User login
Bariatric surgery viable for teens with cognitive disabilities
Adolescents with severe obesity and cognitive impairment or developmental delay (CI/DD) lost as much weight, and at a similar rate, as their typically developing peers following laparoscopic sleeve gastrectomy (LSG), according in an observational study.
“On the basis of these new data, LSG appears to be a viable and successful short-term weight-management tool for adolescents with CI/DD, who are established as particularly vulnerable to obesity and secondary health concerns,” wrote Sarah E. Hornack, PhD, a psychologist with Children’s National Health System and George Washington University, both in Washington, and her associates.
“In fact, there may be advantages to undergoing surgery during adolescence rather than waiting until adulthood for this population,” they wrote in the journal Pediatrics. With more supports likely in place for teens undergoing this surgery, they won’t be “going it alone,” the authors noted, which “could translate to better cooperation with parental guidance regarding surgery requirements, including diet and exercise recommendations.”
Despite higher rates of obesity and related medical issues among youth with CI/DD, little research explores effective interventions in this population, the authors wrote.
They therefore compared outcomes among a group of 63 teens with obesity who underwent bariatric surgery during 2010-2017. The adolescents, who had a body mass index (BMI) of at least 40 kg/m2 or one of at least 35 kg/m2 with a medical comorbidity, first underwent preoperative psychological evaluations involving a cognitive assessment. The 17 adolescents with an IQ less than 80 were classified as having CI/DD, leaving 46 without CI/DD. Three teens had Down syndrome.
Age, sex, and BMI before surgery were similar in those with CI/DD versus those without. The majority of participants overall were female (65%) and black (57%) with an average age of 17 years and an average BMI of 51.2. Whites comprised 24% of participants while 17% were Hispanic and 1% another race/ethnicity.
The findings revealed that IQ did not predict weight loss. The percentage of excess BMI lost (%EBMIL) and rate of excess weight loss remained similar between those with and without CI/DD, though “a trend for a higher rate of change in %EBMIL for those individuals with CI/DD” suggested “they may experience greater rates of weight loss over time than their typically developing peers,” the authors reported. However, the proportion of participants assessed decreased with each follow-up, from 59 at 3 months to 14 at 24 months.
In addition to the small population, short-term follow-up and loss to follow-up, another study limitation is the lack of a control group of CI/DD patients who did not undergo bariatric surgery and instead received a behavioral intervention or other therapy.
But the authors noted existing evidence that “younger children respond better to behavioral interventions than adolescents do, suggesting that older youth may require a different treatment approach.” In addition, “bariatric surgery performed earlier in the trajectory of large weight gain has also been shown to lead to greater resolution of obesity, suggesting that waiting for adulthood can be detrimental,” they wrote.
SOURCE: Hornack SE et al. Pediatrics. 2019 Apr 15. doi: 10.1542/peds.2018-2908.
Despite increasing evidence to support the safety and effectiveness of bariatric surgery in confronting the challenge of increasing obesity rates among adolescents, access to care remains limited for many such teens.
Prominent examples include a significant disparity in insurance authorization for bariatric surgical care when comparing pediatric patients to their adult counterparts, low rates of referral from primary caregivers, and general uncertainty regarding potential exclusionary criteria.
The researchers should be commended for exploring bariatric surgery outcomes in an understudied population. However, both the likely importance of social supports to the participants’ success and, especially, the need to approach the issue of informed thoughtfully, perhaps with additional institutional guidance are crucial to success.
Although literature addressing ethical concerns specifically associated with bariatric surgery for children with intellectual or developmental disability is limited, previous attempts to offer a logical clinical framework highlight the importance of using a case-by-case approach predicated on the need to establish a well-defined risk/benefit ratio.
As an important part of efforts to tackle such challenges, bariatric surgical care providers should strongly consider the routine use of available resources (i.e., institutional ethics committees) to assist in complex medical decision making.”
These comments are adapted from an accompanying editorial by Marc P. Michalsky, MD, of the Ohio State University and Nationwide Children’s Hospital, both in Columbus (Pediatrics. 15 April 2019; doi: 10.1542/peds.2018-4112). He reported having no disclosures.
Despite increasing evidence to support the safety and effectiveness of bariatric surgery in confronting the challenge of increasing obesity rates among adolescents, access to care remains limited for many such teens.
Prominent examples include a significant disparity in insurance authorization for bariatric surgical care when comparing pediatric patients to their adult counterparts, low rates of referral from primary caregivers, and general uncertainty regarding potential exclusionary criteria.
The researchers should be commended for exploring bariatric surgery outcomes in an understudied population. However, both the likely importance of social supports to the participants’ success and, especially, the need to approach the issue of informed thoughtfully, perhaps with additional institutional guidance are crucial to success.
Although literature addressing ethical concerns specifically associated with bariatric surgery for children with intellectual or developmental disability is limited, previous attempts to offer a logical clinical framework highlight the importance of using a case-by-case approach predicated on the need to establish a well-defined risk/benefit ratio.
As an important part of efforts to tackle such challenges, bariatric surgical care providers should strongly consider the routine use of available resources (i.e., institutional ethics committees) to assist in complex medical decision making.”
These comments are adapted from an accompanying editorial by Marc P. Michalsky, MD, of the Ohio State University and Nationwide Children’s Hospital, both in Columbus (Pediatrics. 15 April 2019; doi: 10.1542/peds.2018-4112). He reported having no disclosures.
Despite increasing evidence to support the safety and effectiveness of bariatric surgery in confronting the challenge of increasing obesity rates among adolescents, access to care remains limited for many such teens.
Prominent examples include a significant disparity in insurance authorization for bariatric surgical care when comparing pediatric patients to their adult counterparts, low rates of referral from primary caregivers, and general uncertainty regarding potential exclusionary criteria.
The researchers should be commended for exploring bariatric surgery outcomes in an understudied population. However, both the likely importance of social supports to the participants’ success and, especially, the need to approach the issue of informed thoughtfully, perhaps with additional institutional guidance are crucial to success.
Although literature addressing ethical concerns specifically associated with bariatric surgery for children with intellectual or developmental disability is limited, previous attempts to offer a logical clinical framework highlight the importance of using a case-by-case approach predicated on the need to establish a well-defined risk/benefit ratio.
As an important part of efforts to tackle such challenges, bariatric surgical care providers should strongly consider the routine use of available resources (i.e., institutional ethics committees) to assist in complex medical decision making.”
These comments are adapted from an accompanying editorial by Marc P. Michalsky, MD, of the Ohio State University and Nationwide Children’s Hospital, both in Columbus (Pediatrics. 15 April 2019; doi: 10.1542/peds.2018-4112). He reported having no disclosures.
Adolescents with severe obesity and cognitive impairment or developmental delay (CI/DD) lost as much weight, and at a similar rate, as their typically developing peers following laparoscopic sleeve gastrectomy (LSG), according in an observational study.
