User login
FDA approves siponimod for relapsing forms of MS
(MS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS.
Siponimod is a selective sphingosine 1-phosphate (S1P) receptor modulator that binds to S1P1 and S1P5 receptors. Its binding to the S1P1 receptor prevents lymphocytes from leaving the lymph nodes, which contributes to the treatment’s anti-inflammatory effects. Its binding to the S1P5 and S1P1 subreceptors on oligodendrocytes and astrocytes is intended to promote remyelination and prevent inflammation.
The treatment’s approval is based on the results of the phase 3 EXPAND study, according to the agency’s March 26 announcement. This randomized, double-blind study compared siponimod with placebo among 1,651 patients with secondary progressive MS. At baseline, the population’s mean age was 48 years, and mean disease duration was approximately 16 years. More than half the study population had a median Expanded Disability Status Scale score of 6.0 and relied on a walking aid.
Siponimod reduced the risk of 3-month confirmed disability progression (CDP) by 21%, compared with placebo (P = .013). Among participants with relapse activity in the 2 years prior to screening, siponimod reduced the risk of this outcome by 33%, compared with placebo (P = .0100). Siponimod delayed the risk of 6-month CDP by 26%, compared with placebo (P = .0058) and reduced the annualized relapse rate by 55%. In addition, the data suggested beneficial effects of siponimod on cognition, MRI disease activity, and brain volume loss. Siponimod did not provide significant improvements in patients with nonactive secondary progressive MS.
Common adverse events included headache, hypertension, and transaminase increase. The FDA requires siponimod to be dispensed with a medication guide that describes the treatment’s associated risks of infection, macular edema, decreased heart rate, and impaired lung function.
Novartis manufactures the drug. The company expects the drug to be available within 1 week, according to its press release.
(MS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS.
Siponimod is a selective sphingosine 1-phosphate (S1P) receptor modulator that binds to S1P1 and S1P5 receptors. Its binding to the S1P1 receptor prevents lymphocytes from leaving the lymph nodes, which contributes to the treatment’s anti-inflammatory effects. Its binding to the S1P5 and S1P1 subreceptors on oligodendrocytes and astrocytes is intended to promote remyelination and prevent inflammation.
The treatment’s approval is based on the results of the phase 3 EXPAND study, according to the agency’s March 26 announcement. This randomized, double-blind study compared siponimod with placebo among 1,651 patients with secondary progressive MS. At baseline, the population’s mean age was 48 years, and mean disease duration was approximately 16 years. More than half the study population had a median Expanded Disability Status Scale score of 6.0 and relied on a walking aid.
Siponimod reduced the risk of 3-month confirmed disability progression (CDP) by 21%, compared with placebo (P = .013). Among participants with relapse activity in the 2 years prior to screening, siponimod reduced the risk of this outcome by 33%, compared with placebo (P = .0100). Siponimod delayed the risk of 6-month CDP by 26%, compared with placebo (P = .0058) and reduced the annualized relapse rate by 55%. In addition, the data suggested beneficial effects of siponimod on cognition, MRI disease activity, and brain volume loss. Siponimod did not provide significant improvements in patients with nonactive secondary progressive MS.
Common adverse events included headache, hypertension, and transaminase increase. The FDA requires siponimod to be dispensed with a medication guide that describes the treatment’s associated risks of infection, macular edema, decreased heart rate, and impaired lung function.
Novartis manufactures the drug. The company expects the drug to be available within 1 week, according to its press release.
(MS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary progressive MS.
Siponimod is a selective sphingosine 1-phosphate (S1P) receptor modulator that binds to S1P1 and S1P5 receptors. Its binding to the S1P1 receptor prevents lymphocytes from leaving the lymph nodes, which contributes to the treatment’s anti-inflammatory effects. Its binding to the S1P5 and S1P1 subreceptors on oligodendrocytes and astrocytes is intended to promote remyelination and prevent inflammation.
The treatment’s approval is based on the results of the phase 3 EXPAND study, according to the agency’s March 26 announcement. This randomized, double-blind study compared siponimod with placebo among 1,651 patients with secondary progressive MS. At baseline, the population’s mean age was 48 years, and mean disease duration was approximately 16 years. More than half the study population had a median Expanded Disability Status Scale score of 6.0 and relied on a walking aid.
Siponimod reduced the risk of 3-month confirmed disability progression (CDP) by 21%, compared with placebo (P = .013). Among participants with relapse activity in the 2 years prior to screening, siponimod reduced the risk of this outcome by 33%, compared with placebo (P = .0100). Siponimod delayed the risk of 6-month CDP by 26%, compared with placebo (P = .0058) and reduced the annualized relapse rate by 55%. In addition, the data suggested beneficial effects of siponimod on cognition, MRI disease activity, and brain volume loss. Siponimod did not provide significant improvements in patients with nonactive secondary progressive MS.
Common adverse events included headache, hypertension, and transaminase increase. The FDA requires siponimod to be dispensed with a medication guide that describes the treatment’s associated risks of infection, macular edema, decreased heart rate, and impaired lung function.
Novartis manufactures the drug. The company expects the drug to be available within 1 week, according to its press release.
