User login
Treatment for pediatric low-grade glioma is associated with poor cognitive and socioeconomic outcomes
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
Children who underwent surgery alone had better neuropsychologic and socioeconomic outcomes than those who also underwent radiotherapy, but their outcomes were worse than those of unaffected siblings. These findings were published online June 24 in Cancer.
“Late effects in adulthood are evident even for children with the least malignant types of brain tumors who were treated with the least toxic therapies available at the time,” said M. Douglas Ris, PhD, professor of pediatrics and psychology at Baylor College of Medicine in Houston, in a press release. “As pediatric brain tumors become more survivable with continued advances in treatments, we need to improve surveillance of these populations so that survivors continue to receive the best interventions during their transition to adulthood and well beyond.”
Clinicians generally have assumed that children with low-grade CNS tumors who receive less toxic treatment will have fewer long-term effects than survivors of more malignant tumors who undergo neurotoxic therapies. Yet research has indicated that the former patients can have lasting neurobehavioral or functional morbidity.
Dr. Ris and colleagues invited survivors of pediatric low-grade gliomas participating in the Childhood Cancer Survivor Study (CCSS) and a sibling comparison group to undergo a direct, comprehensive neurocognitive assessment. Of 495 eligible survivors, 257 participated. Seventy-six patients did not travel to a study site, but completed a questionnaire, and the researchers did not include data for this group in their analysis. Dr. Ris and colleagues obtained information about surgery and radiotherapy from participants’ medical records. Patients underwent standardized, age-normed neuropsychologic tests. The primary neuropsychologic outcomes were the Composite Neuropsychological Index (CNI) and estimated IQ. To evaluate socioeconomic outcomes, Dr. Ris and colleagues measured participants’ educational attainment, income, and occupational prestige.
After the researchers adjusted the data for age and sex, they found that siblings had higher mean scores than survivors treated with surgery plus radiotherapy or surgery alone on all neuropsychologic outcomes, including the CNI (siblings, 106.8; surgery only, 95.6; surgery plus radiotherapy, 88.3) and estimated IQ. Survivors who had been diagnosed at younger ages had low scores for all outcomes except for attention/processing speed.
Furthermore, surgery plus radiotherapy was associated with a 7.7-fold higher risk of having an occupation in the lowest sibling quartile, compared with siblings. Survivors who underwent surgery alone had a 2.8-fold higher risk than siblings of having an occupation in the lowest quartile. Surgery plus radiotherapy was associated with a 2.6-fold increased risk of a low occupation score, compared with survivors who underwent surgery alone.
Compared with siblings, surgery plus radiotherapy was associated with a 4.5-fold risk of an annual income of less than $20,000, while the risk for survivors who underwent surgery alone did not differ significantly from that for siblings. Surgery plus radiotherapy was associated with a 2.6-fold higher risk than surgery alone. Surgery plus radiotherapy was also associated with a significantly increased risk for an education level lower than a bachelor’s degree, compared with siblings, but surgery alone was not.
The National Cancer Institute supported the study. The authors had no disclosures.
SOURCE: Ris MD et al. Cancer. 2019 Jun 24. doi: 10.1002/cncr.32186.
FROM CANCER
Long-term trend: Women receiving fewer pelvic exams
according to the National Center for Health Statistics.
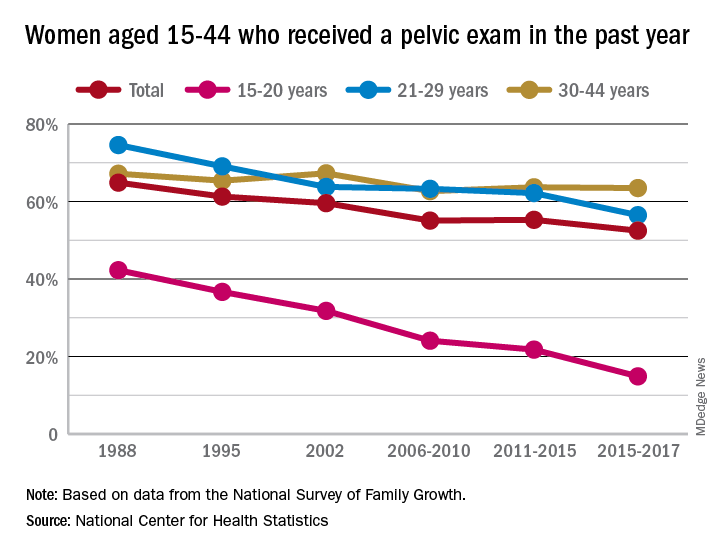
Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
according to the National Center for Health Statistics.

Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
according to the National Center for Health Statistics.

Sixty-five percent of women aged 15-44 years had received a pelvic examination in the past year when asked in 1988 as part of the National Survey of Family Growth, but the 3-year average for the 2015-2017 surveys was 53%, a significant decline, the NCHS said in a recent report.
The decrease was seen in all three of the age subgroups – 15-20 years, 21-29 years, and 30-44 years – over the length of the study period, with the trend in only the oldest women not reaching significance. The 30-44 group also was the only one of the three in which the rate ever increased at any point, the survey data show.
