL-2, MCP-1, IL-8, IL-10, MIP-1beta, IP-10, and eotaxin. The first cytokines to respond to injection were IL-2 and IL-8, rising within 2 hours, prior to symptoms. At 4 hours, when symptoms were present, peak IL-2 elevations were most dramatic, with a 272-fold elevation, followed by IL-8 (11-fold) and IL-10 (1.2-fold).
“IL-2 is both the earliest and most sensitive marker for the coordinated cytokine release that was almost universal in HLA-DQ2.5 + [celiac disease] patients administered gluten peptides,” the investigators wrote.
Similar to intradermal testing, oral challenge with gluten caused IL-2, IL-8, and IL-10 to elevate within 2 hours, and peak within 4-6 hours. Again, IL-2 was most sensitive, with a 15-fold increase at 4 hours. This increase in IL-2 correlated with IL-8 and IL-10 elevations, although IL-2 increases were at least six times greater than the other two cytokines.
“Together, the serum cytokine profile following gluten ingestion is less prominent but qualitatively similar and over a corresponding time course to that after injecting gluten peptides, which is consistent with activated CD4+ T cells being the driver of cytokine release in both scenarios,” the investigators wrote.
Further testing showed that, after gluten challenge, plasma levels of IL-2, IL-8, and IL-10 negatively correlated with duodenal villous height-to-crypt depth ratios. In addition, high levels of IL-2 correlated with severe nausea and vomiting, adding to the evidence that celiac symptoms were linked with specific cytokine elevations.
“The link between immune activation and symptoms was further strengthened by showing that postdose symptoms and cytokine release were both lessened after three weekly doses and absent after 16 twice-weekly injections of gluten peptides,” the investigators wrote. “These findings are consistent with the difference in severity of symptoms after gluten ingestion compared to gluten peptide injection being related to potency of the antigen challenge and T-cell activation measured by circulating IL-2 concentration at 4 hours.”
Even though IL-2 elevations appeared to drive celiac symptoms, the source of IL-2 was initially unknown. “Activated T cells are the primary source of IL-2, but [dendritic cells] can also secrete IL-2 following ligation of specific pathogen recognition receptors; mast cells also secrete IL-2 following exposure to IL-33 or IL-9,” the investigators explained. Still, CD4+ T cells are known to be key players in celiac disease, and the timing and magnitude of IL-2 release made T cells the most likely candidates. To test this hypothesis, the investigators collected blood from patients 6 days after gluten food challenge and incubated these samples for 24 hours with gluten peptides. Results of this test suggested that gluten-specific CD4+ T cells were the most likely source of IL-2.
The connection between particular cytokines and gastrointestinal symptoms is now supported with evidence; however, the investigators pointed out that a relationship between cytokines and other symptoms of celiac disease remains to be seen. “Whether cytokines elevated in blood after injecting gluten peptides or ingesting gluten have any direct extraintestinal effects is unclear,” the investigators wrote. “Fatigue, headache, and ‘brain fog’ are the commonly reported extraintestinal symptoms in [celiac disease] patients. However, symptoms being focused on the upper gastrointestinal tract suggest that cytokines increased in blood have clinical and immunological effects that selectively affect the tissue from which they originate.”
“Future studies should test whether cytokine concentrations are substantially higher in gut mucosal tissue than in blood after gluten challenge or injection of gluten peptides and determine whether alterations in local cytokine levels are matched by immune and inflammatory cell infiltration,” the investigators wrote.
The study was supported by the University of Chicago Celiac Disease Center and the University of Oslo KG Jebsen Coeliac Disease Research Centre. The investigators reported additional relationships The investigators reported additional relationships with several government and nonprofit organizations. Multiple investigators are employees of ImmusanT, which is developing Nexvax2.
SOURCE: Goel G et al. Science Advances. 2019 Aug 7. doi: 10.1126/sciadv.aaw7756.
CD4+ T-cell reactivation and interleukin (IL)–2 release are responsible for acute gastrointestinal symptoms when patients with celiac disease are exposed to gluten, according to investigators.
