User login
Too much to lose from office visit recording or filming
A common phrase you see on inspirational posters is “sing like nobody’s listening, dance like nobody’s watching.”
In medicine, it should be “speak as if everyone is recording, behave as if everyone is filming.”
In this day and age, you’d think that would be obvious. Every few hours there’s a viral video of someone getting upset, then losing their temper and saying something most of us would regret. A few years ago it would be a private matter, but today things are rapidly spread over Facebook and Twitter. Even if it’s entirely false, that doesn’t matter. It’s easy for anyone with a smartphone and apps to edit the clip to make it entirely different from what really happened. People go with their first reaction. By the time the facts come out, they’ve moved on and don’t care about the truth.
Occasionally, I get a request to record what I’m saying. In most cases I decline, and never allow myself to be filmed. I do this because anything can be altered, and unless I go to the effort to record it myself, I have no way to prove who’s telling the truth. So it’s easier just to not do it at all.
Unfortunately, this is often taken as “proof” of me trying to hide something. I’m certainly not. Being open and honest with patients is always something I focus on. But the truth of what happened in a 30- to 60-minute visit can be misconstrued in an edited, and possibly altered, sound bite of 5-10 seconds. People who want to do such things have their own motives and aren’t interested in reason or honesty.
Doctors, like everyone else, are susceptible to human emotions and reactions, but a big part of the job is keeping them controlled and hidden when working with patients. It’s the best way to make reasoned decisions and work with someone who’s frightened, angry, or irrational.
If you find yourself losing the battle to stay in control, sometimes it’s good to remember that your words and actions could be being recorded and posted on Facebook in an hour, whether you permitted it or not. Because you don’t want to learn the hard way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A common phrase you see on inspirational posters is “sing like nobody’s listening, dance like nobody’s watching.”
In medicine, it should be “speak as if everyone is recording, behave as if everyone is filming.”
In this day and age, you’d think that would be obvious. Every few hours there’s a viral video of someone getting upset, then losing their temper and saying something most of us would regret. A few years ago it would be a private matter, but today things are rapidly spread over Facebook and Twitter. Even if it’s entirely false, that doesn’t matter. It’s easy for anyone with a smartphone and apps to edit the clip to make it entirely different from what really happened. People go with their first reaction. By the time the facts come out, they’ve moved on and don’t care about the truth.
Occasionally, I get a request to record what I’m saying. In most cases I decline, and never allow myself to be filmed. I do this because anything can be altered, and unless I go to the effort to record it myself, I have no way to prove who’s telling the truth. So it’s easier just to not do it at all.
Unfortunately, this is often taken as “proof” of me trying to hide something. I’m certainly not. Being open and honest with patients is always something I focus on. But the truth of what happened in a 30- to 60-minute visit can be misconstrued in an edited, and possibly altered, sound bite of 5-10 seconds. People who want to do such things have their own motives and aren’t interested in reason or honesty.
Doctors, like everyone else, are susceptible to human emotions and reactions, but a big part of the job is keeping them controlled and hidden when working with patients. It’s the best way to make reasoned decisions and work with someone who’s frightened, angry, or irrational.
If you find yourself losing the battle to stay in control, sometimes it’s good to remember that your words and actions could be being recorded and posted on Facebook in an hour, whether you permitted it or not. Because you don’t want to learn the hard way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A common phrase you see on inspirational posters is “sing like nobody’s listening, dance like nobody’s watching.”
In medicine, it should be “speak as if everyone is recording, behave as if everyone is filming.”
In this day and age, you’d think that would be obvious. Every few hours there’s a viral video of someone getting upset, then losing their temper and saying something most of us would regret. A few years ago it would be a private matter, but today things are rapidly spread over Facebook and Twitter. Even if it’s entirely false, that doesn’t matter. It’s easy for anyone with a smartphone and apps to edit the clip to make it entirely different from what really happened. People go with their first reaction. By the time the facts come out, they’ve moved on and don’t care about the truth.
Occasionally, I get a request to record what I’m saying. In most cases I decline, and never allow myself to be filmed. I do this because anything can be altered, and unless I go to the effort to record it myself, I have no way to prove who’s telling the truth. So it’s easier just to not do it at all.
Unfortunately, this is often taken as “proof” of me trying to hide something. I’m certainly not. Being open and honest with patients is always something I focus on. But the truth of what happened in a 30- to 60-minute visit can be misconstrued in an edited, and possibly altered, sound bite of 5-10 seconds. People who want to do such things have their own motives and aren’t interested in reason or honesty.
Doctors, like everyone else, are susceptible to human emotions and reactions, but a big part of the job is keeping them controlled and hidden when working with patients. It’s the best way to make reasoned decisions and work with someone who’s frightened, angry, or irrational.
If you find yourself losing the battle to stay in control, sometimes it’s good to remember that your words and actions could be being recorded and posted on Facebook in an hour, whether you permitted it or not. Because you don’t want to learn the hard way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hyperglycemia drives leaky gut syndrome, inflammation
MIAMI – Hyperglycemia increases intestinal permeability, which facilitates enteric infections and systemic inflammation, reported Christoph Thaiss, PhD.
The findings upend the old idea that intestinal barrier dysfunction leads to diabetes, Dr. Thaiss said during a plenary session at the annual Gut Microbiota for Health World Summit. Multiple mouse models link hyperglycemia to intestinal barrier dysfunction, and hemoglobin A1C (HbA1c) levels in humans “highly correlate with the influx of microbial molecules into the intestinal epithelium.”
Researchers often struggle to decide if apparent causes are really confounders or even downstream results (reverse causation). In the metabolic syndrome, patients are known to have increased intestinal permeability – so-called leaky gut – and microbes crossing the gastrointestinal epithelium have been found to cause both gut mucosal infections and chronic systemic inflammation. But because these mechanisms were poorly understood, some experts posited that intestinal barrier dysfunction induced pancreatic beta cell inflammation, insulin resistance, and diabetes.
To take a deeper dive, Dr. Thaiss and his associates at the University of Pennsylvania, Philadelphia started with a mouse model of morbid obesity. The mice had multiple systemic sites with microbial pattern recognition ligands, signifying microbial influx from the gut. They also had genetic signatures indicating a marked disruption of junctions between epithelial cells, compared with healthy controls.
The obese mice also were much more susceptible to enteric infections with Citrobacter rodentium (a Salmonella analog), but obesity itself did not drive this risk, Dr. Thaiss explained. In fact, two different murine models of nonobese type 1 diabetes mellitus showed “leaky” intestinal epithelial adherence junctions, heightened susceptibility to C. rodentium infection, and showed systemic pathogen spread. Ribosomal DNA sequencing showed that these hyperglycemic (diabetic) mice had shifts in their gut microbiomes; however, translocating the altered microbiota to normal mice did not make them more susceptible to enteric infections or systemic inflammation.
