User login
You can observe a lot by watching
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
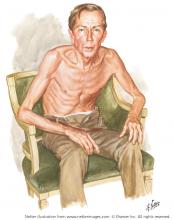
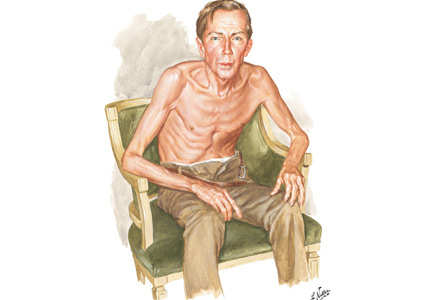
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
"I have trained myself to see what others overlook."
—Sherlock Holmes1
The article by Grandjean and Huber in this issue2 is a timely reminder of the importance of skilled observation in medical care. Osler3 considered observation to represent “the whole art of medicine,” but warned that “for some men it is quite as difficult to record an observation in brief and plain language.” This insight captures not only the never-ending feud between written and visual communication, but also the higher efficiency of images. Leonardo da Vinci, a visual thinker with a touch of dyslexia,4 often boasted in colorful terms about the superiority of the visual. Next to his amazing rendition of a bovine heart he scribbled, “[Writer] how could you describe this heart in words without filling a whole book? So, don’t bother with words unless you are speaking to the blind…you will always be overruled by the painter.”5
See related article and editorial
Ironically, physicians have often preferred the written over the visual. Oliver Wendell Holmes Sr., professor of anatomy at Harvard Medical School and renowned essayist, once wrote a scathing review of a new anatomy textbook that, according to him, had just too many pictures. “Let a student have illustrations,” he thundered “and just so surely will he use them at the expense of the text.”6 The book was Gray’s Anatomy, but Holmes’ tirade exemplifies the conundrum of our profession: to become physicians we must read (and memorize) lots of written text, with little emphasis on how much more efficiently information might be conveyed through a single picture.
This trend is probably worsening. When I first came to the United States 43 years ago, I was amazed at how many of my professors immediately grabbed a sheet of paper and started drawing their explanations to my questions. But I have not seen much of this lately, and that is a pity, since pictures are undoubtedly a better way of communicating.
OBSERVING A PATIENT WITH COPD
Netter’s patient is also exhaling through pursed lips. This reduces the respiratory rate and carbon dioxide level, while improving distribution of ventilation,9,10 oxygen saturation, tidal volume, inspiratory muscle strength, and diaphragmatic efficiency.11,12 Since less inspiratory force is required for each breath, dyspnea is also improved.13,14 Diagnostically, pursed‑lip breathing increases the probability of chronic obstructive pulmonary disease (COPD), with a likelihood ratio of 5.05.15
The man in The Pink Puffer is using accessory respiratory muscles, which not only represents one of the earliest signs of airway obstruction, but also reflects severe disease. In fact, use of accessory respiratory muscles occurs in more than 90% of COPD patients admitted for acute exacerbations.7
Lastly, Netter’s patient exhibits inspiratory retraction of supraclavicular fossae and interspaces (tirage), which indicates increased airway resistance and reduced forced expiratory volume in 1 second (FEV1).16,17 A clavicular “lift” of more than 5 mm correlates with an FEV1 of 0.6 L.18
But what is odd about this patient is what Netter did not portray: clubbing. This goes against the conventional wisdom of the time but is actually correct, since we now know that clubbing is more a feature of chronic bronchitis than emphysema.19 In fact, if present in a “pink puffer,” it should suggest an underlying malignancy. Hence, Netter reminds us that we should never convince ourselves that we see something simply because we know it should be there. Instead, we should always rely on what we see. This is, after all, how Vesalius debunked Galen’s anatomic errors: by seeing for himself. Tom McCrae, Osler’s right-hand man at Johns Hopkins, used to warn his students that one misses more by not seeing than by not knowing. Leonardo put it simply: “Wisdom is the daughter of [visual] experience.”20 In the end, Netter’s drawing reminds us that a picture is truly worth a thousand words.
TEACHING STUDENTS TO OBSERVE
Unfortunately, detecting detail is difficult. It is also very difficult to teach. For the past few months I’ve been asking astute clinicians how they observe, and most of them seem befuddled, as if I had asked which muscles they contract in order to walk. They just walk. And they just observe.
So, how can we rekindle this important but underappreciated component of the physician’s skill set? First of all, by becoming cognizant of its fundamental role in medicine. Second, by accepting that this is something that cannot be easily tested by single-best- answer, black-and-white, multiple-choice exams. Recognizing the complexity of clinical skills reminds us that not all that counts in medicine can be counted, and not all that can be counted counts. Yet it also provides a hurdle, since testing typically drives curriculum. If we cannot assess observation, how can we reincorporate it in the curriculum? Lastly, we need to regain ownership of the teaching of this skill. No art instructor can properly identify and interpret clinical findings. Hence, physicians ought to teach it. In the end, learning how to properly observe is a personal and lifelong effort. As Osler put it, “There is no more difficult art to acquire than the art of observation.”21
Leonardo used to quip that “There are three classes of people: those who see, those who see when they are shown, and those who do not see.”22 Yet this time Leonardo might have been wrong. There are really only two kinds of people: those who have been taught how to observe and those who have not. Leonardo was lucky enough to have been apprenticed to an artist whose nickname was Verrocchio, which resembles the Italian words vero occhio, a “fine eye.” Without Verrocchio, even Leonardo might not have become such a skilled observer. How many Verrocchios are around today?
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
- Doyle AC. A case of identity. In: The Adventures of Sherlock Holmes. London, UK: George Newnes; 1892.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Osler W. The natural method of teaching the subject of medicine. JAMA 1901; 36(24):1673–1679. doi:10.1001/jama.1901.52470240001001
- Mangione S, Del Maestro R. Was Leonardo da Vinci dyslexic? Am J Med 2019 Mar 7; pii:S0002-9343(19)30214-1. Epub ahead of print. doi:10.1016/j.amjmed.2019.02.019
- Leonardo Da Vinci. Studies of the Heart of an Ox, Great Vessels and Bronchial Tree (c. 1513); pen and ink on blue paper, Windsor, London, UK Royal Library (19071r).
- Holmes OW Sr. Gray’s Anatomy. The Boston Medical and Surgical Journal 1859; 60(25):489–496.
- O’Neill S, McCarthy DS. Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strength. Thorax 1983; 38(8):595–600. pmid:6612651
- Banzett RB, Topulos GP, Leith DE, Nations CS. Bracing arms increases the capacity for sustained hyperpnea. Am Rev Respir Dis 1988; 138(1):106–109. doi:10.1164/ajrccm/138.1.106
- Mueller RE, Petty TL, Filley GF. Ventilation and arterial blood gas changes induced by pursed lips breathing. J Appl Physiol 1970; 28(6):784–789. doi:10.1152/jappl.1970.28.6.784
- Thoman RL, Stoker GL, Ross JC. The efficacy of pursed-lips breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1966; 93(1):100–106.
- Breslin EH. The pattern of respiratory muscle recruitment during pursed-lip breathing. Chest 1992; 101(1):75–78. pmid:1729114
- Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther 2003; 83(5):424–431. pmid:12718708
- el-Manshawi A, Killian KJ, Summers E, Jones NL. Breathlessness during exercise with and without resistive loading. J Appl Physiol (1985) 1986; 61(3):896–905. doi:10.1152/jappl.1986.61.3.896
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev 2007; 27(4):237–244. doi:10.1097/01.HCR.0000281770.82652.cb
- Mattos WL, Signori LG, Borges FK, Bergamin JA, Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J Bras Pneumol 2009; 35(5):404–408. pmid:19547847
- Stubbing DG. Physical signs in the evaluation of patients with chronic obstructive pulmonary disease. Pract Cardiol 1984;10:114–120.
