User login
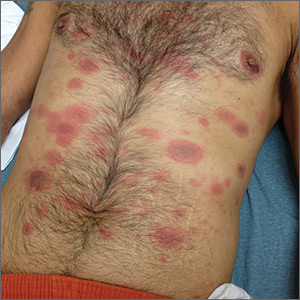
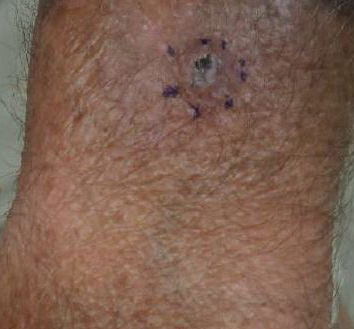
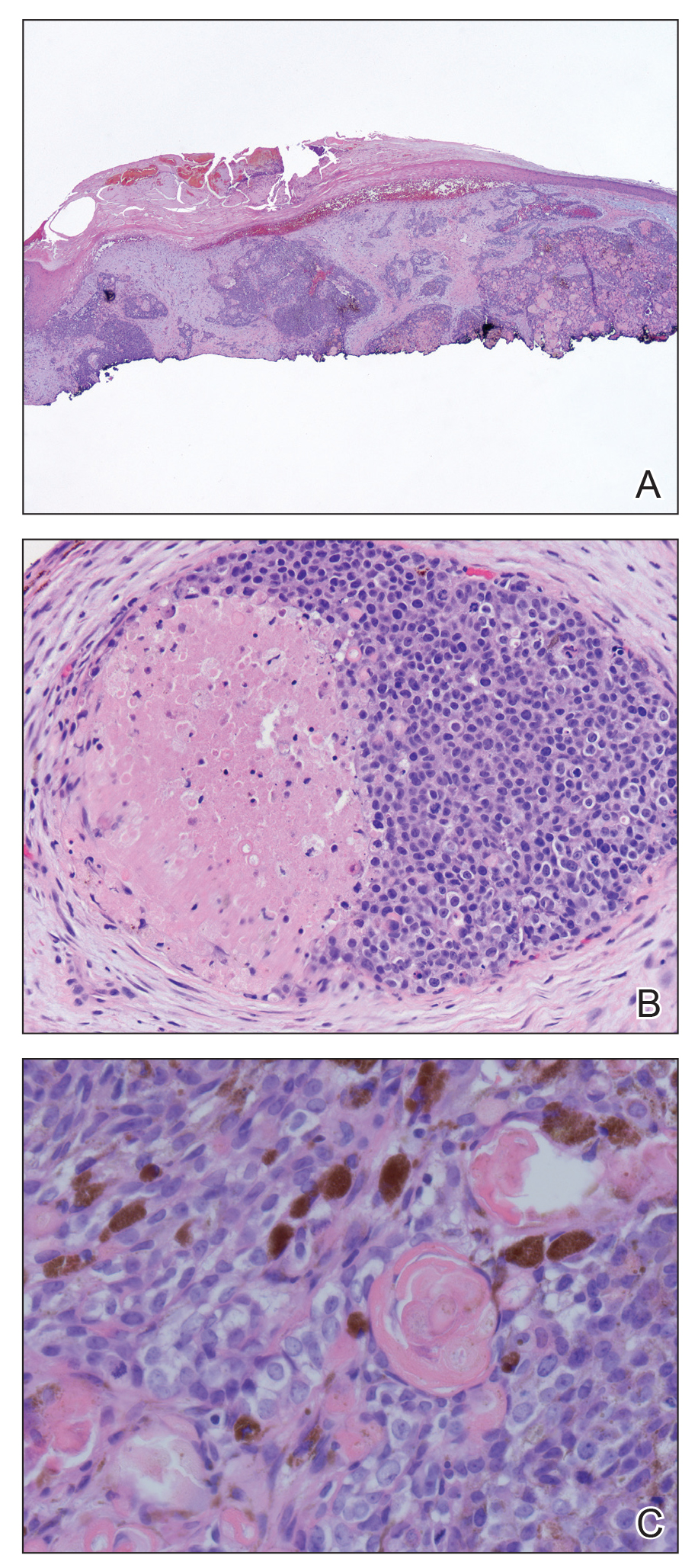
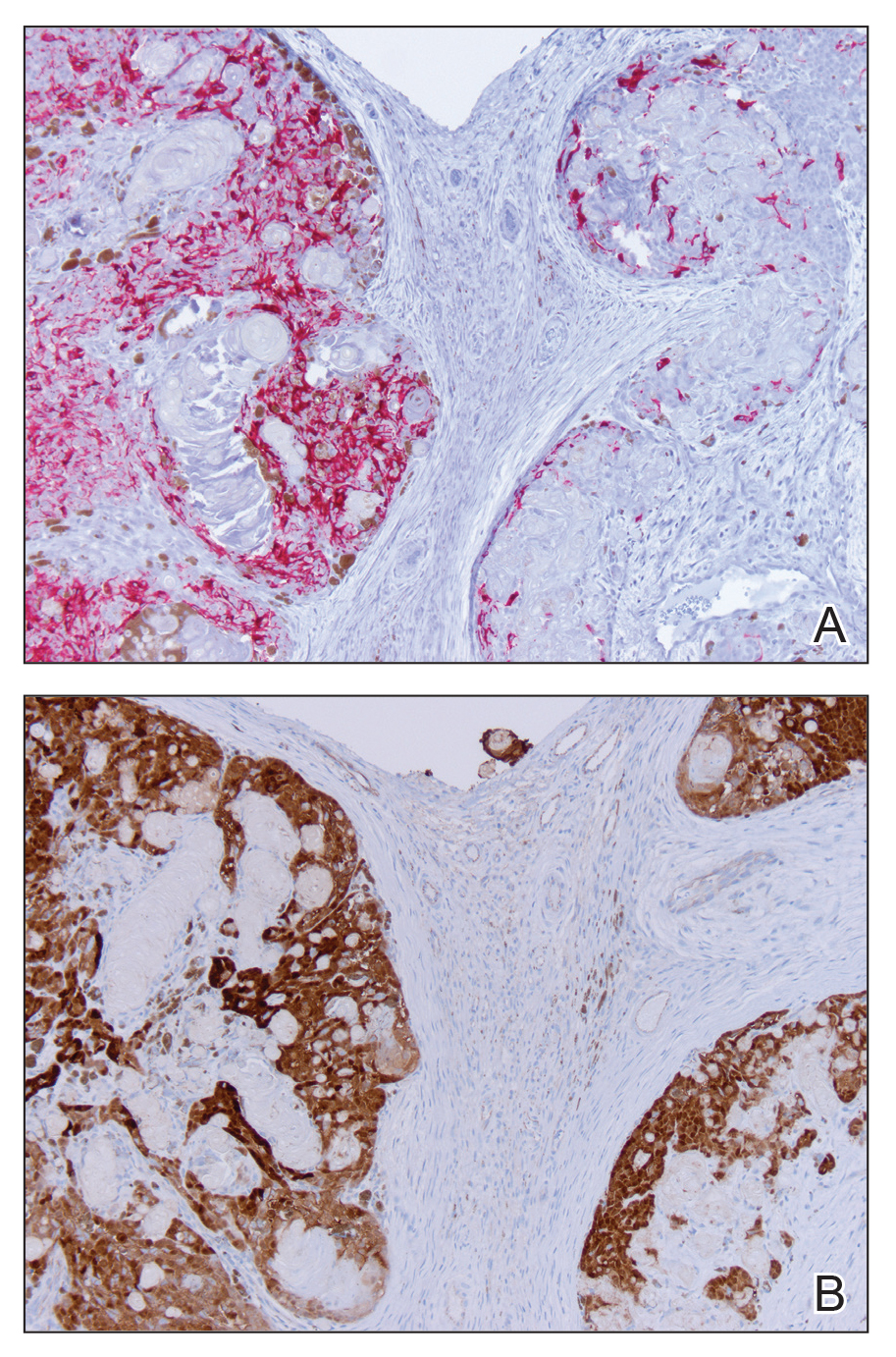
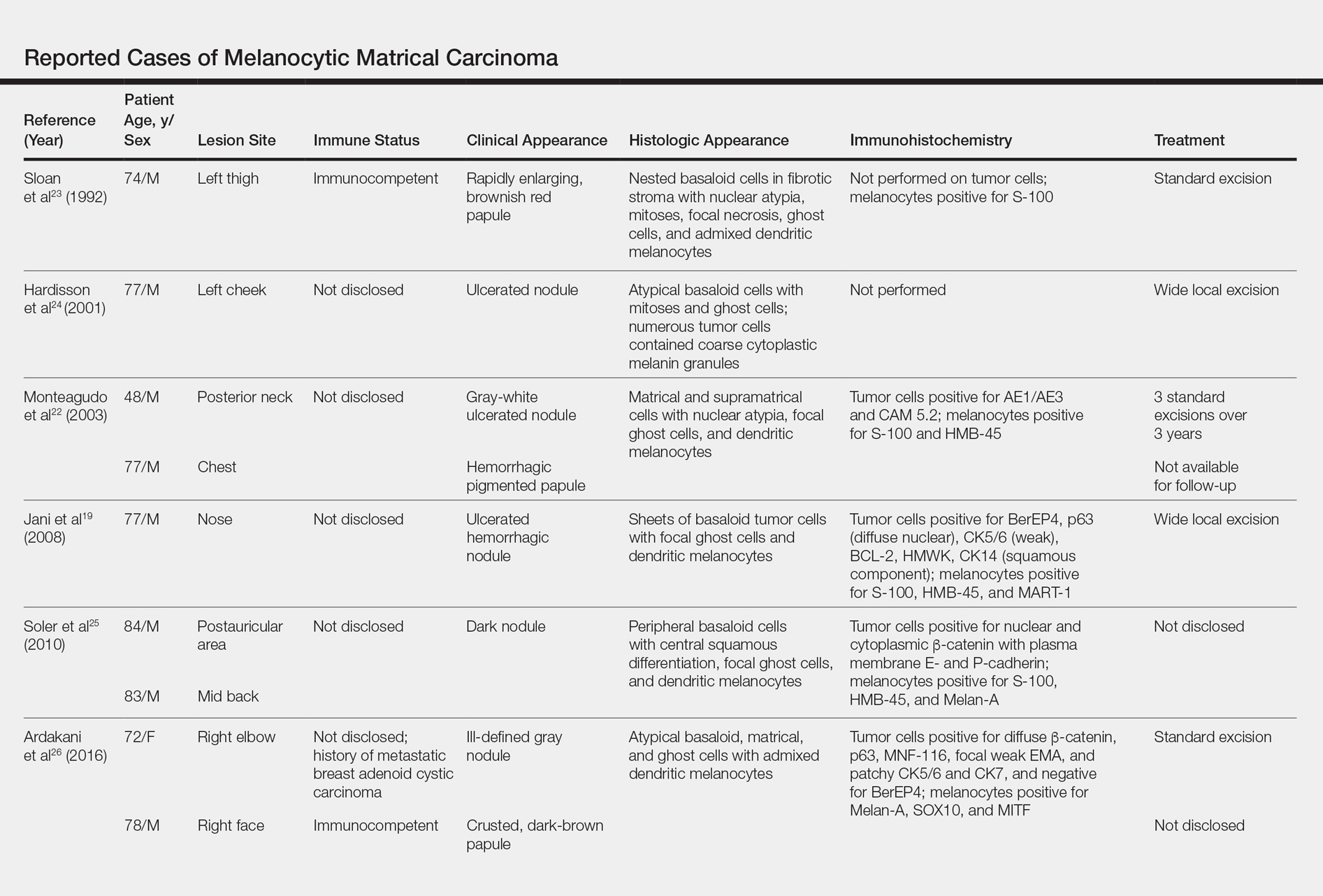
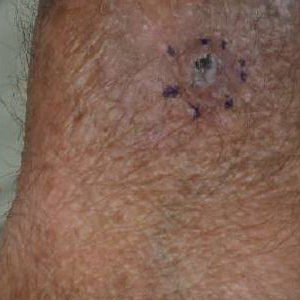
Generalized rash following ankle ulceration
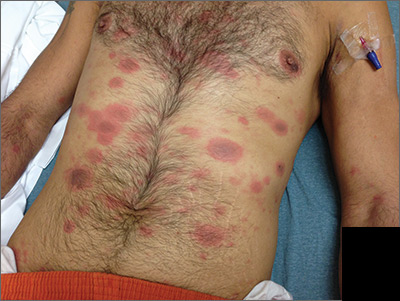
At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.

At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.

At the hospital, the physicians noted that there were no lesions on the mucous membranes of the patient’s eyes, ears, nose, mouth or anus and his vital signs were within normal limits. He was empirically treated with 1 dose of methylprednisolone (125 mg intravenous [IV]) and started on IV piperacillin-tazobactam and vancomycin. The patient subsequently revealed that he’d had a similar experience a year earlier after being treated with TMP-SMX for cellulitis. During the previous episode, he said the lesions were located on the exact same areas of his glans penis and chin. Based on the morphologic characteristics of the eruption and the history of similar lesions that appeared following previous treatment with TMP-SMX, the physician diagnosed disseminated fixed-drug eruption in this patient.
A fixed-drug eruption is an adverse cutaneous reaction to a drug that is defined by a dusky red or violaceous macule, which evolves into a patch, and eventually, an edematous plaque. Fixed-drug eruptions are typically solitary, but may be generalized (as was the case with this patient). The pathophysiology of the disease involves resident intra-epidermal CD8+ T-cells resembling effector memory T-cells. These T-cells are increased in number at the dermoepidermal junction of normal appearing skin; their aberrant activation leads to an inflammatory response, stimulating tissue destruction and formation of the classic fixed-drug lesion.
This diagnosis usually is made based on a history of similar lesions recurring at the same location in response to a specific drug and the classic physical exam findings of well-demarcated, edematous, and violaceous plaques. A skin biopsy may be performed to confirm a fixed-drug eruption in the case of clinical equipoise.
Fixed-drug eruptions occasionally exhibit bullae and erosions and must be differentiated from more serious generalized bullous diseases, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The differential diagnosis also includes erythema multiforme, early bullous drug eruption, and bullous arthropod assault, which may leave similar hyperpigmented patches.
Management of a disseminated fixed-drug eruption requires a thorough history to identify the causative agent (including over-the-counter drugs, herbals, topicals, and eye drops). Most patients are asymptomatic, but some (like this patient) are symptomatic and experience generalized pruritus, cutaneous burning, and/or pain. Symptomatic therapy includes oral antihistamines and potent topical glucocorticoid ointment for non-eroded lesions. Additionally, if not medically contraindicated, oral steroids may be used for generalized or extremely painful mucosal lesions at a dose of 0.5 mg/kg daily for 3 to 5 days.
Local wound care of eroded lesions includes keeping the site moist with a bland emollient and bandaging. The inciting agent must be added to the patient’s allergy list and avoided in the future. In equivocal cases, it is prudent to admit the patient for observation to ensure that the eruption is not a nascent SJS or TEN eruption.
