User login
Atmospheric fluctuations tied to lupus flares
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
SAN FRANCISCO – , according to investigators from Johns Hopkins University, Baltimore.
The work helps solve a longstanding mystery in systemic lupus erythematosus (SLE): why symptoms seem to come and go with the seasons.
Johns Hopkins University research previously has shown that renal flares are more common in the winter; double-stranded DNA antibodies more common in late fall; and rashes more likely in late spring.
However, “the exact reasons of why the seasonality was there remained a big question,” said lead investigator George Stojan, MD, an assistant professor of rheumatology and the codirector of the Hopkins Lupus Center.
To get a handle on the matter, Dr. Stojan and his team reviewed 1,628 patients treated at the university during 1999-2017. Using Environmental Protection Agency data, they examined atmospheric conditions within 350 km of Baltimore in the 10 days leading up to lupus visits for flares; the researchers adjusted for age, sex, income, ethnicity, rural versus urban residence, and how close patients lived to highways and airports.
“We [found] specific, strong associations between atmospheric variables and fine particulate matter concentrations ... and organ-specific lupus flares,” Dr. Stojan said. He explained why that matters in a video interview at an international congress on SLE.
In short, rash was directly associated with concentrations of ozone and inhalable, fine particulate matter less than 2.5 mcm in diameter (PM 2.5). Joint flares were associated with PM 2.5, ozone, resultant wind, and humidity.
Renal flares were inversely associated with temperature, and directly associated with wind and humidity. Pulmonary flares and serositis were associated with PM 2.5, and both hematologic and neurologic flares with wind and temperature.
The analysis was based on a per-unit basis. For example, each mcg/m3 increase in PM 2.5 increased the odds of a pulmonary flare about 4% (odds ratio, 1.042, P = .026). The other findings were mostly of smaller magnitude, but still statistically significant.
The National Institutes of Health funded the research. Dr. Stojan had no disclosures.
SOURCE: Stojan G et al. LUPUS 2019, Abstract M31.
REPORTING FROM LUPUS 2019
How to handle negative online reviews
It happens to all of us: You log onto the Internet one day and discover a scathing review from a disgruntled patient or family member, usually complaining about something totally irrelevant to the excellent care they received.
![]()
Your first impulse may be to post a response, but wait – it turns out that “protected health information” is more liberally defined than most of us think. If you include any information that could be used to identify the patient, you can be considered in violation of HIPAA. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights; and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
In 2013, a California hospital paid $275,000 to settle claims that it violated HIPAA when it disclosed a patient’s health information in response to a negative review. And the Department of Health & Human Services, which enforces HIPAA, has sent warning letters to a variety of physicians and dentists who divulged patient information while responding to reviews. (An HHS spokesperson couldn’t tell me how many such warnings have been issued, because they “don’t track complaints that way.”)
All of that said, :
- Ignore them. This is your best choice 90% of the time. Most negative reviews have minimal impact and simply do not deserve a response, and responding may simply pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site, and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated. If your total number of reviews on that site is too small – for example, there are only 4, and 2 are bad – you have what I call a denominator problem. The solution in those cases is to increase the denominator – that is, increase the total number of reviews. The more you can obtain, the less impact the complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, in your e-mail reminders, or simply leave a stack of requests at your check-out desk and tell your receptionist to hand them out. To be clear, you must encourage all reviews, good or bad, not just favorable ones; if you specify that all reviews must be favorable, you are “filtering,” which can be perceived as false or deceptive advertising.
- Respond generically. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies, or direct readers to positive reviews without referencing any individual cases. You might point out that HIPAA prevents you from disclosing information in response. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. In select situations, this has been very effective for me; in one case, the patient not only removed the negative post, but also became a loyal supporter. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can at least explain that on the site, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely, and I will address that in next month’s column.
Regardless of how you handle your negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It happens to all of us: You log onto the Internet one day and discover a scathing review from a disgruntled patient or family member, usually complaining about something totally irrelevant to the excellent care they received.
![]()
Your first impulse may be to post a response, but wait – it turns out that “protected health information” is more liberally defined than most of us think. If you include any information that could be used to identify the patient, you can be considered in violation of HIPAA. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights; and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
In 2013, a California hospital paid $275,000 to settle claims that it violated HIPAA when it disclosed a patient’s health information in response to a negative review. And the Department of Health & Human Services, which enforces HIPAA, has sent warning letters to a variety of physicians and dentists who divulged patient information while responding to reviews. (An HHS spokesperson couldn’t tell me how many such warnings have been issued, because they “don’t track complaints that way.”)
All of that said, :
- Ignore them. This is your best choice 90% of the time. Most negative reviews have minimal impact and simply do not deserve a response, and responding may simply pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site, and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated. If your total number of reviews on that site is too small – for example, there are only 4, and 2 are bad – you have what I call a denominator problem. The solution in those cases is to increase the denominator – that is, increase the total number of reviews. The more you can obtain, the less impact the complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, in your e-mail reminders, or simply leave a stack of requests at your check-out desk and tell your receptionist to hand them out. To be clear, you must encourage all reviews, good or bad, not just favorable ones; if you specify that all reviews must be favorable, you are “filtering,” which can be perceived as false or deceptive advertising.
- Respond generically. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies, or direct readers to positive reviews without referencing any individual cases. You might point out that HIPAA prevents you from disclosing information in response. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. In select situations, this has been very effective for me; in one case, the patient not only removed the negative post, but also became a loyal supporter. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can at least explain that on the site, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely, and I will address that in next month’s column.
Regardless of how you handle your negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It happens to all of us: You log onto the Internet one day and discover a scathing review from a disgruntled patient or family member, usually complaining about something totally irrelevant to the excellent care they received.
![]()
Your first impulse may be to post a response, but wait – it turns out that “protected health information” is more liberally defined than most of us think. If you include any information that could be used to identify the patient, you can be considered in violation of HIPAA. This is true even if the patient has already disclosed information, because doing so does not nullify their HIPAA rights; and HIPAA provides no exceptions for responses. Even acknowledging that the reviewer was in fact your patient could, in some cases, be considered a violation.
In 2013, a California hospital paid $275,000 to settle claims that it violated HIPAA when it disclosed a patient’s health information in response to a negative review. And the Department of Health & Human Services, which enforces HIPAA, has sent warning letters to a variety of physicians and dentists who divulged patient information while responding to reviews. (An HHS spokesperson couldn’t tell me how many such warnings have been issued, because they “don’t track complaints that way.”)
