User login
Crizanlizumab shows posttreatment effect in sickle cell
FORT LAUDERDALE, FLA. – Sickle cell patients who received high-dose crizanlizumab had fewer vaso-occlusive crises (VOCs) than patients on a low-dose regimen a year after stopping the treatment, findings from a real-world follow-up study suggest.
But hospitalization rates and use of other health care resources were similar during and after therapy, regardless of dose, Nirmish Shah, MD, of Duke Health in Durham, N.C., reported at the annual meeting of the Foundation for Sickle Cell Disease Research.
“This is our attempt at a real-world study of patients after being in a big study that has good results and what happens to them afterward in regard to VOCs and health care utilization,” Dr. Shah said.
He reported results from the SUCCESSOR trial – a multicenter, retrospective cohort study – that evaluated a subset of 48 adult patients up to a year after they completed the SUSTAIN placebo-controlled phase 2 trial of crizanlizumab, a P-selectin inhibitor designed to control sickle cell pain crises.
In SUSTAIN, researchers evaluated two different dosages of crizanlizumab: 5 mg/kg and 2.5 mg/kg.
In the follow-up study, researchers obtained data from medical records over the study period November 2014 to March 2017. Crizanlizumab was not administered in the 52 weeks following the SUSTAIN trial.
They found that the subset of patients on the high dose of crizanlizumab had annual VOC rates of 2.7, compared with 4.0 among patients on the low dose of the drug. By comparison, VOC rates in SUSTAIN were 1.7 per year for the 5-mg/kg–dose group and 3.2 per year for the 2.5-mg/kg–dose group.
Overall, at least 60% of patients in the follow-up study had at least one hospitalization in the year after SUSTAIN. In the higher-dose group, the rates of hospitalization were similar in both SUSTAIN and SUCCESSOR – 53%. In the lower-dose group, hospitalization rates were 67% and 61% in SUCCESSOR and SUSTAIN, respectively.
“Among the previously treated patients with crizanlizumab, the total number of emergency department visits did seem to be higher in SUCCESSOR,” Dr. Shah said.
The study was limited by its retrospective nature and the fact that full data sets and follow-up were available on only a small number of patients, Dr. Shah said. “It was underpowered for a statistical analysis between groups,” he said. The goal was to determine the drug’s effect after treatment is discontinued, he said.
Dr. Shah reported a financial relationship with Novartis, which is developing crizanlizumab.
SOURCE: Shah N et al. FSCDR 2019, Abstract JSCDH-D-19-00031.
FORT LAUDERDALE, FLA. – Sickle cell patients who received high-dose crizanlizumab had fewer vaso-occlusive crises (VOCs) than patients on a low-dose regimen a year after stopping the treatment, findings from a real-world follow-up study suggest.
But hospitalization rates and use of other health care resources were similar during and after therapy, regardless of dose, Nirmish Shah, MD, of Duke Health in Durham, N.C., reported at the annual meeting of the Foundation for Sickle Cell Disease Research.
“This is our attempt at a real-world study of patients after being in a big study that has good results and what happens to them afterward in regard to VOCs and health care utilization,” Dr. Shah said.
He reported results from the SUCCESSOR trial – a multicenter, retrospective cohort study – that evaluated a subset of 48 adult patients up to a year after they completed the SUSTAIN placebo-controlled phase 2 trial of crizanlizumab, a P-selectin inhibitor designed to control sickle cell pain crises.
In SUSTAIN, researchers evaluated two different dosages of crizanlizumab: 5 mg/kg and 2.5 mg/kg.
In the follow-up study, researchers obtained data from medical records over the study period November 2014 to March 2017. Crizanlizumab was not administered in the 52 weeks following the SUSTAIN trial.
They found that the subset of patients on the high dose of crizanlizumab had annual VOC rates of 2.7, compared with 4.0 among patients on the low dose of the drug. By comparison, VOC rates in SUSTAIN were 1.7 per year for the 5-mg/kg–dose group and 3.2 per year for the 2.5-mg/kg–dose group.
Overall, at least 60% of patients in the follow-up study had at least one hospitalization in the year after SUSTAIN. In the higher-dose group, the rates of hospitalization were similar in both SUSTAIN and SUCCESSOR – 53%. In the lower-dose group, hospitalization rates were 67% and 61% in SUCCESSOR and SUSTAIN, respectively.
“Among the previously treated patients with crizanlizumab, the total number of emergency department visits did seem to be higher in SUCCESSOR,” Dr. Shah said.
The study was limited by its retrospective nature and the fact that full data sets and follow-up were available on only a small number of patients, Dr. Shah said. “It was underpowered for a statistical analysis between groups,” he said. The goal was to determine the drug’s effect after treatment is discontinued, he said.
Dr. Shah reported a financial relationship with Novartis, which is developing crizanlizumab.
SOURCE: Shah N et al. FSCDR 2019, Abstract JSCDH-D-19-00031.
FORT LAUDERDALE, FLA. – Sickle cell patients who received high-dose crizanlizumab had fewer vaso-occlusive crises (VOCs) than patients on a low-dose regimen a year after stopping the treatment, findings from a real-world follow-up study suggest.
But hospitalization rates and use of other health care resources were similar during and after therapy, regardless of dose, Nirmish Shah, MD, of Duke Health in Durham, N.C., reported at the annual meeting of the Foundation for Sickle Cell Disease Research.
“This is our attempt at a real-world study of patients after being in a big study that has good results and what happens to them afterward in regard to VOCs and health care utilization,” Dr. Shah said.
He reported results from the SUCCESSOR trial – a multicenter, retrospective cohort study – that evaluated a subset of 48 adult patients up to a year after they completed the SUSTAIN placebo-controlled phase 2 trial of crizanlizumab, a P-selectin inhibitor designed to control sickle cell pain crises.
In SUSTAIN, researchers evaluated two different dosages of crizanlizumab: 5 mg/kg and 2.5 mg/kg.
In the follow-up study, researchers obtained data from medical records over the study period November 2014 to March 2017. Crizanlizumab was not administered in the 52 weeks following the SUSTAIN trial.
They found that the subset of patients on the high dose of crizanlizumab had annual VOC rates of 2.7, compared with 4.0 among patients on the low dose of the drug. By comparison, VOC rates in SUSTAIN were 1.7 per year for the 5-mg/kg–dose group and 3.2 per year for the 2.5-mg/kg–dose group.
Overall, at least 60% of patients in the follow-up study had at least one hospitalization in the year after SUSTAIN. In the higher-dose group, the rates of hospitalization were similar in both SUSTAIN and SUCCESSOR – 53%. In the lower-dose group, hospitalization rates were 67% and 61% in SUCCESSOR and SUSTAIN, respectively.
“Among the previously treated patients with crizanlizumab, the total number of emergency department visits did seem to be higher in SUCCESSOR,” Dr. Shah said.
The study was limited by its retrospective nature and the fact that full data sets and follow-up were available on only a small number of patients, Dr. Shah said. “It was underpowered for a statistical analysis between groups,” he said. The goal was to determine the drug’s effect after treatment is discontinued, he said.
Dr. Shah reported a financial relationship with Novartis, which is developing crizanlizumab.
SOURCE: Shah N et al. FSCDR 2019, Abstract JSCDH-D-19-00031.