In this case, the patient was admitted to the observation unit overnight to monitor for the appearance of systemic symptoms and to assess the evolution of the rash for further mucosal involvement that could have indicated SJS. Upon reassessment the next day, his older lesions had evolved into vesiculated and necrotic areas as per the natural history of severe fixed-drug eruption. He was prescribed prednisone 40 mg/d for 3 days to help with local inflammation, pain, and itching. TMP-SMX was added to his allergy list and he was given local wound care instructions. He was told to return if he developed any systemic symptoms.
This case was adapted from: Bucher J, Rahnama-Moghadam S, Osswald S. Generalized rash follows ankle ulceration. J Fam Pract. 2016;65:489-491.
Children’s book effectively assesses literary skills during well-child visits
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
FROM PEDIATRICS
AAD’s augmented intelligence position statement provides look to the future
A blueprint for the future of into clinical settings, and postmarketing surveillance – is the focus of a newly published American Academy of Dermatology (AAD) position statement.
The statement describes AuI as “a concept that focuses on artificial intelligence’s (AI) assistive role,” emphasizing that AuI is “designed to enhance human intelligence and the physician/patient relationship,” not to replace that relationship. The statement, available on the AAD website, also emphasizes the importance of the human-centered nature of this concept as dermatologists integrate AuI into their practice, noting that “patients should feel well cared for even when interacting with software.”
“We are at the precipice of seeing the beginnings of augmented intelligence tools influence our practice,” statement coauthor Justin M. Ko, MD, MBA, said in an interview. “At the outset, it will likely be systems and tools that are working on practice management processes, insights, and analytics. However, I don’t believe we’re far from seeing the mainstream integration say, of triage, point of care diagnostic support, or chronic disease management capabilities that rely in some part on augmented intelligence models.”
“The key is that we recognize that this is on the horizon, and that we actively work to shape the development and deployment of these tools in a responsible way that enhances patient care and ensures that patient safety is held paramount,” added Dr. Ko, director and chief of medical dermatology at Stanford (Calif.) University.
“We want patients and clinicians to recognize the potential of such tools to augment the core care relationship and improve the delivery of such care,” he explained. “At the same time, we know that, from the technology that exists all around us in all aspects of our lives, that despite the best intentions, there [may be] unintended consequences in the medical realm. We must be cognizant of possibilities, for example, of amplifying bias and increasing health inequity, and take an active approach to mitigating these risks.”
The AAD position statement notes that high-quality AuI will be “designed and evaluated in a manner that enables the delivery of high-quality care to patients.” During development and clinical deployment, the statement highlighted the importance of collaboration with practitioners, and that “AuI must integrate into physician and provider workflows.” The statement advises that AuI tools should be regulated appropriately, and that postmarketing surveillance will be necessary after clinical launch of these tools, measuring outcomes such as “quality, cost, and/or efficiency of care delivery.”
While the first half of the statement provides a roadmap for AuI development and implementation, the second half further emphasizes collaboration and communication with a number of stakeholders. “Effective and ethical development and implementation of AuI will require continuous engagement, education, exploration of privacy and medical-legal issues, and advocacy,” the statement says. “Although the promise of AuI to improve health and wellness holds significant potential,” the statement says, “issues related to privacy and medical-legal complications are amplified by technology that requires transmission of data beyond the confines of a providers’ institution. Protected Health Information must be managed with effective safeguards to prevent inadvertent exposure.”
The conclusion of the statement revisits the game-changing nature of AuI while asserting the AAD’s commitment to guiding integration with dermatology practice. “AuI has the potential to transform our collective and personal experience of health, health care, and wellness,” the AAD wrote. “To achieve this potential, deliberate and diligent efforts must be taken to engage and collaborate with stakeholders and policy makers. The Academy hopes to work with administrative and legislative colleagues to create policies that promote AuI that is high quality, inclusive, equitable, and accessible. Through collaboration and research, the Academy strives to guide the design, implementation, and regulation of these technologies and augment care for all.”
The statement also includes a glossary of terms used in this area. The AAD’s Ad Hoc Taskforce on Augmented Intelligence wrote the position statement; Dr. Ko is on the task force and serves as its chairman.
A blueprint for the future of into clinical settings, and postmarketing surveillance – is the focus of a newly published American Academy of Dermatology (AAD) position statement.
The statement describes AuI as “a concept that focuses on artificial intelligence’s (AI) assistive role,” emphasizing that AuI is “designed to enhance human intelligence and the physician/patient relationship,” not to replace that relationship. The statement, available on the AAD website, also emphasizes the importance of the human-centered nature of this concept as dermatologists integrate AuI into their practice, noting that “patients should feel well cared for even when interacting with software.”
“We are at the precipice of seeing the beginnings of augmented intelligence tools influence our practice,” statement coauthor Justin M. Ko, MD, MBA, said in an interview. “At the outset, it will likely be systems and tools that are working on practice management processes, insights, and analytics. However, I don’t believe we’re far from seeing the mainstream integration say, of triage, point of care diagnostic support, or chronic disease management capabilities that rely in some part on augmented intelligence models.”
“The key is that we recognize that this is on the horizon, and that we actively work to shape the development and deployment of these tools in a responsible way that enhances patient care and ensures that patient safety is held paramount,” added Dr. Ko, director and chief of medical dermatology at Stanford (Calif.) University.
“We want patients and clinicians to recognize the potential of such tools to augment the core care relationship and improve the delivery of such care,” he explained. “At the same time, we know that, from the technology that exists all around us in all aspects of our lives, that despite the best intentions, there [may be] unintended consequences in the medical realm. We must be cognizant of possibilities, for example, of amplifying bias and increasing health inequity, and take an active approach to mitigating these risks.”
The AAD position statement notes that high-quality AuI will be “designed and evaluated in a manner that enables the delivery of high-quality care to patients.” During development and clinical deployment, the statement highlighted the importance of collaboration with practitioners, and that “AuI must integrate into physician and provider workflows.” The statement advises that AuI tools should be regulated appropriately, and that postmarketing surveillance will be necessary after clinical launch of these tools, measuring outcomes such as “quality, cost, and/or efficiency of care delivery.”
While the first half of the statement provides a roadmap for AuI development and implementation, the second half further emphasizes collaboration and communication with a number of stakeholders. “Effective and ethical development and implementation of AuI will require continuous engagement, education, exploration of privacy and medical-legal issues, and advocacy,” the statement says. “Although the promise of AuI to improve health and wellness holds significant potential,” the statement says, “issues related to privacy and medical-legal complications are amplified by technology that requires transmission of data beyond the confines of a providers’ institution. Protected Health Information must be managed with effective safeguards to prevent inadvertent exposure.”
The conclusion of the statement revisits the game-changing nature of AuI while asserting the AAD’s commitment to guiding integration with dermatology practice. “AuI has the potential to transform our collective and personal experience of health, health care, and wellness,” the AAD wrote. “To achieve this potential, deliberate and diligent efforts must be taken to engage and collaborate with stakeholders and policy makers. The Academy hopes to work with administrative and legislative colleagues to create policies that promote AuI that is high quality, inclusive, equitable, and accessible. Through collaboration and research, the Academy strives to guide the design, implementation, and regulation of these technologies and augment care for all.”
The statement also includes a glossary of terms used in this area. The AAD’s Ad Hoc Taskforce on Augmented Intelligence wrote the position statement; Dr. Ko is on the task force and serves as its chairman.
A blueprint for the future of into clinical settings, and postmarketing surveillance – is the focus of a newly published American Academy of Dermatology (AAD) position statement.
The statement describes AuI as “a concept that focuses on artificial intelligence’s (AI) assistive role,” emphasizing that AuI is “designed to enhance human intelligence and the physician/patient relationship,” not to replace that relationship. The statement, available on the AAD website, also emphasizes the importance of the human-centered nature of this concept as dermatologists integrate AuI into their practice, noting that “patients should feel well cared for even when interacting with software.”
“We are at the precipice of seeing the beginnings of augmented intelligence tools influence our practice,” statement coauthor Justin M. Ko, MD, MBA, said in an interview. “At the outset, it will likely be systems and tools that are working on practice management processes, insights, and analytics. However, I don’t believe we’re far from seeing the mainstream integration say, of triage, point of care diagnostic support, or chronic disease management capabilities that rely in some part on augmented intelligence models.”
“The key is that we recognize that this is on the horizon, and that we actively work to shape the development and deployment of these tools in a responsible way that enhances patient care and ensures that patient safety is held paramount,” added Dr. Ko, director and chief of medical dermatology at Stanford (Calif.) University.
“We want patients and clinicians to recognize the potential of such tools to augment the core care relationship and improve the delivery of such care,” he explained. “At the same time, we know that, from the technology that exists all around us in all aspects of our lives, that despite the best intentions, there [may be] unintended consequences in the medical realm. We must be cognizant of possibilities, for example, of amplifying bias and increasing health inequity, and take an active approach to mitigating these risks.”
The AAD position statement notes that high-quality AuI will be “designed and evaluated in a manner that enables the delivery of high-quality care to patients.” During development and clinical deployment, the statement highlighted the importance of collaboration with practitioners, and that “AuI must integrate into physician and provider workflows.” The statement advises that AuI tools should be regulated appropriately, and that postmarketing surveillance will be necessary after clinical launch of these tools, measuring outcomes such as “quality, cost, and/or efficiency of care delivery.”
While the first half of the statement provides a roadmap for AuI development and implementation, the second half further emphasizes collaboration and communication with a number of stakeholders. “Effective and ethical development and implementation of AuI will require continuous engagement, education, exploration of privacy and medical-legal issues, and advocacy,” the statement says. “Although the promise of AuI to improve health and wellness holds significant potential,” the statement says, “issues related to privacy and medical-legal complications are amplified by technology that requires transmission of data beyond the confines of a providers’ institution. Protected Health Information must be managed with effective safeguards to prevent inadvertent exposure.”
The conclusion of the statement revisits the game-changing nature of AuI while asserting the AAD’s commitment to guiding integration with dermatology practice. “AuI has the potential to transform our collective and personal experience of health, health care, and wellness,” the AAD wrote. “To achieve this potential, deliberate and diligent efforts must be taken to engage and collaborate with stakeholders and policy makers. The Academy hopes to work with administrative and legislative colleagues to create policies that promote AuI that is high quality, inclusive, equitable, and accessible. Through collaboration and research, the Academy strives to guide the design, implementation, and regulation of these technologies and augment care for all.”
The statement also includes a glossary of terms used in this area. The AAD’s Ad Hoc Taskforce on Augmented Intelligence wrote the position statement; Dr. Ko is on the task force and serves as its chairman.
New tickborne virus emerges in China
A new virus has been associated with febrile illness in China in patients with histories of tick bites. The data on the discovery, isolation, and characterization of the virus were reported in the New England Journal of Medicine.
The segmented RNA virus now known as Alongshan virus (ALSV) “belongs to the unclassified jingmenvirus group in the family Flaviviridae, which includes the genera flavivirus, pestivirus, hepacivirus, and pegivirus,” wrote Ze-Dong Wang, PhD, of Foshan (China) University, and colleagues.
The index patient with ALSV was a 42-year-old female farmer from the town of Alongshan, China, who presented to a regional hospital in April 2017 with fever, headache, and a history of tick bites. The initial clinical features were similar to those seen in tickborne diseases, but a blood sample showed no RNA or antibodies for tickborne encephalitis virus. Investigators obtained a blood specimen from the index patient 4 days after the onset of illness. After culturing the sample, the investigators extracted the viral RNA genome and sequenced it.
Sequence analysis found that the new pathogen was related to segmented viruses in the jingmenvirus group of the family Flaviviridae; however, “comparison of the amino acids further confirmed that ALSV is genetically distinct from other jingmenviruses,” the investigators said.
The investigators identified 374 patients who presented to the hospital with fever, headache, and a history of tick bites during May 2017–September 2017; 86 patients had confirmed ALSV infections via nested reverse-transcription polymerase chain reaction testing. Of these, 63 were men and 84 were farmers or forestry workers. Although ticks were common in the patients’ environments, no other evidence of tickborne diseases was noted. The patients ranged in age from 24 to 77 years, and the average duration of the infection was 3-7 days.
Symptoms were nonspecific and included fever, headache, fatigue, nausea, cough, and sore throat. All 86 patients were treated with intravenous ribavirin (0.5 g/day), and intramuscular benzylpenicillin sodium (2 million U/day) for 3-5 days. The median hospital stay was 11 days, and no deaths or long-term clinical complications occurred in the confirmed ALSV patients.
ALSV is similar to other jingmenviruses, but is distinct from other infections in part because of the absence of a rash or jaundice, the investigators said.
Although the investigators said they suspected the disease was carried by ticks, they would not rule out mosquitoes as a possible carrier because ALSV RNA was found in mosquitoes in a Northeastern province of China, and the RNA from those mosquitoes was found to be genetically related to the RNA assessed in this study.
Overall, “our findings suggest that ALSV may be the cause of a previously unknown febrile disease, and more studies should be conducted to determine the geographic distribution of this disease outside its current areas of identification,” they said.
The research was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
SOURCE: Wang Z et al. N Engl J Med. 2019 May 29. doi: 10.1056/NEJMoa1805068.
New technology and genomic surveys will continue to help identify emerging pathogens, however, “they may provide limited value in understanding the mechanisms of disease emergence,” wrote Nikos Vasilakis, PhD, and David H. Walker, MD, in an accompanying editorial. An active surveillance program allowed the investigators of the previously unknown tickborne pathogen in China to identify a group of patients with similar history. The new pathogen was classified as one of the jingmenviruses, which “reveal that RNA virus segmentation is an evolutionary process that has occurred in previously unanticipated circumstances.” This study by Wang et al. shows that these viruses are not limited to arthropod hosts but can be dangerous to humans.
The new pathogen had likely been evolving for some time before it was discovered, the editorialists said. “The key to making such discoveries is the study of ill persons, isolation of the etiologic agent, use of tools that will reveal the nature of the agent (e.g., electron microscopy), and application of the appropriate tools for definitive characterization (e.g., sequencing of the RNA genome),” they emphasized. However, to mitigate outbreaks, “proactive, real-time surveillance” may be more cost effective than extensive genomic surveys, they noted (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1901212).
Dr. Vasilakis and Dr. Walker are affiliated with the department of pathology, Center for Biodefense and Emerging Infectious Diseases, Center for Tropical Diseases, and the Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston. They had no financial conflicts to disclose.
New technology and genomic surveys will continue to help identify emerging pathogens, however, “they may provide limited value in understanding the mechanisms of disease emergence,” wrote Nikos Vasilakis, PhD, and David H. Walker, MD, in an accompanying editorial. An active surveillance program allowed the investigators of the previously unknown tickborne pathogen in China to identify a group of patients with similar history. The new pathogen was classified as one of the jingmenviruses, which “reveal that RNA virus segmentation is an evolutionary process that has occurred in previously unanticipated circumstances.” This study by Wang et al. shows that these viruses are not limited to arthropod hosts but can be dangerous to humans.
