User login
Data sharing to third parties prevalent in depression, smoking cessation apps
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
FROM JAMA NETWORK OPEN
More cognitive rigidity found in patients with depression plus fibromyalgia
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
Increasing cognitive complexity cited as a possible therapeutic target
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
FROM THE INTERNATIONAL JOURNAL OF CLINICAL AND HEALTH PSYCHOLOGY
Hyperthyroid? She sure doesn’t look like it
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at imnews@mdedge.com.
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at imnews@mdedge.com.
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at imnews@mdedge.com.
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
I don’t have much use for evidence
.
Of course I am not against evidence. I respect and value evidence. I just have little day-to-day use for it. Maybe your practices are different from mine, but I spend my working days seeing cases ...
Where evidence is irrelevant:
- there is nothing wrong but the patient thinks there might be (an itch, a burning sensation, a mole that may or may not have changed).
- the condition is self-limiting and needs no therapy (viral rashes).
Where evidence could help, but there isn’t any:
- there is no useful available treatment (age-related hair thinning in women).
Where evidence may be statistically significant but clinically trivial:
- pills that make herpes simplex cold sores go away in 6 days instead of 7, acne cream that reduces lesions by 70% instead of 40% (so that after 12 weeks the patient has five pimples instead of eight).
Where evidence is unimpressive or unconvincing (to the patient, who often stops treatment because “it wasn’t working” or “it made me worse”):
- the condition is recurrent, and the patient interprets recurrence as failure (eczema, psoriasis).
- the condition (psoriasis, eczema, acne ) moves around, and the patient interprets success (fewer spots) as failure. (“It’s come in a new place it never came before” or “I never used to get pimples on my jawline.”)
- slam-dunk clearances are few, and instead, every possible permutation in the condition’s course happens – persistence, recurrence, extension, spontaneous involution, going away in one place while proliferating in another, etc. (warts, alopecia areata).
Where patients find other kinds of evidence more compelling than mine:
- “I stopped your cream because calendula/tea tree oil/Vicks VapoRub/apple cider vinegar/avoiding gluten works better” (eczema, rosacea, onychomycosis, etc.). This dynamic is not limited to dermatology. How many Crohn’s patients have I met who say they left their conventional physicians with their standard treatments and now see a naturopath or acupuncturist? Their doctors don’t know they left, or why, because it’s impossible to remember someone who isn’t there, and their patients never told them why they left. These patients tell me they are now “doing better.”
Where evidence is outweighed by other patient considerations:
- topical 5-fluorouracil or imiquimod for superficial basal cells or noninvasive squamous cells in a patient who can’t reach the spot, doesn’t want to be bothered, or just wants the damned thing cut off.
Then, of course, there are cases where evidence is crucial. It’s just that, at the moment, I can’t think of many. Besides which, I’ve lost count of all the patients who had exhaustive food or patch testing, been found sensitive to any number of chemicals and foods – exotic or otherwise – dutifully avoided all of same, yet still break out intermittently and inscrutably, just like all my other atopics.
I’ve concluded that it must be me, or at least the small slice of the planet I work in. Maybe if I practice for 70 years instead of 40, or on five continents instead of the corner of just one, I will figure it all out.
To be clear: I am not a therapeutic nihilist. I want to use verifiably effective treatments for my patients, just as I wish such to be used on me when I need treatment. It’s just that instances in which compelling, decisive evidence makes a crucial difference don’t come up all that often. Evidence applies to populations, whereas I treat people, one at a time.
Meantime, I will muddle along, concerned that one drawback of the emphasis on “evidence” (i.e. statistics) not often noted is its contribution to depersonalizing medical practice, reducing the therapeutic interaction between two people to iterated instances of quantifiable throughput. If you can’t measure it, you didn’t do it and it doesn’t exist. But it does exist. Like every clinician, I see and do it every day.
As Hippocrates supposedly said: Life is short, and art long, opportunity fleeting, experimentations perilous, and judgment difficult.
Evidence notwithstanding, judgment remains difficult, mine and the patient’s.
Now that I’m done with evidence, please stay tuned for future columns where I take on motherhood (unacceptably gendered!) and apple pie (fattening!)
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
.
Of course I am not against evidence. I respect and value evidence. I just have little day-to-day use for it. Maybe your practices are different from mine, but I spend my working days seeing cases ...