Based on these findings, the researchers hypothesized that hyperglycemia itself drove susceptibility to enteric infections. They confirmed this by administering insulin to the mice with type 1 diabetes, which restored intestinal epithelial adherence junctions and stopped the systemic spread of pathogens. In vitro, exposing intestinal epithelial cells to glucose-induced barrier dysfunctions that increased over time and with higher glucose concentrations. RNA sequencing demonstrated that hyperglycemia markedly changed expression of genes that encode proteins that regulate intestinal barrier function. Moreover, hyperglycemic mice lacking the bidirectional glucose transporter GLUT2 showed no intestinal barrier dysfunction and were not susceptible to C. rodentium infection and systemic spread.
Finally, the investigators studied more than 30 clinical measures and microbial products in the systemic circulation of 27 healthy human volunteers. “Of all the variables we measured, HbA1c showed the strongest correlation with the influx of microbial molecules,” said Dr. Thaiss. Serum HbA1c correlated highly (P = .008) with levels of toll-like receptor 4, an indicator of systemic pathogens, but not with body mass index (P = .76).
The findings in humans confirm those in mice and indicate that hyperglycemia is a direct cause of intestinal barrier dysfunction and susceptibility to enteric infection, Dr. Thaiss said, adding that the systemic influx of microbial products might explain the wide range of otherwise unrelated inflammatory conditions seen in patients with metabolic syndrome. Future studies of therapies for enteric infection and systemic inflammation might focus on glucose as a modifier of intestinal barrier function.
These findings, reported at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, were also published in Science.
The work was supported by a Boehringer Ingelheim Funds PhD fellowship, the Leona M. and Harry B. Helmsley Charitable Trust, the Adelis Foundation, the Gurwin Family Fund for Scientific Research, the Crown Endowment Fund for Immunological Research, and others. Dr. Thaiss and his coinvestigators reported having no conflicts of interest.
SOURCE: Thaiss CA et al. Science. 2018;359(6382):1376-83.
MIAMI – Hyperglycemia increases intestinal permeability, which facilitates enteric infections and systemic inflammation, reported Christoph Thaiss, PhD.
The findings upend the old idea that intestinal barrier dysfunction leads to diabetes, Dr. Thaiss said during a plenary session at the annual Gut Microbiota for Health World Summit. Multiple mouse models link hyperglycemia to intestinal barrier dysfunction, and hemoglobin A1C (HbA1c) levels in humans “highly correlate with the influx of microbial molecules into the intestinal epithelium.”
Researchers often struggle to decide if apparent causes are really confounders or even downstream results (reverse causation). In the metabolic syndrome, patients are known to have increased intestinal permeability – so-called leaky gut – and microbes crossing the gastrointestinal epithelium have been found to cause both gut mucosal infections and chronic systemic inflammation. But because these mechanisms were poorly understood, some experts posited that intestinal barrier dysfunction induced pancreatic beta cell inflammation, insulin resistance, and diabetes.
To take a deeper dive, Dr. Thaiss and his associates at the University of Pennsylvania, Philadelphia started with a mouse model of morbid obesity. The mice had multiple systemic sites with microbial pattern recognition ligands, signifying microbial influx from the gut. They also had genetic signatures indicating a marked disruption of junctions between epithelial cells, compared with healthy controls.
The obese mice also were much more susceptible to enteric infections with Citrobacter rodentium (a Salmonella analog), but obesity itself did not drive this risk, Dr. Thaiss explained. In fact, two different murine models of nonobese type 1 diabetes mellitus showed “leaky” intestinal epithelial adherence junctions, heightened susceptibility to C. rodentium infection, and showed systemic pathogen spread. Ribosomal DNA sequencing showed that these hyperglycemic (diabetic) mice had shifts in their gut microbiomes; however, translocating the altered microbiota to normal mice did not make them more susceptible to enteric infections or systemic inflammation.
Based on these findings, the researchers hypothesized that hyperglycemia itself drove susceptibility to enteric infections. They confirmed this by administering insulin to the mice with type 1 diabetes, which restored intestinal epithelial adherence junctions and stopped the systemic spread of pathogens. In vitro, exposing intestinal epithelial cells to glucose-induced barrier dysfunctions that increased over time and with higher glucose concentrations. RNA sequencing demonstrated that hyperglycemia markedly changed expression of genes that encode proteins that regulate intestinal barrier function. Moreover, hyperglycemic mice lacking the bidirectional glucose transporter GLUT2 showed no intestinal barrier dysfunction and were not susceptible to C. rodentium infection and systemic spread.
Finally, the investigators studied more than 30 clinical measures and microbial products in the systemic circulation of 27 healthy human volunteers. “Of all the variables we measured, HbA1c showed the strongest correlation with the influx of microbial molecules,” said Dr. Thaiss. Serum HbA1c correlated highly (P = .008) with levels of toll-like receptor 4, an indicator of systemic pathogens, but not with body mass index (P = .76).
The findings in humans confirm those in mice and indicate that hyperglycemia is a direct cause of intestinal barrier dysfunction and susceptibility to enteric infection, Dr. Thaiss said, adding that the systemic influx of microbial products might explain the wide range of otherwise unrelated inflammatory conditions seen in patients with metabolic syndrome. Future studies of therapies for enteric infection and systemic inflammation might focus on glucose as a modifier of intestinal barrier function.
These findings, reported at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, were also published in Science.
The work was supported by a Boehringer Ingelheim Funds PhD fellowship, the Leona M. and Harry B. Helmsley Charitable Trust, the Adelis Foundation, the Gurwin Family Fund for Scientific Research, the Crown Endowment Fund for Immunological Research, and others. Dr. Thaiss and his coinvestigators reported having no conflicts of interest.
SOURCE: Thaiss CA et al. Science. 2018;359(6382):1376-83.
MIAMI – Hyperglycemia increases intestinal permeability, which facilitates enteric infections and systemic inflammation, reported Christoph Thaiss, PhD.
The findings upend the old idea that intestinal barrier dysfunction leads to diabetes, Dr. Thaiss said during a plenary session at the annual Gut Microbiota for Health World Summit. Multiple mouse models link hyperglycemia to intestinal barrier dysfunction, and hemoglobin A1C (HbA1c) levels in humans “highly correlate with the influx of microbial molecules into the intestinal epithelium.”
Researchers often struggle to decide if apparent causes are really confounders or even downstream results (reverse causation). In the metabolic syndrome, patients are known to have increased intestinal permeability – so-called leaky gut – and microbes crossing the gastrointestinal epithelium have been found to cause both gut mucosal infections and chronic systemic inflammation. But because these mechanisms were poorly understood, some experts posited that intestinal barrier dysfunction induced pancreatic beta cell inflammation, insulin resistance, and diabetes.
To take a deeper dive, Dr. Thaiss and his associates at the University of Pennsylvania, Philadelphia started with a mouse model of morbid obesity. The mice had multiple systemic sites with microbial pattern recognition ligands, signifying microbial influx from the gut. They also had genetic signatures indicating a marked disruption of junctions between epithelial cells, compared with healthy controls.
