User login
Mayo Clinic takes honors as top hospital
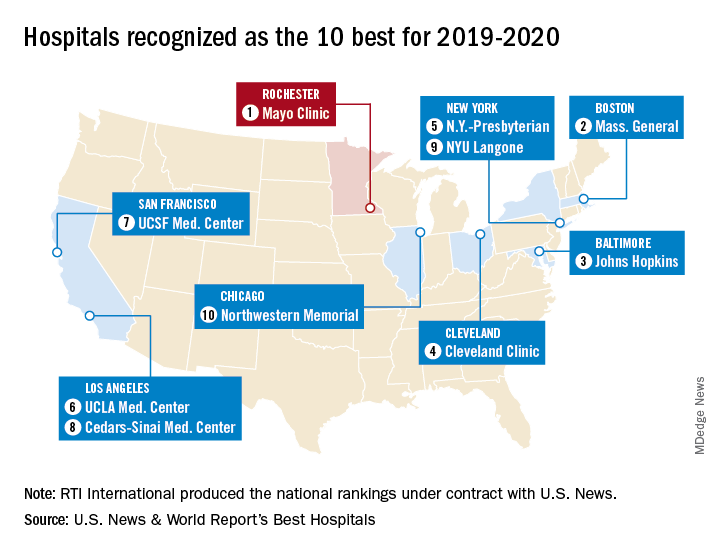
The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
FDA accepts dasotraline NDA for binge-eating disorder
The Food and Drug Administration has accepted the new drug application for dasotraline for the treatment of moderate to severe binge-eating disorder, the drug’s developer, Sunovion, announced July 30.
Dasotraline, a dopamine and norepinephrine reuptake inhibitor, demonstrated significant efficacy in a pair of 12-week, randomized, placebo-controlled studies (SEP360-221 and SEP360-321). The drug also was found to be well tolerated by patients with binge-eating disorder (BED), both in those studies and in a long-term safety study that followed patients for up to a year (SEP360-322).
The medication – characterized by an extended half-life – is to be taken once a day. The most common adverse events reported by patients who took dasotraline include insomnia, dry mouth, decreased appetite, anxiety, nausea, and decreased weight.
BED is more common than any other eating disorder, with an estimated lifetime prevalence among U.S. adults of 1.25% for women and 0.42% for men (CNS Spectr. 2019 Jun 14. doi: 10.1017/S109285291900103). The condition also might run in families. BED often is comorbid with other psychiatric and behavioral disorders, such as depression, substance use, and PTSD, noted Antony Loebel, MD, president and CEO of Sunovion, in a press release. He also said BED often is underrecognized and undertreated.
Meta-analytic reviews show that cognitive-behavioral therapy is considered first-line treatment for BED. However, limited access to such psychological treatments makes the development of medication options such as dasotraline important.
Last year, the agency rejected a new drug application for dasotraline for the treatment of ADHD, citing a need for additional data.
The Food and Drug Administration has accepted the new drug application for dasotraline for the treatment of moderate to severe binge-eating disorder, the drug’s developer, Sunovion, announced July 30.
Dasotraline, a dopamine and norepinephrine reuptake inhibitor, demonstrated significant efficacy in a pair of 12-week, randomized, placebo-controlled studies (SEP360-221 and SEP360-321). The drug also was found to be well tolerated by patients with binge-eating disorder (BED), both in those studies and in a long-term safety study that followed patients for up to a year (SEP360-322).
The medication – characterized by an extended half-life – is to be taken once a day. The most common adverse events reported by patients who took dasotraline include insomnia, dry mouth, decreased appetite, anxiety, nausea, and decreased weight.
BED is more common than any other eating disorder, with an estimated lifetime prevalence among U.S. adults of 1.25% for women and 0.42% for men (CNS Spectr. 2019 Jun 14. doi: 10.1017/S109285291900103). The condition also might run in families. BED often is comorbid with other psychiatric and behavioral disorders, such as depression, substance use, and PTSD, noted Antony Loebel, MD, president and CEO of Sunovion, in a press release. He also said BED often is underrecognized and undertreated.
Meta-analytic reviews show that cognitive-behavioral therapy is considered first-line treatment for BED. However, limited access to such psychological treatments makes the development of medication options such as dasotraline important.
Last year, the agency rejected a new drug application for dasotraline for the treatment of ADHD, citing a need for additional data.
The Food and Drug Administration has accepted the new drug application for dasotraline for the treatment of moderate to severe binge-eating disorder, the drug’s developer, Sunovion, announced July 30.