Data for other subgroups focused on the last 3-year period. From 2015 to 2017, non-Hispanic black women were more likely to have received a pelvic examination in the past year (60%) than were non-Hispanic white (54%) or Hispanic women (45%). An association with education level also was seen: Women with a bachelor’s degree or higher were most likely to get an exam (69%), and those with less than a high-school degree were least likely (52%), the researchers reported.
In 2018, the American College of Obstetricians and Gynecologists altered its recommendation that annual pelvic examinations be part of the well-woman visit for those aged 21 years and over, advising instead “that pelvic examinations be performed when indicated by medical history or symptoms,” the NCHS authors explained. They also suggested that their data “could provide a benchmark for estimates of the prevalence of pelvic examinations before the 2018 ACOG-updated guidelines.”
Siblings of bipolar disorder patients at higher cardiometabolic disease risk
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Age does not influence cladribine’s efficacy in MS
SEATTLE – In addition, age does not affect the likelihood that a patient who receives cladribine will achieve no evidence of disease activity (NEDA), according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In the phase 3 CLARITY study, a cumulative dose of 3.5 mg/kg of cladribine over 2 years was associated with significantly reduced relapse rate and disability progression and improved MRI outcomes, compared with placebo. The drug’s efficacy persisted in patients who were switched to placebo in a 96-week extension study.
A post hoc analysis
A 2017 study by Weideman et al. suggested that disease-modifying treatment (DMT) is less effective in older patients. For this reason, Gavin Giovannoni, MBBCh, PhD, professor of neurology at Queen Mary University of London, and colleagues decided to investigate the effect of age on the efficacy of treatment with 3.5 mg/kg of cladribine. The investigators performed a post hoc analysis of the CLARITY and CLARITY extension studies of patients with relapsing-remitting MS. They categorized patients as older than 45 years or age 45 years or younger.
Patients enrolled in CLARITY were between ages 18 years and 65 years. They underwent MRI at pretrial assessment and at weeks 24, 48, and 96 or early termination. The investigators defined a qualifying relapse as one associated with changes in Kurtzke Functional Systems score and other specified clinical parameters. Qualifying relapses were confirmed by an independent evaluating physician who was blinded to treatment assignment.
In the CLARITY extension study, 98 participants who had received cladribine tablets 3.5 mg/kg in CLARITY were randomized to placebo for 2 additional years. Participants who continued on placebo in the CLARITY extension were evaluated according to age at entry into CLARITY.
Dr. Giovannoni and colleagues performed efficacy analyses for qualifying relapses; all relapses; and mean and cumulative numbers of new T1 gadolinium-enhancing lesions, active T2 lesions, and combined unique lesions. They defined NEDA as freedom from qualifying relapses, 6-month confirmed disability progression (as measured by the Expanded Disability Status Scale [EDSS] score), T1 gadolinium-enhancing lesions, and active T2 lesions. The investigators performed equivalent analyses for patients who received placebo in the CLARITY extension.
Age did not influence efficacy
Within each age group, participants in both treatment arms had similar baseline demographic and disease characteristics. In CLARITY, 221 patients were older than 45 years, and 649 were age 45 years or younger. In the CLARITY extension, 22 patients were older than 45 years, and 76 were age 45 years or younger. In CLARITY, but not the extension, the proportion of women was higher in the older group than in the younger group (77.7% vs. 66.1%). In CLARITY, patients aged 45 years or younger had a higher number of T1 gadolinium-enhancing lesions at baseline, compared with older patients.
At week 96 in CLARITY, the annual rate of qualifying relapses among patients older than 45 years was 0.14 for cladribine and 0.28 for placebo. Among patients aged 45 or younger, the annual rate of qualifying relapses was 0.15 for cladribine and 0.37 for placebo. For patients older than 45 years, the annual rate of all relapses was 0.28 for cladribine and 0.55 for placebo. For patients aged 45 years or younger, the annual rate of all relapses was 0.26 for cladribine and 0.65 for placebo. The treatment effect of cladribine, compared with placebo, on qualifying relapses and all relapses was similar for both age groups. In the CLARITY extension, the annualized relapse rate (ARR) was 0.17 in patients aged 45 years or younger and 0.05 in patients older than 45 years.
The mean number of new T1 gadolinium-enhancing lesions and cumulative new T1 gadolinium-enhancing lesions was reduced with cladribine, compared with placebo, in both age groups at week 96 in CLARITY. The mean number of active T2 lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.167 in patients older than 45 years and 0.667 in patients aged 45 years and younger. In addition, the mean number of combined unique lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.333 in patients older than 45 years and 0.667 in patients aged 45 years or younger.
The proportion of participants who achieved NEDA in CLARITY was 55.6% among patients older than 45 years who received cladribine, 39.6% among patients aged 45 years or younger who received cladribine, 28.2% among patients older than 45 years who received placebo, and 9.5% of patients aged 45 years or younger who received placebo. The odds ratio for achieving NEDA was significantly more favorable for the cladribine group, compared with the placebo group, in both age groups (3.19 for patients older than 45 years and 6.23 for patients aged 45 years or younger). In the CLARITY extension, the proportion of participants who achieved NEDA was 40.9% among patients older than 45 years and 28.2% among patients aged 45 years or younger.