The new pathogen had likely been evolving for some time before it was discovered, the editorialists said. “The key to making such discoveries is the study of ill persons, isolation of the etiologic agent, use of tools that will reveal the nature of the agent (e.g., electron microscopy), and application of the appropriate tools for definitive characterization (e.g., sequencing of the RNA genome),” they emphasized. However, to mitigate outbreaks, “proactive, real-time surveillance” may be more cost effective than extensive genomic surveys, they noted (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1901212).
Dr. Vasilakis and Dr. Walker are affiliated with the department of pathology, Center for Biodefense and Emerging Infectious Diseases, Center for Tropical Diseases, and the Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston. They had no financial conflicts to disclose.
New technology and genomic surveys will continue to help identify emerging pathogens, however, “they may provide limited value in understanding the mechanisms of disease emergence,” wrote Nikos Vasilakis, PhD, and David H. Walker, MD, in an accompanying editorial. An active surveillance program allowed the investigators of the previously unknown tickborne pathogen in China to identify a group of patients with similar history. The new pathogen was classified as one of the jingmenviruses, which “reveal that RNA virus segmentation is an evolutionary process that has occurred in previously unanticipated circumstances.” This study by Wang et al. shows that these viruses are not limited to arthropod hosts but can be dangerous to humans.
The new pathogen had likely been evolving for some time before it was discovered, the editorialists said. “The key to making such discoveries is the study of ill persons, isolation of the etiologic agent, use of tools that will reveal the nature of the agent (e.g., electron microscopy), and application of the appropriate tools for definitive characterization (e.g., sequencing of the RNA genome),” they emphasized. However, to mitigate outbreaks, “proactive, real-time surveillance” may be more cost effective than extensive genomic surveys, they noted (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1901212).
Dr. Vasilakis and Dr. Walker are affiliated with the department of pathology, Center for Biodefense and Emerging Infectious Diseases, Center for Tropical Diseases, and the Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston. They had no financial conflicts to disclose.
A new virus has been associated with febrile illness in China in patients with histories of tick bites. The data on the discovery, isolation, and characterization of the virus were reported in the New England Journal of Medicine.
The segmented RNA virus now known as Alongshan virus (ALSV) “belongs to the unclassified jingmenvirus group in the family Flaviviridae, which includes the genera flavivirus, pestivirus, hepacivirus, and pegivirus,” wrote Ze-Dong Wang, PhD, of Foshan (China) University, and colleagues.
The index patient with ALSV was a 42-year-old female farmer from the town of Alongshan, China, who presented to a regional hospital in April 2017 with fever, headache, and a history of tick bites. The initial clinical features were similar to those seen in tickborne diseases, but a blood sample showed no RNA or antibodies for tickborne encephalitis virus. Investigators obtained a blood specimen from the index patient 4 days after the onset of illness. After culturing the sample, the investigators extracted the viral RNA genome and sequenced it.
Sequence analysis found that the new pathogen was related to segmented viruses in the jingmenvirus group of the family Flaviviridae; however, “comparison of the amino acids further confirmed that ALSV is genetically distinct from other jingmenviruses,” the investigators said.
The investigators identified 374 patients who presented to the hospital with fever, headache, and a history of tick bites during May 2017–September 2017; 86 patients had confirmed ALSV infections via nested reverse-transcription polymerase chain reaction testing. Of these, 63 were men and 84 were farmers or forestry workers. Although ticks were common in the patients’ environments, no other evidence of tickborne diseases was noted. The patients ranged in age from 24 to 77 years, and the average duration of the infection was 3-7 days.
Symptoms were nonspecific and included fever, headache, fatigue, nausea, cough, and sore throat. All 86 patients were treated with intravenous ribavirin (0.5 g/day), and intramuscular benzylpenicillin sodium (2 million U/day) for 3-5 days. The median hospital stay was 11 days, and no deaths or long-term clinical complications occurred in the confirmed ALSV patients.
ALSV is similar to other jingmenviruses, but is distinct from other infections in part because of the absence of a rash or jaundice, the investigators said.
Although the investigators said they suspected the disease was carried by ticks, they would not rule out mosquitoes as a possible carrier because ALSV RNA was found in mosquitoes in a Northeastern province of China, and the RNA from those mosquitoes was found to be genetically related to the RNA assessed in this study.
Overall, “our findings suggest that ALSV may be the cause of a previously unknown febrile disease, and more studies should be conducted to determine the geographic distribution of this disease outside its current areas of identification,” they said.
The research was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
SOURCE: Wang Z et al. N Engl J Med. 2019 May 29. doi: 10.1056/NEJMoa1805068.
A new virus has been associated with febrile illness in China in patients with histories of tick bites. The data on the discovery, isolation, and characterization of the virus were reported in the New England Journal of Medicine.
The segmented RNA virus now known as Alongshan virus (ALSV) “belongs to the unclassified jingmenvirus group in the family Flaviviridae, which includes the genera flavivirus, pestivirus, hepacivirus, and pegivirus,” wrote Ze-Dong Wang, PhD, of Foshan (China) University, and colleagues.
The index patient with ALSV was a 42-year-old female farmer from the town of Alongshan, China, who presented to a regional hospital in April 2017 with fever, headache, and a history of tick bites. The initial clinical features were similar to those seen in tickborne diseases, but a blood sample showed no RNA or antibodies for tickborne encephalitis virus. Investigators obtained a blood specimen from the index patient 4 days after the onset of illness. After culturing the sample, the investigators extracted the viral RNA genome and sequenced it.
Sequence analysis found that the new pathogen was related to segmented viruses in the jingmenvirus group of the family Flaviviridae; however, “comparison of the amino acids further confirmed that ALSV is genetically distinct from other jingmenviruses,” the investigators said.
The investigators identified 374 patients who presented to the hospital with fever, headache, and a history of tick bites during May 2017–September 2017; 86 patients had confirmed ALSV infections via nested reverse-transcription polymerase chain reaction testing. Of these, 63 were men and 84 were farmers or forestry workers. Although ticks were common in the patients’ environments, no other evidence of tickborne diseases was noted. The patients ranged in age from 24 to 77 years, and the average duration of the infection was 3-7 days.
Symptoms were nonspecific and included fever, headache, fatigue, nausea, cough, and sore throat. All 86 patients were treated with intravenous ribavirin (0.5 g/day), and intramuscular benzylpenicillin sodium (2 million U/day) for 3-5 days. The median hospital stay was 11 days, and no deaths or long-term clinical complications occurred in the confirmed ALSV patients.
ALSV is similar to other jingmenviruses, but is distinct from other infections in part because of the absence of a rash or jaundice, the investigators said.
Although the investigators said they suspected the disease was carried by ticks, they would not rule out mosquitoes as a possible carrier because ALSV RNA was found in mosquitoes in a Northeastern province of China, and the RNA from those mosquitoes was found to be genetically related to the RNA assessed in this study.
Overall, “our findings suggest that ALSV may be the cause of a previously unknown febrile disease, and more studies should be conducted to determine the geographic distribution of this disease outside its current areas of identification,” they said.
The research was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
SOURCE: Wang Z et al. N Engl J Med. 2019 May 29. doi: 10.1056/NEJMoa1805068.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Venetoclax plus ibrutinib appears to suit elderly and high-risk patients with CLL
A combination of venetoclax and ibrutinib may be a safe and effective treatment option for previously untreated elderly and high-risk patients with chronic lymphocytic leukemia (CLL), according to investigators of a phase 2 trial of the combination.
About 88% of patients achieved complete remission or complete remission with incomplete count recovery after 12 cycles of treatment, reported lead author Nitin Jain, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
There were no new safety signals for the combination of ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase, and venetoclax, a B-cell lymphoma 2 protein inhibitor, the investigators noted.
“This combination was reported to be safe and active in patients with mantle cell lymphoma,” they wrote in the New England Journal of Medicine. “Given the clinically complementary activity, preclinical synergism, and nonoverlapping toxic effects, we examined the safety and efficacy of combined ibrutinib and venetoclax treatment in previously untreated patients with CLL.”
In particular, the investigators recruited older patients, as this is a common population that can be challenging to treat. “Because CLL typically occurs in older adults, the majority of patients who need treatment are older than 65 years of age,” the investigators wrote. “This group of patients often has unacceptable side effects and has a lower rate of complete remission and undetectable minimal residual disease with chemoimmunotherapy than younger patients.”
The open-label, phase 2 trial enrolled 80 elderly and high-risk patients with previously untreated CLL. Eligibility required an age of at least 65 years or presence of at least one high-risk genetic feature; namely, mutated TP53, unmutated IgVH, or chromosome 11q deletion.
In order to reduce the risk of tumor lysis syndrome, ibrutinib (420 mg once daily) was given as monotherapy for three 28-day cycles. From the fourth cycle onward, venetoclax was also given, with weekly dose escalations to a target dose of 400 mg once daily. The combination was given for 24 cycles, with treatment continuation offered to patients who were still positive for minimal residual disease.
The median patient age was 65 years, with 30% of the population aged 70 years or older. A large majority (92%) had at least one high-risk genetic feature.
Following initiation with three cycles of ibrutinib, most patients had partial responses, the investigators wrote; however, with the addition of venetoclax, responses improved over time. Of all 80 patients, 59 (74%) had a best response of complete remission or complete remission with incomplete count recovery.
After six cycles, 51 out of 70 patients (73%) achieved this marker. After 12 cycles, 29 of 33 patients (88%) had this response, with 61% of the same group demonstrating undetectable minimal residual disease in bone marrow.
After 18 cycles, 25 of 26 patients (96%) had complete remission or complete remission with incomplete count recovery, 18 of which (69%) were negative for minimal residual disease. Three patients completed 24 cycles of combined therapy, all of whom achieved complete remission or complete remission with incomplete count recovery and undetectable minimal residual disease.
Focusing on patients aged 65 years or older, 74% had complete remission or complete remission with incomplete count recovery after six cycles of therapy and nearly half (44%) had undetectable minimal residual disease. After 12 cycles, these rates increased to 94% and 76%, respectively. Responses were also seen across genetically high-risk subgroups.
One patient died from a cryptococcal infection of the central nervous system; this was deemed unrelated to treatment, as symptoms began prior to initiation of treatment and only one dose of ibrutinib was given.
The estimated 1-year progression-free survival rate was 98% and the estimated overall survival rate was 99%. At the time of publication, no patients had disease progression.
Among all patients, 60% experienced grade 3 or higher adverse events, the most common being neutropenia (48%).
Almost half of the patient population (44%) required dose reductions of ibrutinib, most commonly because of atrial fibrillation, and 24% required dose reductions of venetoclax, most often because of neutropenia.
“Our data showed that combination therapy with ibrutinib and venetoclax was effective in patients with CLL, with no new toxic effects from the combination that were not reported previously for the individual agents,” the investigators wrote, adding that the efficacy findings were also “substantially better” than what has been reported with monotherapy for each of the agents in patients with CLL.
The study was funded by AbbVie, the University of Texas MD Anderson Cancer Center Chronic Lymphocytic Leukemia Moon Shot program, the Andrew Sabin Family Foundation, and the CLL Global Research Foundation. The investigators reported relationships with AbbVie, Incyte, Celgene, and other companies.
SOURCE: Jain N et al. N Engl J Med. 2019;380:2095-103.
In addition to noting the “impressive” results from combining venetoclax and ibrutinib as frontline CLL therapy, Adrian Wiestner, MD, PhD, highlighted the lack of a Kaplan-Meier curve in the paper published by Jain et al. in the New England Journal of Medicine.
“Here, assessment of minimal residual disease has replaced the progression-free survival curve of old, indicating a possible shift in focus away from traditional clinical trial endpoints and toward even more stringent measures of clinical efficacy that may be central to regulatory decisions,” Dr. Wiestner wrote.
Dr. Wiestner of the National Institutes of Health made his remarks in an accompanying editorial (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1904362). He reported grants from with Merck, Pharmacyclics (an AbbVie company), and Acerta Pharma.
In addition to noting the “impressive” results from combining venetoclax and ibrutinib as frontline CLL therapy, Adrian Wiestner, MD, PhD, highlighted the lack of a Kaplan-Meier curve in the paper published by Jain et al. in the New England Journal of Medicine.
“Here, assessment of minimal residual disease has replaced the progression-free survival curve of old, indicating a possible shift in focus away from traditional clinical trial endpoints and toward even more stringent measures of clinical efficacy that may be central to regulatory decisions,” Dr. Wiestner wrote.
Dr. Wiestner of the National Institutes of Health made his remarks in an accompanying editorial (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1904362). He reported grants from with Merck, Pharmacyclics (an AbbVie company), and Acerta Pharma.
In addition to noting the “impressive” results from combining venetoclax and ibrutinib as frontline CLL therapy, Adrian Wiestner, MD, PhD, highlighted the lack of a Kaplan-Meier curve in the paper published by Jain et al. in the New England Journal of Medicine.
“Here, assessment of minimal residual disease has replaced the progression-free survival curve of old, indicating a possible shift in focus away from traditional clinical trial endpoints and toward even more stringent measures of clinical efficacy that may be central to regulatory decisions,” Dr. Wiestner wrote.
Dr. Wiestner of the National Institutes of Health made his remarks in an accompanying editorial (N Engl J Med. 2019 May 29. doi: 10.1056/NEJMe1904362). He reported grants from with Merck, Pharmacyclics (an AbbVie company), and Acerta Pharma.
A combination of venetoclax and ibrutinib may be a safe and effective treatment option for previously untreated elderly and high-risk patients with chronic lymphocytic leukemia (CLL), according to investigators of a phase 2 trial of the combination.
About 88% of patients achieved complete remission or complete remission with incomplete count recovery after 12 cycles of treatment, reported lead author Nitin Jain, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
There were no new safety signals for the combination of ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase, and venetoclax, a B-cell lymphoma 2 protein inhibitor, the investigators noted.
“This combination was reported to be safe and active in patients with mantle cell lymphoma,” they wrote in the New England Journal of Medicine. “Given the clinically complementary activity, preclinical synergism, and nonoverlapping toxic effects, we examined the safety and efficacy of combined ibrutinib and venetoclax treatment in previously untreated patients with CLL.”
In particular, the investigators recruited older patients, as this is a common population that can be challenging to treat. “Because CLL typically occurs in older adults, the majority of patients who need treatment are older than 65 years of age,” the investigators wrote. “This group of patients often has unacceptable side effects and has a lower rate of complete remission and undetectable minimal residual disease with chemoimmunotherapy than younger patients.”
The open-label, phase 2 trial enrolled 80 elderly and high-risk patients with previously untreated CLL. Eligibility required an age of at least 65 years or presence of at least one high-risk genetic feature; namely, mutated TP53, unmutated IgVH, or chromosome 11q deletion.
In order to reduce the risk of tumor lysis syndrome, ibrutinib (420 mg once daily) was given as monotherapy for three 28-day cycles. From the fourth cycle onward, venetoclax was also given, with weekly dose escalations to a target dose of 400 mg once daily. The combination was given for 24 cycles, with treatment continuation offered to patients who were still positive for minimal residual disease.
The median patient age was 65 years, with 30% of the population aged 70 years or older. A large majority (92%) had at least one high-risk genetic feature.