The obese mice also were much more susceptible to enteric infections with Citrobacter rodentium (a Salmonella analog), but obesity itself did not drive this risk, Dr. Thaiss explained. In fact, two different murine models of nonobese type 1 diabetes mellitus showed “leaky” intestinal epithelial adherence junctions, heightened susceptibility to C. rodentium infection, and showed systemic pathogen spread. Ribosomal DNA sequencing showed that these hyperglycemic (diabetic) mice had shifts in their gut microbiomes; however, translocating the altered microbiota to normal mice did not make them more susceptible to enteric infections or systemic inflammation.
Based on these findings, the researchers hypothesized that hyperglycemia itself drove susceptibility to enteric infections. They confirmed this by administering insulin to the mice with type 1 diabetes, which restored intestinal epithelial adherence junctions and stopped the systemic spread of pathogens. In vitro, exposing intestinal epithelial cells to glucose-induced barrier dysfunctions that increased over time and with higher glucose concentrations. RNA sequencing demonstrated that hyperglycemia markedly changed expression of genes that encode proteins that regulate intestinal barrier function. Moreover, hyperglycemic mice lacking the bidirectional glucose transporter GLUT2 showed no intestinal barrier dysfunction and were not susceptible to C. rodentium infection and systemic spread.
Finally, the investigators studied more than 30 clinical measures and microbial products in the systemic circulation of 27 healthy human volunteers. “Of all the variables we measured, HbA1c showed the strongest correlation with the influx of microbial molecules,” said Dr. Thaiss. Serum HbA1c correlated highly (P = .008) with levels of toll-like receptor 4, an indicator of systemic pathogens, but not with body mass index (P = .76).
The findings in humans confirm those in mice and indicate that hyperglycemia is a direct cause of intestinal barrier dysfunction and susceptibility to enteric infection, Dr. Thaiss said, adding that the systemic influx of microbial products might explain the wide range of otherwise unrelated inflammatory conditions seen in patients with metabolic syndrome. Future studies of therapies for enteric infection and systemic inflammation might focus on glucose as a modifier of intestinal barrier function.
These findings, reported at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, were also published in Science.
The work was supported by a Boehringer Ingelheim Funds PhD fellowship, the Leona M. and Harry B. Helmsley Charitable Trust, the Adelis Foundation, the Gurwin Family Fund for Scientific Research, the Crown Endowment Fund for Immunological Research, and others. Dr. Thaiss and his coinvestigators reported having no conflicts of interest.
SOURCE: Thaiss CA et al. Science. 2018;359(6382):1376-83.
REPORTING FROM GMFH 2019
The 2018 AHA/ACC cholesterol guidelines: What’s changed?
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
References
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of The American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. doi: 10.1016/j.jacc.2018.11.003. [Epub ahead of print].
2. Alenghat FJ, Davis AM. Management of blood cholesterol. JAMA. 2019;321:800-801.
3. Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069-1080. [ ]
4. US Preventive Services Task Force. Cardiovascular disease: risk assessment with nontraditional risk factors. July 2018. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cardiovascular-disease-screening-using-nontraditional-risk-assessment. Accessed March 26, 2019.
5. American Academy of Family Practitioners. Clinical Practice Guideline: Cholesterol. February 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/cholesterol.html. Accessed March 26, 2019.
Prophylaxis maintains high FIX trough levels in hemophilia B
The recombinant factor IX (FIX) product rIX‐FP (albutrepenonacog alfa) can maintain high steady‐state FIX trough levels in both adult and pediatric patients with severe hemophilia B, according to a pharmacokinetic study.
“rIX‐FP is a fusion protein genetically linking recombinant human coagulation FIX with recombinant human albumin and has an extended half‐life compared with standard products, allowing a prolonged dosing interval,” wrote Joan C. Gill, MD, of the Medical College of Wisconsin, Milwaukee, along with her colleagues. The findings of the study were published in Haemophilia.
Safety and efficacy of rIX-FP was previously demonstrated for both adults/adolescents and children in two phase 3 trials. In the current analysis, the researchers evaluated mean steady state and observed trough FIX:C levels during prophylaxis with rIX-FP in the two previous trials and assessed the impact on hemophilia B patients.
The researchers studied 90 patients with severe hemophilia B, which included both adult/adolescent (n = 63) and pediatric (n = 27) patients. The adult/adolescent group was administered 35‐50 IU/kg or 50‐75 IU/kg of rIX‐FP every 7 and 10 or 14 days, respectively, while pediatric participants (younger than 12 years old) were given 35‐50 IU/kg of rIX‐FP every 7 days. Only the 7‐ and 14‐day dosing intervals were included in the analysis.
After analysis, the researchers reported that steady‐state FIX trough levels were higher than 5% across all doses in 96.2% and 97.9% of adult/adolescent and pediatric patients, respectively.
Among adults/adolescents, including all dose levels, the mean FIX:C trough levels were 22.26% for 7-day regimens and 12.48% for 14-day regimens. Among children in the study, the mean steady-state FIX:C trough level was 12.80%.
The team reported that these results, which are consistent with low median annualized bleeding rates observed, indicate that sustaining high FIX trough levels may successfully change a case of severe disease into a mild bleeding phenotype.
The authors acknowledged a key limitation of the study was the unknown effects of rIX‐FP remaining within the extravascular space. Further biodistribution studies are required to fully understand these effects.
“Patients may find that an extended dosing regimen would still provide protection from bleeds but be easier to maintain,” they concluded.
The study was funded by CSL Behring. The authors reported financial disclosures related to Bayer, CSL Behring, Kedrion Biopharma, Novo Nordisk, Pfizer, and others.
SOURCE: Gill JC et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13735.
The recombinant factor IX (FIX) product rIX‐FP (albutrepenonacog alfa) can maintain high steady‐state FIX trough levels in both adult and pediatric patients with severe hemophilia B, according to a pharmacokinetic study.
“rIX‐FP is a fusion protein genetically linking recombinant human coagulation FIX with recombinant human albumin and has an extended half‐life compared with standard products, allowing a prolonged dosing interval,” wrote Joan C. Gill, MD, of the Medical College of Wisconsin, Milwaukee, along with her colleagues. The findings of the study were published in Haemophilia.
Safety and efficacy of rIX-FP was previously demonstrated for both adults/adolescents and children in two phase 3 trials. In the current analysis, the researchers evaluated mean steady state and observed trough FIX:C levels during prophylaxis with rIX-FP in the two previous trials and assessed the impact on hemophilia B patients.
The researchers studied 90 patients with severe hemophilia B, which included both adult/adolescent (n = 63) and pediatric (n = 27) patients. The adult/adolescent group was administered 35‐50 IU/kg or 50‐75 IU/kg of rIX‐FP every 7 and 10 or 14 days, respectively, while pediatric participants (younger than 12 years old) were given 35‐50 IU/kg of rIX‐FP every 7 days. Only the 7‐ and 14‐day dosing intervals were included in the analysis.
After analysis, the researchers reported that steady‐state FIX trough levels were higher than 5% across all doses in 96.2% and 97.9% of adult/adolescent and pediatric patients, respectively.