REPORTING FROM FSCDR 2019
Once-weekly teriparatide still achieves bone mineral density gains
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
FROM OSTEOPOROSIS AND SARCOPENIA
Low-fat dairy associated with decreased risk of type 2 diabetes
SAN FRANCISCO – in a review of almost 200,000 participants presented at the annual scientific sessions at the American Diabetes Association.
Increasing yogurt consumption was also independently associated with a moderately lower risk of type 2 diabetes, and increasing cheese consumption with a moderately higher risk.
It might have been that yogurt and low-fat milk were simply indicators of healthier living and that cheese consumption – in the study, most commonly on pizza or as processed slices on cheeseburgers – indicated a less healthy way of life, but “we tried our best to control for confounders,” said study lead Jean-Philippe Drouin-Chartier, PhD, of the Harvard School of Public Health, Boston.
And it is possible, he said, that lactic acid bacteria in yogurt could have some effect on the gut microbiome that protects against type 2 disease.
Total dairy consumption has not changed much over the past few decades, but people are drinking less milk and eating more cheese and yogurt. Dr. Drouin-Chartier and colleagues wondered how that affected the risk of type 2 diabetes.
The investigators correlated changes in dairy consumption during 4-year intervals with the incidence of type 2 diabetes in subsequent 4-year intervals using three large, prospective cohort studies that all started about 30 years ago: the Health Professionals Follow-Up Study, Nurses’ Health Study I, and Nurses’ Health Study II.
There were almost 200,000 participants in the pooled analysis, with 2.9 million person-years of follow-up and 12,007 new cases of type 2 diabetes. Participants completed food-frequency questionnaires every 4 years. About 82% of the participants were women. At baseline, they reported consuming about one to four servings of dairy a day.
After adjustment for race, body mass index, calorie intake, family history, physical activity, and other factors associated with type 2 diabetes, the investigators found that increasing yogurt intake by one 4-ounce serving a day while decreasing cheese intake by one 1-ounce serving a day was associated with a 16% (95% confidence interval, 10%-22%) reduction in subsequent risk of type 2 disease. There was an 11% (95% CI, 7%-15%) reduction in risk when a cheese serving was subbed out for a daily 8-ounce serving of reduced-fat milk.
An extra 2 ounces of yogurt a day was associated with a 13% (95% CI, 6%-19%) lower risk of type 2 diabetes, compared with stable consumption, while an extra half ounce of cheese was associated with an 8% (95% CI, 2%-16%) increase in risk.
Overall, substitution of low-fat products (such as 0%-2% milk and low-fat cheese, yogurt, or sherbet) for high-fat products (such as whole milk, ice cream, and high-fat cheese) was associated with a 4% decrease in risk (hazard ratio, 0.96; 95% CI, 0.93-0.99).
However, substitution of reduced-fat milk for whole milk or low-fat cheese for high-fat cheese did not influence the risk, possibly because there is actually not much difference in fat content, Dr. Drouin-Chartier said.
The National Institutes of Health funded the study. Dr. Drouin-Chartier has served as a speaker and consultant for Dairy Farmers of Canada, one other author has advised the group, another has advised the U.S. Department of Agriculture, and the remaining authors had no disclosures.
SOURCE: Drouin-Chartier J et al. ADA 2019, Abstract 159-OR.
SAN FRANCISCO – in a review of almost 200,000 participants presented at the annual scientific sessions at the American Diabetes Association.
Increasing yogurt consumption was also independently associated with a moderately lower risk of type 2 diabetes, and increasing cheese consumption with a moderately higher risk.
It might have been that yogurt and low-fat milk were simply indicators of healthier living and that cheese consumption – in the study, most commonly on pizza or as processed slices on cheeseburgers – indicated a less healthy way of life, but “we tried our best to control for confounders,” said study lead Jean-Philippe Drouin-Chartier, PhD, of the Harvard School of Public Health, Boston.
And it is possible, he said, that lactic acid bacteria in yogurt could have some effect on the gut microbiome that protects against type 2 disease.
Total dairy consumption has not changed much over the past few decades, but people are drinking less milk and eating more cheese and yogurt. Dr. Drouin-Chartier and colleagues wondered how that affected the risk of type 2 diabetes.
The investigators correlated changes in dairy consumption during 4-year intervals with the incidence of type 2 diabetes in subsequent 4-year intervals using three large, prospective cohort studies that all started about 30 years ago: the Health Professionals Follow-Up Study, Nurses’ Health Study I, and Nurses’ Health Study II.
There were almost 200,000 participants in the pooled analysis, with 2.9 million person-years of follow-up and 12,007 new cases of type 2 diabetes. Participants completed food-frequency questionnaires every 4 years. About 82% of the participants were women. At baseline, they reported consuming about one to four servings of dairy a day.
After adjustment for race, body mass index, calorie intake, family history, physical activity, and other factors associated with type 2 diabetes, the investigators found that increasing yogurt intake by one 4-ounce serving a day while decreasing cheese intake by one 1-ounce serving a day was associated with a 16% (95% confidence interval, 10%-22%) reduction in subsequent risk of type 2 disease. There was an 11% (95% CI, 7%-15%) reduction in risk when a cheese serving was subbed out for a daily 8-ounce serving of reduced-fat milk.
An extra 2 ounces of yogurt a day was associated with a 13% (95% CI, 6%-19%) lower risk of type 2 diabetes, compared with stable consumption, while an extra half ounce of cheese was associated with an 8% (95% CI, 2%-16%) increase in risk.
Overall, substitution of low-fat products (such as 0%-2% milk and low-fat cheese, yogurt, or sherbet) for high-fat products (such as whole milk, ice cream, and high-fat cheese) was associated with a 4% decrease in risk (hazard ratio, 0.96; 95% CI, 0.93-0.99).
However, substitution of reduced-fat milk for whole milk or low-fat cheese for high-fat cheese did not influence the risk, possibly because there is actually not much difference in fat content, Dr. Drouin-Chartier said.
The National Institutes of Health funded the study. Dr. Drouin-Chartier has served as a speaker and consultant for Dairy Farmers of Canada, one other author has advised the group, another has advised the U.S. Department of Agriculture, and the remaining authors had no disclosures.
SOURCE: Drouin-Chartier J et al. ADA 2019, Abstract 159-OR.
SAN FRANCISCO – in a review of almost 200,000 participants presented at the annual scientific sessions at the American Diabetes Association.
Increasing yogurt consumption was also independently associated with a moderately lower risk of type 2 diabetes, and increasing cheese consumption with a moderately higher risk.
It might have been that yogurt and low-fat milk were simply indicators of healthier living and that cheese consumption – in the study, most commonly on pizza or as processed slices on cheeseburgers – indicated a less healthy way of life, but “we tried our best to control for confounders,” said study lead Jean-Philippe Drouin-Chartier, PhD, of the Harvard School of Public Health, Boston.
And it is possible, he said, that lactic acid bacteria in yogurt could have some effect on the gut microbiome that protects against type 2 disease.
Total dairy consumption has not changed much over the past few decades, but people are drinking less milk and eating more cheese and yogurt. Dr. Drouin-Chartier and colleagues wondered how that affected the risk of type 2 diabetes.