“These data are consistent with previous analyses from CLARITY using a different age cutoff and with results from the overall study population,” said Dr. Giovannoni and colleagues.
Merck KGaA, which manufactures and markets cladribine, supported the study. Dr. Giovannoni and several of his coinvestigators have received speaker honoraria, consulting fees, or other funding from companies including Merck KGaA.
SEATTLE – In addition, age does not affect the likelihood that a patient who receives cladribine will achieve no evidence of disease activity (NEDA), according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In the phase 3 CLARITY study, a cumulative dose of 3.5 mg/kg of cladribine over 2 years was associated with significantly reduced relapse rate and disability progression and improved MRI outcomes, compared with placebo. The drug’s efficacy persisted in patients who were switched to placebo in a 96-week extension study.
A post hoc analysis
A 2017 study by Weideman et al. suggested that disease-modifying treatment (DMT) is less effective in older patients. For this reason, Gavin Giovannoni, MBBCh, PhD, professor of neurology at Queen Mary University of London, and colleagues decided to investigate the effect of age on the efficacy of treatment with 3.5 mg/kg of cladribine. The investigators performed a post hoc analysis of the CLARITY and CLARITY extension studies of patients with relapsing-remitting MS. They categorized patients as older than 45 years or age 45 years or younger.
Patients enrolled in CLARITY were between ages 18 years and 65 years. They underwent MRI at pretrial assessment and at weeks 24, 48, and 96 or early termination. The investigators defined a qualifying relapse as one associated with changes in Kurtzke Functional Systems score and other specified clinical parameters. Qualifying relapses were confirmed by an independent evaluating physician who was blinded to treatment assignment.
In the CLARITY extension study, 98 participants who had received cladribine tablets 3.5 mg/kg in CLARITY were randomized to placebo for 2 additional years. Participants who continued on placebo in the CLARITY extension were evaluated according to age at entry into CLARITY.
Dr. Giovannoni and colleagues performed efficacy analyses for qualifying relapses; all relapses; and mean and cumulative numbers of new T1 gadolinium-enhancing lesions, active T2 lesions, and combined unique lesions. They defined NEDA as freedom from qualifying relapses, 6-month confirmed disability progression (as measured by the Expanded Disability Status Scale [EDSS] score), T1 gadolinium-enhancing lesions, and active T2 lesions. The investigators performed equivalent analyses for patients who received placebo in the CLARITY extension.
Age did not influence efficacy
Within each age group, participants in both treatment arms had similar baseline demographic and disease characteristics. In CLARITY, 221 patients were older than 45 years, and 649 were age 45 years or younger. In the CLARITY extension, 22 patients were older than 45 years, and 76 were age 45 years or younger. In CLARITY, but not the extension, the proportion of women was higher in the older group than in the younger group (77.7% vs. 66.1%). In CLARITY, patients aged 45 years or younger had a higher number of T1 gadolinium-enhancing lesions at baseline, compared with older patients.
At week 96 in CLARITY, the annual rate of qualifying relapses among patients older than 45 years was 0.14 for cladribine and 0.28 for placebo. Among patients aged 45 or younger, the annual rate of qualifying relapses was 0.15 for cladribine and 0.37 for placebo. For patients older than 45 years, the annual rate of all relapses was 0.28 for cladribine and 0.55 for placebo. For patients aged 45 years or younger, the annual rate of all relapses was 0.26 for cladribine and 0.65 for placebo. The treatment effect of cladribine, compared with placebo, on qualifying relapses and all relapses was similar for both age groups. In the CLARITY extension, the annualized relapse rate (ARR) was 0.17 in patients aged 45 years or younger and 0.05 in patients older than 45 years.
The mean number of new T1 gadolinium-enhancing lesions and cumulative new T1 gadolinium-enhancing lesions was reduced with cladribine, compared with placebo, in both age groups at week 96 in CLARITY. The mean number of active T2 lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.167 in patients older than 45 years and 0.667 in patients aged 45 years and younger. In addition, the mean number of combined unique lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.333 in patients older than 45 years and 0.667 in patients aged 45 years or younger.
The proportion of participants who achieved NEDA in CLARITY was 55.6% among patients older than 45 years who received cladribine, 39.6% among patients aged 45 years or younger who received cladribine, 28.2% among patients older than 45 years who received placebo, and 9.5% of patients aged 45 years or younger who received placebo. The odds ratio for achieving NEDA was significantly more favorable for the cladribine group, compared with the placebo group, in both age groups (3.19 for patients older than 45 years and 6.23 for patients aged 45 years or younger). In the CLARITY extension, the proportion of participants who achieved NEDA was 40.9% among patients older than 45 years and 28.2% among patients aged 45 years or younger.
“These data are consistent with previous analyses from CLARITY using a different age cutoff and with results from the overall study population,” said Dr. Giovannoni and colleagues.
Merck KGaA, which manufactures and markets cladribine, supported the study. Dr. Giovannoni and several of his coinvestigators have received speaker honoraria, consulting fees, or other funding from companies including Merck KGaA.