Dasotraline, a dopamine and norepinephrine reuptake inhibitor, demonstrated significant efficacy in a pair of 12-week, randomized, placebo-controlled studies (SEP360-221 and SEP360-321). The drug also was found to be well tolerated by patients with binge-eating disorder (BED), both in those studies and in a long-term safety study that followed patients for up to a year (SEP360-322).
The medication – characterized by an extended half-life – is to be taken once a day. The most common adverse events reported by patients who took dasotraline include insomnia, dry mouth, decreased appetite, anxiety, nausea, and decreased weight.
BED is more common than any other eating disorder, with an estimated lifetime prevalence among U.S. adults of 1.25% for women and 0.42% for men (CNS Spectr. 2019 Jun 14. doi: 10.1017/S109285291900103). The condition also might run in families. BED often is comorbid with other psychiatric and behavioral disorders, such as depression, substance use, and PTSD, noted Antony Loebel, MD, president and CEO of Sunovion, in a press release. He also said BED often is underrecognized and undertreated.
Meta-analytic reviews show that cognitive-behavioral therapy is considered first-line treatment for BED. However, limited access to such psychological treatments makes the development of medication options such as dasotraline important.
Last year, the agency rejected a new drug application for dasotraline for the treatment of ADHD, citing a need for additional data.
Clues to eczematous cheilitis may lie in the history
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
koakes@mdedge.com
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
koakes@mdedge.com
NEW YORK – , but patients may be slow to seek help, Bethanee Schlosser, MD, PhD, said at the American Academy of Dermatology summer meeting.
One of the challenges in helping patients with lip problems is that lips are constantly in motion and constantly interacting with the outside world, said Dr. Schlosser, of the department of dermatology, Northwestern University, Chicago. There’s ongoing low-level trauma with phonation, eating, drinking, and general environmental exposure, she said. Eczematous cheilitis will present with scaling and erythema of the vermilion lips, with lower lip involvement often more pronounced than symptoms on the upper lip. Fissuring and erosion are sometimes, but not always, present as well.
In addition to flaking and redness, Dr. Schlosser noted that patients will complain of dry lips, irritation, itching, and sometimes tingling.
Sorting out the etiology of eczematous cheilitis requires a thorough history. “Ask about habits, such as lip licking, picking, or biting,” she said. Recent dental work, braces, or other appliances for alignment or temporomandibular joint problems can introduce both mechanical irritation and potential allergens, and even musical instruments can be culprits, such as when an oboe reed causes an allergic reaction.
Personal hygiene products, cosmetics, gum chewing, and candy consumption can be the irritant culprits, noted Dr. Schlosser. Careful questioning of patients and examination of the products used can provide clues, since dyes and pigments in cosmetics and gum may provoke reactions.
History taking should also include questions about tobacco in all forms, marijuana, and prescription medication, which can cause lip problems. And it’s important to ask about skin disease in general, to determine if symptoms are present in other anatomic locations, and to ask about any family history of skin disease, she said.
Endogenous contributors can include true atopic dermatitis, psoriasis, and nutritional deficiencies. Psoriatic cheilitis can have prominent crusting and exfoliation. In a Brazilian study that evaluated patents with cutaneous psoriasis and age-, race-, and sex-matched controls with no history of skin disease, psoriasis was associated with geographic tongue, with an odds ratio of 5.0 (95% CI 1.5-16.8). Geographic stomatitis can also be seen, said Dr. Schlosser. Tongue fissures were also more common among those with psoriasis cheilitis (OR 2.7, 95% confidence interval, 1.3-5.6) in the same study (Med Oral Patol Oral Cir Bucal. 2009 Aug 1;14[8]:e371-5).
For psoriatic cheilitis, looking beyond the lips can help refine the diagnosis, she noted. There may be intra-oral signs or signs of extra-oral involvement, especially on the scalp, ears, and genitalia. Koebnerization may be difficult to detect on the lips, but may be present elsewhere. A family history of psoriasis may also tip the scales toward this diagnosis.
Exogenous causes of eczematous cheilitis are much more common and can include contact with irritants and allergens, factitial cheilitis, and cheilitis medicamentosa, Dr Schlosser pointed out.
Allergic contact dermatitis can come from local exposure (to cosmetics and other personal care items, for example) or from incidental exposures. Components of saliva can become concentrated when saliva dries outside the oral cavity, so for chronic lip lickers, saliva alone can be sufficiently irritating to provoke a cheilitis, Dr. Schlosser said.