- Godfrey S, Edwards RH, Campbell EJ, Newton-Howes J. Clinical and physiological associations of some physical signs observed in patients with chronic airways obstruction. Thorax 1970; 25(3):285–287. pmid:5452279
- Anderson CL, Shankar PS, Scott JH. Physiological significance of sternomastoid muscle contraction in chronic obstructive pulmonary disease. Respir Care 1980; 25(9):937–939.
- Myers KA, Farquhar DR. The rational clinical examination. Does this patient have clubbing? JAMA 2001; 286(3):341–347. pmid:11466101
- Richter JP. The Notebooks of Leonardo Da Vinci. New York: Dover Books; 1970.
- Osler W. On the educational value of the medical society. Yale Medical Journal 1903; 9(10):325.
- Goodreads. Leonardo da Vinci Quotable Quote. http://www.goodreads.com/quotes/243423-there-are-three-classes-of-people-those-whosee-those. Accessed April 15, 2019.
If a picture is worth a thousand words, a patient is worth ten thousand
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
Today’s most prominent medical journals have a “clinical images” section. High- quality, readily accessible digital photography can transport a patient to the journal’s pages, as demonstrated by Grandjean and Huber’s “Thinker sign” images in this issue of the Journal.1 Images challenge healthcare practitioners to recall diseases via pattern recognition, or to deduce them by higher-order cognition. Images can reinforce prior learning, change perspective, and challenge preconceived notions.
See related article and editorial
I have used clinical images—physical examination findings, skin rashes, blood smears, radiography—for more than 20 years as a medical educator. I have dimmed the lights in conference rooms and lecture halls from Maine to Northern California, challenging students, residents, and faculty to contemplate a snippet of history and describe what they see to arrive at a diagnosis. Images are compelling teaching tools for first-year medical students beginning to make clinical observations, and for seasoned clinicians who have seen thousands of patients.
In my experience, clinical image presentations are consistently engaging. Introducing an audience to 8 to 10 patients in an hour loosely mimics the experience of seeing patients over the course of morning hospital rounds or clinic. The images I use are assembled from a collection of images of patients I have seen during my career in medical education. Showing images of patients I’ve personally cared for consistently prompts people to engage. “Here is a patient I saw last week on the medicine wards” reignites the sagging eyes and fading attention of the audience. In retelling a patient encounter, I create a human connection between a picture on the screen—my patient—and the listener. My patient becomes a patient of anyone in the room, a patient someone might see tomorrow on hospital rounds or in clinic.
Sometimes, instead of presenting a brief clinical history or select physical findings, I tell a story about the patient in the image. Whether sad or funny, these stories often bring learners together, prompting them to wonder how there could ever be a better job than the one they have. A prominent educator once approached me after a clinical images presentation to opine, “What you did with us today is the cure for physician burnout.” Hyperbole, perhaps, but I understood what he meant. Over the course of an hour, the audience had been transported to numerous bedsides and examination rooms, witnessing the interesting and delightfully mundane jewels our patients often bring—true pearls, indeed.
However, as educational, fun, and intellectually challenging as clinical images can be, they can never replace the experience of being at the bedside. There is nothing as engaging as the stories the patients themselves tell us. Unfiltered musings come to life, physical findings are indelibly seared into memory.
But unfortunately, even as trainees spend less time than ever before with their patients,2,3 bedside rounding has dramatically faded, replaced by rounds in conference rooms and hospital hallways.4 The underlying cause is multifactorial—declining physical examination skills, increasing use of radiography and other advanced imaging, the electronic health record, and the overwhelming volume of clinical tasks carried out at a distance from the patient.
But this is not the whole story. I also believe that teachers and leaders fear the “thin ice” of rounding at the patient’s bedside. One never knows what will happen there—what will be said, what will be asked, what will be uncovered. What if, while talking to and examining the patient with the Dahl sign shown in Grandjean and Huber,1 the patient’s condition would suddenly deteriorate, urgently requiring nebulized beta-2 agonists and transfer to the medical intensive care unit? What if the patient rambles for 5 minutes about extraneous details not relevant to his or her disease? What if the nurse needs to dispense scheduled medications or hang the next dose of antibiotics? What if the patient asks to use the bedpan at the moment digital clubbing was to be pointed out and discussed?
Of course, the patient may have lots to say, or nothing at all. But in those moments when the ice does not break, when the patient is not suddenly wheeled away to radiology, key clinical findings are seen and remembered, often for an entire career. If the ice does not break, the patient, the story, and the clinical finding—otherwise seen on a large screen in a dark room or on a page in a textbook or journal—come together in that moment, in a way nothing else ever quite can.
In this golden age of technology, we must remember that these images portray real patients with stories to tell, sometimes mundane and sometimes profound, but always worth hearing.
Acknowledgment: The author wishes to thank Mark C. Henderson, MD, for his helpful comments on this manuscript.
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
- Grandjean R, Huber LC. Thinker’s sign. Cleve Clin J Med 2019; 86(7):439. doi:10.3949/ccjm.86a.19036
- Chaiyachati KH, Shea JA, Asch DA, et al. Assessment of inpatient time allocation among first-year internal medicine residents using time-motion observations. JAMA Intern Med 2019. Epub ahead of print. doi:10.1001/jamainternmed.2019.0095
- Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med 2013; 28(8):1042–1047. doi:10.1007/s11606-013-2376-6
- Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med 2009; 4(5):304–307. doi:10.1002/jhm.540
Apply for the Research Career Development Travel Award
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
Whose needs come first – the patient’s or the trial’s?
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Debra Banks (not her real name) had hope. There was a clinical trial open at an academic hospital 200 miles from where she lived. She would commute or find local housing. It would cost her, but this is what her savings were for, she reasoned. What expense could be more important than her life?
Next came the tests. Blood tests, an ultrasound of her heart, breathing tests. She gave vials of blood, lay in scanners, and eagerly jumped through every hoop placed before her. Then came the call from the trial coordinator. Her heart ultrasound showed a mild dysfunction in how it pumped. It excluded her from the trial.
“Not eligible.” The two words that took away everything reverberated in her mind. Her heart had never caused her any problems before. So after the shock wore off, she tried to bargain with the trial coordinator: Had the study drug been shown to cause or worsen heart damage? Could they repeat the ultrasound? Did this blip in her heart function really matter?
When the trial coordinator couldn’t answer all these questions, she encouraged Debra to come into the clinic and talk to the doctors directly. That’s where I met her.
Debra found herself in the middle of a painful crossroads she had no interest being in. What happens when the needs of an individual patient and the needs of medical research are at odds? From Debra’s perspective, she had one goal. She wanted the therapy that would give her the best chance of living.
But the aim of the trial was not to help Debra – not directly, at least. Clinical trials help patient populations. The goal is to add to a body of knowledge: To study new therapies, demonstrate safety and efficacy, and ultimately find better treatments. The bulk of benefit goes to future patients, not individual participants. If an individual participant does benefit, all the better. But this is a bonus, not a requirement.
In order to meet these goals, trials come with inclusion and exclusion criteria. These are often strict. Individuals with certain other medical conditions are frequently excluded, as the person needs to be able to tolerate the toxicities of the drug being tested.
This, of course, is very different from our usual approach to patient care. Outside of trials, the needs of the individual patient are our North Star. Instead of inclusion and exclusion criteria, we have guidelines: general goalposts that hint at the right answer, but are able to be bent based on individual circumstances. It’s something I love about medicine. Part science, part art. Part algorithmic, part creative.
I can give chemotherapy to a patient with a low platelet count, if I think it’s best. I can override an elevated bilirubin. I can simply not check a heart ultrasound in the first place, if I don’t believe it will change my management.
I understand why trial criteria exist. I fully support investing in novel therapies that will help future patients on a large scale. There will invariably be individuals for whom a clinical trial is unsafe or inappropriate for a multitude of reasons, and our job as oncologists is to make that call and convey that news.
Still, that can be hard to square with the human being sitting in front of you. Debra was only in her mid-50s. She was an artist, an educator, a parent. She was a person who was so, so not ready to die. That she would because of a glitch in her heart function – the significance of which nobody knew – was excruciating.