SEATTLE – In addition, age does not affect the likelihood that a patient who receives cladribine will achieve no evidence of disease activity (NEDA), according to a study presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In the phase 3 CLARITY study, a cumulative dose of 3.5 mg/kg of cladribine over 2 years was associated with significantly reduced relapse rate and disability progression and improved MRI outcomes, compared with placebo. The drug’s efficacy persisted in patients who were switched to placebo in a 96-week extension study.
A post hoc analysis
A 2017 study by Weideman et al. suggested that disease-modifying treatment (DMT) is less effective in older patients. For this reason, Gavin Giovannoni, MBBCh, PhD, professor of neurology at Queen Mary University of London, and colleagues decided to investigate the effect of age on the efficacy of treatment with 3.5 mg/kg of cladribine. The investigators performed a post hoc analysis of the CLARITY and CLARITY extension studies of patients with relapsing-remitting MS. They categorized patients as older than 45 years or age 45 years or younger.
Patients enrolled in CLARITY were between ages 18 years and 65 years. They underwent MRI at pretrial assessment and at weeks 24, 48, and 96 or early termination. The investigators defined a qualifying relapse as one associated with changes in Kurtzke Functional Systems score and other specified clinical parameters. Qualifying relapses were confirmed by an independent evaluating physician who was blinded to treatment assignment.
In the CLARITY extension study, 98 participants who had received cladribine tablets 3.5 mg/kg in CLARITY were randomized to placebo for 2 additional years. Participants who continued on placebo in the CLARITY extension were evaluated according to age at entry into CLARITY.
Dr. Giovannoni and colleagues performed efficacy analyses for qualifying relapses; all relapses; and mean and cumulative numbers of new T1 gadolinium-enhancing lesions, active T2 lesions, and combined unique lesions. They defined NEDA as freedom from qualifying relapses, 6-month confirmed disability progression (as measured by the Expanded Disability Status Scale [EDSS] score), T1 gadolinium-enhancing lesions, and active T2 lesions. The investigators performed equivalent analyses for patients who received placebo in the CLARITY extension.
Age did not influence efficacy
Within each age group, participants in both treatment arms had similar baseline demographic and disease characteristics. In CLARITY, 221 patients were older than 45 years, and 649 were age 45 years or younger. In the CLARITY extension, 22 patients were older than 45 years, and 76 were age 45 years or younger. In CLARITY, but not the extension, the proportion of women was higher in the older group than in the younger group (77.7% vs. 66.1%). In CLARITY, patients aged 45 years or younger had a higher number of T1 gadolinium-enhancing lesions at baseline, compared with older patients.
At week 96 in CLARITY, the annual rate of qualifying relapses among patients older than 45 years was 0.14 for cladribine and 0.28 for placebo. Among patients aged 45 or younger, the annual rate of qualifying relapses was 0.15 for cladribine and 0.37 for placebo. For patients older than 45 years, the annual rate of all relapses was 0.28 for cladribine and 0.55 for placebo. For patients aged 45 years or younger, the annual rate of all relapses was 0.26 for cladribine and 0.65 for placebo. The treatment effect of cladribine, compared with placebo, on qualifying relapses and all relapses was similar for both age groups. In the CLARITY extension, the annualized relapse rate (ARR) was 0.17 in patients aged 45 years or younger and 0.05 in patients older than 45 years.
The mean number of new T1 gadolinium-enhancing lesions and cumulative new T1 gadolinium-enhancing lesions was reduced with cladribine, compared with placebo, in both age groups at week 96 in CLARITY. The mean number of active T2 lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.167 in patients older than 45 years and 0.667 in patients aged 45 years and younger. In addition, the mean number of combined unique lesions per patient per scan also was significantly reduced with cladribine, compared with placebo, in both age groups. The reduction was 0.333 in patients older than 45 years and 0.667 in patients aged 45 years or younger.
The proportion of participants who achieved NEDA in CLARITY was 55.6% among patients older than 45 years who received cladribine, 39.6% among patients aged 45 years or younger who received cladribine, 28.2% among patients older than 45 years who received placebo, and 9.5% of patients aged 45 years or younger who received placebo. The odds ratio for achieving NEDA was significantly more favorable for the cladribine group, compared with the placebo group, in both age groups (3.19 for patients older than 45 years and 6.23 for patients aged 45 years or younger). In the CLARITY extension, the proportion of participants who achieved NEDA was 40.9% among patients older than 45 years and 28.2% among patients aged 45 years or younger.
“These data are consistent with previous analyses from CLARITY using a different age cutoff and with results from the overall study population,” said Dr. Giovannoni and colleagues.
Merck KGaA, which manufactures and markets cladribine, supported the study. Dr. Giovannoni and several of his coinvestigators have received speaker honoraria, consulting fees, or other funding from companies including Merck KGaA.