“On the basis of these new data, LSG appears to be a viable and successful short-term weight-management tool for adolescents with CI/DD, who are established as particularly vulnerable to obesity and secondary health concerns,” wrote Sarah E. Hornack, PhD, a psychologist with Children’s National Health System and George Washington University, both in Washington, and her associates.
“In fact, there may be advantages to undergoing surgery during adolescence rather than waiting until adulthood for this population,” they wrote in the journal Pediatrics. With more supports likely in place for teens undergoing this surgery, they won’t be “going it alone,” the authors noted, which “could translate to better cooperation with parental guidance regarding surgery requirements, including diet and exercise recommendations.”
Despite higher rates of obesity and related medical issues among youth with CI/DD, little research explores effective interventions in this population, the authors wrote.
They therefore compared outcomes among a group of 63 teens with obesity who underwent bariatric surgery during 2010-2017. The adolescents, who had a body mass index (BMI) of at least 40 kg/m2 or one of at least 35 kg/m2 with a medical comorbidity, first underwent preoperative psychological evaluations involving a cognitive assessment. The 17 adolescents with an IQ less than 80 were classified as having CI/DD, leaving 46 without CI/DD. Three teens had Down syndrome.
Age, sex, and BMI before surgery were similar in those with CI/DD versus those without. The majority of participants overall were female (65%) and black (57%) with an average age of 17 years and an average BMI of 51.2. Whites comprised 24% of participants while 17% were Hispanic and 1% another race/ethnicity.
The findings revealed that IQ did not predict weight loss. The percentage of excess BMI lost (%EBMIL) and rate of excess weight loss remained similar between those with and without CI/DD, though “a trend for a higher rate of change in %EBMIL for those individuals with CI/DD” suggested “they may experience greater rates of weight loss over time than their typically developing peers,” the authors reported. However, the proportion of participants assessed decreased with each follow-up, from 59 at 3 months to 14 at 24 months.
In addition to the small population, short-term follow-up and loss to follow-up, another study limitation is the lack of a control group of CI/DD patients who did not undergo bariatric surgery and instead received a behavioral intervention or other therapy.
But the authors noted existing evidence that “younger children respond better to behavioral interventions than adolescents do, suggesting that older youth may require a different treatment approach.” In addition, “bariatric surgery performed earlier in the trajectory of large weight gain has also been shown to lead to greater resolution of obesity, suggesting that waiting for adulthood can be detrimental,” they wrote.
SOURCE: Hornack SE et al. Pediatrics. 2019 Apr 15. doi: 10.1542/peds.2018-2908.
Adolescents with severe obesity and cognitive impairment or developmental delay (CI/DD) lost as much weight, and at a similar rate, as their typically developing peers following laparoscopic sleeve gastrectomy (LSG), according in an observational study.
“On the basis of these new data, LSG appears to be a viable and successful short-term weight-management tool for adolescents with CI/DD, who are established as particularly vulnerable to obesity and secondary health concerns,” wrote Sarah E. Hornack, PhD, a psychologist with Children’s National Health System and George Washington University, both in Washington, and her associates.
“In fact, there may be advantages to undergoing surgery during adolescence rather than waiting until adulthood for this population,” they wrote in the journal Pediatrics. With more supports likely in place for teens undergoing this surgery, they won’t be “going it alone,” the authors noted, which “could translate to better cooperation with parental guidance regarding surgery requirements, including diet and exercise recommendations.”
Despite higher rates of obesity and related medical issues among youth with CI/DD, little research explores effective interventions in this population, the authors wrote.
They therefore compared outcomes among a group of 63 teens with obesity who underwent bariatric surgery during 2010-2017. The adolescents, who had a body mass index (BMI) of at least 40 kg/m2 or one of at least 35 kg/m2 with a medical comorbidity, first underwent preoperative psychological evaluations involving a cognitive assessment. The 17 adolescents with an IQ less than 80 were classified as having CI/DD, leaving 46 without CI/DD. Three teens had Down syndrome.
Age, sex, and BMI before surgery were similar in those with CI/DD versus those without. The majority of participants overall were female (65%) and black (57%) with an average age of 17 years and an average BMI of 51.2. Whites comprised 24% of participants while 17% were Hispanic and 1% another race/ethnicity.
The findings revealed that IQ did not predict weight loss. The percentage of excess BMI lost (%EBMIL) and rate of excess weight loss remained similar between those with and without CI/DD, though “a trend for a higher rate of change in %EBMIL for those individuals with CI/DD” suggested “they may experience greater rates of weight loss over time than their typically developing peers,” the authors reported. However, the proportion of participants assessed decreased with each follow-up, from 59 at 3 months to 14 at 24 months.
In addition to the small population, short-term follow-up and loss to follow-up, another study limitation is the lack of a control group of CI/DD patients who did not undergo bariatric surgery and instead received a behavioral intervention or other therapy.
But the authors noted existing evidence that “younger children respond better to behavioral interventions than adolescents do, suggesting that older youth may require a different treatment approach.” In addition, “bariatric surgery performed earlier in the trajectory of large weight gain has also been shown to lead to greater resolution of obesity, suggesting that waiting for adulthood can be detrimental,” they wrote.
SOURCE: Hornack SE et al. Pediatrics. 2019 Apr 15. doi: 10.1542/peds.2018-2908.
FROM PEDIATRICS
Atorvastatin appears to lower cardiovascular risk in RA patients
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
FROM ARTHRITIS & RHEUMATOLOGY
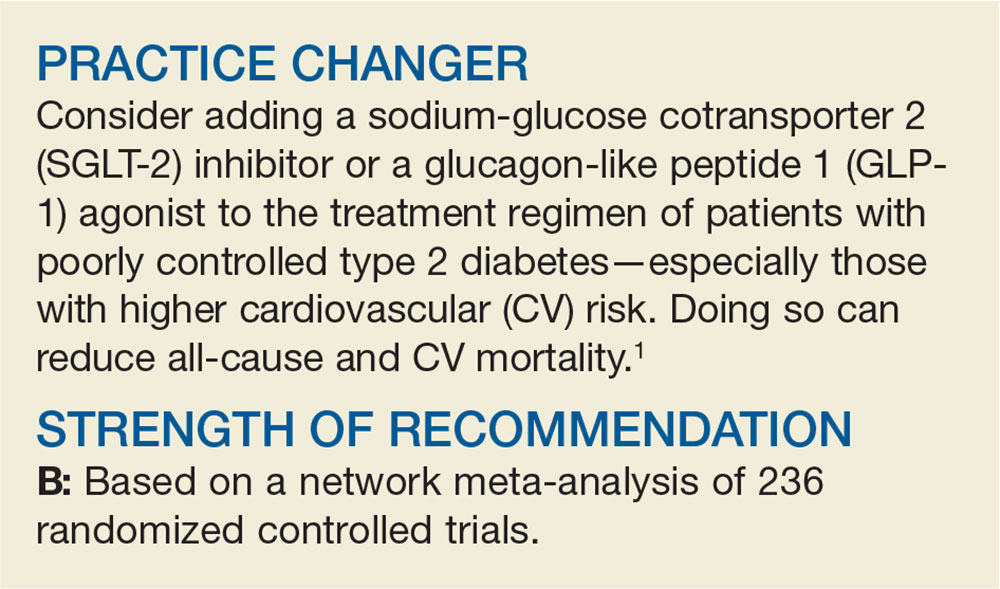
How Do These 3 Diabetes Agents Compare in Reducing Mortality?