Following initiation with three cycles of ibrutinib, most patients had partial responses, the investigators wrote; however, with the addition of venetoclax, responses improved over time. Of all 80 patients, 59 (74%) had a best response of complete remission or complete remission with incomplete count recovery.
After six cycles, 51 out of 70 patients (73%) achieved this marker. After 12 cycles, 29 of 33 patients (88%) had this response, with 61% of the same group demonstrating undetectable minimal residual disease in bone marrow.
After 18 cycles, 25 of 26 patients (96%) had complete remission or complete remission with incomplete count recovery, 18 of which (69%) were negative for minimal residual disease. Three patients completed 24 cycles of combined therapy, all of whom achieved complete remission or complete remission with incomplete count recovery and undetectable minimal residual disease.
Focusing on patients aged 65 years or older, 74% had complete remission or complete remission with incomplete count recovery after six cycles of therapy and nearly half (44%) had undetectable minimal residual disease. After 12 cycles, these rates increased to 94% and 76%, respectively. Responses were also seen across genetically high-risk subgroups.
One patient died from a cryptococcal infection of the central nervous system; this was deemed unrelated to treatment, as symptoms began prior to initiation of treatment and only one dose of ibrutinib was given.
The estimated 1-year progression-free survival rate was 98% and the estimated overall survival rate was 99%. At the time of publication, no patients had disease progression.
Among all patients, 60% experienced grade 3 or higher adverse events, the most common being neutropenia (48%).
Almost half of the patient population (44%) required dose reductions of ibrutinib, most commonly because of atrial fibrillation, and 24% required dose reductions of venetoclax, most often because of neutropenia.
“Our data showed that combination therapy with ibrutinib and venetoclax was effective in patients with CLL, with no new toxic effects from the combination that were not reported previously for the individual agents,” the investigators wrote, adding that the efficacy findings were also “substantially better” than what has been reported with monotherapy for each of the agents in patients with CLL.
The study was funded by AbbVie, the University of Texas MD Anderson Cancer Center Chronic Lymphocytic Leukemia Moon Shot program, the Andrew Sabin Family Foundation, and the CLL Global Research Foundation. The investigators reported relationships with AbbVie, Incyte, Celgene, and other companies.
SOURCE: Jain N et al. N Engl J Med. 2019;380:2095-103.
A combination of venetoclax and ibrutinib may be a safe and effective treatment option for previously untreated elderly and high-risk patients with chronic lymphocytic leukemia (CLL), according to investigators of a phase 2 trial of the combination.
About 88% of patients achieved complete remission or complete remission with incomplete count recovery after 12 cycles of treatment, reported lead author Nitin Jain, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues.
There were no new safety signals for the combination of ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase, and venetoclax, a B-cell lymphoma 2 protein inhibitor, the investigators noted.
“This combination was reported to be safe and active in patients with mantle cell lymphoma,” they wrote in the New England Journal of Medicine. “Given the clinically complementary activity, preclinical synergism, and nonoverlapping toxic effects, we examined the safety and efficacy of combined ibrutinib and venetoclax treatment in previously untreated patients with CLL.”
In particular, the investigators recruited older patients, as this is a common population that can be challenging to treat. “Because CLL typically occurs in older adults, the majority of patients who need treatment are older than 65 years of age,” the investigators wrote. “This group of patients often has unacceptable side effects and has a lower rate of complete remission and undetectable minimal residual disease with chemoimmunotherapy than younger patients.”
The open-label, phase 2 trial enrolled 80 elderly and high-risk patients with previously untreated CLL. Eligibility required an age of at least 65 years or presence of at least one high-risk genetic feature; namely, mutated TP53, unmutated IgVH, or chromosome 11q deletion.
In order to reduce the risk of tumor lysis syndrome, ibrutinib (420 mg once daily) was given as monotherapy for three 28-day cycles. From the fourth cycle onward, venetoclax was also given, with weekly dose escalations to a target dose of 400 mg once daily. The combination was given for 24 cycles, with treatment continuation offered to patients who were still positive for minimal residual disease.
The median patient age was 65 years, with 30% of the population aged 70 years or older. A large majority (92%) had at least one high-risk genetic feature.
Following initiation with three cycles of ibrutinib, most patients had partial responses, the investigators wrote; however, with the addition of venetoclax, responses improved over time. Of all 80 patients, 59 (74%) had a best response of complete remission or complete remission with incomplete count recovery.
After six cycles, 51 out of 70 patients (73%) achieved this marker. After 12 cycles, 29 of 33 patients (88%) had this response, with 61% of the same group demonstrating undetectable minimal residual disease in bone marrow.
After 18 cycles, 25 of 26 patients (96%) had complete remission or complete remission with incomplete count recovery, 18 of which (69%) were negative for minimal residual disease. Three patients completed 24 cycles of combined therapy, all of whom achieved complete remission or complete remission with incomplete count recovery and undetectable minimal residual disease.
Focusing on patients aged 65 years or older, 74% had complete remission or complete remission with incomplete count recovery after six cycles of therapy and nearly half (44%) had undetectable minimal residual disease. After 12 cycles, these rates increased to 94% and 76%, respectively. Responses were also seen across genetically high-risk subgroups.
One patient died from a cryptococcal infection of the central nervous system; this was deemed unrelated to treatment, as symptoms began prior to initiation of treatment and only one dose of ibrutinib was given.
The estimated 1-year progression-free survival rate was 98% and the estimated overall survival rate was 99%. At the time of publication, no patients had disease progression.
Among all patients, 60% experienced grade 3 or higher adverse events, the most common being neutropenia (48%).
Almost half of the patient population (44%) required dose reductions of ibrutinib, most commonly because of atrial fibrillation, and 24% required dose reductions of venetoclax, most often because of neutropenia.
“Our data showed that combination therapy with ibrutinib and venetoclax was effective in patients with CLL, with no new toxic effects from the combination that were not reported previously for the individual agents,” the investigators wrote, adding that the efficacy findings were also “substantially better” than what has been reported with monotherapy for each of the agents in patients with CLL.
The study was funded by AbbVie, the University of Texas MD Anderson Cancer Center Chronic Lymphocytic Leukemia Moon Shot program, the Andrew Sabin Family Foundation, and the CLL Global Research Foundation. The investigators reported relationships with AbbVie, Incyte, Celgene, and other companies.
SOURCE: Jain N et al. N Engl J Med. 2019;380:2095-103.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: After 12 cycles of treatment with venetoclax and ibrutinib, 88% of patients had complete remission or complete remission with incomplete count recovery.
Study details: A randomized, open-label, phase 2 study involving 80 elderly and high-risk patients with chronic lymphocytic leukemia.
Disclosures: The study was funded by AbbVie, the University of Texas MD Anderson Cancer Center Chronic Lymphocytic Leukemia Moon Shot program, the Andrew Sabin Family Foundation, and the CLL Global Research Foundation. The investigators reported relationships with AbbVie, Incyte, Celgene, and other companies.
Source: Jain N et al. N Engl J Med. 2019;380:2095-103.
Daratumumab regimen shows benefit in transplant-ineligible myeloma
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
The findings from the phase 3 MAIA trial highlight the “superior efficacy” of adding daratumumab to lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, Jacob Laubach, MD, commented in an accompanying editorial.
Dr. Laubach noted several important clinical implications of the study findings, including that the use of CD38-targeting monoclonal antibody therapy was associated with a significant improvement in the number of patients who had a complete response to therapy and who were negative for minimal residual disease.
However, with daratumumab as a component of induction and maintenance therapy for patients with multiple myeloma who are ineligible for transplantation, it is important to consider the feasibility of retreatment with CD38-targeting therapy in patients who become resistant to daratumumab-containing regimens.
Jacob Laubach, MD, is at the Dana-Farber Cancer Institute in Boston. He reported having no financial disclosures. He made his remarks in an editorial in the New England Journal of Medicine (2019;380:2172-3).
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation (ASCT), adding daratumumab to lenalidomide and dexamethasone provides better outcomes than standard therapy alone, based on an interim analysis from the phase 3 MAIA trial.
A greater proportion of patients in the daratumumab group had complete responses and were alive without disease progression after a median follow-up of 28 months, reported lead author Thierry Facon, MD, of the University of Lille (France) and colleagues, who also noted that daratumumab was associated with higher rates of grade 3 or 4 pneumonia, neutropenia, and lymphopenia.
“For patients who are ineligible for stem-cell transplantation, multiagent regimens, including alkylating agents, glucocorticoids, immunomodulatory drugs, proteasome inhibitors, and new agents, are the standard of care,” the investigators wrote in the New England Journal of Medicine.
The findings from MAIA add clarity to the efficacy and safety of daratumumab in this setting, building on previous phase 3 myeloma trials in the same area, such as ALCYONE, CASTOR, and POLLUX, the investigators noted.
MAIA was an open-label, international trial involving 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT. Patients were randomized in a 1:1 ratio to receive either daratumumab, lenalidomide, and dexamethasone (daratumumab group; n = 368) or lenalidomide and dexamethasone alone (control group; n = 369).
On a 28-day cycle, all patients received oral lenalidomide 25 mg on days 1-21 and oral dexamethasone 40 mg on days 1, 8, 15, and 22. Patients in the daratumumab group received intravenous daratumumab dosed at 16 mg/kg once a week for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks thereafter. Treatment was continued until unacceptable toxic effects or disease progression occurred.
The primary end point was progression-free survival (PFS). Various secondary end points were also evaluated, including time to progression, complete responses, overall survival, and others.
Among the 737 randomized patients, 729 ultimately underwent treatment. The median patient age was 73 years.
Generally, efficacy measures favored adding daratumumab. After a median follow-up of 28.0 months, disease progression or death had occurred in 26.4% of patients in the daratumumab group, compared with 38.8% in the control group.
The median PFS was not reached in the daratumumab group, compared with 31.9 months in the control group. There was a 44% lower risk of disease progression or death among patients who received daratumumab, compared with the control group (hazard ratio, 0.56, P less than .001).
This PFS trend was consistent across most subgroups, including those for sex, age, and race, with the exception of patients with baseline hepatic impairment.
Additional efficacy measures added weight to the apparent benefit of adding daratumumab. For instance, more patients in the daratumumab group achieved a complete response or better (47.6% vs. 24.9%) and were negative for minimum residual disease (24.2% vs. 7.3%).
In terms of safety, more patients in the daratumumab group than the control group developed grade 3 or higher neutropenia (50% vs. 35.3%), lymphopenia (15.1% vs. 10.7%), infections (32.1% vs. 23.3%) or pneumonia (13.7% vs. 7.9%).
In contrast, grade 3 or 4 anemia was less common in the daratumumab group than the control group (11.8% vs. 19.7%). Overall, the rate of serious adverse events was similar for both groups (approximately 63%), as was the rate of adverse events resulting in death (approximately 6%-7%).
“In this trial involving patients with newly diagnosed multiple myeloma who were ineligible for stem-cell transplantation, the addition of daratumumab to lenalidomide and dexamethasone resulted in significantly longer progression-free survival, a higher response rate, an increased depth of response, and a longer duration of response than lenalidomide and dexamethasone alone,” the investigators concluded.
The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
SOURCE: Facon T et al. N Engl J Med. 2019;380:2104-15.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: For patients with newly diagnosed multiple myeloma who are ineligible for autologous stem cell transplantation, adding daratumumab to lenalidomide and dexamethasone standard therapy provides better outcomes than standard therapy alone.
Major finding: After 28-month follow-up, 26.4% of patients in the daratumumab group had disease progression or died, compared with 38.8% in the control group.
Study details: A randomized, open-label, phase 3 trial involving 737 patients with newly diagnosed multiple myeloma.
Disclosures: The study was funded by Janssen Research and Development. The investigators reported relationships with Janssen, Celgene, Takeda, Sanofi, and other companies.
Source: Facon T et al. N Engl J Med. 2019;380:2104-15.
FFR changes coronary management in one-third of patients
LAS VEGAS – Interventional cardiologists who used fractional flow reserve to assess coronary lesions with an uncertain hemodynamic impact by angiography alone changed their initial therapeutic decision based on angiography for 35% of patients, and for 30% of all lesions examined in a real-world registry with more than 2,200 patients enrolled at 70 worldwide centers.
“Use of fractional flow reserve in contemporary, real-world, global clinical practice changed treatment plans for more than one-third of all comers,” including both patients with stable coronary artery disease and those with acute coronary syndrome, Erick Schampaert, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The impact of fractional flow reserve (FFR) was greatest when operators used it to assess nonculprit lesions among the 31% of the 2,217 total patients enrolled who presented with acute coronary syndrome. In this subgroup, FFR changed the treatment plan for nonculprit lesions that had been based on angiography and clinical status for 36% of these lesions. The changes included an increase in lesions identified to receive medical management, rising from 53% of the nonculprit lesions before FFR to 65% after, while treatment with percutaneous coronary intervention (PCI) fell from 37% of nonculprit lesions before FFR to 28% after, with the remaining lesions designated for coronary artery bypass grafting. Among patients with stable coronary disease the angiography-based treatment decision changed for 28% of nonculprit lesions after FFR.
“These results may provide support to increase use of FFR,” said Dr. Schampaert, an interventional cardiologist and head of cardiology at Hôpital du Sacré-Cœur in Montreal. The analysis “was an attempt to see the current impact of FFR at places where its use is established,” when it’s routinely used to assess the need to treat nonculprit lesions with an uncertain impact on blood flow through a coronary artery. Dr. Schampaert estimated that about one-quarter of patients who present for angiography have nonculprit lesions that leave operators uncertain about their hemodynamic significance after angiography and are candidates for FFR assessment.
The findings “are a call to do more FFR,” agreed M. Chadi Alraies, MD, an interventional cardiologist at the Detroit Medical Center Heart Hospital. “We are underusing FFR and overstenting people, and that worsens outcomes. We don’t do enough FFR,” Dr. Alraies commented.
“We’ve known for some time that angiography alone can lead to overtreatment,” commented Philippe Généreux, MD, an interventional cardiologist at Morristown (N.J.) Medical Center. “With FFR, physiology is the key to optimizing outcomes.”
The PRESSUREwire study included 2,217 consecutive patients who underwent FFR assessment at 70 centers in 15 countries during October 2016–February 2018. The only exclusions were patients with extremely tortuous or calcified arteries or patients with a bypass graft to the target vessel. Enrolled patients averaged 65 years of age, and three-quarters were men; 63% had stable coronary disease, 31% had acute coronary syndrome, and the remainder had silent ischemia documented by noninvasive testing. A stenosis of 50%-69% occluded 54% of the tested coronaries; 24% had a 70%-90% occlusion; 20% had an occlusion of less than 50%; and the remaining patients had an occlusion of more than 90%.
While the overall percentage of patients whose treatment plan changed following FFR assessment shifted moderately, the changes within each treatment category were more striking. For example, among the 62% of all patients initially designated for medical management based on angiography, the FFR findings changed the management plan to PCI in 19% of this subgroup. Conversely, among the 33% of all patients initially designated for PCI based on angiography, 52% instead received medical management based only on their FFR results. Because shifts in treatment strategy following FFR had some patients go from medical management to PCI, and others went from PCI to medical, overall the percentage of patients who received medical management without immediate revascularization had just a modest up-tick, from 62% before FFR to 67% after, Dr. Schampaert said.