Transfer of an irritant or allergen is also possible from other body sites, as when a nail-chewer develops allergic cheilitis from an ingredient in nail polish. Transfer from products used on other facial areas and the hair is also possible, as is “connubial transfer,” when an allergen is transferred from an intimate partner.
Cutaneous patch tests can be helpful in pinpointing the offending agent, or agents, according to Dr. Schlosser. She cited a study of 91 patients (77% of whom were female) who underwent patch testing for eczematous cheilitis. The researchers determined that 45% of patients had allergic contact cheilitis (Int J Dermatol. 2016 Jul;55[7]:e386-91).
The patch testing revealed that fragrances, balsam of Peru (Myroxylon pereirae resin), preservatives, and even metals such as nickel and gold were common allergens. The findings echo those in another database review that showed fragrances, M. pereirae, and nickel as the top three allergens on patch testing for lip cheilitis.
Dr. Schlosser said that the most common offending sources are lipsticks, makeup, other cosmetic products, and moisturizer, which are responsible for 10% or more of reactions.
Whatever the etiology, the treatment of eczematous cheilitis can be divided conceptually into two phases. During the induction phase, use of a low- to mid-potency topical corticosteroid ointment quiets inflammation. Examples include alclometasone 0.05%, desonide 0.05%, fluticasone 0.005%, or triamcinolone 0.1%. “Ointment formulations are preferred,” said Dr. Schlosser, since they won’t dissolve so easily with lip licking and will adhere well to the surface of the vermilion lip.
Next, a topical calcineurin inhibitor such as tacrolimus 0.1% can be used for maintenance. Other topical medications, especially topical anesthetics, should be used with caution, she said.
For psoriatic cheilitis, induction with 5% salicylic acid ointment can be followed by the topical calcineurin inhibitor phase, said Dr. Schlosser.
Dr. Schlosser disclosed financial relationships with Beiersdorf, Decision Support in Medicine, and UpToDate.
koakes@mdedge.com
EXPERT ANALYSIS FROM SUMMER AAD 2019
What’s the best way to sort out red nail discoloration?
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
NEW YORK – Nail discoloration, in all its variety, has a wide differential. And while that differential narrows when a patient presents with concerns about nails with red discoloration, there’s still a long list of diagnoses to consider.
During a nail-focused session at the American Academy of Dermatology summer meeting, Shari Lipner, MD, PhD, took attendees through a presentation-based approach that gets to the root etiology of erythronychia and guides diagnosis and treatment options.
“However, regardless of the etiology, erythronychia shares a common pathogenesis,” said Dr. Lipner, a dermatologist at New York–Presbyterian Hospital and Weil Cornell Medicine. The process begins in the distal nail matrix, resulting in a thin long strip of ventral nail becoming discolored, with the nail bed filling in the concavity. The engorged nail bed also makes the affected nail unit prone to splinter hemorrhages, and the thinned, transparent nail makes the erythema more visible, she explained.
Polydactylous longitudinal erythronychia
For erythronychia affecting several nails, onychotillomania is among the possible causes. This condition “often goes hand in hand with onychophagia,” and trichotillomania, skin-picking, or other self-mutilating disorders may also be present, she said. In both the adult and pediatric population, onychotillomania can accompany psychiatric disorders, including depression and obsessive-compulsive disorder, and may be associated with suicidal ideation, she added.
When onychotillomania is the cause, erythronychia may be accompanied by paronychia, and patients will often have a shortened nail bed and an atrophic nail plate. Dorsal pterygium may also be present.
Dermoscopy can provide some clues that onychotillomania is the culprit, said Dr. Lipner, citing a study that looked at dermoscopic images of 36 cases, which found scales in 94%, absence of the nail plate in 83%, and characteristic wavy lines in 69% (J Am Acad Dermatol. 2018 Oct;79[4]:702-5). Other frequent dermoscopy findings included hemorrhages (64%), crusts (61%), nail bed pigmentation (47%), and speckled dots (39%).
Lichen planus can also affect the nails, with erythronychia among its manifestations, she noted. Though lichen planus is thought of as a disease of middle or older age, usually affecting those aged 50-70 years, “15% of those affected are less than 20 years old,” she said.
The erythronychia of lichen planus is often accompanied by longitudinal riding, splitting, and atrophy of the nail plate, she said. Pterygium can also be present, representing a scar in the nail matrix. Dermatopathology will reveal a patchy, bandlike lichenoid infiltrate, with variable sawtooth hypergranulosis and hyperplasia.