Although T cells have been well studied in previous celiac disease research, clinical symptoms after acute gluten exposure have never been linked with specific cytokine changes, reported lead author Gautam Goel, PhD, of Massachusetts General Hospital in Boston, and colleagues.
“If treated [celiac disease] patients, i.e., those following a strict [gluten-free diet], are exposed to gluten-containing food, they typically suffer from gastrointestinal reactions occurring 1 to 2 hours after the gluten exposure,” the investigators wrote in Science Advances. “There is currently no explanation for the acute gluten-induced symptoms seen in treated [celiac disease] patients.”
The current study was prompted by two phase 1 trials involving the therapeutic vaccine Nexvax2, which uses peptide fragments of gluten proteins to desensitize celiac patients to gluten, the investigators explained. During those trials, intradermal injections of Nexvax2 above a certain dose threshold led to gastrointestinal symptoms within 2-5 hours, but not injection site reactions, which would have been indicative of a cutaneous response to recall antigen.
“Our observations from these phase 1 studies led us to hypothesize that cytokine release occurs following natural gluten exposure and could be used to implicate which arms of the immune system drive early symptoms.”
Of the 28 patients in the two trials, all underwent intradermal testing, while 19 also participated in an oral gluten challenge. Following intradermal injection of gluten peptides, patients exhibited gastrointestinal symptoms, along with coordinated elevations of least 15 plasma cytokines; most significantly IL-2, MCP-1, IL-8, IL-10, MIP-1beta, IP-10, and eotaxin. The first cytokines to respond to injection were IL-2 and IL-8, rising within 2 hours, prior to symptoms. At 4 hours, when symptoms were present, peak IL-2 elevations were most dramatic, with a 272-fold elevation, followed by IL-8 (11-fold) and IL-10 (1.2-fold).
“IL-2 is both the earliest and most sensitive marker for the coordinated cytokine release that was almost universal in HLA-DQ2.5 + [celiac disease] patients administered gluten peptides,” the investigators wrote.
Similar to intradermal testing, oral challenge with gluten caused IL-2, IL-8, and IL-10 to elevate within 2 hours, and peak within 4-6 hours. Again, IL-2 was most sensitive, with a 15-fold increase at 4 hours. This increase in IL-2 correlated with IL-8 and IL-10 elevations, although IL-2 increases were at least six times greater than the other two cytokines.
“Together, the serum cytokine profile following gluten ingestion is less prominent but qualitatively similar and over a corresponding time course to that after injecting gluten peptides, which is consistent with activated CD4+ T cells being the driver of cytokine release in both scenarios,” the investigators wrote.
Further testing showed that, after gluten challenge, plasma levels of IL-2, IL-8, and IL-10 negatively correlated with duodenal villous height-to-crypt depth ratios. In addition, high levels of IL-2 correlated with severe nausea and vomiting, adding to the evidence that celiac symptoms were linked with specific cytokine elevations.
“The link between immune activation and symptoms was further strengthened by showing that postdose symptoms and cytokine release were both lessened after three weekly doses and absent after 16 twice-weekly injections of gluten peptides,” the investigators wrote. “These findings are consistent with the difference in severity of symptoms after gluten ingestion compared to gluten peptide injection being related to potency of the antigen challenge and T-cell activation measured by circulating IL-2 concentration at 4 hours.”
Even though IL-2 elevations appeared to drive celiac symptoms, the source of IL-2 was initially unknown. “Activated T cells are the primary source of IL-2, but [dendritic cells] can also secrete IL-2 following ligation of specific pathogen recognition receptors; mast cells also secrete IL-2 following exposure to IL-33 or IL-9,” the investigators explained. Still, CD4+ T cells are known to be key players in celiac disease, and the timing and magnitude of IL-2 release made T cells the most likely candidates. To test this hypothesis, the investigators collected blood from patients 6 days after gluten food challenge and incubated these samples for 24 hours with gluten peptides. Results of this test suggested that gluten-specific CD4+ T cells were the most likely source of IL-2.