While we can’t enroll every patient in every trial, the least we can do is comb through trial criteria thoughtfully. With the role of clinical investigator comes great responsibility. Are we choosing a cutoff because it makes clinical sense – or because that’s how it was done before? Is there a medical justification behind each and every exclusion criterion? A careless cutoff is not just a line on a protocol. It can be the difference between someone’s last hope – and no more options.
Every time I saw Debra in clinic, she asked about the trial. Then one day she stopped asking. She was distracted by more pressing problems. Her breathing had worsened and her energy levels were so low she could hardly get out of bed. Debra became sicker and sicker until she could no longer request the last hope that might make her better.
A wonderful physician-scientist I worked with once said she split her time between patient care and medical research because they complement each other. Whenever she lost a patient, she turned that pain into motivation to delve deeper into her research. She coped with individual loss by helping to make small, incremental improvements for the needs of many.
I think about this, months later, as I look around the empty exam room where I first met Debra. I imagine a roomful of patients, alive and healthy, for whom the research she was excluded from has benefited.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
NIH Study Will Test New Preventive Drug for Multidrug-Resistant TB
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Tuberculosis (TB) kills more people each year than any other infectious disease. Not only the patients, but their nearest and dearest are at risk, as well. They are more likely to acquire latent TB infection and many will progress to active TB.
NIH is launching a study to compare delamanid, a new drug for multidrug-resistant TB (MDR-TB) with isoniazid, the long-time standard. The study hypothesis is that prophylactic delamanid will better protect family and other household members of patients with MDR-TB. Existing treatments for MDR-TB are often highly toxic and poorly tolerated, putting patients at risk while curing them only about half the time. Delamanid is one of the first drugs available specifically to treat people with MDR-TB and the first formulation suitable for children.
“A highly effective preventive TB therapy for vulnerable household members of people with active MDR-TB disease would be a game-changer in TB care,” says Dr. Anneke Hesseling, MD, PhD, one of the study leaders.
The phase 3 trial, Protecting Households on Exposure to Newly Diagnosed Index Multidrug-Resistant Tuberculosis Patients (PHOENIx MDR-TB), will take place at > 27 sites in at ≥ 12 countries. The researchers plan to enroll 2,158 adults being treated for confirmed active MDR-TB and 3,452 members of their households who are at high risk for developing active TB. The household members will be assigned randomly to receive oral delamanid daily for 26 weeks or oral isoniazid plus vitamin B6 daily for 26 weeks. All at-risk members of the same household will receive the same drug regimen.
Every 2 to 12 weeks, participating household contacts will have physical exams and other health assessments. The researchers will follow them for 96 weeks. Final results are expected in 2024.
TB is the leading cause of death among people with HIV. Both delamanid and isoniazid have minimal potential for interacting with antiretroviral drugs. Study participants with HIV who have not yet begun treatment will be referred to local health care providers for antiretroviral treatment.
Federal Health Care Data Trends 2019
Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References

Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References

Click here to access Federal Health Care Data Trends 2019
Table of Contents
- American Indian/Alaska Native
- Women's Health
- Department of Veterans Affairs
- Neurologic Disorders
- Diabetes Mellitus
- Infectious Disease
- Hematology/Oncology
- Mental Health
- Respiratory Disorders
- Gastrointestinal Disorders
- Department of Defense
- References

AGA Clinical Practice Update: Coagulation in cirrhosis
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
FROM GASTROENTEROLOGY
Stable COPD: Initiating and Optimizing Therapy
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects.1-4 It is a major cause of morbidity and mortality, affecting 5% of the population in the United States and ranking as the third leading cause of death in 2008.5,6 The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this 3-part review, we discuss the management of stable COPD in the context of 3 common clinical scenarios: initiating and optimizing therapy, managing acute exacerbations, and managing advanced disease.
Case Presentation
A 65-year-old man with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect: forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC), 0.45; FEV1, 2 L (65% of predicted); and diffusing capacity of the lung for carbon monoxide, 15 mL/min/mm Hg (65% of predicted). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the past year, and this was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
COPD management is guided by disease severity that is measured using a multimodal staging system developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The initial classification adopted by the GOLD 2011 report encompassed 4 categories based on symptoms, number of exacerbations, and degree of airflow limitation on PFT. However, in 2017 the GOLD ABCD classification was modified to consider only symptoms and risk of exacerbation in classifying patients, regardless of performance on spirometry and FEV1 (Figure 1).7,8 This approach was intended to make therapy more individualized based on the patient clinical profile. The Table provides a summary of the recommended treatments according to classification based on the GOLD 2017 report.
The patient in our clinical scenario can be classified as GOLD category B.
What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta-agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta- agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Figure 2 presents an approach to building a treatment plan for the patient with stable COPD.
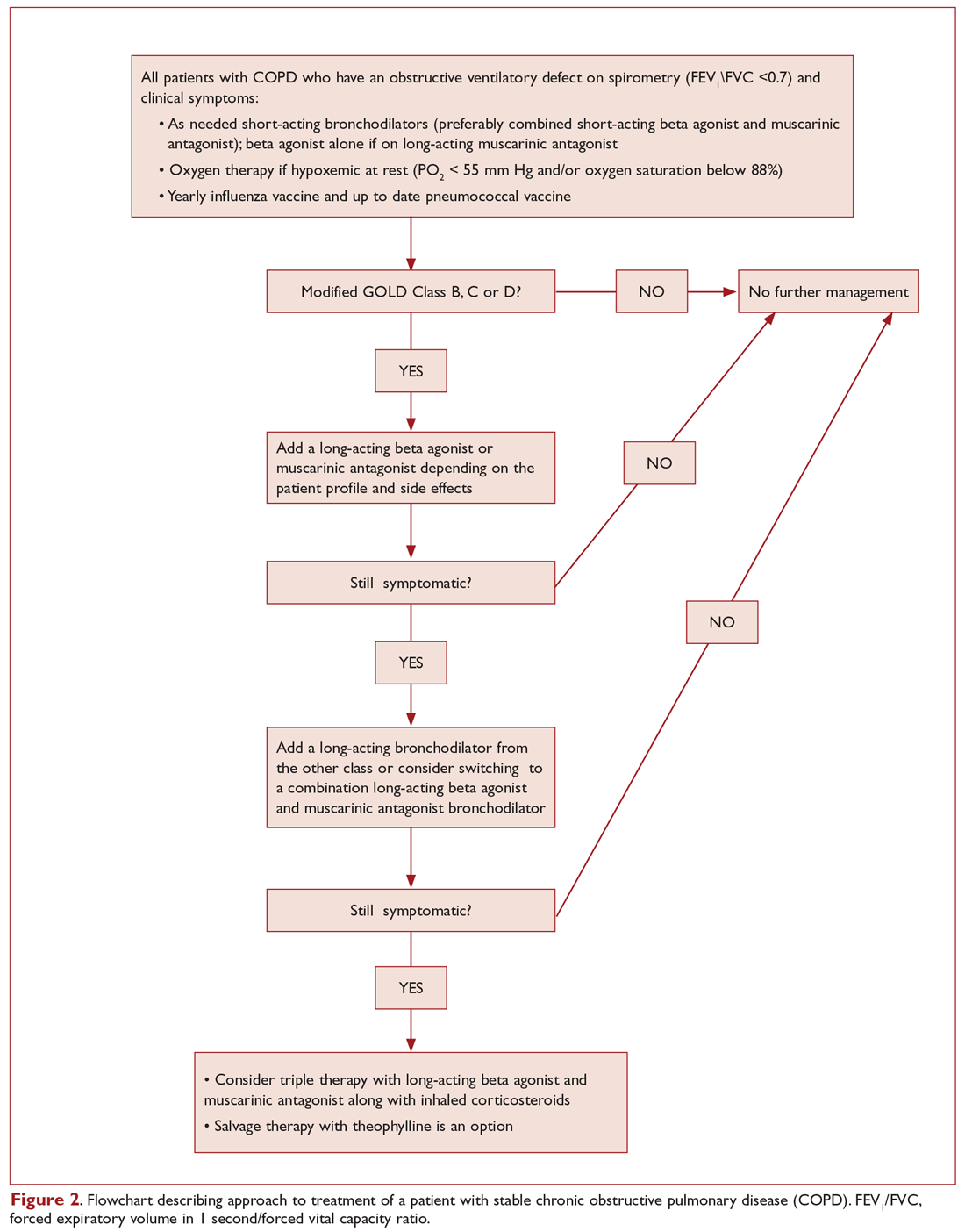
Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms.7 Both short-acting beta-agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD9,10 and seem to have comparative efficacy when compared head-to-head in trials.11 However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with fewer exacerbations compared to albuterol alone.12 On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate.11-13 Inhaled short-acting beta-agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function.14
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta-agonists increased the risk of severe cardiac arrhythmias.15 Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia.16 Beta-agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side effects but achieve less than maximal bronchodilation.17 Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant, especially when combined with long-acting anticholinergic agents.18
In light of the above discussion, a combination of a short-acting beta-agonist and a muscarinic antagonist is recommended in all patients with COPD, unless the patient is on a long-acting muscarinic antagonist (LAMA).7,18 In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator, as he was using the LAMA tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator:7,19 either a long-acting beta-agonist (LABA) or a LAMA. Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extended-duration of action.20 Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist.21 Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in an extended duration of action.21 Revefenacin is a new lung-selective LAMA that is under development and has shown promise among those with moderate to very severe COPD. Results are only limited to phase 3 trials, and clinical studies are still underway.22
The currently available LABAs include salmeterol, formoterol, arformoterol, olodaterol, and indacaterol. The last 2 have the advantage of once-daily dosing rather than twice daily.23,24 LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials.23,25 Vilanterol is another LABA that has a long duration of action and can be used once daily,26 but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium, in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate.27-30
Patients can be started on either a LAMA or LABA depending on the individual patient's needs and the agent's adverse effects.7 Both have comparable adverse effects and efficacy, as detailed below. Concerning adverse effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk,25,31,32 several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators.33,34 There was no difference in risk for adverse cardiovascular events between LABA and LAMA in 1 Canadian study, and slightly more with LABA in a study using an American database.33,34 Wang et al reported that the risk of cardiovascular adverse effects, defined as hospitalizations and emergency room visits from heart failure, arrythmia, stroke, or ischemia, was 1.5 times the baseline risk in the first 30 days of starting a LABA or LAMA.35 The risk was subsequently the same as baseline or even lower after that period. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies, but not clinical trials; the possibility of urinary retention should be discussed with patients upon initiation.36,37 Concerns about increased mortality with the soft mist formulation of tiotropium were put to rest by the Tiotropium Safety and Performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler.38
