User login
Breast density alone should not prompt supplemental imaging discussions
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
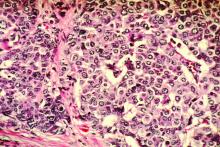
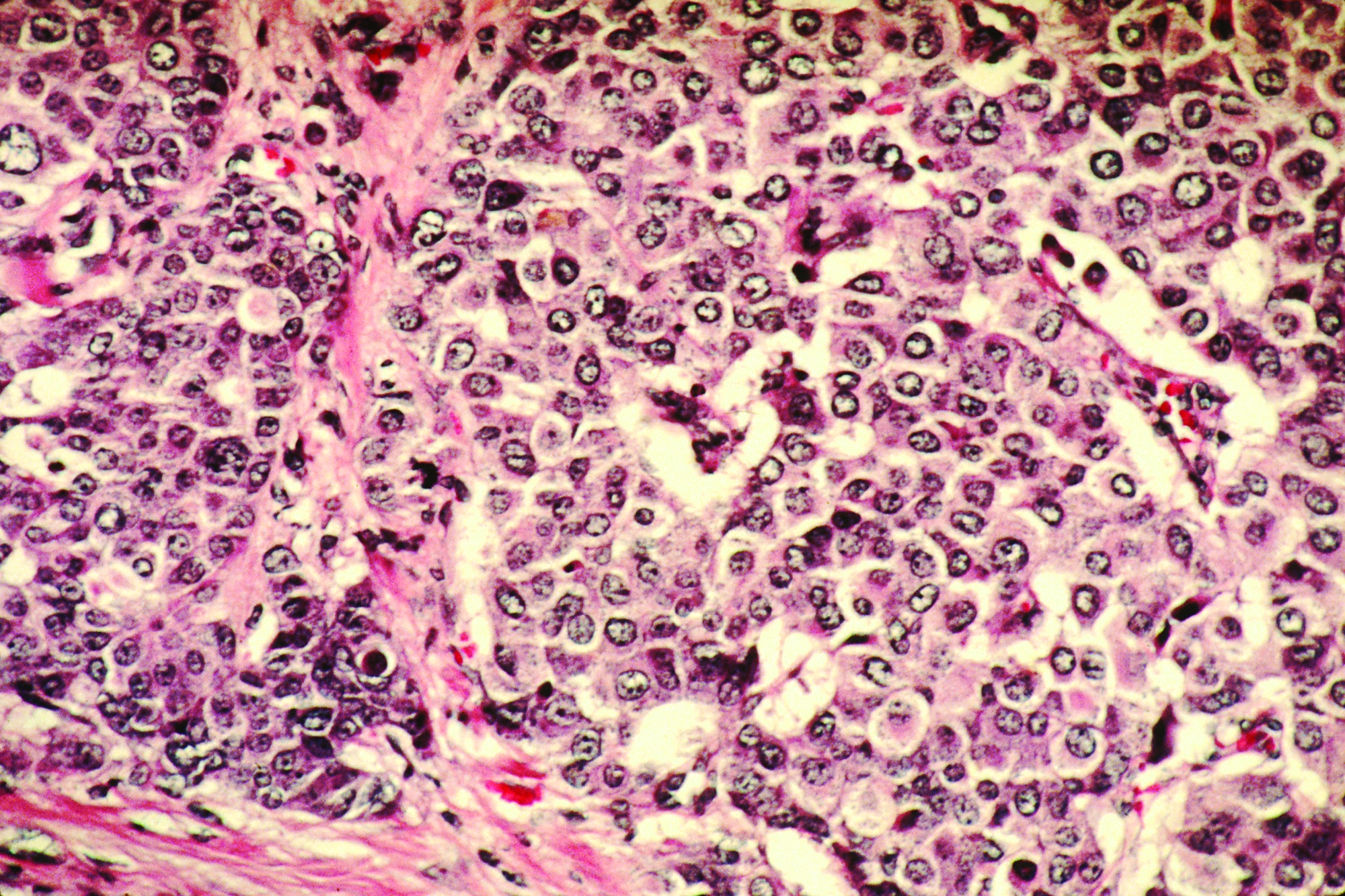
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Identifying women at risk of breast cancer is key, but physicians and policymakers should pause and reassess how exactly to go about it, according to Ilana B. Richman, MD, and Susan H. Busch, PhD of the Yale School of Medicine.