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
Canagliflozin lowers kidney failure risk in T2D: CREDENCE
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Challenges in outpatient psychiatry: When patients don’t pay
Editor’s note: This is the second in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
Mr. A lived a life that seemed glamorous to me. There were lunches with professional athletes, swank social events, and designer everything from clothes to cars. It was a world that I was not familiar with, and while I knew my patient worried about money, it seemed he had it. When he started therapy with me, Mr. A came to every session with a check. At some point, I realized that I had missed a switch in his mood. Despite his therapeutic level of lithium, Mr. A had become manic, and the expression of this mania took the form of even more spending. What started as an exciting lifestyle suddenly became tens of thousands of dollars of debt. I felt guilty that I initially did not see this as pathology, and as a young psychiatrist, I sought consultation with an older and wiser mentor.
After discussing the case, the consulting psychiatrist said to me, “Make sure you get paid; these cases are difficult.” In fact, in the midst of the chaos, Mr. A had stopped bringing a check to the sessions. I sent monthly statements, and they went unpaid. He didn’t have the money for his living expenses and I let this go on without addressing it for much too long. Soon, Mr. A’s debt to me was just one more stress in his life and while I knew I would not pursue reimbursement through legal channels, I did encourage him to find a psychiatrist who was in his insurance network, something he did not want to do. I was too embarrassed to tell the consulting psychiatrist that Mr. A had already accrued well over a thousand dollars in unpaid professional fees. In my mind, I was offering pro bono care because the patient’s financial circumstances had changed, and because I felt guilty that I had not recognized this as mania sooner.
In a 2011 Shrink Rap blog post, Jesse Hellman, MD, a psychiatrist in private practice in Towson, Md., wrote about the meaning of payment in psychotherapy:
“Money is something loaded with meaning to most people. What does it mean that the patient forgets to pay? Does it mean ‘if you really cared about me you would not charge me’? Is it a reflection of anger for something that occurred in the last session? Is it a displacement of feelings from something else (‘my boss didn’t give me the raise I expected’)? Is it completely inadvertent (Freud famously said ‘Sometimes a cigar is only a cigar’)?”
There are so many possibilities, and the psychodynamic therapist wants to understand them. How the patient relates to the therapist is some part of how he relates to others. The patient hopefully starts to watch his own actions and attitudes and also tries to understand them. A nonjudgmental stance helps the patient do this.
The therapist himself needs to be comfortable dealing with the subject of money. Sometimes beginning physicians fluctuate between feeling they are too inexperienced to be paid and feeling that they deserve anything they ask. We physicians might even (unfortunately) take on the attitudes of the insurance companies themselves (“Identification with the Aggressor”).
The blog post was flooded with comments –120 in total, and more comments than any other single Shrink Rap post received for the blog’s 12-year run.
Steven Reidbord, MD, is a psychiatrist in private practice in San Francisco and also a blogger. Dr. Reidbord conversed with me through email about patients who don’t pay.
“In years past, I’ve had a few patients who met with me a few times, always ‘forgetting’ their payment and offering it ‘next time,’ until after three or four sessions, I refused to see them. I always wondered what such patients were thinking, as obviously this arrangement wouldn’t last long. Did they tell themselves they’d pay me at some point, in effect fooling themselves? Was it conscious theft of my services? A couple years ago, I started accepting charge cards, and perhaps as a result, this hasn’t happened. While it’s always useful to consider individual dynamics in explaining such behavior, it’s also important to consider normative psychology: Make it easier to pay, and more people will.”
While payment for out-of-network services is often clear cut – the patients pay and then requests reimbursement from their insurer – the logistics often are confusing for the patient. He or she may believe that she has excellent coverage, only to learn that the out-of-network deductible is very high, or that reimbursement is based on “usual and customary fees” that are much lower than his psychiatrist’s fees. Sometimes people take on the cost of psychiatric care and discover that it costs more than they assumed, or they have a change in their financial circumstances, as my patient did. Sometimes a parent is paying for treatment and decides he can no longer afford it.
“When someone’s financial circumstances change, they often let me know by proposing we meet less often, for example, every other week,” Dr. Reidbord wrote. “I ask to hear more and often offer to adjust my fee to allow weekly meetings to continue.”
Not all patients pay for psychiatric services, and that may make the discussion even harder. When psychiatrists participate with insurance, the patients are responsible for paying only their deductible and then a copay. The patients may unexpectedly be billed for the entire fee if their insurance terminates, or if it does not pay for a submitted claim. And patients who carry public insurance may be seen at sites where there is no out-of-pocket cost to the patient; salaried clinicians often never know if the insurance has paid. In both of these settings, finances are usually discussed with administrative personnel and not with clinical staff.
Anthony Massey, MD, is founder of Maryland’s Gladstone Psychiatry and Wellness. The group is a multidisciplinary organization, and the clinicians participate with employer-based commercial health insurances. The group accepts payment directly from the insurer, and the patient is responsible for payment of the deductible and a copay.
“We try to understand what someone owes before the first appointment. We do an eligibility check online, and we ask for payment at the time of the appointment,” Massey explained. “Sometimes the insurance changes and we don’t know, or sometimes a patient comes to the appointment without the copay. We try to work with people, but if someone builds up a balance over $500, we tell them they can’t be seen here until it’s paid down. We’ll give patients a 30-day prescription and the names of other psychiatrists who accept insurance, but we don’t keep seeing people who don’t pay for their treatment.”
In all medical settings, unpaid bills present a problem, and while most psychiatrists have a method to deal with these issues, there is no perfect answer for every doctor in every situation. There is this tension between wanting to be kind and understanding of the hardships that people have whether those hardships result from life circumstances or from their own choices and behaviors, and of our own need to make the living we feel we deserve and to pay our own bills.
“The only advice I’d give other psychiatrists is to catch it early,” Reidbord said. “ – and stick with it. If a patient doesn’t pay according to your clearly stated policy, explore it right away. Remember that pragmatic issues like poor budgeting or unexpected expenses are just as likely as intrapsychic conflict and ‘acting out.’ Both should be considered.”