All of that said, :
- Ignore them. This is your best choice 90% of the time. Most negative reviews have minimal impact and simply do not deserve a response, and responding may simply pour fuel on the fire. Besides, an occasional negative review actually lends credibility to a reviewing site, and to the positive reviews posted on that site. Polls show that readers are suspicious of sites that contain only rave reviews. They assume such reviews have been “whitewashed” – or just fabricated. If your total number of reviews on that site is too small – for example, there are only 4, and 2 are bad – you have what I call a denominator problem. The solution in those cases is to increase the denominator – that is, increase the total number of reviews. The more you can obtain, the less impact the complaints will have, since you know the overwhelming majority of your patients are happy with your care and will post a positive review if asked. Solicit them on your website, on social media, in your e-mail reminders, or simply leave a stack of requests at your check-out desk and tell your receptionist to hand them out. To be clear, you must encourage all reviews, good or bad, not just favorable ones; if you specify that all reviews must be favorable, you are “filtering,” which can be perceived as false or deceptive advertising.
- Respond generically. In those rare cases where you feel you must respond, do so without acknowledging that the individual was a patient, or disclosing any information that may be linked to the patient. For example, you can say that you provide excellent and appropriate care, or describe your general policies, or direct readers to positive reviews without referencing any individual cases. You might point out that HIPAA prevents you from disclosing information in response. Be polite, professional, and sensitive to the patient’s position. Readers tend to respect and sympathize with a doctor who responds in a professional, respectful manner and does not trash the complainant in retaliation.
- Take the discussion offline. Sometimes the person posting the review is just frustrated and wants to be heard. In those cases, consider contacting the patient and offering to discuss their concerns privately. In select situations, this has been very effective for me; in one case, the patient not only removed the negative post, but also became a loyal supporter. If you cannot resolve your differences, try to get the patient’s written permission to post a response to their review. If they refuse, you can at least explain that on the site, thereby capturing the moral high ground.
If the review contains false or defamatory content, that’s a different situation entirely, and I will address that in next month’s column.
Regardless of how you handle your negative reviews, be sure to learn from them. Your critics, as the song goes, are not always evil – and not always wrong. Complaints give you a chance to review your office policies and procedures and your own conduct, identify weaknesses, and make changes as necessary. At the very least, the exercise will help you to avoid similar complaints in the future. Don’t let valuable opportunities like that pass you by.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Evidence for endoscopic GERD treatments approaching critical mass
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
SAN FRANCISCO – It is now reasonable to conclude that many of the endoscopic devices and procedures developed for the treatment of gastroesophageal reflux disease (GERD) offer good short-term efficacy, leaving only the task to understand how these fit with competing options to improve quality of life long term, according to a state-of-the-art summary at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“The quality of the data for many of these devices has improved substantially, putting us in a much better place than we were in 4 or 5 years ago in considering their role,” reported Michael F. Vaezi, MD, PhD, professor of medicine, Vanderbilt University, Nashville, Tenn.
Over the past 15 years, an array of endoscopic approaches to treatment of GERD has received FDA approval. Examples of the very different techniques include plication devices that can suture, staple, or otherwise prevent reflux at the gastroesophageal junction (GEJ), and interventions aimed at the lower esophageal sphincter (LES), where placement of magnets or radiofrequency ablation has been employed to achieve a tighter defense against transient reflux episodes.
Despite FDA approval, the supportive evidence for many of these endoscopic interventions was criticized. In some cases, the number of patients evaluated in pivotal studies was considered too small. In others, there were objections to methodology, particularly to the choice of control arm. In all cases, there has been concern that follow-up was insufficient to confirm persistent benefit. Many of these criticisms are dissipating under the weight of more data.
“For most of the currently available, FDA-approved devices, there is now a substantial body of at least short-term data showing efficacy and safety,” reported Dr. Vaezi, who is a coauthor of an expert review now being prepared for publication. “This includes evidence that they improve quality of life, reduce the need for acid-suppressing therapy, and reduce esophageal acid exposure.”
Additional follow-up represents the final hurdle for understanding how these endoscopic interventions fit for extended symptom control. The long-term efficacy of the current standards of chronic proton pump inhibitor (PPI) therapy and surgical fundoplication has been established. Among these options, the choice is indefinite pharmacologic therapy or a surgical procedure. Endoscopic devices add additional options, but not with clear conclusions to be drawn on persistence of benefit.
Patient selection is an important consideration. Dr. Vaezi outlined three groups of patients: Patients who have responded to once-daily PPIs and are doing well, but would prefer not to take them indefinitely; PPI non-responders; and patients with improved heartburn but no improvement in regurgitation. Responders are reasonable candidates for endoscopic interventions, but non-responders are not, according to Dr. Vaezi. “You’re exposing the patient to the risk without the benefit, because they don’t have reflux. It’s something else,” he said.
Patients with improved heartburn but no change in regurgitation may be a candidate for endoscopic devices, as long as the clinician rules out non-reflux causes such as achalasia or gastroparesis.
“In patients being considered for alternative nonmedical therapy, it is essential to show that their symptoms are acid related. Those who do not respond to a PPI have traditionally not been good surgical candidates because the lack of a response suggests that acid reflux is not the source of their complaints. For patients being considered for an endoscopic treatment, we must apply the same time proven strategy. At this point, what is uncertain about the device therapies is the long-term durability for reflux control,” Dr. Vaezi said.
PPIs are effective for acid control, so the reason to consider an invasive treatment strategy is to avoid chronic PPI treatment. This is an increasingly attractive goal for many patients as a result of well-publicized case-control studies associating PPI use with a variety of increased risks, such as osteoporosis, chronic kidney disease, and gastrointestinal infections, but many gastroenterologists have been slow to recommend endoscopic interventions due to enduring concerns about safety and efficacy.
From his survey of the evidence, Dr. Vaezi characterized himself as “cautiously optimistic” that many of the endoscopic interventions will be included among standard options for durable GERD treatment.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Cost gap widens between brand-name, generic drugs
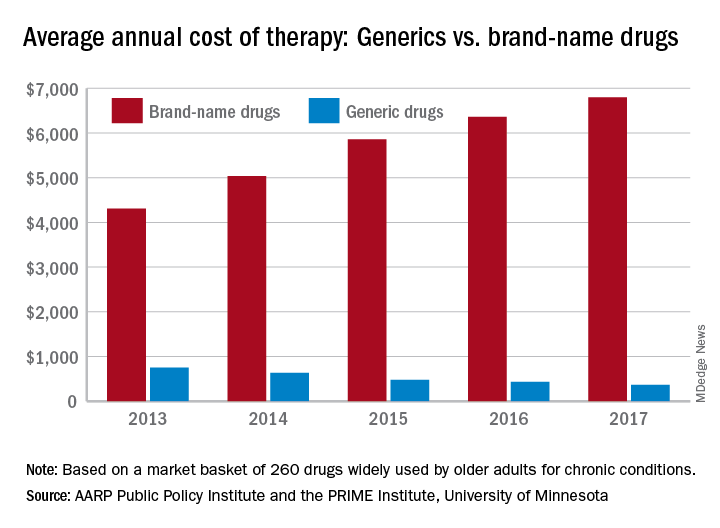
In 2017, the average retail cost of 260 generic drugs widely used by older adults for chronic conditions was $365 for a year of therapy, compared with $6,798 for brand-name drugs. In 2013, that same year of therapy with an average brand-name drug ($4,308) was only 5.7 times more expensive than the generic ($751), the AARP wrote in the report, produced in collaboration with the PRIME Institute at the University of Minnesota, Minneapolis.
“Generics account for nearly 9 out of every 10 prescriptions filled in the U.S. but represent less than a quarter of the country’s drug spending. These results highlight the importance of eliminating anticompetitive behavior by brand-name drug companies so that we get more lower-priced generic drugs on the market,” Debra Whitman, executive vice president and chief public policy officer at AARP, said in a written statement.
The average retail cost of a larger group of 390 generic drugs used by older adults fell by 9.3% from 2016 to 2017, compared with an increase of 8.4% for a group of 267 brand-name prescription drugs. Over that same time, the general inflation rate rose by 2.1%, the AARP noted.
The AARP’s annual Rx Price Watch Report is based on data from the Truven Health MarketScan research databases.

In 2017, the average retail cost of 260 generic drugs widely used by older adults for chronic conditions was $365 for a year of therapy, compared with $6,798 for brand-name drugs. In 2013, that same year of therapy with an average brand-name drug ($4,308) was only 5.7 times more expensive than the generic ($751), the AARP wrote in the report, produced in collaboration with the PRIME Institute at the University of Minnesota, Minneapolis.
“Generics account for nearly 9 out of every 10 prescriptions filled in the U.S. but represent less than a quarter of the country’s drug spending. These results highlight the importance of eliminating anticompetitive behavior by brand-name drug companies so that we get more lower-priced generic drugs on the market,” Debra Whitman, executive vice president and chief public policy officer at AARP, said in a written statement.
The average retail cost of a larger group of 390 generic drugs used by older adults fell by 9.3% from 2016 to 2017, compared with an increase of 8.4% for a group of 267 brand-name prescription drugs. Over that same time, the general inflation rate rose by 2.1%, the AARP noted.
The AARP’s annual Rx Price Watch Report is based on data from the Truven Health MarketScan research databases.

In 2017, the average retail cost of 260 generic drugs widely used by older adults for chronic conditions was $365 for a year of therapy, compared with $6,798 for brand-name drugs. In 2013, that same year of therapy with an average brand-name drug ($4,308) was only 5.7 times more expensive than the generic ($751), the AARP wrote in the report, produced in collaboration with the PRIME Institute at the University of Minnesota, Minneapolis.
“Generics account for nearly 9 out of every 10 prescriptions filled in the U.S. but represent less than a quarter of the country’s drug spending. These results highlight the importance of eliminating anticompetitive behavior by brand-name drug companies so that we get more lower-priced generic drugs on the market,” Debra Whitman, executive vice president and chief public policy officer at AARP, said in a written statement.
The average retail cost of a larger group of 390 generic drugs used by older adults fell by 9.3% from 2016 to 2017, compared with an increase of 8.4% for a group of 267 brand-name prescription drugs. Over that same time, the general inflation rate rose by 2.1%, the AARP noted.
The AARP’s annual Rx Price Watch Report is based on data from the Truven Health MarketScan research databases.
ACP leaders explain why they value telehealth
PHILADELPHIA – according to the recently announced results of a questionnaire by the American College of Physicians.
The survey participants included 233 members of the ACP, who provided their responses between October 2018 and January 2019. ACP President Ana Maria Lopez, MD, as well as Tabassum Salam, MD, vice president for medical education, announced the survey’s results at a press briefing at the annual meeting of the American College of Physicians.
Dr. Lopez and Dr. Salam highlighted some of the findings and expressed their enthusiasm about the recent increases in the use of telemedicine in a video interview. They also explained how telehealth benefits patients and the barriers to more widespread use of telemedicine.
Dr. Lopez and Dr. Salam did not disclose any relevant conflicts of interest.
PHILADELPHIA – according to the recently announced results of a questionnaire by the American College of Physicians.
The survey participants included 233 members of the ACP, who provided their responses between October 2018 and January 2019. ACP President Ana Maria Lopez, MD, as well as Tabassum Salam, MD, vice president for medical education, announced the survey’s results at a press briefing at the annual meeting of the American College of Physicians.
Dr. Lopez and Dr. Salam highlighted some of the findings and expressed their enthusiasm about the recent increases in the use of telemedicine in a video interview. They also explained how telehealth benefits patients and the barriers to more widespread use of telemedicine.
Dr. Lopez and Dr. Salam did not disclose any relevant conflicts of interest.
PHILADELPHIA – according to the recently announced results of a questionnaire by the American College of Physicians.
The survey participants included 233 members of the ACP, who provided their responses between October 2018 and January 2019. ACP President Ana Maria Lopez, MD, as well as Tabassum Salam, MD, vice president for medical education, announced the survey’s results at a press briefing at the annual meeting of the American College of Physicians.
Dr. Lopez and Dr. Salam highlighted some of the findings and expressed their enthusiasm about the recent increases in the use of telemedicine in a video interview. They also explained how telehealth benefits patients and the barriers to more widespread use of telemedicine.
Dr. Lopez and Dr. Salam did not disclose any relevant conflicts of interest.
REPORTING FROM INTERNAL MEDICINE 2019
Gilteritinib prolonged survival in FLT3-mutated AML
ATLANTA – The FLT3 inhibitor gilteritinib (Xospata) significantly prolonged overall survival, compared with salvage chemotherapy, in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia, Alexander E. Perl, MD, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia reported at the 2019 annual meeting of the American Association for Cancer Research (AACR).
In a video interview, Dr. Perl discussed the results of the ADMIRAL global phase 3 randomized trial and described the current state of therapy for patients with relapsed/refractory AML bearing FLT3 mutations. Up to 70% of patients with acute myeloid leukemia will experience a relapse, and up to 40% may have disease that is resistant to induction chemotherapy. Survival for these patients is generally poor.
In particular, patients with acute myeloid leukemia and FLT3-activating mutations are at increased risk for early relapse and poor overall survival.
The ADMIRAL trial is funded by Astellas Pharma. Dr. Perl disclosed advisory board participation, consulting fees, and institutional support from Astellas and others.
ATLANTA – The FLT3 inhibitor gilteritinib (Xospata) significantly prolonged overall survival, compared with salvage chemotherapy, in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia, Alexander E. Perl, MD, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia reported at the 2019 annual meeting of the American Association for Cancer Research (AACR).
In a video interview, Dr. Perl discussed the results of the ADMIRAL global phase 3 randomized trial and described the current state of therapy for patients with relapsed/refractory AML bearing FLT3 mutations. Up to 70% of patients with acute myeloid leukemia will experience a relapse, and up to 40% may have disease that is resistant to induction chemotherapy. Survival for these patients is generally poor.
In particular, patients with acute myeloid leukemia and FLT3-activating mutations are at increased risk for early relapse and poor overall survival.
The ADMIRAL trial is funded by Astellas Pharma. Dr. Perl disclosed advisory board participation, consulting fees, and institutional support from Astellas and others.
ATLANTA – The FLT3 inhibitor gilteritinib (Xospata) significantly prolonged overall survival, compared with salvage chemotherapy, in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia, Alexander E. Perl, MD, from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia reported at the 2019 annual meeting of the American Association for Cancer Research (AACR).
In a video interview, Dr. Perl discussed the results of the ADMIRAL global phase 3 randomized trial and described the current state of therapy for patients with relapsed/refractory AML bearing FLT3 mutations. Up to 70% of patients with acute myeloid leukemia will experience a relapse, and up to 40% may have disease that is resistant to induction chemotherapy. Survival for these patients is generally poor.
In particular, patients with acute myeloid leukemia and FLT3-activating mutations are at increased risk for early relapse and poor overall survival.
The ADMIRAL trial is funded by Astellas Pharma. Dr. Perl disclosed advisory board participation, consulting fees, and institutional support from Astellas and others.
REPORTING FROM AACR 2019
Pooled KEYNOTE data support pembro for elderly patients with NSCLC
GENEVA – Pembrolizumab monotherapy is as safe and effective in elderly patients with non–small cell lung cancer (NSCLC) as it is younger patients, according to investigators.
They reached this conclusion after analyzing pooled data from 264 patients 75 years or older involved in the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 phase 3 trials, reported lead author Kaname Nosaki, MD, of the National Hospital Organization Kyushu Cancer Center in Fukuoka, Japan, and his colleagues.
“Approximately 70% of newly-diagnosed NSCLC cases occur in the elderly, and more than half are locally advanced or metastatic,” the investigators noted in their abstract. Despite this, patients aged 75 years or older are underrepresented in clinical trials, Dr. Nosaki said during a presentation at the European Lung Cancer Conference.
All patients in the three KEYNOTE trials had PD-L1 positive NSCLC, with variations between studies with respect to PD-L1 tumor proportion score (TPS) and dosing regimen. While KEYNOTE-010 and KEYNOTE-042 involved patients with a TPS of at least 1%, KEYNOTE-024 raised the minimum TPS threshold to 50%. KEYNOTE-010 pembrolizumab dose was set at 2 mg/kg or 10 mg/kg, compared with the other two studies, which set a consistent dose of the checkpoint inhibitor at 200 mg.
As with younger patients, higher TPS expression generally predicted better outcomes. Independent of treatment line, elderly patients with a TPS of at least 50% had a hazard ratio of 0.40 in favor of pembrolizumab over chemotherapy, compared with all PD-L1-positive elderly patients, who had a hazard ratio of 0.76.
Generally, adverse events were comparable between age groups, with 68% of elderly patients experiencing at least one treatment-related adverse event, compared with 65% of younger patients. Grade 3 or 4 adverse events were slightly more common among elderly patients than younger patients (23% vs. 16%), with a mild concomitant increase in adverse event–related treatment discontinuations (11% vs. 7%). The rate of immune-mediated adverse events and infusion reactions, however, held steady regardless of age group, occurring in one out of four patients (25%). In contrast with these similarities, almost all elderly patients receiving chemotherapy (94%) had adverse events, compared with two out of three elderly patients receiving pembrolizumab. Rates of grade 3 or 4 adverse events also favored pembrolizumab over chemotherapy (23% vs. 59%).
“These data support the use of pembrolizumab monotherapy in elderly patients more than 75 years old with advanced PD-L1-expressing NSCLC,” Dr. Nosaki concluded.
Invited discussant Sanjay Popat, PhD, of Imperial College London, described the knowledge gap addressed by this study. “If we look at U.S. statistics, we see that lung cancer is the leading cause of death for patients above the age of 80, both for males and females,” Dr. Popat said at the meeting, presented by the European Society for Medical Oncology. “The real question is should this group of patients be getting any form of checkpoint inhibitors at all, and if so, what is the benefit to risk ratio?
“Our patients are getting older, we’re all living slightly longer, and the burden of geriatric oncology is predicted to rise quite markedly with age, so it’s important to get a good feel for how we should be managing our senior population,” he added.
According to Dr. Popat, elderly patients naturally undergo immune senescence, meaning the immune system deteriorates with age, and this phenomenon could theoretically mitigate efficacy of immunotherapies; however, previous studies have not found decreased efficacy among elderly patients. Still, some “so-called elderly population subsets we’ve been analyzing are actually around the median age [of diagnosis with NSCLC],” Dr. Popat said, noting that among these studies, those with wider age ranges offer more reliable data.
“Today we looked at the novel cutoff, this 75-year group cutoff, which I very much welcome,” Dr. Popat said, “because this much more reflects what we see in routine clinical care.”
Regarding the results, Dr. Popat suggested that chemotherapy leads to an “excess of mortality” among elderly patients, “likely due to toxicities,” thereby explaining part of the relative advantage provided by pembrolizumab. Considering these findings in addition to previous experiences with pembrolizumab in the elderly, Dr. Popat said that “if you choose your patient population well, fit patients well enough to go to a trial, they don’t have an excess of toxicities regardless of their age.”
Taken as a whole, the present analysis supports the routine use of pembrolizumab in fit, elderly patients, Dr. Popat said.
The study was funded by MSD. The investigators reported financial relationships with AstraZeneca, Eli Lilly, Taiho, Chugai, and others.
SOURCE: Nosaki et al. ELCC 2019. Abstract 103O_PR.
GENEVA – Pembrolizumab monotherapy is as safe and effective in elderly patients with non–small cell lung cancer (NSCLC) as it is younger patients, according to investigators.
They reached this conclusion after analyzing pooled data from 264 patients 75 years or older involved in the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 phase 3 trials, reported lead author Kaname Nosaki, MD, of the National Hospital Organization Kyushu Cancer Center in Fukuoka, Japan, and his colleagues.
“Approximately 70% of newly-diagnosed NSCLC cases occur in the elderly, and more than half are locally advanced or metastatic,” the investigators noted in their abstract. Despite this, patients aged 75 years or older are underrepresented in clinical trials, Dr. Nosaki said during a presentation at the European Lung Cancer Conference.
All patients in the three KEYNOTE trials had PD-L1 positive NSCLC, with variations between studies with respect to PD-L1 tumor proportion score (TPS) and dosing regimen. While KEYNOTE-010 and KEYNOTE-042 involved patients with a TPS of at least 1%, KEYNOTE-024 raised the minimum TPS threshold to 50%. KEYNOTE-010 pembrolizumab dose was set at 2 mg/kg or 10 mg/kg, compared with the other two studies, which set a consistent dose of the checkpoint inhibitor at 200 mg.
As with younger patients, higher TPS expression generally predicted better outcomes. Independent of treatment line, elderly patients with a TPS of at least 50% had a hazard ratio of 0.40 in favor of pembrolizumab over chemotherapy, compared with all PD-L1-positive elderly patients, who had a hazard ratio of 0.76.
Generally, adverse events were comparable between age groups, with 68% of elderly patients experiencing at least one treatment-related adverse event, compared with 65% of younger patients. Grade 3 or 4 adverse events were slightly more common among elderly patients than younger patients (23% vs. 16%), with a mild concomitant increase in adverse event–related treatment discontinuations (11% vs. 7%). The rate of immune-mediated adverse events and infusion reactions, however, held steady regardless of age group, occurring in one out of four patients (25%). In contrast with these similarities, almost all elderly patients receiving chemotherapy (94%) had adverse events, compared with two out of three elderly patients receiving pembrolizumab. Rates of grade 3 or 4 adverse events also favored pembrolizumab over chemotherapy (23% vs. 59%).
“These data support the use of pembrolizumab monotherapy in elderly patients more than 75 years old with advanced PD-L1-expressing NSCLC,” Dr. Nosaki concluded.
Invited discussant Sanjay Popat, PhD, of Imperial College London, described the knowledge gap addressed by this study. “If we look at U.S. statistics, we see that lung cancer is the leading cause of death for patients above the age of 80, both for males and females,” Dr. Popat said at the meeting, presented by the European Society for Medical Oncology. “The real question is should this group of patients be getting any form of checkpoint inhibitors at all, and if so, what is the benefit to risk ratio?
“Our patients are getting older, we’re all living slightly longer, and the burden of geriatric oncology is predicted to rise quite markedly with age, so it’s important to get a good feel for how we should be managing our senior population,” he added.
According to Dr. Popat, elderly patients naturally undergo immune senescence, meaning the immune system deteriorates with age, and this phenomenon could theoretically mitigate efficacy of immunotherapies; however, previous studies have not found decreased efficacy among elderly patients. Still, some “so-called elderly population subsets we’ve been analyzing are actually around the median age [of diagnosis with NSCLC],” Dr. Popat said, noting that among these studies, those with wider age ranges offer more reliable data.
“Today we looked at the novel cutoff, this 75-year group cutoff, which I very much welcome,” Dr. Popat said, “because this much more reflects what we see in routine clinical care.”
Regarding the results, Dr. Popat suggested that chemotherapy leads to an “excess of mortality” among elderly patients, “likely due to toxicities,” thereby explaining part of the relative advantage provided by pembrolizumab. Considering these findings in addition to previous experiences with pembrolizumab in the elderly, Dr. Popat said that “if you choose your patient population well, fit patients well enough to go to a trial, they don’t have an excess of toxicities regardless of their age.”
Taken as a whole, the present analysis supports the routine use of pembrolizumab in fit, elderly patients, Dr. Popat said.
The study was funded by MSD. The investigators reported financial relationships with AstraZeneca, Eli Lilly, Taiho, Chugai, and others.
SOURCE: Nosaki et al. ELCC 2019. Abstract 103O_PR.
GENEVA – Pembrolizumab monotherapy is as safe and effective in elderly patients with non–small cell lung cancer (NSCLC) as it is younger patients, according to investigators.
They reached this conclusion after analyzing pooled data from 264 patients 75 years or older involved in the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 phase 3 trials, reported lead author Kaname Nosaki, MD, of the National Hospital Organization Kyushu Cancer Center in Fukuoka, Japan, and his colleagues.
“Approximately 70% of newly-diagnosed NSCLC cases occur in the elderly, and more than half are locally advanced or metastatic,” the investigators noted in their abstract. Despite this, patients aged 75 years or older are underrepresented in clinical trials, Dr. Nosaki said during a presentation at the European Lung Cancer Conference.
All patients in the three KEYNOTE trials had PD-L1 positive NSCLC, with variations between studies with respect to PD-L1 tumor proportion score (TPS) and dosing regimen. While KEYNOTE-010 and KEYNOTE-042 involved patients with a TPS of at least 1%, KEYNOTE-024 raised the minimum TPS threshold to 50%. KEYNOTE-010 pembrolizumab dose was set at 2 mg/kg or 10 mg/kg, compared with the other two studies, which set a consistent dose of the checkpoint inhibitor at 200 mg.
As with younger patients, higher TPS expression generally predicted better outcomes. Independent of treatment line, elderly patients with a TPS of at least 50% had a hazard ratio of 0.40 in favor of pembrolizumab over chemotherapy, compared with all PD-L1-positive elderly patients, who had a hazard ratio of 0.76.
Generally, adverse events were comparable between age groups, with 68% of elderly patients experiencing at least one treatment-related adverse event, compared with 65% of younger patients. Grade 3 or 4 adverse events were slightly more common among elderly patients than younger patients (23% vs. 16%), with a mild concomitant increase in adverse event–related treatment discontinuations (11% vs. 7%). The rate of immune-mediated adverse events and infusion reactions, however, held steady regardless of age group, occurring in one out of four patients (25%). In contrast with these similarities, almost all elderly patients receiving chemotherapy (94%) had adverse events, compared with two out of three elderly patients receiving pembrolizumab. Rates of grade 3 or 4 adverse events also favored pembrolizumab over chemotherapy (23% vs. 59%).
“These data support the use of pembrolizumab monotherapy in elderly patients more than 75 years old with advanced PD-L1-expressing NSCLC,” Dr. Nosaki concluded.
Invited discussant Sanjay Popat, PhD, of Imperial College London, described the knowledge gap addressed by this study. “If we look at U.S. statistics, we see that lung cancer is the leading cause of death for patients above the age of 80, both for males and females,” Dr. Popat said at the meeting, presented by the European Society for Medical Oncology. “The real question is should this group of patients be getting any form of checkpoint inhibitors at all, and if so, what is the benefit to risk ratio?
“Our patients are getting older, we’re all living slightly longer, and the burden of geriatric oncology is predicted to rise quite markedly with age, so it’s important to get a good feel for how we should be managing our senior population,” he added.
According to Dr. Popat, elderly patients naturally undergo immune senescence, meaning the immune system deteriorates with age, and this phenomenon could theoretically mitigate efficacy of immunotherapies; however, previous studies have not found decreased efficacy among elderly patients. Still, some “so-called elderly population subsets we’ve been analyzing are actually around the median age [of diagnosis with NSCLC],” Dr. Popat said, noting that among these studies, those with wider age ranges offer more reliable data.
“Today we looked at the novel cutoff, this 75-year group cutoff, which I very much welcome,” Dr. Popat said, “because this much more reflects what we see in routine clinical care.”
Regarding the results, Dr. Popat suggested that chemotherapy leads to an “excess of mortality” among elderly patients, “likely due to toxicities,” thereby explaining part of the relative advantage provided by pembrolizumab. Considering these findings in addition to previous experiences with pembrolizumab in the elderly, Dr. Popat said that “if you choose your patient population well, fit patients well enough to go to a trial, they don’t have an excess of toxicities regardless of their age.”
Taken as a whole, the present analysis supports the routine use of pembrolizumab in fit, elderly patients, Dr. Popat said.
The study was funded by MSD. The investigators reported financial relationships with AstraZeneca, Eli Lilly, Taiho, Chugai, and others.
SOURCE: Nosaki et al. ELCC 2019. Abstract 103O_PR.
REPORTING FROM ELCC 2019
Interest renewed in targeting gluten in schizophrenia
ORLANDO – Going gluten free shows a benefit for a subset of schizophrenia patients, and it offers a new array of potential intervention – suggesting that knowing what some patients consume or interrupting newly identified mechanisms could make real differences in symptom severity, an expert said at the annual congress of the Schizophrenia International Research Society.
Deanna L. Kelly, PharmD, director of the Treatment Research Program at the University of Maryland, Baltimore, said that if she’d been told 10 years ago that she’d be studying links between diet and schizophrenia, “I would have probably not believed you.”
Interest in the link between wheat, which contains gluten, and schizophrenia is not brand new. Research published in the 1960s found that, as wheat consumption fell in Scandinavia during World War II, so did hospital admissions for schizophrenia. In the United States, schizophrenia admissions rose as wheat consumption rose. But interest in the topic died off in the 1980s, when links between a gluten-free diet and schizophrenia symptoms were found to be weak or nonexistent.
Dr. Kelly said that’s because that research looked at all comers without a finely tuned schizophrenia population.
a protein that helps bread rise during baking and is hard to digest. Researchers have found that antibodies to other gluten proteins – such as anti–tissue transglutaminase antibodies, used to diagnose celiac disease – are not elevated in schizophrenia patients, compared with healthy controls (Schizophr Res. 2018 May;195:585-6). But native gliadin antibodies (AGA IgG) are significantly elevated – this is seen in about 30% of patients, compared with about 10% in controls, Dr. Kelly said.
Elevated AGA IgG is also correlated to higher levels of peripheral inflammation and higher levels of peripheral kynurenine, a metabolite of tryptophan linked to schizophrenia.
In a feasibility study with 16 patients published this year, researchers randomized patients with elevated AGA IgG to a gluten-free diet – they were fed with certified gluten-free shakes – or a diet that wasn’t gluten free over 5 weeks. Patients stayed at a hospital to ensure adherence to the diet and for close monitoring. They found that those who were gluten free showed significant improvement in negative symptoms, measured by the Scale for the Assessment of Negative Symptoms, compared with those who continued eating gluten. These symptoms included the inability to experience pleasure, inability to speak, lack of initiative, and inability to express emotion (J Psychiatry Neurosci. 2019 Mar 27;44[3]:1-9).
“Removing gliadin may improve negative symptoms in schizophrenia,” Dr. Kelly said.
Those on the gluten-free diet also showed improvement in gastrointestinal symptoms and improvement in certain cognitive traits, such as attention and verbal learning.
Her research team is now conducting on a larger trial comparing the two diets, this time with the gluten-containing diet involving a higher amount of gluten, which researchers think better reflects real-life diets.
Researchers are still not sure how gliadin intake affects schizophrenia symptoms, but it could involve problems with the blood brain barrier, the permeability of the gut, or the effects on the microbiome, she said. But the importance of gliadin and gluten to this group of schizophrenia patients raises the possibility of treatment with ongoing dietary changes, anti-inflammatory treatments, blocking absorption of gluten, improving how it’s digested or by blocking gliadin antibodies.
“We’re trying to learn about disease states themselves, but each person should find their best lives,” Dr. Kelly said. “Everyone deserves optimized and personalized treatment.”
Dr. Kelly reported financial relationships with Lundbeck and HLS Therapeutics.
ORLANDO – Going gluten free shows a benefit for a subset of schizophrenia patients, and it offers a new array of potential intervention – suggesting that knowing what some patients consume or interrupting newly identified mechanisms could make real differences in symptom severity, an expert said at the annual congress of the Schizophrenia International Research Society.
Deanna L. Kelly, PharmD, director of the Treatment Research Program at the University of Maryland, Baltimore, said that if she’d been told 10 years ago that she’d be studying links between diet and schizophrenia, “I would have probably not believed you.”
Interest in the link between wheat, which contains gluten, and schizophrenia is not brand new. Research published in the 1960s found that, as wheat consumption fell in Scandinavia during World War II, so did hospital admissions for schizophrenia. In the United States, schizophrenia admissions rose as wheat consumption rose. But interest in the topic died off in the 1980s, when links between a gluten-free diet and schizophrenia symptoms were found to be weak or nonexistent.
Dr. Kelly said that’s because that research looked at all comers without a finely tuned schizophrenia population.
a protein that helps bread rise during baking and is hard to digest. Researchers have found that antibodies to other gluten proteins – such as anti–tissue transglutaminase antibodies, used to diagnose celiac disease – are not elevated in schizophrenia patients, compared with healthy controls (Schizophr Res. 2018 May;195:585-6). But native gliadin antibodies (AGA IgG) are significantly elevated – this is seen in about 30% of patients, compared with about 10% in controls, Dr. Kelly said.
Elevated AGA IgG is also correlated to higher levels of peripheral inflammation and higher levels of peripheral kynurenine, a metabolite of tryptophan linked to schizophrenia.
In a feasibility study with 16 patients published this year, researchers randomized patients with elevated AGA IgG to a gluten-free diet – they were fed with certified gluten-free shakes – or a diet that wasn’t gluten free over 5 weeks. Patients stayed at a hospital to ensure adherence to the diet and for close monitoring. They found that those who were gluten free showed significant improvement in negative symptoms, measured by the Scale for the Assessment of Negative Symptoms, compared with those who continued eating gluten. These symptoms included the inability to experience pleasure, inability to speak, lack of initiative, and inability to express emotion (J Psychiatry Neurosci. 2019 Mar 27;44[3]:1-9).
“Removing gliadin may improve negative symptoms in schizophrenia,” Dr. Kelly said.
Those on the gluten-free diet also showed improvement in gastrointestinal symptoms and improvement in certain cognitive traits, such as attention and verbal learning.
Her research team is now conducting on a larger trial comparing the two diets, this time with the gluten-containing diet involving a higher amount of gluten, which researchers think better reflects real-life diets.
Researchers are still not sure how gliadin intake affects schizophrenia symptoms, but it could involve problems with the blood brain barrier, the permeability of the gut, or the effects on the microbiome, she said. But the importance of gliadin and gluten to this group of schizophrenia patients raises the possibility of treatment with ongoing dietary changes, anti-inflammatory treatments, blocking absorption of gluten, improving how it’s digested or by blocking gliadin antibodies.
“We’re trying to learn about disease states themselves, but each person should find their best lives,” Dr. Kelly said. “Everyone deserves optimized and personalized treatment.”
Dr. Kelly reported financial relationships with Lundbeck and HLS Therapeutics.
ORLANDO – Going gluten free shows a benefit for a subset of schizophrenia patients, and it offers a new array of potential intervention – suggesting that knowing what some patients consume or interrupting newly identified mechanisms could make real differences in symptom severity, an expert said at the annual congress of the Schizophrenia International Research Society.
Deanna L. Kelly, PharmD, director of the Treatment Research Program at the University of Maryland, Baltimore, said that if she’d been told 10 years ago that she’d be studying links between diet and schizophrenia, “I would have probably not believed you.”
Interest in the link between wheat, which contains gluten, and schizophrenia is not brand new. Research published in the 1960s found that, as wheat consumption fell in Scandinavia during World War II, so did hospital admissions for schizophrenia. In the United States, schizophrenia admissions rose as wheat consumption rose. But interest in the topic died off in the 1980s, when links between a gluten-free diet and schizophrenia symptoms were found to be weak or nonexistent.
Dr. Kelly said that’s because that research looked at all comers without a finely tuned schizophrenia population.
a protein that helps bread rise during baking and is hard to digest. Researchers have found that antibodies to other gluten proteins – such as anti–tissue transglutaminase antibodies, used to diagnose celiac disease – are not elevated in schizophrenia patients, compared with healthy controls (Schizophr Res. 2018 May;195:585-6). But native gliadin antibodies (AGA IgG) are significantly elevated – this is seen in about 30% of patients, compared with about 10% in controls, Dr. Kelly said.
Elevated AGA IgG is also correlated to higher levels of peripheral inflammation and higher levels of peripheral kynurenine, a metabolite of tryptophan linked to schizophrenia.
In a feasibility study with 16 patients published this year, researchers randomized patients with elevated AGA IgG to a gluten-free diet – they were fed with certified gluten-free shakes – or a diet that wasn’t gluten free over 5 weeks. Patients stayed at a hospital to ensure adherence to the diet and for close monitoring. They found that those who were gluten free showed significant improvement in negative symptoms, measured by the Scale for the Assessment of Negative Symptoms, compared with those who continued eating gluten. These symptoms included the inability to experience pleasure, inability to speak, lack of initiative, and inability to express emotion (J Psychiatry Neurosci. 2019 Mar 27;44[3]:1-9).
“Removing gliadin may improve negative symptoms in schizophrenia,” Dr. Kelly said.
Those on the gluten-free diet also showed improvement in gastrointestinal symptoms and improvement in certain cognitive traits, such as attention and verbal learning.
Her research team is now conducting on a larger trial comparing the two diets, this time with the gluten-containing diet involving a higher amount of gluten, which researchers think better reflects real-life diets.
Researchers are still not sure how gliadin intake affects schizophrenia symptoms, but it could involve problems with the blood brain barrier, the permeability of the gut, or the effects on the microbiome, she said. But the importance of gliadin and gluten to this group of schizophrenia patients raises the possibility of treatment with ongoing dietary changes, anti-inflammatory treatments, blocking absorption of gluten, improving how it’s digested or by blocking gliadin antibodies.
“We’re trying to learn about disease states themselves, but each person should find their best lives,” Dr. Kelly said. “Everyone deserves optimized and personalized treatment.”
Dr. Kelly reported financial relationships with Lundbeck and HLS Therapeutics.
EXPERT ANALYSIS FROM SIRS 2019
Highlights from the ‘Updates in ACS’ session (VIDEO)
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
Hospital Medicine 2019 attendees outlined their key takeaways from the Updates in Acute Coronary Syndrome session, presented by Jeffrey Trost, MD, of Johns Hopkins University, Baltimore.

Dr. Trost’s discussion focused on the relationship between dual antiplatelet therapy, in-stent thrombosis, and in-stent restenosis. He also explored the diagnostic role of fractional flow reserve, and he outlined effective approaches to PCSK9 inhibitor use.
REPORTING FROM HM19
Tumor-treating fields boost chemo for mesothelioma
GENEVA – For patients with malignant pleural mesothelioma, adding tumor-treating fields (TTFields) to standard pemetrexed plus platinum compound chemotherapy could boost median overall survival by about 6 months, according to final results from the phase 2 STELLAR trial.
The survival benefit of TTFields was greatest among patients with epithelioid mesothelioma, reported lead author Giovanni Luca Ceresoli, MD, of Humanitas Gavazzeni in Bergamo, Italy. According to Dr. Ceresoli, who presented findings at the at the European Lung Cancer Conference, TTFields offer a safe way to improve mesothelioma outcomes without increasing the risk of serious adverse events.
“TTFields are a locoregional treatment comprising low-intensity alternating electric fields delivered through a portable medical device,” Dr. Ceresoli explained at the meeting, presented by the European Society for Medical Oncology. “Their main mode of action is an anti-mitotic mechanism.” He noted that TTFields are already approved by the Food and Drug Administration for newly diagnosed glioblastoma.
The STELLAR trial involved 80 patients with mesothelioma who were treated with TTFields in combination with standard first-line chemotherapy, a combination of pemetrexed with cisplatin or carboplatin. Patients were instructed to self-administer continuous 150 kHz TTFields for at least 18 hours a day. Eligibility required an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Both ECOG status and cancer-related pain were followed with a visual analog scale until disease progression. Median overall survival (OS) was the primary endpoint.
The patient population was predominantly male (84%), with median age of 67 years. About 44% of the patients had an ECOG performance status of 1 and 66% had epithelioid histology. Median treatment time per day was 16.3 hours.
After a minimum follow-up of 1 year, patients treated with TTFields in combination with standard chemotherapy had a median overall survival of 18.2 months, compared with 12.1 months for standard chemotherapy alone, which Dr. Ceresoli cited as the historical benchmark. The survival benefit was 3 months longer among patients with epithelioid mesothelioma, who had a median overall survival of 21.2 months.
In addition to survival benefits, the investigators found that median time to decreased performance status was just over 1 year (13.1 months), and that pain did not increase to a clinically significant degree (33%) until an average of 8.4 months. Although no device-related serious adverse events occurred, 37 patients (46%) experienced TTFields-related dermatitis; 4 of these patients had grade 3 dermatitis. Dr. Ceresoli noted that dermatitis was typically “easily managed” with topical application of a corticosteroid, while patients with severe dermatitis took short treatment breaks.
“In conclusion, in the STELLAR trial, TTFields in combination with standard chemotherapy were effective and safe for first-line treatment of unresectable malignant pleural mesothelioma, and median overall survival was significantly longer as compared to historical controls,” Dr. Ceresoli said, pointing out better survival than in recent trials MAPS and LUME-Meso.