Where evidence is irrelevant:
- there is nothing wrong but the patient thinks there might be (an itch, a burning sensation, a mole that may or may not have changed).
- the condition is self-limiting and needs no therapy (viral rashes).
Where evidence could help, but there isn’t any:
- there is no useful available treatment (age-related hair thinning in women).
Where evidence may be statistically significant but clinically trivial:
- pills that make herpes simplex cold sores go away in 6 days instead of 7, acne cream that reduces lesions by 70% instead of 40% (so that after 12 weeks the patient has five pimples instead of eight).
Where evidence is unimpressive or unconvincing (to the patient, who often stops treatment because “it wasn’t working” or “it made me worse”):
- the condition is recurrent, and the patient interprets recurrence as failure (eczema, psoriasis).
- the condition (psoriasis, eczema, acne ) moves around, and the patient interprets success (fewer spots) as failure. (“It’s come in a new place it never came before” or “I never used to get pimples on my jawline.”)
- slam-dunk clearances are few, and instead, every possible permutation in the condition’s course happens – persistence, recurrence, extension, spontaneous involution, going away in one place while proliferating in another, etc. (warts, alopecia areata).
Where patients find other kinds of evidence more compelling than mine:
- “I stopped your cream because calendula/tea tree oil/Vicks VapoRub/apple cider vinegar/avoiding gluten works better” (eczema, rosacea, onychomycosis, etc.). This dynamic is not limited to dermatology. How many Crohn’s patients have I met who say they left their conventional physicians with their standard treatments and now see a naturopath or acupuncturist? Their doctors don’t know they left, or why, because it’s impossible to remember someone who isn’t there, and their patients never told them why they left. These patients tell me they are now “doing better.”
Where evidence is outweighed by other patient considerations:
- topical 5-fluorouracil or imiquimod for superficial basal cells or noninvasive squamous cells in a patient who can’t reach the spot, doesn’t want to be bothered, or just wants the damned thing cut off.
Then, of course, there are cases where evidence is crucial. It’s just that, at the moment, I can’t think of many. Besides which, I’ve lost count of all the patients who had exhaustive food or patch testing, been found sensitive to any number of chemicals and foods – exotic or otherwise – dutifully avoided all of same, yet still break out intermittently and inscrutably, just like all my other atopics.
I’ve concluded that it must be me, or at least the small slice of the planet I work in. Maybe if I practice for 70 years instead of 40, or on five continents instead of the corner of just one, I will figure it all out.
To be clear: I am not a therapeutic nihilist. I want to use verifiably effective treatments for my patients, just as I wish such to be used on me when I need treatment. It’s just that instances in which compelling, decisive evidence makes a crucial difference don’t come up all that often. Evidence applies to populations, whereas I treat people, one at a time.
Meantime, I will muddle along, concerned that one drawback of the emphasis on “evidence” (i.e. statistics) not often noted is its contribution to depersonalizing medical practice, reducing the therapeutic interaction between two people to iterated instances of quantifiable throughput. If you can’t measure it, you didn’t do it and it doesn’t exist. But it does exist. Like every clinician, I see and do it every day.
As Hippocrates supposedly said: Life is short, and art long, opportunity fleeting, experimentations perilous, and judgment difficult.
Evidence notwithstanding, judgment remains difficult, mine and the patient’s.
Now that I’m done with evidence, please stay tuned for future columns where I take on motherhood (unacceptably gendered!) and apple pie (fattening!)
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
.
Of course I am not against evidence. I respect and value evidence. I just have little day-to-day use for it. Maybe your practices are different from mine, but I spend my working days seeing cases ...
Where evidence is irrelevant:
- there is nothing wrong but the patient thinks there might be (an itch, a burning sensation, a mole that may or may not have changed).
- the condition is self-limiting and needs no therapy (viral rashes).
Where evidence could help, but there isn’t any:
- there is no useful available treatment (age-related hair thinning in women).
Where evidence may be statistically significant but clinically trivial:
- pills that make herpes simplex cold sores go away in 6 days instead of 7, acne cream that reduces lesions by 70% instead of 40% (so that after 12 weeks the patient has five pimples instead of eight).