PRESSUREwire was funded by Abbott Vascular, a company that markets an FFR device. Dr. Schampaert has been a consultant to Abbott Vascular as well as AstraZeneca, Bayer, Medtronic, Volcano-Philips, Sanofi, and Servier. Dr. Alaries had no disclosures. Dr. Généreux has been a consultant to Abbott Vascular and to several other companies, and he has an equity interest in Saranas.
SOURCE: Schampaert E et al. SCAI 2019, Abstract.
LAS VEGAS – Interventional cardiologists who used fractional flow reserve to assess coronary lesions with an uncertain hemodynamic impact by angiography alone changed their initial therapeutic decision based on angiography for 35% of patients, and for 30% of all lesions examined in a real-world registry with more than 2,200 patients enrolled at 70 worldwide centers.
“Use of fractional flow reserve in contemporary, real-world, global clinical practice changed treatment plans for more than one-third of all comers,” including both patients with stable coronary artery disease and those with acute coronary syndrome, Erick Schampaert, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The impact of fractional flow reserve (FFR) was greatest when operators used it to assess nonculprit lesions among the 31% of the 2,217 total patients enrolled who presented with acute coronary syndrome. In this subgroup, FFR changed the treatment plan for nonculprit lesions that had been based on angiography and clinical status for 36% of these lesions. The changes included an increase in lesions identified to receive medical management, rising from 53% of the nonculprit lesions before FFR to 65% after, while treatment with percutaneous coronary intervention (PCI) fell from 37% of nonculprit lesions before FFR to 28% after, with the remaining lesions designated for coronary artery bypass grafting. Among patients with stable coronary disease the angiography-based treatment decision changed for 28% of nonculprit lesions after FFR.
“These results may provide support to increase use of FFR,” said Dr. Schampaert, an interventional cardiologist and head of cardiology at Hôpital du Sacré-Cœur in Montreal. The analysis “was an attempt to see the current impact of FFR at places where its use is established,” when it’s routinely used to assess the need to treat nonculprit lesions with an uncertain impact on blood flow through a coronary artery. Dr. Schampaert estimated that about one-quarter of patients who present for angiography have nonculprit lesions that leave operators uncertain about their hemodynamic significance after angiography and are candidates for FFR assessment.
The findings “are a call to do more FFR,” agreed M. Chadi Alraies, MD, an interventional cardiologist at the Detroit Medical Center Heart Hospital. “We are underusing FFR and overstenting people, and that worsens outcomes. We don’t do enough FFR,” Dr. Alraies commented.
“We’ve known for some time that angiography alone can lead to overtreatment,” commented Philippe Généreux, MD, an interventional cardiologist at Morristown (N.J.) Medical Center. “With FFR, physiology is the key to optimizing outcomes.”
The PRESSUREwire study included 2,217 consecutive patients who underwent FFR assessment at 70 centers in 15 countries during October 2016–February 2018. The only exclusions were patients with extremely tortuous or calcified arteries or patients with a bypass graft to the target vessel. Enrolled patients averaged 65 years of age, and three-quarters were men; 63% had stable coronary disease, 31% had acute coronary syndrome, and the remainder had silent ischemia documented by noninvasive testing. A stenosis of 50%-69% occluded 54% of the tested coronaries; 24% had a 70%-90% occlusion; 20% had an occlusion of less than 50%; and the remaining patients had an occlusion of more than 90%.
While the overall percentage of patients whose treatment plan changed following FFR assessment shifted moderately, the changes within each treatment category were more striking. For example, among the 62% of all patients initially designated for medical management based on angiography, the FFR findings changed the management plan to PCI in 19% of this subgroup. Conversely, among the 33% of all patients initially designated for PCI based on angiography, 52% instead received medical management based only on their FFR results. Because shifts in treatment strategy following FFR had some patients go from medical management to PCI, and others went from PCI to medical, overall the percentage of patients who received medical management without immediate revascularization had just a modest up-tick, from 62% before FFR to 67% after, Dr. Schampaert said.
PRESSUREwire was funded by Abbott Vascular, a company that markets an FFR device. Dr. Schampaert has been a consultant to Abbott Vascular as well as AstraZeneca, Bayer, Medtronic, Volcano-Philips, Sanofi, and Servier. Dr. Alaries had no disclosures. Dr. Généreux has been a consultant to Abbott Vascular and to several other companies, and he has an equity interest in Saranas.
SOURCE: Schampaert E et al. SCAI 2019, Abstract.
LAS VEGAS – Interventional cardiologists who used fractional flow reserve to assess coronary lesions with an uncertain hemodynamic impact by angiography alone changed their initial therapeutic decision based on angiography for 35% of patients, and for 30% of all lesions examined in a real-world registry with more than 2,200 patients enrolled at 70 worldwide centers.
“Use of fractional flow reserve in contemporary, real-world, global clinical practice changed treatment plans for more than one-third of all comers,” including both patients with stable coronary artery disease and those with acute coronary syndrome, Erick Schampaert, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The impact of fractional flow reserve (FFR) was greatest when operators used it to assess nonculprit lesions among the 31% of the 2,217 total patients enrolled who presented with acute coronary syndrome. In this subgroup, FFR changed the treatment plan for nonculprit lesions that had been based on angiography and clinical status for 36% of these lesions. The changes included an increase in lesions identified to receive medical management, rising from 53% of the nonculprit lesions before FFR to 65% after, while treatment with percutaneous coronary intervention (PCI) fell from 37% of nonculprit lesions before FFR to 28% after, with the remaining lesions designated for coronary artery bypass grafting. Among patients with stable coronary disease the angiography-based treatment decision changed for 28% of nonculprit lesions after FFR.
“These results may provide support to increase use of FFR,” said Dr. Schampaert, an interventional cardiologist and head of cardiology at Hôpital du Sacré-Cœur in Montreal. The analysis “was an attempt to see the current impact of FFR at places where its use is established,” when it’s routinely used to assess the need to treat nonculprit lesions with an uncertain impact on blood flow through a coronary artery. Dr. Schampaert estimated that about one-quarter of patients who present for angiography have nonculprit lesions that leave operators uncertain about their hemodynamic significance after angiography and are candidates for FFR assessment.
The findings “are a call to do more FFR,” agreed M. Chadi Alraies, MD, an interventional cardiologist at the Detroit Medical Center Heart Hospital. “We are underusing FFR and overstenting people, and that worsens outcomes. We don’t do enough FFR,” Dr. Alraies commented.
“We’ve known for some time that angiography alone can lead to overtreatment,” commented Philippe Généreux, MD, an interventional cardiologist at Morristown (N.J.) Medical Center. “With FFR, physiology is the key to optimizing outcomes.”
The PRESSUREwire study included 2,217 consecutive patients who underwent FFR assessment at 70 centers in 15 countries during October 2016–February 2018. The only exclusions were patients with extremely tortuous or calcified arteries or patients with a bypass graft to the target vessel. Enrolled patients averaged 65 years of age, and three-quarters were men; 63% had stable coronary disease, 31% had acute coronary syndrome, and the remainder had silent ischemia documented by noninvasive testing. A stenosis of 50%-69% occluded 54% of the tested coronaries; 24% had a 70%-90% occlusion; 20% had an occlusion of less than 50%; and the remaining patients had an occlusion of more than 90%.
While the overall percentage of patients whose treatment plan changed following FFR assessment shifted moderately, the changes within each treatment category were more striking. For example, among the 62% of all patients initially designated for medical management based on angiography, the FFR findings changed the management plan to PCI in 19% of this subgroup. Conversely, among the 33% of all patients initially designated for PCI based on angiography, 52% instead received medical management based only on their FFR results. Because shifts in treatment strategy following FFR had some patients go from medical management to PCI, and others went from PCI to medical, overall the percentage of patients who received medical management without immediate revascularization had just a modest up-tick, from 62% before FFR to 67% after, Dr. Schampaert said.
PRESSUREwire was funded by Abbott Vascular, a company that markets an FFR device. Dr. Schampaert has been a consultant to Abbott Vascular as well as AstraZeneca, Bayer, Medtronic, Volcano-Philips, Sanofi, and Servier. Dr. Alaries had no disclosures. Dr. Généreux has been a consultant to Abbott Vascular and to several other companies, and he has an equity interest in Saranas.
SOURCE: Schampaert E et al. SCAI 2019, Abstract.
REPORTING FROM SCAI 2019
HPV vaccine: Is one dose enough?
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – There is good news and bad news about human papillomavirus (HPV) vaccination as a means of preventing cervical cancer.
The bad news is the HPV vaccines are projected to be in short supply, unable to meet global demand until at least 2024. The good news is that – in one study, for 11 years and counting – which would effectively double the existing supply, Aimee R. Kreimer, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
These data come from post hoc analyses of major phase 3 randomized controlled trials of bivalent HPV vaccine in Costa Rica and quadrivalent vaccine in India. However, these secondary analyses aren’t considered rock solid evidence because the subjects who got a single dose weren’t randomized to that strategy, they simply for one reason or another didn’t receive the recommended additional dose or doses.
“I don’t know if these studies are enough, so several studies have been launched over the past couple of years with an eye toward generating the quality of data that would be sufficient to motivate policy change, if in fact one dose is proven to be effective,” said Dr. Kreimer, a senior scientist at the National Cancer Institute in Bethesda, Md.
The first of these formal randomized, controlled trials – a delayed second-dose study in 9- to 11-year-old U.S. boys and girls – is due to be completed next year. Four other trials ongoing in Africa and Costa Rica, all in females, are expected to report findings in 2022-2025.
Dr. Kreimer is first author of a soon-to-be-published 11-year update from the phase 3 Costa Rica HPV Vaccine Trial, which was launched prior to licensure of the GlaxoSmithKline bivalent HPV vaccine. Previous analyses showed that at both 4 and 7 years of follow-up, a single dose of the vaccine was as effective as two or three in preventing infection with HPV types 16 and 18, which are covered by the vaccine.
“Now the research question has transitioned to, ‘Will one dose be sufficiently durable?’ she explained.
The answer from this study is yes. At 11 years since receipt of the bivalent HPV vaccine, there was no difference in terms of prevalent HPV 16/18 infection between the one-, two-, and three-dose groups. To address the issue of possible selection bias in this post hoc nonrandomized comparison, Dr. Kreimer and her coinvestigators looked at rates of infection with HPV 31 and 45, which aren’t covered by the vaccine. The rates were similar regardless of the number of vaccine doses received 11 years earlier, indicating women in all three dosing groups are at similar risk for acquiring HPV infection, thus bolstering the legitimacy of the conclusion that one dose provides effective long-term protection.
Intriguingly, HPV serum antibody levels in the single-dose group have remained stable for 11 years at a level that’s only about one-quarter of that associated with three doses of the vaccine, albeit an order of magnitude greater than the level induced by natural immunity.
“This really challenges the dogma of the HPV vaccine,” according to Dr. Kreimer. “It suggests that inferior [HPV] antibodies do not necessarily mean inferior protection.”
The explanation for this phenomenon appears to be that HPV subunit vaccine mimics the shell of authentic virions so well that the immune system sees it as dangerous and mounts long-term antibody production. Also, cervical infection by HPV is a relatively slow process, allowing time for vaccine-induced antibodies to interrupt it, she said.
In contrast to the encouraging findings from this post hoc analysis and another from a phase 3 trial of quadrivalent vaccine in India, numerous phase 4 vaccine effectiveness monitoring studies have shown markedly lower vaccine effectiveness for one dose of HPV vaccine. Dr. Kreimer cautioned that this is a flawed conclusion attributable to a methodologic artifact whereby the investigators have lumped together single-dose recipients who were 17 years old or more at the time with those who were younger.
“The problem is that many people who are aged 17-18 years already have HPV infection, so when they are vaccinated it shows up as a vaccine failure. That’s not correct. These are prophylactic HPV vaccines. They’re not meant to help clear an infection,” she noted.
Stepping back, Dr. Kreimer observed that cervical cancer “is really a story of inequality.” Indeed, 90% of cervical cancers occur in low-income countries, where HPV vaccination uptake remains very low even more than a decade after licensure. When modelers project out in the future, they estimate that at current HPV vaccination levels in Sub-Saharan Africa, which has the highest cervical cancer rates in the world, it would take more than 100 years to achieve the World Health Organization goal of eliminating the malignancy.
Asked by an audience member how low a single-dose vaccine effectiveness level she considers acceptable to help reach the goal of eliminating cervical cancer in developing countries, Dr. Kreimer cautioned against the tendency to let ‘perfect’ become the enemy of ‘good.’
“I’ll remind everyone that, in this moment, very few of the target girls in the lower– and upper-lower–income countries are getting any vaccination. So I don’t think it’s a question of whether we should be going from two to one dose, I think it’s really a question of, for those who are at zero doses, how do we get them one dose? And with the HPV vaccine, we’ve even seen suggestions of herd immunity if we have 50% uptake,” she replied.
Dr. Kreimer reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019
Maternal mortality: Critical next steps in addressing the crisis
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1
A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
Melanocytic Matrical Carcinoma in a Solid-Organ Transplant Recipient
To the Editor:
A 68-year-old white man presented with a firm, gradually enlarging, mildly tender, grayish black papule with central ulceration on the left dorsal wrist of 4 months’ duration (Figure 1). His relevant medical history included multiple basal cell carcinomas (BCCs) and squamous cell carcinomas, as well as a single-lung transplant 2 years prior, for which he was on chronic immunosuppressive therapy with azathioprine, everolimus, tacrolimus, and prednisone. The clinical differential diagnosis included pigmented BCC, malignant melanoma, and ulcerated squamous cell carcinoma.
Histologic examination of the lesion (Figure 2) demonstrated irregular nodules of basaloid tumor cells with rounded nuclei, visible nucleoli, and scant cytoplasm involving the dermis. The tumor produced abrupt matrical-type keratinization, forming ghost cells. The lesion also contained frequent mitotic figures, apoptotic cells, focal areas of necrosis, and abundant melanin pigment. Admixed throughout the lesion were pigmented and dendritic melanocytic cells. The overlying epidermis was focally ulcerated with an adjacent localized connection between the tumor and the epidermis. Keratinocyte atypia was found in the surrounding epidermis, which contained melanophages, solar elastosis, and scattered chronic inflammatory cells. An immunohistochemical study (Figure 3) for tyrosinase demonstrated abundant admixed melanocytic cells. β-Catenin expression was shown in both nuclear and cytoplasmic distributions, and there was focal labeling on BerEP4 staining.
The lesion was subsequently treated with wide local excision. The patient has not had recurrence to date.
