User login
ASTRO announces new president-elect, board members
The American Society for Radiation Oncology (ASTRO) has elected a new president-elect and three new officers to its board of directors.
The incoming president-elect is Laura Dawson, MD, of Princess Margaret Cancer Centre and University of Toronto. Dr. Dawson is a professor and radiation oncologist specializing in gastrointestinal malignancies with a focus on hepatobiliary carcinoma and liver metastases, stereotactic body radiation therapy, image-guided radiation therapy, and normal tissue radiation toxicity.
Dr. Dawson will begin her term in September 2019 during ASTRO’s 61st annual meeting. She will serve a 1-year term as president-elect, a 1-year term as president, and a 1-year term as chair of the ASTRO board. During her tenure, Dr. Dawson plans to address issues such as physician burnout, restrictive prior authorization practices, and diversity in the workforce.
Neha Vapiwala, MD, is ASTRO’s new secretary/treasurer-elect. Dr. Vapiwala is an associate professor, vice-chair of education in the department of radiation oncology, and dean of admissions at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
She specializes in the management of patients with genitourinary cancers, and her research is focused on improving the delivery of photon- and proton-based radiation.
Dr. Vapiwala will serve a 1-year term as ASTRO’s secretary/treasurer-elect followed by a 3-year term as secretary/treasurer. In these roles, she plans to address challenges in health care economics and assess the role of artificial intelligence and other new technologies in radiation oncology.
Constantine Mantz, MD, is ASTRO’s new Health Policy Council vice-chair. Dr. Mantz is chief policy officer and a radiation oncologist at 21st Century Oncology in Ft. Meyers, Fla.
He specializes in head and neck, prostate, breast, lung, and colorectal cancers.
Dr. Mantz will serve a 2-year term as vice-chair of the Health Policy Council followed by a 2-year term as chair. Dr. Mantz plans to address payment reform issues, including the implementation of an alternative payment model for radiation oncology.
Brian Marples, PhD, is ASTRO’s new Science Council vice-chair. Dr. Marples is a research professor and director of radiobiology in the department of radiation oncology at the University of Miami Miller School of Medicine. His research is focused on maximizing a tumor’s response to radiation while minimizing damage to normal tissue.
Dr. Marples will serve a 2-year term as vice-chair of the Science Council, followed by a 2-year term as chair. He plans to engage the medical community and the public in radiation oncology innovations, such as new technologies that combine radiation and immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
The American Society for Radiation Oncology (ASTRO) has elected a new president-elect and three new officers to its board of directors.
The incoming president-elect is Laura Dawson, MD, of Princess Margaret Cancer Centre and University of Toronto. Dr. Dawson is a professor and radiation oncologist specializing in gastrointestinal malignancies with a focus on hepatobiliary carcinoma and liver metastases, stereotactic body radiation therapy, image-guided radiation therapy, and normal tissue radiation toxicity.
Dr. Dawson will begin her term in September 2019 during ASTRO’s 61st annual meeting. She will serve a 1-year term as president-elect, a 1-year term as president, and a 1-year term as chair of the ASTRO board. During her tenure, Dr. Dawson plans to address issues such as physician burnout, restrictive prior authorization practices, and diversity in the workforce.
Neha Vapiwala, MD, is ASTRO’s new secretary/treasurer-elect. Dr. Vapiwala is an associate professor, vice-chair of education in the department of radiation oncology, and dean of admissions at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
She specializes in the management of patients with genitourinary cancers, and her research is focused on improving the delivery of photon- and proton-based radiation.
Dr. Vapiwala will serve a 1-year term as ASTRO’s secretary/treasurer-elect followed by a 3-year term as secretary/treasurer. In these roles, she plans to address challenges in health care economics and assess the role of artificial intelligence and other new technologies in radiation oncology.
Constantine Mantz, MD, is ASTRO’s new Health Policy Council vice-chair. Dr. Mantz is chief policy officer and a radiation oncologist at 21st Century Oncology in Ft. Meyers, Fla.
He specializes in head and neck, prostate, breast, lung, and colorectal cancers.
Dr. Mantz will serve a 2-year term as vice-chair of the Health Policy Council followed by a 2-year term as chair. Dr. Mantz plans to address payment reform issues, including the implementation of an alternative payment model for radiation oncology.
Brian Marples, PhD, is ASTRO’s new Science Council vice-chair. Dr. Marples is a research professor and director of radiobiology in the department of radiation oncology at the University of Miami Miller School of Medicine. His research is focused on maximizing a tumor’s response to radiation while minimizing damage to normal tissue.
Dr. Marples will serve a 2-year term as vice-chair of the Science Council, followed by a 2-year term as chair. He plans to engage the medical community and the public in radiation oncology innovations, such as new technologies that combine radiation and immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
The American Society for Radiation Oncology (ASTRO) has elected a new president-elect and three new officers to its board of directors.
The incoming president-elect is Laura Dawson, MD, of Princess Margaret Cancer Centre and University of Toronto. Dr. Dawson is a professor and radiation oncologist specializing in gastrointestinal malignancies with a focus on hepatobiliary carcinoma and liver metastases, stereotactic body radiation therapy, image-guided radiation therapy, and normal tissue radiation toxicity.