Among adults/adolescents, including all dose levels, the mean FIX:C trough levels were 22.26% for 7-day regimens and 12.48% for 14-day regimens. Among children in the study, the mean steady-state FIX:C trough level was 12.80%.
The team reported that these results, which are consistent with low median annualized bleeding rates observed, indicate that sustaining high FIX trough levels may successfully change a case of severe disease into a mild bleeding phenotype.
The authors acknowledged a key limitation of the study was the unknown effects of rIX‐FP remaining within the extravascular space. Further biodistribution studies are required to fully understand these effects.
“Patients may find that an extended dosing regimen would still provide protection from bleeds but be easier to maintain,” they concluded.
The study was funded by CSL Behring. The authors reported financial disclosures related to Bayer, CSL Behring, Kedrion Biopharma, Novo Nordisk, Pfizer, and others.
SOURCE: Gill JC et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13735.
The recombinant factor IX (FIX) product rIX‐FP (albutrepenonacog alfa) can maintain high steady‐state FIX trough levels in both adult and pediatric patients with severe hemophilia B, according to a pharmacokinetic study.
“rIX‐FP is a fusion protein genetically linking recombinant human coagulation FIX with recombinant human albumin and has an extended half‐life compared with standard products, allowing a prolonged dosing interval,” wrote Joan C. Gill, MD, of the Medical College of Wisconsin, Milwaukee, along with her colleagues. The findings of the study were published in Haemophilia.
Safety and efficacy of rIX-FP was previously demonstrated for both adults/adolescents and children in two phase 3 trials. In the current analysis, the researchers evaluated mean steady state and observed trough FIX:C levels during prophylaxis with rIX-FP in the two previous trials and assessed the impact on hemophilia B patients.
The researchers studied 90 patients with severe hemophilia B, which included both adult/adolescent (n = 63) and pediatric (n = 27) patients. The adult/adolescent group was administered 35‐50 IU/kg or 50‐75 IU/kg of rIX‐FP every 7 and 10 or 14 days, respectively, while pediatric participants (younger than 12 years old) were given 35‐50 IU/kg of rIX‐FP every 7 days. Only the 7‐ and 14‐day dosing intervals were included in the analysis.
After analysis, the researchers reported that steady‐state FIX trough levels were higher than 5% across all doses in 96.2% and 97.9% of adult/adolescent and pediatric patients, respectively.
Among adults/adolescents, including all dose levels, the mean FIX:C trough levels were 22.26% for 7-day regimens and 12.48% for 14-day regimens. Among children in the study, the mean steady-state FIX:C trough level was 12.80%.
The team reported that these results, which are consistent with low median annualized bleeding rates observed, indicate that sustaining high FIX trough levels may successfully change a case of severe disease into a mild bleeding phenotype.
The authors acknowledged a key limitation of the study was the unknown effects of rIX‐FP remaining within the extravascular space. Further biodistribution studies are required to fully understand these effects.
“Patients may find that an extended dosing regimen would still provide protection from bleeds but be easier to maintain,” they concluded.
The study was funded by CSL Behring. The authors reported financial disclosures related to Bayer, CSL Behring, Kedrion Biopharma, Novo Nordisk, Pfizer, and others.
SOURCE: Gill JC et al. Haemophilia. 2019 Mar 13. doi: 10.1111/hae.13735.
FROM HAEMOPHILIA
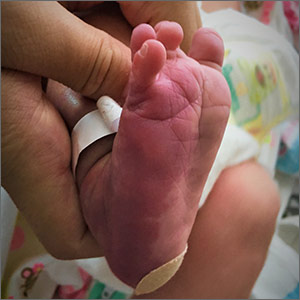
Violaceous patches on baby’s foot/leg
The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
Study eyes biomarkers of regorafenib response in hepatocellular carcinoma
.
“In the absence of established or predefined biomarkers for regorafenib, we performed a broad exploratory biomarker analyses at the DNA, RNA, and protein level that represents a much more comprehensive approach than previous studies of regorafenib or sorafenib,” wrote Michael Teufel, PhD, of Bayer Healthcare Pharmaceuticals in Whippany, N.J., and his associates. The preplanned, retrospective analysis of data from the phase 3 RESOURCE trial was reported in Gastroenterology.
The randomized trial included 567 patients whose hepatocellular carcinoma had progressed on sorafenib. Regorafenib significantly outperformed placebo with regard to overall survival (OS). Dr. Teufel and his associates performed next-generation sequencing on 17 archived tumor samples containing sufficient tissue (all from regorafenib recipients). They also performed immune profiling on 46 tumor samples (32 from regorafenib recipients and 14 from placebo recipients), protein analysis on 499 plasma samples (332 from regorafenib recipients and 167 from placebo recipients), and microRNA analysis on 343 plasma samples (234 regorafenib recipients and 109 placebo recipients).
Among 266 proteins tested, decreased levels of 5 proteins correlated with significantly longer OS on regorafenib therapy. These proteins are involved in inflammation or hepatocellular carcinogenesis, the researchers noted. Importantly, none were associated with survival independent of treatment. These five proteins included angiopoietin 1 (hazard ratio for OS, 0.53; 95% confidence interval, 0.38-0.73), cystatin B (hazard ratio, 0.47; 95% CI, 0.34-0.64); the latency-associated peptide of transforming growth factor beta (HR, 0.46; 95% CI, 0.33-0.64), oxidized low-density lipoprotein receptor 1 (HR, 0.54; 95% CI, 0.41-0.72), and C-C motif chemokine ligand 3 (HR, 0.54; 95% CI, 0.39-0.74).
Additionally, baseline concentrations of 47 of the 266 proteins correlated with a time to progression (TPP) benefit on regorafenib therapy (adjusted P less than or equal to .05 for each). The 47 proteins included all 5 that predicted an OS benefit. All but two proteins (calbindin and gelsolin) showed the same directional effect as for OS (that is, low expression predicted response).
Nine plasma microRNA’s levels correlated with improved OS on regorafenib (adjusted P less than or equal to .05): MIR30A, MIR122, MIR125B, MIR200A, MIR374B, MIR15B, MIR107, MIR320, and MIR645. Notably, expression was linked to longer OS specifically among patients with the Hoshida S3 subtype of hepatocellular carcinoma. Next-generation sequencing of tumor samples also identified 49 variants in 27 oncogenes or tumor-suppressor genes. Mutations in CTNNB1 were found in 3 of 10 patients who progressed on regorafenib, and VEGFA amplification was found in 1 of 7 regorafenib responders.
“Thus far, rational biomarker selection has been unsuccessful in identifying predictive markers for regorafenib in colorectal cancer and gastrointestinal stromal tumors,” the researchers commented. “The broader approach used in this study is not only biologically warranted considering the heterogeneity of hepatocellular carcinoma tumors, but is also needed due to the multiple targets and pathways affected by MKIs such as regorafenib. Levels of these circulating biomarkers and genetic features of tumors might be used to identify patients with hepatocellular carcinoma most likely to respond to regorafenib.”
Bayer funded the study, provided the study drug, and was involved in all aspects of the study. Dr. Teufel and three coinvestigators are Bayer employees. Dr. Teufel and two coinvestigators own stock in Bayer. Three other coinvestigators disclosed ties to Bayer and other pharmaceutical companies.
SOURCE: Teufel M et al. Gastroenterology. 2019 Jan 30. doi: 10.1053/j.gastro.2019.01.261.
.
“In the absence of established or predefined biomarkers for regorafenib, we performed a broad exploratory biomarker analyses at the DNA, RNA, and protein level that represents a much more comprehensive approach than previous studies of regorafenib or sorafenib,” wrote Michael Teufel, PhD, of Bayer Healthcare Pharmaceuticals in Whippany, N.J., and his associates. The preplanned, retrospective analysis of data from the phase 3 RESOURCE trial was reported in Gastroenterology.
The randomized trial included 567 patients whose hepatocellular carcinoma had progressed on sorafenib. Regorafenib significantly outperformed placebo with regard to overall survival (OS). Dr. Teufel and his associates performed next-generation sequencing on 17 archived tumor samples containing sufficient tissue (all from regorafenib recipients). They also performed immune profiling on 46 tumor samples (32 from regorafenib recipients and 14 from placebo recipients), protein analysis on 499 plasma samples (332 from regorafenib recipients and 167 from placebo recipients), and microRNA analysis on 343 plasma samples (234 regorafenib recipients and 109 placebo recipients).
Among 266 proteins tested, decreased levels of 5 proteins correlated with significantly longer OS on regorafenib therapy. These proteins are involved in inflammation or hepatocellular carcinogenesis, the researchers noted. Importantly, none were associated with survival independent of treatment. These five proteins included angiopoietin 1 (hazard ratio for OS, 0.53; 95% confidence interval, 0.38-0.73), cystatin B (hazard ratio, 0.47; 95% CI, 0.34-0.64); the latency-associated peptide of transforming growth factor beta (HR, 0.46; 95% CI, 0.33-0.64), oxidized low-density lipoprotein receptor 1 (HR, 0.54; 95% CI, 0.41-0.72), and C-C motif chemokine ligand 3 (HR, 0.54; 95% CI, 0.39-0.74).
Additionally, baseline concentrations of 47 of the 266 proteins correlated with a time to progression (TPP) benefit on regorafenib therapy (adjusted P less than or equal to .05 for each). The 47 proteins included all 5 that predicted an OS benefit. All but two proteins (calbindin and gelsolin) showed the same directional effect as for OS (that is, low expression predicted response).
Nine plasma microRNA’s levels correlated with improved OS on regorafenib (adjusted P less than or equal to .05): MIR30A, MIR122, MIR125B, MIR200A, MIR374B, MIR15B, MIR107, MIR320, and MIR645. Notably, expression was linked to longer OS specifically among patients with the Hoshida S3 subtype of hepatocellular carcinoma. Next-generation sequencing of tumor samples also identified 49 variants in 27 oncogenes or tumor-suppressor genes. Mutations in CTNNB1 were found in 3 of 10 patients who progressed on regorafenib, and VEGFA amplification was found in 1 of 7 regorafenib responders.
“Thus far, rational biomarker selection has been unsuccessful in identifying predictive markers for regorafenib in colorectal cancer and gastrointestinal stromal tumors,” the researchers commented. “The broader approach used in this study is not only biologically warranted considering the heterogeneity of hepatocellular carcinoma tumors, but is also needed due to the multiple targets and pathways affected by MKIs such as regorafenib. Levels of these circulating biomarkers and genetic features of tumors might be used to identify patients with hepatocellular carcinoma most likely to respond to regorafenib.”
Bayer funded the study, provided the study drug, and was involved in all aspects of the study. Dr. Teufel and three coinvestigators are Bayer employees. Dr. Teufel and two coinvestigators own stock in Bayer. Three other coinvestigators disclosed ties to Bayer and other pharmaceutical companies.
SOURCE: Teufel M et al. Gastroenterology. 2019 Jan 30. doi: 10.1053/j.gastro.2019.01.261.
.
“In the absence of established or predefined biomarkers for regorafenib, we performed a broad exploratory biomarker analyses at the DNA, RNA, and protein level that represents a much more comprehensive approach than previous studies of regorafenib or sorafenib,” wrote Michael Teufel, PhD, of Bayer Healthcare Pharmaceuticals in Whippany, N.J., and his associates. The preplanned, retrospective analysis of data from the phase 3 RESOURCE trial was reported in Gastroenterology.
The randomized trial included 567 patients whose hepatocellular carcinoma had progressed on sorafenib. Regorafenib significantly outperformed placebo with regard to overall survival (OS). Dr. Teufel and his associates performed next-generation sequencing on 17 archived tumor samples containing sufficient tissue (all from regorafenib recipients). They also performed immune profiling on 46 tumor samples (32 from regorafenib recipients and 14 from placebo recipients), protein analysis on 499 plasma samples (332 from regorafenib recipients and 167 from placebo recipients), and microRNA analysis on 343 plasma samples (234 regorafenib recipients and 109 placebo recipients).
Among 266 proteins tested, decreased levels of 5 proteins correlated with significantly longer OS on regorafenib therapy. These proteins are involved in inflammation or hepatocellular carcinogenesis, the researchers noted. Importantly, none were associated with survival independent of treatment. These five proteins included angiopoietin 1 (hazard ratio for OS, 0.53; 95% confidence interval, 0.38-0.73), cystatin B (hazard ratio, 0.47; 95% CI, 0.34-0.64); the latency-associated peptide of transforming growth factor beta (HR, 0.46; 95% CI, 0.33-0.64), oxidized low-density lipoprotein receptor 1 (HR, 0.54; 95% CI, 0.41-0.72), and C-C motif chemokine ligand 3 (HR, 0.54; 95% CI, 0.39-0.74).
Additionally, baseline concentrations of 47 of the 266 proteins correlated with a time to progression (TPP) benefit on regorafenib therapy (adjusted P less than or equal to .05 for each). The 47 proteins included all 5 that predicted an OS benefit. All but two proteins (calbindin and gelsolin) showed the same directional effect as for OS (that is, low expression predicted response).
Nine plasma microRNA’s levels correlated with improved OS on regorafenib (adjusted P less than or equal to .05): MIR30A, MIR122, MIR125B, MIR200A, MIR374B, MIR15B, MIR107, MIR320, and MIR645. Notably, expression was linked to longer OS specifically among patients with the Hoshida S3 subtype of hepatocellular carcinoma. Next-generation sequencing of tumor samples also identified 49 variants in 27 oncogenes or tumor-suppressor genes. Mutations in CTNNB1 were found in 3 of 10 patients who progressed on regorafenib, and VEGFA amplification was found in 1 of 7 regorafenib responders.
“Thus far, rational biomarker selection has been unsuccessful in identifying predictive markers for regorafenib in colorectal cancer and gastrointestinal stromal tumors,” the researchers commented. “The broader approach used in this study is not only biologically warranted considering the heterogeneity of hepatocellular carcinoma tumors, but is also needed due to the multiple targets and pathways affected by MKIs such as regorafenib. Levels of these circulating biomarkers and genetic features of tumors might be used to identify patients with hepatocellular carcinoma most likely to respond to regorafenib.”
Bayer funded the study, provided the study drug, and was involved in all aspects of the study. Dr. Teufel and three coinvestigators are Bayer employees. Dr. Teufel and two coinvestigators own stock in Bayer. Three other coinvestigators disclosed ties to Bayer and other pharmaceutical companies.
SOURCE: Teufel M et al. Gastroenterology. 2019 Jan 30. doi: 10.1053/j.gastro.2019.01.261.
FROM GASTROENTEROLOGY
Hemophilia questionnaire proves valid around the globe
The PROBE (Patient Reported Outcomes Burdens and Experience) questionnaire was found to be a valid instrument to evaluate health status in patients with hemophilia in an cross‐cultural context, according to an international study.
“This study [aimed] to investigate the variation in the PROBE questionnaire–driven measurements across four broad geographical regions,” wrote Chatree Chai‐Adisaksopha, MD, of McMaster University in Hamilton, Ontario, and his colleagues. The results of the study were published in Haemophilia.
The researchers analyzed data from 862 study participants who resided in various geographical regions, including North America, South America, Europe, and the Western Pacific. The majority of participants were male and had greater than 12 years of education.
The team assessed common characteristics of participants through collection of demographic data, including age, gender, years of education, among others. With respect to hemophilia, they evaluated patient‐reported outcome measures across these four regions.
“Outcome measurement in haemophilia has been developed to capture clinically relevant outcomes like bleeding rates, pharmacokinetics, joint pain, joint function scores, radiologic changes and mortality rates,” the researchers wrote.
After analysis, Dr. Chai‐Adisaksopha and his colleagues found that the PROBE questionnaire showed low variability when used on a multinational, cross‐cultural level. In particular, limited variation was found with respect to years of education and geographical region in all subcategories, with the exception of mobility score. In contrast, diagnosis and age had the highest levels of variation.
Region contributed 0.26% of variance in the PROBE score. Similarly, education level contributed 0.34% of the variance. However, age contributed 3.42% and diagnosis contributed 22.42% of the variance in the PROBE score.
The authors acknowledged a key limitation of the study was the inability to include all geographical regions, largely due to an inadequate number of participants in certain areas, such as Africa.
“Despite being used in disparate groups of patients, in 14 countries, in four regions and in 20 languages, the tool produced comparable results, suggesting it can be reliably used across these groups,” the researchers wrote.
The study was funded by Baxalta, Bayer, Bioverativ, CSL Behring, Novo Nordisk, Roche, and Sobi, with additional support from the U.S. National Hemophilia Foundation. Co-author Mark W. Skinner is the principal investigator and no other investigators reported relevant conflicts of interest.
SOURCE: Chai-Adisaksopha C et al. Haemophilia. 2019 Mar 12. doi: 10.1111/hae.13703 .
The PROBE (Patient Reported Outcomes Burdens and Experience) questionnaire was found to be a valid instrument to evaluate health status in patients with hemophilia in an cross‐cultural context, according to an international study.
“This study [aimed] to investigate the variation in the PROBE questionnaire–driven measurements across four broad geographical regions,” wrote Chatree Chai‐Adisaksopha, MD, of McMaster University in Hamilton, Ontario, and his colleagues. The results of the study were published in Haemophilia.
The researchers analyzed data from 862 study participants who resided in various geographical regions, including North America, South America, Europe, and the Western Pacific. The majority of participants were male and had greater than 12 years of education.
The team assessed common characteristics of participants through collection of demographic data, including age, gender, years of education, among others. With respect to hemophilia, they evaluated patient‐reported outcome measures across these four regions.
“Outcome measurement in haemophilia has been developed to capture clinically relevant outcomes like bleeding rates, pharmacokinetics, joint pain, joint function scores, radiologic changes and mortality rates,” the researchers wrote.
After analysis, Dr. Chai‐Adisaksopha and his colleagues found that the PROBE questionnaire showed low variability when used on a multinational, cross‐cultural level. In particular, limited variation was found with respect to years of education and geographical region in all subcategories, with the exception of mobility score. In contrast, diagnosis and age had the highest levels of variation.
Region contributed 0.26% of variance in the PROBE score. Similarly, education level contributed 0.34% of the variance. However, age contributed 3.42% and diagnosis contributed 22.42% of the variance in the PROBE score.
The authors acknowledged a key limitation of the study was the inability to include all geographical regions, largely due to an inadequate number of participants in certain areas, such as Africa.
“Despite being used in disparate groups of patients, in 14 countries, in four regions and in 20 languages, the tool produced comparable results, suggesting it can be reliably used across these groups,” the researchers wrote.
The study was funded by Baxalta, Bayer, Bioverativ, CSL Behring, Novo Nordisk, Roche, and Sobi, with additional support from the U.S. National Hemophilia Foundation. Co-author Mark W. Skinner is the principal investigator and no other investigators reported relevant conflicts of interest.
SOURCE: Chai-Adisaksopha C et al. Haemophilia. 2019 Mar 12. doi: 10.1111/hae.13703 .
The PROBE (Patient Reported Outcomes Burdens and Experience) questionnaire was found to be a valid instrument to evaluate health status in patients with hemophilia in an cross‐cultural context, according to an international study.
“This study [aimed] to investigate the variation in the PROBE questionnaire–driven measurements across four broad geographical regions,” wrote Chatree Chai‐Adisaksopha, MD, of McMaster University in Hamilton, Ontario, and his colleagues. The results of the study were published in Haemophilia.
The researchers analyzed data from 862 study participants who resided in various geographical regions, including North America, South America, Europe, and the Western Pacific. The majority of participants were male and had greater than 12 years of education.
The team assessed common characteristics of participants through collection of demographic data, including age, gender, years of education, among others. With respect to hemophilia, they evaluated patient‐reported outcome measures across these four regions.
“Outcome measurement in haemophilia has been developed to capture clinically relevant outcomes like bleeding rates, pharmacokinetics, joint pain, joint function scores, radiologic changes and mortality rates,” the researchers wrote.
After analysis, Dr. Chai‐Adisaksopha and his colleagues found that the PROBE questionnaire showed low variability when used on a multinational, cross‐cultural level. In particular, limited variation was found with respect to years of education and geographical region in all subcategories, with the exception of mobility score. In contrast, diagnosis and age had the highest levels of variation.
Region contributed 0.26% of variance in the PROBE score. Similarly, education level contributed 0.34% of the variance. However, age contributed 3.42% and diagnosis contributed 22.42% of the variance in the PROBE score.
The authors acknowledged a key limitation of the study was the inability to include all geographical regions, largely due to an inadequate number of participants in certain areas, such as Africa.
“Despite being used in disparate groups of patients, in 14 countries, in four regions and in 20 languages, the tool produced comparable results, suggesting it can be reliably used across these groups,” the researchers wrote.
The study was funded by Baxalta, Bayer, Bioverativ, CSL Behring, Novo Nordisk, Roche, and Sobi, with additional support from the U.S. National Hemophilia Foundation. Co-author Mark W. Skinner is the principal investigator and no other investigators reported relevant conflicts of interest.
SOURCE: Chai-Adisaksopha C et al. Haemophilia. 2019 Mar 12. doi: 10.1111/hae.13703 .
FROM HAEMOPHILIA
Anastrozole/fulvestrant prolongs OS in metastatic ER+ breast cancer
For women with metastatic hormone receptor–positive breast cancer, the addition of the selective estrogen receptor modifier fulvestrant (Faslodex) to the aromatase inhibitor anastrozole (Arimidex and generics) resulted in a small but significant improvement in overall survival, according to final results from a randomized phase 3 trial.
Among 694 patients randomized for whom data were available, the hazard ratio for death with the combination when compared with anastrozole alone was 0.82 (P = .03), reported Rita S. Mehta, MD, from the University of California (Irvine) Medical Center and her colleagues.
The benefit of the combination was highest for patients without prior exposure to adjuvant endocrine therapy.
“Furthermore, sequential therapy with anastrozole and fulvestrant (45% of patients crossed over to fulvestrant alone) did not negate the significance of the long-term overall survival benefit with the combination therapy as compared with anastrozole,” the investigators wrote in The New England Journal of Medicine.
The current report is the final survival analysis of the trial. The primary results were reported in 2012 (N Engl J Med. 2012; 367:435-44). A total of 707 postmenopausal women with previously untreated metastatic disease were randomly assigned to receive either 1 mg of anastrozole orally every day with crossover to fulvestrant alone strongly encouraged if the disease progressed or to anastrozole and fulvestrant in combination. Randomization was stratified according to prior adjuvant tamoxifen use. A total of 694 women had data available for analysis.
The primary analysis, conducted at a median follow-up of 35 months, showed a median progression-free survival (PFS) with anastrozole alone of 13.5 months, compared with 15.0 months for anastrozole/fulvestrant (HR for progression or death 0.80; P = .007). Respective median overall survival was 41.3 months and 47.7 months (HR for death 0.81; P = .05).
The current, final analysis, conducted at a median follow-up of 7 years in patients who did not have disease progression, showed 261 deaths among 345 women (76%) in the anastrozole-only group, compared with 247 deaths among 349 women (71%) in the combination group (HR for death 0.82; P = .03).
Overall survival was longer for those women who had not previously received tamoxifen who were treated with the combination, at a median of 52.2 months versus 40.3 months for women not previously treated with tamoxifen who received anastrozole alone (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92). In contrast, there was no significant difference in OS between the two treatment groups in women who had previously received tamoxifen.
Approximately 45% of patients initially randomized to anastrozole alone were crossed over to fulvestrant.
The incidence of long-term toxic effects and treatment-related deaths was similar between the groups. Previously reported treatment-related deaths with the combination included pulmonary emboli in two patients and a cerebrovascular ischemic event in one patients.
At the time of data cutoff for the final report, 15% of patients in the combination-therapy group and 13% in the anastrozole-only group had experienced grade 3 toxicities.
The study was supported by National Cancer Institute grants and by AstraZeneca. Dr. Mehta reported institutional and personal grants from AstraZeneca and others. Multiple coauthors reported similar relationships.
SOURCE: Mehta RS et al. N Engl J Med. 2019;380:1226-34.
For women with metastatic hormone receptor–positive breast cancer, the addition of the selective estrogen receptor modifier fulvestrant (Faslodex) to the aromatase inhibitor anastrozole (Arimidex and generics) resulted in a small but significant improvement in overall survival, according to final results from a randomized phase 3 trial.
Among 694 patients randomized for whom data were available, the hazard ratio for death with the combination when compared with anastrozole alone was 0.82 (P = .03), reported Rita S. Mehta, MD, from the University of California (Irvine) Medical Center and her colleagues.
The benefit of the combination was highest for patients without prior exposure to adjuvant endocrine therapy.
“Furthermore, sequential therapy with anastrozole and fulvestrant (45% of patients crossed over to fulvestrant alone) did not negate the significance of the long-term overall survival benefit with the combination therapy as compared with anastrozole,” the investigators wrote in The New England Journal of Medicine.
The current report is the final survival analysis of the trial. The primary results were reported in 2012 (N Engl J Med. 2012; 367:435-44). A total of 707 postmenopausal women with previously untreated metastatic disease were randomly assigned to receive either 1 mg of anastrozole orally every day with crossover to fulvestrant alone strongly encouraged if the disease progressed or to anastrozole and fulvestrant in combination. Randomization was stratified according to prior adjuvant tamoxifen use. A total of 694 women had data available for analysis.
The primary analysis, conducted at a median follow-up of 35 months, showed a median progression-free survival (PFS) with anastrozole alone of 13.5 months, compared with 15.0 months for anastrozole/fulvestrant (HR for progression or death 0.80; P = .007). Respective median overall survival was 41.3 months and 47.7 months (HR for death 0.81; P = .05).
The current, final analysis, conducted at a median follow-up of 7 years in patients who did not have disease progression, showed 261 deaths among 345 women (76%) in the anastrozole-only group, compared with 247 deaths among 349 women (71%) in the combination group (HR for death 0.82; P = .03).
Overall survival was longer for those women who had not previously received tamoxifen who were treated with the combination, at a median of 52.2 months versus 40.3 months for women not previously treated with tamoxifen who received anastrozole alone (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92). In contrast, there was no significant difference in OS between the two treatment groups in women who had previously received tamoxifen.
Approximately 45% of patients initially randomized to anastrozole alone were crossed over to fulvestrant.
The incidence of long-term toxic effects and treatment-related deaths was similar between the groups. Previously reported treatment-related deaths with the combination included pulmonary emboli in two patients and a cerebrovascular ischemic event in one patients.
At the time of data cutoff for the final report, 15% of patients in the combination-therapy group and 13% in the anastrozole-only group had experienced grade 3 toxicities.
The study was supported by National Cancer Institute grants and by AstraZeneca. Dr. Mehta reported institutional and personal grants from AstraZeneca and others. Multiple coauthors reported similar relationships.
SOURCE: Mehta RS et al. N Engl J Med. 2019;380:1226-34.
For women with metastatic hormone receptor–positive breast cancer, the addition of the selective estrogen receptor modifier fulvestrant (Faslodex) to the aromatase inhibitor anastrozole (Arimidex and generics) resulted in a small but significant improvement in overall survival, according to final results from a randomized phase 3 trial.
Among 694 patients randomized for whom data were available, the hazard ratio for death with the combination when compared with anastrozole alone was 0.82 (P = .03), reported Rita S. Mehta, MD, from the University of California (Irvine) Medical Center and her colleagues.
The benefit of the combination was highest for patients without prior exposure to adjuvant endocrine therapy.