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations.39 No difference in hospitalization rate or mortality was noted in 1 meta-analysis, although tiotropium was more effective in reducing exacerbations.40 The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA, while a LAMA would be a better first agent for a patient with severe tachycardia induced by albuterol.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation.41 ICS should not be used alone for COPD management and are always combined with a LABA.7 Several ICS formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations, with modest effect on lung function and no change in mortality.42 Side effects include oral candidiasis, dysphonia, and skin bruising.43 There is also an increased risk of pneumonia.44 ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS).45 ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.46
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of LABA, recommendations are to add a bronchodilator from the other class.7 There are also multiple combined LAMA-LABA inhalers that are approved in the United States and can possibly improve adherence to therapy. These include tiotropium-olodaterol, umeclidinium-vilanterol, glycopyrronium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post-bronchodilator FEV1 and quality of life, with no change in hospital admissions, mortality, or adverse effects.47 Interestingly, adding tiotropium to LABA reduced exacerbations, although adding LABA to tiotropium did not.47
Current guidelines recommend that patients in GOLD categories C and D who are not well controlled should receive a combination of LABA-ICS.7 However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS.48 In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life.7,49 Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial.27 In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms.50 A combination of triple therapy with fluticasone furoate, umeclidinium, and vilanterol was recently noted to result in a lower rate of moderate or severe COPD exacerbations, preserve lung function, and maintain health-related quality of life, as compared with fluticasone furoate/vilanterol or umeclidinium/vilanterol combination therapy among those with symptomatic COPD with a history of exacerbations.51
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways.52 Theophylline has a serious adverse-effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor.53 It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials.54,55
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in the treatment of COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief.53 If used, the therapeutic range of theophylline for COPD is 8 to 12 mcg/mL peak level measured 3 to 7 hours after morning dose, and this level is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients.56
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients, regardless of disease severity, as they increase morbidity and mortality without improving symptoms or lung function.57,58 The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alfa, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied.59 There is no consistent evidence of beneficial role of mucolytics in COPD patients.7,59 The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio, 0.78; 95% CI, 0.67-0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers.60
Long-Term Antibiotics
There is no role for long-term antibiotics in the management of COPD.7 Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patients with frequent exacerbations on optimal therapy and will be discussed later in the review.61
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88% or PaO2 ≤ 55 mm Hg), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D.7 It improves symptoms, quality of life, exercise tolerance, and health care utilization. Beneficial effects last for about 2 years.62,63
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther. 2014;18:381-388.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598-604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir. 2010;27:1254-1266.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl). 2010;123:3467-3478.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1-126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648-654.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J. 2002;20:1138-1146.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax. 2003;58:580-584.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med. 1996;100(1A):11S-8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest. 1994;105:1411-1419.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115:635-641.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:85-90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142:305-311.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther. 2003;10:341-347.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990;336:1396-1399.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother. 2012;46:1717-1721.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420-1425.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1640-1645.
22. Ferguson GT, Feldman G, Pudi KK, et al. improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6:154-165.
23. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
24. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697-714.
25. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
26. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest. 2012;142:119-127.
27. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
28. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524-533.
29. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
30. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156.
31. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554. Expert Opin Pharmacother. 2009;10:719–22.
32. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29:2167-2178.
33. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175-1185.
34. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med. 2016;4:2050312116671337.
35. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-Control Study. JAMA Intern Med. 2018;178:229-238.
36. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2009;360:185-186.
37. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695-1703.
38. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491-1501.
39. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
40. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(9):CD009157.
41. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med. 2005;5:3.
42. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(7):CD002991.
43. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126:213-219.
44. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
45. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:2797-2803.
46. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241-1249.
47. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989.
48. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222-2234.
49. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545-555.
50. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741-750.
51. Lipson DA, Barnhart, Brealey N, et al; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671-1680.
52. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther. 2017;11:265-272.
53. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med. 1988;17:135-144.
54. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002(4):CD003902.
55. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med. 1989;320:1521-1525.
56. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials. 2015;16:267.
57. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(3):CD005374.
58. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res. 2014;15:37.
59. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(7):CD001287.
60. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
61. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
62. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
63. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976-983.
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects.1-4 It is a major cause of morbidity and mortality, affecting 5% of the population in the United States and ranking as the third leading cause of death in 2008.5,6 The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this 3-part review, we discuss the management of stable COPD in the context of 3 common clinical scenarios: initiating and optimizing therapy, managing acute exacerbations, and managing advanced disease.
Case Presentation
A 65-year-old man with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect: forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC), 0.45; FEV1, 2 L (65% of predicted); and diffusing capacity of the lung for carbon monoxide, 15 mL/min/mm Hg (65% of predicted). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the past year, and this was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
COPD management is guided by disease severity that is measured using a multimodal staging system developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The initial classification adopted by the GOLD 2011 report encompassed 4 categories based on symptoms, number of exacerbations, and degree of airflow limitation on PFT. However, in 2017 the GOLD ABCD classification was modified to consider only symptoms and risk of exacerbation in classifying patients, regardless of performance on spirometry and FEV1 (Figure 1).7,8 This approach was intended to make therapy more individualized based on the patient clinical profile. The Table provides a summary of the recommended treatments according to classification based on the GOLD 2017 report.