The connection between particular cytokines and gastrointestinal symptoms is now supported with evidence; however, the investigators pointed out that a relationship between cytokines and other symptoms of celiac disease remains to be seen. “Whether cytokines elevated in blood after injecting gluten peptides or ingesting gluten have any direct extraintestinal effects is unclear,” the investigators wrote. “Fatigue, headache, and ‘brain fog’ are the commonly reported extraintestinal symptoms in [celiac disease] patients. However, symptoms being focused on the upper gastrointestinal tract suggest that cytokines increased in blood have clinical and immunological effects that selectively affect the tissue from which they originate.”
“Future studies should test whether cytokine concentrations are substantially higher in gut mucosal tissue than in blood after gluten challenge or injection of gluten peptides and determine whether alterations in local cytokine levels are matched by immune and inflammatory cell infiltration,” the investigators wrote.
The study was supported by the University of Chicago Celiac Disease Center and the University of Oslo KG Jebsen Coeliac Disease Research Centre. The investigators reported additional relationships The investigators reported additional relationships with several government and nonprofit organizations. Multiple investigators are employees of ImmusanT, which is developing Nexvax2.
SOURCE: Goel G et al. Science Advances. 2019 Aug 7. doi: 10.1126/sciadv.aaw7756.
CD4+ T-cell reactivation and interleukin (IL)–2 release are responsible for acute gastrointestinal symptoms when patients with celiac disease are exposed to gluten, according to investigators.
Although T cells have been well studied in previous celiac disease research, clinical symptoms after acute gluten exposure have never been linked with specific cytokine changes, reported lead author Gautam Goel, PhD, of Massachusetts General Hospital in Boston, and colleagues.
“If treated [celiac disease] patients, i.e., those following a strict [gluten-free diet], are exposed to gluten-containing food, they typically suffer from gastrointestinal reactions occurring 1 to 2 hours after the gluten exposure,” the investigators wrote in Science Advances. “There is currently no explanation for the acute gluten-induced symptoms seen in treated [celiac disease] patients.”
The current study was prompted by two phase 1 trials involving the therapeutic vaccine Nexvax2, which uses peptide fragments of gluten proteins to desensitize celiac patients to gluten, the investigators explained. During those trials, intradermal injections of Nexvax2 above a certain dose threshold led to gastrointestinal symptoms within 2-5 hours, but not injection site reactions, which would have been indicative of a cutaneous response to recall antigen.
“Our observations from these phase 1 studies led us to hypothesize that cytokine release occurs following natural gluten exposure and could be used to implicate which arms of the immune system drive early symptoms.”
Of the 28 patients in the two trials, all underwent intradermal testing, while 19 also participated in an oral gluten challenge. Following intradermal injection of gluten peptides, patients exhibited gastrointestinal symptoms, along with coordinated elevations of least 15 plasma cytokines; most significantly IL-2, MCP-1, IL-8, IL-10, MIP-1beta, IP-10, and eotaxin. The first cytokines to respond to injection were IL-2 and IL-8, rising within 2 hours, prior to symptoms. At 4 hours, when symptoms were present, peak IL-2 elevations were most dramatic, with a 272-fold elevation, followed by IL-8 (11-fold) and IL-10 (1.2-fold).
“IL-2 is both the earliest and most sensitive marker for the coordinated cytokine release that was almost universal in HLA-DQ2.5 + [celiac disease] patients administered gluten peptides,” the investigators wrote.
Similar to intradermal testing, oral challenge with gluten caused IL-2, IL-8, and IL-10 to elevate within 2 hours, and peak within 4-6 hours. Again, IL-2 was most sensitive, with a 15-fold increase at 4 hours. This increase in IL-2 correlated with IL-8 and IL-10 elevations, although IL-2 increases were at least six times greater than the other two cytokines.