I wish I could say that in the decades since I treated Mr. A that no patients have ever failed to pay their professional fees and that I have perfectly mastered my own issues with money as it pertains to professional fees. While the vast majority of patients do pay, there are still occasional circumstances in which someone’s financial circumstances change, or very rarely where someone ends his treatment without paying for the last few sessions.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
Editor’s note: This is the second in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
Mr. A lived a life that seemed glamorous to me. There were lunches with professional athletes, swank social events, and designer everything from clothes to cars. It was a world that I was not familiar with, and while I knew my patient worried about money, it seemed he had it. When he started therapy with me, Mr. A came to every session with a check. At some point, I realized that I had missed a switch in his mood. Despite his therapeutic level of lithium, Mr. A had become manic, and the expression of this mania took the form of even more spending. What started as an exciting lifestyle suddenly became tens of thousands of dollars of debt. I felt guilty that I initially did not see this as pathology, and as a young psychiatrist, I sought consultation with an older and wiser mentor.
After discussing the case, the consulting psychiatrist said to me, “Make sure you get paid; these cases are difficult.” In fact, in the midst of the chaos, Mr. A had stopped bringing a check to the sessions. I sent monthly statements, and they went unpaid. He didn’t have the money for his living expenses and I let this go on without addressing it for much too long. Soon, Mr. A’s debt to me was just one more stress in his life and while I knew I would not pursue reimbursement through legal channels, I did encourage him to find a psychiatrist who was in his insurance network, something he did not want to do. I was too embarrassed to tell the consulting psychiatrist that Mr. A had already accrued well over a thousand dollars in unpaid professional fees. In my mind, I was offering pro bono care because the patient’s financial circumstances had changed, and because I felt guilty that I had not recognized this as mania sooner.
In a 2011 Shrink Rap blog post, Jesse Hellman, MD, a psychiatrist in private practice in Towson, Md., wrote about the meaning of payment in psychotherapy:
“Money is something loaded with meaning to most people. What does it mean that the patient forgets to pay? Does it mean ‘if you really cared about me you would not charge me’? Is it a reflection of anger for something that occurred in the last session? Is it a displacement of feelings from something else (‘my boss didn’t give me the raise I expected’)? Is it completely inadvertent (Freud famously said ‘Sometimes a cigar is only a cigar’)?”
There are so many possibilities, and the psychodynamic therapist wants to understand them. How the patient relates to the therapist is some part of how he relates to others. The patient hopefully starts to watch his own actions and attitudes and also tries to understand them. A nonjudgmental stance helps the patient do this.
The therapist himself needs to be comfortable dealing with the subject of money. Sometimes beginning physicians fluctuate between feeling they are too inexperienced to be paid and feeling that they deserve anything they ask. We physicians might even (unfortunately) take on the attitudes of the insurance companies themselves (“Identification with the Aggressor”).
The blog post was flooded with comments –120 in total, and more comments than any other single Shrink Rap post received for the blog’s 12-year run.
Steven Reidbord, MD, is a psychiatrist in private practice in San Francisco and also a blogger. Dr. Reidbord conversed with me through email about patients who don’t pay.
“In years past, I’ve had a few patients who met with me a few times, always ‘forgetting’ their payment and offering it ‘next time,’ until after three or four sessions, I refused to see them. I always wondered what such patients were thinking, as obviously this arrangement wouldn’t last long. Did they tell themselves they’d pay me at some point, in effect fooling themselves? Was it conscious theft of my services? A couple years ago, I started accepting charge cards, and perhaps as a result, this hasn’t happened. While it’s always useful to consider individual dynamics in explaining such behavior, it’s also important to consider normative psychology: Make it easier to pay, and more people will.”
While payment for out-of-network services is often clear cut – the patients pay and then requests reimbursement from their insurer – the logistics often are confusing for the patient. He or she may believe that she has excellent coverage, only to learn that the out-of-network deductible is very high, or that reimbursement is based on “usual and customary fees” that are much lower than his psychiatrist’s fees. Sometimes people take on the cost of psychiatric care and discover that it costs more than they assumed, or they have a change in their financial circumstances, as my patient did. Sometimes a parent is paying for treatment and decides he can no longer afford it.
“When someone’s financial circumstances change, they often let me know by proposing we meet less often, for example, every other week,” Dr. Reidbord wrote. “I ask to hear more and often offer to adjust my fee to allow weekly meetings to continue.”
Not all patients pay for psychiatric services, and that may make the discussion even harder. When psychiatrists participate with insurance, the patients are responsible for paying only their deductible and then a copay. The patients may unexpectedly be billed for the entire fee if their insurance terminates, or if it does not pay for a submitted claim. And patients who carry public insurance may be seen at sites where there is no out-of-pocket cost to the patient; salaried clinicians often never know if the insurance has paid. In both of these settings, finances are usually discussed with administrative personnel and not with clinical staff.
Anthony Massey, MD, is founder of Maryland’s Gladstone Psychiatry and Wellness. The group is a multidisciplinary organization, and the clinicians participate with employer-based commercial health insurances. The group accepts payment directly from the insurer, and the patient is responsible for payment of the deductible and a copay.
“We try to understand what someone owes before the first appointment. We do an eligibility check online, and we ask for payment at the time of the appointment,” Massey explained. “Sometimes the insurance changes and we don’t know, or sometimes a patient comes to the appointment without the copay. We try to work with people, but if someone builds up a balance over $500, we tell them they can’t be seen here until it’s paid down. We’ll give patients a 30-day prescription and the names of other psychiatrists who accept insurance, but we don’t keep seeing people who don’t pay for their treatment.”
In all medical settings, unpaid bills present a problem, and while most psychiatrists have a method to deal with these issues, there is no perfect answer for every doctor in every situation. There is this tension between wanting to be kind and understanding of the hardships that people have whether those hardships result from life circumstances or from their own choices and behaviors, and of our own need to make the living we feel we deserve and to pay our own bills.
“The only advice I’d give other psychiatrists is to catch it early,” Reidbord said. “ – and stick with it. If a patient doesn’t pay according to your clearly stated policy, explore it right away. Remember that pragmatic issues like poor budgeting or unexpected expenses are just as likely as intrapsychic conflict and ‘acting out.’ Both should be considered.”
I wish I could say that in the decades since I treated Mr. A that no patients have ever failed to pay their professional fees and that I have perfectly mastered my own issues with money as it pertains to professional fees. While the vast majority of patients do pay, there are still occasional circumstances in which someone’s financial circumstances change, or very rarely where someone ends his treatment without paying for the last few sessions.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
Editor’s note: This is the second in a series of articles by Dr. Miller about challenges in outpatient psychiatry.
Mr. A lived a life that seemed glamorous to me. There were lunches with professional athletes, swank social events, and designer everything from clothes to cars. It was a world that I was not familiar with, and while I knew my patient worried about money, it seemed he had it. When he started therapy with me, Mr. A came to every session with a check. At some point, I realized that I had missed a switch in his mood. Despite his therapeutic level of lithium, Mr. A had become manic, and the expression of this mania took the form of even more spending. What started as an exciting lifestyle suddenly became tens of thousands of dollars of debt. I felt guilty that I initially did not see this as pathology, and as a young psychiatrist, I sought consultation with an older and wiser mentor.