Dr. Dawson will begin her term in September 2019 during ASTRO’s 61st annual meeting. She will serve a 1-year term as president-elect, a 1-year term as president, and a 1-year term as chair of the ASTRO board. During her tenure, Dr. Dawson plans to address issues such as physician burnout, restrictive prior authorization practices, and diversity in the workforce.
Neha Vapiwala, MD, is ASTRO’s new secretary/treasurer-elect. Dr. Vapiwala is an associate professor, vice-chair of education in the department of radiation oncology, and dean of admissions at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
She specializes in the management of patients with genitourinary cancers, and her research is focused on improving the delivery of photon- and proton-based radiation.
Dr. Vapiwala will serve a 1-year term as ASTRO’s secretary/treasurer-elect followed by a 3-year term as secretary/treasurer. In these roles, she plans to address challenges in health care economics and assess the role of artificial intelligence and other new technologies in radiation oncology.
Constantine Mantz, MD, is ASTRO’s new Health Policy Council vice-chair. Dr. Mantz is chief policy officer and a radiation oncologist at 21st Century Oncology in Ft. Meyers, Fla.
He specializes in head and neck, prostate, breast, lung, and colorectal cancers.
Dr. Mantz will serve a 2-year term as vice-chair of the Health Policy Council followed by a 2-year term as chair. Dr. Mantz plans to address payment reform issues, including the implementation of an alternative payment model for radiation oncology.
Brian Marples, PhD, is ASTRO’s new Science Council vice-chair. Dr. Marples is a research professor and director of radiobiology in the department of radiation oncology at the University of Miami Miller School of Medicine. His research is focused on maximizing a tumor’s response to radiation while minimizing damage to normal tissue.
Dr. Marples will serve a 2-year term as vice-chair of the Science Council, followed by a 2-year term as chair. He plans to engage the medical community and the public in radiation oncology innovations, such as new technologies that combine radiation and immunotherapy.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Most patients with new hypertension under revised BP guidelines won’t need pharmacotherapy
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – An additional 14% of Americans have been reclassified as having hypertension – an increase from 32% to 46% of adults – under the latest guidelines on management of high blood pressure released by the American College of Cardiology and American Heart Association in 2017. However, this does not mean these patients are mandated for pharmacologic therapy, since most of them have been reclassified as having stage 1 hypertension, said Leslie L. Davis, PhD, RN, ANP-BC, FPCNA, FAANP, FAHA, associate professor of nursing at the University of North Carolina, Greensboro.
According to the new guidelines, normal BP is classified as less than 120 mm Hg systolic and less than 80 mm Hg diastolic. Elevated BP is 120-129 mm Hg systolic and under 80 mm Hg diastolic, while patients are classified as having stage 1 hypertension if their systolic BP is 130-139 mm Hg or diastolic BP is 80-90 mm Hg. Patients now have stage 2 hypertension if their systolic BP is higher than 140 mm Hg or diastolic BP is above 90 mm Hg.
This raises the importance of getting an accurate BP measurement from patients. At least two readings over two or more visits should be used before categorizing a patient. To take an ideal reading, patients should be sitting at rest with their back supported, feet positioned on a flat surface (no crossing legs), and their arm at heart level for at least 5 minutes. Patients should also refrain from tobacco or caffeine use 30 minutes before the reading. “These numbers need to be correct, because we’ve got new [BP] categories, and also for managing blood pressure, you’re making decisions based on these numbers,” said Dr. Davis at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
When taking a patient’s BP, neither the patient nor the provider should talk, and constricting clothes on the upper extremity should be removed instead of pushed up. Using a incorrectly sized cuff can artificially raise or lower a patient’s BP level, so clinicians should use one that is 80% of the length and 40% of the width of the patient’s arm circumference. If the BP reading is elevated, confirm the reading in the other arm and use the arm with the higher reading for future measurements. BP measurements taken in different settings, such as ambulatory BP monitoring or home BP monitoring, can help give context to the in-office reading and whether the patient has white-coat hypertension or masked hypertension.
After a patient has their accurate BP reading, they can calculate their atherosclerotic cardiovascular disease (ASCVD) risk using the ACC’s ASCVD Risk Estimator, said Dr. Davis. If a patient has confirmed cardiovascular disease or has a 10% or greater 10-year ASCVD risk, the target to lower BP is 130 based on high-quality evidence, but expert opinion recommends targeting 130/80 for patients with confirmed hypertension as well.
“If somebody is overweight or obese, 10 pounds is 10 points,” said Dr. Davis. “Even if you don’t get them to the appropriate body mass index over 3 months’ time, that’s as much as a low or medium dose of antihypertensive therapy. For you to be able to get double-digit reduction, that’s what a med does.”
Other nonpharmacologic interventions for these patients include a heart-healthy diet such as the DASH diet, lowering sodium and increasing potassium, structuring exercise and physical activity, lowering use of or avoiding alcohol, and smoking cessation. The goal of nonpharmacologic therapy is not only to lower BP, but make the medication work better, said Dr. Davis.
Pharmacologic therapy should be initiated when patients exceed or are above the cutoff values for the new BP categories and if a patient has already had a cardiovascular event. “Basically, if you’re above that line in the sand of your goal, that’s when to start medications,” she said.
For stage 1 hypertension, first-line therapy is lifestyle change plus thiazides, calcium-channel blockers, or ACE inhibitors, with stage 1 hypertension therapy consisting of a combination of two first-line therapy therapies to reduce systolic BP by about 20 mm Hg and diastolic BP by 10 mm Hg. Beta-blockers are not first-line antihypertension therapy, but can be considered in patients with coronary artery disease and heart failure with reduced left ventricular ejection fraction.
With regard to follow-up, patients with low ASCVD risk and stage 1 hypertension can be monitored in 3-6 months after lifestyle changes, while patients with high ASCVD risk and stage 1 hypertension should be followed up in 1 month. Patients with stage 2 hypertension should follow up with their primary care provider 1 month after beginning their therapy, and those with very high BP should promptly be started on drug treatment with lifestyle changes, with upward dose adjustments as needed.
In adults aged 65 years or older, the ACC/AHA guidelines also focused on how to prevent cognitive decline and dementia, said Dr. Davis. The goal for ambulatory, community-dwelling adults is still to have a systolic BP of less than 130 mm Hg, but clinical judgment should prevail because of comorbid conditions and limited life expectancy in these patients. Patient preference should also be considered, and clinicians should use a team-based approach with shared decision making to determine goals for each patient.
Dr. Davis reported no relevant financial disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
AGA remembers Dr. Henry T. Lynch
Henry T. Lynch, MD, came from a humble background, growing up in a rough neighborhood in New York City. He enlisted in the Navy and served in the South Pacific during World War II. Afterward, Dr. Lynch focused his efforts on completing his education, which eventually lead him to the medical field.
After obtaining his high-school equivalency, and completing his undergraduate degree at the University of Oklahoma and his master’s degree in clinical psychology at the University of Denver, his path turned toward the field in which he would make his thrilling and unprecedented discoveries. He studied for a PhD in human genetics at the University of Texas at Austin and received his medical degree from the University of Texas Medical Branch in Galveston. He completed his internship at St. Mary’s Hospital in Evansville, Indiana, and his residency in internal medicine at the University of Nebraska College of Medicine. His first faculty appointment was at The University of Texas MD Anderson Cancer Center.
In 1967, he accepted a position at Creighton, in Omaha, Neb., where he would spend the rest of his storied career. Dr. Lynch was a professor at Creighton University School of Medicine, and the founder and director of the Hereditary Cancer Center at Creighton, established in 1984. He served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research at Creighton.
A patient he encountered in 1962 – an alcoholic that drank because he believed he would die of colon cancer since everyone in his family had – was the catalyst for his groundbreaking work into the possibility of a hereditary component to some forms of cancer. During this time, it was understood that carcinogenic chemicals and viruses were the primary cause of cancer.
Dr. Lynch provided the first complete description of hereditary nonpolyposis colorectal cancer, a form of colon cancer eventually renamed Lynch syndrome. He continued his research, eventually identifying a hereditary form of breast and ovarian cancers, melanoma, and prostate and pancreatic cancers. His efforts also resulted in one of the world’s largest databases of family cancer histories.
Dr. Lynch passed away on June 2, 2019, at the age of 91. AGA members are sharing their stories and the impact Dr. Lynch had on their work in the AGA Community.
Lucas Franki contributed to this report.
Henry T. Lynch, MD, came from a humble background, growing up in a rough neighborhood in New York City. He enlisted in the Navy and served in the South Pacific during World War II. Afterward, Dr. Lynch focused his efforts on completing his education, which eventually lead him to the medical field.
After obtaining his high-school equivalency, and completing his undergraduate degree at the University of Oklahoma and his master’s degree in clinical psychology at the University of Denver, his path turned toward the field in which he would make his thrilling and unprecedented discoveries. He studied for a PhD in human genetics at the University of Texas at Austin and received his medical degree from the University of Texas Medical Branch in Galveston. He completed his internship at St. Mary’s Hospital in Evansville, Indiana, and his residency in internal medicine at the University of Nebraska College of Medicine. His first faculty appointment was at The University of Texas MD Anderson Cancer Center.
In 1967, he accepted a position at Creighton, in Omaha, Neb., where he would spend the rest of his storied career. Dr. Lynch was a professor at Creighton University School of Medicine, and the founder and director of the Hereditary Cancer Center at Creighton, established in 1984. He served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research at Creighton.
A patient he encountered in 1962 – an alcoholic that drank because he believed he would die of colon cancer since everyone in his family had – was the catalyst for his groundbreaking work into the possibility of a hereditary component to some forms of cancer. During this time, it was understood that carcinogenic chemicals and viruses were the primary cause of cancer.
Dr. Lynch provided the first complete description of hereditary nonpolyposis colorectal cancer, a form of colon cancer eventually renamed Lynch syndrome. He continued his research, eventually identifying a hereditary form of breast and ovarian cancers, melanoma, and prostate and pancreatic cancers. His efforts also resulted in one of the world’s largest databases of family cancer histories.
Dr. Lynch passed away on June 2, 2019, at the age of 91. AGA members are sharing their stories and the impact Dr. Lynch had on their work in the AGA Community.
Lucas Franki contributed to this report.
Henry T. Lynch, MD, came from a humble background, growing up in a rough neighborhood in New York City. He enlisted in the Navy and served in the South Pacific during World War II. Afterward, Dr. Lynch focused his efforts on completing his education, which eventually lead him to the medical field.
After obtaining his high-school equivalency, and completing his undergraduate degree at the University of Oklahoma and his master’s degree in clinical psychology at the University of Denver, his path turned toward the field in which he would make his thrilling and unprecedented discoveries. He studied for a PhD in human genetics at the University of Texas at Austin and received his medical degree from the University of Texas Medical Branch in Galveston. He completed his internship at St. Mary’s Hospital in Evansville, Indiana, and his residency in internal medicine at the University of Nebraska College of Medicine. His first faculty appointment was at The University of Texas MD Anderson Cancer Center.
In 1967, he accepted a position at Creighton, in Omaha, Neb., where he would spend the rest of his storied career. Dr. Lynch was a professor at Creighton University School of Medicine, and the founder and director of the Hereditary Cancer Center at Creighton, established in 1984. He served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research at Creighton.
A patient he encountered in 1962 – an alcoholic that drank because he believed he would die of colon cancer since everyone in his family had – was the catalyst for his groundbreaking work into the possibility of a hereditary component to some forms of cancer. During this time, it was understood that carcinogenic chemicals and viruses were the primary cause of cancer.
Dr. Lynch provided the first complete description of hereditary nonpolyposis colorectal cancer, a form of colon cancer eventually renamed Lynch syndrome. He continued his research, eventually identifying a hereditary form of breast and ovarian cancers, melanoma, and prostate and pancreatic cancers. His efforts also resulted in one of the world’s largest databases of family cancer histories.
Dr. Lynch passed away on June 2, 2019, at the age of 91. AGA members are sharing their stories and the impact Dr. Lynch had on their work in the AGA Community.
Lucas Franki contributed to this report.
Top AGA Community patient cases
Physicians with difficult patient scenarios bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, Infliximab and liver abscess
(http://ow.ly/MehK30p2UZr)
2. Positive Cologuard testing in patient on blood thinners
(http://ow.ly/lJXF30p2V12)
3. Recombinant zoster vaccine in IBD patients on biologics
(http://ow.ly/FWGA30p2V1F)
4. Hair loss and Crohn’s disease
(http://ow.ly/C6Sa30p2V2h)
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, Infliximab and liver abscess
(http://ow.ly/MehK30p2UZr)
2. Positive Cologuard testing in patient on blood thinners
(http://ow.ly/lJXF30p2V12)
3. Recombinant zoster vaccine in IBD patients on biologics
(http://ow.ly/FWGA30p2V1F)
4. Hair loss and Crohn’s disease
(http://ow.ly/C6Sa30p2V2h)
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios bring their questions to the AGA Community (https://community.gastro.org) to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Crohn’s disease, Infliximab and liver abscess
(http://ow.ly/MehK30p2UZr)
2. Positive Cologuard testing in patient on blood thinners
(http://ow.ly/lJXF30p2V12)
3. Recombinant zoster vaccine in IBD patients on biologics
(http://ow.ly/FWGA30p2V1F)
4. Hair loss and Crohn’s disease
(http://ow.ly/C6Sa30p2V2h)
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Remember the AGA Research Foundation in your will or living trust
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence. By including a gift to the AGA Research Foundation in your will, you can support our mission tomorrow without giving away any of your assets today.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- Versatile. You can give a specific item, a set amount of money or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org/.
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence. By including a gift to the AGA Research Foundation in your will, you can support our mission tomorrow without giving away any of your assets today.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- Versatile. You can give a specific item, a set amount of money or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org/.
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence. By including a gift to the AGA Research Foundation in your will, you can support our mission tomorrow without giving away any of your assets today.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- Versatile. You can give a specific item, a set amount of money or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org/.
Consider adding chemical peels for your acne patients
MILAN – according to Dee Anna Glaser, MD.
Speaking at the World Congress of Dermatology, Dr. Glaser, director of clinical research and interim chair of the department of dermatology at Saint Louis University, said that in her practice, acne and actinic keratosis are the most common medical indications for chemical peels and that “acne is just a winner all the way around.”
For acne, she added: “Chemical peels can help both the comedonal and the inflammatory component. It should probably be combined with other therapies, and it does produce both an exfoliative and an anti-inflammatory benefit,.”
A variety of chemical peel formulations can be considered for acne, Dr. Glaser noted. “Typically, you’re going to use a light chemical peel,” such as glycolic or salicylic acid. Other options include Jessner’s solution or a light trichloroacetic acid formulation, she said, adding that tretinoin alone can also be considered.
In choosing between glycolic and salicylic acid, Dr. Glaser said, “salicylic acid should theoretically be the best agent because it is lipophilic and the glycolic acid is hydrophilic.” The reality of how these agents perform clinically, though, may sort out differently.
Dr. Glaser pointed to a double-blind, randomized controlled trial of the two agents in 20 women with facial acne. The severity of participants’ inflammatory acne was mild to moderate, with an average of 27 inflammatory lesions, and they had been on a stable prescription or over-the-counter acne regimen for at least 2 months (Dermatol Surg. 2008 Jan;34[1]:45-50).
Patients received six peels – one every 2 weeks – with 30% glycolic acid (an alpha-hydroxy acid) and 30% salicylic acid (a beta-hydroxy acid) in the split-face study.
All participants started at 4 minutes of exposure and increased up to 5 minutes as tolerated, although timing is only really important for glycolic acid, the same duration of exposure was maintained for each agent for the sake of consistency between arms, said Dr. Glaser, one of the investigators.
Sharing photographs of study participants, she observed that after six peels, “there really isn’t a significant difference.” Therefore, she added, “even though salicylic acid should be better, you can see that glycolic acid really held its own in this study.”
Dr. Glaser pointed out that a trend was seen for slightly better results with salicylic acid and results with this agent were more durable than those seen with glycolic acid. Patients reported fewer side effects on the beta-hydroxy–treated side as well.
She referred to another study, conducted in Japan, that used a double-blind, split-face design to compare 40% glycolic acid with a placebo that had a similarly low pH of 2.0. The 26 patients with moderate acne received five peels on a biweekly schedule, with glycolic acid significantly outperforming placebo. Among acne subtypes, noninflammatory acne improved more than inflammatory acne with glycolic acid (Dermatol Surg. 2014 Mar;40[3]:314-22).
Dr. Glaser said that in her own practice, she still tries to use salicylic acid for her acne patients,” though some patients prefer the experience of a glycolic acid peel, with which there’s likely to be less pain. “So if you have a preference, or your patient has a preference, you will probably be able to use the acid that works best for you,” she said.
Whatever peel is chosen, it should be considered an adjuvant to other topical and systemic acne therapies, Dr. Glaser stressed. “To maintain the results, you really do need to maintain the patient on some sort of standard acne therapy that you would normally do.”
Peels can also be an effective part of a multipronged approach that includes laser therapy and intralesional steroids, she said. However, peels can be considered for monotherapy in patients who don’t tolerate other acne therapies, and they can be used safely in pregnancy, she said.
As with all such treatments, dermatologists should remember to consider and counsel about herpes simplex virus prophylaxis and sun protection.
Dr. Glaser reported financial relationships with Galderma, Ulthera, Ortho, Allergan, Cellgene, and other pharmaceutical companies.
MILAN – according to Dee Anna Glaser, MD.
Speaking at the World Congress of Dermatology, Dr. Glaser, director of clinical research and interim chair of the department of dermatology at Saint Louis University, said that in her practice, acne and actinic keratosis are the most common medical indications for chemical peels and that “acne is just a winner all the way around.”
For acne, she added: “Chemical peels can help both the comedonal and the inflammatory component. It should probably be combined with other therapies, and it does produce both an exfoliative and an anti-inflammatory benefit,.”
A variety of chemical peel formulations can be considered for acne, Dr. Glaser noted. “Typically, you’re going to use a light chemical peel,” such as glycolic or salicylic acid. Other options include Jessner’s solution or a light trichloroacetic acid formulation, she said, adding that tretinoin alone can also be considered.
In choosing between glycolic and salicylic acid, Dr. Glaser said, “salicylic acid should theoretically be the best agent because it is lipophilic and the glycolic acid is hydrophilic.” The reality of how these agents perform clinically, though, may sort out differently.
Dr. Glaser pointed to a double-blind, randomized controlled trial of the two agents in 20 women with facial acne. The severity of participants’ inflammatory acne was mild to moderate, with an average of 27 inflammatory lesions, and they had been on a stable prescription or over-the-counter acne regimen for at least 2 months (Dermatol Surg. 2008 Jan;34[1]:45-50).
Patients received six peels – one every 2 weeks – with 30% glycolic acid (an alpha-hydroxy acid) and 30% salicylic acid (a beta-hydroxy acid) in the split-face study.
All participants started at 4 minutes of exposure and increased up to 5 minutes as tolerated, although timing is only really important for glycolic acid, the same duration of exposure was maintained for each agent for the sake of consistency between arms, said Dr. Glaser, one of the investigators.
Sharing photographs of study participants, she observed that after six peels, “there really isn’t a significant difference.” Therefore, she added, “even though salicylic acid should be better, you can see that glycolic acid really held its own in this study.”
Dr. Glaser pointed out that a trend was seen for slightly better results with salicylic acid and results with this agent were more durable than those seen with glycolic acid. Patients reported fewer side effects on the beta-hydroxy–treated side as well.
She referred to another study, conducted in Japan, that used a double-blind, split-face design to compare 40% glycolic acid with a placebo that had a similarly low pH of 2.0. The 26 patients with moderate acne received five peels on a biweekly schedule, with glycolic acid significantly outperforming placebo. Among acne subtypes, noninflammatory acne improved more than inflammatory acne with glycolic acid (Dermatol Surg. 2014 Mar;40[3]:314-22).
Dr. Glaser said that in her own practice, she still tries to use salicylic acid for her acne patients,” though some patients prefer the experience of a glycolic acid peel, with which there’s likely to be less pain. “So if you have a preference, or your patient has a preference, you will probably be able to use the acid that works best for you,” she said.
Whatever peel is chosen, it should be considered an adjuvant to other topical and systemic acne therapies, Dr. Glaser stressed. “To maintain the results, you really do need to maintain the patient on some sort of standard acne therapy that you would normally do.”
Peels can also be an effective part of a multipronged approach that includes laser therapy and intralesional steroids, she said. However, peels can be considered for monotherapy in patients who don’t tolerate other acne therapies, and they can be used safely in pregnancy, she said.
As with all such treatments, dermatologists should remember to consider and counsel about herpes simplex virus prophylaxis and sun protection.
Dr. Glaser reported financial relationships with Galderma, Ulthera, Ortho, Allergan, Cellgene, and other pharmaceutical companies.
MILAN – according to Dee Anna Glaser, MD.
Speaking at the World Congress of Dermatology, Dr. Glaser, director of clinical research and interim chair of the department of dermatology at Saint Louis University, said that in her practice, acne and actinic keratosis are the most common medical indications for chemical peels and that “acne is just a winner all the way around.”
For acne, she added: “Chemical peels can help both the comedonal and the inflammatory component. It should probably be combined with other therapies, and it does produce both an exfoliative and an anti-inflammatory benefit,.”
A variety of chemical peel formulations can be considered for acne, Dr. Glaser noted. “Typically, you’re going to use a light chemical peel,” such as glycolic or salicylic acid. Other options include Jessner’s solution or a light trichloroacetic acid formulation, she said, adding that tretinoin alone can also be considered.
In choosing between glycolic and salicylic acid, Dr. Glaser said, “salicylic acid should theoretically be the best agent because it is lipophilic and the glycolic acid is hydrophilic.” The reality of how these agents perform clinically, though, may sort out differently.
Dr. Glaser pointed to a double-blind, randomized controlled trial of the two agents in 20 women with facial acne. The severity of participants’ inflammatory acne was mild to moderate, with an average of 27 inflammatory lesions, and they had been on a stable prescription or over-the-counter acne regimen for at least 2 months (Dermatol Surg. 2008 Jan;34[1]:45-50).
Patients received six peels – one every 2 weeks – with 30% glycolic acid (an alpha-hydroxy acid) and 30% salicylic acid (a beta-hydroxy acid) in the split-face study.
All participants started at 4 minutes of exposure and increased up to 5 minutes as tolerated, although timing is only really important for glycolic acid, the same duration of exposure was maintained for each agent for the sake of consistency between arms, said Dr. Glaser, one of the investigators.
Sharing photographs of study participants, she observed that after six peels, “there really isn’t a significant difference.” Therefore, she added, “even though salicylic acid should be better, you can see that glycolic acid really held its own in this study.”
Dr. Glaser pointed out that a trend was seen for slightly better results with salicylic acid and results with this agent were more durable than those seen with glycolic acid. Patients reported fewer side effects on the beta-hydroxy–treated side as well.
She referred to another study, conducted in Japan, that used a double-blind, split-face design to compare 40% glycolic acid with a placebo that had a similarly low pH of 2.0. The 26 patients with moderate acne received five peels on a biweekly schedule, with glycolic acid significantly outperforming placebo. Among acne subtypes, noninflammatory acne improved more than inflammatory acne with glycolic acid (Dermatol Surg. 2014 Mar;40[3]:314-22).
Dr. Glaser said that in her own practice, she still tries to use salicylic acid for her acne patients,” though some patients prefer the experience of a glycolic acid peel, with which there’s likely to be less pain. “So if you have a preference, or your patient has a preference, you will probably be able to use the acid that works best for you,” she said.
Whatever peel is chosen, it should be considered an adjuvant to other topical and systemic acne therapies, Dr. Glaser stressed. “To maintain the results, you really do need to maintain the patient on some sort of standard acne therapy that you would normally do.”
Peels can also be an effective part of a multipronged approach that includes laser therapy and intralesional steroids, she said. However, peels can be considered for monotherapy in patients who don’t tolerate other acne therapies, and they can be used safely in pregnancy, she said.
As with all such treatments, dermatologists should remember to consider and counsel about herpes simplex virus prophylaxis and sun protection.
Dr. Glaser reported financial relationships with Galderma, Ulthera, Ortho, Allergan, Cellgene, and other pharmaceutical companies.
EXPERT ANALYSIS FROM WCD2019
Washington State removes exemption for MMR vaccine
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.
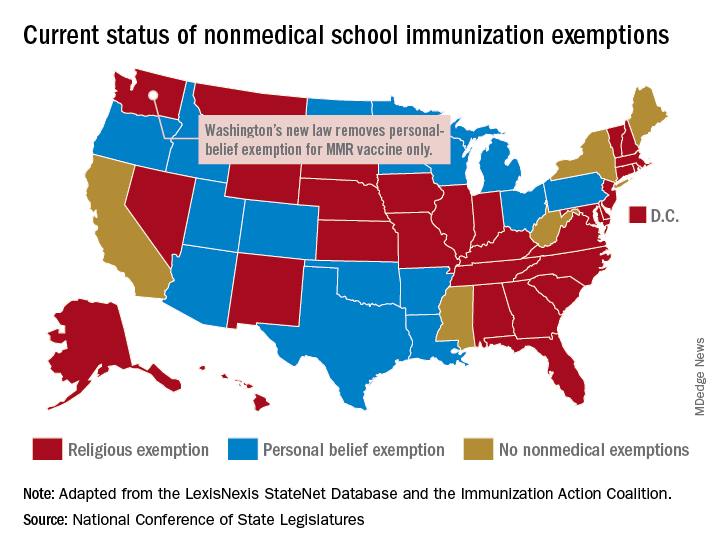
“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Inflammation diminishes quality of life in NAFLD, not fibrosis
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
RNA interference drug fitusiran looks effective in both hemophilia A and B
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
REPORTING FROM 2019 ISTH CONGRESS
Gram-negative bacteremia: Cultures, drugs, and duration
Are we doing it right?
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.
Are we doing it right?
Are we doing it right?
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.
Case
A 42-year-old woman with uncontrolled diabetes presents to the ED with fever, chills, dysuria, and flank pain for 3 days. On exam, she is febrile and tachycardic. Lab results show leukocytosis and urinalysis is consistent with infection. CT scan shows acute pyelonephritis without complication. She is admitted to the hospital and started on ceftriaxone 2 g/24 hrs. On hospital day 2, her blood cultures show gram-negative bacteria.
Brief overview
Management of gram-negative (GN) bacteremia remains a challenging clinical situation for inpatient providers. With the push for high-value care and reductions in length of stay, recent literature has focused on reviewing current practices and attempting to standardize care. Despite this, no overarching guidelines exist to direct practice and clinicians are left to make decisions based on prior experience and expert opinion. Three key clinical questions exist when caring for a hospitalized patient with GN bacteremia: Should blood cultures be repeated? When is transition to oral antibiotics appropriate? And for what duration should antibiotics be given?
Overview of the data
When considering repeating blood cultures, it is important to understand that current literature does not support the practice for all GN bacteremias.
Canzoneri et al. retrospectively studied GN bacteremia and found that it took 17 repeat blood cultures being drawn to yield 1 positive result, which suggests that they are not necessary in all cases.1 Furthermore, repeat blood cultures increase cost of hospitalization, length of stay, and inconvenience to patients.2
However, Mushtaq et al. noted that repeating blood cultures can provide valuable information to confirm the response to treatment in patients with endovascular infection. Furthermore, they found that repeated blood cultures are also reasonable when the following scenarios are suspected: endocarditis or central line–associated infection, concern for multidrug resistant GN bacilli, and ongoing evidence of sepsis or patient decompensation.3
Consideration of a transition from intravenous to oral antibiotics is a key decision point in the care of GN bacteremia. Without guidelines, clinicians are left to evaluate patients on a case-by-case basis.4 Studies have suggested that the transition should be guided by the condition of the patient, the type of infection, and the culture-derived sensitivities.5 Additionally, bioavailability of antibiotics (see Table 1) is an important consideration and a recent examination of oral antibiotic failure rates demonstrated that lower bioavailability antibiotics have an increased risk of failure (2% vs. 16%).6
In their study, Kutob et al. highlighted the importance of choosing not only an antibiotic of high bioavailability, but also an antibiotic dose which will support a high concentration of the antibiotic in the bloodstream.6 For example, they identify ciprofloxacin as a moderate bioavailability medication, but note that most cases they examined utilized 500 mg b.i.d., where the concentration-dependent killing and dose-dependent bioavailability would advocate for the use of 750 mg b.i.d. or 500 mg every 8 hours.
The heterogeneity of GN bloodstream infections also creates difficulty in standardization of care. The literature suggests that infection source plays a significant role in the type of GN bacteria isolated.6,7 The best data for the transition to oral antibiotics exists with urologic sources and it remains unclear whether bacteria from other sources have higher risks of oral antibiotic failure.8
One recent study of 66 patients examined bacteremia in the setting of cholangitis and found that, once patients had stabilized, a switch from intravenous to oral antibiotics was noninferior, but randomized, prospective trials have not been performed. Notably, patients were transitioned to orals only after they were found to have a fluoroquinolone-sensitive infection, allowing the study authors to use higher-bioavailability agents for the transition to orals.9 Multiple studies have highlighted the unique care required for certain infections, such as pseudomonal infections, which most experts agree requires a more conservative approach.5,6
Fluoroquinolones are the bedrock of therapy for GN bacteremia because of historic in vivo experience and in vitro findings about bioavailability and dose-dependent killing, but they are also the antibiotic class associated with the highest hospitalization rates for antibiotic-associated adverse events.8 A recent noninferiority trial comparing the use of beta-lactams with fluoroquinolones found that beta-lactams were noninferior, though the study was flawed by the limited number of beta-lactam–using patients identified.8 It is clear that more investigation is needed before recommendations can be made regarding ideal oral antibiotics for GN bacteremia.
The transition to oral is reasonable given the following criteria: the patient has improved on intravenous antibiotics and source control has been achieved; the culture data have demonstrated sensitivity to the oral antibiotic of choice, with special care given to higher-risk bacteria such as Pseudomonas; the patient is able to take the oral antibiotic; and the oral antibiotic of choice has the highest bioavailability possible and is given at an appropriate dose to reach its highest killing and bioavailability concentrations.7
After evaluating the appropriateness of transition to oral antibiotics, the final decision is about duration of antibiotic therapy. Current Infectious Disease Society of America guidelines are based on expert opinion and recommend 7-14 days of therapy. As with many common infections, recent studies have focused on evaluating reduction in antibiotic durations.
Chotiprasitsakul et al. demonstrated no difference in mortality or morbidity in 385 propensity-matched pairs with treatment of Enterobacteriaceae bacteremia for 8 versus 15 days.10 A mixed meta-analysis performed in 2011 evaluated 24 randomized, controlled trials and found shorter durations (5-7 days) had similar outcomes to prolonged durations (7-21 days).11 Recently, Yahav et al. performed a randomized control trial comparing 7- and 14-day regimens for uncomplicated GN bacteremia and found a 7-day course to be noninferior if patients were clinically stable by day 5 and had source control.12
It should be noted that not all studies have found that reduced durations are without harm. Nelson et al. performed a retrospective cohort analysis and found that reduced durations of antibiotics (7-10 days) increased mortality and recurrent infection when compared with a longer course (greater than 10 days).13 These contrary findings highlight the need for provider discretion in selecting a course of antibiotics as well as the need for further studies about optimal duration of antibiotics.
Application of the data
Returning to our case, on day 3, the patient’s fever had resolved and leukocytosis improved. In the absence of concern for persistent infection, repeat blood cultures were not performed. On day 4 initial blood cultures showed pan-sensitive Escherichia coli. The patient was transitioned to 750 mg oral ciprofloxacin b.i.d. to complete a 10-day course from first dose of ceftriaxone and was discharged from the hospital.
Bottom line
Management of GN bacteremia requires individualized care based on clinical presentation, but the data presented above can be used as broad guidelines to help reduce excess blood cultures, avoid prolonged use of intravenous antibiotics, and limit the duration of antibiotic exposure.
Dr. Imber is an assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, and director of the Internal Medicine Simulation Education and Hospitalist Procedural Certification. Dr. Burns is an assistant professor in the division of hospital medicine at the University of New Mexico. Dr. Chan is currently a chief resident in the department of internal medicine at the University of New Mexico.
References
1. Canzoneri CN et al. Follow-up blood cultures in gram-negative bacteremia: Are they needed? Clin Infect Dis. 2017;65(11):1776-9. doi: 10.1093/cid/cix648.
2. Kang CK et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365. doi: 10.1186/1471-2334-13-365.
3. Mushtaq A et al. Repeating blood cultures after an initial bacteremia: When and how often? Cleve Clin J Med. 2019;86(2):89-92. doi: 10.3949/ccjm.86a.18001.
4. Nimmich EB et al. Development of institutional guidelines for management of gram-negative bloodstream infections: Incorporating local evidence. Hosp Pharm. 2017;52(10):691-7. doi: 10.1177/0018578717720506.
5. Hale AJ et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018 May. doi: 10.12788/jhm.2949.
6. Kutob LF et al. Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents. 2016. doi: 10.1016/j.ijantimicag.2016.07.013.
7. Tamma PD et al. Association of 30-day mortality with oral step-down vs. continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.6226.
8. Mercuro NJ et al. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones vs. B-lactams. Int J Antimicrob Agents. 2017. doi: 10.1016/j.ijantimicag.2017.12.007.
9. Park TY et al. Early oral antibiotic switch compared with conventional intravenous antibiotic therapy for acute cholangitis with bacteremia. Dig Dis Sci. 2014;59:2790-6. doi: 10.1007/s10620-014-3233-0.
10. Chotiprasitsakul D et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172-7. doi:10.1093/cid/cix767.
11. Havey TC et al. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15(6):R267. doi:10.1186/cc10545.
12. Yahav D et al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2018 Dec. doi:10.1093/cid/ciy1054.
13. Nelson AN et al. Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection. 2017;45(5):613-20. doi:10.1007/s15010-017-1020-5.