“Together, the serum cytokine profile following gluten ingestion is less prominent but qualitatively similar and over a corresponding time course to that after injecting gluten peptides, which is consistent with activated CD4+ T cells being the driver of cytokine release in both scenarios,” the investigators wrote.
Further testing showed that, after gluten challenge, plasma levels of IL-2, IL-8, and IL-10 negatively correlated with duodenal villous height-to-crypt depth ratios. In addition, high levels of IL-2 correlated with severe nausea and vomiting, adding to the evidence that celiac symptoms were linked with specific cytokine elevations.
“The link between immune activation and symptoms was further strengthened by showing that postdose symptoms and cytokine release were both lessened after three weekly doses and absent after 16 twice-weekly injections of gluten peptides,” the investigators wrote. “These findings are consistent with the difference in severity of symptoms after gluten ingestion compared to gluten peptide injection being related to potency of the antigen challenge and T-cell activation measured by circulating IL-2 concentration at 4 hours.”
Even though IL-2 elevations appeared to drive celiac symptoms, the source of IL-2 was initially unknown. “Activated T cells are the primary source of IL-2, but [dendritic cells] can also secrete IL-2 following ligation of specific pathogen recognition receptors; mast cells also secrete IL-2 following exposure to IL-33 or IL-9,” the investigators explained. Still, CD4+ T cells are known to be key players in celiac disease, and the timing and magnitude of IL-2 release made T cells the most likely candidates. To test this hypothesis, the investigators collected blood from patients 6 days after gluten food challenge and incubated these samples for 24 hours with gluten peptides. Results of this test suggested that gluten-specific CD4+ T cells were the most likely source of IL-2.
The connection between particular cytokines and gastrointestinal symptoms is now supported with evidence; however, the investigators pointed out that a relationship between cytokines and other symptoms of celiac disease remains to be seen. “Whether cytokines elevated in blood after injecting gluten peptides or ingesting gluten have any direct extraintestinal effects is unclear,” the investigators wrote. “Fatigue, headache, and ‘brain fog’ are the commonly reported extraintestinal symptoms in [celiac disease] patients. However, symptoms being focused on the upper gastrointestinal tract suggest that cytokines increased in blood have clinical and immunological effects that selectively affect the tissue from which they originate.”
“Future studies should test whether cytokine concentrations are substantially higher in gut mucosal tissue than in blood after gluten challenge or injection of gluten peptides and determine whether alterations in local cytokine levels are matched by immune and inflammatory cell infiltration,” the investigators wrote.
The study was supported by the University of Chicago Celiac Disease Center and the University of Oslo KG Jebsen Coeliac Disease Research Centre. The investigators reported additional relationships The investigators reported additional relationships with several government and nonprofit organizations. Multiple investigators are employees of ImmusanT, which is developing Nexvax2.
SOURCE: Goel G et al. Science Advances. 2019 Aug 7. doi: 10.1126/sciadv.aaw7756.
FROM SCIENCE ADVANCES
Vitamin D supplementation may improve ulcerative colitis
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
Vitamin D supplementation may lead to significant improvements in ulcerative colitis (UC), based on a placebo-controlled trial involving 60 patients with active disease.
Those who achieved vitamin D levels greater than 40 ng/mL were most likely to benefit, reported lead author Rizwan Ahamed Z, MD, of the Postgraduate Institute of Medical Education and Research in Chandigarh, India, and colleagues. They noted that the findings contribute much-needed clinical data to a largely theoretical subject area.
“[T]he discovery of vitamin D receptors on lymphocytes, monocytes, and dendritic cells initiated various studies which have highlighted the role of vitamin D in regulating gut mucosal immunity and gut barrier,” the investigators wrote in Journal of Clinical Gastroenterology. “In experimental interleukin (IL)-10 knockout mice models, vitamin D deficiency was found to result in severe colitis, progressive wasting, and high mortality. However, vitamin D supplementation not only prevented but also ameliorated symptoms of colitis in the mice model.”