Melanocytic matricoma (MM), a rare adnexal tumor, was first described in 1999 by Carlson et al.1 A PubMed search of articles indexed for MEDLINE using the terms melanocytic and matricoma yielded 24 reported cases in the English-language literature.1-17 It consists of an admixed population of basaloid matrical and supramatrical cells, ghost cells, and dendritic melanocytes in a well-circumscribed dermal nodule, typically without epidermal or adnexal connection. In comparison to the more commonly described pilomatricoma, which can be uncommonly pigmented, MM typically has only focal areas of ghost cells and lacks cystic architecture.1,9,10,18 A granulomatous reaction to keratinaceous debris is variably present.1,9,10 Histologically, the scattered dendritic melanocytes are classically benign, but cases demonstrating melanocyte atypia have been reported.10,13 Melanocytic matricoma appears most commonly as a black or gray papule on sun-damaged skin in older men and tends not to recur following complete excision; thus, MM is considered to be a clinically benign neoplasm. Given the demographics and distribution of the lesions, exposure to UV radiation is thought to play a contributory role in the pathogenesis.2,10,19 Melanocytic matricoma is believed to recapitulate the hair follicle in the anagen phase, where there is close interplay between matrical keratinocytes and melanocytes prior to cessation of melanogenesis during the catagen phase.5,6,8,20,21 Evidence demonstrating highly conserved β-catenin and downstream lymphoid enhancer binding factor 1 (LEF1) expression, as well as pleckstrin homology-like domain, family A, member 1 (PHLDA1) expression (as a marker for follicular stem cells), points to constitutive activity in the Wnt signaling pathway in follicular stem cells of the bulge area as a major agent of tumorigenesis.12
Melanocytic matrical carcinoma, also known as malignant MM or matrical carcinoma with melanocytic hyperplasia, may be considered the malignant counterpart to MM.22 A PubMed search of articles indexed for MEDLINE using the terms melanocytic matrical carcinoma, malignant melanocytic matricoma, and matrical carcinoma with melanocytic hyperplasia, with review of references to identify additional citations, yielded 13 reported cases of MMC in the English-language literature (Table).19,22-30 As with MM, MMC is a biphasic tumor with basaloid matrical and supramatrical cells; focal areas of ghost cells; and admixed, banal-appearing dendritic melanocytes. However, the basaloid component also demonstrates nuclear atypia, mitoses, occasional ulceration, and variably poor circumscription. Clinically these lesions can mimic pigmented BCC, malignant melanoma, or other malignant adnexal tumors.25 Their natural history is unknown due to few reported cases, but they can be correlated with matrical carcinomas, which were first described by Weedon et al31 in 1980. A summary of more than 130 cases of matrical carcinomas in the English-language literature found that MMCs have high rates of local recurrence and metastasize in approximately 13% of cases. Wide local excision demonstrated lower rates of recurrence than simple excision (23% vs 83%), but there were insufficient cases to determine the incidence following Mohs micrographic surgery.32 Melanocytic matrical carcinomas also demonstrate mutations in the β-catenin pathway,pointing to a similar pathogenesis as their benign counterparts or perhaps direct malignant transformation.25,33,34
A subset of MMCs are combined cutaneous tumors (CCTs) consisting of epithelial neoplasms in close association with malignant melanocytes. Two of the more common variants include dermal squamomelanocytic tumors, a term first used by Pool et al,35 and malignant basomelanocytic tumors, as named by Erickson et al,36 but trichoblastomelanomas and other types have been documented.37 Although CCTs typically occur in the same patient populations as MMCs, namely elderly white men with chronically sun-damaged skin,they exhibit several important distinctions.37-39 By definition, CCTs have a malignant melanocytic component, whereas melanocytes are nonneoplastic in MMCs. The pathogenesis may differ as well. Various mechanisms for the close association of epithelial tumors and melanoma have been proposed, including field cancerization, tumor collision, tumor-tumor metastases, tumor colonization, and others, though CCTs likely arise through combinations of these processes depending upon their subtype.37-39 Paracrine signaling may play an important role in the pathogenesis of both tumors.5,6,8,38 As with MMCs, the prognosis of CCTs is limited by relatively few reported cases. Despite advanced Breslow depths in many cases, these tumors display more indolent behavior suggestive of melanoma in situ rather than invasive melanoma, perhaps due to dependence upon epithelial paracrine factors.37,39-42
Solid-organ transplant recipients have higher rates of more aggressive malignancies, of which skin cancer is the most common.43-49 Squamous cell carcinoma of the skin accounts for 95% of cutaneous malignancies in this population and occurs at approximately 65 times the rate of the general population.50 The risk of other skin cancers also is increased, though less dramatically, including BCC (10-fold increased risk) and melanoma (2- to 8-fold increased risk).46,50-53 The cause likely is multifactorial, including older age, history of skin cancer pretransplant, more than 5 years posttransplant, male sex, and incrementally as Fitzpatrick skin type decreases from VI to I.54-56 Immunosuppressive therapy also plays a role in tumorigenesis. Azathioprine metabolites have specifically been implicated in UVA radiation–induced promutagenic oxidative damage to DNA.57 Other studies have found no significant differences in the type of immunosuppressant used but instead have correlated rates of skin cancer to overall immunosuppression.48,55,58 Lung transplant recipients in particular demonstrate high rates of cutaneous malignancy, likely due in part to the necessity of more potent immunosuppressive regimens. Nearly one-third of patients develop a cutaneous malignancy by 5 years and nearly half by 10 years posttransplant.55
We report a rare case of MMC in a solid-organ transplant recipient. We hypothesize that the combination of UV radiation exposure–induced photodamage acquired pretransplant in addition to an aggressive immunosuppressive regimen with azathioprine and other agents posttransplant contributed to the development of this patient’s rare malignancy. Although rare, these tumors should remain in the differential diagnosis of clinicians and pathologists caring for this unique patient population.
- Carlson JA, Healy K, Slominski A, et al. Melanocytic matricoma: a report of two cases of a new entity. Am J Dermatopathol. 1999;21:344-349.
- Rizzardi C, Brollo A, Colonna A, et al. A tumor with composite pilo-folliculosebaceous differentiation harboring a recently described new entity—melanocytic matricoma. Am J Dermatopathol. 2002;24:493-497.
- Williams CM, Bozner P, Oliveri CV, et al. Melanocytic matricoma: case confirmation of a recently described entity. J Cutan Pathol. 2003;30:275-278.
- Horenstein MG, Kahn AG. Pathologic quiz case: a 69-year-old man with a brown-black facial papule. melanocytic matricoma. Arch Pathol Lab Med. 2004;128:e163-e164.
- Soler AP, Burchette JL, Bellet JS, et al. Cell adhesion protein expression in melanocytic matricoma. J Cutan Pathol. 2007;34:456-460.
- Islam MN, Bhattacharyya I, Proper SA, et al. Melanocytic matricoma: a distinctive clinicopathologic entity. Dermatol Surg. 2007;33:857-863.
- Monteagudo B, Requena L, Used-Aznar MM, et al. Melanocytic matricoma. Actas Dermosifiliogr. 2008;99:573-582.
- Cartaginese F, Sidoni A. Melanocytic matricoma. report of a further case with clinicopathological and immunohistochemical findings, differential diagnosis and review of the literature. Histol Histopathol. 2010;25:713-717.
- Tallon B, Cerroni L. Where pigmented pilomatricoma and melanocytic matricoma collide. Am J Dermatopathol. 2010;32:769-773.
- Zussman J, Sheth S, Ra SH, et al. Melanocytic matricoma with melanocytic atypia: report of a unique case and review of the literature. Am J Dermatopathol. 2011;33:508-512.
- Tanboon J, Manonukul J, Pattanaprichakul P. Melanocytic matricoma: two cases of a rare entity in women. J Cutan Pathol. 2014;41:775-782.
- Battistella M, Carlson JA, Oslo A, et al. Skin tumors with matrical differentiation: lessons from hair keratins, beta-catenin and PHLDA-1 expression. J Cutan Pathol. 2014;41:427-436.
- Barrado-Solis N, Moles-Poveda P, Roca-Estelles MJ, et al. Melanocytic matricoma with melanocytic atypia: report of a new case [published online February 11, 2015]. J Eur Acad Dermatol Venereol. 2016;30:859-860.
- Pagliarello C, Stanganelli I, Ricci R, et al. A pinkish-blue exophytic nodule on the arm of an elderly man: a quiz. melanocytic matricoma. Acta Derm Venereol. 2017;97:1261-1262.
- Winslow CY, Camacho I, Nousari CH. Melanocytic matricoma with consumption of the epidermis: an atypical histologic attribute or a malignant variant? Am J Dermatopathol. 2017;39:907-909.
- Sangiorgio V, Moneghini L, Tosi D, et al. A case of melanocytic matricoma with prominent mitotic activity and melanocytic hyperplasia. Int J Dermatol. 2018;57:e78-e81.
- Song J, Lu S, Wu Z. An unusual case of melanocytic matricoma in a young pregnant woman. Australas J Dermatol. 2019;60:140-141.
- Ishida M, Okabe H. Pigmented pilomatricoma: an underrecognized variant. Int J Clin Exp Pathol. 2013;6:1890-1893.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S-97S.
- De Berker D, Higgins CA, Jahada C, et al. Biology of hair and nails. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1075-1092.
- Monteagudo C, Fernandez-Figueras MT, San Juan J, et al. Matrical carcinoma with prominent melanocytc hyperplasia (malignant melanocytic matricoma?). Am J Dermatopathol. 2003;25:485-489.
- Sloan JB, Sueki H, Jaworsky C. Pigmented malignant pilomatrixoma: report of a case and review of the literature. J Cutan Pathol. 1992;19:240-246.
- Hardisson D, Linares MD, Cuevas-Santos J, et al. Pilomatrix carcinoma: a clinicopathologic study of six cases and review of the literature. Am J Dermatopathol. 2001;23:394-401.
- Soler AP, Kindel SE, McCloskey G, et al. Cell-cell adhesion proteins in melanocytic pilomatrix carcinoma. Rare Tumors. 2010;2:e43-e45.
- Ardakani NM, Palmer DL, Wood BA. Malignant melanocytic matricoma: a report of 2 cases and review of the literature. Am J Dermatopathol. 2016;38:33-38.
- Villada G, Romagosa R, Miteva M, et al. Matrical carcinoma with melanocytic proliferation and prominent squamoid whorls. Am J Dermatopathol. 2016;38:e11-e14.
- Ji C, Zhang Y, Heller P, et al. Melanocytic matrical carcinoma mimicking melanoma. Am J Dermatopathol. 2017;39:903-906.
- Nielson CB, Vincek V. Malignant melanocytic matricoma and criteria for malignancy. Open J Pathol. 2018;8:94-100.
- Lehmer L, Carly SK, de Feraudy S. Matrical carcinoma with melanocytic hyperplasia mimicking nodular melanoma in an elderly Mexican male. J Cutan Pathol. 2019;46:442-446.
- Weedon D, Bell J, Mayze J. Matrical carcinoma of the skin. J Cutan Pathol. 1980;7:39-42.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Hassanein AM, Glanz SM. Beta-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol. 2004;150:511-516.
- Pool SE, Manieei F, Clark WH Jr, et al. Dermal squamo-melanocytic tumor: a unique biphenotypic neoplasm of uncertain biological potential. Hum Pathol. 1999;30:525-529.
- Erickson LA, Myers JL, Mihm MC, et al. Malignant basomelanocytic tumor manifesting as metastatic melanoma. Am J Surg Pathol. 2004;28:1393-1396.
- Amin SM, Cooper C, Yelamos O, et al. Combined cutaneous tumors with a melanoma component: a clinical, histologic, and molecular study. J Am Acad Dermatol. 2015;73:451-460.
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603.
- Satter EK, Metcalf J, Lountzis N, et al. Tumors composed of malignant epithelial and melanocytic populations: a case series and review of the literature. J Cutan Pathol. 2009;36:211-219.
- Pouryazdanparast P, Yu L, Johnson T, et al. An unusual squamo-melanocytic tumor of uncertain biologic behavior: a variant of melanoma? Am J Dermatopathol. 2009;31:457-461.
- Burkhalter A, White W. Malignant melanoma in situ colonizing basal cell carcinoma: a simulator of invasive melanoma. Am J Dermatopathol. 1997;19:303-307.
- Papa G, Grandi G, Pascone M. Collision tumor of malignant skin cancers: a case of melanoma in basal cell carcinoma. Pathol Res Pract. 2006;202:691-694.
- Miao Y, Everly JJ, Gross TG, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347-1359.
- Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. a follow-up study. Transplantation. 1996;61:715-721.
- Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1-17.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:253-261.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-273.
- DePry JL, Reed KB, Cook-Harris RH, et al. Iatrogenic immunosuppression and cutaneous malignancy. Clin Dermatol. 2011;29:602-613.
- Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622-1630.
- Jensen P, Hansen S, Møller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177-186.
- Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-913.
- Hollenbeak CS, Todd MM, Billingsley EM, et al. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005;104:1962-1967.
- Le Mire L, Hollowood K, Gray D, et al. Melanomas in renal transplant recipients. Br J Dermatol. 2006;154:472-477.
- Gogia R, Binstock M, Hirose R, et al. Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol. 2013;68:585-591.
- Rashtak S, Dierkhising RA, Kremers WK, et al. Incidence and risk factors for skin cancer following lung transplantation. J Am Acad Dermatol. 2015;72:92-98.
- Ruiz DE, Luzuriaga AM, Hsieh C. Yearly burden of skin cancer in non-Caucasian and Caucasian solid-organ transplant recipients. J Clin Aesthet Dermatol. 2015;8:16-19.
- Perrett CM, Walker SL, O’Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol. 2008;159:198-204.
- Abou Ayache R, Thierry A, Bridoux F, et al. Long-term maintenance of calcineurin inhibitor monotherapy reduces the risk for squamous cell carcinomas after kidney transplantation compared with bi- or tritherapy. Transplant Proc. 2007;39:2592-2594.
To the Editor:
A 68-year-old white man presented with a firm, gradually enlarging, mildly tender, grayish black papule with central ulceration on the left dorsal wrist of 4 months’ duration (Figure 1). His relevant medical history included multiple basal cell carcinomas (BCCs) and squamous cell carcinomas, as well as a single-lung transplant 2 years prior, for which he was on chronic immunosuppressive therapy with azathioprine, everolimus, tacrolimus, and prednisone. The clinical differential diagnosis included pigmented BCC, malignant melanoma, and ulcerated squamous cell carcinoma.
Histologic examination of the lesion (Figure 2) demonstrated irregular nodules of basaloid tumor cells with rounded nuclei, visible nucleoli, and scant cytoplasm involving the dermis. The tumor produced abrupt matrical-type keratinization, forming ghost cells. The lesion also contained frequent mitotic figures, apoptotic cells, focal areas of necrosis, and abundant melanin pigment. Admixed throughout the lesion were pigmented and dendritic melanocytic cells. The overlying epidermis was focally ulcerated with an adjacent localized connection between the tumor and the epidermis. Keratinocyte atypia was found in the surrounding epidermis, which contained melanophages, solar elastosis, and scattered chronic inflammatory cells. An immunohistochemical study (Figure 3) for tyrosinase demonstrated abundant admixed melanocytic cells. β-Catenin expression was shown in both nuclear and cytoplasmic distributions, and there was focal labeling on BerEP4 staining.