“There’s not much evidence about how to treat lichen planus of the nails,” noted Dr. Lipner. Options can include intralesional corticosteroid injections at the nail matrix, topical corticosteroids, oral methotrexate, and retinoids.
Darier disease, an inherited condition caused by mutations in ATP2A2, has both skin and nail manifestations. Characteristic skin signs include hyperkeratotic papules, cobblestone papules, and palmar pits, she said. When nails are affected – as they are in up to 95% of Darier disease patients – they can have a characteristic “candy cane” appearance, with bands of longitudinal erythronychia alternating with normal-colored nail. The nails can also have V-shaped notching, she added.
Patients with systemic lupus erythematosus can also have longitudinal erythronychia; here, dermoscopy will show the characteristic prominent capillary loops in the proximal nail folds, she said.
Foreign substances such as nail polish and dyes, when they’re the source of erythronychia in one or several nails, can usually be wiped off with alcohol or acetone; also, “the proximal margin of discoloration will follow the same pattern as the nail fold,” said Dr. Lipner.
Localized longitudinal erythronychia
When the red discoloration is limited to a single nail, the differential shifts, said Dr. Lipner. With a hematoma, there’s often a history of trauma, and dermoscopy will show characteristic globules and streaks.
The first clue that erythronychia caused by onychopapilloma can be seen at the lunula: There will often be a comet- or pencil-shaped point to the discoloration in that region. But, she said, “the key is to do dermoscopy at the free edge of the nail plate. In 100 percent of cases, there will be a subungual hyperkeratotic papule” that will solidify the diagnosis.
Histopathology of onychopapillomas – a relatively recently recognized entity – will show nail bed and distal matrix acanthosis, with the distal nail bed showing matrix metaplasia, Dr. Lipner said.
Glomus tumors arise from cells of the glomus body, a specialized vascular apparatus that is involved in temperature regulation. “These structures are abundant in the digits and the subungual region,” said Dr. Lipner, which means that glomus tumors – a benign lesion – may develop subungually. Glomus tumors can be idiopathic, but she added, “think neurofibromatosis type 1 if you see multiple glomus tumors.”
Glomus tumors will present with longitudinal erythronychia, with a distal split of the nail sometimes accompanying the discoloration. A round bluish to gray nodule can also be seen. A thorough history and exam can help solidify the diagnosis, said Dr. Lipner; there’s a characteristic triad of point tenderness with the application of pressure to the nail, pain, and cold hypersensitivity.
Classically, the point tenderness is assessed by Love’s test, which elicits severe pain when the subungual tumor is pressed with a small object like the end of paper clip or a ballpoint pen. Application of a tourniquet to the arm after elevation, termed Hildreth’s test, should alleviate subungual glomus tumor pain, and release of the tourniquet causes an abrupt and marked recurrence of pain. Immersing the patient’s affected hand in cold water also increases glomus tumor pain.
“Imaging can be quite helpful” to confirm a glomus tumor diagnosis, said Dr. Lipner. “X-ray is cheap, and you can see erosions in 50% of patients.” However, she added, doppler ultrasound and magnetic resonance imaging are more sensitive, detecting tumors as small as 2 mm.
“Biopsy with histopathology is the gold standard in making the diagnosis” of glomus tumor, though, she noted. Pathologic examination will show monomorphic cells with small caliber vascular channels.
Malignancy is actually uncommon with erythronychia, though it’s always in the differential diagnosis, she said.
In one case series examining subungual squamous cell carcinomas, common findings included onycholysis and localized hyperkeratosis, along with longitudinal erythronychia. “This may be subtle; splinter hemorrhages can also be present,” noted Dr. Lipner, adding, “If there are any symptoms, or an evolving band, a biopsy is indicated.”
“Even with erythronychia, the presentation can be quite variable,” she said. Nail unit melanoma can count erythronychia among the presenting signs. Clues that should raise suspicion for melanoma can include a wide band and prominent onycholysis, especially distal onycholysis and splintering. Again, she said, “biopsy with histopathology is the gold standard in making the diagnosis”; any symptoms or evidence of an evolving band should trigger a biopsy.
Dr. Lipner had no conflicts of interest relevant to her presentation.
SOURCE: Lipner S. Summer AAD 2019, Presentation F035.