Human studies have revealed similar associations between vitamin D supplementation and inflammatory bowel disease, such as a study by Jørgensen and colleagues that found a lower risk of relapse in Crohn’s disease, and another by Sharifi and colleagues that showed injectable vitamin D could reduce erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with UC. Still, the investigators suggested that more clinical data are needed, particularly for outcomes after vitamin D therapy. In addition to providing such data, the present trial was also the first of its kind to test oral nano vitamin D3, which may have better bioavailability than conventional supplements.
The investigators initially recruited 110 patients with active UC who had an ulcerative colitis disease activity index (UCDAI) of at least 3. After screening, 50 patients were excluded because they had vitamin D levels greater than 40 ng/mL, were already taking a vitamin D supplement, had severe UC requiring hospitalization, or exhibited severe systemic illness. The remaining 60 patients were randomized in a 1:1 ratio to receive either 60,000 IU nano vitamin D3 once daily for 8 days, or placebo. Disease parameters, which were measured at baseline and then again at 4 weeks, included UCDAI, ESR, CRP, and fecal calprotectin. The primary outcome was response, defined as a UCDAI reduction of at least 3 points. Secondary outcome measures included stool frequency, stool consistency, and remission (UCDAI less than 3); in addition, the investigators evaluated histologic, endoscopic, fecal, and serum inflammatory markers.
The majority of patients in the study were men (60%), with a mean age of 36 years. Most patients had moderate UC (73.3%), while smaller proportions had severe (18%) or mild (8%) disease. All patients were taking a 5-aminosalicylic acid oral compound and some (16.6%) were also taking azathioprine. At baseline, the mean vitamin D level was 14 ng/mL. Most patients (70%) were diagnosed with vitamin D deficiency, based on measurements below 20 ng/mL. The remaining patients were diagnosed with insufficiency (13%; 20-30 ng/mL) or suboptimal levels (17%; 30-40 ng/mL).
From baseline to 4-week follow-up, median vitamin D level in the supplement group increased from 15.4 to 40.83 ng/mL, compared with a much smaller increase in the placebo group, from 13.45 to 18.85 ng/mL. Compared with the placebo group, significantly more patients given nano vitamin D3 achieved a UCDAI 3-point reduction (53% vs 13%; P = .001); this translated to a Pearson correlation coefficient (rho) of –0.713, between vitamin D level and UCDAI. Similar, albeit less strong, inverse relationships were detected between vitamin D level and CRP (rho = −0.603) and calprotectin (rho = −0.368).
Benefits observed in the supplement group also extended to stool frequency, stool consistency, and histologic measures. Those who achieved a vitamin D level greater than 40 ng/mL were 4 times more likely to have a UCDAI 3-point reduction than those who did not meet the same criteria (80% vs 20%; P = .038). Independent predictors of response included baseline histologic activity (odds ratio, 1.92), and to a greater extent, vitamin D supplementation (OR, 9.17). No patients achieved remission, which the investigators attributed to the relatively short study duration.
Minor, self-limiting side effects occurred in 13.3% and 10% of patients given the vitamin D supplement and placebo, respectively.
“[T]he present study showed significant improvement in all inflammatory parameters of the disease including clinical, endoscopic, histopathologic, and serum and fecal markers of inflammation, all of which paralleled each other in showing [the benefit of] oral nano vitamin D supplementation,” the investigators concluded. They advised that larger, longer-term studies are needed before the findings can be generalized to all patients with active UC.
The investigators disclosed no external funding or conflicts of interest.
SOURCE: Ahamed R et al. J Clin Gastroenterol. 2019 Jul 24. doi: 10.1097/MCG.0000000000001233.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Hospital slashes S. aureus vancomycin resistance
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: Staphylococcus aureus vancomycin MIC creep is reversible through dedicated antimicrobial stewardship.
Major finding: The prevalence of hGISA in MRSA and MSSA specimens improved from 25% during 2002-2009 to 6% during 2010-2017 at one German tertiary children’s hospital.
Study details: This was a retrospective single-center analysis of vancomycin resistance trends over time in 540 S. aureus specimens gathered in 2002-2017.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.