Where evidence is unimpressive or unconvincing (to the patient, who often stops treatment because “it wasn’t working” or “it made me worse”):
- the condition is recurrent, and the patient interprets recurrence as failure (eczema, psoriasis).
- the condition (psoriasis, eczema, acne ) moves around, and the patient interprets success (fewer spots) as failure. (“It’s come in a new place it never came before” or “I never used to get pimples on my jawline.”)
- slam-dunk clearances are few, and instead, every possible permutation in the condition’s course happens – persistence, recurrence, extension, spontaneous involution, going away in one place while proliferating in another, etc. (warts, alopecia areata).
Where patients find other kinds of evidence more compelling than mine:
- “I stopped your cream because calendula/tea tree oil/Vicks VapoRub/apple cider vinegar/avoiding gluten works better” (eczema, rosacea, onychomycosis, etc.). This dynamic is not limited to dermatology. How many Crohn’s patients have I met who say they left their conventional physicians with their standard treatments and now see a naturopath or acupuncturist? Their doctors don’t know they left, or why, because it’s impossible to remember someone who isn’t there, and their patients never told them why they left. These patients tell me they are now “doing better.”
Where evidence is outweighed by other patient considerations:
- topical 5-fluorouracil or imiquimod for superficial basal cells or noninvasive squamous cells in a patient who can’t reach the spot, doesn’t want to be bothered, or just wants the damned thing cut off.
Then, of course, there are cases where evidence is crucial. It’s just that, at the moment, I can’t think of many. Besides which, I’ve lost count of all the patients who had exhaustive food or patch testing, been found sensitive to any number of chemicals and foods – exotic or otherwise – dutifully avoided all of same, yet still break out intermittently and inscrutably, just like all my other atopics.
I’ve concluded that it must be me, or at least the small slice of the planet I work in. Maybe if I practice for 70 years instead of 40, or on five continents instead of the corner of just one, I will figure it all out.
To be clear: I am not a therapeutic nihilist. I want to use verifiably effective treatments for my patients, just as I wish such to be used on me when I need treatment. It’s just that instances in which compelling, decisive evidence makes a crucial difference don’t come up all that often. Evidence applies to populations, whereas I treat people, one at a time.
Meantime, I will muddle along, concerned that one drawback of the emphasis on “evidence” (i.e. statistics) not often noted is its contribution to depersonalizing medical practice, reducing the therapeutic interaction between two people to iterated instances of quantifiable throughput. If you can’t measure it, you didn’t do it and it doesn’t exist. But it does exist. Like every clinician, I see and do it every day.
As Hippocrates supposedly said: Life is short, and art long, opportunity fleeting, experimentations perilous, and judgment difficult.
Evidence notwithstanding, judgment remains difficult, mine and the patient’s.
Now that I’m done with evidence, please stay tuned for future columns where I take on motherhood (unacceptably gendered!) and apple pie (fattening!)
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
New research in otitis media
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
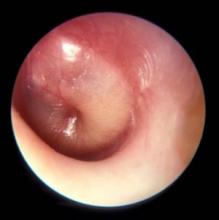
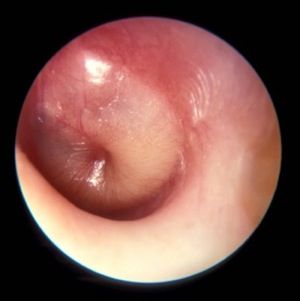
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
Quality standards aim to improve worldwide spondyloarthritis care
MADRID – Referral, treatment, and rapid access to care are three of nine new quality standards developed by a multidisciplinary task force of the Assessment of SpondyloArthritis international Society (ASAS) with the aim of improving the management of adults with axial spondyloarthritis (axSpA).
The other quality standards look at how to improve patient education and self-management and call for annual review, Uta Kiltz, MD, said at the European Congress of Rheumatology.
“Several unmet needs such as delayed diagnosis and restricted access to treatment have been described in patients with axSpA worldwide,” Dr. Kiltz observed in an interview. Results from the ASAS-COMOSPA study (Ann Rheum Dis. 2018;77[3]:405-11), for example, highlighted inequity in the prescription of biologic disease-modifying antirheumatic drugs across the globe.