EXPERT ANALYSIS FROM SUMMER AAD 2019
Lumateperone schizophrenia drug seems to hit snag
FDA cancels lumateperone advisory panel
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
FDA cancels lumateperone advisory panel
FDA cancels lumateperone advisory panel
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
U.S. regulators canceled a July 31, 2019, advisory committee about lumateperone, an experimental schizophrenia drug that has had some mixed results in testing.
On July 23, the Food and Drug Administration announced the cancellation of the Psychopharmacologic Drugs Advisory Committee meeting it had previously called for to review the new drug application for lumateperone. The agency said the meeting was canceled because of “new information regarding the application.” The FDA said it was continuing to evaluate the application and would, as needed, announce a future meeting on it.
The developer of lumateperone, Intra-Cellular Therapies, issued its own statement on July 23, noting that a meeting had been scheduled with the FDA “shortly” and that an update would be provided after the meeting. The New York–based firm also said it recently had provided additional information to the FDA to meet agency requests. This information was related to “nonclinical studies.”
“The FDA canceled the advisory committee meeting to allow sufficient time to review this new and any forthcoming information as they continue” to review the new drug application for lumateperone, Intra-Cellular said in the July 23 statement. The company also said there may be an extension of the FDA’s Sept. 27, 2019, target action date on the lumateperone application.
Investors viewed this as bad news. Shares of Intra-Cellular on July 23 dropped from an opening price of $11.90 to a closing one of $8.19. On July 29, they closed at $8.12.
Still, it is unclear how the FDA will decide on the lumateperone application and whether the agency will call another advisory committee meeting on it.
Last year, the FDA accepted the application for lumateperone, a once-daily treatment, Intra-Cellular said. The agency had in 2017 given a fast-track designation to lumateperone for the treatment of schizophrenia.
Lumateperone is the lead product for the company.
On the company’s website, Intra-Cellular says three large randomized, double-blind, placebo-controlled trials have been done for lumateperone as a schizophrenia drug. In two of these studies, results for lumateperone at a 60-mg dose showed a “statistically significant separation from placebo on the primary endpoint, the Positive and Negative Syndrome Scale or PANSS total score.”
In a recent routine filing with the Securities and Exchange Commission, Intra-Cellular said it was having an “ongoing dialogue” with the FDA about lumateperone. The company in 2016 had announced that, in a phase 3 study known as ITI-007-302, lumateperone had not separated from placebo on the primary endpoint, change from baseline on the PANSS total score, in the predefined patient population. The active control for ITI-007-302, risperidone, did separate from placebo.
In the recent SEC filing, Intra-Cellular said the FDA already has confirmed that the results of ITI-007-302 did not preclude the submission of a new drug application.
Intra-Cellular also said “lumateperone was statistically significantly better than risperidone on key safety and tolerability parameters, and exhibited a safety profile similar to placebo” in the 302 study. Lumateperone’s failure to best placebo in the 302 test was “in part due to an unusually high placebo response at certain sites.”
Pantoprazole not needed for most patients on anticoagulant/antiplatelet therapies
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
For most patients taking antiplatelet and/or anticoagulant therapies, the proton pump inhibitor (PPI) pantoprazole is unnecessary, based on findings from the prospective COMPASS trial, which involved more than 17,000 participants.
Pantoprazole may reduce the risk of bleeding from gastroduodenal lesions, but it is unlikely to prevent upper-gastrointestinal events, reported lead author Paul Moayyedi, MB ChB, PhD, of McMaster University in Hamilton, Canada, and colleagues.
The investigators wrote in Gastroenterology, “Guidelines suggest that patients receiving the combination of antiplatelet and anticoagulant therapy should receive PPIs to reduce the risk of upper-GI bleeding. However … there are no randomized data to support the use of PPI therapy in patients taking oral anticoagulants, and a paucity of data relating to aspirin.”
To fill this knowledge gap, the investigators recruited 17,598 participants from 33 countries who had stable peripheral artery disease and cardiovascular disease. Participants were randomized to one of three groups: 100-mg aspirin once daily, 5-mg rivaroxaban twice daily, or a combination of 2.5-mg rivaroxaban twice daily with 100-mg aspirin once daily. This part of the trial was discontinued before completion because of early cardiovascular advantages associated with combination therapy over aspirin alone, and related findings were reported previously. While combination therapy did reduce cardiovascular risks, it had less favorable effects on gut health, highlighted by an associated increase in major GI bleeding events. Despite early cessation of the cardiovascular portion of the trial, the pantoprazole regimen was continued, offering a look at the effect of long-term PPI use on gut health.