REPORTING FROM CMSC 2019
Daily aspirin use may not improve CV outcomes in healthy elderly
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Severe Acne Fulminans Following Low-Dose Isotretinoin and Testosterone Use
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
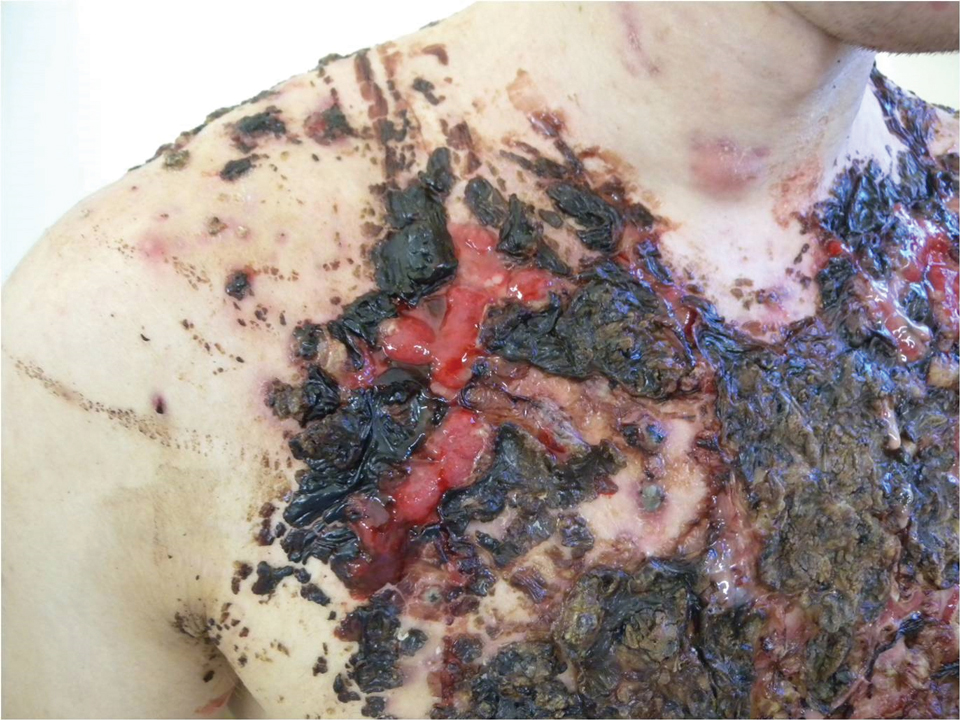
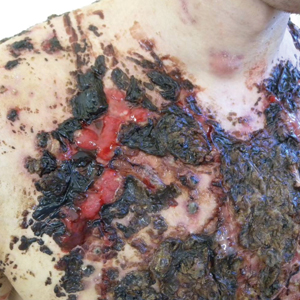
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.
At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
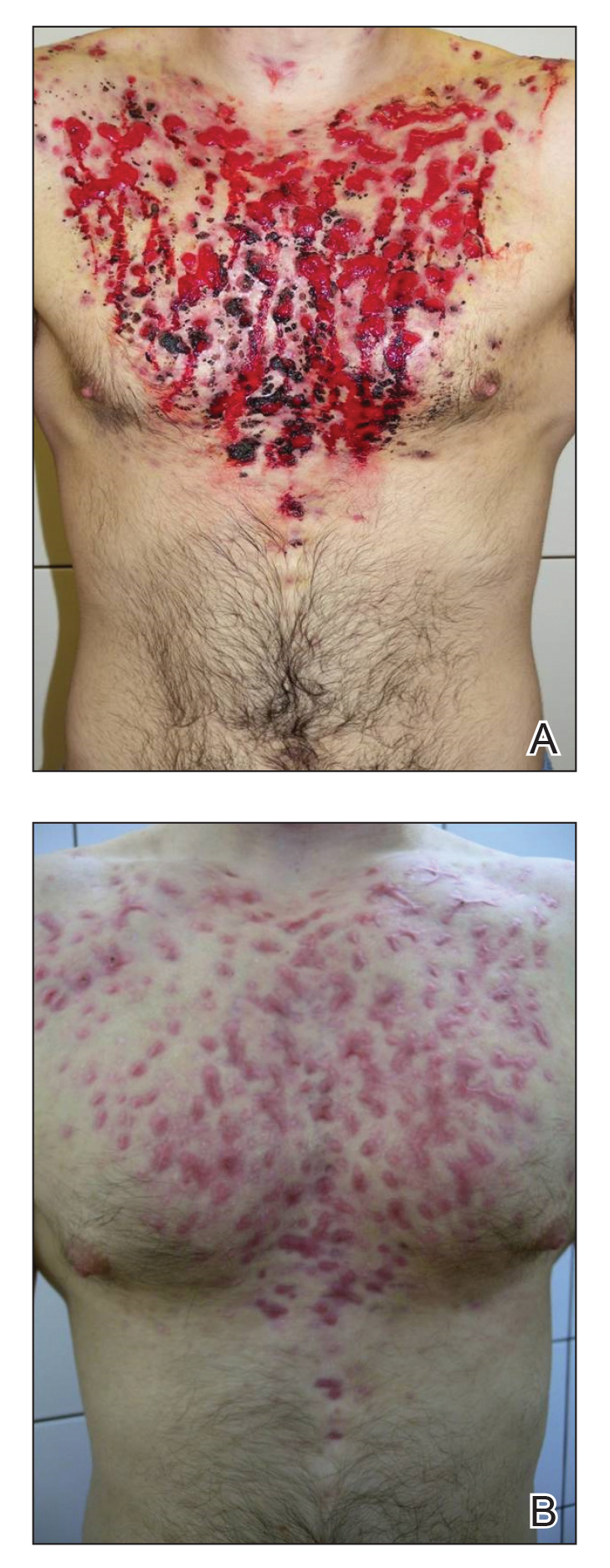
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.