After discussing the case, the consulting psychiatrist said to me, “Make sure you get paid; these cases are difficult.” In fact, in the midst of the chaos, Mr. A had stopped bringing a check to the sessions. I sent monthly statements, and they went unpaid. He didn’t have the money for his living expenses and I let this go on without addressing it for much too long. Soon, Mr. A’s debt to me was just one more stress in his life and while I knew I would not pursue reimbursement through legal channels, I did encourage him to find a psychiatrist who was in his insurance network, something he did not want to do. I was too embarrassed to tell the consulting psychiatrist that Mr. A had already accrued well over a thousand dollars in unpaid professional fees. In my mind, I was offering pro bono care because the patient’s financial circumstances had changed, and because I felt guilty that I had not recognized this as mania sooner.
In a 2011 Shrink Rap blog post, Jesse Hellman, MD, a psychiatrist in private practice in Towson, Md., wrote about the meaning of payment in psychotherapy:
“Money is something loaded with meaning to most people. What does it mean that the patient forgets to pay? Does it mean ‘if you really cared about me you would not charge me’? Is it a reflection of anger for something that occurred in the last session? Is it a displacement of feelings from something else (‘my boss didn’t give me the raise I expected’)? Is it completely inadvertent (Freud famously said ‘Sometimes a cigar is only a cigar’)?”
There are so many possibilities, and the psychodynamic therapist wants to understand them. How the patient relates to the therapist is some part of how he relates to others. The patient hopefully starts to watch his own actions and attitudes and also tries to understand them. A nonjudgmental stance helps the patient do this.
The therapist himself needs to be comfortable dealing with the subject of money. Sometimes beginning physicians fluctuate between feeling they are too inexperienced to be paid and feeling that they deserve anything they ask. We physicians might even (unfortunately) take on the attitudes of the insurance companies themselves (“Identification with the Aggressor”).
The blog post was flooded with comments –120 in total, and more comments than any other single Shrink Rap post received for the blog’s 12-year run.
Steven Reidbord, MD, is a psychiatrist in private practice in San Francisco and also a blogger. Dr. Reidbord conversed with me through email about patients who don’t pay.
“In years past, I’ve had a few patients who met with me a few times, always ‘forgetting’ their payment and offering it ‘next time,’ until after three or four sessions, I refused to see them. I always wondered what such patients were thinking, as obviously this arrangement wouldn’t last long. Did they tell themselves they’d pay me at some point, in effect fooling themselves? Was it conscious theft of my services? A couple years ago, I started accepting charge cards, and perhaps as a result, this hasn’t happened. While it’s always useful to consider individual dynamics in explaining such behavior, it’s also important to consider normative psychology: Make it easier to pay, and more people will.”
While payment for out-of-network services is often clear cut – the patients pay and then requests reimbursement from their insurer – the logistics often are confusing for the patient. He or she may believe that she has excellent coverage, only to learn that the out-of-network deductible is very high, or that reimbursement is based on “usual and customary fees” that are much lower than his psychiatrist’s fees. Sometimes people take on the cost of psychiatric care and discover that it costs more than they assumed, or they have a change in their financial circumstances, as my patient did. Sometimes a parent is paying for treatment and decides he can no longer afford it.
“When someone’s financial circumstances change, they often let me know by proposing we meet less often, for example, every other week,” Dr. Reidbord wrote. “I ask to hear more and often offer to adjust my fee to allow weekly meetings to continue.”
Not all patients pay for psychiatric services, and that may make the discussion even harder. When psychiatrists participate with insurance, the patients are responsible for paying only their deductible and then a copay. The patients may unexpectedly be billed for the entire fee if their insurance terminates, or if it does not pay for a submitted claim. And patients who carry public insurance may be seen at sites where there is no out-of-pocket cost to the patient; salaried clinicians often never know if the insurance has paid. In both of these settings, finances are usually discussed with administrative personnel and not with clinical staff.
Anthony Massey, MD, is founder of Maryland’s Gladstone Psychiatry and Wellness. The group is a multidisciplinary organization, and the clinicians participate with employer-based commercial health insurances. The group accepts payment directly from the insurer, and the patient is responsible for payment of the deductible and a copay.
“We try to understand what someone owes before the first appointment. We do an eligibility check online, and we ask for payment at the time of the appointment,” Massey explained. “Sometimes the insurance changes and we don’t know, or sometimes a patient comes to the appointment without the copay. We try to work with people, but if someone builds up a balance over $500, we tell them they can’t be seen here until it’s paid down. We’ll give patients a 30-day prescription and the names of other psychiatrists who accept insurance, but we don’t keep seeing people who don’t pay for their treatment.”
In all medical settings, unpaid bills present a problem, and while most psychiatrists have a method to deal with these issues, there is no perfect answer for every doctor in every situation. There is this tension between wanting to be kind and understanding of the hardships that people have whether those hardships result from life circumstances or from their own choices and behaviors, and of our own need to make the living we feel we deserve and to pay our own bills.
“The only advice I’d give other psychiatrists is to catch it early,” Reidbord said. “ – and stick with it. If a patient doesn’t pay according to your clearly stated policy, explore it right away. Remember that pragmatic issues like poor budgeting or unexpected expenses are just as likely as intrapsychic conflict and ‘acting out.’ Both should be considered.”
I wish I could say that in the decades since I treated Mr. A that no patients have ever failed to pay their professional fees and that I have perfectly mastered my own issues with money as it pertains to professional fees. While the vast majority of patients do pay, there are still occasional circumstances in which someone’s financial circumstances change, or very rarely where someone ends his treatment without paying for the last few sessions.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore. Patient details were altered to preserve confidentiality.
Combined laser-topical therapy improves erythema associated with rosacea
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
DENVER – Among patients with facial erythema associated with erythrotelangiectatic rosacea, combining a long-pulsed 532 nm laser with daily application of a topical skin care regimen achieved equivalent to superior results in fewer treatments, compared with long-pulsed laser treatment alone.
The findings come from a pilot trial that Brian S. Biesman, MD, presented at the annual conference of the American Society for Laser Medicine and Surgery.
“Vascular laser therapy is the standard of care for reduction of facial erythema associated with erythrotelangiectatic rosacea,” said Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn. “The question was, if we combine topicals plus laser, can we get an enhanced outcome relative to laser treatment alone?”
To find out, he and his colleagues conducted a blinded, controlled prospective study of 30 subjects with mild to moderate erythrotelangiectatic rosacea who were evenly split into two groups. Those in group 1 received three treatments with the Excel V 532 nm long-pulsed laser by Cutera. Those in group 2 received two laser treatments with the Excel V long-pulsed 532 nm long-pulsed laser plus concurrent daily use of the topical Jan Marini Skin Care Management System, which included a glycolic acid cleanser, vitamin C serum, active containing glycolic, salicylic and azelaic acids, peptide, and growth factor moisturizer and a broad-spectrum sunscreen. It also contained RosaLieve, a proprietary redness-reducing complex.
The researchers performed laser treatments at 4-week intervals and evaluated subjects at baseline, 4, 8, and 12 weeks by physician and subject self-assessment using 5-point (0-4) standardized scales: the Clinician Erythema Assessment (CEA) and patient self-assessment as well as a dermatology Quality of Life Assessment. In both treatment groups, reduction in facial erythema as assessed by CEA and patient self-assessment showed statistically significant improvement at all measured intervals. Specifically, average CEA scores improved from 3.00 to 1.87 among patients in group 1, and from 3.07 to 1.64 among those in group 2. “These were both statistically significant from baseline,” Dr. Biesman said. “What does it really say? The laser plus topical was superior to the laser-only treatment at all measured intervals. I didn’t expect to see that. There was continued improvement noted from week 8 to week 12. That was more of a trend; it was not statistically significant. There were no complications or adverse reactions in either group. The study data indicate that best results may be achieved with a combination of laser and home care.”
He acknowledged certain limitations of the study, including its small sample size and relatively short course of follow-up. “We didn’t have standardization of topical therapy in the laser-only group,” Dr. Biesman said. “Those patients were told to use their usual topical regimen. They were not allowed to use retinoids. We also didn’t have a control arm.”
He disclosed that he has received grant funding from Jan Marini Skin Research and Cutera.
REPORTING FROM ASLMS 2019
Novel body contouring device targets muscle, not fat
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
DENVER –
The device, known as CoolTone, is being developed by Allergan and uses high-powered coil electromagnetic stimulation applicators to induce eddy currents in the muscle tissue. CoolTone is pending Food and Drug Administration clearance and is not yet commercially available.
“Fat reduction is just one part of body contouring,” Mathew M. Avram, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “You have skin, fat, and muscle. More and more we’re targeting all three areas for patients’ best body contouring outcomes.”
According to Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston, CoolTone provides high-frequency electromagnetic muscle stimulation that triggers muscle contractions that cannot be achieved by normal exercise to increase muscle mass and strength. “You’re doing super physiological amounts of contractions with this stimulation – the equivalent of doing thousands of sit-ups, if you’re treating the abdomen,” he said. “It strengthens, tones, and firms muscles in abdomen, buttocks, arms, and legs. There is a history of this type of technology for athletes and other indications in physical therapy.”
The current FDA clearance for a predicate electromagnetic stimulation system for muscle conditioning is for the abdomen, buttocks, thighs, and arms. “This is for improvement of abdominal tone, strengthening of the abdominal muscles, and development of a firmer abdomen,” said Dr. Avram, who also is director of dermatologic surgery at Mass General. “It’s for strengthening, toning, and firming of buttocks and thighs, and for improvement of muscle tone and firmness, and for strengthening muscle in arms.”
The electrical current induced by the CoolTone device flows readily into muscle and not into fat, he continued. This brings the current to nearby motor nerve structures that stimulate contraction once the action potential is reached. “You’re getting maximal contractions that are extreme for a full range of muscle fibers,” explained Dr. Avram, who is the immediate past president of the ASLMS. “This requires an external electrical stimulus; it’s not something you do with normal exercise. With mild exercise, only the slow-twitch muscle fibers are activated, not the fast-twitch muscle fibers. Also, the pulsing sequences are designed to preferentially excite motor nerves rather than sensory nerves. So it’s really going after the ability for you to contract your muscles as much as possible.”
Dr. Avram has received consulting fees from Merz and Alastin and holds ownership interests with ZALEA, InMode, and Cytrellis. He has served on the advisory boards for ZELTIQ Aesthetics, Soliton, Sciton, and Sienna Biopharmaceuticals, and he has intellectual property rights with Cytrellis.
EXPERT ANALYSIS FROM ASLMS 2019
TNF-alpha, adiponectin potential biomarkers for PsA, psoriasis differentiation
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
What is medical marijuana actually useful for?
PHILADELPHIA – , some insights into adverse effects, and “a lot of the Wild West,” Ellie Grossman, MD, MPH, said here at the annual meeting of the American College of Physicians.
The opioid-sparing effects of medical marijuana have been highlighted in recent reports suggesting that cannabis users may use less opioids, and that states with medical marijuana laws have seen drops in opioid overdose mortality, Dr. Grossman said.
“That’s kind of a story on pain and cannabinoids, and that’s really the biggest story there is in terms of medical evidence and effectiveness for this agent,” said Dr. Grossman, an instructor at Harvard Medical School and Primary Care Lead for Behavioral Health Integration, Cambridge Health Alliance, Somerville, Mass.
However, being the top story in medical marijuana may not be a very high bar in 2019, given current issues with research in this area, including inconsistencies in medical marijuana formulations, relatively small numbers of patients enrolled in studies, and meta-analyses that have produced equivocal results.
“Unfortunately, this is an area where there’s a lot of, shall I say, ‘squishiness’ in the data, through no fault of the researchers involved – it’s just an area that’s really hard to study,” Dr. Goodman said in her update on medical marijuana use at the meeting.
Most studies of cannabinoids for chronic pain have compared these agents to placebo, rather than the long list of other medications that might be used to treat pain, Dr. Grossman said.
There are several meta-analyses available, including a recently published Cochrane review in which authors concluded that, for neuropathic pain, the potential benefits of cannabis-based medicines may outweigh their potential harms.
“The upshot here is that there may be some evidence for neuropathic pain, but the evidence is generally of poor quality and kind of mixed,” said Dr. Grossman.
State-level medical cannabis laws were linked to significantly lower opioid overdose mortality rates in a 2014 study (JAMA Intern Med. 2014;174[10]:1668-73). In more recent studies, states with medical cannabis laws were found to have lower Medicare Part D opioid-prescribing rates, and in another study, legalization of medical marijuana was linked to lower rates of chronic and high-risk opioid use.
“It certainly seems like maybe we as prescribers are prescribing [fewer] opioids if there’s medical cannabis around,” Dr. Grossman said. “What this means for our patients in the short term and long term, we don’t totally know. But clearly, fewer opioid overdoses is a way better thing than more, so there could be something here.”
The cannabinoids approved by the Food and Drug Administration include nabilone (Cesamet) and dronabinol (Marinol), both synthetic cannabinoids indicated for cancer chemotherapy–related nausea and vomiting, along with cannabidiol (Epidiolex), just approved in June 2018 for treatment of some rare pediatric refractory epilepsy syndromes, Dr. Grossman said.
For chemotherapy-induced nausea and vomiting, evidence suggests oral cannabinoids are more effective than placebo, but there’s mixed evidence as to whether they are better than other antiemetics, Dr. Grossman said, while in terms of spasticity related to multiple sclerosis, research has shown small improvements in patient-reported symptoms.
Long-term adverse event data specific to medical marijuana are scant, with much of the evidence coming from studies of recreational marijuana users, Dr. Grossman said.
Those long-term effects include increased risk of pulmonary effects such as cough, wheeze, and phlegm that improve with discontinuation; case reports of unintentional pediatric ingestions; and lower neonatal birth weight, which should be discussed with women of reproductive age who are using or considering medical marijuana, Dr. Grossman said.
Motor vehicle accidents, development of psychiatric symptoms, and psychosis relapse also have been linked to use, she said.
Some real-world adverse event data specific to medical marijuana data are available through the Minnesota medical cannabis program. They found 16% of surveyed users reported an adverse event within the first 4 months, including dry mouth, fatigue, mental clouding, and drowsiness, Dr. Grossman told attendees.
Dr. Grossman reported that she has no relationship with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
SOURCE: Grossman E. ACP 2019, Presentation MTP 010.
PHILADELPHIA – , some insights into adverse effects, and “a lot of the Wild West,” Ellie Grossman, MD, MPH, said here at the annual meeting of the American College of Physicians.
The opioid-sparing effects of medical marijuana have been highlighted in recent reports suggesting that cannabis users may use less opioids, and that states with medical marijuana laws have seen drops in opioid overdose mortality, Dr. Grossman said.
“That’s kind of a story on pain and cannabinoids, and that’s really the biggest story there is in terms of medical evidence and effectiveness for this agent,” said Dr. Grossman, an instructor at Harvard Medical School and Primary Care Lead for Behavioral Health Integration, Cambridge Health Alliance, Somerville, Mass.
However, being the top story in medical marijuana may not be a very high bar in 2019, given current issues with research in this area, including inconsistencies in medical marijuana formulations, relatively small numbers of patients enrolled in studies, and meta-analyses that have produced equivocal results.
“Unfortunately, this is an area where there’s a lot of, shall I say, ‘squishiness’ in the data, through no fault of the researchers involved – it’s just an area that’s really hard to study,” Dr. Goodman said in her update on medical marijuana use at the meeting.
Most studies of cannabinoids for chronic pain have compared these agents to placebo, rather than the long list of other medications that might be used to treat pain, Dr. Grossman said.
There are several meta-analyses available, including a recently published Cochrane review in which authors concluded that, for neuropathic pain, the potential benefits of cannabis-based medicines may outweigh their potential harms.
“The upshot here is that there may be some evidence for neuropathic pain, but the evidence is generally of poor quality and kind of mixed,” said Dr. Grossman.
State-level medical cannabis laws were linked to significantly lower opioid overdose mortality rates in a 2014 study (JAMA Intern Med. 2014;174[10]:1668-73). In more recent studies, states with medical cannabis laws were found to have lower Medicare Part D opioid-prescribing rates, and in another study, legalization of medical marijuana was linked to lower rates of chronic and high-risk opioid use.
“It certainly seems like maybe we as prescribers are prescribing [fewer] opioids if there’s medical cannabis around,” Dr. Grossman said. “What this means for our patients in the short term and long term, we don’t totally know. But clearly, fewer opioid overdoses is a way better thing than more, so there could be something here.”
The cannabinoids approved by the Food and Drug Administration include nabilone (Cesamet) and dronabinol (Marinol), both synthetic cannabinoids indicated for cancer chemotherapy–related nausea and vomiting, along with cannabidiol (Epidiolex), just approved in June 2018 for treatment of some rare pediatric refractory epilepsy syndromes, Dr. Grossman said.
For chemotherapy-induced nausea and vomiting, evidence suggests oral cannabinoids are more effective than placebo, but there’s mixed evidence as to whether they are better than other antiemetics, Dr. Grossman said, while in terms of spasticity related to multiple sclerosis, research has shown small improvements in patient-reported symptoms.
Long-term adverse event data specific to medical marijuana are scant, with much of the evidence coming from studies of recreational marijuana users, Dr. Grossman said.
Those long-term effects include increased risk of pulmonary effects such as cough, wheeze, and phlegm that improve with discontinuation; case reports of unintentional pediatric ingestions; and lower neonatal birth weight, which should be discussed with women of reproductive age who are using or considering medical marijuana, Dr. Grossman said.
Motor vehicle accidents, development of psychiatric symptoms, and psychosis relapse also have been linked to use, she said.
Some real-world adverse event data specific to medical marijuana data are available through the Minnesota medical cannabis program. They found 16% of surveyed users reported an adverse event within the first 4 months, including dry mouth, fatigue, mental clouding, and drowsiness, Dr. Grossman told attendees.
Dr. Grossman reported that she has no relationship with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
SOURCE: Grossman E. ACP 2019, Presentation MTP 010.
PHILADELPHIA – , some insights into adverse effects, and “a lot of the Wild West,” Ellie Grossman, MD, MPH, said here at the annual meeting of the American College of Physicians.
The opioid-sparing effects of medical marijuana have been highlighted in recent reports suggesting that cannabis users may use less opioids, and that states with medical marijuana laws have seen drops in opioid overdose mortality, Dr. Grossman said.
“That’s kind of a story on pain and cannabinoids, and that’s really the biggest story there is in terms of medical evidence and effectiveness for this agent,” said Dr. Grossman, an instructor at Harvard Medical School and Primary Care Lead for Behavioral Health Integration, Cambridge Health Alliance, Somerville, Mass.
However, being the top story in medical marijuana may not be a very high bar in 2019, given current issues with research in this area, including inconsistencies in medical marijuana formulations, relatively small numbers of patients enrolled in studies, and meta-analyses that have produced equivocal results.
“Unfortunately, this is an area where there’s a lot of, shall I say, ‘squishiness’ in the data, through no fault of the researchers involved – it’s just an area that’s really hard to study,” Dr. Goodman said in her update on medical marijuana use at the meeting.
Most studies of cannabinoids for chronic pain have compared these agents to placebo, rather than the long list of other medications that might be used to treat pain, Dr. Grossman said.
There are several meta-analyses available, including a recently published Cochrane review in which authors concluded that, for neuropathic pain, the potential benefits of cannabis-based medicines may outweigh their potential harms.
“The upshot here is that there may be some evidence for neuropathic pain, but the evidence is generally of poor quality and kind of mixed,” said Dr. Grossman.
State-level medical cannabis laws were linked to significantly lower opioid overdose mortality rates in a 2014 study (JAMA Intern Med. 2014;174[10]:1668-73). In more recent studies, states with medical cannabis laws were found to have lower Medicare Part D opioid-prescribing rates, and in another study, legalization of medical marijuana was linked to lower rates of chronic and high-risk opioid use.
“It certainly seems like maybe we as prescribers are prescribing [fewer] opioids if there’s medical cannabis around,” Dr. Grossman said. “What this means for our patients in the short term and long term, we don’t totally know. But clearly, fewer opioid overdoses is a way better thing than more, so there could be something here.”