When asked by the invited discussant about future research, Dr. Ceresoli described a narrower focus for upcoming TTFields studies for mesothelioma. “As you well know, most patients have epithelioid histology, and in our hands, the patients with epithelioid histology had better prognoses,” he said. “So, in the future, I think we will focus on epithelioid tumors.”
Dr. Ceresoli disclosed travel funding from Novocure.
SOURCE: Ceresoli et al. ELCC 2019. Abstract 55O.
GENEVA – For patients with malignant pleural mesothelioma, adding tumor-treating fields (TTFields) to standard pemetrexed plus platinum compound chemotherapy could boost median overall survival by about 6 months, according to final results from the phase 2 STELLAR trial.
The survival benefit of TTFields was greatest among patients with epithelioid mesothelioma, reported lead author Giovanni Luca Ceresoli, MD, of Humanitas Gavazzeni in Bergamo, Italy. According to Dr. Ceresoli, who presented findings at the at the European Lung Cancer Conference, TTFields offer a safe way to improve mesothelioma outcomes without increasing the risk of serious adverse events.
“TTFields are a locoregional treatment comprising low-intensity alternating electric fields delivered through a portable medical device,” Dr. Ceresoli explained at the meeting, presented by the European Society for Medical Oncology. “Their main mode of action is an anti-mitotic mechanism.” He noted that TTFields are already approved by the Food and Drug Administration for newly diagnosed glioblastoma.
The STELLAR trial involved 80 patients with mesothelioma who were treated with TTFields in combination with standard first-line chemotherapy, a combination of pemetrexed with cisplatin or carboplatin. Patients were instructed to self-administer continuous 150 kHz TTFields for at least 18 hours a day. Eligibility required an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Both ECOG status and cancer-related pain were followed with a visual analog scale until disease progression. Median overall survival (OS) was the primary endpoint.
The patient population was predominantly male (84%), with median age of 67 years. About 44% of the patients had an ECOG performance status of 1 and 66% had epithelioid histology. Median treatment time per day was 16.3 hours.
After a minimum follow-up of 1 year, patients treated with TTFields in combination with standard chemotherapy had a median overall survival of 18.2 months, compared with 12.1 months for standard chemotherapy alone, which Dr. Ceresoli cited as the historical benchmark. The survival benefit was 3 months longer among patients with epithelioid mesothelioma, who had a median overall survival of 21.2 months.
In addition to survival benefits, the investigators found that median time to decreased performance status was just over 1 year (13.1 months), and that pain did not increase to a clinically significant degree (33%) until an average of 8.4 months. Although no device-related serious adverse events occurred, 37 patients (46%) experienced TTFields-related dermatitis; 4 of these patients had grade 3 dermatitis. Dr. Ceresoli noted that dermatitis was typically “easily managed” with topical application of a corticosteroid, while patients with severe dermatitis took short treatment breaks.
“In conclusion, in the STELLAR trial, TTFields in combination with standard chemotherapy were effective and safe for first-line treatment of unresectable malignant pleural mesothelioma, and median overall survival was significantly longer as compared to historical controls,” Dr. Ceresoli said, pointing out better survival than in recent trials MAPS and LUME-Meso.
When asked by the invited discussant about future research, Dr. Ceresoli described a narrower focus for upcoming TTFields studies for mesothelioma. “As you well know, most patients have epithelioid histology, and in our hands, the patients with epithelioid histology had better prognoses,” he said. “So, in the future, I think we will focus on epithelioid tumors.”
Dr. Ceresoli disclosed travel funding from Novocure.
SOURCE: Ceresoli et al. ELCC 2019. Abstract 55O.
GENEVA – For patients with malignant pleural mesothelioma, adding tumor-treating fields (TTFields) to standard pemetrexed plus platinum compound chemotherapy could boost median overall survival by about 6 months, according to final results from the phase 2 STELLAR trial.
The survival benefit of TTFields was greatest among patients with epithelioid mesothelioma, reported lead author Giovanni Luca Ceresoli, MD, of Humanitas Gavazzeni in Bergamo, Italy. According to Dr. Ceresoli, who presented findings at the at the European Lung Cancer Conference, TTFields offer a safe way to improve mesothelioma outcomes without increasing the risk of serious adverse events.
“TTFields are a locoregional treatment comprising low-intensity alternating electric fields delivered through a portable medical device,” Dr. Ceresoli explained at the meeting, presented by the European Society for Medical Oncology. “Their main mode of action is an anti-mitotic mechanism.” He noted that TTFields are already approved by the Food and Drug Administration for newly diagnosed glioblastoma.
The STELLAR trial involved 80 patients with mesothelioma who were treated with TTFields in combination with standard first-line chemotherapy, a combination of pemetrexed with cisplatin or carboplatin. Patients were instructed to self-administer continuous 150 kHz TTFields for at least 18 hours a day. Eligibility required an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Both ECOG status and cancer-related pain were followed with a visual analog scale until disease progression. Median overall survival (OS) was the primary endpoint.
The patient population was predominantly male (84%), with median age of 67 years. About 44% of the patients had an ECOG performance status of 1 and 66% had epithelioid histology. Median treatment time per day was 16.3 hours.
After a minimum follow-up of 1 year, patients treated with TTFields in combination with standard chemotherapy had a median overall survival of 18.2 months, compared with 12.1 months for standard chemotherapy alone, which Dr. Ceresoli cited as the historical benchmark. The survival benefit was 3 months longer among patients with epithelioid mesothelioma, who had a median overall survival of 21.2 months.
In addition to survival benefits, the investigators found that median time to decreased performance status was just over 1 year (13.1 months), and that pain did not increase to a clinically significant degree (33%) until an average of 8.4 months. Although no device-related serious adverse events occurred, 37 patients (46%) experienced TTFields-related dermatitis; 4 of these patients had grade 3 dermatitis. Dr. Ceresoli noted that dermatitis was typically “easily managed” with topical application of a corticosteroid, while patients with severe dermatitis took short treatment breaks.
“In conclusion, in the STELLAR trial, TTFields in combination with standard chemotherapy were effective and safe for first-line treatment of unresectable malignant pleural mesothelioma, and median overall survival was significantly longer as compared to historical controls,” Dr. Ceresoli said, pointing out better survival than in recent trials MAPS and LUME-Meso.
When asked by the invited discussant about future research, Dr. Ceresoli described a narrower focus for upcoming TTFields studies for mesothelioma. “As you well know, most patients have epithelioid histology, and in our hands, the patients with epithelioid histology had better prognoses,” he said. “So, in the future, I think we will focus on epithelioid tumors.”
Dr. Ceresoli disclosed travel funding from Novocure.
SOURCE: Ceresoli et al. ELCC 2019. Abstract 55O.
At ELCC 2019











