User login
Announcing the 2019 chapter awards and grant recipients
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustainability, and innovation within their activities, which are then recognized at SHM’s annual conference.
Please join SHM in congratulating the following chapters on their year of success in 2018!
Platinum Chapters: Iowa; Knoxville; Maryland; Michigan; Minnesota; New Mexico; North Carolina Triangle; Pacific Northwest; Southwest Florida; Wiregrass.
Gold Chapters: Houston; NYC/Westchester; Piedmont Triad; San Francisco Bay Area
Silver Chapters: Boston/Eastern Massachusetts; Charlotte Metro Area; Gulf States; Hampton Roads; Kentucky; Maine; Nebraska; North Jersey; Rocky Mountain; South Central Pennsylvania; South Texas; St. Louis.
Outstanding Chapter of the Year
Michigan. The Outstanding Chapter of the Year Award goes to one chapter that exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2018 is the Michigan chapter of SHM.
The Michigan chapter continues to embrace the mission of our society and nurtures a vibrant, multidisciplinary membership. It is currently the largest chapter in the program, representing more than 750 SHM members.
Using a technology-enabled platform, the Michigan chapter has expanded its meetings to four different sites, leveraging expertise from across the state. The chapter recently held its largest meeting focused on provider burnout, with more than 100 attendees across four different sites. It featured a main speaker as well as a multidisciplinary panel of leaders from eight different health systems.
The chapter has successfully incorporated advocacy into its annual planning and actively responds to new legislation affecting hospital medicine. It continues to be an active and valued member of the Michigan medical community and the SHM chapter community at large.
In addition to the service to members, the chapter strives to serve the SHM chapter community at large by collaborating and sharing best practices.
The chapter’s level of originality is not only a benefit to the chapter, but also to SHM’s Chapter program as a whole. Congratulations to the Michigan chapter on being named the Outstanding Chapter of 2018.
Rising Star Chapter
Knoxville. The Rising Star Chapter Award goes to one chapter that has been active for 2 years or less and in the past 12 months has made improvements to its leadership, stability and growth, and membership.
The recipient of the Rising Star Chapter Award for 2018 is the Knoxville (Tenn.) Chapter of SHM, which has made significant strides since its launch in the spring of 2017. The chapter assembled a group of local hospitalists from Knoxville and the surrounding region to encourage participation and drive quality initiatives in area hospitals.
The Knoxville chapter developed a leadership framework, including officers and board members, and just completed its first formal chapter leadership election. In 2018, the chapter held four meetings, including an event steered toward residents and students. Membership in the chapter has grown by more than 20% since inception. The chapter has engaged in statewide quality initiatives with the Tennessee Hospital Association and has engaged with other chapters across the state.
The Knoxville’s Chapter is an active, enthusiastic organization that is rapidly growing and thriving. Congratulations to the Knoxville chapter on being named the Rising Star Chapter for 2018.
Student Hospitalist Scholar Grant recipients
SHM is proud to acknowledge the latest winners of its Student Hospitalist Scholar Grant. These medical students were awarded grants to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other areas relevant to the field of hospital medicine.
Sandeep Bala
University of Chicago Pritzker School of Medicine
Poster 382 – The impact of plain language open medical notes on patient activation
Location: Denver (Colo.) Health Hospital
Monisha Bhatia
Vanderbilt University School of Medicine
Poster 23 – Using electronic medical record phenotypic data to predict discharge destination
Location: Vanderbilt University Medical Center, Nashville, Tenn.
Maximilian Hemmrich
University of Chicago Pritzker School of Medicine
Oral presentation, 11:45 a.m. – Noon, Tuesday, March 26
Project: Derivation and validation of a COPD readmission risk prediction tool
Location: University of Chicago
Ilana Scandariato Lavina
Weill Cornell Medical College
Poster 424 – Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Yun Li
Geisel School of Medicine at Dartmouth
Poster 320 – Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae
Location: Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
Resident Travel Grant recipients
We would like to congratulate the latest winners of SHM’s Resident Travel Grant. To qualify for this award, residents submitted an abstract for consideration in the RIV session at HM19 as first authors. Each of them produced outstanding work worthy of recognition:
Daniel Choi, MD – New York Presbyterian Hospital–Weill Cornell
Poster 277 – Improving rates of appropriate ICD deactivation discussions in admitted patients made DNR and/or comfort care
Armond Esmaili, MD – University of California, San Francisco, and the San Francisco VA Medical Center
Poster 649 – Early isolated hypotension, a sepsis “Canary in the Coal Mine”: Timing of antibiotics according to hypotension identifies different sepsis subtypes at differing risks of treatment delay
Poster 8 – Who’s waiting? Predictors of antibiotic delays in hypotensive patients with sepsis
Geoffroy Fauchet, MD – University of Colorado at Denver, Aurora – Rocky Mountain Regional VA Medical Center
Poster 288 – Tackling opioid prescriptions through resident engagement
Nick Zessis, MD – Washington University School of Medicine, St. Louis Children’s Hospital
Poster 101 – Smartphone-based teaching app increases frequency of residents teaching medical students
David Sterken, MD – University of California, San Francisco
Poster 230 – Safety of antimotility agent use during treatment for Clostridioides difficile infection in malignant hepatology patients
James Anstey, MD – University of California, San Francisco
Poster 57 – The POCUS supervision safety gap: Attending physician knowledge in point-of-care ultrasound lags behind that of internal medicine residents
Poster 147 – Association of post paracentesis albumin dosage and acute kidney injury in hospitalized patients
Nicholas Iverson, MD – University of California, San Francisco
Poster 408 – Implications of using an alternative measure to assess opiate days supplied at discharge
Marwah Shahid, MD – Baylor College of Medicine, Houston
Poster 22 – Topic modeling to evaluate hospital Google reviews
Abhishek Chaturvedi, MD – Allegheny Health Network, Pittsburgh
Poster 152 – Association of socioeconomic and racial disparities with health care utilization and outcomes in opioid overdose–related hospitalizations in the United States: Insights from National Inpatient Sample from 2012 through 2014
Pratyusha Tirumanisetty, MD – Unity Hospital, Rochester (N.Y.) Regional Health
Poster 9 – Does hospital-onset Clostridium difficile infection increase the risk of hospital discharge to skilled nursing facilities? A retrospective case-control study from a community hospital.
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustainability, and innovation within their activities, which are then recognized at SHM’s annual conference.
Please join SHM in congratulating the following chapters on their year of success in 2018!
Platinum Chapters: Iowa; Knoxville; Maryland; Michigan; Minnesota; New Mexico; North Carolina Triangle; Pacific Northwest; Southwest Florida; Wiregrass.
Gold Chapters: Houston; NYC/Westchester; Piedmont Triad; San Francisco Bay Area
Silver Chapters: Boston/Eastern Massachusetts; Charlotte Metro Area; Gulf States; Hampton Roads; Kentucky; Maine; Nebraska; North Jersey; Rocky Mountain; South Central Pennsylvania; South Texas; St. Louis.
Outstanding Chapter of the Year
Michigan. The Outstanding Chapter of the Year Award goes to one chapter that exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2018 is the Michigan chapter of SHM.
The Michigan chapter continues to embrace the mission of our society and nurtures a vibrant, multidisciplinary membership. It is currently the largest chapter in the program, representing more than 750 SHM members.
Using a technology-enabled platform, the Michigan chapter has expanded its meetings to four different sites, leveraging expertise from across the state. The chapter recently held its largest meeting focused on provider burnout, with more than 100 attendees across four different sites. It featured a main speaker as well as a multidisciplinary panel of leaders from eight different health systems.
The chapter has successfully incorporated advocacy into its annual planning and actively responds to new legislation affecting hospital medicine. It continues to be an active and valued member of the Michigan medical community and the SHM chapter community at large.
In addition to the service to members, the chapter strives to serve the SHM chapter community at large by collaborating and sharing best practices.
The chapter’s level of originality is not only a benefit to the chapter, but also to SHM’s Chapter program as a whole. Congratulations to the Michigan chapter on being named the Outstanding Chapter of 2018.
Rising Star Chapter
Knoxville. The Rising Star Chapter Award goes to one chapter that has been active for 2 years or less and in the past 12 months has made improvements to its leadership, stability and growth, and membership.
The recipient of the Rising Star Chapter Award for 2018 is the Knoxville (Tenn.) Chapter of SHM, which has made significant strides since its launch in the spring of 2017. The chapter assembled a group of local hospitalists from Knoxville and the surrounding region to encourage participation and drive quality initiatives in area hospitals.
The Knoxville chapter developed a leadership framework, including officers and board members, and just completed its first formal chapter leadership election. In 2018, the chapter held four meetings, including an event steered toward residents and students. Membership in the chapter has grown by more than 20% since inception. The chapter has engaged in statewide quality initiatives with the Tennessee Hospital Association and has engaged with other chapters across the state.
The Knoxville’s Chapter is an active, enthusiastic organization that is rapidly growing and thriving. Congratulations to the Knoxville chapter on being named the Rising Star Chapter for 2018.
Student Hospitalist Scholar Grant recipients
SHM is proud to acknowledge the latest winners of its Student Hospitalist Scholar Grant. These medical students were awarded grants to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other areas relevant to the field of hospital medicine.
Sandeep Bala
University of Chicago Pritzker School of Medicine
Poster 382 – The impact of plain language open medical notes on patient activation
Location: Denver (Colo.) Health Hospital
Monisha Bhatia
Vanderbilt University School of Medicine
Poster 23 – Using electronic medical record phenotypic data to predict discharge destination
Location: Vanderbilt University Medical Center, Nashville, Tenn.
Maximilian Hemmrich
University of Chicago Pritzker School of Medicine
Oral presentation, 11:45 a.m. – Noon, Tuesday, March 26
Project: Derivation and validation of a COPD readmission risk prediction tool
Location: University of Chicago
Ilana Scandariato Lavina
Weill Cornell Medical College
Poster 424 – Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Yun Li
Geisel School of Medicine at Dartmouth
Poster 320 – Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae
Location: Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
Resident Travel Grant recipients
We would like to congratulate the latest winners of SHM’s Resident Travel Grant. To qualify for this award, residents submitted an abstract for consideration in the RIV session at HM19 as first authors. Each of them produced outstanding work worthy of recognition:
Daniel Choi, MD – New York Presbyterian Hospital–Weill Cornell
Poster 277 – Improving rates of appropriate ICD deactivation discussions in admitted patients made DNR and/or comfort care
Armond Esmaili, MD – University of California, San Francisco, and the San Francisco VA Medical Center
Poster 649 – Early isolated hypotension, a sepsis “Canary in the Coal Mine”: Timing of antibiotics according to hypotension identifies different sepsis subtypes at differing risks of treatment delay
Poster 8 – Who’s waiting? Predictors of antibiotic delays in hypotensive patients with sepsis
Geoffroy Fauchet, MD – University of Colorado at Denver, Aurora – Rocky Mountain Regional VA Medical Center
Poster 288 – Tackling opioid prescriptions through resident engagement
Nick Zessis, MD – Washington University School of Medicine, St. Louis Children’s Hospital
Poster 101 – Smartphone-based teaching app increases frequency of residents teaching medical students
David Sterken, MD – University of California, San Francisco
Poster 230 – Safety of antimotility agent use during treatment for Clostridioides difficile infection in malignant hepatology patients
James Anstey, MD – University of California, San Francisco
Poster 57 – The POCUS supervision safety gap: Attending physician knowledge in point-of-care ultrasound lags behind that of internal medicine residents
Poster 147 – Association of post paracentesis albumin dosage and acute kidney injury in hospitalized patients
Nicholas Iverson, MD – University of California, San Francisco
Poster 408 – Implications of using an alternative measure to assess opiate days supplied at discharge
Marwah Shahid, MD – Baylor College of Medicine, Houston
Poster 22 – Topic modeling to evaluate hospital Google reviews
Abhishek Chaturvedi, MD – Allegheny Health Network, Pittsburgh
Poster 152 – Association of socioeconomic and racial disparities with health care utilization and outcomes in opioid overdose–related hospitalizations in the United States: Insights from National Inpatient Sample from 2012 through 2014
Pratyusha Tirumanisetty, MD – Unity Hospital, Rochester (N.Y.) Regional Health
Poster 9 – Does hospital-onset Clostridium difficile infection increase the risk of hospital discharge to skilled nursing facilities? A retrospective case-control study from a community hospital.
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustainability, and innovation within their activities, which are then recognized at SHM’s annual conference.
Please join SHM in congratulating the following chapters on their year of success in 2018!
Platinum Chapters: Iowa; Knoxville; Maryland; Michigan; Minnesota; New Mexico; North Carolina Triangle; Pacific Northwest; Southwest Florida; Wiregrass.
Gold Chapters: Houston; NYC/Westchester; Piedmont Triad; San Francisco Bay Area
Silver Chapters: Boston/Eastern Massachusetts; Charlotte Metro Area; Gulf States; Hampton Roads; Kentucky; Maine; Nebraska; North Jersey; Rocky Mountain; South Central Pennsylvania; South Texas; St. Louis.
Outstanding Chapter of the Year
Michigan. The Outstanding Chapter of the Year Award goes to one chapter that exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2018 is the Michigan chapter of SHM.
The Michigan chapter continues to embrace the mission of our society and nurtures a vibrant, multidisciplinary membership. It is currently the largest chapter in the program, representing more than 750 SHM members.
Using a technology-enabled platform, the Michigan chapter has expanded its meetings to four different sites, leveraging expertise from across the state. The chapter recently held its largest meeting focused on provider burnout, with more than 100 attendees across four different sites. It featured a main speaker as well as a multidisciplinary panel of leaders from eight different health systems.
The chapter has successfully incorporated advocacy into its annual planning and actively responds to new legislation affecting hospital medicine. It continues to be an active and valued member of the Michigan medical community and the SHM chapter community at large.
In addition to the service to members, the chapter strives to serve the SHM chapter community at large by collaborating and sharing best practices.
The chapter’s level of originality is not only a benefit to the chapter, but also to SHM’s Chapter program as a whole. Congratulations to the Michigan chapter on being named the Outstanding Chapter of 2018.
Rising Star Chapter
Knoxville. The Rising Star Chapter Award goes to one chapter that has been active for 2 years or less and in the past 12 months has made improvements to its leadership, stability and growth, and membership.
The recipient of the Rising Star Chapter Award for 2018 is the Knoxville (Tenn.) Chapter of SHM, which has made significant strides since its launch in the spring of 2017. The chapter assembled a group of local hospitalists from Knoxville and the surrounding region to encourage participation and drive quality initiatives in area hospitals.
The Knoxville chapter developed a leadership framework, including officers and board members, and just completed its first formal chapter leadership election. In 2018, the chapter held four meetings, including an event steered toward residents and students. Membership in the chapter has grown by more than 20% since inception. The chapter has engaged in statewide quality initiatives with the Tennessee Hospital Association and has engaged with other chapters across the state.
The Knoxville’s Chapter is an active, enthusiastic organization that is rapidly growing and thriving. Congratulations to the Knoxville chapter on being named the Rising Star Chapter for 2018.
Student Hospitalist Scholar Grant recipients
SHM is proud to acknowledge the latest winners of its Student Hospitalist Scholar Grant. These medical students were awarded grants to complete scholarly work with an active SHM mentor in a project related to patient safety, quality improvement, or other areas relevant to the field of hospital medicine.
Sandeep Bala
University of Chicago Pritzker School of Medicine
Poster 382 – The impact of plain language open medical notes on patient activation
Location: Denver (Colo.) Health Hospital
Monisha Bhatia
Vanderbilt University School of Medicine
Poster 23 – Using electronic medical record phenotypic data to predict discharge destination
Location: Vanderbilt University Medical Center, Nashville, Tenn.
Maximilian Hemmrich
University of Chicago Pritzker School of Medicine
Oral presentation, 11:45 a.m. – Noon, Tuesday, March 26
Project: Derivation and validation of a COPD readmission risk prediction tool
Location: University of Chicago
Ilana Scandariato Lavina
Weill Cornell Medical College
Poster 424 – Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Yun Li
Geisel School of Medicine at Dartmouth
Poster 320 – Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae
Location: Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
Resident Travel Grant recipients
We would like to congratulate the latest winners of SHM’s Resident Travel Grant. To qualify for this award, residents submitted an abstract for consideration in the RIV session at HM19 as first authors. Each of them produced outstanding work worthy of recognition:
Daniel Choi, MD – New York Presbyterian Hospital–Weill Cornell
Poster 277 – Improving rates of appropriate ICD deactivation discussions in admitted patients made DNR and/or comfort care
Armond Esmaili, MD – University of California, San Francisco, and the San Francisco VA Medical Center
Poster 649 – Early isolated hypotension, a sepsis “Canary in the Coal Mine”: Timing of antibiotics according to hypotension identifies different sepsis subtypes at differing risks of treatment delay
Poster 8 – Who’s waiting? Predictors of antibiotic delays in hypotensive patients with sepsis
Geoffroy Fauchet, MD – University of Colorado at Denver, Aurora – Rocky Mountain Regional VA Medical Center
Poster 288 – Tackling opioid prescriptions through resident engagement
Nick Zessis, MD – Washington University School of Medicine, St. Louis Children’s Hospital
Poster 101 – Smartphone-based teaching app increases frequency of residents teaching medical students
David Sterken, MD – University of California, San Francisco
Poster 230 – Safety of antimotility agent use during treatment for Clostridioides difficile infection in malignant hepatology patients
James Anstey, MD – University of California, San Francisco
Poster 57 – The POCUS supervision safety gap: Attending physician knowledge in point-of-care ultrasound lags behind that of internal medicine residents
Poster 147 – Association of post paracentesis albumin dosage and acute kidney injury in hospitalized patients
Nicholas Iverson, MD – University of California, San Francisco
Poster 408 – Implications of using an alternative measure to assess opiate days supplied at discharge
Marwah Shahid, MD – Baylor College of Medicine, Houston
Poster 22 – Topic modeling to evaluate hospital Google reviews
Abhishek Chaturvedi, MD – Allegheny Health Network, Pittsburgh
Poster 152 – Association of socioeconomic and racial disparities with health care utilization and outcomes in opioid overdose–related hospitalizations in the United States: Insights from National Inpatient Sample from 2012 through 2014
Pratyusha Tirumanisetty, MD – Unity Hospital, Rochester (N.Y.) Regional Health
Poster 9 – Does hospital-onset Clostridium difficile infection increase the risk of hospital discharge to skilled nursing facilities? A retrospective case-control study from a community hospital.
Many EMS protocols for status epilepticus do not follow evidence-based guidelines
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
FROM JAMA
Key clinical point: Many emergency medical services (EMS) system protocols may not follow evidence-based guidelines or accurately define generalized convulsive status epilepticus.
Major finding: In all, 18.2% of the protocols recommended at least one medication by the route and at the dose suggested in clinical trials or in an evidence-based guideline.
Study details: A review of treatment protocols from 33 EMS systems that cover the 58 counties in California.
Disclosures: The authors reported personal compensation from JAMA Neurology and Continuum Audio unrelated to the present study and grants from the National Institutes of Health.
Source: Betjemann JP et al. JAMA. 2019 March 26.
Swedish strategies improve survival for premature infants
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
FROM JAMA
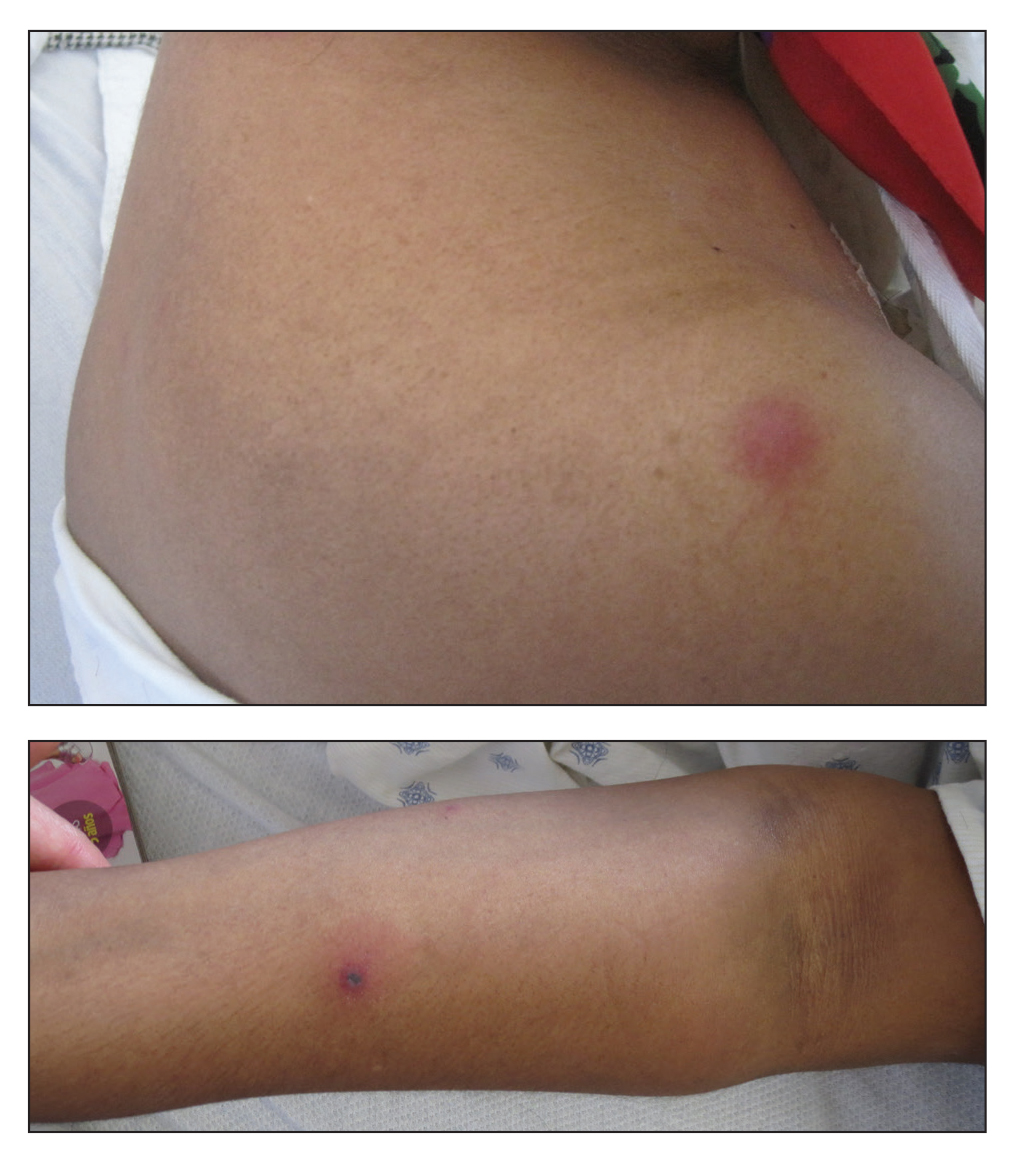
Erythematous and Necrotic Papules in an Immunosuppressed Woman
The Diagnosis: Disseminated Fusariosis
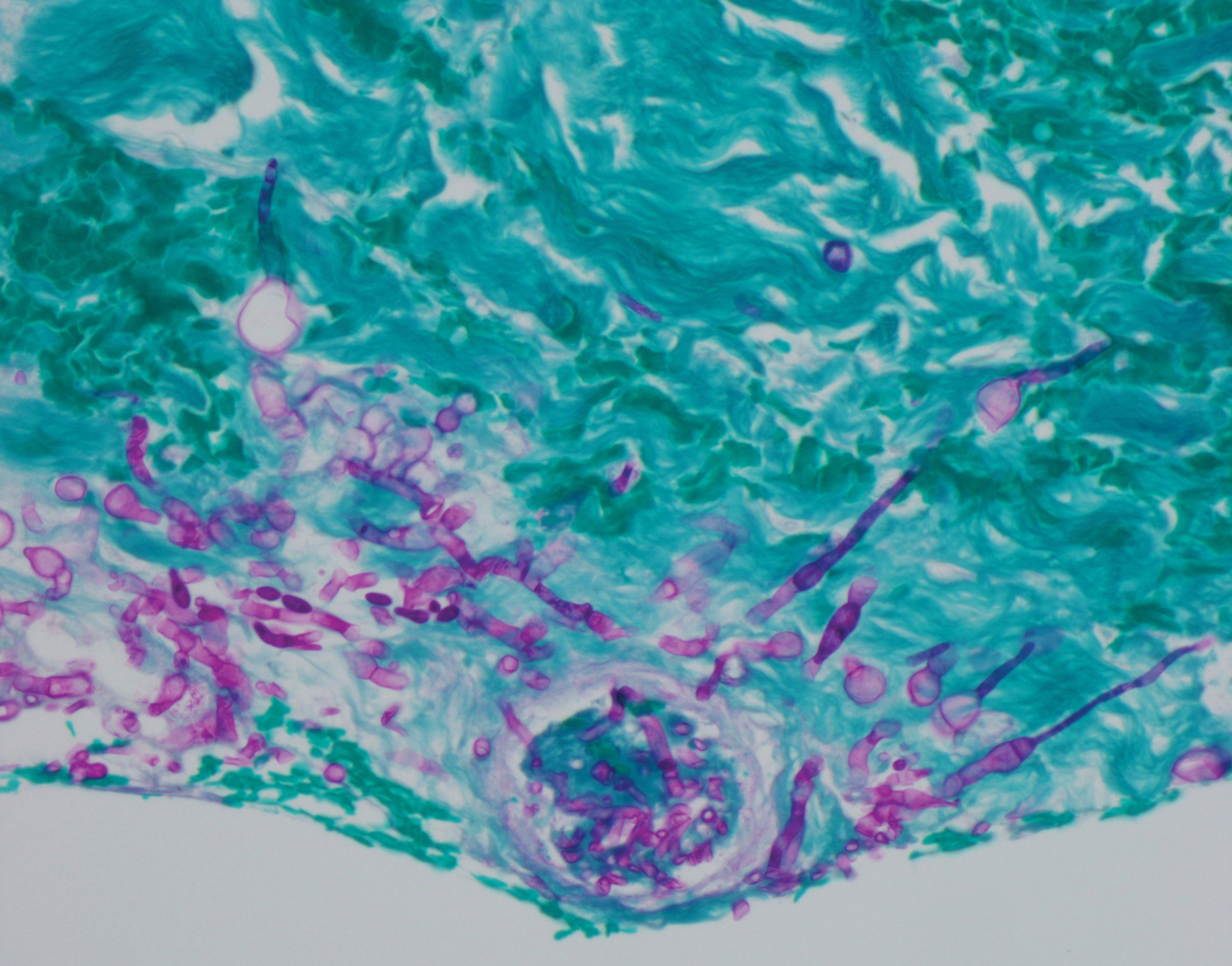
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.
Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
The Diagnosis: Disseminated Fusariosis
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.
Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
The Diagnosis: Disseminated Fusariosis
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.
Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
For endometrial cancer, postop taxane/platinum competes with standard therapy
For patients with endometrial cancer at high risk of progression, receiving a combination of taxane and platinum chemotherapy after surgery offers similar efficacy and tolerability as doxorubicin and cisplatin, the standard therapy, according to a recent phase 3 trial.
Progression-free and overall survival rates were similar across treatment types, reported lead author Hiroyuki Nomura, MD, of Keio University, Tokyo, and his colleagues. The findings maintain doxorubicin/cisplatin as standard therapy; however, taxane/platinum chemotherapy could be a possible alternative for some patients because of similar efficacy and tolerability with a distinct toxicity profile.
“Establishment of evidence and validation of the optimal postoperative adjuvant chemotherapy regimen for endometrial cancer are important issues,” the investigators wrote in JAMA Oncology.
The multicenter, open-label study involved 788 patients with endometrial cancer at risk of progression, based on histologic findings. Eligibility required that patients have a residual tumor of at least 2 cm without extension beyond the abdominal cavity. Patients were randomly grouped into one of three treatment groups: doxorubicin/cisplatin, paclitaxel/carboplatin, or docetaxel/cisplatin. If tolerated, six 3-week cycles were given. The median follow-up period was 7 years. The primary and secondary endpoints were 5-year progression free survival and overall survival, respectively.
Survival rates were statistically similar between groups. The 5-year progression-free survival rate was 73.3% for doxorubicin/cisplatin, 73.9% for paclitaxel/carboplatin, and 79.0% for docetaxel/cisplatin (P = .12); the 5-year overall survival rate was 82.7% for doxorubicin/cisplatin, 86.1% for paclitaxel/carboplatin, and 88.1% for docetaxel/cisplatin (P = .67). Tolerability was also comparable, with a small range of discontinuation rates across treatment types, from 20.2% to 25.5% (P = .14).
“Although the superiority of docetaxel plus cisplatin and paclitaxel plus carboplatin over doxorubicin plus cisplatin was not demonstrated, we found that the three regimens were comparable in therapeutic effect,” the investigators concluded. “[C]onsidering efficacy and tolerability, taxane plus platinum regimens may be an alternative to treatment with doxorubicin plus cisplatin.”
The study was funded by a Health Labour Sciences Research Grant with nonspecific funding from AstraZeneca, Eisai, Bristol-Myers Squibb, and others. The investigators reported financial relationships with Chugai, Sanofi, Takeda, AbbVie, and others.
SOURCE: Nomura H et al. JAMA Oncol. 2019 Mar 21. doi: 10.1001/jamaoncol.2019.0001.
For patients with endometrial cancer at high risk of progression, receiving a combination of taxane and platinum chemotherapy after surgery offers similar efficacy and tolerability as doxorubicin and cisplatin, the standard therapy, according to a recent phase 3 trial.
Progression-free and overall survival rates were similar across treatment types, reported lead author Hiroyuki Nomura, MD, of Keio University, Tokyo, and his colleagues. The findings maintain doxorubicin/cisplatin as standard therapy; however, taxane/platinum chemotherapy could be a possible alternative for some patients because of similar efficacy and tolerability with a distinct toxicity profile.
“Establishment of evidence and validation of the optimal postoperative adjuvant chemotherapy regimen for endometrial cancer are important issues,” the investigators wrote in JAMA Oncology.
The multicenter, open-label study involved 788 patients with endometrial cancer at risk of progression, based on histologic findings. Eligibility required that patients have a residual tumor of at least 2 cm without extension beyond the abdominal cavity. Patients were randomly grouped into one of three treatment groups: doxorubicin/cisplatin, paclitaxel/carboplatin, or docetaxel/cisplatin. If tolerated, six 3-week cycles were given. The median follow-up period was 7 years. The primary and secondary endpoints were 5-year progression free survival and overall survival, respectively.
Survival rates were statistically similar between groups. The 5-year progression-free survival rate was 73.3% for doxorubicin/cisplatin, 73.9% for paclitaxel/carboplatin, and 79.0% for docetaxel/cisplatin (P = .12); the 5-year overall survival rate was 82.7% for doxorubicin/cisplatin, 86.1% for paclitaxel/carboplatin, and 88.1% for docetaxel/cisplatin (P = .67). Tolerability was also comparable, with a small range of discontinuation rates across treatment types, from 20.2% to 25.5% (P = .14).
“Although the superiority of docetaxel plus cisplatin and paclitaxel plus carboplatin over doxorubicin plus cisplatin was not demonstrated, we found that the three regimens were comparable in therapeutic effect,” the investigators concluded. “[C]onsidering efficacy and tolerability, taxane plus platinum regimens may be an alternative to treatment with doxorubicin plus cisplatin.”
The study was funded by a Health Labour Sciences Research Grant with nonspecific funding from AstraZeneca, Eisai, Bristol-Myers Squibb, and others. The investigators reported financial relationships with Chugai, Sanofi, Takeda, AbbVie, and others.
SOURCE: Nomura H et al. JAMA Oncol. 2019 Mar 21. doi: 10.1001/jamaoncol.2019.0001.
For patients with endometrial cancer at high risk of progression, receiving a combination of taxane and platinum chemotherapy after surgery offers similar efficacy and tolerability as doxorubicin and cisplatin, the standard therapy, according to a recent phase 3 trial.
Progression-free and overall survival rates were similar across treatment types, reported lead author Hiroyuki Nomura, MD, of Keio University, Tokyo, and his colleagues. The findings maintain doxorubicin/cisplatin as standard therapy; however, taxane/platinum chemotherapy could be a possible alternative for some patients because of similar efficacy and tolerability with a distinct toxicity profile.
“Establishment of evidence and validation of the optimal postoperative adjuvant chemotherapy regimen for endometrial cancer are important issues,” the investigators wrote in JAMA Oncology.
The multicenter, open-label study involved 788 patients with endometrial cancer at risk of progression, based on histologic findings. Eligibility required that patients have a residual tumor of at least 2 cm without extension beyond the abdominal cavity. Patients were randomly grouped into one of three treatment groups: doxorubicin/cisplatin, paclitaxel/carboplatin, or docetaxel/cisplatin. If tolerated, six 3-week cycles were given. The median follow-up period was 7 years. The primary and secondary endpoints were 5-year progression free survival and overall survival, respectively.
Survival rates were statistically similar between groups. The 5-year progression-free survival rate was 73.3% for doxorubicin/cisplatin, 73.9% for paclitaxel/carboplatin, and 79.0% for docetaxel/cisplatin (P = .12); the 5-year overall survival rate was 82.7% for doxorubicin/cisplatin, 86.1% for paclitaxel/carboplatin, and 88.1% for docetaxel/cisplatin (P = .67). Tolerability was also comparable, with a small range of discontinuation rates across treatment types, from 20.2% to 25.5% (P = .14).
“Although the superiority of docetaxel plus cisplatin and paclitaxel plus carboplatin over doxorubicin plus cisplatin was not demonstrated, we found that the three regimens were comparable in therapeutic effect,” the investigators concluded. “[C]onsidering efficacy and tolerability, taxane plus platinum regimens may be an alternative to treatment with doxorubicin plus cisplatin.”
The study was funded by a Health Labour Sciences Research Grant with nonspecific funding from AstraZeneca, Eisai, Bristol-Myers Squibb, and others. The investigators reported financial relationships with Chugai, Sanofi, Takeda, AbbVie, and others.
SOURCE: Nomura H et al. JAMA Oncol. 2019 Mar 21. doi: 10.1001/jamaoncol.2019.0001.
FROM JAMA ONCOLOGY
SHM Chapter leaders highlight their local programs (VIDEO)
Leaders of SHM chapters give their take-away points from the Chapter Leader Training Program and highlights from their own local programs.

Leaders of SHM chapters give their take-away points from the Chapter Leader Training Program and highlights from their own local programs.

Leaders of SHM chapters give their take-away points from the Chapter Leader Training Program and highlights from their own local programs.

Highlights from the "Updates in Sepsis" session (VIDEO)

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.
Adapting to change key to hospitalists’ future, SHM president says
Society of Hospital Medicine President Nasim Afsar, MD, SFHM, told a packed ballroom of hospitalists at HM19 on Monday that it’s not change to the health care industry that is most central to their future, but it’s how they assume a role within it and how they spark it themselves.
With a tone that was, at times, almost ebullient about change, Dr. Afsar characterized the flux of health care as a series of opportunities to improve patient care.
“Run toward change,” said Dr. Afsar, chief ambulatory officer and chief medical officer for accountable care organizations at University of California, Irvine. “And be a force of positive change.”
The push toward affordability and value has made for some “unlikely partners,” she noted, including the health care venture launched by Amazon, Berkshire Hathaway, and JPMorgan Chase, as well as some newer corporations stepping into the health care sphere, such as Uber with its UberHealth and the creation of giants like the CVS-Aetna merger.
She acknowledged it brings “uncertainty and risk” but suggested that hospitalists are equipped to cope, saying that “we’ve all experienced this in our personal and professional lives.”
Dr. Afsar described four major themes of change to the health care landscape that will affect how hospitalists do their jobs.
- A new setting of care. “The care of the patients is moving from the hospital to the ambulatory setting,” she said. “Some of the surgeries that we used to do in the ER are now being done in ambulatory surgery centers. Antibiotics are being infused via IVs at patients’ homes.”
- Focus on health and well-being. “There’s a transition as a society on focusing on the sick to prevention of disease,” she said. “How can we prevent chronic illness once it occurs? How can we limit its progression? This is a very new focus for us in health care.”
- An increasing role of patient care teams – including primary care doctors, pharmacists, and case managers – rather than hospital-based teams.
- A new focus on patient-centered care. “It’s a focus about how we can be everywhere the patient is, at anytime that the patient needs us,” she said.
A sense of the way forward, Dr. Afsar said, came out of recent strategic meetings of the SHM board of directors, in which they talked about the role and future of hospitalists in population health management and value-based care. They agreed hospitalists should define themselves by their values and competencies, not by the hospital building itself. Hospitalists should use the acute care episode to make sure patients are connected to a larger system of care with wellness and prevention in mind.
“It’s not the strongest of the species that survive, nor the most intelligent,” Dr. Afsar said. “But the ones who are most adaptable to change. While there’s debate on the Internet about who originally said this, there’s absolutely no debate that the theme in life and in health care is adaptability in the face of constant change.”
In his own address at the Annual Conference of the Society of Hospital Medicine, Christopher Frost, MD, SFHM, the president-elect of SHM and national medical director of hospital-based services for LifePoint Health in Brentwood, Tenn., echoed Dr. Afsar’s theme of action in the context of change.
A key word, he said, is “multifarious” – the health care industry changes and the ways hospitalists are tackling these changes come in many and various types.
“We will not just react to – but actually help author – aspects of this change,” he said, including the continued move from fee for service to value-based and risk-based models of payment, and how to put new insights into disease processes to use and how they’re linked to social factors.
Increasing the diversity of hospitalist teams, maximizing the use of technology, and improving LGBTQ care are all themes of change being addressed at the meeting, he noted.
“When we summit one mountain of our own professional Alps,” Dr. Frost said, “and we see another on the horizon, we say, ‘Let’s climb that one. Let’s go there.’ ”
Society of Hospital Medicine President Nasim Afsar, MD, SFHM, told a packed ballroom of hospitalists at HM19 on Monday that it’s not change to the health care industry that is most central to their future, but it’s how they assume a role within it and how they spark it themselves.
With a tone that was, at times, almost ebullient about change, Dr. Afsar characterized the flux of health care as a series of opportunities to improve patient care.
“Run toward change,” said Dr. Afsar, chief ambulatory officer and chief medical officer for accountable care organizations at University of California, Irvine. “And be a force of positive change.”
The push toward affordability and value has made for some “unlikely partners,” she noted, including the health care venture launched by Amazon, Berkshire Hathaway, and JPMorgan Chase, as well as some newer corporations stepping into the health care sphere, such as Uber with its UberHealth and the creation of giants like the CVS-Aetna merger.
She acknowledged it brings “uncertainty and risk” but suggested that hospitalists are equipped to cope, saying that “we’ve all experienced this in our personal and professional lives.”
Dr. Afsar described four major themes of change to the health care landscape that will affect how hospitalists do their jobs.
- A new setting of care. “The care of the patients is moving from the hospital to the ambulatory setting,” she said. “Some of the surgeries that we used to do in the ER are now being done in ambulatory surgery centers. Antibiotics are being infused via IVs at patients’ homes.”
- Focus on health and well-being. “There’s a transition as a society on focusing on the sick to prevention of disease,” she said. “How can we prevent chronic illness once it occurs? How can we limit its progression? This is a very new focus for us in health care.”
- An increasing role of patient care teams – including primary care doctors, pharmacists, and case managers – rather than hospital-based teams.
- A new focus on patient-centered care. “It’s a focus about how we can be everywhere the patient is, at anytime that the patient needs us,” she said.
A sense of the way forward, Dr. Afsar said, came out of recent strategic meetings of the SHM board of directors, in which they talked about the role and future of hospitalists in population health management and value-based care. They agreed hospitalists should define themselves by their values and competencies, not by the hospital building itself. Hospitalists should use the acute care episode to make sure patients are connected to a larger system of care with wellness and prevention in mind.
“It’s not the strongest of the species that survive, nor the most intelligent,” Dr. Afsar said. “But the ones who are most adaptable to change. While there’s debate on the Internet about who originally said this, there’s absolutely no debate that the theme in life and in health care is adaptability in the face of constant change.”
In his own address at the Annual Conference of the Society of Hospital Medicine, Christopher Frost, MD, SFHM, the president-elect of SHM and national medical director of hospital-based services for LifePoint Health in Brentwood, Tenn., echoed Dr. Afsar’s theme of action in the context of change.
A key word, he said, is “multifarious” – the health care industry changes and the ways hospitalists are tackling these changes come in many and various types.
“We will not just react to – but actually help author – aspects of this change,” he said, including the continued move from fee for service to value-based and risk-based models of payment, and how to put new insights into disease processes to use and how they’re linked to social factors.
Increasing the diversity of hospitalist teams, maximizing the use of technology, and improving LGBTQ care are all themes of change being addressed at the meeting, he noted.
“When we summit one mountain of our own professional Alps,” Dr. Frost said, “and we see another on the horizon, we say, ‘Let’s climb that one. Let’s go there.’ ”
Society of Hospital Medicine President Nasim Afsar, MD, SFHM, told a packed ballroom of hospitalists at HM19 on Monday that it’s not change to the health care industry that is most central to their future, but it’s how they assume a role within it and how they spark it themselves.
With a tone that was, at times, almost ebullient about change, Dr. Afsar characterized the flux of health care as a series of opportunities to improve patient care.
“Run toward change,” said Dr. Afsar, chief ambulatory officer and chief medical officer for accountable care organizations at University of California, Irvine. “And be a force of positive change.”
The push toward affordability and value has made for some “unlikely partners,” she noted, including the health care venture launched by Amazon, Berkshire Hathaway, and JPMorgan Chase, as well as some newer corporations stepping into the health care sphere, such as Uber with its UberHealth and the creation of giants like the CVS-Aetna merger.
She acknowledged it brings “uncertainty and risk” but suggested that hospitalists are equipped to cope, saying that “we’ve all experienced this in our personal and professional lives.”
Dr. Afsar described four major themes of change to the health care landscape that will affect how hospitalists do their jobs.
- A new setting of care. “The care of the patients is moving from the hospital to the ambulatory setting,” she said. “Some of the surgeries that we used to do in the ER are now being done in ambulatory surgery centers. Antibiotics are being infused via IVs at patients’ homes.”
- Focus on health and well-being. “There’s a transition as a society on focusing on the sick to prevention of disease,” she said. “How can we prevent chronic illness once it occurs? How can we limit its progression? This is a very new focus for us in health care.”
- An increasing role of patient care teams – including primary care doctors, pharmacists, and case managers – rather than hospital-based teams.
- A new focus on patient-centered care. “It’s a focus about how we can be everywhere the patient is, at anytime that the patient needs us,” she said.
A sense of the way forward, Dr. Afsar said, came out of recent strategic meetings of the SHM board of directors, in which they talked about the role and future of hospitalists in population health management and value-based care. They agreed hospitalists should define themselves by their values and competencies, not by the hospital building itself. Hospitalists should use the acute care episode to make sure patients are connected to a larger system of care with wellness and prevention in mind.
“It’s not the strongest of the species that survive, nor the most intelligent,” Dr. Afsar said. “But the ones who are most adaptable to change. While there’s debate on the Internet about who originally said this, there’s absolutely no debate that the theme in life and in health care is adaptability in the face of constant change.”
In his own address at the Annual Conference of the Society of Hospital Medicine, Christopher Frost, MD, SFHM, the president-elect of SHM and national medical director of hospital-based services for LifePoint Health in Brentwood, Tenn., echoed Dr. Afsar’s theme of action in the context of change.
A key word, he said, is “multifarious” – the health care industry changes and the ways hospitalists are tackling these changes come in many and various types.
“We will not just react to – but actually help author – aspects of this change,” he said, including the continued move from fee for service to value-based and risk-based models of payment, and how to put new insights into disease processes to use and how they’re linked to social factors.
Increasing the diversity of hospitalist teams, maximizing the use of technology, and improving LGBTQ care are all themes of change being addressed at the meeting, he noted.
“When we summit one mountain of our own professional Alps,” Dr. Frost said, “and we see another on the horizon, we say, ‘Let’s climb that one. Let’s go there.’ ”
FDA panel leans toward more robust breast implant surveillance
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Interview with John Corboy, MD, on discontinuing disease modifying therapy in elderly patients with MS
Discontinuing
How would you characterize the prevalence of MS in the elderly?
DR. CORBOY: A recent large demographic study put together by the National MS Society found that there’s almost a million individuals diagnosed with MS over the course of the last 40 to 50 years. The largest population segment was those aged 55 to 64 years. People with MS aged 55 or older constituted 46% of all those with MS.
What disease-modifying therapies (DMTs) are approved by the FDA for the elderly?
DR. CORBOY: Of the drugs that have received FDA approval, most are for individuals over the age of 18 and there’s no specific age cutoff. However, there’s no data supporting DMT use in people over the age of 55 because they were excluded from the studies.
There’s one DMT, fingolimod, that was approved for use in patients under the age of 18; all others are approved for 18 and above. However, none of them are explicitly approved for people over the age of 55, because there is no data to support it.
What is the goal of your study, the DISCO MS trial?
DR. CORBOY: The DISCO MS trial will be the first randomized, controlled, blinded discontinuation trial in the MS space. The objective is to assess the benefit of DMTs in patients over the age of 55.
Part of the rationale for the trial is that prior subgroup analyses have shown that the vast majority of the benefit that we’ve been able to measure with all of these DMTs is seen in those who are under age 45.
A number of studies have examined existing databases and individuals who were either randomly or deliberately taken off of their medication as they age, including people who were felt to be stable with no recent relapses and no recent changes on their MRI brain. These studies reinforced that when discontinuing medications, the individuals who were much more likely to have recurrence of disease activity were younger patients.
Pathological studies clearly show the number of acutely inflamed plaques in the white matter is dramatically lower in autopsies of older vs younger patients. There are different changes in older patients, with lymphocytic nodules in the meninges, gray matter plaques related to these meningeal nodules, microglial activation, and smaller numbers of active, or mostly, inactive, white matter plaques. It’s been difficult to show any substantial benefit in slowing disability progression, much of which is felt to not be associated with acute inflammatory disease in the aging patient. All of these medicines, which can be thought of as anti-inflammatory medicines, are very beneficial when patients are young but less so as they age.
Would you describe the DISCO MS study design?
DR. CORBOY: Our study looks at individuals who are 55 and older who have not had a relapse for at least 5 years, and who’ve not had a change on their brain scan for at least 3 years.
Individuals will be randomized to either stay on the medication that they’re currently taking or discontinue that medicine. They will be followed then for 2 years. The primary outcome will be either a new relapse or a new scan change. The examining investigators are blinded to whether the patient is currently taking a MS disease modifying therapy.
Secondary outcomes include progression of disability as measured by confirmed change on the Extended Disability Status Scale (EDSS).
The enrollment goal is about 300 patients. There are presently 15 sites. The goal is to have the study completed in about 3 years. We’re presently over halfway through enrollment.
We also have a number of patient-reported outcomes because we’re particularly interested in the patient’s view of what’s going on in terms of how they feel. Understanding that dynamic will be extremely important.
We are including both patients with relapsing MS and progressive forms of MS, noting that they should have no relapse and no scan change at study entry.
What are the challenges with this study?
DR. CORBOY: One challenge is interpreting the information with the assumption that the hypothesis is validated. The hypothesis is that in a stable population of older patients that we can safely discontinue DMTs.
If that is found to be true, the question is how many people will be affected? We know that about 46% of people with MS are 55 and older, but there are not really good estimates of the number of individuals 55 and older who remain on a DMT and who are stable by the definition I just described.
It can be safely said, I think, that a substantial number of the individuals 55 and older are still on DMTs. If there’s almost a million people with MS and 46% are 55 and older, that means around 400,000 people with MS in the United States are aged 55 and older. If only half of those are on a DMT, that leaves 200,000. If only half of those are stable and could go off therapy, that would mean perhaps 100,000 people could discontinue DMTs in the United States. If all those assumptions are true, that would be a substantial savings in the health care burden of the United States from a relatively small population of individuals.
Beyond the cost, there are adverse events associated with using these medications. Older patients are more likely to be at risk of complications of MS DMTs. There also are doctor visits, blood monitoring, and other things that are done over time, and the inconvenience of taking a medicine on a routine basis if, indeed, it’s really not necessary because there is no benefit. Moreover, older individuals have other conditions (eg diabetes, hypertension, arrhythmias, cancer, etc) that may limit their ability to use medications due to risk. We’re very interested to see the outcome.
Discontinuing
How would you characterize the prevalence of MS in the elderly?
DR. CORBOY: A recent large demographic study put together by the National MS Society found that there’s almost a million individuals diagnosed with MS over the course of the last 40 to 50 years. The largest population segment was those aged 55 to 64 years. People with MS aged 55 or older constituted 46% of all those with MS.
What disease-modifying therapies (DMTs) are approved by the FDA for the elderly?
DR. CORBOY: Of the drugs that have received FDA approval, most are for individuals over the age of 18 and there’s no specific age cutoff. However, there’s no data supporting DMT use in people over the age of 55 because they were excluded from the studies.
There’s one DMT, fingolimod, that was approved for use in patients under the age of 18; all others are approved for 18 and above. However, none of them are explicitly approved for people over the age of 55, because there is no data to support it.
What is the goal of your study, the DISCO MS trial?
DR. CORBOY: The DISCO MS trial will be the first randomized, controlled, blinded discontinuation trial in the MS space. The objective is to assess the benefit of DMTs in patients over the age of 55.
Part of the rationale for the trial is that prior subgroup analyses have shown that the vast majority of the benefit that we’ve been able to measure with all of these DMTs is seen in those who are under age 45.
A number of studies have examined existing databases and individuals who were either randomly or deliberately taken off of their medication as they age, including people who were felt to be stable with no recent relapses and no recent changes on their MRI brain. These studies reinforced that when discontinuing medications, the individuals who were much more likely to have recurrence of disease activity were younger patients.
Pathological studies clearly show the number of acutely inflamed plaques in the white matter is dramatically lower in autopsies of older vs younger patients. There are different changes in older patients, with lymphocytic nodules in the meninges, gray matter plaques related to these meningeal nodules, microglial activation, and smaller numbers of active, or mostly, inactive, white matter plaques. It’s been difficult to show any substantial benefit in slowing disability progression, much of which is felt to not be associated with acute inflammatory disease in the aging patient. All of these medicines, which can be thought of as anti-inflammatory medicines, are very beneficial when patients are young but less so as they age.
Would you describe the DISCO MS study design?
DR. CORBOY: Our study looks at individuals who are 55 and older who have not had a relapse for at least 5 years, and who’ve not had a change on their brain scan for at least 3 years.
Individuals will be randomized to either stay on the medication that they’re currently taking or discontinue that medicine. They will be followed then for 2 years. The primary outcome will be either a new relapse or a new scan change. The examining investigators are blinded to whether the patient is currently taking a MS disease modifying therapy.
Secondary outcomes include progression of disability as measured by confirmed change on the Extended Disability Status Scale (EDSS).
The enrollment goal is about 300 patients. There are presently 15 sites. The goal is to have the study completed in about 3 years. We’re presently over halfway through enrollment.
We also have a number of patient-reported outcomes because we’re particularly interested in the patient’s view of what’s going on in terms of how they feel. Understanding that dynamic will be extremely important.
We are including both patients with relapsing MS and progressive forms of MS, noting that they should have no relapse and no scan change at study entry.
What are the challenges with this study?
DR. CORBOY: One challenge is interpreting the information with the assumption that the hypothesis is validated. The hypothesis is that in a stable population of older patients that we can safely discontinue DMTs.
If that is found to be true, the question is how many people will be affected? We know that about 46% of people with MS are 55 and older, but there are not really good estimates of the number of individuals 55 and older who remain on a DMT and who are stable by the definition I just described.
It can be safely said, I think, that a substantial number of the individuals 55 and older are still on DMTs. If there’s almost a million people with MS and 46% are 55 and older, that means around 400,000 people with MS in the United States are aged 55 and older. If only half of those are on a DMT, that leaves 200,000. If only half of those are stable and could go off therapy, that would mean perhaps 100,000 people could discontinue DMTs in the United States. If all those assumptions are true, that would be a substantial savings in the health care burden of the United States from a relatively small population of individuals.
Beyond the cost, there are adverse events associated with using these medications. Older patients are more likely to be at risk of complications of MS DMTs. There also are doctor visits, blood monitoring, and other things that are done over time, and the inconvenience of taking a medicine on a routine basis if, indeed, it’s really not necessary because there is no benefit. Moreover, older individuals have other conditions (eg diabetes, hypertension, arrhythmias, cancer, etc) that may limit their ability to use medications due to risk. We’re very interested to see the outcome.
Discontinuing
How would you characterize the prevalence of MS in the elderly?
DR. CORBOY: A recent large demographic study put together by the National MS Society found that there’s almost a million individuals diagnosed with MS over the course of the last 40 to 50 years. The largest population segment was those aged 55 to 64 years. People with MS aged 55 or older constituted 46% of all those with MS.
What disease-modifying therapies (DMTs) are approved by the FDA for the elderly?
DR. CORBOY: Of the drugs that have received FDA approval, most are for individuals over the age of 18 and there’s no specific age cutoff. However, there’s no data supporting DMT use in people over the age of 55 because they were excluded from the studies.
There’s one DMT, fingolimod, that was approved for use in patients under the age of 18; all others are approved for 18 and above. However, none of them are explicitly approved for people over the age of 55, because there is no data to support it.
What is the goal of your study, the DISCO MS trial?
DR. CORBOY: The DISCO MS trial will be the first randomized, controlled, blinded discontinuation trial in the MS space. The objective is to assess the benefit of DMTs in patients over the age of 55.
Part of the rationale for the trial is that prior subgroup analyses have shown that the vast majority of the benefit that we’ve been able to measure with all of these DMTs is seen in those who are under age 45.
A number of studies have examined existing databases and individuals who were either randomly or deliberately taken off of their medication as they age, including people who were felt to be stable with no recent relapses and no recent changes on their MRI brain. These studies reinforced that when discontinuing medications, the individuals who were much more likely to have recurrence of disease activity were younger patients.
Pathological studies clearly show the number of acutely inflamed plaques in the white matter is dramatically lower in autopsies of older vs younger patients. There are different changes in older patients, with lymphocytic nodules in the meninges, gray matter plaques related to these meningeal nodules, microglial activation, and smaller numbers of active, or mostly, inactive, white matter plaques. It’s been difficult to show any substantial benefit in slowing disability progression, much of which is felt to not be associated with acute inflammatory disease in the aging patient. All of these medicines, which can be thought of as anti-inflammatory medicines, are very beneficial when patients are young but less so as they age.
Would you describe the DISCO MS study design?
DR. CORBOY: Our study looks at individuals who are 55 and older who have not had a relapse for at least 5 years, and who’ve not had a change on their brain scan for at least 3 years.
Individuals will be randomized to either stay on the medication that they’re currently taking or discontinue that medicine. They will be followed then for 2 years. The primary outcome will be either a new relapse or a new scan change. The examining investigators are blinded to whether the patient is currently taking a MS disease modifying therapy.
Secondary outcomes include progression of disability as measured by confirmed change on the Extended Disability Status Scale (EDSS).
The enrollment goal is about 300 patients. There are presently 15 sites. The goal is to have the study completed in about 3 years. We’re presently over halfway through enrollment.
We also have a number of patient-reported outcomes because we’re particularly interested in the patient’s view of what’s going on in terms of how they feel. Understanding that dynamic will be extremely important.
We are including both patients with relapsing MS and progressive forms of MS, noting that they should have no relapse and no scan change at study entry.
What are the challenges with this study?
DR. CORBOY: One challenge is interpreting the information with the assumption that the hypothesis is validated. The hypothesis is that in a stable population of older patients that we can safely discontinue DMTs.
If that is found to be true, the question is how many people will be affected? We know that about 46% of people with MS are 55 and older, but there are not really good estimates of the number of individuals 55 and older who remain on a DMT and who are stable by the definition I just described.
It can be safely said, I think, that a substantial number of the individuals 55 and older are still on DMTs. If there’s almost a million people with MS and 46% are 55 and older, that means around 400,000 people with MS in the United States are aged 55 and older. If only half of those are on a DMT, that leaves 200,000. If only half of those are stable and could go off therapy, that would mean perhaps 100,000 people could discontinue DMTs in the United States. If all those assumptions are true, that would be a substantial savings in the health care burden of the United States from a relatively small population of individuals.
Beyond the cost, there are adverse events associated with using these medications. Older patients are more likely to be at risk of complications of MS DMTs. There also are doctor visits, blood monitoring, and other things that are done over time, and the inconvenience of taking a medicine on a routine basis if, indeed, it’s really not necessary because there is no benefit. Moreover, older individuals have other conditions (eg diabetes, hypertension, arrhythmias, cancer, etc) that may limit their ability to use medications due to risk. We’re very interested to see the outcome.