The cannabinoids approved by the Food and Drug Administration include nabilone (Cesamet) and dronabinol (Marinol), both synthetic cannabinoids indicated for cancer chemotherapy–related nausea and vomiting, along with cannabidiol (Epidiolex), just approved in June 2018 for treatment of some rare pediatric refractory epilepsy syndromes, Dr. Grossman said.
For chemotherapy-induced nausea and vomiting, evidence suggests oral cannabinoids are more effective than placebo, but there’s mixed evidence as to whether they are better than other antiemetics, Dr. Grossman said, while in terms of spasticity related to multiple sclerosis, research has shown small improvements in patient-reported symptoms.
Long-term adverse event data specific to medical marijuana are scant, with much of the evidence coming from studies of recreational marijuana users, Dr. Grossman said.
Those long-term effects include increased risk of pulmonary effects such as cough, wheeze, and phlegm that improve with discontinuation; case reports of unintentional pediatric ingestions; and lower neonatal birth weight, which should be discussed with women of reproductive age who are using or considering medical marijuana, Dr. Grossman said.
Motor vehicle accidents, development of psychiatric symptoms, and psychosis relapse also have been linked to use, she said.
Some real-world adverse event data specific to medical marijuana data are available through the Minnesota medical cannabis program. They found 16% of surveyed users reported an adverse event within the first 4 months, including dry mouth, fatigue, mental clouding, and drowsiness, Dr. Grossman told attendees.
Dr. Grossman reported that she has no relationship with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
SOURCE: Grossman E. ACP 2019, Presentation MTP 010.
AT INTERNAL MEDICINE 2019
PIONEER-HF Extension: Don’t stall starting sacubitril/valsartan
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
NEW ORLEANS – Waiting a few months after a patient has been hospitalized for acute decompensated heart failure before launching a switch from enalapril to sacubitril/valsartan imposes a steep price in terms of extra major cardiovascular events, compared with starting the angiotensin-neprilysin inhibitor during the initial hospitalization, according to the open-label extension of the PIONEER-HF trial.
“We think these data have important clinical implications: While sacubitril/valsartan decreases NT-proBNP compared with enalapril regardless of when it is initiated, the early improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with acute decompensated heart failure,” Adam D. DeVore, MD, declared in presenting the PIONEER-HF Extension results at the annual meeting of the American College of Cardiology.
PIONEER-HF was a landmark, practice-changing, double-blind clinical trial in which 881 patients were randomized to initiation of sacubitril/valsartan (Entresto) or enalapril during hospitalization for acute decompensated heart failure. In the previously reported main outcome, 8 weeks after discharge the sacubitril/valsartan group had a 29% greater reduction in NT-proBNP (the N-terminal prohormone of brain natriuretic peptide) and a 42% lower rate of the composite clinical endpoint of cardiovascular death or heart failure rehospitalization than the enalapril group (N Engl J Med. 2019 Feb 7;380[6]:539-48).
The 4-week open-label extension of PIONEER-HF began at week 8, when participants initially randomized to enalapril during the double-blind phase were switched to sacubitril/valsartan, while those assigned to in-hospital initiation of the angiotensin-neprilysin inhibitor (ARNI) stayed the course.
At week 12, after 4 weeks of open-label treatment, patients on sacubitril/valsartan from the start experienced an additional 18.5% drop in NT-proBNP from their week-8 baseline of 1,218 pg/mL. Meanwhile, the NT-proBNP level in the switch group plunged by 35.8% from a week-8 baseline of 1,630 pg/mL. As a result, both groups ended up at the same much-improved biomarker level at week 12, observed Dr. DeVore, a cardiologist at Duke University in Durham, N.C.
Clinical event rates, however, were another story altogether. The clinical event gap between the two study arms documented at week 8 in the double-blind phase of the trial didn’t close significantly in the 4 weeks after the enalapril group crossed over to open-label sacubitril/valsartan. Indeed, the relative risk of the composite endpoint of cardiovascular death, heart failure rehospitalization, or left ventricular assist device implantation during the 4-week extension phase was 33% lower in the continuous sacubitril/valsartan group than in the switchers. The absolute risk reduction was 5.6%, with a favorable number needed to treat of 18.
This difference was driven mainly by less rehospitalization for heart failure. Few cardiovascular deaths or LVAD implantations occurred during the relatively brief 4-week extension phase of the trial.
“But this is an important thing as we think about what we’re trying to accomplish in heart failure: trying to find tools that improve rehospitalization rates after people leave the hospital is extremely important,” Dr. DeVore said. “We do know that the really vulnerable period for rehospitalization is early on, so my sus```picion – though I can’t prove it – is that’s the important part. That’s when we need to have patients on the best therapy.”
He was asked how practical it is to initiate sacubitril/valsartan during hospitalization for acute decompensated heart failure in real-world clinical practice, given that it can be done only after patients achieve hemodynamic stability.
“I think the definition of hemodynamic stability we used in the trial was a fairly straightforward one, very clinical, and one we can translate to the bedside,” Dr. DeVore replied. “Patients had to have a systolic blood pressure of 100 mm Hg or greater for 6 hours, which is easily documented in the hospital, no changes in IV diuretics or IV vasodilators for 6 hours, and no IV inotropes for the last 24 hours. That’s how we defined hemodynamic stability. I think we should be able to find these patients.”
Average length of stay in the index hospitalization in PIONEER-HF was just over 5 days, but the study protocol actually resulted in longer-than-needed hospitalization because it required that patients had to receive three double-blind doses of their study medication before discharge. In routine practice, it’s unlikely that in-hospital initiation of sacubitril/valsartan will result in a length of stay greater than the national average of about 4.5 days, according the cardiologist.
Current ACC/American Heart Association/Heart Failure Society of American guidelines on management of heart failure include a Class Ia recommendation to switch patients from an ACE inhibitor or angiotensin inhibitor to sacubitril/valsartan (Circulation. 2017 Aug 8;136[6]:e137-61).
However, heart failure specialists are concerned by national data showing that sacubitril/valsartan remains widely underprescribed.
Dr. DeVore reported serving as a consultant to Novartis and receiving research grants from a half dozen pharmaceutical companies as well as the American Heart Association, National Heart, Lung, and Blood Institute, and the Patient-Centered Outcomes Research Institute .
REPORTING FROM ACC 19
Key clinical point: over the next 12 weeks, compared with initiation of enalapril followed by a delayed switch to sacubitril/valsartan at 8 weeks.
Major finding: The number needed to treat with in-hospital initiation of sacubitril/valsartan instead of enalapril to avoid one cardiovascular death, heart failure rehospitalization, or implantation of a left ventricular assist device was 18.
Study details: The PIONEER-HF Extension study included 881 heart failure patients, all on open-label sacubitril/valsartan during the 4-week extension phase.
Disclosures: The study was sponsored by AstraZeneca. The presenter reported receiving research grants from and serving as a consultant to the company.