The latest proposal from the U.S. Food and Drug Administration focuses on recommending additional screening for women with dense breasts, but that can be too broad of a stroke. “Breast density is only one aspect of breast cancer risk,” the coauthors noted, and limiting supplemental screening recommendations to women with dense breasts may leave out many others at legitimate risk.
So how should supplemental screening be handled moving forward? In their accompanying study, Kerlikowske et al. rejected 2 strategies while embracing elements of 3 others, but none of them were recognized as the proper path to take.
At the same time, the coauthors asked, “Why legislate this particular area of medicine?” And what is the exact opportunity cost of supplemental screening? There is no simple answer, which highlights “both the overall inefficiency of supplemental screening and the insensitivity of a targeted approach.” In short, more work is needed.
These comments are adapted from an accompanying editorial (JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1737 ). Dr. Richman reported receiving funding from the Centers for Medicare and Medicaid Services to develop quality measures, along with funding from the National Center for Advancing Translational Sciences.
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
Breast density should be a factor in assessing breast cancer risk and recommending supplemental imaging, but not the primary factor, according to a study of women who were screened for the disease.
“Counseling strategies that identified women for supplemental imaging based on breast density and BCSC 5-year risk were more efficient compared with strategies based on age and density or density alone,” wrote Karla Kerlikowske, MD, of the Department of Veterans Affairs, and her coauthors. The study was published in JAMA Internal Medicine.
To assess breast cancer risk and strategies for recommending supplemental screening, the researchers assembled a cohort of 638,856 women aged 40 to 74 years who received mammograms at Breast Cancer Surveillance Consortium (BCSC) facilities from Jan. 3, 2005, to Dec. 31, 2014. Participants were identified as high risk via combinations of Breast Imaging Reporting and Data System (BI-RADS) breast density, BCSC 5-year breast cancer risk, and age.
Women with dense breasts made up 47% of those screened, and 60% of those with advanced cancers. Low advanced cancer rates (less than .61 per 1,000 mammograms) occurred in 34.5% of women with dense breasts, while high advanced cancer rates (greater than or equal to .61 cases per 1,000 mammograms) occurred in women with heterogeneously dense breasts and a 5-year risk of 2.5% or higher (6.0% of screened women) and those with extremely dense breasts and a 5-year risk of 1.0% or higher (6.5% of screened women).
In a hypothetical cohort of 100,000 women, supplemental imaging for all 47,012 women with dense breasts would mean a ratio of 1,866 supplemental imaging discussions per potential advanced breast cancer prevented. If imaging was considered based on a combination of density plus BCSC 5-year risk, the number of women screened would be reduced to 12,506 and the ratio would become 1,097 supplemental imaging discussions per potential advanced cancer prevented.
The coauthors acknowledged their study’s limitations, including their lack of ability to determine if women at high risk of advanced cancer would benefit from supplemental screening. In addition, they were unable to evaluate digital breast tomosynthesis outcomes, though they noted that, to their knowledge, “no published evidence indicates that advanced cancer rates differ for digital mammography vs. tomosynthesis according to breast density.”
The study was funded by the Patient-Centered Outcomes Research Institute, the Breast Cancer Surveillance Consortium, the National Cancer Institute, the Agency for Health Research and Quality, and the Lake Champlain Cancer Research Organization. The authors reported several potential conflicts of interest, including being members of various working groups, advisory boards, committees, task forces, and panels.
SOURCE: Kerlikowske K et al. JAMA Intern Med. 2019 Jul 1. doi:10.1001/jamainternmed.2019.1758 .
FROM JAMA INTERNAL MEDICINE
To help patients stay on diabetes regimens: Communicate, educate, and use technology
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
REPORTING FROM ADA 2019
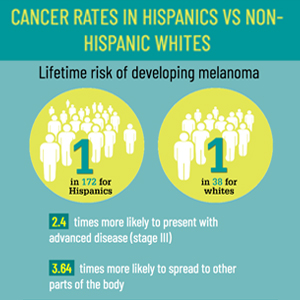
Infographic: Skin Cancer Stats in Hispanic Patients
Multigene panel sequencing is moderately cost-effective in NSCLC
Testing for targetable alterations in a panel of genes is moderately more cost-effective than testing for single genetic markers in patients with advanced non–small cell lung cancer (NSCLC), finds a retrospective cohort study.
“Targeted therapies are now a therapeutic cornerstone for patients with [advanced NSCLC], but the best diagnostic approach for identifying those who are eligible for treatment remains uncertain,” noted the investigators, led by Lotte Steuten, PhD, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Research Center, Seattle.
Using the Flatiron Health database, the investigators identified 5,688 patients with stage IIIB or IV NSCLC who were tested for targetable genetic alterations. Overall, 15.4% of the patients had multigene panel sequencing (MGPS) entailing evaluation of at least 30 genes, while the rest had single-marker genetic testing (SMGT) entailing evaluation of markers for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) or a smaller panel of variants.
Study results, reported in JCO Clinical Cancer Informatics, showed that 22% of the entire study cohort tested positive for EGFR mutations (18.5% with MGPS, 17.3% with SMGT) or ALK mutations (3.59% with MGPS, 3.78% with SMGT). In addition, 8% of those in the MGPS-tested group were found to have BRAF, RET, ROS1, HER2, or MET mutations.
The proportion of patients receiving treatments targeted to the specific mutations was 21% in the MGPS-tested group and 19% in the SMGT-tested group. The patients tested with MGPS had an expected survival that was 0.06 life-years longer (1.20 vs. 1.14) with lifetime total costs that were $8,814 higher per patient ($67,110 vs. $58,297).
Compared with SMGT, MGPS had an incremental cost-effectiveness ratio of $148,478 per life-year gained. This value can be characterized as moderate, given commonly cited threshold values in the United States that range from $50,000 to $200,000 per life-year gained, according to the investigators.
In sensitivity analyses, the expected incremental cost-effectiveness fell (improved) to $110,000 per life-year gained in a scenario in which all patients with an actionable mutation received a targeted treatment.
“Our cost-effectiveness analysis of MGPS versus SMGT provides evidence for health insurers who must consider both value and budget impact when weighing coverage policies for MGPS,” Dr. Steuten and colleagues wrote, noting that such evaluations are limited at present by reliance on retrospective data.
“To reduce decision uncertainty regarding insurance coverage of MGPS, our study highlights the need for prospective studies directly comparing the management of [advanced NSCLC] with MGPS versus SMGT that include both clinical and economic end points,” they concluded. “As this analysis represents a snapshot in time, the model developed should be updated as new clinical or cost information becomes available.”
Dr. Steuten disclosed having a consulting or advisory role with Agendia and Roche (immediate family member), and receiving research funding from Thermo Fisher Scientific, EMD Serono (institutional), Nohla Therapeutics (institutional), and the Personalized Medicine Coalition (institutional), which funded the study.
SOURCE: Steuten L et al. JCO Clin Cancer Inform. 2019 June 3. doi: 10.1200/CCI.19.00002.
Testing for targetable alterations in a panel of genes is moderately more cost-effective than testing for single genetic markers in patients with advanced non–small cell lung cancer (NSCLC), finds a retrospective cohort study.
“Targeted therapies are now a therapeutic cornerstone for patients with [advanced NSCLC], but the best diagnostic approach for identifying those who are eligible for treatment remains uncertain,” noted the investigators, led by Lotte Steuten, PhD, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Research Center, Seattle.
Using the Flatiron Health database, the investigators identified 5,688 patients with stage IIIB or IV NSCLC who were tested for targetable genetic alterations. Overall, 15.4% of the patients had multigene panel sequencing (MGPS) entailing evaluation of at least 30 genes, while the rest had single-marker genetic testing (SMGT) entailing evaluation of markers for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) or a smaller panel of variants.
Study results, reported in JCO Clinical Cancer Informatics, showed that 22% of the entire study cohort tested positive for EGFR mutations (18.5% with MGPS, 17.3% with SMGT) or ALK mutations (3.59% with MGPS, 3.78% with SMGT). In addition, 8% of those in the MGPS-tested group were found to have BRAF, RET, ROS1, HER2, or MET mutations.
The proportion of patients receiving treatments targeted to the specific mutations was 21% in the MGPS-tested group and 19% in the SMGT-tested group. The patients tested with MGPS had an expected survival that was 0.06 life-years longer (1.20 vs. 1.14) with lifetime total costs that were $8,814 higher per patient ($67,110 vs. $58,297).
Compared with SMGT, MGPS had an incremental cost-effectiveness ratio of $148,478 per life-year gained. This value can be characterized as moderate, given commonly cited threshold values in the United States that range from $50,000 to $200,000 per life-year gained, according to the investigators.
In sensitivity analyses, the expected incremental cost-effectiveness fell (improved) to $110,000 per life-year gained in a scenario in which all patients with an actionable mutation received a targeted treatment.
“Our cost-effectiveness analysis of MGPS versus SMGT provides evidence for health insurers who must consider both value and budget impact when weighing coverage policies for MGPS,” Dr. Steuten and colleagues wrote, noting that such evaluations are limited at present by reliance on retrospective data.
“To reduce decision uncertainty regarding insurance coverage of MGPS, our study highlights the need for prospective studies directly comparing the management of [advanced NSCLC] with MGPS versus SMGT that include both clinical and economic end points,” they concluded. “As this analysis represents a snapshot in time, the model developed should be updated as new clinical or cost information becomes available.”
Dr. Steuten disclosed having a consulting or advisory role with Agendia and Roche (immediate family member), and receiving research funding from Thermo Fisher Scientific, EMD Serono (institutional), Nohla Therapeutics (institutional), and the Personalized Medicine Coalition (institutional), which funded the study.
SOURCE: Steuten L et al. JCO Clin Cancer Inform. 2019 June 3. doi: 10.1200/CCI.19.00002.
Testing for targetable alterations in a panel of genes is moderately more cost-effective than testing for single genetic markers in patients with advanced non–small cell lung cancer (NSCLC), finds a retrospective cohort study.
“Targeted therapies are now a therapeutic cornerstone for patients with [advanced NSCLC], but the best diagnostic approach for identifying those who are eligible for treatment remains uncertain,” noted the investigators, led by Lotte Steuten, PhD, Hutchinson Institute for Cancer Outcomes Research, Fred Hutchinson Cancer Research Center, Seattle.
Using the Flatiron Health database, the investigators identified 5,688 patients with stage IIIB or IV NSCLC who were tested for targetable genetic alterations. Overall, 15.4% of the patients had multigene panel sequencing (MGPS) entailing evaluation of at least 30 genes, while the rest had single-marker genetic testing (SMGT) entailing evaluation of markers for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) or a smaller panel of variants.
Study results, reported in JCO Clinical Cancer Informatics, showed that 22% of the entire study cohort tested positive for EGFR mutations (18.5% with MGPS, 17.3% with SMGT) or ALK mutations (3.59% with MGPS, 3.78% with SMGT). In addition, 8% of those in the MGPS-tested group were found to have BRAF, RET, ROS1, HER2, or MET mutations.
The proportion of patients receiving treatments targeted to the specific mutations was 21% in the MGPS-tested group and 19% in the SMGT-tested group. The patients tested with MGPS had an expected survival that was 0.06 life-years longer (1.20 vs. 1.14) with lifetime total costs that were $8,814 higher per patient ($67,110 vs. $58,297).
Compared with SMGT, MGPS had an incremental cost-effectiveness ratio of $148,478 per life-year gained. This value can be characterized as moderate, given commonly cited threshold values in the United States that range from $50,000 to $200,000 per life-year gained, according to the investigators.
In sensitivity analyses, the expected incremental cost-effectiveness fell (improved) to $110,000 per life-year gained in a scenario in which all patients with an actionable mutation received a targeted treatment.
“Our cost-effectiveness analysis of MGPS versus SMGT provides evidence for health insurers who must consider both value and budget impact when weighing coverage policies for MGPS,” Dr. Steuten and colleagues wrote, noting that such evaluations are limited at present by reliance on retrospective data.
“To reduce decision uncertainty regarding insurance coverage of MGPS, our study highlights the need for prospective studies directly comparing the management of [advanced NSCLC] with MGPS versus SMGT that include both clinical and economic end points,” they concluded. “As this analysis represents a snapshot in time, the model developed should be updated as new clinical or cost information becomes available.”
Dr. Steuten disclosed having a consulting or advisory role with Agendia and Roche (immediate family member), and receiving research funding from Thermo Fisher Scientific, EMD Serono (institutional), Nohla Therapeutics (institutional), and the Personalized Medicine Coalition (institutional), which funded the study.
SOURCE: Steuten L et al. JCO Clin Cancer Inform. 2019 June 3. doi: 10.1200/CCI.19.00002.
FROM JCO CLINICAL CANCER INFORMATICS
Pediatric cholestatic liver disease: Successful transition of care
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
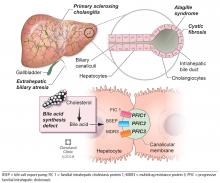
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
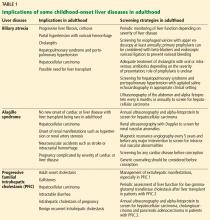
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
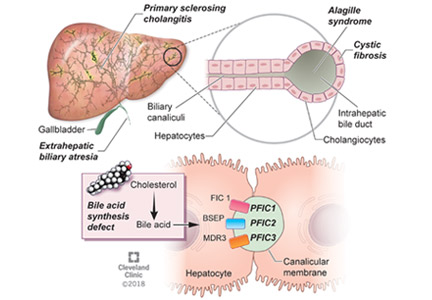
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
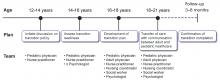
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
KEY POINTS
- The causes of cholestasis in children are different from those in adults, with genetic inherited causes more common in childhood.
- Cholestasis in children can be caused by biliary tract obstruction such as in biliary atresia or defects in forming and excreting bile acids and other components of bile.
- With the growing number of people with childhood-onset liver disease surviving into adulthood, it is important for internists to be aware of unique problems and challenges in continuing management of this population.
- In addition to medical comorbidities, these patients may also have impaired psychosocial functioning and quality of life.
Giant cell arteritis: An updated review of an old disease
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
KEY POINTS
- Giant cell arteritis can present with cranial symptoms, extracranial large-vessel involvement, or polymyalgia rheumatica.
- Temporal artery biopsy is the standard for diagnosis.
- Adverse effects of glucocorticoid treatment, particularly bone loss, need to be managed.
- In patients treated with glucocorticoids alone, the relapse rate is high when the drugs are tapered; thus, prolonged treatment is required.
Infertility: A practical framework
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
KEY POINTS
- A primary care physician can provide advice and testing regarding most fertility concerns.
- Female reproductive aging is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 and older.
- Male factor infertility can now often be overcome with assisted reproductive technologies.
- Polycystic ovary syndrome can cause anovulation and has metabolic effects that can evolve into metabolic syndrome, with serious health consequences.
Ambulatory ECG monitoring in the age of smartphones
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
KEY POINTS
- Ambulatory ECG monitoring is commonly used to correlate symptoms with arrhythmia, confirm occult atrial fibrillation, and assess the efficacy of antiarrhythmic therapy.
- Devices have features such as access to the full monitoring time (“full disclosure”), extended monitoring, and telemetry, each with advantages and limitations.
- Consumer-oriented wearable devices are aimed at arrhythmia monitoring, which could lead to increased arrhythmia detection, but at the risk of more false-positive results and excessive use of healthcare resources.
Type 2 diabetes: Evolving concepts and treatment
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
KEY POINTS
- At least 11 pathways lead to hyperglycemia; of these, beta-cell dysfunction is central.
- As different classes of diabetes drugs act on different pathways, we can target the pathways contributing to hyperglycemia in the individual patient, using fewer agents and lessening the risk of hypoglycemic episodes.
- In selecting treatment, we should favor drugs that are “gentle” on beta cells, do not cause dangerous hypoglycemia, and improve long-term outcomes as shown in randomized clinical trials.
Clinical trials: More to learn than the results
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
The clinical update of giant cell arteritis (GCA) by Rinden et al in this issue of the Journal reminded me of just how much of our management of this disease has, for decades, been based on retrospective studies (we owe a lot to clinicians from the Mayo Clinic for their compiled observations) tempered by our own recalled experiences, which we may at times twist a bit to fit prevailing paradigms. Several prospective interventional studies, perhaps most importantly the Giant-Cell Arteritis Actemra (GIACTA) trial,1 evaluated the ability of the interleukin 6 (IL-6) antagonist tocilizumab to supplant the protracted use of glucocorticoids in the treatment of GCA. But I learned much more from this trial, in the form of collected clinical tidbits, than just the bottom-line abstract conclusion that IL-6 antagonism is at least a promising approach in many patients with GCA.
As teachers, we tell students to read the entire published clinical trial report, not just the abstract and conclusions. Over the years, I have been impatient with those who violated this dictum, but I now often find myself among the ranks of those who would have been targets of my disapproval. Usually, the articles that I merely skim lie outside my subsubspecialty areas of interest, as time constraints make this abridged reading a necessity for survival, but that excuse does not diminish the self-recognition of my often less-than-complete understanding of the clinical condition being reported. Delving into the nuances of GIACTA truly emphasized that point.
The external validity of any trial rests on understanding the trial’s methods. In the case of GIACTA, there was much more to be learned and affirmed from the trial1 than that 1 year of tocilizumab treatment met the primary end point of increasing the percent of patients achieving sustained remission at week 52 after a rapid 26-week tapering off of prednisone compared with placebo.
One treatment group in the GIACTA trial underwent an aggressive 6-month tapering of prednisone, while another underwent a more protracted tapering over 12 months (more in line with common practice). Patients tapered over 6 months also received either the IL-6 antagonist or placebo for the full year. The concept was that if IL-6 blockade is a correct approach, then it will maintain remission in more patients, and significantly reduce the total amount of steroid needed to control the disease, despite rapid, aggressive steroid tapering. This turned out to be correct, although more than 20% of the drug-treated patients still experienced a flare of GCA (vs 68% of the placebo-treated group).
Somewhat surprising was that almost 20% of the entered patients did not achieve an initial remission despite receiving high-dose prednisone. The traditional teaching is that if a patient diagnosed with GCA does not respond to high-dose steroids, the diagnosis should be questioned.
Another interesting facet of the study relates to the diagnosis. We are becoming more aware of the different GCA phenotypes, which include prominent polymyalgia rheumatica or constitutional features, “classic” GCA with cranial symptoms, and dominant large-vessel vasculitis (aortitis and major aortic branch disease). In GIACTA, even though imaging was not mandated, 37% of participants were enrolled based in part on imaging results (CT, MRI, angiography, or PET-CT), not on the results of temporal artery biopsy. This forces us to think more broadly about diagnosing and staging GCA, and to wonder if we should even modify our approach to other clinical challenges, including unexplained fever and wasting in older patients.
Another tidbit that came out of the study relates to the relationship between the acute-phase response and clinical flares. We already knew that a rise in the erythrocyte sedimentation rate is a nonspecific sign and does not equate with a flare. In this trial one-third of patients in the placebo group who had a flare had a normal sedimentation rate or C-reactive protein during the flare, and about one-third of patients in the placebo group were receiving more than 10 mg of prednisone. In preliminary reports of follow-up after 52 weeks of treatment,2 patients who had achieved complete remission with the IL-6 antagonist and were off of prednisone were still not out of the woods; when the drug was discontinued, many flares continued to occur over the 2-year study extension. We have more to learn about what triggers and drives flares in this disease.
Thus, in addition to informing us of a successful “steroid-sparing” and rescue drug option for our patients with GCA, the details of this well-conducted trial both challenge and reaffirm some of our clinical impressions. Clearly, GCA must be defined for many patients as a very chronic disease, perhaps with occult vascular reservoirs, the biologic basis of which remains to be defined.
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Stone JH, Bao M, Han J, et al. Long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the GIACTA trial (abstract). Ann Rheum Dis 2019; 78:145–146. doi:10.1136/annrheumdis-2019-eular.2099