It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.
At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.
It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
To the Editor:
Acne fulminans (AF), the most severe form of acne, is a rare condition with an incidence of less than 1% of total acne cases.1 Adolescent boys are the most susceptible group of patients.2 Painful inflammatory pustules that transform into deep ulcerations covered by abundant hemorrhagic crust are typical of AF. Commonly affected areas include the face, back, neck, and chest. Additionally, fever and polyarthralgia may be present, and there often is myopathy due to rapid weight loss.3,4 Less often, erythema nodosum and splenomegaly may be observed.5 Laboratory testing also may reveal markers of systemic inflammation such as leukocytosis with neutrophilia, elevated C-reactive protein levels, increased erythrocyte sedimentation rate, and thrombocytosis. Anemia and elevated hepatic enzyme levels also may be present in AF.2 It is suspected that AF may be induced by low doses of isotretinoin therapy with concomitant inherited susceptibility.6
We report the case of a 21-year-old man who was referred to the Department of Dermatology by his primary care physician for evaluation of severe hemorrhagic lesions on the trunk following use of oral isotretinoin (Figure 1). Prior to development of the lesions, the patient had started weekly intramuscular injections of testosterone 500 mg, which he purchased online without consulting a physician, to address muscle mass reduction associated with sudden weight loss from intense physical training. After 8 months of testosterone supplementation along with continued physical training, the patient presented to his primary care physician for treatment of acne vulgaris on the back and trunk of 2 months’ duration. Oral isotretinoin 20 mg once daily was initiated; however, the patient reported that the acne lesions showed progression after 1 month of treatment. Isotretinoin was increased to a more weight-appropriate dosage of 60 mg once daily 2 weeks before admission to our dermatology clinic.
At the current presentation, dermatologic examination revealed numerous inflamed ulcerations covered by a hemorrhagic crust on the back and trunk. The patient also reported knee, elbow, and inguinal pain, especially at night. No fever or loss of appetite was reported. The patient was otherwise healthy and had no remarkable family history of acne or other dermatologic diseases.
Laboratory testing showed leukocytosis (11,000/µL [reference range, 4500–11,000/µL]), an elevated C-reactive protein level (66 mg/L [reference range, 0.08–3.1 mg/L]), and an elevated erythrocyte sedimentation rate (46 mm/h [reference range, 0–20 mm/h]). There were laboratory and clinical signs of a secondary bacterial infection in the affected areas, and a culture of secretions collected from lesions on the back grew Staphylococcus aureus with sensitivity to erythromycin, clindamycin, doxycycline, and trimethoprim-sulfamethoxazole and resistance to penicillin. A diagnosis of AF was made based on the clinical presentation and systemic symptoms, and anabolic-androgenic steroids and low-dose isotretinoin were identified as etiologic factors.
Treatment initially included cessation of isotretinoin and administration of prednisone, omeprazole, clindamycin, and doxycycline. Prednisone was given at a dosage of 40 mg once daily for 1 week, then decreased by 5 mg every 7 days. Omeprazole was given concurrently as prophylaxis for the gastrointestinal tract side effects of long-term prednisone use. Clindamycin was given at a dosage of 300 mg 3 times daily. Doxycycline was given for 6 weeks at a dosage of 100 mg twice daily. Topical octenidine dihydrochloride also was given.
Marked improvement was noted after 24 hours (Figure 2) as well as on the third day of treatment (Figure 3A). After 6 weeks, only disfiguring scars were visible (Figure 3B). Oral isotretinoin was reincorporated after 8 weeks and was subsequently discontinued after 5 months of therapy with a cumulative dose of 150 mg/kg.
It is important to differentiate AF from exacerbation of acne vulgaris because patients typically have mild or moderate acne vulgaris before the onset of acute symptoms.1 Acne fulminans is characterized by systemic symptoms such as myalgia, polyarthralgia, fatigue, and osteolytic bone lesions.1,7 Additionally, hematologic symptoms such as fever, leukocytosis, anemia, splenomegaly, and hepatomegaly may be present.1,5,7 Our patient demonstrated the polysymptomatic form of AF. The patient had severe acne with a tendency to scar. There also were some systemic manifestations such as polyarthralgia, weight loss, leukocytosis, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level.
The clinical diagnosis in our patient also was supported by the hypothesis that heredity, overactive immune reactions, bacterial infections, and use of some drugs (eg, isotretinoin, tetracycline, testosterone) can trigger AF.8 The most well-known theory is that low doses of isotretinoin induce AF.6 The majority of cases are caused by doses of less than 20 mg/kg once daily, but there have been reports of patients using full doses and developing this condition.9 The fact that the use of low- and high-dose isotretinoin can provoke AF suggests an idiosyncratic reaction that is not clearly dose related. The most dangerous triggering factor of AF is concomitant usage of testosterone and isotretinoin.10 Our patient used testosterone injections to increase muscle mass and underwent treatment with isotretinoin for acne.