At baseline, about two-thirds of participants (64%) were not taking a PPI, requiring randomization to either 40-mg pantoprazole once daily or matching placebo. The primary efficacy outcome was time to first upper-GI clinical event, defined as a composite of the following: upper-GI obstruction, perforation, at least five gastroduodenal erosions with at least 3 days of GI pain, symptomatic gastroduodenal ulcer involving at least 3 days of GI pain, overt upper-GI bleeding of unknown origin, occult bleeding (drop in hemoglobin of at least 2 g/dL), overt bleeding with a gastroduodenal lesion (active bleeding during endoscopy), or a symptomatic gastroduodenal ulcer involving at least 3 days of GI pain. In addition to this measure, the investigators evaluated a post-hoc endpoint with a looser definition of peptic ulcer events, most notably eliminating the requirement that a lesion be actively bleeding during endoscopy.
Most patients in the trial (78%) were male, and 23% were current smokers. Smaller proportions of the population were taking a nonsteroidal anti-inflammatory drug (5%) and/or had a history of peptic ulcer disease (2.6%). The median follow-up was 3.01 years, ranging from 2.49 to 3.59 years. Permanent discontinuations occurred at approximately equal rates in the pantoprazole (21%) and placebo (22%) group, after a median of 11 months (338 days). In both groups, more than 96% of participants who continued treatment took their medications as prescribed at least 80% of the time.
Analysis showed that upper-GI events occurred marginally less often in the pantoprazole group than the placebo group, but without statistical significance (1.2% vs. 1.3%; P = .35). Of the outcomes measured, only overt bleeding of gastroduodenal origin detected by radiography or endoscopy was statistically less common in the pantoprazole group than the placebo group, with a 48% reduced rate (0.2% vs. 0.4%; P = .03). No statistical efficacy differences or statistical interactions were detected between population subgroups.
“The data suggest that routine use of PPI therapy is not warranted for patients receiving low-dose rivaroxaban with or without aspirin for the prevention of atherothrombotic events in patients with stable coronary artery disease or symptomatic peripheral artery disease, as there was no overall impact on clinical upper-GI events or upper-GI bleeding,” the investigators wrote. “This is in contrast to previous systematic reviews of randomized trials reporting that PPIs were associated with a 50%-70% reduction in bleeding and symptomatic peptic ulcers related to nonsteroidal anti-inflammatory drugs, including in the critical care setting.”
Post-hoc analysis, which allowed for a broader definition of upper-GI events related to gastroduodenal ulcers, revealed a slightly greater reduction in risk of bleeding lesions in patients taking pantoprazole, compared with placebo (hazard ratio, 0.45), and additional risk reductions for peptic ulcers (HR, 0.46) and erosions (HR, 0.33). Ultimately, pantoprazole reduced the combined rate of post-hoc events by 56%.
The investigators noted that these ulcer- and erosion-reducing effects of pantoprazole align with previous reports. “It is therefore possible that PPIs might be beneficial for patients at particularly high risk for peptic ulcer disease who are also taking aspirin and/or anticoagulants,” the investigators concluded.
The COMPASS trial was funded by Bayer AG. The investigators disclosed additional relationships with Allergan, Takeda, Janssen, and others.
SOURCE: Moayyedi P et al. Gastro. 2019 May 2. doi: 10.1053/j.gastro.2019.04.041.