The lesion was subsequently treated with wide local excision. The patient has not had recurrence to date.
Melanocytic matricoma (MM), a rare adnexal tumor, was first described in 1999 by Carlson et al.1 A PubMed search of articles indexed for MEDLINE using the terms melanocytic and matricoma yielded 24 reported cases in the English-language literature.1-17 It consists of an admixed population of basaloid matrical and supramatrical cells, ghost cells, and dendritic melanocytes in a well-circumscribed dermal nodule, typically without epidermal or adnexal connection. In comparison to the more commonly described pilomatricoma, which can be uncommonly pigmented, MM typically has only focal areas of ghost cells and lacks cystic architecture.1,9,10,18 A granulomatous reaction to keratinaceous debris is variably present.1,9,10 Histologically, the scattered dendritic melanocytes are classically benign, but cases demonstrating melanocyte atypia have been reported.10,13 Melanocytic matricoma appears most commonly as a black or gray papule on sun-damaged skin in older men and tends not to recur following complete excision; thus, MM is considered to be a clinically benign neoplasm. Given the demographics and distribution of the lesions, exposure to UV radiation is thought to play a contributory role in the pathogenesis.2,10,19 Melanocytic matricoma is believed to recapitulate the hair follicle in the anagen phase, where there is close interplay between matrical keratinocytes and melanocytes prior to cessation of melanogenesis during the catagen phase.5,6,8,20,21 Evidence demonstrating highly conserved β-catenin and downstream lymphoid enhancer binding factor 1 (LEF1) expression, as well as pleckstrin homology-like domain, family A, member 1 (PHLDA1) expression (as a marker for follicular stem cells), points to constitutive activity in the Wnt signaling pathway in follicular stem cells of the bulge area as a major agent of tumorigenesis.12
Melanocytic matrical carcinoma, also known as malignant MM or matrical carcinoma with melanocytic hyperplasia, may be considered the malignant counterpart to MM.22 A PubMed search of articles indexed for MEDLINE using the terms melanocytic matrical carcinoma, malignant melanocytic matricoma, and matrical carcinoma with melanocytic hyperplasia, with review of references to identify additional citations, yielded 13 reported cases of MMC in the English-language literature (Table).19,22-30 As with MM, MMC is a biphasic tumor with basaloid matrical and supramatrical cells; focal areas of ghost cells; and admixed, banal-appearing dendritic melanocytes. However, the basaloid component also demonstrates nuclear atypia, mitoses, occasional ulceration, and variably poor circumscription. Clinically these lesions can mimic pigmented BCC, malignant melanoma, or other malignant adnexal tumors.25 Their natural history is unknown due to few reported cases, but they can be correlated with matrical carcinomas, which were first described by Weedon et al31 in 1980. A summary of more than 130 cases of matrical carcinomas in the English-language literature found that MMCs have high rates of local recurrence and metastasize in approximately 13% of cases. Wide local excision demonstrated lower rates of recurrence than simple excision (23% vs 83%), but there were insufficient cases to determine the incidence following Mohs micrographic surgery.32 Melanocytic matrical carcinomas also demonstrate mutations in the β-catenin pathway,pointing to a similar pathogenesis as their benign counterparts or perhaps direct malignant transformation.25,33,34
A subset of MMCs are combined cutaneous tumors (CCTs) consisting of epithelial neoplasms in close association with malignant melanocytes. Two of the more common variants include dermal squamomelanocytic tumors, a term first used by Pool et al,35 and malignant basomelanocytic tumors, as named by Erickson et al,36 but trichoblastomelanomas and other types have been documented.37 Although CCTs typically occur in the same patient populations as MMCs, namely elderly white men with chronically sun-damaged skin,they exhibit several important distinctions.37-39 By definition, CCTs have a malignant melanocytic component, whereas melanocytes are nonneoplastic in MMCs. The pathogenesis may differ as well. Various mechanisms for the close association of epithelial tumors and melanoma have been proposed, including field cancerization, tumor collision, tumor-tumor metastases, tumor colonization, and others, though CCTs likely arise through combinations of these processes depending upon their subtype.37-39 Paracrine signaling may play an important role in the pathogenesis of both tumors.5,6,8,38 As with MMCs, the prognosis of CCTs is limited by relatively few reported cases. Despite advanced Breslow depths in many cases, these tumors display more indolent behavior suggestive of melanoma in situ rather than invasive melanoma, perhaps due to dependence upon epithelial paracrine factors.37,39-42
Solid-organ transplant recipients have higher rates of more aggressive malignancies, of which skin cancer is the most common.43-49 Squamous cell carcinoma of the skin accounts for 95% of cutaneous malignancies in this population and occurs at approximately 65 times the rate of the general population.50 The risk of other skin cancers also is increased, though less dramatically, including BCC (10-fold increased risk) and melanoma (2- to 8-fold increased risk).46,50-53 The cause likely is multifactorial, including older age, history of skin cancer pretransplant, more than 5 years posttransplant, male sex, and incrementally as Fitzpatrick skin type decreases from VI to I.54-56 Immunosuppressive therapy also plays a role in tumorigenesis. Azathioprine metabolites have specifically been implicated in UVA radiation–induced promutagenic oxidative damage to DNA.57 Other studies have found no significant differences in the type of immunosuppressant used but instead have correlated rates of skin cancer to overall immunosuppression.48,55,58 Lung transplant recipients in particular demonstrate high rates of cutaneous malignancy, likely due in part to the necessity of more potent immunosuppressive regimens. Nearly one-third of patients develop a cutaneous malignancy by 5 years and nearly half by 10 years posttransplant.55
We report a rare case of MMC in a solid-organ transplant recipient. We hypothesize that the combination of UV radiation exposure–induced photodamage acquired pretransplant in addition to an aggressive immunosuppressive regimen with azathioprine and other agents posttransplant contributed to the development of this patient’s rare malignancy. Although rare, these tumors should remain in the differential diagnosis of clinicians and pathologists caring for this unique patient population.
To the Editor:
A 68-year-old white man presented with a firm, gradually enlarging, mildly tender, grayish black papule with central ulceration on the left dorsal wrist of 4 months’ duration (Figure 1). His relevant medical history included multiple basal cell carcinomas (BCCs) and squamous cell carcinomas, as well as a single-lung transplant 2 years prior, for which he was on chronic immunosuppressive therapy with azathioprine, everolimus, tacrolimus, and prednisone. The clinical differential diagnosis included pigmented BCC, malignant melanoma, and ulcerated squamous cell carcinoma.
Histologic examination of the lesion (Figure 2) demonstrated irregular nodules of basaloid tumor cells with rounded nuclei, visible nucleoli, and scant cytoplasm involving the dermis. The tumor produced abrupt matrical-type keratinization, forming ghost cells. The lesion also contained frequent mitotic figures, apoptotic cells, focal areas of necrosis, and abundant melanin pigment. Admixed throughout the lesion were pigmented and dendritic melanocytic cells. The overlying epidermis was focally ulcerated with an adjacent localized connection between the tumor and the epidermis. Keratinocyte atypia was found in the surrounding epidermis, which contained melanophages, solar elastosis, and scattered chronic inflammatory cells. An immunohistochemical study (Figure 3) for tyrosinase demonstrated abundant admixed melanocytic cells. β-Catenin expression was shown in both nuclear and cytoplasmic distributions, and there was focal labeling on BerEP4 staining.
The lesion was subsequently treated with wide local excision. The patient has not had recurrence to date.
Melanocytic matricoma (MM), a rare adnexal tumor, was first described in 1999 by Carlson et al.1 A PubMed search of articles indexed for MEDLINE using the terms melanocytic and matricoma yielded 24 reported cases in the English-language literature.1-17 It consists of an admixed population of basaloid matrical and supramatrical cells, ghost cells, and dendritic melanocytes in a well-circumscribed dermal nodule, typically without epidermal or adnexal connection. In comparison to the more commonly described pilomatricoma, which can be uncommonly pigmented, MM typically has only focal areas of ghost cells and lacks cystic architecture.1,9,10,18 A granulomatous reaction to keratinaceous debris is variably present.1,9,10 Histologically, the scattered dendritic melanocytes are classically benign, but cases demonstrating melanocyte atypia have been reported.10,13 Melanocytic matricoma appears most commonly as a black or gray papule on sun-damaged skin in older men and tends not to recur following complete excision; thus, MM is considered to be a clinically benign neoplasm. Given the demographics and distribution of the lesions, exposure to UV radiation is thought to play a contributory role in the pathogenesis.2,10,19 Melanocytic matricoma is believed to recapitulate the hair follicle in the anagen phase, where there is close interplay between matrical keratinocytes and melanocytes prior to cessation of melanogenesis during the catagen phase.5,6,8,20,21 Evidence demonstrating highly conserved β-catenin and downstream lymphoid enhancer binding factor 1 (LEF1) expression, as well as pleckstrin homology-like domain, family A, member 1 (PHLDA1) expression (as a marker for follicular stem cells), points to constitutive activity in the Wnt signaling pathway in follicular stem cells of the bulge area as a major agent of tumorigenesis.12
Melanocytic matrical carcinoma, also known as malignant MM or matrical carcinoma with melanocytic hyperplasia, may be considered the malignant counterpart to MM.22 A PubMed search of articles indexed for MEDLINE using the terms melanocytic matrical carcinoma, malignant melanocytic matricoma, and matrical carcinoma with melanocytic hyperplasia, with review of references to identify additional citations, yielded 13 reported cases of MMC in the English-language literature (Table).19,22-30 As with MM, MMC is a biphasic tumor with basaloid matrical and supramatrical cells; focal areas of ghost cells; and admixed, banal-appearing dendritic melanocytes. However, the basaloid component also demonstrates nuclear atypia, mitoses, occasional ulceration, and variably poor circumscription. Clinically these lesions can mimic pigmented BCC, malignant melanoma, or other malignant adnexal tumors.25 Their natural history is unknown due to few reported cases, but they can be correlated with matrical carcinomas, which were first described by Weedon et al31 in 1980. A summary of more than 130 cases of matrical carcinomas in the English-language literature found that MMCs have high rates of local recurrence and metastasize in approximately 13% of cases. Wide local excision demonstrated lower rates of recurrence than simple excision (23% vs 83%), but there were insufficient cases to determine the incidence following Mohs micrographic surgery.32 Melanocytic matrical carcinomas also demonstrate mutations in the β-catenin pathway,pointing to a similar pathogenesis as their benign counterparts or perhaps direct malignant transformation.25,33,34
A subset of MMCs are combined cutaneous tumors (CCTs) consisting of epithelial neoplasms in close association with malignant melanocytes. Two of the more common variants include dermal squamomelanocytic tumors, a term first used by Pool et al,35 and malignant basomelanocytic tumors, as named by Erickson et al,36 but trichoblastomelanomas and other types have been documented.37 Although CCTs typically occur in the same patient populations as MMCs, namely elderly white men with chronically sun-damaged skin,they exhibit several important distinctions.37-39 By definition, CCTs have a malignant melanocytic component, whereas melanocytes are nonneoplastic in MMCs. The pathogenesis may differ as well. Various mechanisms for the close association of epithelial tumors and melanoma have been proposed, including field cancerization, tumor collision, tumor-tumor metastases, tumor colonization, and others, though CCTs likely arise through combinations of these processes depending upon their subtype.37-39 Paracrine signaling may play an important role in the pathogenesis of both tumors.5,6,8,38 As with MMCs, the prognosis of CCTs is limited by relatively few reported cases. Despite advanced Breslow depths in many cases, these tumors display more indolent behavior suggestive of melanoma in situ rather than invasive melanoma, perhaps due to dependence upon epithelial paracrine factors.37,39-42
Solid-organ transplant recipients have higher rates of more aggressive malignancies, of which skin cancer is the most common.43-49 Squamous cell carcinoma of the skin accounts for 95% of cutaneous malignancies in this population and occurs at approximately 65 times the rate of the general population.50 The risk of other skin cancers also is increased, though less dramatically, including BCC (10-fold increased risk) and melanoma (2- to 8-fold increased risk).46,50-53 The cause likely is multifactorial, including older age, history of skin cancer pretransplant, more than 5 years posttransplant, male sex, and incrementally as Fitzpatrick skin type decreases from VI to I.54-56 Immunosuppressive therapy also plays a role in tumorigenesis. Azathioprine metabolites have specifically been implicated in UVA radiation–induced promutagenic oxidative damage to DNA.57 Other studies have found no significant differences in the type of immunosuppressant used but instead have correlated rates of skin cancer to overall immunosuppression.48,55,58 Lung transplant recipients in particular demonstrate high rates of cutaneous malignancy, likely due in part to the necessity of more potent immunosuppressive regimens. Nearly one-third of patients develop a cutaneous malignancy by 5 years and nearly half by 10 years posttransplant.55
We report a rare case of MMC in a solid-organ transplant recipient. We hypothesize that the combination of UV radiation exposure–induced photodamage acquired pretransplant in addition to an aggressive immunosuppressive regimen with azathioprine and other agents posttransplant contributed to the development of this patient’s rare malignancy. Although rare, these tumors should remain in the differential diagnosis of clinicians and pathologists caring for this unique patient population.
- Carlson JA, Healy K, Slominski A, et al. Melanocytic matricoma: a report of two cases of a new entity. Am J Dermatopathol. 1999;21:344-349.
- Rizzardi C, Brollo A, Colonna A, et al. A tumor with composite pilo-folliculosebaceous differentiation harboring a recently described new entity—melanocytic matricoma. Am J Dermatopathol. 2002;24:493-497.
- Williams CM, Bozner P, Oliveri CV, et al. Melanocytic matricoma: case confirmation of a recently described entity. J Cutan Pathol. 2003;30:275-278.
- Horenstein MG, Kahn AG. Pathologic quiz case: a 69-year-old man with a brown-black facial papule. melanocytic matricoma. Arch Pathol Lab Med. 2004;128:e163-e164.
- Soler AP, Burchette JL, Bellet JS, et al. Cell adhesion protein expression in melanocytic matricoma. J Cutan Pathol. 2007;34:456-460.
- Islam MN, Bhattacharyya I, Proper SA, et al. Melanocytic matricoma: a distinctive clinicopathologic entity. Dermatol Surg. 2007;33:857-863.
- Monteagudo B, Requena L, Used-Aznar MM, et al. Melanocytic matricoma. Actas Dermosifiliogr. 2008;99:573-582.
- Cartaginese F, Sidoni A. Melanocytic matricoma. report of a further case with clinicopathological and immunohistochemical findings, differential diagnosis and review of the literature. Histol Histopathol. 2010;25:713-717.
- Tallon B, Cerroni L. Where pigmented pilomatricoma and melanocytic matricoma collide. Am J Dermatopathol. 2010;32:769-773.
- Zussman J, Sheth S, Ra SH, et al. Melanocytic matricoma with melanocytic atypia: report of a unique case and review of the literature. Am J Dermatopathol. 2011;33:508-512.
- Tanboon J, Manonukul J, Pattanaprichakul P. Melanocytic matricoma: two cases of a rare entity in women. J Cutan Pathol. 2014;41:775-782.