Treatment of AF is controversial, as there is no standard therapy. Currently, steroids and isotretinoin are the treatments of choice. Antibiotic use is controversial because of a lack of randomized trials.11
In the first stage of treatment, prednisone 0.5 to 1 mg/kg once daily is recommended as an initial anti-inflammatory therapy, with gradual dose reduction. According to evidence-based recommendations, a low dose of isotretinoin can be introduced after crusted lesions have healed. The overlapping therapy with steroids and isotretinoin should be provided for at least 4 weeks. High-potency topical corticosteroids can be used on granulation tissue, which can shorten the systemic treatment with prednisone or can be an alternative treatment for patients with contraindications to systemic corticosteroids.11
Additionally, local care of the lesions including compresses and topical emollients is crucial. There are some case reports in which there is introduction of high doses of isotretinoin, subsequently with systemic steroids.7,8,12 Seukeran and Cunliffe5 proved that it is beneficial to give acne prophylaxis to prevent further episodes. Our patient was similarly treated with systemic steroids and isotretinoin. Treatment guidelines for AF do not recommend oral antibiotics,11 but data are limited in the case of isotretinoin-induced AF. Our patient was given doxycycline concomitant with systemic steroids, which was necessary due to signs of secondary infection from a lesion culture. Doxycycline was stopped when isotretinoin treatment was initiated to prevent pseudotumor cerebri. The patient achieved good clinical improvement with no relapse.
Using isotretinoin to treat acne vulgaris has many benefits, despite the possibility of developing AF as an extremely rare complication. Clinicians should be aware of the risk of this complication to make the diagnosis and provide appropriate care, especially in young men. It is particularly important to consider the possibility of concomitant testosterone and isotretinoin when documenting the patient’s medical history.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
- Romiti R, Jansen T, Plewig G. Acne fulminans. An Bras Dermatol. 2000;75:611-617.
- Karvonen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. 1993;28:572-579.
- Kelly AP, Burns RE. Acute febrile ulcerative conglobate acne with polyarthralgia. Arch Dermatol. 1971;104:182-187.
- Plewig G, Kligman AM. Vitamin A acid in acneiform dermatoses. Acta Derm Venereol Suppl. 1975;74:119-127.
- Seukeran DC, Cunliffe WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. 1999;141:307-309.
- Kraus SL, Emmert S, Schön MP, et al. The dark side of beauty: acne fulminans induced by anabolic steroids in a male bodybuilder. Arch Dermatol. 2012;148:1210-1212.
- Jansen T, Plewig G. Acne fulminans. Int J Dermatol. 1998;37:254-257.
- Zanelato TP, Gontijo GM, Alves CA, et al. Disabling acne fulminans. An Bras Dermatol. 2011;86:9-12.
- Azulay DR, Abulafia LA, Costa JAN, et al. Tecido de granulação exuberante. efeito colateral da terapêutica com isotretinoína. An Bras Dermatol. 1985;60:179-182.
- Traupe H, von Mühlendahl KE, Brämswig J, et al. Acne of the fulminans type following testosterone therapy in three excessively tall boys. Arch Dermatol. 1988;124:414-417.
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117.
- Honma M, Murakami M, Iinuma S, et al. Acne fulminans following measles infection. J Dermatol. 2009;36:471-473.
Practice Points
- Acne fulminans, the most severe form of acne, is characterized by deep ulcerations covered by a hemorrhagic crust. It is commonly associated with fever, polyarthralgia, and myopathy caused by rapid weight loss.
- This rare condition is recognized as a potential complication of oral isotretinoin therapy.
Genetic variant could dictate rituximab response in lupus
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
MADRID – Response to rituximab in patients with systemic lupus erythematosus (SLE) might be dictated by the presence of a genetic variant that encodes the Fc gamma receptors (FcGRs), expressed on natural killer (NK) cells, according to findings from a single-center, longitudinal cohort study.

It is well known that not everyone with SLE will respond well to rituximab, but that some will, first author Md Yuzaiful Md Yusof, MBChB, PhD, explained in an interview at the European Congress of Rheumatology.
Although data from clinical trials with rituximab in this patient setting have been essentially negative, the methodology of those trials has since been disputed, he observed. Indeed, subsequent data (Ann Rheum Dis. 2017;76:1829-36) have suggested that as many as 80% of patients could achieve a response with rituximab, particularly if there is complete B-cell depletion.
Previous researchers (Ann Rheum Dis. 2012;71:875-7) have shown that a polymorphism (158V) in the Fc gamma receptor IIIA (FCGR3A) gene is associated with the response to rituximab-based therapy in patients with rheumatoid arthritis (RA). This gene is important for antibody-dependent cellular-mediated cytotoxicity (ADCC).
The objective of the current study – an observational, prospective, longitudinal cohort study conducted in Leeds (England) – was therefore to see if the FCGR3A-158V polymorphism might influence response in patients with SLE.
“We were trying to find pretreatment biomarkers that could predict response to rituximab in SLE,” Dr. Md Yusof explained.
For the study, 85 patients who were treated with rituximab were assessed. The cohort was predominantly female (96%), with a mean age of 40 years. All of the patients had antinuclear antibodies, with just over half having anti–double-stranded DNA antibodies, and two-thirds having extractable nuclear antigens. One-third had low complement (C3/C4) levels.