The investigators correlated changes in dairy consumption during 4-year intervals with the incidence of type 2 diabetes in subsequent 4-year intervals using three large, prospective cohort studies that all started about 30 years ago: the Health Professionals Follow-Up Study, Nurses’ Health Study I, and Nurses’ Health Study II.
There were almost 200,000 participants in the pooled analysis, with 2.9 million person-years of follow-up and 12,007 new cases of type 2 diabetes. Participants completed food-frequency questionnaires every 4 years. About 82% of the participants were women. At baseline, they reported consuming about one to four servings of dairy a day.
After adjustment for race, body mass index, calorie intake, family history, physical activity, and other factors associated with type 2 diabetes, the investigators found that increasing yogurt intake by one 4-ounce serving a day while decreasing cheese intake by one 1-ounce serving a day was associated with a 16% (95% confidence interval, 10%-22%) reduction in subsequent risk of type 2 disease. There was an 11% (95% CI, 7%-15%) reduction in risk when a cheese serving was subbed out for a daily 8-ounce serving of reduced-fat milk.
An extra 2 ounces of yogurt a day was associated with a 13% (95% CI, 6%-19%) lower risk of type 2 diabetes, compared with stable consumption, while an extra half ounce of cheese was associated with an 8% (95% CI, 2%-16%) increase in risk.
Overall, substitution of low-fat products (such as 0%-2% milk and low-fat cheese, yogurt, or sherbet) for high-fat products (such as whole milk, ice cream, and high-fat cheese) was associated with a 4% decrease in risk (hazard ratio, 0.96; 95% CI, 0.93-0.99).
However, substitution of reduced-fat milk for whole milk or low-fat cheese for high-fat cheese did not influence the risk, possibly because there is actually not much difference in fat content, Dr. Drouin-Chartier said.
The National Institutes of Health funded the study. Dr. Drouin-Chartier has served as a speaker and consultant for Dairy Farmers of Canada, one other author has advised the group, another has advised the U.S. Department of Agriculture, and the remaining authors had no disclosures.
SOURCE: Drouin-Chartier J et al. ADA 2019, Abstract 159-OR.
REPORTING FROM ADA 2019
Patients with COPD at heightened risk for community-acquired pneumonia requiring hospitalization
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
Patients with chronic obstructive pulmonary disease are at a significantly increased risk for hospitalization for community-acquired pneumonia (CAP), compared with patients without COPD, a large prospective study has found.
Jose Bordon, MD, and colleagues aimed to define incidence and outcomes of COPD patients hospitalized with pneumonia in the city of Louisville, Ky., and to extrapolate the burden of disease in the U.S. population. They conducted a secondary analysis of data from the University of Louisville Pneumonia Study, a prospective population-based cohort study of all hospitalized adults with CAP who were residents in the city of Louisville, Ky., from June 1, 2014, to May 31, 2016.
COPD prevalence in the city of Louisville was derived via data from the 2014 Behavioral Risk Factor Surveillance System (BRFSS) as well as from the 2014 National Health Interview Survey (NHIS). In addition, the researchers analyzed clinical outcomes including time to clinical stability (TCS), length of hospital stay (LOS), and mortality, according to Dr. Bordon, an infectious disease specialist at Providence Health Center, Washington, and colleagues on behalf of the University of Louisville Pneumonia Study Group.
The researchers found an 18-fold greater incidence of community-acquired pneumonia in patients with COPD, compared with non-COPD patients.
A total of 18,246 individuals aged 40 and older with COPD were estimated to live in Louisville, Ky. The researchers found that 3,419 COPD patients were hospitalized due to CAP in Louisville during the 2-year study period. COPD patients, compared with non-COPD patients, were more likely to have a history of heart failure, more ICU admissions, and use of mechanical ventilation, compared with patients without COPD. The two groups had similar pneumonia severity index scores, and 17% received oral steroids prior to admission. COPD patients had more pneumococcal pneumonia, despite receiving pneumococcal vaccine significantly more often than non-COPD patients.
The annual incidence of hospitalized CAP was 9,369 cases per 100,000 COPD patients in the city of Louisville. In the same period, the incidence of CAP in patients without COPD was 509 per 100,000, a more than 18-fold difference.
Although the incidence of CAP in COPD patients was much higher than in those without, the difference didn’t appear to have an impact on clinical outcomes. There were no clinical differences among patients with vs. without COPD in regard to time to reach clinical improvement and time of hospital discharge, and in-hospital mortality was not statistically significantly different between the groups, the authors reported. The mortality of COPD patients during hospitalization, at 30 days, at 6 months, and at 1 year was 5.6% of patients, 11.9%, 24.3%, and 33.0%, respectively vs. 6.6%, 14.2%, 24.2%, and 30.1% in non-COPD patients. However, 1-year all-cause mortality was a significant 25% greater among COPD patients, as might be expected by the progression and effects of the underlying disease.
“[Our] observations mean that nearly 1 in 10 persons with COPD will be hospitalized annually due to CAP. This translates into approximately 500,000 COPD patients hospitalized with CAP every year in the U.S., resulting in a substantial burden of approximately 5 billion U.S. dollars in hospitalization costs,” the researchers stated.
“Modifiable factors associated with CAP such as tobacco smoking and immunizations should be health interventions to prevent the burden of CAP in COPD patients,” even though “pneumococcal vaccination was used more often in the COPD population than in other CAP patients, but pneumococcal pneumonia still occurred at a numerically higher rate,” they noted.
The study was supported by the University of Louisville, Ky., with partial support from Pfizer. The authors reported having no conflicts.
SOURCE: Bordon JM et al. Clin Microbiol Infect. 2019 Jun 26; doi: 10.1016/j.cmi.2019.06.025.
FROM CLINICAL MICROBIOLOGY AND INFECTION
Comorbidities drive excess mortality after breast cancer diagnosis in childhood cancer survivors
Among women with breast cancer, risk of death is more than twice as high for those who are childhood cancer survivors than for those in whom this cancer is their first, found a retrospective cohort study. However, the excess deaths are mainly from comorbidities related to previous therapies.
Breast cancer is among the leading subsequent malignancies in adult survivors of pediatric cancers, note the investigators, who were led by Chaya S. Moskowitz, PhD, of the department of epidemiology and biostatistics at Memorial Sloan Kettering Cancer Center in New York. But outcomes after this diagnosis are not well characterized.
The investigators used the Childhood Cancer Survivor Study to identify 274 female 5-year survivors of cancer diagnosed before age 21 years who received a subsequent breast cancer diagnosis at a median age of 38 years. They then used Surveillance, Epidemiology, and End Results data to identify a control group of 1,095 female patients with de novo breast cancer matched on age, race, stage, and year of breast cancer diagnosis.
The 10-year overall survival was 73% among the childhood cancer survivors, investigators reported in the Journal of Clinical Oncology.
Compared with the control women whose breast cancer was their first cancer, the women with breast cancer who were childhood cancer survivors had an elevated risk of death from any cause (hazard ratio, 2.2) that persisted after analyses were adjusted for receipt of chemotherapy and radiation therapy (HR, 2.4). In addition, findings were similar in analyses restricted to women with ductal carcinoma in situ and women with stage 1-3 breast cancer.
The childhood cancer survivors had a modestly elevated risk of dying from breast cancer (HR, 1.3) but a sharply elevated risk of dying from other health-related causes, including other subsequent malignancies and cardiovascular or pulmonary disease often related to previous therapies (HR, 5.5).
In addition, the childhood cancer survivors had a higher cumulative incidence of diagnosis of second asynchronous breast cancers a year or more later, relative to the women in whom breast cancer was their first cancer (P less than .001). The 5-year cumulative incidence was 8.0% among the childhood cancer survivors and just 2.7% among the control women.
“Although BC [breast cancer]-specific mortality was modestly higher in childhood cancer survivors, deaths attributable to health conditions other than BC seem to be the driving force in the elevated all-cause mortality,” Dr. Moskowitz and colleagues wrote.
“To change the dismal outcomes of these women, our results suggest that it is imperative that at the time of a secondary BC diagnosis, they have a comprehensive evaluation that extends beyond a singular focus of the BC,” they concluded. “This should include an assessment of existing cardiopulmonary disease and a plan for future cancer screening to optimize the management of comorbidities and cardiopulmonary disease and prolong the lifespan of these survivors.”
Dr. Moskowitz reported that she has a consulting or advisory role with Bioclinica. The study was supported by the National Cancer Institute, a Memorial Sloan Kettering Cancer Center Core grant, the Meg Berté Owen Foundation, and the American Lebanese Syrian Associated Charities.
SOURCE: Moskowitz CS et al. J Clin Oncol. 2019 Jul 1. doi: 10.1200/JCO.18.02219.
Among women with breast cancer, risk of death is more than twice as high for those who are childhood cancer survivors than for those in whom this cancer is their first, found a retrospective cohort study. However, the excess deaths are mainly from comorbidities related to previous therapies.
Breast cancer is among the leading subsequent malignancies in adult survivors of pediatric cancers, note the investigators, who were led by Chaya S. Moskowitz, PhD, of the department of epidemiology and biostatistics at Memorial Sloan Kettering Cancer Center in New York. But outcomes after this diagnosis are not well characterized.
The investigators used the Childhood Cancer Survivor Study to identify 274 female 5-year survivors of cancer diagnosed before age 21 years who received a subsequent breast cancer diagnosis at a median age of 38 years. They then used Surveillance, Epidemiology, and End Results data to identify a control group of 1,095 female patients with de novo breast cancer matched on age, race, stage, and year of breast cancer diagnosis.
The 10-year overall survival was 73% among the childhood cancer survivors, investigators reported in the Journal of Clinical Oncology.
Compared with the control women whose breast cancer was their first cancer, the women with breast cancer who were childhood cancer survivors had an elevated risk of death from any cause (hazard ratio, 2.2) that persisted after analyses were adjusted for receipt of chemotherapy and radiation therapy (HR, 2.4). In addition, findings were similar in analyses restricted to women with ductal carcinoma in situ and women with stage 1-3 breast cancer.
The childhood cancer survivors had a modestly elevated risk of dying from breast cancer (HR, 1.3) but a sharply elevated risk of dying from other health-related causes, including other subsequent malignancies and cardiovascular or pulmonary disease often related to previous therapies (HR, 5.5).
In addition, the childhood cancer survivors had a higher cumulative incidence of diagnosis of second asynchronous breast cancers a year or more later, relative to the women in whom breast cancer was their first cancer (P less than .001). The 5-year cumulative incidence was 8.0% among the childhood cancer survivors and just 2.7% among the control women.
“Although BC [breast cancer]-specific mortality was modestly higher in childhood cancer survivors, deaths attributable to health conditions other than BC seem to be the driving force in the elevated all-cause mortality,” Dr. Moskowitz and colleagues wrote.
“To change the dismal outcomes of these women, our results suggest that it is imperative that at the time of a secondary BC diagnosis, they have a comprehensive evaluation that extends beyond a singular focus of the BC,” they concluded. “This should include an assessment of existing cardiopulmonary disease and a plan for future cancer screening to optimize the management of comorbidities and cardiopulmonary disease and prolong the lifespan of these survivors.”
Dr. Moskowitz reported that she has a consulting or advisory role with Bioclinica. The study was supported by the National Cancer Institute, a Memorial Sloan Kettering Cancer Center Core grant, the Meg Berté Owen Foundation, and the American Lebanese Syrian Associated Charities.
SOURCE: Moskowitz CS et al. J Clin Oncol. 2019 Jul 1. doi: 10.1200/JCO.18.02219.
Among women with breast cancer, risk of death is more than twice as high for those who are childhood cancer survivors than for those in whom this cancer is their first, found a retrospective cohort study. However, the excess deaths are mainly from comorbidities related to previous therapies.
Breast cancer is among the leading subsequent malignancies in adult survivors of pediatric cancers, note the investigators, who were led by Chaya S. Moskowitz, PhD, of the department of epidemiology and biostatistics at Memorial Sloan Kettering Cancer Center in New York. But outcomes after this diagnosis are not well characterized.
The investigators used the Childhood Cancer Survivor Study to identify 274 female 5-year survivors of cancer diagnosed before age 21 years who received a subsequent breast cancer diagnosis at a median age of 38 years. They then used Surveillance, Epidemiology, and End Results data to identify a control group of 1,095 female patients with de novo breast cancer matched on age, race, stage, and year of breast cancer diagnosis.
The 10-year overall survival was 73% among the childhood cancer survivors, investigators reported in the Journal of Clinical Oncology.
Compared with the control women whose breast cancer was their first cancer, the women with breast cancer who were childhood cancer survivors had an elevated risk of death from any cause (hazard ratio, 2.2) that persisted after analyses were adjusted for receipt of chemotherapy and radiation therapy (HR, 2.4). In addition, findings were similar in analyses restricted to women with ductal carcinoma in situ and women with stage 1-3 breast cancer.
The childhood cancer survivors had a modestly elevated risk of dying from breast cancer (HR, 1.3) but a sharply elevated risk of dying from other health-related causes, including other subsequent malignancies and cardiovascular or pulmonary disease often related to previous therapies (HR, 5.5).
In addition, the childhood cancer survivors had a higher cumulative incidence of diagnosis of second asynchronous breast cancers a year or more later, relative to the women in whom breast cancer was their first cancer (P less than .001). The 5-year cumulative incidence was 8.0% among the childhood cancer survivors and just 2.7% among the control women.
“Although BC [breast cancer]-specific mortality was modestly higher in childhood cancer survivors, deaths attributable to health conditions other than BC seem to be the driving force in the elevated all-cause mortality,” Dr. Moskowitz and colleagues wrote.
“To change the dismal outcomes of these women, our results suggest that it is imperative that at the time of a secondary BC diagnosis, they have a comprehensive evaluation that extends beyond a singular focus of the BC,” they concluded. “This should include an assessment of existing cardiopulmonary disease and a plan for future cancer screening to optimize the management of comorbidities and cardiopulmonary disease and prolong the lifespan of these survivors.”
Dr. Moskowitz reported that she has a consulting or advisory role with Bioclinica. The study was supported by the National Cancer Institute, a Memorial Sloan Kettering Cancer Center Core grant, the Meg Berté Owen Foundation, and the American Lebanese Syrian Associated Charities.
SOURCE: Moskowitz CS et al. J Clin Oncol. 2019 Jul 1. doi: 10.1200/JCO.18.02219.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Ill-Defined Macule on the Abdomen
The Diagnosis: Microvenular Hemangioma
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
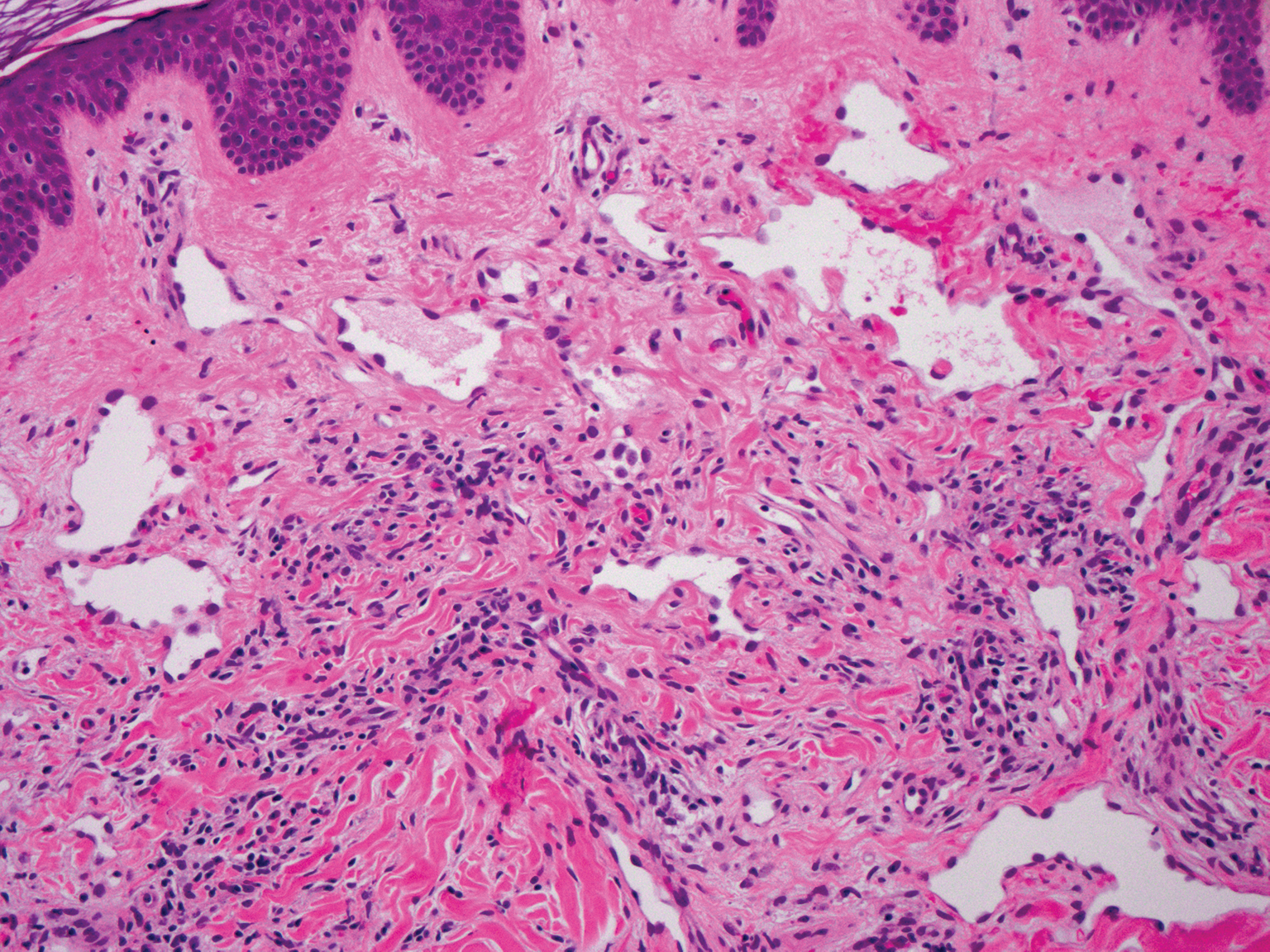
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
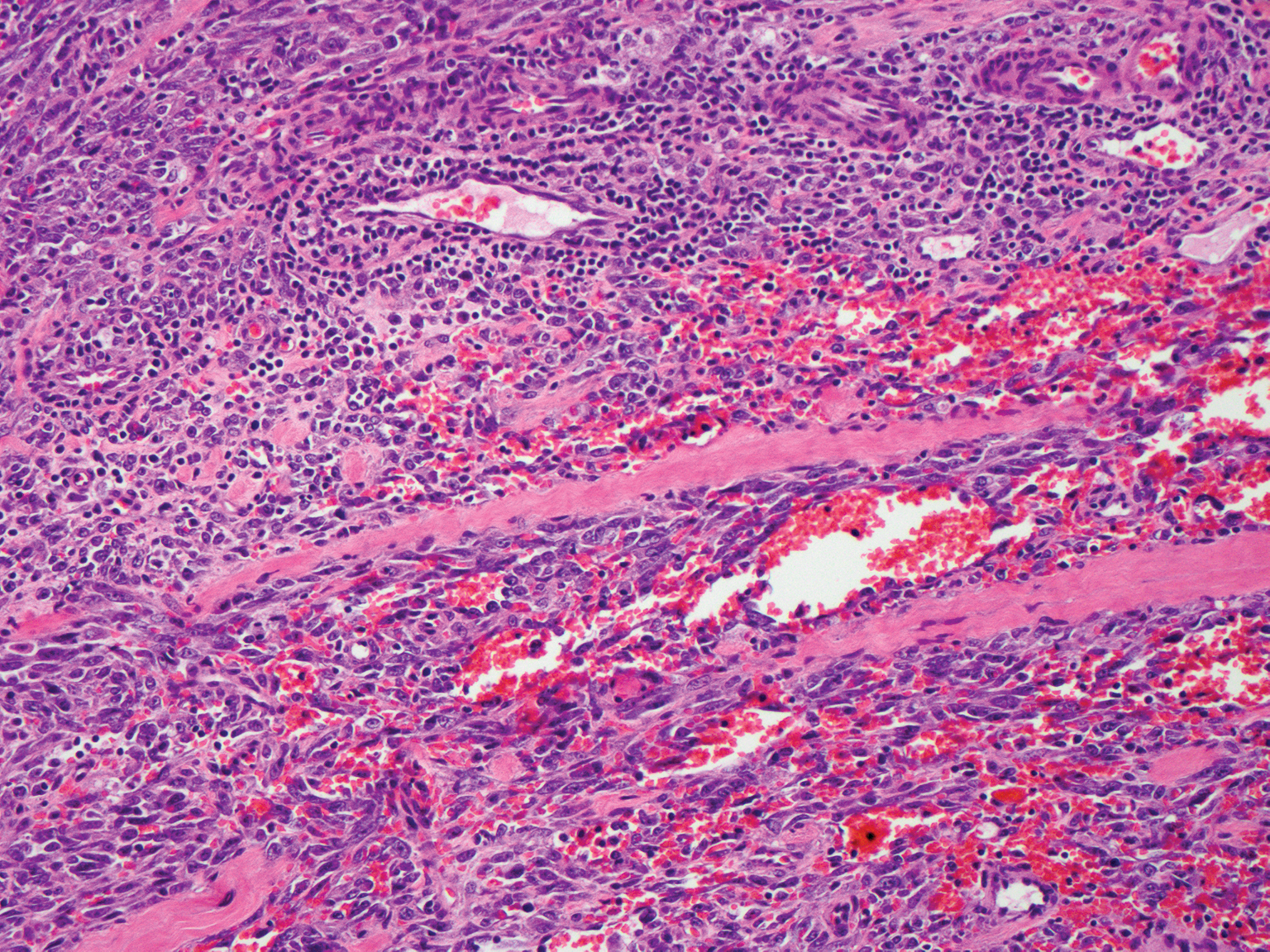
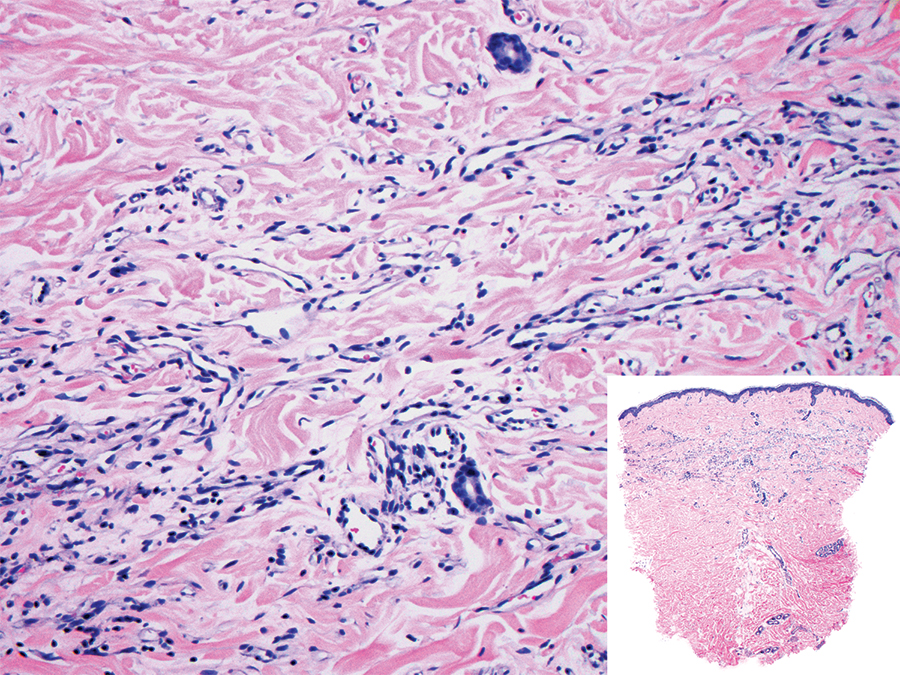
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
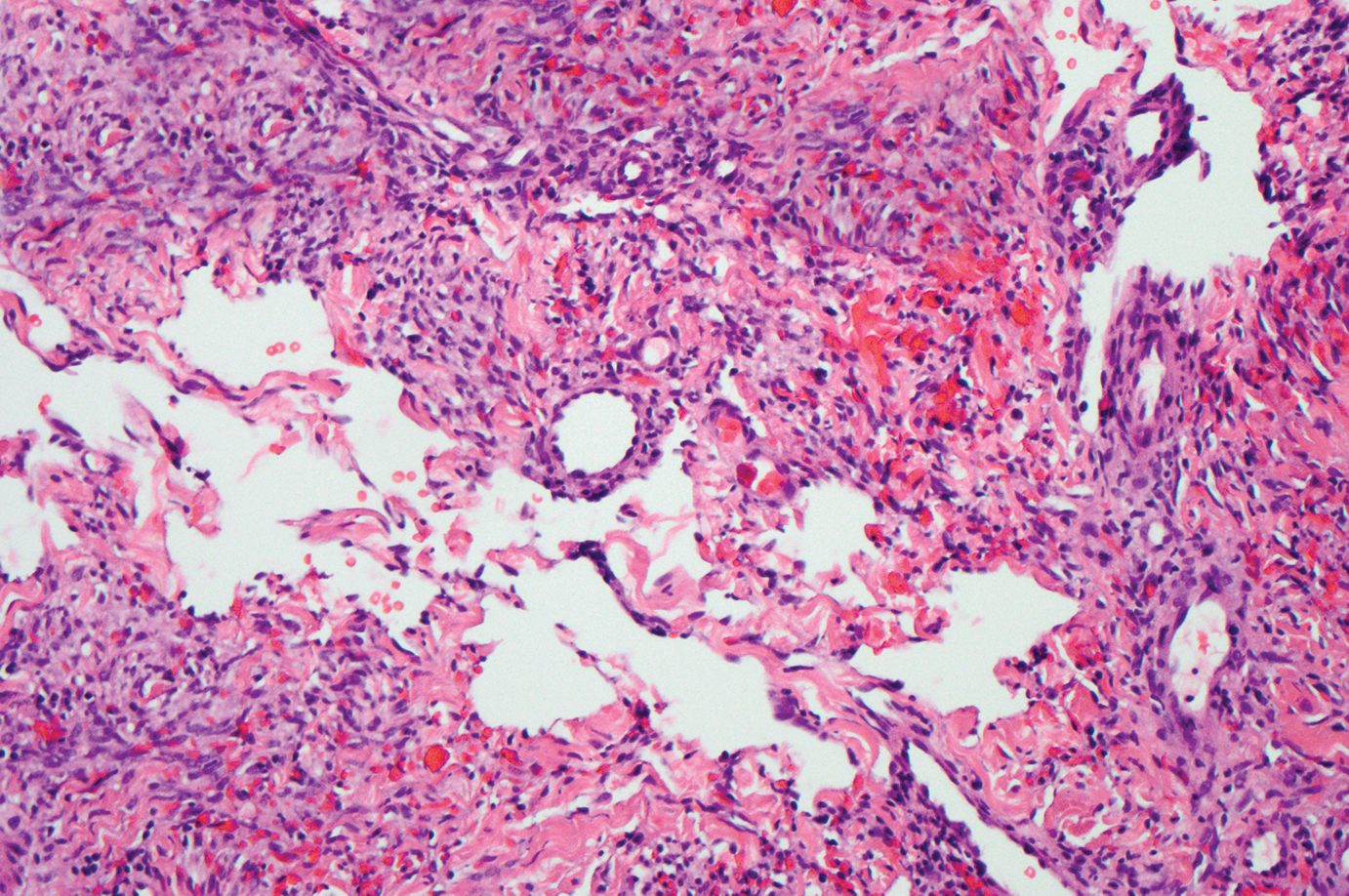
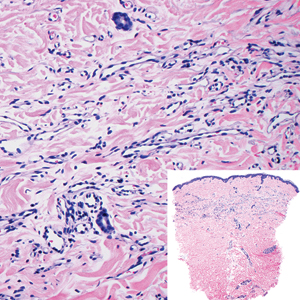
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
The Diagnosis: Microvenular Hemangioma
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
The Diagnosis: Microvenular Hemangioma
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
A 38-year-old woman presented with an asymptomatic lesion on the abdomen. On physical examination, there was a 5×2-mm, solitary, ill-defined pink macule on the right side of the abdomen. The patient denied recent change in size or color of the lesion, prior trauma, or a personal or family history of similar lesions. Due to the uncertain diagnostic appearance, a punch biopsy was performed.
SC daratumumab deemed feasible for every multiple myeloma patient
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
CHICAGO – Subcutaneous (SC) daratumumab is noninferior to intravenous (IV) daratumumab for patients with relapsed or refractory multiple myeloma (MM), according findings from a phase 3 trial.
In the COLUMBA trial, SC daratumumab proved noninferior to IV daratumumab with regard to overall response rate and maximum trough concentration (Ctrough).
The safety profiles of the two formulations were similar, although patients who received SC daratumumab had a lower rate of infusion-related reactions. SC daratumumab also had a lower treatment burden.
“The COLUMBA study shows that [SC daratumumab] can be used in every myeloma patient [as a] single agent or, maybe in the future, in combination with the different backbones,” said Maria-Victoria Mateos, MD, PhD, of University Hospital of Salamanca (Spain).
Dr. Mateos presented results from the COLUMBA trial at the annual meeting of the American Society of Clinical Oncology.
Dr. Mateos cited a previous phase 1b study that had suggested that SC daratumumab might produce similar results as IV daratumumab (Blood. 2017;130:838) while providing a more convenient delivery method. She pointed out that infusions of IV daratumumab can last hours, while the SC formulation can be delivered in minutes.
The aim of the phase 3 COLUMBA study was to compare the IV and SC formulations head-to-head. The trial enrolled 522 patients with relapsed/refractory multiple myeloma. They were randomized to receive daratumumab SC (n = 263) or IV (n = 259).
The median patient age was 68 years (range, 33-92 years) in the IV arm and 65 years (range, 42-84 years) in the SC arm. Patients had received a median of four prior lines of therapy (range, 1-15 in the IV arm and 2-12 in the SC arm). Most patients were refractory to their last line of therapy – 85% in the IV arm and 80% in the SC arm – and most patients had standard-risk cytogenetics – 83% and 74%, respectively.
Treatment
Patients received SC daratumumab at 1,800 mg and IV daratumumab at 16 mg/kg. Both were given weekly for cycles 1-2, every 2 weeks for cycles 3-6, and every 4 weeks thereafter until disease progression.
The median duration of the first infusion was 421 minutes in the IV arm and 5 minutes in the SC arm. The median duration of the second infusion was 255 minutes and 5 minutes, respectively, and the median duration of subsequent infusions was 205 minutes and 5 minutes, respectively.
At a median follow-up of 7.46 months, 57% of patients in each arm had discontinued the study treatment. The most common reasons for discontinuation were progression – 44% of the IV arm and 43% of the SC arm – and adverse events (AEs) – 8% and 7%, respectively.
Safety
Dr. Mateos said the safety profiles of IV and SC daratumumab were comparable. However, infusion-related reactions were significantly less likely in the SC arm, occurring in 12.7% of those patients and 34.5% of patients in the IV arm (P less than .0001).
Grade 3 or higher treatment-emergent AEs occurred in 49% of patients in the IV arm and 46% of those in the SC arm. Rates of grade 5 AEs were 7% and 5%, respectively. The most common grade 3/4 AEs (in the IV and SC arms, respectively) were anemia (14% and 13%), thrombocytopenia (14% for both), neutropenia (8% and 13%), lymphopenia (6% and 5%), and hypertension (6% and 3%).
Efficacy
One of the study’s primary endpoints was overall response rate, which was 37.1% in the IV arm and 41.1% in the SC arm (relative risk, 1.11; 95% CI, 0.89-1.37; P less than .0001). This met the criteria for noninferiority, and overall response rates were comparable across all patient subgroups, Dr. Mateos noted.
The rates of complete response or stringent complete response were also comparable at 2.7% in the IV arm and 1.9% in the SC arm. Rates of very good partial response were 17.0% and 19.0%, respectively.
The study’s other primary endpoint was maximum Ctrough predose on day 1 of cycle 3. The ratio of maximum Ctrough for daratumumab SC over IV was 107.93% (90% CI, 95.74%-121.67%), which met the noninferiority criterion.
Survival outcomes were also similar between the IV and SC arms. The median progression-free survival was 6.1 months and 5.6 months, respectively (P = .9258). The rate of overall survival at 6 months was 83.0% and 87.5%, respectively (P = .6032).
Considering these results together, Dr. Mateos and colleagues concluded that SC daratumumab is noninferior to IV daratumumab.
“[SC daratumumab] has a reduced treatment burden due to a considerably shorter administration duration, and patients treated with [SC daratumumab] reported higher satisfaction with therapy,” Dr. Mateos said.
The results support the use of flat-dose 1,800-mg SC daratumumab, which is comparable with the IV formulation, she said.
The COLUMBA trial was sponsored by Janssen Research & Development. Dr. Mateos reported relationships with Amgen, Celgene, Janssen-Cilag, and Takeda.
SOURCE: Mateos MV et al. ASCO 2019, Abstract 8005.
REPORTING FROM ASCO 2019
Interview with Mary Alissa Willis, MD, on MS and Mental Health
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Mary Alissa Willis, MD, is the Medical Director for the Mellen Center for Multiple Sclerosis at the Cleveland Clinic. We spoke with Dr. Willis about mental health issues in persons with MS and what health care facilities can do to better care for these patients.
What is the prevalence of depression in patients with MS?
Depression and anxiety are common in persons with multiple sclerosis (PwMS). In a systematic review, Ruth Ann Marrie, MD, PhD, and colleagues estimated the prevalence of depression in PwMS to be 23.7%. Approximately half of all PwMS experience depression at some point after their diagnosis.1-3 This makes depression nearly twice as common in MS as in the general population.
What are some reasons patients with MS might begin to experience depression? What are some warning signs to look out for?
Many people assume that PwMS experience depression because of psychosocial stressors, unpredictability of disease progression, or poor coping strategies. Although these factors do contribute to depression, there is increasing evidence that immune dysregulation and structural changes in the brain caused by MS disease activity make some PwMS uniquely vulnerable to depression. Functional MRI studies have shown abnormalities in the prefrontal-subcortical network connectivity, which is involved in mood regulation. In addition, persistent somatic symptoms such as severe pain and fatigue limit daily activities and social participation—generally considered to be protective factors in depression.4 Warning signs for depression include loss of interest in activities, weight loss or weight gain, changes in sleep—too much or too little, an increase in fatigue, expressions of hopelessness or guilt, or increasing drug or alcohol use.
What are some special considerations for patients with MS battling suicidal ideation?
Anthony Feinstein, MBBCh, MPhil, PhD, and colleagues reported that more than 28% of PwMS had suicidal ideation at some point.5 This is a huge number of people struggling with thoughts of suicide. Depression is a risk factor for suicide but other risks specific to PwMS include perceived loss of control, loss of job/income/social roles, loss of driving privileges, and marked physical or cognitive difficulties. While we are doing a better job screening for depression with quick screening tools such as the PHQ9, we could do better in suicide risk assessment.
What can health care providers do better to address depression and risk for suicide? What safety/preventative measures can they take?
The first step in better addressing depression and risk for suicide is to directly ask patients. Pay attention to changes in appearance, behavior, requests for prescription refills, frequency of appointments, and who accompanies patients to appointments. Explore the reasons for these changes. It is important to respond proactively when comments or behavior suggest a patient at risk. Ask specific questions about plan, access to means, previous suicide attempts, and support network. Refer promptly for emergency or mental health services when appropriate. Familiarity with mental health colleagues, local crisis centers, and helplines can be helpful in engaging a team of people to provide assistance to a patient in need.
References
1. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979.
2. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis. Review and recommendations for clinical research. Arch Neurol. 1990;47(1):98-104.
3. Marrie RA, Walld R, Bolton JM, et al; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Estimating annual prevalence of depression and anxiety disorder in multiple sclerosis using administrative data. BMC Res Notes. 2017;10(1):619.
4. Passamonti L. Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(pt 12):3380-3391.
5. Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59(5):674-678.
Labor outcomes unchanged by alternate anesthesia in bleeding disorder patients
Among pregnant women with inherited bleeding disorders, having a contraindication to regional anesthesia appears not to impact labor outcomes, according to a retrospective analysis.
“The purpose of this study was to determine the anesthetic use in labour in a cohort of women with inherited bleeding disorders,” wrote Sean C. Boyd, of Coombe Women & Infants University Hospital in Dublin, Ireland, and colleagues. The findings were reported in the European Journal of Obstetrics & Gynecology and Reproductive Biology.
The study comprised 97 pregnant women with an inherited bleeding disorder and outcomes related to 130 delivered newborns.
The researchers reviewed medical records of patients with a variety of inherited bleeding disorders: type 1 von Willebrand disease (VWD), deficiencies of factors VII, VIII, IX, X, and XI, combined deficiencies, and others.
Various clinical data, including both obstetric and anesthetic outcomes, were collected from January 2011 to December 2016.
When researchers compared pregnancies where regional anesthesia was contraindicated to those in which it was considered safe, women with a contraindication were more likely to receive general anesthesia for cesarean section (20% vs. 1%), more likely to use a remifentanil infusion (31% vs. 0), and more likely to require prophylactic hemostatic support for delivery (61% vs. 1%).
Vaginal (71% vs. 65%; P = .4) and caesarean section (29% vs. 32%; P = .28) delivery rates were similar between the two groups.
Rates of postpartum hemorrhage were greater in pregnancies where regional anesthesia was contraindicated (24% vs. 12%), but not significantly different (P = .07). No cases of vertebral canal hematoma or neonatal hemorrhage were reported among participants.
“Women are anxious about analgesia and anesthesia in labour and understandably, more so, when they are aware that they are unable to have an epidural or spinal,” the researchers wrote. “This study shows what alternative analgesia is used in labour and, reassuringly, that labour outcome is the same.”
Two key limitations of the study were the small sample size and the wide range of included bleeding disorders.
No funding sources were reported. The authors did not report conflicts of interest.
SOURCE: Boyd SC et al. Eur J Obstet Gynecol Reprod Biol. 2019 Jun 3. doi: 10.1016/j.ejogrb.2019.05.043.
Among pregnant women with inherited bleeding disorders, having a contraindication to regional anesthesia appears not to impact labor outcomes, according to a retrospective analysis.
“The purpose of this study was to determine the anesthetic use in labour in a cohort of women with inherited bleeding disorders,” wrote Sean C. Boyd, of Coombe Women & Infants University Hospital in Dublin, Ireland, and colleagues. The findings were reported in the European Journal of Obstetrics & Gynecology and Reproductive Biology.
The study comprised 97 pregnant women with an inherited bleeding disorder and outcomes related to 130 delivered newborns.
The researchers reviewed medical records of patients with a variety of inherited bleeding disorders: type 1 von Willebrand disease (VWD), deficiencies of factors VII, VIII, IX, X, and XI, combined deficiencies, and others.
Various clinical data, including both obstetric and anesthetic outcomes, were collected from January 2011 to December 2016.
When researchers compared pregnancies where regional anesthesia was contraindicated to those in which it was considered safe, women with a contraindication were more likely to receive general anesthesia for cesarean section (20% vs. 1%), more likely to use a remifentanil infusion (31% vs. 0), and more likely to require prophylactic hemostatic support for delivery (61% vs. 1%).
Vaginal (71% vs. 65%; P = .4) and caesarean section (29% vs. 32%; P = .28) delivery rates were similar between the two groups.
Rates of postpartum hemorrhage were greater in pregnancies where regional anesthesia was contraindicated (24% vs. 12%), but not significantly different (P = .07). No cases of vertebral canal hematoma or neonatal hemorrhage were reported among participants.
“Women are anxious about analgesia and anesthesia in labour and understandably, more so, when they are aware that they are unable to have an epidural or spinal,” the researchers wrote. “This study shows what alternative analgesia is used in labour and, reassuringly, that labour outcome is the same.”
Two key limitations of the study were the small sample size and the wide range of included bleeding disorders.
No funding sources were reported. The authors did not report conflicts of interest.
SOURCE: Boyd SC et al. Eur J Obstet Gynecol Reprod Biol. 2019 Jun 3. doi: 10.1016/j.ejogrb.2019.05.043.
Among pregnant women with inherited bleeding disorders, having a contraindication to regional anesthesia appears not to impact labor outcomes, according to a retrospective analysis.
“The purpose of this study was to determine the anesthetic use in labour in a cohort of women with inherited bleeding disorders,” wrote Sean C. Boyd, of Coombe Women & Infants University Hospital in Dublin, Ireland, and colleagues. The findings were reported in the European Journal of Obstetrics & Gynecology and Reproductive Biology.
The study comprised 97 pregnant women with an inherited bleeding disorder and outcomes related to 130 delivered newborns.
The researchers reviewed medical records of patients with a variety of inherited bleeding disorders: type 1 von Willebrand disease (VWD), deficiencies of factors VII, VIII, IX, X, and XI, combined deficiencies, and others.
Various clinical data, including both obstetric and anesthetic outcomes, were collected from January 2011 to December 2016.
When researchers compared pregnancies where regional anesthesia was contraindicated to those in which it was considered safe, women with a contraindication were more likely to receive general anesthesia for cesarean section (20% vs. 1%), more likely to use a remifentanil infusion (31% vs. 0), and more likely to require prophylactic hemostatic support for delivery (61% vs. 1%).
Vaginal (71% vs. 65%; P = .4) and caesarean section (29% vs. 32%; P = .28) delivery rates were similar between the two groups.
Rates of postpartum hemorrhage were greater in pregnancies where regional anesthesia was contraindicated (24% vs. 12%), but not significantly different (P = .07). No cases of vertebral canal hematoma or neonatal hemorrhage were reported among participants.
“Women are anxious about analgesia and anesthesia in labour and understandably, more so, when they are aware that they are unable to have an epidural or spinal,” the researchers wrote. “This study shows what alternative analgesia is used in labour and, reassuringly, that labour outcome is the same.”
Two key limitations of the study were the small sample size and the wide range of included bleeding disorders.
No funding sources were reported. The authors did not report conflicts of interest.
SOURCE: Boyd SC et al. Eur J Obstet Gynecol Reprod Biol. 2019 Jun 3. doi: 10.1016/j.ejogrb.2019.05.043.
FROM THE EUROPEAN JOURNAL OF OBSTETRICS & GYNECOLOGY AND REPRODUCTIVE BIOLOGY
Another study supports safety of 2-cm margins for thick melanomas
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
FROM THE LANCET