Pigmented Fungiform Papillae of the Tongue in an Indian Male
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
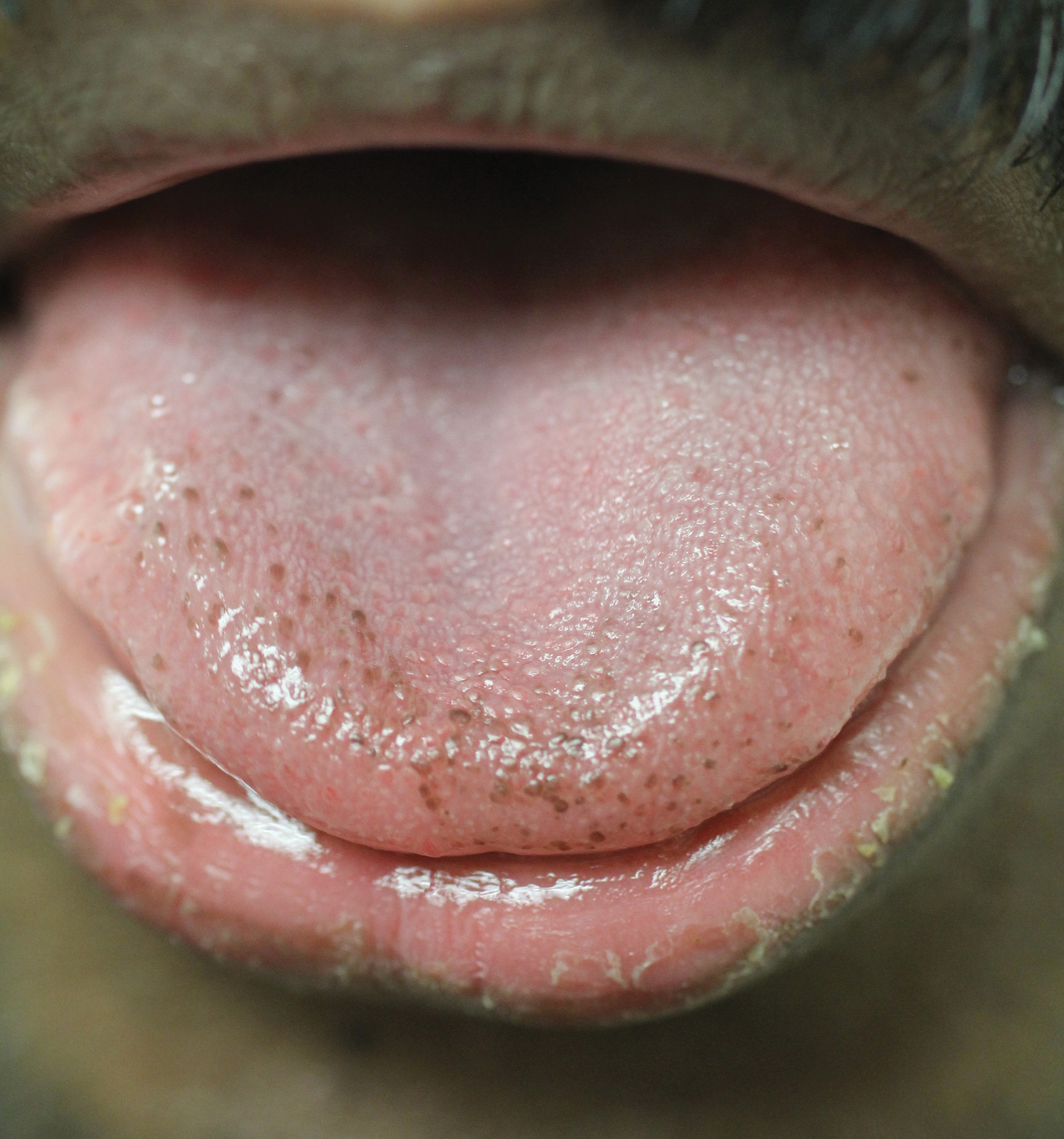
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.
Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.
Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.
Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
Practice Points
- Pigmented fungiform papillae of the tongue are common lingual hyperpigmented macules in patients with skin of color.
- It is important to be aware of this benign entity to provide reassurance to patients and avoid unnecessary testing.
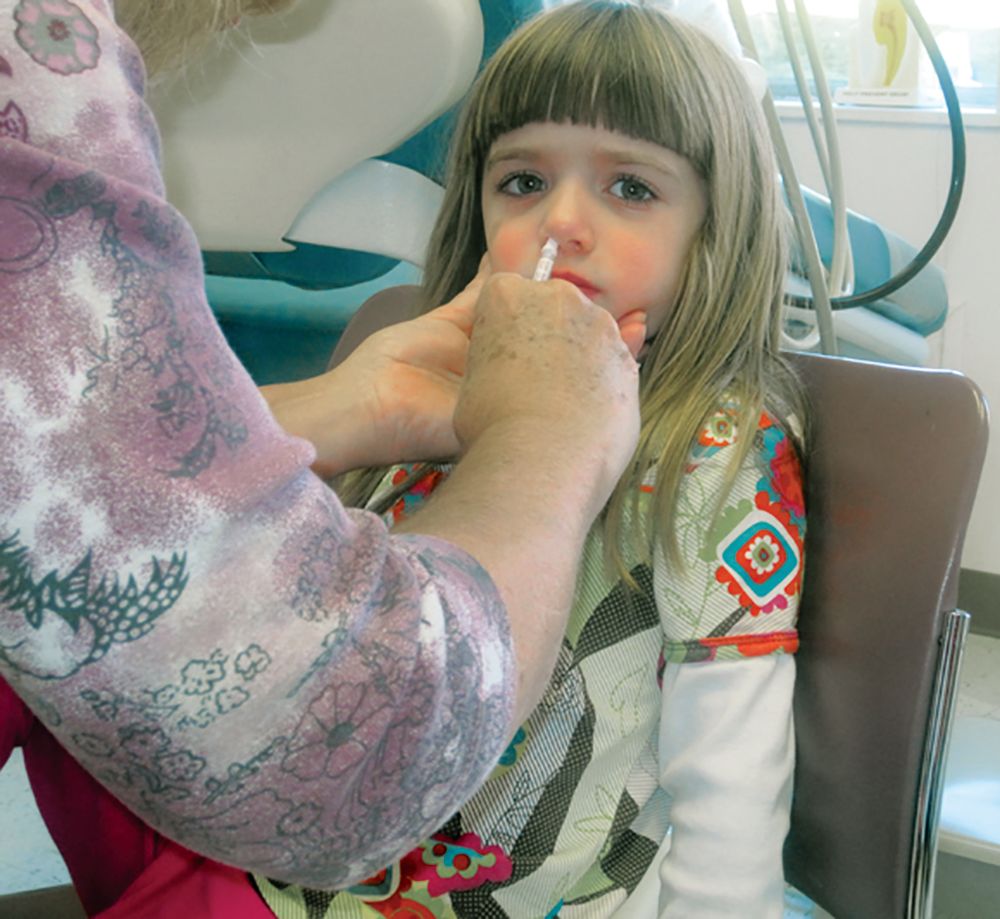
AAP updates 2019-2020 flu vaccine recommendations to include nasal spray
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Study launched to further evaluate the central vein sign in MS
DALLAS –
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Daniel Ontaneda, MD, said that up to 20% of individuals referred for a diagnosis of multiple sclerosis (MS) are incorrectly diagnosed with the disease, and about two-thirds of misdiagnosed patients are exposed to unnecessary and sometimes life-threatening risks associated with disease-modifying therapies. “MRI is a sensitive tool for diagnosis of MS and is an integral component of the diagnostic criteria for MS,” said Dr. Ontaneda, a neurologist at the Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research. “However, there are problems with its implementation. Approximately half of individuals referred to an MS clinic present with atypical symptoms [fatigue, cognitive disturbance, pain] and not typical syndromes [unilateral optic neuritis, brain stem syndromes, partial myelitis]. Increasing diagnostic sensitivity may have come at the price of decreased specificity. MRI criteria have a specificity of 32% for dissemination in space and 42% for dissemination in time.”
While misdiagnosis appears to be mainly caused by overinterpretation of abnormal MRI findings, the central vein sign (CVS) is an effective method to overcome such challenges. Recent studies have demonstrated that CVS may help to identify MS, as 85% of white matter lesions in MS have a central vein, compared with only 8% of small vessel ischemic disease, 34% of migraine, and 14% of other inflammatory or autoimmune diseases.
“We think there is a significant and unmet need for more specific and accurate diagnostic tests to facilitate early confirmation of a diagnosis of MS,” Dr. Ontaneda said. “We propose a prospective evaluation of the central vein sign, which we hypothesize will reduce misdiagnosis, hasten early diagnosis, and simplify clinical decision making.”
With funding from the Race to Erase MS Foundation, he and his associates have designed CAVS-MS (Central Vein Sign in MS), a multicenter, prospective, observational trial being conducted at 10 sites. The first phase of the study is a cross-sectional pilot at the 10 sites. The primary objective is to establish the contrast-to-noise ratio of lesion to normal-appearing white matter and central vein to lesion across the 10 sites using 3-tesla FLAIR imaging in subjects with a clinical or radiologic suspicion of MS. The secondary objectives are to investigate the difference in contrast-to-noise ratio identified in the primary objective between pre- and postcontrast FLAIR imaging to identify whether gadolinium injection improves central vein detection, to determine the reproducibility of different methods for detection of positive CVS across sites, and to determine the sensitivity and specificity of the different methods for the diagnosis of MS, compared with the McDonald 2010 MS criteria.
The study population will consist of 100 individuals referred to an MS center based on clinical or radiologic suspicion of MS; 30 participants are currently enrolled. The 10 sites include the Cleveland Clinic; Johns Hopkins University, Baltimore; the University of California, San Francisco; the University of Texas, Houston; the University of Toronto; the University of Vermont, Burlington; the University of Southern California, Los Angeles; Cedars-Sinai Medical Center, Los Angeles; Yale University, New Haven, Conn.; and the University of Pennsylvania, Philadelphia.
CAVS-MS includes development of a software platform for rating of central veins through an imaging software partner, QMENTA. “We are going to have the individual clinicians at each site rate the lesions, so we will have information from 10 different raters,” Dr. Ontaneda said. The study will be coordinated at the Cleveland Clinic, central image analysis will be conducted at the National Institutes of Health, and statistical analysis will be performed at the University of Pennsylvania.
The researchers also hope to perform a prospective study with three objectives. The first is to determine if incorporation of CVS for the diagnosis of MS improves diagnostic accuracy and hastens diagnosis in individuals presenting with typical first clinical events. The second objective “is to determine if incorporation of CVS for the diagnosis of MS improves specificity among individuals presenting with atypical syndromes,” Dr. Ontaneda said. “The third aim is to look at central vein volume as a predictor of clinical/MRI disease activity associated with disability in MS.”
He concluded his remarks by describing the CVS as “a tool that offers promise both for increasing specificity and perhaps enabling earlier diagnosis of MS. Studies will determine if the central vein sign can be incorporated into the diagnostic criteria. The NIH is working with MRI manufacturers to make sequences available for disseminated clinical use.”
Dr. Ontaneda reported that he has received grant support from the National Institutes of Health, the Race to Erase MS Foundation, the Patient-Centered Outcomes Research Institute, the National Multiple Sclerosis Society, Genentech, Genzyme, and Novartis. He has also received consulting fees from Biogen, Genentech, and Novartis.
DALLAS –
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Daniel Ontaneda, MD, said that up to 20% of individuals referred for a diagnosis of multiple sclerosis (MS) are incorrectly diagnosed with the disease, and about two-thirds of misdiagnosed patients are exposed to unnecessary and sometimes life-threatening risks associated with disease-modifying therapies. “MRI is a sensitive tool for diagnosis of MS and is an integral component of the diagnostic criteria for MS,” said Dr. Ontaneda, a neurologist at the Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research. “However, there are problems with its implementation. Approximately half of individuals referred to an MS clinic present with atypical symptoms [fatigue, cognitive disturbance, pain] and not typical syndromes [unilateral optic neuritis, brain stem syndromes, partial myelitis]. Increasing diagnostic sensitivity may have come at the price of decreased specificity. MRI criteria have a specificity of 32% for dissemination in space and 42% for dissemination in time.”
While misdiagnosis appears to be mainly caused by overinterpretation of abnormal MRI findings, the central vein sign (CVS) is an effective method to overcome such challenges. Recent studies have demonstrated that CVS may help to identify MS, as 85% of white matter lesions in MS have a central vein, compared with only 8% of small vessel ischemic disease, 34% of migraine, and 14% of other inflammatory or autoimmune diseases.
“We think there is a significant and unmet need for more specific and accurate diagnostic tests to facilitate early confirmation of a diagnosis of MS,” Dr. Ontaneda said. “We propose a prospective evaluation of the central vein sign, which we hypothesize will reduce misdiagnosis, hasten early diagnosis, and simplify clinical decision making.”
With funding from the Race to Erase MS Foundation, he and his associates have designed CAVS-MS (Central Vein Sign in MS), a multicenter, prospective, observational trial being conducted at 10 sites. The first phase of the study is a cross-sectional pilot at the 10 sites. The primary objective is to establish the contrast-to-noise ratio of lesion to normal-appearing white matter and central vein to lesion across the 10 sites using 3-tesla FLAIR imaging in subjects with a clinical or radiologic suspicion of MS. The secondary objectives are to investigate the difference in contrast-to-noise ratio identified in the primary objective between pre- and postcontrast FLAIR imaging to identify whether gadolinium injection improves central vein detection, to determine the reproducibility of different methods for detection of positive CVS across sites, and to determine the sensitivity and specificity of the different methods for the diagnosis of MS, compared with the McDonald 2010 MS criteria.
The study population will consist of 100 individuals referred to an MS center based on clinical or radiologic suspicion of MS; 30 participants are currently enrolled. The 10 sites include the Cleveland Clinic; Johns Hopkins University, Baltimore; the University of California, San Francisco; the University of Texas, Houston; the University of Toronto; the University of Vermont, Burlington; the University of Southern California, Los Angeles; Cedars-Sinai Medical Center, Los Angeles; Yale University, New Haven, Conn.; and the University of Pennsylvania, Philadelphia.
CAVS-MS includes development of a software platform for rating of central veins through an imaging software partner, QMENTA. “We are going to have the individual clinicians at each site rate the lesions, so we will have information from 10 different raters,” Dr. Ontaneda said. The study will be coordinated at the Cleveland Clinic, central image analysis will be conducted at the National Institutes of Health, and statistical analysis will be performed at the University of Pennsylvania.
The researchers also hope to perform a prospective study with three objectives. The first is to determine if incorporation of CVS for the diagnosis of MS improves diagnostic accuracy and hastens diagnosis in individuals presenting with typical first clinical events. The second objective “is to determine if incorporation of CVS for the diagnosis of MS improves specificity among individuals presenting with atypical syndromes,” Dr. Ontaneda said. “The third aim is to look at central vein volume as a predictor of clinical/MRI disease activity associated with disability in MS.”
He concluded his remarks by describing the CVS as “a tool that offers promise both for increasing specificity and perhaps enabling earlier diagnosis of MS. Studies will determine if the central vein sign can be incorporated into the diagnostic criteria. The NIH is working with MRI manufacturers to make sequences available for disseminated clinical use.”
Dr. Ontaneda reported that he has received grant support from the National Institutes of Health, the Race to Erase MS Foundation, the Patient-Centered Outcomes Research Institute, the National Multiple Sclerosis Society, Genentech, Genzyme, and Novartis. He has also received consulting fees from Biogen, Genentech, and Novartis.
DALLAS –
At the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, Daniel Ontaneda, MD, said that up to 20% of individuals referred for a diagnosis of multiple sclerosis (MS) are incorrectly diagnosed with the disease, and about two-thirds of misdiagnosed patients are exposed to unnecessary and sometimes life-threatening risks associated with disease-modifying therapies. “MRI is a sensitive tool for diagnosis of MS and is an integral component of the diagnostic criteria for MS,” said Dr. Ontaneda, a neurologist at the Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research. “However, there are problems with its implementation. Approximately half of individuals referred to an MS clinic present with atypical symptoms [fatigue, cognitive disturbance, pain] and not typical syndromes [unilateral optic neuritis, brain stem syndromes, partial myelitis]. Increasing diagnostic sensitivity may have come at the price of decreased specificity. MRI criteria have a specificity of 32% for dissemination in space and 42% for dissemination in time.”
While misdiagnosis appears to be mainly caused by overinterpretation of abnormal MRI findings, the central vein sign (CVS) is an effective method to overcome such challenges. Recent studies have demonstrated that CVS may help to identify MS, as 85% of white matter lesions in MS have a central vein, compared with only 8% of small vessel ischemic disease, 34% of migraine, and 14% of other inflammatory or autoimmune diseases.
“We think there is a significant and unmet need for more specific and accurate diagnostic tests to facilitate early confirmation of a diagnosis of MS,” Dr. Ontaneda said. “We propose a prospective evaluation of the central vein sign, which we hypothesize will reduce misdiagnosis, hasten early diagnosis, and simplify clinical decision making.”
With funding from the Race to Erase MS Foundation, he and his associates have designed CAVS-MS (Central Vein Sign in MS), a multicenter, prospective, observational trial being conducted at 10 sites. The first phase of the study is a cross-sectional pilot at the 10 sites. The primary objective is to establish the contrast-to-noise ratio of lesion to normal-appearing white matter and central vein to lesion across the 10 sites using 3-tesla FLAIR imaging in subjects with a clinical or radiologic suspicion of MS. The secondary objectives are to investigate the difference in contrast-to-noise ratio identified in the primary objective between pre- and postcontrast FLAIR imaging to identify whether gadolinium injection improves central vein detection, to determine the reproducibility of different methods for detection of positive CVS across sites, and to determine the sensitivity and specificity of the different methods for the diagnosis of MS, compared with the McDonald 2010 MS criteria.
The study population will consist of 100 individuals referred to an MS center based on clinical or radiologic suspicion of MS; 30 participants are currently enrolled. The 10 sites include the Cleveland Clinic; Johns Hopkins University, Baltimore; the University of California, San Francisco; the University of Texas, Houston; the University of Toronto; the University of Vermont, Burlington; the University of Southern California, Los Angeles; Cedars-Sinai Medical Center, Los Angeles; Yale University, New Haven, Conn.; and the University of Pennsylvania, Philadelphia.
CAVS-MS includes development of a software platform for rating of central veins through an imaging software partner, QMENTA. “We are going to have the individual clinicians at each site rate the lesions, so we will have information from 10 different raters,” Dr. Ontaneda said. The study will be coordinated at the Cleveland Clinic, central image analysis will be conducted at the National Institutes of Health, and statistical analysis will be performed at the University of Pennsylvania.
The researchers also hope to perform a prospective study with three objectives. The first is to determine if incorporation of CVS for the diagnosis of MS improves diagnostic accuracy and hastens diagnosis in individuals presenting with typical first clinical events. The second objective “is to determine if incorporation of CVS for the diagnosis of MS improves specificity among individuals presenting with atypical syndromes,” Dr. Ontaneda said. “The third aim is to look at central vein volume as a predictor of clinical/MRI disease activity associated with disability in MS.”
He concluded his remarks by describing the CVS as “a tool that offers promise both for increasing specificity and perhaps enabling earlier diagnosis of MS. Studies will determine if the central vein sign can be incorporated into the diagnostic criteria. The NIH is working with MRI manufacturers to make sequences available for disseminated clinical use.”
Dr. Ontaneda reported that he has received grant support from the National Institutes of Health, the Race to Erase MS Foundation, the Patient-Centered Outcomes Research Institute, the National Multiple Sclerosis Society, Genentech, Genzyme, and Novartis. He has also received consulting fees from Biogen, Genentech, and Novartis.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2019
Report calls for focus on ‘subpopulations’ to fight opioid epidemic
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Better communication with pharmacists can improve postop pain control
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
LAS VEGAS – . Watch out for overlapping medication orders. Beware of gabapentin mishaps, and embrace Tylenol – but not always.
April Smith, PharmD, associate professor of pharmacy practice at Creighton University, Omaha, offered these tips about postoperative care to surgeons at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“We’re probably one of the most underutilized professions you have on your team,” she said, adding that “we have to know what you’re doing to help you.”
As she explained, “if you’re going to have a new order set, let us know that, so we can be your allies in helping nurses and other people understand why we’re doing what we’re doing. I’m on the same floor, and the nurses are coming up to me and asking me questions. If I can explain to them why we’re doing these things, they’ll get on board a lot faster and save you a lot of phone calls. I know you’re surgeons and you hate that [phone calls].”
Better communication with pharmacists can also boost the stocking of enhanced-recovery medications in automatic dispensing machines, she said, so they’re ready when patients need them.
Dr. Smith offered these tips about specific postsurgery medications:
- Scopolamine is a “great drug for post-op vomiting and nausea,” Dr. Smith said. But do not use it in patients over 65, and it’s contraindicated in glaucoma. Beware of these notable side effects: Blurry vision, constipation, and urinary retention. Dexamethasone and ondansetron can be used as an alternative, she said.
- Use of the blood thinner enoxaparin after discharge may become more common as surgical stays become shorter, Dr. Smith said. She urged surgeons to keep its cost in mind: a 10-day course can be as little as $2 with Medicaid or as much as $140 (a cash price for patients without coverage).
- Make sure to adjust medications based on preoperative or intraoperative doses, she said, to avoid endangering patients by inadvertently doubling up on doses. And watch out for previous use of gabapentin, which is part of enhanced-recovery protocols. Patients who take the drug at home should be put back on their typical dose.
- Also, she warned, “don’t give gabapentin to someone who’s never had it before plus an opioid.” This, she said, can cause delirium.
- Consider starting liquids the night of surgery so patients can begin taking their home medications such as sleep, chronic pain, and psychiatric drugs. Patients will be more stable and satisfied, Dr. Smith said.
- Don’t prescribe hard-to-find medications like oxycodone oral solution or oral ketorolac. These drugs will send patients from pharmacy to pharmacy in search of them, Dr. Smith said.
- Embrace a “Meds to Beds” program if possible. These programs enlist on-site pharmacies to deliver medications to bedside for patients to take home.
- Consider Tylenol as a postoperative painkiller with scheduled doses and be aware that you can prescribe the over-the-counter adult liquid form. However, Dr. Smith cautioned that Tylenol is “not great” on an as-needed basis. Gabapentin and celecoxib (unless contraindicated) are also helpful for postop pain relief, and they’re inexpensive, she said. Three to five days should be enough in most minimally invasive surgeries.
- Don’t overprescribe opioids. “The more we prescribe, the more they will consume,” Dr. Smith said. Check the American College of Surgeons guidelines regarding the ideal number of postsurgery, 5-mg doses of oxycodone to prescribe to opioid-naive patients at discharge. No more than 10 or 15 pills are recommended for several types of general surgery (J Amer Coll Surg. 2018;227:411-8).
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Smith reports no relevant disclosures.
EXPERT ANALYSIS FROM MISS
VA Community Living Centers Health Care Reports Are Now Public
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
Although VA nursing homes, on the whole, have sicker patients than do those in private sector nursing homes, they compare closely in terms of quality of care—and in some cases, VA health care gets higher marks. VA has more higher performing facilities (17% vs 11%) and fewer low-performing facilities (17% vs 20%).
Those figures come from the health care inspection reports and staffing data for its 134 community living centers (CLCs) that the VA is, for the first time, posting publicly. So far, VA has posted 101 health inspection reports; the remainder are scheduled for later this year. The reports cover April 2018 to the present.
The VA reports are based on yearly, unannounced inspections conducted by an outside contracted agency. The survey teams assess a variety of aspects of life at VA nursing homes, such as the care of residents and the processes used to give that care, how the staff and residents interact, and the nursing home environment. The surveyors also review residents’ clinical records and interview residents, family members, caregivers, and staff.
VA nursing homes also had a significantly lower percentage (6%) of 1-star (lowest rated) nursing homes compared with 15,487 private sector nursing homes rated by the Centers for Medicare and Medicaid Services. Both Medicare-certified skilled nursing facilities and VA CLCs must meet federal standards, such as having enough staff to provide adequate care. “There is significant evidence of a relationship between resident outcomes and staffing levels in nursing homes,” the VA says in its description of survey criteria.
Many VA nursing home residents are being treated for conditions rarely seen in private sector nursing homes, the VA says, including veteran-specific conditions, such as posttraumatic stress disorder (12% vs 0.5%) and traumatic brain injury (2% vs 0.8%). In 2018, 42% of 41,076 VA CLC residents had a service-connected disability rating of ≥ 50%. CLCs also provide more hospice care and care for conditions related to homelessness.
However, the VA notes that “quality measures are not the same as quality standards.” According to Medicare Nursing Home Compare, the quality of resident care measures are not benchmarks, thresholds, guidelines, or standards of care—they are a “snapshot at a point in time” of the average condition of residents. For instance, individual CLCs may serve special populations and have a higher rate of certain conditions. A CLC that specializes in complex skin and wound care may admit veterans with severe pressure ulcers that occurred at home or another hospital.
Detailed information on individual quality measures and how VA facilities compare with others in their areas are available at www.accesstocare.va.gov/healthcare/qualityofcare. That site also has an interactive searchable map that can be used to locate CLCs by zip code or distance. The health inspection reports are available at www.va.gov/qualityofcare/apps/aspire/clcsurvey.aspx.
It’s not too early to get ready for HM20
Hospitalist Benji K. Mathews, MD, SFHM, CLHM, will bring a unique commitment to medical education to HM20, which will be held next year on April 16-18 in San Diego.
Dr. Mathews enjoys receiving technological gadgets periodically at his home. Just ask his elementary school–age children: They’ve learned how to use handheld ultrasound devices on each other.
“They’re able to find their siblings’ kidneys and hearts,” said Dr. Mathews, an assistant professor of medicine at the University of Minnesota, Minneapolis, and a hospitalist with HealthPartners in Saint Paul, Minn. “I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more!”
Society of Hospital Medicine members and nonmembers who would like to submit proposals for workshops and didactic sessions at HM20 must move quickly. “The open call for content opened in January 2019, providing enough time to prepare and submit,” Dr. Mathews said. “The HM20 call for content will stay open for 2 weeks after HM19 is wrapped up.”
Dr. Mathews expects HM20 will build upon the successes of this year’s conference and support SHM’s commitment to diversity in voices and programming. More than 4,000 attendees are expected.
“HM20 is a team effort with a diverse group serving on the annual meeting planning committee,” he said. “In conjunction with the submissions we receive from the open call, the Annual Conference Committee really builds on the momentum and feedback from attendees from the previous year’s annual meeting. We will identify popular sessions and topics and also review the data we receive from attendees about how they rated sessions and speakers. The chair and committee members will review all of these metrics and use them to plan HM20.”
Dr. Mathews said several topics will get special emphasis in 2020. “We would like to have more content for nurse practitioners and physician assistants and continued representation from the broad range of hospitalists throughout the nation in academic and community settings,” he said.
“We’re also hoping to provide more credit offerings in addition to those we now offer via the American Academy of Family Physicians and the American Osteopathic Association. Next year, we’re hoping to offer pharmacology credit.”
In addition, he said, “we hope to have focused content on diversity issues such as women in hospital medicine and gender and racial bias. We also plan to provide a continued focus on integration of work and life and topics in technology such as bedside ultrasound and telemedicine.”
Technology will be more than a topic at HM20. SHM plans to embrace it in the conference itself to a greater extent than ever before. “We hope to build an online interactive schedule so that attendees may search tracks by day and credit type and schedule their sessions ahead of time,” Dr. Mathews said. “There will still be a PDF schedule, but we hope to push a more interactive, paperless version. We also hope to have e-posters for the first time at HM20.”
The emphasis on technology is a perfect fit for Dr. Mathews, who’s a pioneer in the use of bedside ultrasound. “I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine,” he said. “The group and mentors provided opportunities to develop further niches in my practice. I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound. Now, I continue to teach clinicians, educators, and learners.”
Dr. Mathews has no relevant disclosures.
Hospitalist Benji K. Mathews, MD, SFHM, CLHM, will bring a unique commitment to medical education to HM20, which will be held next year on April 16-18 in San Diego.
Dr. Mathews enjoys receiving technological gadgets periodically at his home. Just ask his elementary school–age children: They’ve learned how to use handheld ultrasound devices on each other.
“They’re able to find their siblings’ kidneys and hearts,” said Dr. Mathews, an assistant professor of medicine at the University of Minnesota, Minneapolis, and a hospitalist with HealthPartners in Saint Paul, Minn. “I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more!”
Society of Hospital Medicine members and nonmembers who would like to submit proposals for workshops and didactic sessions at HM20 must move quickly. “The open call for content opened in January 2019, providing enough time to prepare and submit,” Dr. Mathews said. “The HM20 call for content will stay open for 2 weeks after HM19 is wrapped up.”
Dr. Mathews expects HM20 will build upon the successes of this year’s conference and support SHM’s commitment to diversity in voices and programming. More than 4,000 attendees are expected.
“HM20 is a team effort with a diverse group serving on the annual meeting planning committee,” he said. “In conjunction with the submissions we receive from the open call, the Annual Conference Committee really builds on the momentum and feedback from attendees from the previous year’s annual meeting. We will identify popular sessions and topics and also review the data we receive from attendees about how they rated sessions and speakers. The chair and committee members will review all of these metrics and use them to plan HM20.”
Dr. Mathews said several topics will get special emphasis in 2020. “We would like to have more content for nurse practitioners and physician assistants and continued representation from the broad range of hospitalists throughout the nation in academic and community settings,” he said.
“We’re also hoping to provide more credit offerings in addition to those we now offer via the American Academy of Family Physicians and the American Osteopathic Association. Next year, we’re hoping to offer pharmacology credit.”
In addition, he said, “we hope to have focused content on diversity issues such as women in hospital medicine and gender and racial bias. We also plan to provide a continued focus on integration of work and life and topics in technology such as bedside ultrasound and telemedicine.”
Technology will be more than a topic at HM20. SHM plans to embrace it in the conference itself to a greater extent than ever before. “We hope to build an online interactive schedule so that attendees may search tracks by day and credit type and schedule their sessions ahead of time,” Dr. Mathews said. “There will still be a PDF schedule, but we hope to push a more interactive, paperless version. We also hope to have e-posters for the first time at HM20.”
The emphasis on technology is a perfect fit for Dr. Mathews, who’s a pioneer in the use of bedside ultrasound. “I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine,” he said. “The group and mentors provided opportunities to develop further niches in my practice. I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound. Now, I continue to teach clinicians, educators, and learners.”
Dr. Mathews has no relevant disclosures.
Hospitalist Benji K. Mathews, MD, SFHM, CLHM, will bring a unique commitment to medical education to HM20, which will be held next year on April 16-18 in San Diego.
Dr. Mathews enjoys receiving technological gadgets periodically at his home. Just ask his elementary school–age children: They’ve learned how to use handheld ultrasound devices on each other.
“They’re able to find their siblings’ kidneys and hearts,” said Dr. Mathews, an assistant professor of medicine at the University of Minnesota, Minneapolis, and a hospitalist with HealthPartners in Saint Paul, Minn. “I often show an image of this to encourage hospitalists that, if children can pick it up, highly educated providers can do the same and more!”
Society of Hospital Medicine members and nonmembers who would like to submit proposals for workshops and didactic sessions at HM20 must move quickly. “The open call for content opened in January 2019, providing enough time to prepare and submit,” Dr. Mathews said. “The HM20 call for content will stay open for 2 weeks after HM19 is wrapped up.”
Dr. Mathews expects HM20 will build upon the successes of this year’s conference and support SHM’s commitment to diversity in voices and programming. More than 4,000 attendees are expected.
“HM20 is a team effort with a diverse group serving on the annual meeting planning committee,” he said. “In conjunction with the submissions we receive from the open call, the Annual Conference Committee really builds on the momentum and feedback from attendees from the previous year’s annual meeting. We will identify popular sessions and topics and also review the data we receive from attendees about how they rated sessions and speakers. The chair and committee members will review all of these metrics and use them to plan HM20.”
Dr. Mathews said several topics will get special emphasis in 2020. “We would like to have more content for nurse practitioners and physician assistants and continued representation from the broad range of hospitalists throughout the nation in academic and community settings,” he said.
“We’re also hoping to provide more credit offerings in addition to those we now offer via the American Academy of Family Physicians and the American Osteopathic Association. Next year, we’re hoping to offer pharmacology credit.”
In addition, he said, “we hope to have focused content on diversity issues such as women in hospital medicine and gender and racial bias. We also plan to provide a continued focus on integration of work and life and topics in technology such as bedside ultrasound and telemedicine.”
Technology will be more than a topic at HM20. SHM plans to embrace it in the conference itself to a greater extent than ever before. “We hope to build an online interactive schedule so that attendees may search tracks by day and credit type and schedule their sessions ahead of time,” Dr. Mathews said. “There will still be a PDF schedule, but we hope to push a more interactive, paperless version. We also hope to have e-posters for the first time at HM20.”
The emphasis on technology is a perfect fit for Dr. Mathews, who’s a pioneer in the use of bedside ultrasound. “I was fortunate to be a part of a great residency program at the University of Minnesota Medical School, which started a hospital medicine pathway that had several nationally recognized hospital medicine leaders as mentors. I was lucky to work with several of them through the HealthPartners organization in Saint Paul, and that developed in me a further desire to practice hospital medicine,” he said. “The group and mentors provided opportunities to develop further niches in my practice. I took an interest in the field of improving diagnosis and combined it with the 21st-century innovative tool of bedside ultrasound. Now, I continue to teach clinicians, educators, and learners.”
Dr. Mathews has no relevant disclosures.
Welcome to Day 3 of HM19
We have reached the final day of another SHM annual conference. What a spectacular ride it has been! On the first day, we heard about the success of the first National Hospitalist Day and how hospital medicine continues to evolve with the health care landscape in the United States and beyond. Throughout the course of the meeting, you have learned about critical updates in our specialty that will support hospitalists’ quest to lead the change. As health care transforms, we are well positioned to innovate in support of our peers and patients.
The importance of high-value care has been a theme of HM19, balanced with the high value of physician well-being. With inspiring keynotes from Marc Harrison, MD, on influencing lives more effectively and more affordably to approaches to fighting burnout from Tait Shanafelt, MD, hospital medicine as a specialty has the power to transform health care for patients and providers alike. I hope you also had the chance to attend the sessions on clinical updates, diagnostic reasoning, practice management, and career development, to name just a few.
The final day of the meeting is no exception when it comes to impactful topics and memorable sessions. Beginning at 7:30 a.m., you’ll find a number of sessions and workshops to round out your conference experience. Our popular “Stump the Professor” series is back, focused on challenging clinical unknowns. In addition, “Medical Jeopardy,” tips on being a successful teaching attending, best practices for promoting diversity in HMGs, the history of hospitals, and updates on LGBTQ health are just a few of the topics you’ll have a chance to immerse yourself in today.
Because of the proximity to the nation’s capital, we also are looking forward to Hill Day today, when hospitalists will travel to Capitol Hill to meet with legislators to discuss issues and policy affecting hospital medicine. This is yet another example of how hospitalists and SHM are partnering to be a voice for clinicians in important health care policy conversations.
As the conference concludes, I thank you for joining us and being a part of the hospital medicine movement. We hope you will continue to engage with SHM throughout the year as you further connect with colleagues via special interest groups, chapters, and committees. If you’re new to SHM, we invite you to discover all the options that your membership offers. We look forward to seeing you next year at Hospital Medicine 2020 (HM20) in San Diego, from April 15-18!
Dr. Frost is the incoming president of the Society of Hospital Medicine and the national medical director of hospital-based services at LifePoint Health in Brentwood, Tennessee.
We have reached the final day of another SHM annual conference. What a spectacular ride it has been! On the first day, we heard about the success of the first National Hospitalist Day and how hospital medicine continues to evolve with the health care landscape in the United States and beyond. Throughout the course of the meeting, you have learned about critical updates in our specialty that will support hospitalists’ quest to lead the change. As health care transforms, we are well positioned to innovate in support of our peers and patients.
The importance of high-value care has been a theme of HM19, balanced with the high value of physician well-being. With inspiring keynotes from Marc Harrison, MD, on influencing lives more effectively and more affordably to approaches to fighting burnout from Tait Shanafelt, MD, hospital medicine as a specialty has the power to transform health care for patients and providers alike. I hope you also had the chance to attend the sessions on clinical updates, diagnostic reasoning, practice management, and career development, to name just a few.
The final day of the meeting is no exception when it comes to impactful topics and memorable sessions. Beginning at 7:30 a.m., you’ll find a number of sessions and workshops to round out your conference experience. Our popular “Stump the Professor” series is back, focused on challenging clinical unknowns. In addition, “Medical Jeopardy,” tips on being a successful teaching attending, best practices for promoting diversity in HMGs, the history of hospitals, and updates on LGBTQ health are just a few of the topics you’ll have a chance to immerse yourself in today.
Because of the proximity to the nation’s capital, we also are looking forward to Hill Day today, when hospitalists will travel to Capitol Hill to meet with legislators to discuss issues and policy affecting hospital medicine. This is yet another example of how hospitalists and SHM are partnering to be a voice for clinicians in important health care policy conversations.
As the conference concludes, I thank you for joining us and being a part of the hospital medicine movement. We hope you will continue to engage with SHM throughout the year as you further connect with colleagues via special interest groups, chapters, and committees. If you’re new to SHM, we invite you to discover all the options that your membership offers. We look forward to seeing you next year at Hospital Medicine 2020 (HM20) in San Diego, from April 15-18!
Dr. Frost is the incoming president of the Society of Hospital Medicine and the national medical director of hospital-based services at LifePoint Health in Brentwood, Tennessee.
We have reached the final day of another SHM annual conference. What a spectacular ride it has been! On the first day, we heard about the success of the first National Hospitalist Day and how hospital medicine continues to evolve with the health care landscape in the United States and beyond. Throughout the course of the meeting, you have learned about critical updates in our specialty that will support hospitalists’ quest to lead the change. As health care transforms, we are well positioned to innovate in support of our peers and patients.
The importance of high-value care has been a theme of HM19, balanced with the high value of physician well-being. With inspiring keynotes from Marc Harrison, MD, on influencing lives more effectively and more affordably to approaches to fighting burnout from Tait Shanafelt, MD, hospital medicine as a specialty has the power to transform health care for patients and providers alike. I hope you also had the chance to attend the sessions on clinical updates, diagnostic reasoning, practice management, and career development, to name just a few.
The final day of the meeting is no exception when it comes to impactful topics and memorable sessions. Beginning at 7:30 a.m., you’ll find a number of sessions and workshops to round out your conference experience. Our popular “Stump the Professor” series is back, focused on challenging clinical unknowns. In addition, “Medical Jeopardy,” tips on being a successful teaching attending, best practices for promoting diversity in HMGs, the history of hospitals, and updates on LGBTQ health are just a few of the topics you’ll have a chance to immerse yourself in today.
Because of the proximity to the nation’s capital, we also are looking forward to Hill Day today, when hospitalists will travel to Capitol Hill to meet with legislators to discuss issues and policy affecting hospital medicine. This is yet another example of how hospitalists and SHM are partnering to be a voice for clinicians in important health care policy conversations.
As the conference concludes, I thank you for joining us and being a part of the hospital medicine movement. We hope you will continue to engage with SHM throughout the year as you further connect with colleagues via special interest groups, chapters, and committees. If you’re new to SHM, we invite you to discover all the options that your membership offers. We look forward to seeing you next year at Hospital Medicine 2020 (HM20) in San Diego, from April 15-18!
Dr. Frost is the incoming president of the Society of Hospital Medicine and the national medical director of hospital-based services at LifePoint Health in Brentwood, Tennessee.
Things We Do For No Reason’ session highlights stress tests, VTE chemoprophylaxis
Stress testing for patients with low-risk chest pain and use of venous thromboembolism chemoprophylaxis in low-risk patients are the latest practices to come under scrutiny and were discussed during a special session at HM19.
Anthony Breu, MD, a hospitalist and director of resident education in the Veterans Affairs Boston Healthcare System, presented “common practices that have low or no value.” He provided arguments against these practices based in both evidence and pathophysiology.
“Things We Do For No Reason: The 2019 Clinical Update for Hospitalists,” presented Tuesday morning, was the latest in a series of such sessions held during SHM’s Annual Conference. Leonard S. Feldman, MD, SFHM, from Johns Hopkins Medicine in Baltimore, presented the first “Things We Do For No Reason” session at the 2012 conference in San Diego, analyzing the 30% target hematocrit for cardiac patients, naso-gastric lavage in gastrointestinal bleeds, fractional excretion of sodium and urea in evaluating acute kidney injuries, and the use of daily chest x-rays in ICUs.
“For patients with low-risk chest pain, stress tests do not add value,” said Dr. Breu. There are a number of ways a hospitalist can determine whether a patient has low-risk chest pain, and the rates of acute MI and mortality at 30 days are “well below 1%” for these patients, he noted.
“Stress tests may lower this rate a bit, but they are unfortunately unable to identify all patients who will experience subsequent events,” he said. “Also, given that pretest probability of coronary artery disease is low in these patients, the false-positive rate approaches 50%.”
Dr. Breu attributed the continued use of stress tests in patients with low-risk chest pain to recent American College of Cardiology and American Heart Association statements on appropriate use of stress testing, which should occur during initial hospitalization or within 72 hours of discharge. In addition, many hospitalists believe a negative stress test result can help them rule out a diagnosis like coronary artery disease.
In a second example of suspect practices, the need for venous thromboembolism (VTE) chemoprophylaxis, such as with subcutaneous enoxaparin, in high-risk patients is based on evidence that shows a reduction in asymptomatic deep vein thrombosis (DVT). However, “the evidence is not as clear” on whether VTE chemoprophylaxis reduces symptomatic DVT or pulmonary embolism (PE), Dr. Breu said.
“When this is coupled with the fact that bleeding is increased with chemoprophylaxis, its use in low-risk patients should be avoided,” Dr. Breu said. “Unfortunately, there is evidence that many of us administer VTE prophylaxis to low-risk patients just as often as we do for high-risk patients.”
Dr. Breu said he suspects many providers “overestimate the benefits” of VTE chemoprophylaxis. “I suspect that if someone is on the fence about whether to administer VTE [chemoprophylaxis], many err on the side of administering, fearing that they will miss an opportunity to prevent a DVT or PE while not realizing the risk of bleeding is real.”
Dr. Breu presented evidence from the literature as support for his argument against the practice of ordering stress tests and administering VTE chemoprophylaxis in low-risk patients. The oldest study goes back to 1967, while the most recent study was published in February 2019, he said.
“My hope is that attendees will be less inclined to order stress tests in patients with low-risk chest pain,” Dr. Breu said in an interview. “I also hope that they will apply scoring systems to better identify patients who are at high risk for VTE and not administer chemoprophylaxis to all others.”
Dr. Breu reported having no relevant financial disclosures.
Stress testing for patients with low-risk chest pain and use of venous thromboembolism chemoprophylaxis in low-risk patients are the latest practices to come under scrutiny and were discussed during a special session at HM19.
Anthony Breu, MD, a hospitalist and director of resident education in the Veterans Affairs Boston Healthcare System, presented “common practices that have low or no value.” He provided arguments against these practices based in both evidence and pathophysiology.
“Things We Do For No Reason: The 2019 Clinical Update for Hospitalists,” presented Tuesday morning, was the latest in a series of such sessions held during SHM’s Annual Conference. Leonard S. Feldman, MD, SFHM, from Johns Hopkins Medicine in Baltimore, presented the first “Things We Do For No Reason” session at the 2012 conference in San Diego, analyzing the 30% target hematocrit for cardiac patients, naso-gastric lavage in gastrointestinal bleeds, fractional excretion of sodium and urea in evaluating acute kidney injuries, and the use of daily chest x-rays in ICUs.
“For patients with low-risk chest pain, stress tests do not add value,” said Dr. Breu. There are a number of ways a hospitalist can determine whether a patient has low-risk chest pain, and the rates of acute MI and mortality at 30 days are “well below 1%” for these patients, he noted.
“Stress tests may lower this rate a bit, but they are unfortunately unable to identify all patients who will experience subsequent events,” he said. “Also, given that pretest probability of coronary artery disease is low in these patients, the false-positive rate approaches 50%.”
Dr. Breu attributed the continued use of stress tests in patients with low-risk chest pain to recent American College of Cardiology and American Heart Association statements on appropriate use of stress testing, which should occur during initial hospitalization or within 72 hours of discharge. In addition, many hospitalists believe a negative stress test result can help them rule out a diagnosis like coronary artery disease.
In a second example of suspect practices, the need for venous thromboembolism (VTE) chemoprophylaxis, such as with subcutaneous enoxaparin, in high-risk patients is based on evidence that shows a reduction in asymptomatic deep vein thrombosis (DVT). However, “the evidence is not as clear” on whether VTE chemoprophylaxis reduces symptomatic DVT or pulmonary embolism (PE), Dr. Breu said.
“When this is coupled with the fact that bleeding is increased with chemoprophylaxis, its use in low-risk patients should be avoided,” Dr. Breu said. “Unfortunately, there is evidence that many of us administer VTE prophylaxis to low-risk patients just as often as we do for high-risk patients.”
Dr. Breu said he suspects many providers “overestimate the benefits” of VTE chemoprophylaxis. “I suspect that if someone is on the fence about whether to administer VTE [chemoprophylaxis], many err on the side of administering, fearing that they will miss an opportunity to prevent a DVT or PE while not realizing the risk of bleeding is real.”
Dr. Breu presented evidence from the literature as support for his argument against the practice of ordering stress tests and administering VTE chemoprophylaxis in low-risk patients. The oldest study goes back to 1967, while the most recent study was published in February 2019, he said.
“My hope is that attendees will be less inclined to order stress tests in patients with low-risk chest pain,” Dr. Breu said in an interview. “I also hope that they will apply scoring systems to better identify patients who are at high risk for VTE and not administer chemoprophylaxis to all others.”
Dr. Breu reported having no relevant financial disclosures.
Stress testing for patients with low-risk chest pain and use of venous thromboembolism chemoprophylaxis in low-risk patients are the latest practices to come under scrutiny and were discussed during a special session at HM19.
Anthony Breu, MD, a hospitalist and director of resident education in the Veterans Affairs Boston Healthcare System, presented “common practices that have low or no value.” He provided arguments against these practices based in both evidence and pathophysiology.
“Things We Do For No Reason: The 2019 Clinical Update for Hospitalists,” presented Tuesday morning, was the latest in a series of such sessions held during SHM’s Annual Conference. Leonard S. Feldman, MD, SFHM, from Johns Hopkins Medicine in Baltimore, presented the first “Things We Do For No Reason” session at the 2012 conference in San Diego, analyzing the 30% target hematocrit for cardiac patients, naso-gastric lavage in gastrointestinal bleeds, fractional excretion of sodium and urea in evaluating acute kidney injuries, and the use of daily chest x-rays in ICUs.
“For patients with low-risk chest pain, stress tests do not add value,” said Dr. Breu. There are a number of ways a hospitalist can determine whether a patient has low-risk chest pain, and the rates of acute MI and mortality at 30 days are “well below 1%” for these patients, he noted.
“Stress tests may lower this rate a bit, but they are unfortunately unable to identify all patients who will experience subsequent events,” he said. “Also, given that pretest probability of coronary artery disease is low in these patients, the false-positive rate approaches 50%.”
Dr. Breu attributed the continued use of stress tests in patients with low-risk chest pain to recent American College of Cardiology and American Heart Association statements on appropriate use of stress testing, which should occur during initial hospitalization or within 72 hours of discharge. In addition, many hospitalists believe a negative stress test result can help them rule out a diagnosis like coronary artery disease.
In a second example of suspect practices, the need for venous thromboembolism (VTE) chemoprophylaxis, such as with subcutaneous enoxaparin, in high-risk patients is based on evidence that shows a reduction in asymptomatic deep vein thrombosis (DVT). However, “the evidence is not as clear” on whether VTE chemoprophylaxis reduces symptomatic DVT or pulmonary embolism (PE), Dr. Breu said.
“When this is coupled with the fact that bleeding is increased with chemoprophylaxis, its use in low-risk patients should be avoided,” Dr. Breu said. “Unfortunately, there is evidence that many of us administer VTE prophylaxis to low-risk patients just as often as we do for high-risk patients.”
Dr. Breu said he suspects many providers “overestimate the benefits” of VTE chemoprophylaxis. “I suspect that if someone is on the fence about whether to administer VTE [chemoprophylaxis], many err on the side of administering, fearing that they will miss an opportunity to prevent a DVT or PE while not realizing the risk of bleeding is real.”
Dr. Breu presented evidence from the literature as support for his argument against the practice of ordering stress tests and administering VTE chemoprophylaxis in low-risk patients. The oldest study goes back to 1967, while the most recent study was published in February 2019, he said.
“My hope is that attendees will be less inclined to order stress tests in patients with low-risk chest pain,” Dr. Breu said in an interview. “I also hope that they will apply scoring systems to better identify patients who are at high risk for VTE and not administer chemoprophylaxis to all others.”
Dr. Breu reported having no relevant financial disclosures.