Complete B-cell depletion occurred in 63% of patients with the FCGR3A-158V allele, a significantly higher rate than the 40% observed among those with 158 FF genotype (odds ratio, 2.73; P = .041). A significantly higher percentage of patients with the FCGR3A-158V allele also achieved a major BILAG (British Isles Lupus Assessment Group) response when compared against patients with the 158 FF variant (48% vs. 23%), with an odds ratio of 3.06 (P = .033).
Rituximab’s effect on NK cell-mediated B-cell killing may have played a key role in treatment response. Carrying the FCGR3A-158V allele was associated with greater degranulation activity versus the 158 FF variant.
Lastly, patients were more likely to remain on treatment with rituximab over a 10-year period if they had the FCGR3A-158V allele, compared with the 158 FF variant.
“These data suggest one mechanism by which patients with SLE might become resistant to the effects of rituximab, and could be used to guide therapy in the future,” Dr. Md Yusof suggested.
“Once this finding is validated, the clinical implication is that this genetic testing could be done prior to rituximab to identify those who will respond to therapy,” he postulated. “People with SLE who have this genetic variant with high affinity for rituximab are the ones that are better suited for rituximab therapy,” he added, otherwise a different CD20-directed antibody or alternative B-cell blockade therapies should be used.
The U.K. National Institute for Health Research funded the study. Dr. Md Yusof had no conflicts of interest to disclose; some coauthors disclosed ties to Roche, GlaxoSmithKline, and AstraZeneca, among other companies.
SOURCE: Md Yusof MY et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):1069-70. Abstract SAT0009, doi: 10.1136/annrheumdis-2019-eular.6919.
REPORTING FROM EULAR 2019 CONGRESS
PsA Fast Facts: PsA and psoriasis links



Minimally invasive cosmetic surgery: Steady growth in 2018
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.
The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
that brought the total to nearly 16 million procedures, according to the American Society of Plastic Surgeons.

The most popular form of minimally invasive cosmetic surgery among the estimated 15.9 million procedures performed in 2018 was, once again, onabotulinumtoxinA injection, which represented almost half of the total for the year with 7.4 million anatomic sites injected (up by 2.9%), the ASPS said in its 2018 Plastic Surgery Statistics Report.
Soft-tissue-filler injections, the next most popular type of surgery, were up 1.7% to almost 2.7 million procedures, while chemical peels rose 0.6% to nearly 1.4 million procedures. Numbers for 2018 were down, however, for the two other top-five surgeries: Laser hair removal slipped 0.9% from 2017 and microdermabrasion fell 4.2%, the ASPS reported.
Going back quite a bit further in time – the year 2000, to be exact – reveals 21st-century growth of 228% for the minimally invasive sector as a whole, but the long-term trend for cosmetic surgery was not quite as rosy – down by 4.7% since 2000. From 2017 to 2018, though, cosmetic surgery procedures were up by 1.2%, with breast augmentation the most popular, followed by liposuction, rhinoplasty, blepharoplasty, and abdominoplasty, according to the ASPS.
The 2018 statistics report was based on analysis of the society’s Tracking Operations and Outcomes for Plastic Surgeons database and an annual survey of board-certified dermatologists, otolaryngologists, and plastic surgeons (final sample = 724).
ACIP approves meningococcal booster for persons at increased risk
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The committee voted unanimously in favor of a booster dose of MenB vaccine 1 year after completion of the primary series, with additional boosters every 2-3 years “for as long as risk remains” for high-risk persons, including microbiologists and persons with complement deficiency, complement inhibitor use, or asplenia.
The committee also voted unanimously in favor of a one-time MenB booster for individuals aged 10 years and older who are at least a year beyond completion of a MenB primary series and deemed at increased risk by public health officials in an outbreak situation.
In addition, “a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccine strategy, and projected duration of elevated risk” according to the language, which was included in the unanimously approved statement “Meningococcal Vaccination: Recommendations of The Advisory Committee on Immunization Practices.”
The updated statement on meningococcal vaccination was developed in 2019 “to consolidate all existing ACIP recommendations for MenACWY and MenB vaccines in a single document,” said Sarah Mbaeyi, MD, of the CDC’s National Center for Immunization and Respiratory Diseases, who presented immunogenicity data and the proposed recommendations.
The statement includes the recommendation of a MenB primary series for individuals aged 16-23 years based on shared clinical decision making. Kelly Moore, MD, of Vanderbilt University, Nashville, Tenn., noted the importance of ongoing data collection, and said clinicians must make clear to patients that, “if they want protection, they need the booster.”
Approximately 7% of serogroup B cases in the United States are related to disease outbreaks, mainly among college students, Dr. Mbaeyi said. All 13 universities that experienced outbreaks between 2013 and 2019 have implemented a MenB primary series, and one university has implemented an off-label booster program.
The work group concluded that a MenB booster dose is necessary to sustain protection against serogroup B disease in persons at increased risk during an outbreak, and that the potential benefits outweighed the harms given the seriousness of meningococcal disease.
Paul Hunter, MD, of the City of Milwaukee Health Department, noted that “the booster recommendation gives more flexibility” in an outbreak response.
The committee also voted unanimously to approve the Vaccines for Children resolution for the meningococcal vaccine that updates language to align with the new recommendations.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING