User login
Medicare going in ‘right direction’ on opioid epidemic
About 174,000 Medicare beneficiaries received such a medication – either buprenorphine or naltrexone – to help them with recovery in 2018, according to the Office of Inspector General in the Department of Health & Human Services.
In addition, prescriptions for naloxone – the drug that can reverse an opioid overdose – spiked since 2016, rising 501% – and that is likely an underestimate because it doesn’t include doses of the nasal spray Medicare members might have received through local programs, the OIG said.
“For now, the numbers are going in the right direction,” said Miriam Anderson, lead investigator on the report. “But this is a national crisis and we must remain vigilant and continue to fight this epidemic and ensure that opioids are prescribed and used appropriately.”
During the 2 years studied, the threat of new addictions appeared to slow. Prescriptions for an opioid through Medicare Part D decreased by 11%. The numbers of the beneficiaries considered at serious risk for misuse or overdose ― either because they received extreme amounts of opioids or appeared to be “doctor shopping” – dropped 46%. And there were 51% fewer doctors or other providers flagged for prescribing opioids to patients at serious risk from 2016 to 2018.
The report says the OIG and other law enforcement agencies will investigate the highest-level prescribers for possible fraud and signs that some providers operate pill mills. The report mentions a physician in Florida who provided 104 high-risk Medicare patients with 2,619 opioid prescriptions.
It will be up to Medicare to follow up with patients whose opioid use suggests addiction, recreational use, or resale. In one case, a Pennsylvania woman received 10,728 oxycodone pills and 570 fentanyl patches from a single physician during 2018. A Medicare member in Alabama acquired 56 opioid prescriptions from 25 different prescribers within 1 year.
In a statement, the Centers for Medicare & Medicaid Services said: “Fighting the opioid epidemic has been a top priority for the Trump administration. We are encouraged by the OIG’s conclusion which finds significant progress has been made in our efforts to decrease opioid misuse while simultaneously increasing medication-assisted treatment in the Medicare Part D program.”
The agency points to recent efforts to curb opioid misuse including a 7-day limit on first-time opioid prescriptions, pharmacy alerts about Medicare beneficiaries who receive high doses of pain medication, and drug management programs that may restrict a patient’s supply. CMS says it does not use a “one-size-fits-all” approach. Medicare patients in long-term care facilities or hospice care and those in cancer treatment are exempt from the opioid-prescribing restrictions.
The opioid-prescribing limits are raising alarms among some Medicare recipients, especially those who qualify based on a long-term disability and deal with severe, chronic pain.
Jae Kennedy, PhD, a disability policy expert at Washington State University, Spokane, said cutting back on opioid prescriptions is generally a good development.
“But we hear from people in the disability community who feel like they’re being victimized by this new, very stringent set of dispensing limits,” said Dr. Kennedy. “People have been managing their pain, in some cases for many years without a problem, and now they’re being kind of criminalized by this new bureaucratic backlash.”
Ms. Anderson said the OIG agrees that “some patients need opioids and they should receive those needed for their condition. This report raises concerns that some patients may be receiving opioids above and beyond those needs.”
While most Medicare beneficiaries are 65 years or older, the 15% who are under 65 and disabled may be the key piece of this report. Dr. Kennedy’s research shows they are up to three times more likely to describe persistent pain than are other adults and 50% more likely to report opioid misuse. A 2017 OIG report found that 74% of Medicare beneficiaries at serious risk for addiction and overdose deaths were under age 65 years.
Dr. Kennedy said it’s good to see Medicare expanding access to medication-assisted treatment, known as MAT, for addiction, but the agency needs to make sure that more buprenorphine prescribers accept all patients, not just the ones who are easiest to manage. Patients with disabilities often need many different medications for multiple physical and mental health conditions.
“Saying, ‘Well, because you’ve got schizophrenia or manic depressive disorder, we can’t treat you,’ I think is discriminatory,” Dr. Kennedy said. “It’s happening with private buprenorphine prescribers in this country because there are so few.”
Americans 65 years or older have the lowest rates of opioid overdose deaths. Even so, the Centers for Disease Control and Prevention says the number of deaths among seniors increased by 279% from 1999 to 2017.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. WBUR, a public radio station owned by Boston University, is a member station of NPR.
About 174,000 Medicare beneficiaries received such a medication – either buprenorphine or naltrexone – to help them with recovery in 2018, according to the Office of Inspector General in the Department of Health & Human Services.
In addition, prescriptions for naloxone – the drug that can reverse an opioid overdose – spiked since 2016, rising 501% – and that is likely an underestimate because it doesn’t include doses of the nasal spray Medicare members might have received through local programs, the OIG said.
“For now, the numbers are going in the right direction,” said Miriam Anderson, lead investigator on the report. “But this is a national crisis and we must remain vigilant and continue to fight this epidemic and ensure that opioids are prescribed and used appropriately.”
During the 2 years studied, the threat of new addictions appeared to slow. Prescriptions for an opioid through Medicare Part D decreased by 11%. The numbers of the beneficiaries considered at serious risk for misuse or overdose ― either because they received extreme amounts of opioids or appeared to be “doctor shopping” – dropped 46%. And there were 51% fewer doctors or other providers flagged for prescribing opioids to patients at serious risk from 2016 to 2018.
The report says the OIG and other law enforcement agencies will investigate the highest-level prescribers for possible fraud and signs that some providers operate pill mills. The report mentions a physician in Florida who provided 104 high-risk Medicare patients with 2,619 opioid prescriptions.
It will be up to Medicare to follow up with patients whose opioid use suggests addiction, recreational use, or resale. In one case, a Pennsylvania woman received 10,728 oxycodone pills and 570 fentanyl patches from a single physician during 2018. A Medicare member in Alabama acquired 56 opioid prescriptions from 25 different prescribers within 1 year.
In a statement, the Centers for Medicare & Medicaid Services said: “Fighting the opioid epidemic has been a top priority for the Trump administration. We are encouraged by the OIG’s conclusion which finds significant progress has been made in our efforts to decrease opioid misuse while simultaneously increasing medication-assisted treatment in the Medicare Part D program.”
The agency points to recent efforts to curb opioid misuse including a 7-day limit on first-time opioid prescriptions, pharmacy alerts about Medicare beneficiaries who receive high doses of pain medication, and drug management programs that may restrict a patient’s supply. CMS says it does not use a “one-size-fits-all” approach. Medicare patients in long-term care facilities or hospice care and those in cancer treatment are exempt from the opioid-prescribing restrictions.
The opioid-prescribing limits are raising alarms among some Medicare recipients, especially those who qualify based on a long-term disability and deal with severe, chronic pain.
Jae Kennedy, PhD, a disability policy expert at Washington State University, Spokane, said cutting back on opioid prescriptions is generally a good development.
“But we hear from people in the disability community who feel like they’re being victimized by this new, very stringent set of dispensing limits,” said Dr. Kennedy. “People have been managing their pain, in some cases for many years without a problem, and now they’re being kind of criminalized by this new bureaucratic backlash.”
Ms. Anderson said the OIG agrees that “some patients need opioids and they should receive those needed for their condition. This report raises concerns that some patients may be receiving opioids above and beyond those needs.”
While most Medicare beneficiaries are 65 years or older, the 15% who are under 65 and disabled may be the key piece of this report. Dr. Kennedy’s research shows they are up to three times more likely to describe persistent pain than are other adults and 50% more likely to report opioid misuse. A 2017 OIG report found that 74% of Medicare beneficiaries at serious risk for addiction and overdose deaths were under age 65 years.
Dr. Kennedy said it’s good to see Medicare expanding access to medication-assisted treatment, known as MAT, for addiction, but the agency needs to make sure that more buprenorphine prescribers accept all patients, not just the ones who are easiest to manage. Patients with disabilities often need many different medications for multiple physical and mental health conditions.
“Saying, ‘Well, because you’ve got schizophrenia or manic depressive disorder, we can’t treat you,’ I think is discriminatory,” Dr. Kennedy said. “It’s happening with private buprenorphine prescribers in this country because there are so few.”
Americans 65 years or older have the lowest rates of opioid overdose deaths. Even so, the Centers for Disease Control and Prevention says the number of deaths among seniors increased by 279% from 1999 to 2017.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. WBUR, a public radio station owned by Boston University, is a member station of NPR.
About 174,000 Medicare beneficiaries received such a medication – either buprenorphine or naltrexone – to help them with recovery in 2018, according to the Office of Inspector General in the Department of Health & Human Services.
In addition, prescriptions for naloxone – the drug that can reverse an opioid overdose – spiked since 2016, rising 501% – and that is likely an underestimate because it doesn’t include doses of the nasal spray Medicare members might have received through local programs, the OIG said.
“For now, the numbers are going in the right direction,” said Miriam Anderson, lead investigator on the report. “But this is a national crisis and we must remain vigilant and continue to fight this epidemic and ensure that opioids are prescribed and used appropriately.”
During the 2 years studied, the threat of new addictions appeared to slow. Prescriptions for an opioid through Medicare Part D decreased by 11%. The numbers of the beneficiaries considered at serious risk for misuse or overdose ― either because they received extreme amounts of opioids or appeared to be “doctor shopping” – dropped 46%. And there were 51% fewer doctors or other providers flagged for prescribing opioids to patients at serious risk from 2016 to 2018.
The report says the OIG and other law enforcement agencies will investigate the highest-level prescribers for possible fraud and signs that some providers operate pill mills. The report mentions a physician in Florida who provided 104 high-risk Medicare patients with 2,619 opioid prescriptions.
It will be up to Medicare to follow up with patients whose opioid use suggests addiction, recreational use, or resale. In one case, a Pennsylvania woman received 10,728 oxycodone pills and 570 fentanyl patches from a single physician during 2018. A Medicare member in Alabama acquired 56 opioid prescriptions from 25 different prescribers within 1 year.
In a statement, the Centers for Medicare & Medicaid Services said: “Fighting the opioid epidemic has been a top priority for the Trump administration. We are encouraged by the OIG’s conclusion which finds significant progress has been made in our efforts to decrease opioid misuse while simultaneously increasing medication-assisted treatment in the Medicare Part D program.”
The agency points to recent efforts to curb opioid misuse including a 7-day limit on first-time opioid prescriptions, pharmacy alerts about Medicare beneficiaries who receive high doses of pain medication, and drug management programs that may restrict a patient’s supply. CMS says it does not use a “one-size-fits-all” approach. Medicare patients in long-term care facilities or hospice care and those in cancer treatment are exempt from the opioid-prescribing restrictions.
The opioid-prescribing limits are raising alarms among some Medicare recipients, especially those who qualify based on a long-term disability and deal with severe, chronic pain.
Jae Kennedy, PhD, a disability policy expert at Washington State University, Spokane, said cutting back on opioid prescriptions is generally a good development.
“But we hear from people in the disability community who feel like they’re being victimized by this new, very stringent set of dispensing limits,” said Dr. Kennedy. “People have been managing their pain, in some cases for many years without a problem, and now they’re being kind of criminalized by this new bureaucratic backlash.”
Ms. Anderson said the OIG agrees that “some patients need opioids and they should receive those needed for their condition. This report raises concerns that some patients may be receiving opioids above and beyond those needs.”
While most Medicare beneficiaries are 65 years or older, the 15% who are under 65 and disabled may be the key piece of this report. Dr. Kennedy’s research shows they are up to three times more likely to describe persistent pain than are other adults and 50% more likely to report opioid misuse. A 2017 OIG report found that 74% of Medicare beneficiaries at serious risk for addiction and overdose deaths were under age 65 years.
Dr. Kennedy said it’s good to see Medicare expanding access to medication-assisted treatment, known as MAT, for addiction, but the agency needs to make sure that more buprenorphine prescribers accept all patients, not just the ones who are easiest to manage. Patients with disabilities often need many different medications for multiple physical and mental health conditions.
“Saying, ‘Well, because you’ve got schizophrenia or manic depressive disorder, we can’t treat you,’ I think is discriminatory,” Dr. Kennedy said. “It’s happening with private buprenorphine prescribers in this country because there are so few.”
Americans 65 years or older have the lowest rates of opioid overdose deaths. Even so, the Centers for Disease Control and Prevention says the number of deaths among seniors increased by 279% from 1999 to 2017.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. WBUR, a public radio station owned by Boston University, is a member station of NPR.
Acalabrutinib extends PFS in advanced CLL
AMSTERDAM – For patients with relapsed or refractory chronic lymphocytic leukemia (CLL), monotherapy with the Bruton tyrosine kinase (BTK) inhibitor acalabrutinib (Calquence) was associated with better progression-free survival and a more tolerable safety profile than rituximab combined with either idelalisib (Zydelig) or bendamustine, an interim analysis from the phase 3 ASCEND trial showed.
Among 310 patients with previously treated CLL followed for a median of 16.1 months, the primary endpoint of median progression-free survival (PFS), as assessed by independent reviewers, had not been reached for patients treated with acalabrutinib, compared with 16.5 months for patients treated with idelalisib and rituximab (IdR) or bendamustine and rituximab (BR), reported Paolo Ghia, MD, PhD, from Università Vita-Salute San Raffaele in Milan.
“We show that acalabrutinib improved progression-free survival across all groups, including those with high-risk features,” he said at the annual congress of the European Hematology Association.
Acalabrutinib is approved in the United States for treatment of mantle cell lymphoma that has progressed on at least one prior therapy. It has been shown in preclinical studies to be more selective for BTK than the first-in-class agent ibrutinib (Imbruvica), with less off-target kinase inhibition, Dr. Ghia said.
ASCEND was designed to see whether acalabrutinib monotherapy could offer superior PFS to IdR or BR in patients with CLL who had progressed or were refractory to at least one prior line of therapy.
Patients were randomly assigned, with 155 patients in each arm, to either acalabrutinib 100 mg orally twice daily or the investigator’s choice of either idelalisib 150 mg orally twice daily plus IV rituximab at an initial dose of 375 mg/m2, followed by up to seven doses at 500 mg/m2 delivered every 2 weeks for four infusions, then every 4 weeks for the remaining three infusions or IV bendamustine 70 mg/m2 on days 1 and 2 of each cycle, plus rituximab at the 375 mg/m2 dose on day 1 for the first cycle, followed by 500 mg/m2 for up to six total cycles.
Dr. Ghia presented results of an interim analysis planned for when two-thirds of the predicted PFS events (approximately 79) had occurred.
The baseline patient characteristics were generally similar, with a median age of 68 years in the acalabrutinib arm and 67 years in the comparison arm. Almost half of all patients in each arm had bulky disease, defined as 5 cm or greater. The majority of patients had two or more prior lines of therapy.
The primary endpoint of PFS as assessed by independent review favored acalabrutinib, with a hazard ratio of 0.31 (P less than .0001). Results were similar when acalabrutinib was compared with each of the regimens in the comparison arm (HR, 0.29 vs. IdR, 0.36 vs. BR; P less than .001 for each comparison).
Acalabrutinib was also superior in patients with high-risk cytogenetic features, compared with the other two regimens combined (HR, 0.27; P less than .001).
The benefit of the BTK inhibitor was consistent across all subgroups, including age, sex, performance status, Rai stage at screening, bulky/nonbulky disease, number of prior therapies, presence or absence of deletion 17p or TP53 mutation, mutated or unmutated immunoglobulin heavy chain, and complex/noncomplex karyotype.
Reviewer-assessed objective response rates were similar, occurring in 81% of patients on acalabrutinib and 76% of patients on other regimens.
There were no complete responses in the acalabrutinib arm, compared with two complete responses in the comparison arm. The majority of responses in each arm were partial responses (81% and 74%, respectively).
The median duration of response was not reached with acalabrutinib, compared with 13.6 months with the other therapies (HR, 0.33; P less than .0001).
In all, 85% of patients on acalabrutinib had a response lasting at least 12 months, compared with 60% of patients on the other regimens. There was no difference in overall survival at the 16.1-month median follow-up.
Adverse events of any grade occurred in 94% of patients on acalabrutinib, 99% on IdR, and 80% on BR; the respective incidences of serious adverse events were 29%, 56%, and 26%. Grade 3-4 adverse events occurred in 45%, 86%, and 43% of patients, respectively.
There were 13 treatment-related deaths. Six deaths in the acalabrutinib arm were caused by brain neoplasm, cachexia, cerebral ischemia, malignant lung tumor, neuroendocrine carcinoma, and sepsis. Five deaths among IdR-treated patients included chronic heart failure, cardiopulmonary disease, interstitial lung disease, MI, and pseudomonal pneumonia. Two deaths in BR-treated patients were attributed to acute cardiac failure and a gastric neoplasm.
The results show that “acalabrutinib has demonstrated efficacy in previously untreated and relapsed/refractory CLL and may be considered as an option in the future treatment paradigm,” Dr. Ghia said.
Acalabrutinib monotherapy is currently being compared with ibrutinib monotherapy in patients with relapsed/refractory CLL; in addition, the phase 3 ELEVATE-TN study investigating acalabrutinib in combination with obinutuzumab (Gazyva) versus obinutuzumab plus chlorambucil has reached its primary PFS endpoint and will be reported soon, Dr. Ghia said.
The ASCEND trial is sponsored by Acerta Pharma; AstraZeneca holds majority shares in the company. Dr. Ghia reported consulting fees and honoraria from AstraZeneca and other companies, and research funding from several different companies.
SOURCE: Ghia P et al. EHA Congress, Abstract LB2606.
AMSTERDAM – For patients with relapsed or refractory chronic lymphocytic leukemia (CLL), monotherapy with the Bruton tyrosine kinase (BTK) inhibitor acalabrutinib (Calquence) was associated with better progression-free survival and a more tolerable safety profile than rituximab combined with either idelalisib (Zydelig) or bendamustine, an interim analysis from the phase 3 ASCEND trial showed.
Among 310 patients with previously treated CLL followed for a median of 16.1 months, the primary endpoint of median progression-free survival (PFS), as assessed by independent reviewers, had not been reached for patients treated with acalabrutinib, compared with 16.5 months for patients treated with idelalisib and rituximab (IdR) or bendamustine and rituximab (BR), reported Paolo Ghia, MD, PhD, from Università Vita-Salute San Raffaele in Milan.
“We show that acalabrutinib improved progression-free survival across all groups, including those with high-risk features,” he said at the annual congress of the European Hematology Association.
Acalabrutinib is approved in the United States for treatment of mantle cell lymphoma that has progressed on at least one prior therapy. It has been shown in preclinical studies to be more selective for BTK than the first-in-class agent ibrutinib (Imbruvica), with less off-target kinase inhibition, Dr. Ghia said.
ASCEND was designed to see whether acalabrutinib monotherapy could offer superior PFS to IdR or BR in patients with CLL who had progressed or were refractory to at least one prior line of therapy.
Patients were randomly assigned, with 155 patients in each arm, to either acalabrutinib 100 mg orally twice daily or the investigator’s choice of either idelalisib 150 mg orally twice daily plus IV rituximab at an initial dose of 375 mg/m2, followed by up to seven doses at 500 mg/m2 delivered every 2 weeks for four infusions, then every 4 weeks for the remaining three infusions or IV bendamustine 70 mg/m2 on days 1 and 2 of each cycle, plus rituximab at the 375 mg/m2 dose on day 1 for the first cycle, followed by 500 mg/m2 for up to six total cycles.
Dr. Ghia presented results of an interim analysis planned for when two-thirds of the predicted PFS events (approximately 79) had occurred.
The baseline patient characteristics were generally similar, with a median age of 68 years in the acalabrutinib arm and 67 years in the comparison arm. Almost half of all patients in each arm had bulky disease, defined as 5 cm or greater. The majority of patients had two or more prior lines of therapy.
The primary endpoint of PFS as assessed by independent review favored acalabrutinib, with a hazard ratio of 0.31 (P less than .0001). Results were similar when acalabrutinib was compared with each of the regimens in the comparison arm (HR, 0.29 vs. IdR, 0.36 vs. BR; P less than .001 for each comparison).
Acalabrutinib was also superior in patients with high-risk cytogenetic features, compared with the other two regimens combined (HR, 0.27; P less than .001).
The benefit of the BTK inhibitor was consistent across all subgroups, including age, sex, performance status, Rai stage at screening, bulky/nonbulky disease, number of prior therapies, presence or absence of deletion 17p or TP53 mutation, mutated or unmutated immunoglobulin heavy chain, and complex/noncomplex karyotype.
Reviewer-assessed objective response rates were similar, occurring in 81% of patients on acalabrutinib and 76% of patients on other regimens.
There were no complete responses in the acalabrutinib arm, compared with two complete responses in the comparison arm. The majority of responses in each arm were partial responses (81% and 74%, respectively).
The median duration of response was not reached with acalabrutinib, compared with 13.6 months with the other therapies (HR, 0.33; P less than .0001).
In all, 85% of patients on acalabrutinib had a response lasting at least 12 months, compared with 60% of patients on the other regimens. There was no difference in overall survival at the 16.1-month median follow-up.
Adverse events of any grade occurred in 94% of patients on acalabrutinib, 99% on IdR, and 80% on BR; the respective incidences of serious adverse events were 29%, 56%, and 26%. Grade 3-4 adverse events occurred in 45%, 86%, and 43% of patients, respectively.
There were 13 treatment-related deaths. Six deaths in the acalabrutinib arm were caused by brain neoplasm, cachexia, cerebral ischemia, malignant lung tumor, neuroendocrine carcinoma, and sepsis. Five deaths among IdR-treated patients included chronic heart failure, cardiopulmonary disease, interstitial lung disease, MI, and pseudomonal pneumonia. Two deaths in BR-treated patients were attributed to acute cardiac failure and a gastric neoplasm.
The results show that “acalabrutinib has demonstrated efficacy in previously untreated and relapsed/refractory CLL and may be considered as an option in the future treatment paradigm,” Dr. Ghia said.
Acalabrutinib monotherapy is currently being compared with ibrutinib monotherapy in patients with relapsed/refractory CLL; in addition, the phase 3 ELEVATE-TN study investigating acalabrutinib in combination with obinutuzumab (Gazyva) versus obinutuzumab plus chlorambucil has reached its primary PFS endpoint and will be reported soon, Dr. Ghia said.
The ASCEND trial is sponsored by Acerta Pharma; AstraZeneca holds majority shares in the company. Dr. Ghia reported consulting fees and honoraria from AstraZeneca and other companies, and research funding from several different companies.
SOURCE: Ghia P et al. EHA Congress, Abstract LB2606.
AMSTERDAM – For patients with relapsed or refractory chronic lymphocytic leukemia (CLL), monotherapy with the Bruton tyrosine kinase (BTK) inhibitor acalabrutinib (Calquence) was associated with better progression-free survival and a more tolerable safety profile than rituximab combined with either idelalisib (Zydelig) or bendamustine, an interim analysis from the phase 3 ASCEND trial showed.
Among 310 patients with previously treated CLL followed for a median of 16.1 months, the primary endpoint of median progression-free survival (PFS), as assessed by independent reviewers, had not been reached for patients treated with acalabrutinib, compared with 16.5 months for patients treated with idelalisib and rituximab (IdR) or bendamustine and rituximab (BR), reported Paolo Ghia, MD, PhD, from Università Vita-Salute San Raffaele in Milan.
“We show that acalabrutinib improved progression-free survival across all groups, including those with high-risk features,” he said at the annual congress of the European Hematology Association.
Acalabrutinib is approved in the United States for treatment of mantle cell lymphoma that has progressed on at least one prior therapy. It has been shown in preclinical studies to be more selective for BTK than the first-in-class agent ibrutinib (Imbruvica), with less off-target kinase inhibition, Dr. Ghia said.
ASCEND was designed to see whether acalabrutinib monotherapy could offer superior PFS to IdR or BR in patients with CLL who had progressed or were refractory to at least one prior line of therapy.
Patients were randomly assigned, with 155 patients in each arm, to either acalabrutinib 100 mg orally twice daily or the investigator’s choice of either idelalisib 150 mg orally twice daily plus IV rituximab at an initial dose of 375 mg/m2, followed by up to seven doses at 500 mg/m2 delivered every 2 weeks for four infusions, then every 4 weeks for the remaining three infusions or IV bendamustine 70 mg/m2 on days 1 and 2 of each cycle, plus rituximab at the 375 mg/m2 dose on day 1 for the first cycle, followed by 500 mg/m2 for up to six total cycles.
Dr. Ghia presented results of an interim analysis planned for when two-thirds of the predicted PFS events (approximately 79) had occurred.
The baseline patient characteristics were generally similar, with a median age of 68 years in the acalabrutinib arm and 67 years in the comparison arm. Almost half of all patients in each arm had bulky disease, defined as 5 cm or greater. The majority of patients had two or more prior lines of therapy.
The primary endpoint of PFS as assessed by independent review favored acalabrutinib, with a hazard ratio of 0.31 (P less than .0001). Results were similar when acalabrutinib was compared with each of the regimens in the comparison arm (HR, 0.29 vs. IdR, 0.36 vs. BR; P less than .001 for each comparison).
Acalabrutinib was also superior in patients with high-risk cytogenetic features, compared with the other two regimens combined (HR, 0.27; P less than .001).
The benefit of the BTK inhibitor was consistent across all subgroups, including age, sex, performance status, Rai stage at screening, bulky/nonbulky disease, number of prior therapies, presence or absence of deletion 17p or TP53 mutation, mutated or unmutated immunoglobulin heavy chain, and complex/noncomplex karyotype.
Reviewer-assessed objective response rates were similar, occurring in 81% of patients on acalabrutinib and 76% of patients on other regimens.
There were no complete responses in the acalabrutinib arm, compared with two complete responses in the comparison arm. The majority of responses in each arm were partial responses (81% and 74%, respectively).
The median duration of response was not reached with acalabrutinib, compared with 13.6 months with the other therapies (HR, 0.33; P less than .0001).
In all, 85% of patients on acalabrutinib had a response lasting at least 12 months, compared with 60% of patients on the other regimens. There was no difference in overall survival at the 16.1-month median follow-up.
Adverse events of any grade occurred in 94% of patients on acalabrutinib, 99% on IdR, and 80% on BR; the respective incidences of serious adverse events were 29%, 56%, and 26%. Grade 3-4 adverse events occurred in 45%, 86%, and 43% of patients, respectively.
There were 13 treatment-related deaths. Six deaths in the acalabrutinib arm were caused by brain neoplasm, cachexia, cerebral ischemia, malignant lung tumor, neuroendocrine carcinoma, and sepsis. Five deaths among IdR-treated patients included chronic heart failure, cardiopulmonary disease, interstitial lung disease, MI, and pseudomonal pneumonia. Two deaths in BR-treated patients were attributed to acute cardiac failure and a gastric neoplasm.
The results show that “acalabrutinib has demonstrated efficacy in previously untreated and relapsed/refractory CLL and may be considered as an option in the future treatment paradigm,” Dr. Ghia said.
Acalabrutinib monotherapy is currently being compared with ibrutinib monotherapy in patients with relapsed/refractory CLL; in addition, the phase 3 ELEVATE-TN study investigating acalabrutinib in combination with obinutuzumab (Gazyva) versus obinutuzumab plus chlorambucil has reached its primary PFS endpoint and will be reported soon, Dr. Ghia said.
The ASCEND trial is sponsored by Acerta Pharma; AstraZeneca holds majority shares in the company. Dr. Ghia reported consulting fees and honoraria from AstraZeneca and other companies, and research funding from several different companies.
SOURCE: Ghia P et al. EHA Congress, Abstract LB2606.
REPORTING FROM EHA CONGRESS
Establishing the Diagnosis of Rosacea in Skin of Color Patients
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
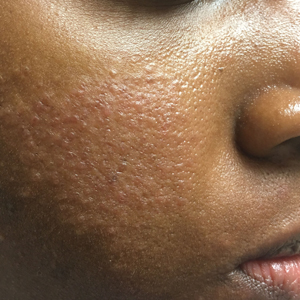
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
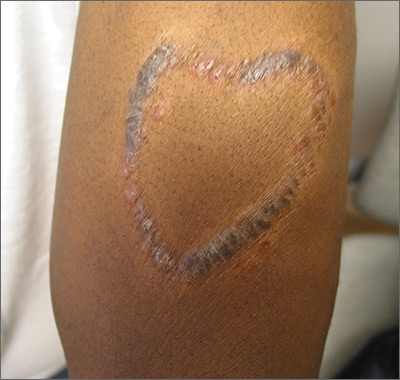
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Practice Points
- The clinical signs of rosacea may be similar in all skin types; however, dermatologists must have a high clinical index of suspicion for rosacea in patients with skin of color (SOC).
- Dermatologists should consider a wide differential diagnosis when presented with an SOC patient with facial erythema and/or papules and pustules.
Rash over homemade tattoo
Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Although the FP had never seen anything like this before, he was aware that dyes in tattoos could cause an allergic reaction. His research also suggested that sarcoidosis could occur in a tattoo, so this was part of his differential diagnosis written on the pathology form. He suggested a 4 mm punch biopsy to determine what was going on.
(See the Watch & Learn video on “Punch biopsy.”)
The pathology came back consistent with sarcoidosis. On the follow-up visit, the FP explained the diagnosis and suggested that the patient have a chest x-ray. The chest x-ray showed bilateral hilar adenopathy consistent with stage I sarcoidosis. The FP prescribed a 15-g tube of 0.05% clobetasol to treat the lesion and referred the patient to Dermatology and Pulmonology.
When the patient visited Dermatology 2 months later, most of the sarcoid plaques were flat but some remained raised and pruritic. The dermatologist offered intralesional triamcinolone for the stubborn plaques, and the patient consented. This intralesional steroid in conjunction with the topical steroid provided a good result that treated the itching and flattened the lesions. The remaining tattoo and color of the skin were not normal, but the patient was happy with the result. She had no pulmonary symptoms, and her pulmonary function tests showed a mild decrease in her diffusing capacity. She continued to see the dermatologist and pulmonologist for ongoing care of her sarcoidosis.
Photo courtesy of Amor Khachemoune, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Bae E, Bae Y, Sarabi K, et al. Sarcoidosis. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1153-1160.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
This month in the journal CHEST®
Editor’s picks
EDITORIAL
The CHEST Editorial Team: Serving Our Contributors and Readers
By Dr. P. J. Mazzone
ORIGINAL RESEARCH
Pulmonary Arterial Histologic Lesions in Patients With COPD With Severe Pulmonary Hypertension
By Dr. V. Bunel, et al.
Pulmonary Edema Following Initiation of Parenteral Prostacyclin Therapy for Pulmonary Arterial Hypertension: A Retrospective Study
By Dr. N. A. Khan, et al.
Lung Allocation Score Thresholds Prioritize Survival After Lung Transplantation
By Dr. S. S. Li, et al.
EVIDENCE-BASED MEDICINE
Chronic Cough and Gastroesophageal Reflux in Children: CHEST Guideline and Expert Panel Report
By Dr. A. B. Chang, et al.
Editor’s picks
Editor’s picks
EDITORIAL
The CHEST Editorial Team: Serving Our Contributors and Readers
By Dr. P. J. Mazzone
ORIGINAL RESEARCH
Pulmonary Arterial Histologic Lesions in Patients With COPD With Severe Pulmonary Hypertension
By Dr. V. Bunel, et al.
Pulmonary Edema Following Initiation of Parenteral Prostacyclin Therapy for Pulmonary Arterial Hypertension: A Retrospective Study
By Dr. N. A. Khan, et al.
Lung Allocation Score Thresholds Prioritize Survival After Lung Transplantation
By Dr. S. S. Li, et al.
EVIDENCE-BASED MEDICINE
Chronic Cough and Gastroesophageal Reflux in Children: CHEST Guideline and Expert Panel Report
By Dr. A. B. Chang, et al.
EDITORIAL
The CHEST Editorial Team: Serving Our Contributors and Readers
By Dr. P. J. Mazzone
ORIGINAL RESEARCH
Pulmonary Arterial Histologic Lesions in Patients With COPD With Severe Pulmonary Hypertension
By Dr. V. Bunel, et al.
Pulmonary Edema Following Initiation of Parenteral Prostacyclin Therapy for Pulmonary Arterial Hypertension: A Retrospective Study
By Dr. N. A. Khan, et al.
Lung Allocation Score Thresholds Prioritize Survival After Lung Transplantation
By Dr. S. S. Li, et al.
EVIDENCE-BASED MEDICINE
Chronic Cough and Gastroesophageal Reflux in Children: CHEST Guideline and Expert Panel Report
By Dr. A. B. Chang, et al.
Biologics. NetWork name change. Rapid sequence intubation. Competitive bidding. Genomic classifier.
Airways disorders
Asthma biologics: which patients?
Biologic therapies targeting specific inflammatory pathways promise “precision” medicine for severe asthma. Because these therapies are expensive and have different mechanisms of action, appropriate patient selection is crucial. To date, the biologics have been primarily used in severe asthma.
Severe asthma has been defined as “asthma which remains uncontrolled on high-dose inhaled corticosteroids plus a second controller for the previous year or systemic corticosteroids (for 50% or more of the previous year) to prevent it from becoming uncontrolled, or which remains uncontrolled despite this therapy” (Chung, et al. Eur Respir J. 2014;43:343).
Severe asthma is an infrequent to rare occurrence. Only 5% to 10 % of patients have severe asthma (Varsano, et al. Respir Med. 2017;123:131). Indeed, one study suggests that only 3.6% of patients meet criteria for it (Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896).
Not all difficult to control asthma is severe. With aggressive management of comorbidities and appropriate assessment of medication adherence/inhaler technique, up to 50% of uncontrolled asthmatics can reach therapeutic goals with traditional stepwise inhaler-based therapies (Tay, et al. J Allergy Clin Immunol Pract. 2017;5[4]:956; Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896). Yet, incorrect inhaler technique (MDI and DPI) is unacceptably frequent and has not improved over the past 40 years (Sanchis, et al. Chest. 2016;150[2]:394). Furthermore, correct inhaler technique was found in only 15.5% of health-care providers and has worsened in recent years (Plaza, et al. J Allergy Clin Immunol Pract. 2018;6[3]:987).
After establishing appropriate diagnosis, control of comorbidities, proper inhaler technique, and medication adherence, evaluation of a severe asthmatic’s inflammatory phenotype is necessary. Several phenotypes have emerged, including the severe allergic asthma phenotype and the severe eosinophilic asthma phenotype. Molecular phenotyping allows stratification into type–2–high vs type–2-low patients, which helps guide selection of the appropriate biologic. Options include: (1) anti-IgE (omalizumab); (2) anti-interleukin–5 (mepolizumab and reslizumab); (3) anti-interleukin-5 receptor alpha (benralizumab); and (4) anti-interleukin-4 receptor alpha and interleukin-13 (dupilumab).
Targeted biologics for specific severe asthma phenotypes may be cost effective long-term. However, long-term side effects need to be assessed and pharmaco-economic studies need to be performed.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Stuart M. Garay, MD, FCCP
Steering Committee Chair
Clinical research and quality improvement
Anew, redefined, and enriched
CHEST Physician readers may not know that Clinical Research recently changed its name to include quality improvement (QI). Its new mission is “to provide a forum for clinical research, QI, research ethics, and regulatory aspects, as topics of multidisciplinary discussion and collaboration.” Medical education has become a natural addition to our NetWork’s scope, as this field of scholarly activity has emerged and grown tremendously in the past decade. Interestingly, the number of session submissions to CHEST 2019 increased by a whopping 32% vs 2018, while our NetWork saw an even more impressive increase of 42% in submissions deemed Clinical, Education Research, or QI.
The initial concept of a CHEST Clinical Research NetWork was narrower, aiming to fill gaps between our members’ interests and activities with those of pharmaceutical industry partners and equipment and device manufacturers. Its scope evolved, and our NetWork became the home of all clinical research endeavors not pinned to a specific condition, disease class, or membership category.
QI emerged in other industries long ago, using scientific methods and known to be foundational for any organization’s capacity to survive in competitive environments, become more efficient, satisfy customers, improve outcomes, and develop better work flows and conditions for employees and business partners.
If the QI world strives to achieve certainty with confidence levels less than 0.0001, it is interesting that in our scientific quest we settle for P less than .05. Self-indulgence? Simplistically speaking, are we tolerating “defect rates” of 5%, while others aim for 6 sigma thresholds? These are a few thoughts on how health care can learn from other industries and apply more stringent standards for scholarly activities in clinical research, education, and QI.
In conclusion, while it continues to strive to build the infrastructure of future CHEST clinical research nodes for randomized or observational multicentric studies, Clinical Research and QI NetWork enthusiastically embraces the fields of medical education and QI into its enriched activity scope and scale.
Octavian C. Ioachimescu, MD, PhD, FCCP
Steering Committee Member
Critical care
Rapid sequence intubation
Casey and colleagues recently published a study (N Engl J Med. 2019;380[9]:811) that challenges the long-held view that rapid sequence intubation (RSI) should not include ventilation attempts between induction and laryngoscopy. Airway management purists will say that true RSI is pre-oxygenate, give a sedation agent followed immediately by a paralytic agent, and immediate laryngoscopy as soon as the patient is paralyzed. However, RSI has come to mean the use of a sedation agent and a paralytic agent without specific timing of when to give the paralytic.
Purists would also say RSI is done for patients who are at a high risk for aspiration. In this study, the amount of subjectively reported aspiration was actually lower in the YES BVM group: 2.5% vs 4.0%. The presence of a new opacity on chest radiograph within 48 hours was 16% vs 15%, suggesting that there is no significant difference in the incidence of aspiration.
In this study, 40% in the NO BVM group and 30% in the YES BVM group had O2 desaturations below 90%. These statistics highlight the fact that it is imperative to pre-oxygenate all patients who will undergo intubation. Critically ill patients have little reserve. These patients are on the steep portion of the oxygen dissociation curve. The saturations will drop quickly. It is better to avoid any desaturation if possible.
This study demonstrates that bag-mask ventilation between induction and laryngoscopy can help prevent severe desaturation with a number needed to treat to prevent one severe hypoxic event is nine.
John Gaillard, MD, FCCP
Steering Committee Member
Home-based mechanical ventilation and neuromuscular disease
Pressures of competitive bidding process
Advancements in invasive and noninvasive ventilator technology have allowed patients with neuromuscular conditions and severe COPD to transition from institutional care to living at home. Ventilator support is reserved for severe or progressive respiratory impairment where interruption would lead to serious negative consequences. Access to this technology does entail significant cost, as monthly rental fees range from $660 to $1,352 and yearly ventilator claims for chronic respiratory failure have increased from 29% in 2009 to 85% in 2015 (US Dept HHS, OIG Data brief 2016). There is a current proposal to include home mechanical ventilators with oxygen and other services in competitive bidding programs (CBP). Since oxygen was included in CBP, access to liquid oxygen systems and payments for oxygen have decreased significantly. Of patients using home oxygen since July 1, 2016, 59% reported difficulties with access to oxygen-related equipment and services (American Association for Respiratory Care Comment on Federal policies, aarc.org).
Ventilator-dependent patients should not be subjected to the pressures of CBP when trying to obtain the equipment, supplies, and access to experienced medical providers that are necessary to remain in their homes. Beyond denying ventilatory support to some, CBP may also result in other unintended consequences, including the increased use of otherwise avoidable tracheostomies to ensure coverage for appropriate services. CHEST, including the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork and other patient groups, has advocated that home mechanical ventilators should be permanently excluded from the CBP to protect these fragile and vulnerable patients.
Jeanette Brown, MD, PhD
Steering Committee Member
Interstitial and diffuse lung disease
New genomic classifier
A confident diagnosis of idiopathic pulmonary fibrosis (IPF) relies on the radiographic pattern of usual interstitial pneumonitis (UIP), although in some cases, histologic confirmation is warranted. Transbronchial biopsy (TBBx) does not provide adequate tissue for diagnosis, thus patients are subjected to the risk of a surgical biopsy. A promising new test can increase confidence in the diagnosis of IPF. The Envisia Genomic Classifier (Veracyte) is a recently approved test to aid in the diagnosis of IPF. It utilizes 190 genes and RNA sequencing, combined with machine learning, to create an algorithm that determines the presence of UIP on samples derived from TBBx.
A proof-of-principle study described the characteristics of the genomic classifier in distinguishing UIP for 53 training subjects and 31 test subjects. To ensure validation, this new test was compared with a diagnosis determined by histopathologic review from expert pathologists. Specificity was 86% and sensitivity 63% in distinguishing UIP vs non-UIP patterns. Although false-negatives were a concern due to IPFs heterogeneous involvement of the lung, combining multiple specimens from a single patient increased accuracy.
The recently-published BRAVE study was a validation and utilization study, proving the test’s success in identifying UIP on TBBx samples. The test was again compared with diagnostic histopathology, demonstrating 88% specificity and 70% sensitivity. In addition, two multidisciplinary teams had an 86% agreement on diagnoses when using pathology vs the genomic classifier. The classifier is commercially available and is covered by Medicare in the United States.
As with all new technology, it is expected that its use will increase in the future and that we will learn more about how, and in whom, to best utilize this tool.
Samantha D’Annunzio, MD
Steering Committee Member
References
Pankratz DG, et al. Ann Am Thorac Soc. 2017;14[11]1646.
Raghu G, et al. Lancet Respir Med. 2019 Jun;7(6):487.
Airways disorders
Asthma biologics: which patients?
Biologic therapies targeting specific inflammatory pathways promise “precision” medicine for severe asthma. Because these therapies are expensive and have different mechanisms of action, appropriate patient selection is crucial. To date, the biologics have been primarily used in severe asthma.
Severe asthma has been defined as “asthma which remains uncontrolled on high-dose inhaled corticosteroids plus a second controller for the previous year or systemic corticosteroids (for 50% or more of the previous year) to prevent it from becoming uncontrolled, or which remains uncontrolled despite this therapy” (Chung, et al. Eur Respir J. 2014;43:343).
Severe asthma is an infrequent to rare occurrence. Only 5% to 10 % of patients have severe asthma (Varsano, et al. Respir Med. 2017;123:131). Indeed, one study suggests that only 3.6% of patients meet criteria for it (Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896).
Not all difficult to control asthma is severe. With aggressive management of comorbidities and appropriate assessment of medication adherence/inhaler technique, up to 50% of uncontrolled asthmatics can reach therapeutic goals with traditional stepwise inhaler-based therapies (Tay, et al. J Allergy Clin Immunol Pract. 2017;5[4]:956; Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896). Yet, incorrect inhaler technique (MDI and DPI) is unacceptably frequent and has not improved over the past 40 years (Sanchis, et al. Chest. 2016;150[2]:394). Furthermore, correct inhaler technique was found in only 15.5% of health-care providers and has worsened in recent years (Plaza, et al. J Allergy Clin Immunol Pract. 2018;6[3]:987).
After establishing appropriate diagnosis, control of comorbidities, proper inhaler technique, and medication adherence, evaluation of a severe asthmatic’s inflammatory phenotype is necessary. Several phenotypes have emerged, including the severe allergic asthma phenotype and the severe eosinophilic asthma phenotype. Molecular phenotyping allows stratification into type–2–high vs type–2-low patients, which helps guide selection of the appropriate biologic. Options include: (1) anti-IgE (omalizumab); (2) anti-interleukin–5 (mepolizumab and reslizumab); (3) anti-interleukin-5 receptor alpha (benralizumab); and (4) anti-interleukin-4 receptor alpha and interleukin-13 (dupilumab).
Targeted biologics for specific severe asthma phenotypes may be cost effective long-term. However, long-term side effects need to be assessed and pharmaco-economic studies need to be performed.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Stuart M. Garay, MD, FCCP
Steering Committee Chair
Clinical research and quality improvement
Anew, redefined, and enriched
CHEST Physician readers may not know that Clinical Research recently changed its name to include quality improvement (QI). Its new mission is “to provide a forum for clinical research, QI, research ethics, and regulatory aspects, as topics of multidisciplinary discussion and collaboration.” Medical education has become a natural addition to our NetWork’s scope, as this field of scholarly activity has emerged and grown tremendously in the past decade. Interestingly, the number of session submissions to CHEST 2019 increased by a whopping 32% vs 2018, while our NetWork saw an even more impressive increase of 42% in submissions deemed Clinical, Education Research, or QI.
The initial concept of a CHEST Clinical Research NetWork was narrower, aiming to fill gaps between our members’ interests and activities with those of pharmaceutical industry partners and equipment and device manufacturers. Its scope evolved, and our NetWork became the home of all clinical research endeavors not pinned to a specific condition, disease class, or membership category.
QI emerged in other industries long ago, using scientific methods and known to be foundational for any organization’s capacity to survive in competitive environments, become more efficient, satisfy customers, improve outcomes, and develop better work flows and conditions for employees and business partners.
If the QI world strives to achieve certainty with confidence levels less than 0.0001, it is interesting that in our scientific quest we settle for P less than .05. Self-indulgence? Simplistically speaking, are we tolerating “defect rates” of 5%, while others aim for 6 sigma thresholds? These are a few thoughts on how health care can learn from other industries and apply more stringent standards for scholarly activities in clinical research, education, and QI.
In conclusion, while it continues to strive to build the infrastructure of future CHEST clinical research nodes for randomized or observational multicentric studies, Clinical Research and QI NetWork enthusiastically embraces the fields of medical education and QI into its enriched activity scope and scale.
Octavian C. Ioachimescu, MD, PhD, FCCP
Steering Committee Member
Critical care
Rapid sequence intubation
Casey and colleagues recently published a study (N Engl J Med. 2019;380[9]:811) that challenges the long-held view that rapid sequence intubation (RSI) should not include ventilation attempts between induction and laryngoscopy. Airway management purists will say that true RSI is pre-oxygenate, give a sedation agent followed immediately by a paralytic agent, and immediate laryngoscopy as soon as the patient is paralyzed. However, RSI has come to mean the use of a sedation agent and a paralytic agent without specific timing of when to give the paralytic.
Purists would also say RSI is done for patients who are at a high risk for aspiration. In this study, the amount of subjectively reported aspiration was actually lower in the YES BVM group: 2.5% vs 4.0%. The presence of a new opacity on chest radiograph within 48 hours was 16% vs 15%, suggesting that there is no significant difference in the incidence of aspiration.
In this study, 40% in the NO BVM group and 30% in the YES BVM group had O2 desaturations below 90%. These statistics highlight the fact that it is imperative to pre-oxygenate all patients who will undergo intubation. Critically ill patients have little reserve. These patients are on the steep portion of the oxygen dissociation curve. The saturations will drop quickly. It is better to avoid any desaturation if possible.
This study demonstrates that bag-mask ventilation between induction and laryngoscopy can help prevent severe desaturation with a number needed to treat to prevent one severe hypoxic event is nine.
John Gaillard, MD, FCCP
Steering Committee Member
Home-based mechanical ventilation and neuromuscular disease
Pressures of competitive bidding process
Advancements in invasive and noninvasive ventilator technology have allowed patients with neuromuscular conditions and severe COPD to transition from institutional care to living at home. Ventilator support is reserved for severe or progressive respiratory impairment where interruption would lead to serious negative consequences. Access to this technology does entail significant cost, as monthly rental fees range from $660 to $1,352 and yearly ventilator claims for chronic respiratory failure have increased from 29% in 2009 to 85% in 2015 (US Dept HHS, OIG Data brief 2016). There is a current proposal to include home mechanical ventilators with oxygen and other services in competitive bidding programs (CBP). Since oxygen was included in CBP, access to liquid oxygen systems and payments for oxygen have decreased significantly. Of patients using home oxygen since July 1, 2016, 59% reported difficulties with access to oxygen-related equipment and services (American Association for Respiratory Care Comment on Federal policies, aarc.org).
Ventilator-dependent patients should not be subjected to the pressures of CBP when trying to obtain the equipment, supplies, and access to experienced medical providers that are necessary to remain in their homes. Beyond denying ventilatory support to some, CBP may also result in other unintended consequences, including the increased use of otherwise avoidable tracheostomies to ensure coverage for appropriate services. CHEST, including the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork and other patient groups, has advocated that home mechanical ventilators should be permanently excluded from the CBP to protect these fragile and vulnerable patients.
Jeanette Brown, MD, PhD
Steering Committee Member
Interstitial and diffuse lung disease
New genomic classifier
A confident diagnosis of idiopathic pulmonary fibrosis (IPF) relies on the radiographic pattern of usual interstitial pneumonitis (UIP), although in some cases, histologic confirmation is warranted. Transbronchial biopsy (TBBx) does not provide adequate tissue for diagnosis, thus patients are subjected to the risk of a surgical biopsy. A promising new test can increase confidence in the diagnosis of IPF. The Envisia Genomic Classifier (Veracyte) is a recently approved test to aid in the diagnosis of IPF. It utilizes 190 genes and RNA sequencing, combined with machine learning, to create an algorithm that determines the presence of UIP on samples derived from TBBx.
A proof-of-principle study described the characteristics of the genomic classifier in distinguishing UIP for 53 training subjects and 31 test subjects. To ensure validation, this new test was compared with a diagnosis determined by histopathologic review from expert pathologists. Specificity was 86% and sensitivity 63% in distinguishing UIP vs non-UIP patterns. Although false-negatives were a concern due to IPFs heterogeneous involvement of the lung, combining multiple specimens from a single patient increased accuracy.
The recently-published BRAVE study was a validation and utilization study, proving the test’s success in identifying UIP on TBBx samples. The test was again compared with diagnostic histopathology, demonstrating 88% specificity and 70% sensitivity. In addition, two multidisciplinary teams had an 86% agreement on diagnoses when using pathology vs the genomic classifier. The classifier is commercially available and is covered by Medicare in the United States.
As with all new technology, it is expected that its use will increase in the future and that we will learn more about how, and in whom, to best utilize this tool.
Samantha D’Annunzio, MD
Steering Committee Member
References
Pankratz DG, et al. Ann Am Thorac Soc. 2017;14[11]1646.
Raghu G, et al. Lancet Respir Med. 2019 Jun;7(6):487.
Airways disorders
Asthma biologics: which patients?
Biologic therapies targeting specific inflammatory pathways promise “precision” medicine for severe asthma. Because these therapies are expensive and have different mechanisms of action, appropriate patient selection is crucial. To date, the biologics have been primarily used in severe asthma.
Severe asthma has been defined as “asthma which remains uncontrolled on high-dose inhaled corticosteroids plus a second controller for the previous year or systemic corticosteroids (for 50% or more of the previous year) to prevent it from becoming uncontrolled, or which remains uncontrolled despite this therapy” (Chung, et al. Eur Respir J. 2014;43:343).
Severe asthma is an infrequent to rare occurrence. Only 5% to 10 % of patients have severe asthma (Varsano, et al. Respir Med. 2017;123:131). Indeed, one study suggests that only 3.6% of patients meet criteria for it (Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896).
Not all difficult to control asthma is severe. With aggressive management of comorbidities and appropriate assessment of medication adherence/inhaler technique, up to 50% of uncontrolled asthmatics can reach therapeutic goals with traditional stepwise inhaler-based therapies (Tay, et al. J Allergy Clin Immunol Pract. 2017;5[4]:956; Hekking, et al. J Allergy Clin Immunol. 2015;135[4]:896). Yet, incorrect inhaler technique (MDI and DPI) is unacceptably frequent and has not improved over the past 40 years (Sanchis, et al. Chest. 2016;150[2]:394). Furthermore, correct inhaler technique was found in only 15.5% of health-care providers and has worsened in recent years (Plaza, et al. J Allergy Clin Immunol Pract. 2018;6[3]:987).
After establishing appropriate diagnosis, control of comorbidities, proper inhaler technique, and medication adherence, evaluation of a severe asthmatic’s inflammatory phenotype is necessary. Several phenotypes have emerged, including the severe allergic asthma phenotype and the severe eosinophilic asthma phenotype. Molecular phenotyping allows stratification into type–2–high vs type–2-low patients, which helps guide selection of the appropriate biologic. Options include: (1) anti-IgE (omalizumab); (2) anti-interleukin–5 (mepolizumab and reslizumab); (3) anti-interleukin-5 receptor alpha (benralizumab); and (4) anti-interleukin-4 receptor alpha and interleukin-13 (dupilumab).
Targeted biologics for specific severe asthma phenotypes may be cost effective long-term. However, long-term side effects need to be assessed and pharmaco-economic studies need to be performed.
Megan Conroy, MD
Steering Committee Fellow-in-Training
Stuart M. Garay, MD, FCCP
Steering Committee Chair
Clinical research and quality improvement
Anew, redefined, and enriched
CHEST Physician readers may not know that Clinical Research recently changed its name to include quality improvement (QI). Its new mission is “to provide a forum for clinical research, QI, research ethics, and regulatory aspects, as topics of multidisciplinary discussion and collaboration.” Medical education has become a natural addition to our NetWork’s scope, as this field of scholarly activity has emerged and grown tremendously in the past decade. Interestingly, the number of session submissions to CHEST 2019 increased by a whopping 32% vs 2018, while our NetWork saw an even more impressive increase of 42% in submissions deemed Clinical, Education Research, or QI.
The initial concept of a CHEST Clinical Research NetWork was narrower, aiming to fill gaps between our members’ interests and activities with those of pharmaceutical industry partners and equipment and device manufacturers. Its scope evolved, and our NetWork became the home of all clinical research endeavors not pinned to a specific condition, disease class, or membership category.
QI emerged in other industries long ago, using scientific methods and known to be foundational for any organization’s capacity to survive in competitive environments, become more efficient, satisfy customers, improve outcomes, and develop better work flows and conditions for employees and business partners.
If the QI world strives to achieve certainty with confidence levels less than 0.0001, it is interesting that in our scientific quest we settle for P less than .05. Self-indulgence? Simplistically speaking, are we tolerating “defect rates” of 5%, while others aim for 6 sigma thresholds? These are a few thoughts on how health care can learn from other industries and apply more stringent standards for scholarly activities in clinical research, education, and QI.
In conclusion, while it continues to strive to build the infrastructure of future CHEST clinical research nodes for randomized or observational multicentric studies, Clinical Research and QI NetWork enthusiastically embraces the fields of medical education and QI into its enriched activity scope and scale.
Octavian C. Ioachimescu, MD, PhD, FCCP
Steering Committee Member
Critical care
Rapid sequence intubation
Casey and colleagues recently published a study (N Engl J Med. 2019;380[9]:811) that challenges the long-held view that rapid sequence intubation (RSI) should not include ventilation attempts between induction and laryngoscopy. Airway management purists will say that true RSI is pre-oxygenate, give a sedation agent followed immediately by a paralytic agent, and immediate laryngoscopy as soon as the patient is paralyzed. However, RSI has come to mean the use of a sedation agent and a paralytic agent without specific timing of when to give the paralytic.
Purists would also say RSI is done for patients who are at a high risk for aspiration. In this study, the amount of subjectively reported aspiration was actually lower in the YES BVM group: 2.5% vs 4.0%. The presence of a new opacity on chest radiograph within 48 hours was 16% vs 15%, suggesting that there is no significant difference in the incidence of aspiration.
In this study, 40% in the NO BVM group and 30% in the YES BVM group had O2 desaturations below 90%. These statistics highlight the fact that it is imperative to pre-oxygenate all patients who will undergo intubation. Critically ill patients have little reserve. These patients are on the steep portion of the oxygen dissociation curve. The saturations will drop quickly. It is better to avoid any desaturation if possible.
This study demonstrates that bag-mask ventilation between induction and laryngoscopy can help prevent severe desaturation with a number needed to treat to prevent one severe hypoxic event is nine.
John Gaillard, MD, FCCP
Steering Committee Member
Home-based mechanical ventilation and neuromuscular disease
Pressures of competitive bidding process
Advancements in invasive and noninvasive ventilator technology have allowed patients with neuromuscular conditions and severe COPD to transition from institutional care to living at home. Ventilator support is reserved for severe or progressive respiratory impairment where interruption would lead to serious negative consequences. Access to this technology does entail significant cost, as monthly rental fees range from $660 to $1,352 and yearly ventilator claims for chronic respiratory failure have increased from 29% in 2009 to 85% in 2015 (US Dept HHS, OIG Data brief 2016). There is a current proposal to include home mechanical ventilators with oxygen and other services in competitive bidding programs (CBP). Since oxygen was included in CBP, access to liquid oxygen systems and payments for oxygen have decreased significantly. Of patients using home oxygen since July 1, 2016, 59% reported difficulties with access to oxygen-related equipment and services (American Association for Respiratory Care Comment on Federal policies, aarc.org).
Ventilator-dependent patients should not be subjected to the pressures of CBP when trying to obtain the equipment, supplies, and access to experienced medical providers that are necessary to remain in their homes. Beyond denying ventilatory support to some, CBP may also result in other unintended consequences, including the increased use of otherwise avoidable tracheostomies to ensure coverage for appropriate services. CHEST, including the Home-Based Mechanical Ventilation and Neuromuscular Disease NetWork and other patient groups, has advocated that home mechanical ventilators should be permanently excluded from the CBP to protect these fragile and vulnerable patients.
Jeanette Brown, MD, PhD
Steering Committee Member
Interstitial and diffuse lung disease
New genomic classifier
A confident diagnosis of idiopathic pulmonary fibrosis (IPF) relies on the radiographic pattern of usual interstitial pneumonitis (UIP), although in some cases, histologic confirmation is warranted. Transbronchial biopsy (TBBx) does not provide adequate tissue for diagnosis, thus patients are subjected to the risk of a surgical biopsy. A promising new test can increase confidence in the diagnosis of IPF. The Envisia Genomic Classifier (Veracyte) is a recently approved test to aid in the diagnosis of IPF. It utilizes 190 genes and RNA sequencing, combined with machine learning, to create an algorithm that determines the presence of UIP on samples derived from TBBx.
A proof-of-principle study described the characteristics of the genomic classifier in distinguishing UIP for 53 training subjects and 31 test subjects. To ensure validation, this new test was compared with a diagnosis determined by histopathologic review from expert pathologists. Specificity was 86% and sensitivity 63% in distinguishing UIP vs non-UIP patterns. Although false-negatives were a concern due to IPFs heterogeneous involvement of the lung, combining multiple specimens from a single patient increased accuracy.
The recently-published BRAVE study was a validation and utilization study, proving the test’s success in identifying UIP on TBBx samples. The test was again compared with diagnostic histopathology, demonstrating 88% specificity and 70% sensitivity. In addition, two multidisciplinary teams had an 86% agreement on diagnoses when using pathology vs the genomic classifier. The classifier is commercially available and is covered by Medicare in the United States.
As with all new technology, it is expected that its use will increase in the future and that we will learn more about how, and in whom, to best utilize this tool.
Samantha D’Annunzio, MD
Steering Committee Member
References
Pankratz DG, et al. Ann Am Thorac Soc. 2017;14[11]1646.
Raghu G, et al. Lancet Respir Med. 2019 Jun;7(6):487.
From the EVP/CEO: Opportunities for CHEST to broaden its reach across the globe
Our recent congress in Bangkok, Thailand, was just the beginning of our plans to expand the CHEST brand and share our innovative education across the globe. The congress held this past April served as a successful launch pad that included attendance of over 1,000 delegates who represented 57 countries and featured innovative and diverse educational opportunities that incorporated the best of the CHEST Annual Meeting, including interactive lectures, recent advances in clinical practice and science, guided poster presentations, and hands-on simulation opportunities. The exceptional program is attributed to the partnership with the Thoracic Society of Thailand; the Chair, Dr. David Schulman; the faculty, for delivering such an innovative and engaging educational event; and many others who planned, supported, and participated in this impressive event.
In order to have a greater impact on the way that lung diseases, critical care conditions, and sleep disorders are diagnosed and treated, CHEST has been actively expanding its reach and the way it plans, develops, and executes its international educational strategy. This was CHEST’s first venture into a new model designed around hosting one large congress outside of the United States and one smaller regional congress each year. This year, Athens, Greece, followed in June as the site of the regional congress. In subsequent years, we will be increasing the offerings and the options for these regional international events.
Newly announced, we are planning a regional program in Singapore for late fall of 2020 or early 2021. This program will be conducted in collaboration with the National University Health System and Dr. Pyng Lee. Also, next year, the CHEST Congress will be held in Bologna, Italy, June 25-27, in collaboration with the CHEST delegation from Italy, led by Dr. Francesco de Blasio. The program chairs for this event are Drs. John Studdard and William Kelly.
We are excited to broaden our international educational reach through this plan. By refining, growing, and building upon this new model and developing more live learning, hands-on simulation, CHEST gamification, and other interactive components, we continually provide the most cutting-edge learning opportunities available across the globe. We are accomplishing this through partnering with global societies and CHEST delegations to identify unmet educational needs by region and patient base. Through this expansion, we hope to continue our fight to “Crush” lung disease wherever it exists.
Our recent congress in Bangkok, Thailand, was just the beginning of our plans to expand the CHEST brand and share our innovative education across the globe. The congress held this past April served as a successful launch pad that included attendance of over 1,000 delegates who represented 57 countries and featured innovative and diverse educational opportunities that incorporated the best of the CHEST Annual Meeting, including interactive lectures, recent advances in clinical practice and science, guided poster presentations, and hands-on simulation opportunities. The exceptional program is attributed to the partnership with the Thoracic Society of Thailand; the Chair, Dr. David Schulman; the faculty, for delivering such an innovative and engaging educational event; and many others who planned, supported, and participated in this impressive event.
In order to have a greater impact on the way that lung diseases, critical care conditions, and sleep disorders are diagnosed and treated, CHEST has been actively expanding its reach and the way it plans, develops, and executes its international educational strategy. This was CHEST’s first venture into a new model designed around hosting one large congress outside of the United States and one smaller regional congress each year. This year, Athens, Greece, followed in June as the site of the regional congress. In subsequent years, we will be increasing the offerings and the options for these regional international events.
Newly announced, we are planning a regional program in Singapore for late fall of 2020 or early 2021. This program will be conducted in collaboration with the National University Health System and Dr. Pyng Lee. Also, next year, the CHEST Congress will be held in Bologna, Italy, June 25-27, in collaboration with the CHEST delegation from Italy, led by Dr. Francesco de Blasio. The program chairs for this event are Drs. John Studdard and William Kelly.
We are excited to broaden our international educational reach through this plan. By refining, growing, and building upon this new model and developing more live learning, hands-on simulation, CHEST gamification, and other interactive components, we continually provide the most cutting-edge learning opportunities available across the globe. We are accomplishing this through partnering with global societies and CHEST delegations to identify unmet educational needs by region and patient base. Through this expansion, we hope to continue our fight to “Crush” lung disease wherever it exists.
Our recent congress in Bangkok, Thailand, was just the beginning of our plans to expand the CHEST brand and share our innovative education across the globe. The congress held this past April served as a successful launch pad that included attendance of over 1,000 delegates who represented 57 countries and featured innovative and diverse educational opportunities that incorporated the best of the CHEST Annual Meeting, including interactive lectures, recent advances in clinical practice and science, guided poster presentations, and hands-on simulation opportunities. The exceptional program is attributed to the partnership with the Thoracic Society of Thailand; the Chair, Dr. David Schulman; the faculty, for delivering such an innovative and engaging educational event; and many others who planned, supported, and participated in this impressive event.
In order to have a greater impact on the way that lung diseases, critical care conditions, and sleep disorders are diagnosed and treated, CHEST has been actively expanding its reach and the way it plans, develops, and executes its international educational strategy. This was CHEST’s first venture into a new model designed around hosting one large congress outside of the United States and one smaller regional congress each year. This year, Athens, Greece, followed in June as the site of the regional congress. In subsequent years, we will be increasing the offerings and the options for these regional international events.
Newly announced, we are planning a regional program in Singapore for late fall of 2020 or early 2021. This program will be conducted in collaboration with the National University Health System and Dr. Pyng Lee. Also, next year, the CHEST Congress will be held in Bologna, Italy, June 25-27, in collaboration with the CHEST delegation from Italy, led by Dr. Francesco de Blasio. The program chairs for this event are Drs. John Studdard and William Kelly.
We are excited to broaden our international educational reach through this plan. By refining, growing, and building upon this new model and developing more live learning, hands-on simulation, CHEST gamification, and other interactive components, we continually provide the most cutting-edge learning opportunities available across the globe. We are accomplishing this through partnering with global societies and CHEST delegations to identify unmet educational needs by region and patient base. Through this expansion, we hope to continue our fight to “Crush” lung disease wherever it exists.
In Memoriam
CHEST has been notified of the following deaths.
We extend our sincere condolences.
John W. Thomas, MD (2018)
Frederick J Curley, MD (2018)
CHEST has been notified of the following deaths.
We extend our sincere condolences.
John W. Thomas, MD (2018)
Frederick J Curley, MD (2018)
CHEST has been notified of the following deaths.
We extend our sincere condolences.
John W. Thomas, MD (2018)
Frederick J Curley, MD (2018)
Restless legs syndrome: Update on evaluation and treatment
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
Restless legs syndrome (RLS) is a very common disease affecting about 10% of Caucasian adults with about one third of them having RLS symptoms severe enough to require treatment.
Although many patients still go undiagnosed or misdiagnosed, the diagnosis is easily established with the five diagnostic criteria that are simplified by the acronym URGES:
1. Urge to move the legs associated with unpleasant leg sensations.
2. Rest induces symptoms.
3. Gets better with activity.
4. Evening and nighttime worsening.
5. Solely not accounted by another medical or behavioral condition.
The diagnosis is based completely upon the history. However, supplemental tests can be helpful to rule out underlying conditions that increase the risk of RLS. Routine lab tests, such as serum creatinine (to rule out renal disease), TSH (to rule out thyroid disease), and a CBC/ferritin/iron with transferrin saturation (to rule out low iron stores) should be ordered if not done recently.
A polysomnographic sleep study should not be ordered unless there is a strong suspicion that sleep apnea is present. Even very frequent PLM (periodic limb movements) are not that helpful in confirming the diagnosis of RLS since they are nonspecific and often occurring with drug treatment (SSRIs, SNRIs) and many medical conditions such as sleep apnea, narcolepsy, and REM behavior disorder.
The paradigm for treating RLS has been presented in the consensus article published in 2013 (Silber MH, et al. Mayo Clin Proc. 2013 Sep;88[9]:977). Since 2013, there has been a gradual shift of that paradigm that recommended starting an approved dopamine agonist (pramipexole, ropinirole, or rotigotine) or an alpha-2-delta ligand (gabapentin enacarbil, gabapentin, or pregabalin) as first-line treatment. Although dopamine agonists provide excellent relief of RLS symptoms initially, with time, they tend to markedly worsen RLS. This process is called RLS augmentation and has become one of the most common causes of refractory RLS and difficult-to-treat patients.
RLS augmentation typically onsets a few months to several years after starting a short-acting dopamine agonist (DA) like pramipexole or ropinirole. It presents with symptoms occurring a few hours earlier than prior to starting the medication, symptoms becoming more intense with less rest time needed to trigger RLS symptoms, drugs becoming less effective both in effectiveness and duration of action, and spread of symptoms to other body parts (arms, trunk, and even head). The majority of physicians mistake this worsening of RLS for the natural progression of the disease and, thus, increase the dose of the DA, which provides temporary improvement. Further increases become progressively necessary until the patient is receiving very large doses, often exceeding 10 times the FDA maximum recommended doses. Eventually, further dose increments provide minimal additional benefit, leaving patients with severe, around the clock RLS symptoms causing extreme misery. To be more aware of augmentation, physicians should consider augmentation may be occurring whenever a patient who has been on a regimen of stable dopamine agonist treatment for at least 6 months requests more medication.
The incidence of augmentation for patients taking short-acting DA drugs is about 7% to 8% per year so that by 10 years, the vast majority of these patients with RLS are experiencing augmentation. Since it has been over 13 years since pramipexole and ropinirole have been approved for treating RLS, currently, over 75% of patients referred to national RLS experts are referred due to augmentation (although the actual referral diagnosis is often “refractory RLS”). Despite the concerns about augmentation, the short-acting DA drugs are by far the most commonly prescribed medications for initial treatment of RLS.
To help educate doctors about RLS augmentation, a consensus article was published in 2016 promoting guidelines for the prevention and treatment of RLS augmentation (Garcia-Borreguero D, et al. Sleep Med. 2016;21:1-11). Since augmentation occurs only with dopaminergic drugs (with the exception of tramadol), considering the use of nondopaminergic drugs for first-line therapy of RLS would dramatically decrease the occurrence of augmentation. This is a clear shift in the paradigm of choosing equally amongst the approved RLS drugs.
Unless contraindicated, the alpha-2-delta drugs should be the first consideration for treating new RLS patients. These drugs can be as effective as the DA drugs but cannot cause augmentation and, also, do not cause Impulse control disorders, which occur with the use of DAs. Furthermore, they reduce insomnia and anxiety that are both associated with RLS. The use of these drugs may be limited by their side effects, which include CNS depressive effects (sedation, dizziness, decreased balance or cognition) or depression.
When the alpha-2-delta ligands can’t be used due to lack of efficacy, side effects or cost, the DA drugs may then be appropriate. The rotigotine patch has the lowest incidence of augmentation, especially at the approved doses of up to 3 mg. If the rotigotine patch cannot be used (most often due to skin side effects or cost), then the short-acting DA drugs may be employed. Augmentation may be prevented or significantly delayed by starting these drugs at their lowest dose (.125 mg for pramipexole and .25 mg for ropinirole) and increasing the dose as little as possible, definitely not exceeding the approved RLS limits of .5 mg for pramipexole and 4 mg for ropinirole. My personal suggestion is not to exceed .25 mg for pramipexole and 1 mg for ropinirole as augmentation is dose-related but may occur at even the lowest doses. When patients need and request increased treatment for their RLS, rather than increasing the dose of the DA, instead, consider adding other medications, such as the alpha-2-delta ligands or even low dose opioids.
Managing augmentation is typically a very challenging problem for both the physician and patient; this is described in detail in the augmentation article referenced above. Decreasing, or better yet eliminating , the short-acting DA is the preferred method for treating augmentation. However, upon elimination of the DA, there is a short period of 1 to 4 weeks (average of 10-12 days) when the RLS symptoms get dramatically worse. Patients typically experience extremely severe RLS symptoms around the clock and may not be able to sleep at all until the RLS calms down. Most often, only low dose opioid treatment will enable them to get through this transition. The augmentation article (with its algorithm) may help physicians manage augmentation, but patients with severe augmentation may need referral to an RLS specialist who is experienced in this area and who is comfortable managing the disease with opioids.
Low iron levels are often associated with RLS, cause RLS symptoms to worsen, and increase the risk of augmentation (Allen RP, et al, and the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2018;41:27). We typically suggest that patients with ferritin levels under 100 mcg/L should get supplemental iron. However, oral iron absorption is very limited when the patient’s ferritin is above 50 mcg/L and, therefore, most patients may require IV iron to improve their RLS symptoms. There are several IV iron preparations but only iron dextrose, iron carboxymaltose, and ferumoxytol are effective. When the ferritin level is increased to over 200 µg/L, RLS symptoms may be dramatically improved.
With the currently available treatment options, most patients should have their RLS symptoms well controlled without developing augmentation.
Dr. Buchfuhrer is with Stanford University, Department of Psychiatry and Behavioral Sciences in the School of Medicine, Division of Sleep Medicine, Stanford, Calif.
Fill your day in New Orleans
No matter if you’re only in New Orleans during #CHEST2019 for a day or for the entire meeting, we’ve got you covered on how to spend your time in the Big Easy outside of sessions and CHEST events!
Rise and shine! If breakfast is the most important meal of the day, we’ve got the perfect way to start your morning before heading over to the Ernest N. Morial Convention Center to begin a day of learning. For a quick bite, try the ever popular Cafe du Monde Riverwalk next to the convention center for a light breakfast of beignets and café au lait, or Fulton Street Cafe.
Lunchtime: For something a little more hearty, head to Green Goddess in the French Quarter for southern comfort food. There’s something for everyone, as you’ll even find some vegan dishes on the menu. If you have time for a longer mid-day break, check out a Garden District Tour, Steam Boat on the River, or relax in Jackson Square.
Evening: You’ve had a long day of sessions, lectures, and exploring the exhibit hall, and now you want to wind down with a good meal (and maybe a drink!). For a slower vibe and space to linger and enjoy yourself, take an Uber/Lyft over to La Petite Grocery on Magazine Street for some tasty, traditional New Orleans dishes.
If you’re a night owl or looking for a late-night activity with a group of your friends and peers, there are plenty of places to find a cocktail on Bourbon Street, or listen to live jazz music along Frenchman Street.
There are many more things you can check out in New Orleans, and we hope you enjoy your stay during CHEST 2019.
*Note: If you’re staying in the hotel block near the convention center, many of the attractions, including the Convention Center, will be a short walking distance. Otherwise, we suggest taking an Uber or Lyft to reach your destination.
No matter if you’re only in New Orleans during #CHEST2019 for a day or for the entire meeting, we’ve got you covered on how to spend your time in the Big Easy outside of sessions and CHEST events!
Rise and shine! If breakfast is the most important meal of the day, we’ve got the perfect way to start your morning before heading over to the Ernest N. Morial Convention Center to begin a day of learning. For a quick bite, try the ever popular Cafe du Monde Riverwalk next to the convention center for a light breakfast of beignets and café au lait, or Fulton Street Cafe.
Lunchtime: For something a little more hearty, head to Green Goddess in the French Quarter for southern comfort food. There’s something for everyone, as you’ll even find some vegan dishes on the menu. If you have time for a longer mid-day break, check out a Garden District Tour, Steam Boat on the River, or relax in Jackson Square.
Evening: You’ve had a long day of sessions, lectures, and exploring the exhibit hall, and now you want to wind down with a good meal (and maybe a drink!). For a slower vibe and space to linger and enjoy yourself, take an Uber/Lyft over to La Petite Grocery on Magazine Street for some tasty, traditional New Orleans dishes.
If you’re a night owl or looking for a late-night activity with a group of your friends and peers, there are plenty of places to find a cocktail on Bourbon Street, or listen to live jazz music along Frenchman Street.
There are many more things you can check out in New Orleans, and we hope you enjoy your stay during CHEST 2019.
*Note: If you’re staying in the hotel block near the convention center, many of the attractions, including the Convention Center, will be a short walking distance. Otherwise, we suggest taking an Uber or Lyft to reach your destination.
No matter if you’re only in New Orleans during #CHEST2019 for a day or for the entire meeting, we’ve got you covered on how to spend your time in the Big Easy outside of sessions and CHEST events!
Rise and shine! If breakfast is the most important meal of the day, we’ve got the perfect way to start your morning before heading over to the Ernest N. Morial Convention Center to begin a day of learning. For a quick bite, try the ever popular Cafe du Monde Riverwalk next to the convention center for a light breakfast of beignets and café au lait, or Fulton Street Cafe.
Lunchtime: For something a little more hearty, head to Green Goddess in the French Quarter for southern comfort food. There’s something for everyone, as you’ll even find some vegan dishes on the menu. If you have time for a longer mid-day break, check out a Garden District Tour, Steam Boat on the River, or relax in Jackson Square.
Evening: You’ve had a long day of sessions, lectures, and exploring the exhibit hall, and now you want to wind down with a good meal (and maybe a drink!). For a slower vibe and space to linger and enjoy yourself, take an Uber/Lyft over to La Petite Grocery on Magazine Street for some tasty, traditional New Orleans dishes.
If you’re a night owl or looking for a late-night activity with a group of your friends and peers, there are plenty of places to find a cocktail on Bourbon Street, or listen to live jazz music along Frenchman Street.
There are many more things you can check out in New Orleans, and we hope you enjoy your stay during CHEST 2019.
*Note: If you’re staying in the hotel block near the convention center, many of the attractions, including the Convention Center, will be a short walking distance. Otherwise, we suggest taking an Uber or Lyft to reach your destination.