- Battistella M, Carlson JA, Oslo A, et al. Skin tumors with matrical differentiation: lessons from hair keratins, beta-catenin and PHLDA-1 expression. J Cutan Pathol. 2014;41:427-436.
- Barrado-Solis N, Moles-Poveda P, Roca-Estelles MJ, et al. Melanocytic matricoma with melanocytic atypia: report of a new case [published online February 11, 2015]. J Eur Acad Dermatol Venereol. 2016;30:859-860.
- Pagliarello C, Stanganelli I, Ricci R, et al. A pinkish-blue exophytic nodule on the arm of an elderly man: a quiz. melanocytic matricoma. Acta Derm Venereol. 2017;97:1261-1262.
- Winslow CY, Camacho I, Nousari CH. Melanocytic matricoma with consumption of the epidermis: an atypical histologic attribute or a malignant variant? Am J Dermatopathol. 2017;39:907-909.
- Sangiorgio V, Moneghini L, Tosi D, et al. A case of melanocytic matricoma with prominent mitotic activity and melanocytic hyperplasia. Int J Dermatol. 2018;57:e78-e81.
- Song J, Lu S, Wu Z. An unusual case of melanocytic matricoma in a young pregnant woman. Australas J Dermatol. 2019;60:140-141.
- Ishida M, Okabe H. Pigmented pilomatricoma: an underrecognized variant. Int J Clin Exp Pathol. 2013;6:1890-1893.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S-97S.
- De Berker D, Higgins CA, Jahada C, et al. Biology of hair and nails. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1075-1092.
- Monteagudo C, Fernandez-Figueras MT, San Juan J, et al. Matrical carcinoma with prominent melanocytc hyperplasia (malignant melanocytic matricoma?). Am J Dermatopathol. 2003;25:485-489.
- Sloan JB, Sueki H, Jaworsky C. Pigmented malignant pilomatrixoma: report of a case and review of the literature. J Cutan Pathol. 1992;19:240-246.
- Hardisson D, Linares MD, Cuevas-Santos J, et al. Pilomatrix carcinoma: a clinicopathologic study of six cases and review of the literature. Am J Dermatopathol. 2001;23:394-401.
- Soler AP, Kindel SE, McCloskey G, et al. Cell-cell adhesion proteins in melanocytic pilomatrix carcinoma. Rare Tumors. 2010;2:e43-e45.
- Ardakani NM, Palmer DL, Wood BA. Malignant melanocytic matricoma: a report of 2 cases and review of the literature. Am J Dermatopathol. 2016;38:33-38.
- Villada G, Romagosa R, Miteva M, et al. Matrical carcinoma with melanocytic proliferation and prominent squamoid whorls. Am J Dermatopathol. 2016;38:e11-e14.
- Ji C, Zhang Y, Heller P, et al. Melanocytic matrical carcinoma mimicking melanoma. Am J Dermatopathol. 2017;39:903-906.
- Nielson CB, Vincek V. Malignant melanocytic matricoma and criteria for malignancy. Open J Pathol. 2018;8:94-100.
- Lehmer L, Carly SK, de Feraudy S. Matrical carcinoma with melanocytic hyperplasia mimicking nodular melanoma in an elderly Mexican male. J Cutan Pathol. 2019;46:442-446.
- Weedon D, Bell J, Mayze J. Matrical carcinoma of the skin. J Cutan Pathol. 1980;7:39-42.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Hassanein AM, Glanz SM. Beta-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol. 2004;150:511-516.
- Pool SE, Manieei F, Clark WH Jr, et al. Dermal squamo-melanocytic tumor: a unique biphenotypic neoplasm of uncertain biological potential. Hum Pathol. 1999;30:525-529.
- Erickson LA, Myers JL, Mihm MC, et al. Malignant basomelanocytic tumor manifesting as metastatic melanoma. Am J Surg Pathol. 2004;28:1393-1396.
- Amin SM, Cooper C, Yelamos O, et al. Combined cutaneous tumors with a melanoma component: a clinical, histologic, and molecular study. J Am Acad Dermatol. 2015;73:451-460.
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603.
- Satter EK, Metcalf J, Lountzis N, et al. Tumors composed of malignant epithelial and melanocytic populations: a case series and review of the literature. J Cutan Pathol. 2009;36:211-219.
- Pouryazdanparast P, Yu L, Johnson T, et al. An unusual squamo-melanocytic tumor of uncertain biologic behavior: a variant of melanoma? Am J Dermatopathol. 2009;31:457-461.
- Burkhalter A, White W. Malignant melanoma in situ colonizing basal cell carcinoma: a simulator of invasive melanoma. Am J Dermatopathol. 1997;19:303-307.
- Papa G, Grandi G, Pascone M. Collision tumor of malignant skin cancers: a case of melanoma in basal cell carcinoma. Pathol Res Pract. 2006;202:691-694.
- Miao Y, Everly JJ, Gross TG, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347-1359.
- Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. a follow-up study. Transplantation. 1996;61:715-721.
- Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1-17.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:253-261.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-273.
- DePry JL, Reed KB, Cook-Harris RH, et al. Iatrogenic immunosuppression and cutaneous malignancy. Clin Dermatol. 2011;29:602-613.
- Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622-1630.
- Jensen P, Hansen S, Møller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177-186.
- Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-913.
- Hollenbeak CS, Todd MM, Billingsley EM, et al. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005;104:1962-1967.
- Le Mire L, Hollowood K, Gray D, et al. Melanomas in renal transplant recipients. Br J Dermatol. 2006;154:472-477.
- Gogia R, Binstock M, Hirose R, et al. Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol. 2013;68:585-591.
- Rashtak S, Dierkhising RA, Kremers WK, et al. Incidence and risk factors for skin cancer following lung transplantation. J Am Acad Dermatol. 2015;72:92-98.
- Ruiz DE, Luzuriaga AM, Hsieh C. Yearly burden of skin cancer in non-Caucasian and Caucasian solid-organ transplant recipients. J Clin Aesthet Dermatol. 2015;8:16-19.
- Perrett CM, Walker SL, O’Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol. 2008;159:198-204.
- Abou Ayache R, Thierry A, Bridoux F, et al. Long-term maintenance of calcineurin inhibitor monotherapy reduces the risk for squamous cell carcinomas after kidney transplantation compared with bi- or tritherapy. Transplant Proc. 2007;39:2592-2594.
- Carlson JA, Healy K, Slominski A, et al. Melanocytic matricoma: a report of two cases of a new entity. Am J Dermatopathol. 1999;21:344-349.
- Rizzardi C, Brollo A, Colonna A, et al. A tumor with composite pilo-folliculosebaceous differentiation harboring a recently described new entity—melanocytic matricoma. Am J Dermatopathol. 2002;24:493-497.
- Williams CM, Bozner P, Oliveri CV, et al. Melanocytic matricoma: case confirmation of a recently described entity. J Cutan Pathol. 2003;30:275-278.
- Horenstein MG, Kahn AG. Pathologic quiz case: a 69-year-old man with a brown-black facial papule. melanocytic matricoma. Arch Pathol Lab Med. 2004;128:e163-e164.
- Soler AP, Burchette JL, Bellet JS, et al. Cell adhesion protein expression in melanocytic matricoma. J Cutan Pathol. 2007;34:456-460.
- Islam MN, Bhattacharyya I, Proper SA, et al. Melanocytic matricoma: a distinctive clinicopathologic entity. Dermatol Surg. 2007;33:857-863.
- Monteagudo B, Requena L, Used-Aznar MM, et al. Melanocytic matricoma. Actas Dermosifiliogr. 2008;99:573-582.
- Cartaginese F, Sidoni A. Melanocytic matricoma. report of a further case with clinicopathological and immunohistochemical findings, differential diagnosis and review of the literature. Histol Histopathol. 2010;25:713-717.
- Tallon B, Cerroni L. Where pigmented pilomatricoma and melanocytic matricoma collide. Am J Dermatopathol. 2010;32:769-773.
- Zussman J, Sheth S, Ra SH, et al. Melanocytic matricoma with melanocytic atypia: report of a unique case and review of the literature. Am J Dermatopathol. 2011;33:508-512.
- Tanboon J, Manonukul J, Pattanaprichakul P. Melanocytic matricoma: two cases of a rare entity in women. J Cutan Pathol. 2014;41:775-782.
- Battistella M, Carlson JA, Oslo A, et al. Skin tumors with matrical differentiation: lessons from hair keratins, beta-catenin and PHLDA-1 expression. J Cutan Pathol. 2014;41:427-436.
- Barrado-Solis N, Moles-Poveda P, Roca-Estelles MJ, et al. Melanocytic matricoma with melanocytic atypia: report of a new case [published online February 11, 2015]. J Eur Acad Dermatol Venereol. 2016;30:859-860.
- Pagliarello C, Stanganelli I, Ricci R, et al. A pinkish-blue exophytic nodule on the arm of an elderly man: a quiz. melanocytic matricoma. Acta Derm Venereol. 2017;97:1261-1262.
- Winslow CY, Camacho I, Nousari CH. Melanocytic matricoma with consumption of the epidermis: an atypical histologic attribute or a malignant variant? Am J Dermatopathol. 2017;39:907-909.
- Sangiorgio V, Moneghini L, Tosi D, et al. A case of melanocytic matricoma with prominent mitotic activity and melanocytic hyperplasia. Int J Dermatol. 2018;57:e78-e81.
- Song J, Lu S, Wu Z. An unusual case of melanocytic matricoma in a young pregnant woman. Australas J Dermatol. 2019;60:140-141.
- Ishida M, Okabe H. Pigmented pilomatricoma: an underrecognized variant. Int J Clin Exp Pathol. 2013;6:1890-1893.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S-97S.
- De Berker D, Higgins CA, Jahada C, et al. Biology of hair and nails. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:1075-1092.
- Monteagudo C, Fernandez-Figueras MT, San Juan J, et al. Matrical carcinoma with prominent melanocytc hyperplasia (malignant melanocytic matricoma?). Am J Dermatopathol. 2003;25:485-489.
- Sloan JB, Sueki H, Jaworsky C. Pigmented malignant pilomatrixoma: report of a case and review of the literature. J Cutan Pathol. 1992;19:240-246.
- Hardisson D, Linares MD, Cuevas-Santos J, et al. Pilomatrix carcinoma: a clinicopathologic study of six cases and review of the literature. Am J Dermatopathol. 2001;23:394-401.
- Soler AP, Kindel SE, McCloskey G, et al. Cell-cell adhesion proteins in melanocytic pilomatrix carcinoma. Rare Tumors. 2010;2:e43-e45.
- Ardakani NM, Palmer DL, Wood BA. Malignant melanocytic matricoma: a report of 2 cases and review of the literature. Am J Dermatopathol. 2016;38:33-38.
- Villada G, Romagosa R, Miteva M, et al. Matrical carcinoma with melanocytic proliferation and prominent squamoid whorls. Am J Dermatopathol. 2016;38:e11-e14.
- Ji C, Zhang Y, Heller P, et al. Melanocytic matrical carcinoma mimicking melanoma. Am J Dermatopathol. 2017;39:903-906.
- Nielson CB, Vincek V. Malignant melanocytic matricoma and criteria for malignancy. Open J Pathol. 2018;8:94-100.
- Lehmer L, Carly SK, de Feraudy S. Matrical carcinoma with melanocytic hyperplasia mimicking nodular melanoma in an elderly Mexican male. J Cutan Pathol. 2019;46:442-446.
- Weedon D, Bell J, Mayze J. Matrical carcinoma of the skin. J Cutan Pathol. 1980;7:39-42.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Hassanein AM, Glanz SM. Beta-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol. 2004;150:511-516.
- Pool SE, Manieei F, Clark WH Jr, et al. Dermal squamo-melanocytic tumor: a unique biphenotypic neoplasm of uncertain biological potential. Hum Pathol. 1999;30:525-529.
- Erickson LA, Myers JL, Mihm MC, et al. Malignant basomelanocytic tumor manifesting as metastatic melanoma. Am J Surg Pathol. 2004;28:1393-1396.
- Amin SM, Cooper C, Yelamos O, et al. Combined cutaneous tumors with a melanoma component: a clinical, histologic, and molecular study. J Am Acad Dermatol. 2015;73:451-460.
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603.
- Satter EK, Metcalf J, Lountzis N, et al. Tumors composed of malignant epithelial and melanocytic populations: a case series and review of the literature. J Cutan Pathol. 2009;36:211-219.
- Pouryazdanparast P, Yu L, Johnson T, et al. An unusual squamo-melanocytic tumor of uncertain biologic behavior: a variant of melanoma? Am J Dermatopathol. 2009;31:457-461.
- Burkhalter A, White W. Malignant melanoma in situ colonizing basal cell carcinoma: a simulator of invasive melanoma. Am J Dermatopathol. 1997;19:303-307.
- Papa G, Grandi G, Pascone M. Collision tumor of malignant skin cancers: a case of melanoma in basal cell carcinoma. Pathol Res Pract. 2006;202:691-694.
- Miao Y, Everly JJ, Gross TG, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87:1347-1359.
- Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. a follow-up study. Transplantation. 1996;61:715-721.
- Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1-17.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:253-261.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-273.
- DePry JL, Reed KB, Cook-Harris RH, et al. Iatrogenic immunosuppression and cutaneous malignancy. Clin Dermatol. 2011;29:602-613.
- Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622-1630.
- Jensen P, Hansen S, Møller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177-186.
- Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-913.
- Hollenbeak CS, Todd MM, Billingsley EM, et al. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005;104:1962-1967.
- Le Mire L, Hollowood K, Gray D, et al. Melanomas in renal transplant recipients. Br J Dermatol. 2006;154:472-477.
- Gogia R, Binstock M, Hirose R, et al. Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol. 2013;68:585-591.
- Rashtak S, Dierkhising RA, Kremers WK, et al. Incidence and risk factors for skin cancer following lung transplantation. J Am Acad Dermatol. 2015;72:92-98.
- Ruiz DE, Luzuriaga AM, Hsieh C. Yearly burden of skin cancer in non-Caucasian and Caucasian solid-organ transplant recipients. J Clin Aesthet Dermatol. 2015;8:16-19.
- Perrett CM, Walker SL, O’Donovan P, et al. Azathioprine treatment photosensitizes human skin to ultraviolet A radiation. Br J Dermatol. 2008;159:198-204.
- Abou Ayache R, Thierry A, Bridoux F, et al. Long-term maintenance of calcineurin inhibitor monotherapy reduces the risk for squamous cell carcinomas after kidney transplantation compared with bi- or tritherapy. Transplant Proc. 2007;39:2592-2594.
Practice Points
- Melanocytic matrical carcinoma (MMC) is an extremely rare adnexal malignancy that can present as a hyperpigmented papule with or without ulceration.
- Histologically, the lesion resembles a matrical carcinoma with admixed, banal-appearing dendritic melanocytes.
- Solid-organ transplant recipients are at an increased risk of cutaneous malignancies, including rare cancers such as MMC, and these neoplasms should remain in the clinician’s differential diagnosis.