“The variation in quality of care is noted across rheumatologic diseases,” said Dr. Kiltz, of Ruhr University Bochum and Rheumazentrum Ruhrgebiet in Herne, Germany. “Assessing the quality of care provided to patients with axSpA is important not only to patients and physicians, but also to providers and purchasers of health care.”
A major goal of ASAS is to improve quality of care and health outcomes in patients with axSpA. To address the many gaps in current care, the society set out to develop quality standards to optimize patients’ access to care and their overall treatment.
“A quality standard consists of a quality statement accompanied by a measure. The measure can be used to assess the quality of care or service provision specified in the treatment,” Dr. Kiltz explained.
Quality standards are very different from recommendations or guidelines, she stressed. While the latter imply evidence-based actions that should be done to optimally diagnose and treat the disease, quality standards identify resources or processes that need to be optimized in high-priority areas for quality improvement.
The nine ASAS quality standards cover key areas for quality improvement relating to the care of adults with axSpA that need improvement worldwide. The statements were carefully phrased following a consensus, and the tools by which they could be measured agreed.
The first three standards concern the time to referral from primary to specialist care and state that people with a suspicion of axSpA are referred to a rheumatologist within 3 working days, assessed by a rheumatologist within 3 weeks after referral, and have their diagnostic work up completed within 2 months.
The next two quality standards concern pharmacologic management: Disease activity of people with axSpA is monitored under the supervision of a rheumatologist with validated composite scores at least twice a year, and in people with axSpA and active disease despite conventional therapy, treatment escalation to biologics is discussed.
Nonpharmacologic treatment is also covered, with the sixth quality standard stating: “People with axial SpA are informed about the benefits of regular exercise.”
Quality standard 7 states: “People with axSpA are offered education on the disease including self-management within 2 months of diagnosis,” Dr. Kiltz said. Rapid access to care is the focus of quality statement 8: “People with axSpA and disease flare or possible drug-related side effects receive advice within 2 working days of contacting the rheumatologist.”
The ninth and last quality standard states that people with axSpA should have a comprehensive annual review by a rheumatologist.
“These are the first quality standards applicable worldwide for the improvement of health care for adult patients with axSpA,” Dr. Kiltz said. “The ASAS quality standards are all measurable and achievable and are intended to minimize variation in quality of care.”
Dr. Kiltz had no relevant conflicts of interest.
SOURCE: Kiltz U. Ann Rheum Dis. Jun 2019;78(Suppl 2):1-2. Abstract SP0004, doi: 10.1136/annrheumdis-2019-eular.8514.
MADRID – Referral, treatment, and rapid access to care are three of nine new quality standards developed by a multidisciplinary task force of the Assessment of SpondyloArthritis international Society (ASAS) with the aim of improving the management of adults with axial spondyloarthritis (axSpA).
The other quality standards look at how to improve patient education and self-management and call for annual review, Uta Kiltz, MD, said at the European Congress of Rheumatology.
“Several unmet needs such as delayed diagnosis and restricted access to treatment have been described in patients with axSpA worldwide,” Dr. Kiltz observed in an interview. Results from the ASAS-COMOSPA study (Ann Rheum Dis. 2018;77[3]:405-11), for example, highlighted inequity in the prescription of biologic disease-modifying antirheumatic drugs across the globe.
“The variation in quality of care is noted across rheumatologic diseases,” said Dr. Kiltz, of Ruhr University Bochum and Rheumazentrum Ruhrgebiet in Herne, Germany. “Assessing the quality of care provided to patients with axSpA is important not only to patients and physicians, but also to providers and purchasers of health care.”
A major goal of ASAS is to improve quality of care and health outcomes in patients with axSpA. To address the many gaps in current care, the society set out to develop quality standards to optimize patients’ access to care and their overall treatment.
“A quality standard consists of a quality statement accompanied by a measure. The measure can be used to assess the quality of care or service provision specified in the treatment,” Dr. Kiltz explained.
Quality standards are very different from recommendations or guidelines, she stressed. While the latter imply evidence-based actions that should be done to optimally diagnose and treat the disease, quality standards identify resources or processes that need to be optimized in high-priority areas for quality improvement.
The nine ASAS quality standards cover key areas for quality improvement relating to the care of adults with axSpA that need improvement worldwide. The statements were carefully phrased following a consensus, and the tools by which they could be measured agreed.
The first three standards concern the time to referral from primary to specialist care and state that people with a suspicion of axSpA are referred to a rheumatologist within 3 working days, assessed by a rheumatologist within 3 weeks after referral, and have their diagnostic work up completed within 2 months.
The next two quality standards concern pharmacologic management: Disease activity of people with axSpA is monitored under the supervision of a rheumatologist with validated composite scores at least twice a year, and in people with axSpA and active disease despite conventional therapy, treatment escalation to biologics is discussed.
Nonpharmacologic treatment is also covered, with the sixth quality standard stating: “People with axial SpA are informed about the benefits of regular exercise.”
Quality standard 7 states: “People with axSpA are offered education on the disease including self-management within 2 months of diagnosis,” Dr. Kiltz said. Rapid access to care is the focus of quality statement 8: “People with axSpA and disease flare or possible drug-related side effects receive advice within 2 working days of contacting the rheumatologist.”
The ninth and last quality standard states that people with axSpA should have a comprehensive annual review by a rheumatologist.
“These are the first quality standards applicable worldwide for the improvement of health care for adult patients with axSpA,” Dr. Kiltz said. “The ASAS quality standards are all measurable and achievable and are intended to minimize variation in quality of care.”
Dr. Kiltz had no relevant conflicts of interest.
SOURCE: Kiltz U. Ann Rheum Dis. Jun 2019;78(Suppl 2):1-2. Abstract SP0004, doi: 10.1136/annrheumdis-2019-eular.8514.
MADRID – Referral, treatment, and rapid access to care are three of nine new quality standards developed by a multidisciplinary task force of the Assessment of SpondyloArthritis international Society (ASAS) with the aim of improving the management of adults with axial spondyloarthritis (axSpA).
The other quality standards look at how to improve patient education and self-management and call for annual review, Uta Kiltz, MD, said at the European Congress of Rheumatology.
“Several unmet needs such as delayed diagnosis and restricted access to treatment have been described in patients with axSpA worldwide,” Dr. Kiltz observed in an interview. Results from the ASAS-COMOSPA study (Ann Rheum Dis. 2018;77[3]:405-11), for example, highlighted inequity in the prescription of biologic disease-modifying antirheumatic drugs across the globe.
“The variation in quality of care is noted across rheumatologic diseases,” said Dr. Kiltz, of Ruhr University Bochum and Rheumazentrum Ruhrgebiet in Herne, Germany. “Assessing the quality of care provided to patients with axSpA is important not only to patients and physicians, but also to providers and purchasers of health care.”
A major goal of ASAS is to improve quality of care and health outcomes in patients with axSpA. To address the many gaps in current care, the society set out to develop quality standards to optimize patients’ access to care and their overall treatment.
“A quality standard consists of a quality statement accompanied by a measure. The measure can be used to assess the quality of care or service provision specified in the treatment,” Dr. Kiltz explained.
Quality standards are very different from recommendations or guidelines, she stressed. While the latter imply evidence-based actions that should be done to optimally diagnose and treat the disease, quality standards identify resources or processes that need to be optimized in high-priority areas for quality improvement.
The nine ASAS quality standards cover key areas for quality improvement relating to the care of adults with axSpA that need improvement worldwide. The statements were carefully phrased following a consensus, and the tools by which they could be measured agreed.
The first three standards concern the time to referral from primary to specialist care and state that people with a suspicion of axSpA are referred to a rheumatologist within 3 working days, assessed by a rheumatologist within 3 weeks after referral, and have their diagnostic work up completed within 2 months.
The next two quality standards concern pharmacologic management: Disease activity of people with axSpA is monitored under the supervision of a rheumatologist with validated composite scores at least twice a year, and in people with axSpA and active disease despite conventional therapy, treatment escalation to biologics is discussed.
Nonpharmacologic treatment is also covered, with the sixth quality standard stating: “People with axial SpA are informed about the benefits of regular exercise.”
Quality standard 7 states: “People with axSpA are offered education on the disease including self-management within 2 months of diagnosis,” Dr. Kiltz said. Rapid access to care is the focus of quality statement 8: “People with axSpA and disease flare or possible drug-related side effects receive advice within 2 working days of contacting the rheumatologist.”
The ninth and last quality standard states that people with axSpA should have a comprehensive annual review by a rheumatologist.
“These are the first quality standards applicable worldwide for the improvement of health care for adult patients with axSpA,” Dr. Kiltz said. “The ASAS quality standards are all measurable and achievable and are intended to minimize variation in quality of care.”
Dr. Kiltz had no relevant conflicts of interest.
SOURCE: Kiltz U. Ann Rheum Dis. Jun 2019;78(Suppl 2):1-2. Abstract SP0004, doi: 10.1136/annrheumdis-2019-eular.8514.
REPORTING FROM EULAR 2019 CONGRESS
Mitral valve repair improves prognosis in heart failure patients with secondary MR
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Formal weight loss programs improve NAFLD
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues.
“Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine.“However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease.
The literature search returned 22 eligible studies involving 2,588 patients. The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues.
“Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine.“However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease.
The literature search returned 22 eligible studies involving 2,588 patients. The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues.
“Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine.“However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease.
The literature search returned 22 eligible studies involving 2,588 patients. The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide
FROM JAMA INTERNAL MEDICINE
Key clinical point: Major finding: Weight loss interventions were associated with significantly decreased alanine aminotransferase (-9.81 U/L; I2 = 97%).
Study details: A meta-analysis of randomized clinicals involving weight loss interventions for patients with nonalcoholic fatty liver disease (NAFLD).
Disclosures: The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
Source: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
Formal weight loss programs improve NAFLD
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Weight loss interventions were associated with significantly decreased alanine aminotransferase (-9.81 U/L; I2 = 97%).
Study details: A meta-analysis of randomized clinicals involving weight loss interventions for patients with nonalcoholic fatty liver disease (NAFLD).
Disclosures: The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
Source: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
Increased cancer death linked to radioactive iodine therapy
Radioactive iodine therapy for hyperthyroidism may be associated with an increased risk of death from cancer, according to a longitudinal cohort study published in the July 1 issue of JAMA Internal Medicine.
The study followed 18,805 individuals whose hyperthyroidism was treated with radioactive iodine in the United States and United Kingdom between 1946 and 1964.
Researchers found positive dose-response relationships between radioactive iodine therapy and most of the solid cancers that were evaluated. However these only attained statistical significance in the case of female breast cancer – where there was a 12% increase in the risk of death from breast cancer from a 100-mGy tissue- or organ-absorbed dose – or for all solid cancers combined, where a 100-mGy dose to the stomach was associated with a 6% increase in death from all solid cancers.
Based on this, the authors estimated that 8% of solid cancer deaths, including 14% of breast cancer deaths, could be attributed to the radiation. When combined with current US mortality rates, that translated to around 13 excess solid cancer deaths, including three deaths from breast cancer, for every 1000 patients receiving a 100 mGy absorbed dose to the stomach or breast at age 40 years.
However they noted that patients with Graves disease are now recommended to receive higher doses, and calculated that for 150-mGy, 200-mGy and 250-mGy dosages there would be 19-32 excess solid cancer deaths per 1000 patients treated at age 40 years.
“To our knowledge, this is the first study to characterize the dose-response relationship between RAI treatment and site-specific cancer mortality in patients with hyperthyroidism using reliable estimates of absorbed dose to exposed organs or tissues,” wrote Cari M. Kitahara, PhD, from the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, and co-authors.
Radioactive iodine therapy did not appear to be associated with an increased risk of death from leukemia, non-Hodgkin lymphoma or multiple myeloma.
The authors noted that this was unexpected given previous findings of an elevated risk of leukemia in patients with thyroid cancer who received higher levels of radiation. They suggested that the greater uncertainty in calculation of red bone marrow exposure compared to that of other organs and tissue, as well as the relatively small number of leukemia deaths, may have limited their ability to detect a dose-response relationship.
The study was funded by the National Cancer Institute. One author declared membership of a consortium supported by the pharmaceutical sector.
SOURCE: Kitahara C et al. JAMA Internal Medicine 2019, July 1. DOI:10.1001/jamainternmed.2019.0981.
Radioactive iodine therapy for hyperthyroidism may be associated with an increased risk of death from cancer, according to a longitudinal cohort study published in the July 1 issue of JAMA Internal Medicine.
The study followed 18,805 individuals whose hyperthyroidism was treated with radioactive iodine in the United States and United Kingdom between 1946 and 1964.
Researchers found positive dose-response relationships between radioactive iodine therapy and most of the solid cancers that were evaluated. However these only attained statistical significance in the case of female breast cancer – where there was a 12% increase in the risk of death from breast cancer from a 100-mGy tissue- or organ-absorbed dose – or for all solid cancers combined, where a 100-mGy dose to the stomach was associated with a 6% increase in death from all solid cancers.
Based on this, the authors estimated that 8% of solid cancer deaths, including 14% of breast cancer deaths, could be attributed to the radiation. When combined with current US mortality rates, that translated to around 13 excess solid cancer deaths, including three deaths from breast cancer, for every 1000 patients receiving a 100 mGy absorbed dose to the stomach or breast at age 40 years.
However they noted that patients with Graves disease are now recommended to receive higher doses, and calculated that for 150-mGy, 200-mGy and 250-mGy dosages there would be 19-32 excess solid cancer deaths per 1000 patients treated at age 40 years.
“To our knowledge, this is the first study to characterize the dose-response relationship between RAI treatment and site-specific cancer mortality in patients with hyperthyroidism using reliable estimates of absorbed dose to exposed organs or tissues,” wrote Cari M. Kitahara, PhD, from the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, and co-authors.
Radioactive iodine therapy did not appear to be associated with an increased risk of death from leukemia, non-Hodgkin lymphoma or multiple myeloma.
The authors noted that this was unexpected given previous findings of an elevated risk of leukemia in patients with thyroid cancer who received higher levels of radiation. They suggested that the greater uncertainty in calculation of red bone marrow exposure compared to that of other organs and tissue, as well as the relatively small number of leukemia deaths, may have limited their ability to detect a dose-response relationship.
The study was funded by the National Cancer Institute. One author declared membership of a consortium supported by the pharmaceutical sector.
SOURCE: Kitahara C et al. JAMA Internal Medicine 2019, July 1. DOI:10.1001/jamainternmed.2019.0981.
Radioactive iodine therapy for hyperthyroidism may be associated with an increased risk of death from cancer, according to a longitudinal cohort study published in the July 1 issue of JAMA Internal Medicine.
The study followed 18,805 individuals whose hyperthyroidism was treated with radioactive iodine in the United States and United Kingdom between 1946 and 1964.
Researchers found positive dose-response relationships between radioactive iodine therapy and most of the solid cancers that were evaluated. However these only attained statistical significance in the case of female breast cancer – where there was a 12% increase in the risk of death from breast cancer from a 100-mGy tissue- or organ-absorbed dose – or for all solid cancers combined, where a 100-mGy dose to the stomach was associated with a 6% increase in death from all solid cancers.
Based on this, the authors estimated that 8% of solid cancer deaths, including 14% of breast cancer deaths, could be attributed to the radiation. When combined with current US mortality rates, that translated to around 13 excess solid cancer deaths, including three deaths from breast cancer, for every 1000 patients receiving a 100 mGy absorbed dose to the stomach or breast at age 40 years.
However they noted that patients with Graves disease are now recommended to receive higher doses, and calculated that for 150-mGy, 200-mGy and 250-mGy dosages there would be 19-32 excess solid cancer deaths per 1000 patients treated at age 40 years.
“To our knowledge, this is the first study to characterize the dose-response relationship between RAI treatment and site-specific cancer mortality in patients with hyperthyroidism using reliable estimates of absorbed dose to exposed organs or tissues,” wrote Cari M. Kitahara, PhD, from the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, and co-authors.
Radioactive iodine therapy did not appear to be associated with an increased risk of death from leukemia, non-Hodgkin lymphoma or multiple myeloma.
The authors noted that this was unexpected given previous findings of an elevated risk of leukemia in patients with thyroid cancer who received higher levels of radiation. They suggested that the greater uncertainty in calculation of red bone marrow exposure compared to that of other organs and tissue, as well as the relatively small number of leukemia deaths, may have limited their ability to detect a dose-response relationship.
The study was funded by the National Cancer Institute. One author declared membership of a consortium supported by the pharmaceutical sector.
SOURCE: Kitahara C et al. JAMA Internal Medicine 2019, July 1. DOI:10.1001/jamainternmed.2019.0981.
FROM JAMA INTERNAL MEDICINE