The patient in our clinical scenario can be classified as GOLD category B.

What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta-agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta- agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Figure 2 presents an approach to building a treatment plan for the patient with stable COPD.

Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms.7 Both short-acting beta-agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD9,10 and seem to have comparative efficacy when compared head-to-head in trials.11 However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with fewer exacerbations compared to albuterol alone.12 On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate.11-13 Inhaled short-acting beta-agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function.14
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta-agonists increased the risk of severe cardiac arrhythmias.15 Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia.16 Beta-agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side effects but achieve less than maximal bronchodilation.17 Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant, especially when combined with long-acting anticholinergic agents.18
In light of the above discussion, a combination of a short-acting beta-agonist and a muscarinic antagonist is recommended in all patients with COPD, unless the patient is on a long-acting muscarinic antagonist (LAMA).7,18 In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator, as he was using the LAMA tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator:7,19 either a long-acting beta-agonist (LABA) or a LAMA. Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extended-duration of action.20 Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist.21 Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in an extended duration of action.21 Revefenacin is a new lung-selective LAMA that is under development and has shown promise among those with moderate to very severe COPD. Results are only limited to phase 3 trials, and clinical studies are still underway.22
The currently available LABAs include salmeterol, formoterol, arformoterol, olodaterol, and indacaterol. The last 2 have the advantage of once-daily dosing rather than twice daily.23,24 LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials.23,25 Vilanterol is another LABA that has a long duration of action and can be used once daily,26 but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium, in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate.27-30
Patients can be started on either a LAMA or LABA depending on the individual patient's needs and the agent's adverse effects.7 Both have comparable adverse effects and efficacy, as detailed below. Concerning adverse effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk,25,31,32 several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators.33,34 There was no difference in risk for adverse cardiovascular events between LABA and LAMA in 1 Canadian study, and slightly more with LABA in a study using an American database.33,34 Wang et al reported that the risk of cardiovascular adverse effects, defined as hospitalizations and emergency room visits from heart failure, arrythmia, stroke, or ischemia, was 1.5 times the baseline risk in the first 30 days of starting a LABA or LAMA.35 The risk was subsequently the same as baseline or even lower after that period. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies, but not clinical trials; the possibility of urinary retention should be discussed with patients upon initiation.36,37 Concerns about increased mortality with the soft mist formulation of tiotropium were put to rest by the Tiotropium Safety and Performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler.38
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations.39 No difference in hospitalization rate or mortality was noted in 1 meta-analysis, although tiotropium was more effective in reducing exacerbations.40 The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA, while a LAMA would be a better first agent for a patient with severe tachycardia induced by albuterol.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation.41 ICS should not be used alone for COPD management and are always combined with a LABA.7 Several ICS formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations, with modest effect on lung function and no change in mortality.42 Side effects include oral candidiasis, dysphonia, and skin bruising.43 There is also an increased risk of pneumonia.44 ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS).45 ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.46
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of LABA, recommendations are to add a bronchodilator from the other class.7 There are also multiple combined LAMA-LABA inhalers that are approved in the United States and can possibly improve adherence to therapy. These include tiotropium-olodaterol, umeclidinium-vilanterol, glycopyrronium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post-bronchodilator FEV1 and quality of life, with no change in hospital admissions, mortality, or adverse effects.47 Interestingly, adding tiotropium to LABA reduced exacerbations, although adding LABA to tiotropium did not.47
Current guidelines recommend that patients in GOLD categories C and D who are not well controlled should receive a combination of LABA-ICS.7 However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS.48 In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life.7,49 Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial.27 In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms.50 A combination of triple therapy with fluticasone furoate, umeclidinium, and vilanterol was recently noted to result in a lower rate of moderate or severe COPD exacerbations, preserve lung function, and maintain health-related quality of life, as compared with fluticasone furoate/vilanterol or umeclidinium/vilanterol combination therapy among those with symptomatic COPD with a history of exacerbations.51
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways.52 Theophylline has a serious adverse-effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor.53 It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials.54,55
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in the treatment of COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief.53 If used, the therapeutic range of theophylline for COPD is 8 to 12 mcg/mL peak level measured 3 to 7 hours after morning dose, and this level is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients.56
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients, regardless of disease severity, as they increase morbidity and mortality without improving symptoms or lung function.57,58 The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alfa, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied.59 There is no consistent evidence of beneficial role of mucolytics in COPD patients.7,59 The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio, 0.78; 95% CI, 0.67-0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers.60
Long-Term Antibiotics
There is no role for long-term antibiotics in the management of COPD.7 Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patients with frequent exacerbations on optimal therapy and will be discussed later in the review.61
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88% or PaO2 ≤ 55 mm Hg), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D.7 It improves symptoms, quality of life, exercise tolerance, and health care utilization. Beneficial effects last for about 2 years.62,63
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects.1-4 It is a major cause of morbidity and mortality, affecting 5% of the population in the United States and ranking as the third leading cause of death in 2008.5,6 The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this 3-part review, we discuss the management of stable COPD in the context of 3 common clinical scenarios: initiating and optimizing therapy, managing acute exacerbations, and managing advanced disease.
Case Presentation
A 65-year-old man with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect: forced expiratory volume in 1 second/forced vital capacity ratio (FEV1/FVC), 0.45; FEV1, 2 L (65% of predicted); and diffusing capacity of the lung for carbon monoxide, 15 mL/min/mm Hg (65% of predicted). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the past year, and this was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
COPD management is guided by disease severity that is measured using a multimodal staging system developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The initial classification adopted by the GOLD 2011 report encompassed 4 categories based on symptoms, number of exacerbations, and degree of airflow limitation on PFT. However, in 2017 the GOLD ABCD classification was modified to consider only symptoms and risk of exacerbation in classifying patients, regardless of performance on spirometry and FEV1 (Figure 1).7,8 This approach was intended to make therapy more individualized based on the patient clinical profile. The Table provides a summary of the recommended treatments according to classification based on the GOLD 2017 report.

The patient in our clinical scenario can be classified as GOLD category B.

What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta-agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta- agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Figure 2 presents an approach to building a treatment plan for the patient with stable COPD.

Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms.7 Both short-acting beta-agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD9,10 and seem to have comparative efficacy when compared head-to-head in trials.11 However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with fewer exacerbations compared to albuterol alone.12 On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate.11-13 Inhaled short-acting beta-agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function.14
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta-agonists increased the risk of severe cardiac arrhythmias.15 Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia.16 Beta-agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side effects but achieve less than maximal bronchodilation.17 Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant, especially when combined with long-acting anticholinergic agents.18
In light of the above discussion, a combination of a short-acting beta-agonist and a muscarinic antagonist is recommended in all patients with COPD, unless the patient is on a long-acting muscarinic antagonist (LAMA).7,18 In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator, as he was using the LAMA tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator:7,19 either a long-acting beta-agonist (LABA) or a LAMA. Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extended-duration of action.20 Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist.21 Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in an extended duration of action.21 Revefenacin is a new lung-selective LAMA that is under development and has shown promise among those with moderate to very severe COPD. Results are only limited to phase 3 trials, and clinical studies are still underway.22
The currently available LABAs include salmeterol, formoterol, arformoterol, olodaterol, and indacaterol. The last 2 have the advantage of once-daily dosing rather than twice daily.23,24 LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials.23,25 Vilanterol is another LABA that has a long duration of action and can be used once daily,26 but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium, in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate.27-30
Patients can be started on either a LAMA or LABA depending on the individual patient's needs and the agent's adverse effects.7 Both have comparable adverse effects and efficacy, as detailed below. Concerning adverse effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk,25,31,32 several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators.33,34 There was no difference in risk for adverse cardiovascular events between LABA and LAMA in 1 Canadian study, and slightly more with LABA in a study using an American database.33,34 Wang et al reported that the risk of cardiovascular adverse effects, defined as hospitalizations and emergency room visits from heart failure, arrythmia, stroke, or ischemia, was 1.5 times the baseline risk in the first 30 days of starting a LABA or LAMA.35 The risk was subsequently the same as baseline or even lower after that period. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies, but not clinical trials; the possibility of urinary retention should be discussed with patients upon initiation.36,37 Concerns about increased mortality with the soft mist formulation of tiotropium were put to rest by the Tiotropium Safety and Performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler.38
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations.39 No difference in hospitalization rate or mortality was noted in 1 meta-analysis, although tiotropium was more effective in reducing exacerbations.40 The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA, while a LAMA would be a better first agent for a patient with severe tachycardia induced by albuterol.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation.41 ICS should not be used alone for COPD management and are always combined with a LABA.7 Several ICS formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations, with modest effect on lung function and no change in mortality.42 Side effects include oral candidiasis, dysphonia, and skin bruising.43 There is also an increased risk of pneumonia.44 ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS).45 ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.46
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of LABA, recommendations are to add a bronchodilator from the other class.7 There are also multiple combined LAMA-LABA inhalers that are approved in the United States and can possibly improve adherence to therapy. These include tiotropium-olodaterol, umeclidinium-vilanterol, glycopyrronium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post-bronchodilator FEV1 and quality of life, with no change in hospital admissions, mortality, or adverse effects.47 Interestingly, adding tiotropium to LABA reduced exacerbations, although adding LABA to tiotropium did not.47
Current guidelines recommend that patients in GOLD categories C and D who are not well controlled should receive a combination of LABA-ICS.7 However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS.48 In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life.7,49 Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial.27 In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms.50 A combination of triple therapy with fluticasone furoate, umeclidinium, and vilanterol was recently noted to result in a lower rate of moderate or severe COPD exacerbations, preserve lung function, and maintain health-related quality of life, as compared with fluticasone furoate/vilanterol or umeclidinium/vilanterol combination therapy among those with symptomatic COPD with a history of exacerbations.51
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways.52 Theophylline has a serious adverse-effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor.53 It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials.54,55
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in the treatment of COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief.53 If used, the therapeutic range of theophylline for COPD is 8 to 12 mcg/mL peak level measured 3 to 7 hours after morning dose, and this level is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients.56
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients, regardless of disease severity, as they increase morbidity and mortality without improving symptoms or lung function.57,58 The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alfa, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied.59 There is no consistent evidence of beneficial role of mucolytics in COPD patients.7,59 The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio, 0.78; 95% CI, 0.67-0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers.60
Long-Term Antibiotics
There is no role for long-term antibiotics in the management of COPD.7 Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patients with frequent exacerbations on optimal therapy and will be discussed later in the review.61
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88% or PaO2 ≤ 55 mm Hg), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D.7 It improves symptoms, quality of life, exercise tolerance, and health care utilization. Beneficial effects last for about 2 years.62,63
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther. 2014;18:381-388.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598-604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir. 2010;27:1254-1266.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl). 2010;123:3467-3478.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1-126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648-654.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J. 2002;20:1138-1146.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax. 2003;58:580-584.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med. 1996;100(1A):11S-8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest. 1994;105:1411-1419.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115:635-641.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:85-90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142:305-311.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther. 2003;10:341-347.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990;336:1396-1399.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother. 2012;46:1717-1721.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420-1425.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1640-1645.
22. Ferguson GT, Feldman G, Pudi KK, et al. improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6:154-165.
23. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
24. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697-714.
25. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
26. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest. 2012;142:119-127.
27. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
28. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524-533.
29. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
30. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156.
31. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554. Expert Opin Pharmacother. 2009;10:719–22.
32. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29:2167-2178.
33. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175-1185.
34. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med. 2016;4:2050312116671337.
35. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-Control Study. JAMA Intern Med. 2018;178:229-238.
36. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2009;360:185-186.
37. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695-1703.
38. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491-1501.
39. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
40. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(9):CD009157.
41. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med. 2005;5:3.
42. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(7):CD002991.
43. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126:213-219.
44. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
45. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:2797-2803.
46. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241-1249.
47. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989.
48. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222-2234.
49. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545-555.
50. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741-750.
51. Lipson DA, Barnhart, Brealey N, et al; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671-1680.
52. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther. 2017;11:265-272.
53. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med. 1988;17:135-144.
54. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002(4):CD003902.
55. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med. 1989;320:1521-1525.
56. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials. 2015;16:267.
57. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(3):CD005374.
58. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res. 2014;15:37.
59. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(7):CD001287.
60. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
61. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
62. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
63. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976-983.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther. 2014;18:381-388.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598-604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir. 2010;27:1254-1266.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl). 2010;123:3467-3478.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1-126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648-654.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J. 2002;20:1138-1146.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax. 2003;58:580-584.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med. 1996;100(1A):11S-8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest. 1994;105:1411-1419.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115:635-641.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:85-90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142:305-311.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther. 2003;10:341-347.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990;336:1396-1399.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother. 2012;46:1717-1721.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420-1425.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1640-1645.
22. Ferguson GT, Feldman G, Pudi KK, et al. improvements in lung function with nebulized revefenacin in the treatment of patients with moderate to very severe COPD: results from two replicate phase III clinical trials. Chronic Obstr Pulm Dis. 2019;6:154-165.
23. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
24. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697-714.
25. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
26. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest. 2012;142:119-127.
27. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
28. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524-533.
29. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
30. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156.
31. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554. Expert Opin Pharmacother. 2009;10:719–22.
32. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther. 2007;29:2167-2178.
33. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175-1185.
34. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med. 2016;4:2050312116671337.
35. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-Control Study. JAMA Intern Med. 2018;178:229-238.
36. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2009;360:185-186.
37. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695-1703.
38. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491-1501.
39. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
40. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(9):CD009157.
41. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med. 2005;5:3.
42. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012(7):CD002991.
43. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126:213-219.
44. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
45. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:2797-2803.
46. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241-1249.
47. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989.
48. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222-2234.
49. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545-555.
50. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741-750.
51. Lipson DA, Barnhart, Brealey N, et al; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671-1680.
52. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther. 2017;11:265-272.
53. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med. 1988;17:135-144.
54. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002(4):CD003902.
55. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med. 1989;320:1521-1525.
56. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials. 2015;16:267.
57. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(3):CD005374.
58. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res. 2014;15:37.
59. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(7):CD001287.
60. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
61. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
62. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
63. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000;117:976-983.
Stable COPD: Managing Acute Exacerbations
Case Presentation
A 70-year-old man with severe chronic obstructive pulmonary disease (COPD) on oxygen therapy and obstructive sleep apnea treated with nocturnal continuous positive airway pressure was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 beats/min, oxygen saturation 94% on 2 L/min of oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15 breaths/min, and body mass index 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD.1,2 AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality.3 Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPD are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations.4 Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is AECOPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD.5 Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED.6 Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both.7
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF-α positive cells has also been reported.2 With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD.1 Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane.6
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. Patients with frequent exacerbations have a worse quality of life than patients with a history of less frequent exacerbations.8 Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in forced expiratory volume in 1 second (FEV1) per lower respiratory tract infection per year,9,10 and approximately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations.11
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations.2 Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation.12 Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia.6
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society5 have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory-modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate.13 In 2 large clinical trials,14,15 daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of inhaled corticosteroids (ICS) and long-acting muscarinic antagonists (LAMAs). Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use.14,16 These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use.17 While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations.4 This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant gastrointestinal side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance.18 Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment.6 Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients.5
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions.19 Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well-tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades,20-22 although the largest clinical trial to date was reported in 2014.23 Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.37-0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/forced vital capacity ratio < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD.24,25 But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis.5 One recent study showed that the use of once-daily generic azithromycin 5 days per week appeared to have an impact on AECOPD incidence.26 In this study, the rate of AECOPD was reduced from 1.83 to 1.48 exacerbations per patient-year (relative risk, 0.83; 95% CI, 0.72–0.95; P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99).27 Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing.28 Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. Due to potential adverse effects, however, macrolide therapy is an option rather than a strong recommendation.5 The prescribing clinician also needs to consider potential of prolongation of the QT interval.26
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD.29,30 Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immunostimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis and increasing opsonization when administered to individuals with COPD.6 Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract.29,30 However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A study that evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD31 found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy.6
β-Blockers
Observational studies of β-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; 95% CI, 0.60–0.90) in patients receiving β-blockers versus those not on β-blockers.32 Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing.33
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations.34 Two small, single-center studies,35,36 have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study,6 which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations.37 Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
1. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35:157-163.
2. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786-796.
3. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698-702.
4. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef. 2014;7:98.
5. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
6. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Respirology. 2017;22:575-601.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418-1422.
9. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
10. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358-364.
11. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847-852.
12. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114-1121.
13. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
14. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
15. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long-acting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
16. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology. 2011;16:1249-1257.
17. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375-382.
18. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013(11):CD002309.
19. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis. 1964;90:721-729.
20. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis. 1983;64:405-415.
21. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur J Clin Pharmacol. 1976;9:393-396.
22. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med. 1994;88:531-535.
23. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
24. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ. 1960;1:297-303.
25. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ. 1961;2:979.
26. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
27. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503-1508.
28. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
29. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1719-1724.
30. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age. 2004;67:828-834.
31. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis. 2007;2:335.
32. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71:8-14.
33. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open. 2016;6:e012292.
34. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128-1138.
35. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57:1453-1457.
36. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol. 2016;97:107-113.
37. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883-890.
Case Presentation
A 70-year-old man with severe chronic obstructive pulmonary disease (COPD) on oxygen therapy and obstructive sleep apnea treated with nocturnal continuous positive airway pressure was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 beats/min, oxygen saturation 94% on 2 L/min of oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15 breaths/min, and body mass index 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD.1,2 AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality.3 Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPD are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations.4 Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is AECOPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD.5 Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED.6 Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both.7
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF-α positive cells has also been reported.2 With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD.1 Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane.6
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. Patients with frequent exacerbations have a worse quality of life than patients with a history of less frequent exacerbations.8 Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in forced expiratory volume in 1 second (FEV1) per lower respiratory tract infection per year,9,10 and approximately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations.11
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations.2 Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation.12 Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia.6
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society5 have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory-modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate.13 In 2 large clinical trials,14,15 daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of inhaled corticosteroids (ICS) and long-acting muscarinic antagonists (LAMAs). Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use.14,16 These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use.17 While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations.4 This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant gastrointestinal side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance.18 Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment.6 Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients.5
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions.19 Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well-tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades,20-22 although the largest clinical trial to date was reported in 2014.23 Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.37-0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/forced vital capacity ratio < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD.24,25 But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis.5 One recent study showed that the use of once-daily generic azithromycin 5 days per week appeared to have an impact on AECOPD incidence.26 In this study, the rate of AECOPD was reduced from 1.83 to 1.48 exacerbations per patient-year (relative risk, 0.83; 95% CI, 0.72–0.95; P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99).27 Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing.28 Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. Due to potential adverse effects, however, macrolide therapy is an option rather than a strong recommendation.5 The prescribing clinician also needs to consider potential of prolongation of the QT interval.26
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD.29,30 Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immunostimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis and increasing opsonization when administered to individuals with COPD.6 Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract.29,30 However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A study that evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD31 found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy.6
β-Blockers
Observational studies of β-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; 95% CI, 0.60–0.90) in patients receiving β-blockers versus those not on β-blockers.32 Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing.33
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations.34 Two small, single-center studies,35,36 have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study,6 which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations.37 Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
Case Presentation
A 70-year-old man with severe chronic obstructive pulmonary disease (COPD) on oxygen therapy and obstructive sleep apnea treated with nocturnal continuous positive airway pressure was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 beats/min, oxygen saturation 94% on 2 L/min of oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15 breaths/min, and body mass index 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD.1,2 AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality.3 Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPD are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations.4 Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is AECOPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD.5 Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED.6 Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both.7
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF-α positive cells has also been reported.2 With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD.1 Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane.6
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. Patients with frequent exacerbations have a worse quality of life than patients with a history of less frequent exacerbations.8 Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in forced expiratory volume in 1 second (FEV1) per lower respiratory tract infection per year,9,10 and approximately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations.11
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations.2 Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation.12 Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia.6
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society5 have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory-modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate.13 In 2 large clinical trials,14,15 daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of inhaled corticosteroids (ICS) and long-acting muscarinic antagonists (LAMAs). Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use.14,16 These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use.17 While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations.4 This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant gastrointestinal side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance.18 Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment.6 Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients.5
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions.19 Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well-tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades,20-22 although the largest clinical trial to date was reported in 2014.23 Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.37-0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/forced vital capacity ratio < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD.24,25 But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis.5 One recent study showed that the use of once-daily generic azithromycin 5 days per week appeared to have an impact on AECOPD incidence.26 In this study, the rate of AECOPD was reduced from 1.83 to 1.48 exacerbations per patient-year (relative risk, 0.83; 95% CI, 0.72–0.95; P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99).27 Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing.28 Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. Due to potential adverse effects, however, macrolide therapy is an option rather than a strong recommendation.5 The prescribing clinician also needs to consider potential of prolongation of the QT interval.26
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD.29,30 Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immunostimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis and increasing opsonization when administered to individuals with COPD.6 Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract.29,30 However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A study that evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD31 found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy.6
β-Blockers
Observational studies of β-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; 95% CI, 0.60–0.90) in patients receiving β-blockers versus those not on β-blockers.32 Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing.33
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations.34 Two small, single-center studies,35,36 have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study,6 which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations.37 Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
1. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35:157-163.
2. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786-796.
3. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698-702.
4. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef. 2014;7:98.
5. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
6. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Respirology. 2017;22:575-601.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418-1422.
9. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
10. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358-364.
11. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847-852.
12. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114-1121.
13. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
14. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
15. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long-acting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
16. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology. 2011;16:1249-1257.
17. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375-382.
18. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013(11):CD002309.
19. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis. 1964;90:721-729.
20. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis. 1983;64:405-415.
21. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur J Clin Pharmacol. 1976;9:393-396.
22. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med. 1994;88:531-535.
23. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
24. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ. 1960;1:297-303.
25. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ. 1961;2:979.
26. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
27. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503-1508.
28. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
29. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1719-1724.
30. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age. 2004;67:828-834.
31. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis. 2007;2:335.
32. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71:8-14.
33. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open. 2016;6:e012292.
34. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128-1138.
35. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57:1453-1457.
36. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol. 2016;97:107-113.
37. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883-890.
1. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35:157-163.
2. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786-796.
3. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698-702.
4. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef. 2014;7:98.
5. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
6. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Respirology. 2017;22:575-601.
7. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181.
8. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418-1422.
9. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
10. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358-364.
11. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847-852.
12. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114-1121.
13. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53-67.
14. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
15. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long-acting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
16. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology. 2011;16:1249-1257.
17. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis. 2012;7:375-382.
18. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013(11):CD002309.
19. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis. 1964;90:721-729.
20. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis. 1983;64:405-415.
21. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur J Clin Pharmacol. 1976;9:393-396.
22. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med. 1994;88:531-535.
23. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187-194.
24. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ. 1960;1:297-303.
25. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ. 1961;2:979.
26. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
27. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189:1503-1508.
28. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
29. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1719-1724.
30. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age. 2004;67:828-834.
31. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis. 2007;2:335.
32. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71:8-14.
33. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open. 2016;6:e012292.
34. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128-1138.
35. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57:1453-1457.
36. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol. 2016;97:107-113.
37. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21:883-890.
Stable COPD: Managing Advanced Disease
Case Presentation
A 65-year-old man with severe chronic obstructive disease (COPD; forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 27; FEV1 25% of predicted; residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a long-acting beta agonist (LABA)/long-acting muscarinic antagonist (LAMA) combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest computed tomography (CT) scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively.1 Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population, as compared with pharmacologic management alone.2 However, the procedure is associated with risks, including higher short-term morbidity and mortality.2 Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone.2 On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone.2 Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery.2,3
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction.4-9 Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics.10,11 The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis, and increased frequency of COPD exacerbation in the following 30 days. The fact that a high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy.12 The HRCT scans also help in identifying those with complete fissures, a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic LVRS.13 Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction.11,14 Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status.13
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for more than 15 hours a day is thought to reduce mortality among patients with COPD and severe resting hypoxemia.15-18 More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status.19
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease.20 Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources.21 A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders.22
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment.23 The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to improve his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is an indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 additional year after transplant, but only 11% of patients with an FEV1 > 25% will do so.24 The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify patients who will benefit from lung transplantation.25,26 International guidelines for the selection of lung transplant candidates identify the following patient characteristics:27
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy;
- The patient is not a candidate for endoscopic or surgical LVRS;
- BODE index is 5 to 6;
- The PCO2 is greater than 50 mm Hg (6.6 kPa) and/or PO2 is less than 60 mm Hg (8 kPa);
- FEV1 is 25% predicted.
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery.28
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year.29 COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members, and palliative care is recommended by multiple international societies for patients with advanced COPD.30,31 In spite of these recommendations, the utilization of palliative care resources has been dismally low.32,33 Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
1. Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg. 1998;39:237.
2. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med. 2001;345:1075-1083.
3. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073.
4. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg. 2014;148:2651-2658.
5. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA. 2016;315:175-184.
6. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology. 2015;20:319-326.
7. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88:1993-1998.
8. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med. 2003;167:771-778.
9. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med. 2001;163:1068-1073.
10. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997-1005.
11. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233-1244.
12. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006;129:518-526.
13. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration. 2016;92:397-403.
14. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J. 2015;46:651-662.
15. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391-398.
16. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet. 1981;1:681-686.
17. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
18. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365.
19. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-1627.
20. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(2):CD003793.
21. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362-368.
22. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772-778.
23. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
24. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1156-1163.
25. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J. 2010;36:74-80.
26. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:109-114.
27. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1-15.
28. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol. 2008;3:1404-1409.
29. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax. 2015;70:294-296.
30. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care. 2016;15:18.
31. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
32. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med. 2016;19:360-372.
33. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151:41-46.
Case Presentation
A 65-year-old man with severe chronic obstructive disease (COPD; forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 27; FEV1 25% of predicted; residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a long-acting beta agonist (LABA)/long-acting muscarinic antagonist (LAMA) combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest computed tomography (CT) scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively.1 Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population, as compared with pharmacologic management alone.2 However, the procedure is associated with risks, including higher short-term morbidity and mortality.2 Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone.2 On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone.2 Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery.2,3
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction.4-9 Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics.10,11 The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis, and increased frequency of COPD exacerbation in the following 30 days. The fact that a high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy.12 The HRCT scans also help in identifying those with complete fissures, a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic LVRS.13 Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction.11,14 Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status.13
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for more than 15 hours a day is thought to reduce mortality among patients with COPD and severe resting hypoxemia.15-18 More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status.19
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease.20 Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources.21 A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders.22
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment.23 The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to improve his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is an indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 additional year after transplant, but only 11% of patients with an FEV1 > 25% will do so.24 The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify patients who will benefit from lung transplantation.25,26 International guidelines for the selection of lung transplant candidates identify the following patient characteristics:27
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy;
- The patient is not a candidate for endoscopic or surgical LVRS;
- BODE index is 5 to 6;
- The PCO2 is greater than 50 mm Hg (6.6 kPa) and/or PO2 is less than 60 mm Hg (8 kPa);
- FEV1 is 25% predicted.
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery.28
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year.29 COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members, and palliative care is recommended by multiple international societies for patients with advanced COPD.30,31 In spite of these recommendations, the utilization of palliative care resources has been dismally low.32,33 Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
Case Presentation
A 65-year-old man with severe chronic obstructive disease (COPD; forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 27; FEV1 25% of predicted; residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a long-acting beta agonist (LABA)/long-acting muscarinic antagonist (LAMA) combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest computed tomography (CT) scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively.1 Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population, as compared with pharmacologic management alone.2 However, the procedure is associated with risks, including higher short-term morbidity and mortality.2 Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone.2 On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone.2 Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery.2,3
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction.4-9 Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics.10,11 The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis, and increased frequency of COPD exacerbation in the following 30 days. The fact that a high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy.12 The HRCT scans also help in identifying those with complete fissures, a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic LVRS.13 Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction.11,14 Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status.13
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for more than 15 hours a day is thought to reduce mortality among patients with COPD and severe resting hypoxemia.15-18 More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status.19
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease.20 Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources.21 A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders.22
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment.23 The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to improve his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is an indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 additional year after transplant, but only 11% of patients with an FEV1 > 25% will do so.24 The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify patients who will benefit from lung transplantation.25,26 International guidelines for the selection of lung transplant candidates identify the following patient characteristics:27
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy;
- The patient is not a candidate for endoscopic or surgical LVRS;
- BODE index is 5 to 6;
- The PCO2 is greater than 50 mm Hg (6.6 kPa) and/or PO2 is less than 60 mm Hg (8 kPa);
- FEV1 is 25% predicted.
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery.28
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year.29 COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members, and palliative care is recommended by multiple international societies for patients with advanced COPD.30,31 In spite of these recommendations, the utilization of palliative care resources has been dismally low.32,33 Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
1. Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg. 1998;39:237.
2. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med. 2001;345:1075-1083.
3. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073.
4. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg. 2014;148:2651-2658.
5. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA. 2016;315:175-184.
6. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology. 2015;20:319-326.
7. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88:1993-1998.
8. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med. 2003;167:771-778.
9. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med. 2001;163:1068-1073.
10. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997-1005.
11. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233-1244.
12. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006;129:518-526.
13. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration. 2016;92:397-403.
14. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J. 2015;46:651-662.
15. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391-398.
16. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet. 1981;1:681-686.
17. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
18. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365.
19. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-1627.
20. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(2):CD003793.
21. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362-368.
22. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772-778.
23. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
24. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1156-1163.
25. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J. 2010;36:74-80.
26. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:109-114.
27. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1-15.
28. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol. 2008;3:1404-1409.
29. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax. 2015;70:294-296.
30. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care. 2016;15:18.
31. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
32. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med. 2016;19:360-372.
33. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151:41-46.
1. Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg. 1998;39:237.
2. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med. 2001;345:1075-1083.
3. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073.
4. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg. 2014;148:2651-2658.
5. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA. 2016;315:175-184.
6. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology. 2015;20:319-326.
7. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg. 2009;88:1993-1998.
8. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med. 2003;167:771-778.
9. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med. 2001;163:1068-1073.
10. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997-1005.
11. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233-1244.
12. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006;129:518-526.
13. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration. 2016;92:397-403.
14. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J. 2015;46:651-662.
15. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391-398.
16. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet. 1981;1:681-686.
17. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
18. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365.
19. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-1627.
20. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(2):CD003793.
21. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362-368.
22. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772-778.
23. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. www.goldcopd.org. Accessed July 9, 2019.
24. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1156-1163.
25. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J. 2010;36:74-80.
26. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:109-114.
27. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1-15.
28. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol. 2008;3:1404-1409.
29. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax. 2015;70:294-296.
30. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care. 2016;15:18.
31. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932-946.
32. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med. 2016;19:360-372.
33. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151:41-46.