FROM GASTROENTEROLOGY
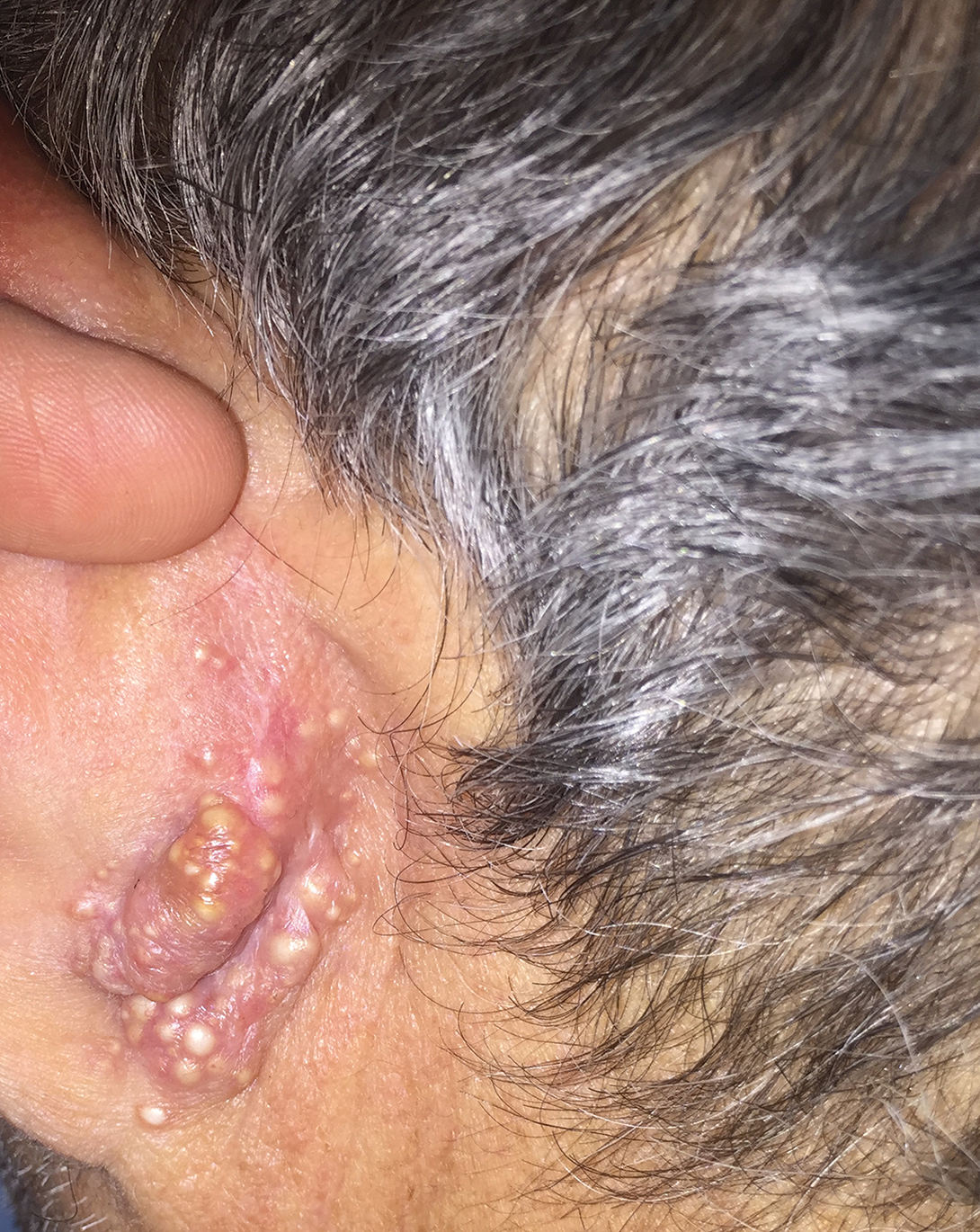
Rapidly Growing Retroauricular Tumor
The Diagnosis: Milia En Plaque
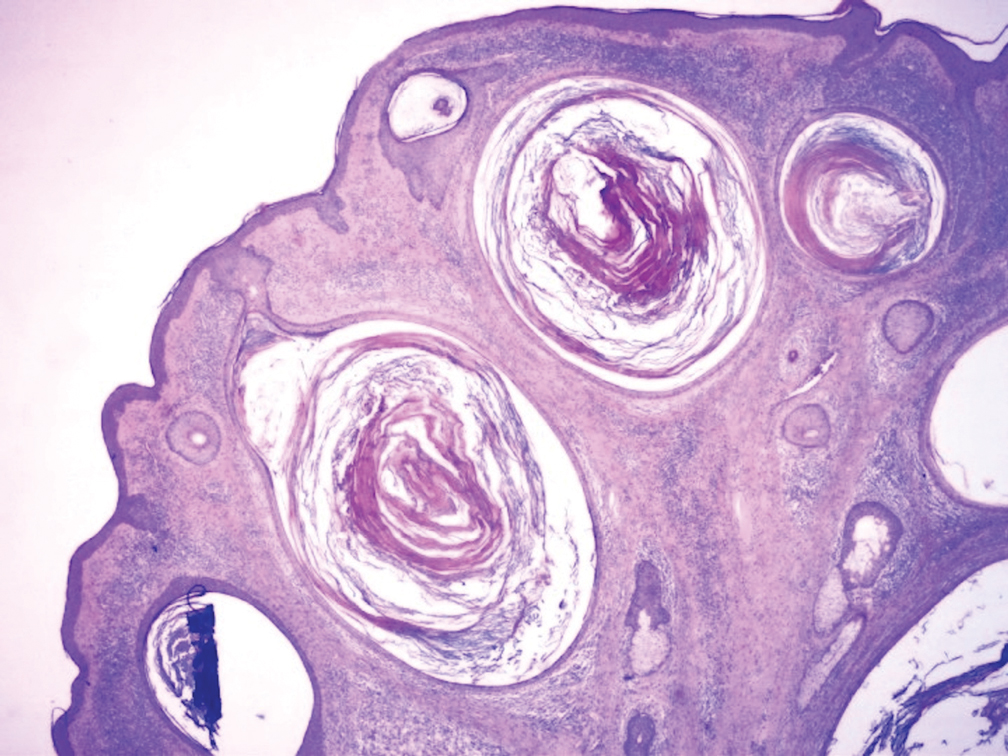
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
A 72-year-old man with a history of hypertension presented with a rapidly growing left retroauricular tumor of 3 months' duration. When manipulated, whitish material with a foul-smelling odor was expressed from the lesion. Physical examination showed an erythematous 3.2 ×1-cm tumor on the left posterior ear with multiple 1- to 2-mm white-yellow papules on its surface. A biopsy of the lesion was performed.
Don’t Mix Off-label Use With Off-the-rack Pills
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
Short Takes
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Acid-suppressing drug use associated with increased antiallergy drug use
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
Gastric acid–suppressing medications such as proton pump inhibitors are associated with a significant increase in subsequent antiallergy medication use, particularly in older individuals.
In a population-based study of health insurance data from 8.2 million people, Austrian researchers looked for prescriptions of gastric acid inhibitors, antiallergy drugs, or other commonly prescribed (lipid-modifying and antihypertensive) drugs as controls from 2009-2013.
According to results published in Nature Communications, gastric acid–suppressing drugs were associated with an overall 96% higher rate of subsequent prescriptions for antiallergy medications than among the general population not taking gastric acid–suppressing drugs (P less than .001). Among individuals aged 60 years or older, the rate of allergy medication prescriptions after acid-suppressing treatment was more than five times higher than that in the general population.
The rate of antiallergy medication use after acid-suppressing medication prescription was threefold higher than the rate seen after lipid-modifying or antihypertensive drug prescription.
“Our findings confirm an epidemiological association between gastric acid suppression and development of allergic symptoms, in line with previous mechanistic animal trials and human observational studies,” wrote Galateja Jordakieva, PhD, of the department of physical medicine, rehabilitation, and occupational medicine at the Medical University of Vienna, and coauthors.
All groups of acid-inhibiting medications were associated with a higher rate of subsequent antiallergy medication prescriptions. The only exception was prostaglandin E2 medications, but the authors said that here the numbers were too low to draw conclusions.
The hazard rate for antiallergy medications also increased with increasing numbers of daily doses of acid-suppressing medication, the study showed. The hazard rate for the lowest quartile – up to 20 daily doses per year – was a significant 28% higher than that of the general population, while the third quartile (68-213 daily dose per year) was associated with a 2.67-fold higher hazard. A similar increase was seen for the fourth quartile of acid suppression–medication dosing.
The authors established that just six daily doses of acid-suppressing drugs in a year were associated with significantly earlier prescriptions of antiallergy medication.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, emphasized in an associated commentary that the findings reflect association, not causation. “There are many possible explanations for the observed association ... In fact, the data make other explanations more than likely to be the cause of allergies.”
Coprescriptions of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) are common among PPI users and “are among the drugs that are very well known to increase the risk of an allergic reaction.” The data show that antiallergy medicines are prescribed at a relatively similar rate at all ages, while PPI prescribing increases in the elderly.
“If the rate of prescription of PPIs shows a steep rise with age and they are a significant cause of allergies, then the anti-allergy medicines ... should also show a steep rise with age. The fact that they don’t, either means they show no relation or that any relation has a minimal effect on allergies,” Dr. Evans said. “Nearly all drugs can have very rare allergic reactions, including PPIs, but this paper does not help to show what the true rate is of these very rare reactions, or whether they are caused by PPIs alone. The design and analysis methods in the paper are likely to exaggerate their apparent occurrence.”
The study was supported by Burgenländische Gebietskrankenkasse and the Austrian Science Fund. No conflicts of interest were declared.
SOURCE: Jordakieva G et al. Nat Commun. 2019 Jul 30. doi: 10.1038/s41467-019-10914-6.
FROM NATURE COMMUNICATIONS