“Furthermore, sequential therapy with anastrozole and fulvestrant (45% of patients crossed over to fulvestrant alone) did not negate the significance of the long-term overall survival benefit with the combination therapy as compared with anastrozole,” the investigators wrote in The New England Journal of Medicine.
The current report is the final survival analysis of the trial. The primary results were reported in 2012 (N Engl J Med. 2012; 367:435-44). A total of 707 postmenopausal women with previously untreated metastatic disease were randomly assigned to receive either 1 mg of anastrozole orally every day with crossover to fulvestrant alone strongly encouraged if the disease progressed or to anastrozole and fulvestrant in combination. Randomization was stratified according to prior adjuvant tamoxifen use. A total of 694 women had data available for analysis.
The primary analysis, conducted at a median follow-up of 35 months, showed a median progression-free survival (PFS) with anastrozole alone of 13.5 months, compared with 15.0 months for anastrozole/fulvestrant (HR for progression or death 0.80; P = .007). Respective median overall survival was 41.3 months and 47.7 months (HR for death 0.81; P = .05).
The current, final analysis, conducted at a median follow-up of 7 years in patients who did not have disease progression, showed 261 deaths among 345 women (76%) in the anastrozole-only group, compared with 247 deaths among 349 women (71%) in the combination group (HR for death 0.82; P = .03).
Overall survival was longer for those women who had not previously received tamoxifen who were treated with the combination, at a median of 52.2 months versus 40.3 months for women not previously treated with tamoxifen who received anastrozole alone (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92). In contrast, there was no significant difference in OS between the two treatment groups in women who had previously received tamoxifen.
Approximately 45% of patients initially randomized to anastrozole alone were crossed over to fulvestrant.
The incidence of long-term toxic effects and treatment-related deaths was similar between the groups. Previously reported treatment-related deaths with the combination included pulmonary emboli in two patients and a cerebrovascular ischemic event in one patients.
At the time of data cutoff for the final report, 15% of patients in the combination-therapy group and 13% in the anastrozole-only group had experienced grade 3 toxicities.
The study was supported by National Cancer Institute grants and by AstraZeneca. Dr. Mehta reported institutional and personal grants from AstraZeneca and others. Multiple coauthors reported similar relationships.
SOURCE: Mehta RS et al. N Engl J Med. 2019;380:1226-34.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Anastrozole/fulvestrant improved survival when compared with anastrozole alone.
Major finding: The hazard ratio for death with the combination was 0.82 (P = .03).
Study details: Final survival analysis of a phase 3, randomized trial in 694 women with metastatic hormone receptor–positive breast cancer.
Disclosures: The study was supported by National Cancer Institute grants and by AstraZeneca. Dr. Mehta reported institutional and personal grants from AstraZeneca and others. Multiple coauthors reported similar relationships.
Source: Mehta RS et al. N Engl J Med. 2019;380:1226-34.
Mucosal impedance contour rapidly distinguished GERD, non-GERD, and eosinophilic esophagitis
.
Source: American Gastroenterological Association
Each group showed a significantly different (P less than .01) pattern of mucosal impedance (MI), or disruption of mucosal integrity, along the esophageal axis, wrote Dhyanesh A. Patel, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates. Patients without GERD had higher MI values along all esophageal segments, while GERD was characterized by below-normal values in the distal esophagus only, and eosinophilic esophagitis led to low values throughout the esophagus.
The findings were validated in a separate patient cohort, and the only reported adverse event was an episode of mild chest pain. “This contour heatmap could easily be employed to establish a diagnosis during endoscopy, independent of biopsy or pH monitoring,” the investigators wrote in Gastroenterology. They cautioned that the balloon catheter cannot be safely used in patients with severe fibrostenotic disease.
Current definitive diagnostics for GERD leave much to be desired. Transnasal probes are imprecise and uncomfortable, and they can be insensitive if discomfort causes patients to vary normal activity or skip meals. Wireless ambulatory pH monitoring is more tolerable but unreliable and measures only acidity of refluxed material at a single point along the esophagus. These tests also “fail to account for day-to-day variability of reflux, as they only provide a 24- to 48-hour snapshot of a disease process that is chronic in nature,” the researchers wrote. Eosinophilic esophagitis is becoming more common and usually requires proximal and distal biopsies for diagnosis.
Mucosal impedance contour pattern testing is based on the fact that both GERD and eosinophilic esophagitis involve increased distance between esophageal epithelial cells. The amount of intercellular dilatation correlates inversely with MI values. In proof-of-concept studies, individuals with GERD, non-GERD, eosinophilic esophagitis, and achalasia had distinct MI patterns. However, these studies tested a single-channel catheter system that took only point measurements and was subject to interoperator variability. To improve on this concept, Dr. Patel and his associates mounted radial and axial sensors on a balloon catheter to measure MI at 180-degree intervals along a 10-cm esophageal segment.
They tested the new device prospectively in 69 patients undergoing esophagogastroduodenoscopy with or without pH monitoring (which was used as the standard). In all, 24 patients had GERD, 21 had eosinophilic esophagitis, and 24 had normal findings. By using the intercept and slope of the balloon MI measurements, the researchers detected GERD with an area under the receiver operating characteristic curve (AUC) of 0.67, eosinophilic esophagitis with an AUC of 0.84, and non-GERD with an AUC of 0.83.
These findings held up in a separate validation cohort of 36 patients (28 with GERD and eight with eosinophilic esophagitis) from three tertiary care centers. The probability of eosinophilic esophagitis was highest in patients with low distal MI values (that is, a low intercept) and a low slope (showing that MI values remained low proximally). A low distal MI intercept with a steeper positive slope suggested GERD, while a higher distal MI intercept with a steep slope signified non-GERD.
The system “potentially obviates the need for 24- to 48-hour ambulatory wireless pH monitoring or esophageal biopsies for histopathology,” the researchers concluded. “This can help reduce diagnostic and treatment latency and might allow for monitoring disease activity over time.”
The National Institutes of Health funded the external validation analysis. Diversatek Healthcare, which patented the device together with Vanderbilt University, gave research funding to four coinvestigators, including the senior author. Dr. Patel and the other five coinvestigators reported having no conflicts of interest.
SOURCE: Patel DA et al. Gastroenterology. 2019 Jan 31. doi: 10.1053/j.gastro.2019.01.253.
Evaluating esophageal disorders such as GERD or eosinophilic esophagitis can be time consuming for patients in clinical practice and requires multiple visits to complete testing and obtain results. Other than visualizing complications of reflux such as erosive esophagitis or Barrett’s esophagus, there has been no immediate option to diagnose GERD in standard practice during routine endoscopy. Furthermore, the decision to pursue long-term medication or surgery for GERD relies on a brief pH assessment to be truly representative of a patient’s everyday symptoms. Follow-up of eosinophilic esophagitis requires repeated upper endoscopies with biopsies after every incremental change in medication or diet, which unsurprisingly, can reduce compliance with ongoing management for what is often a readily treatable condition.
Both GERD and eosinophilic esophagitis can be characterized by changes in esophageal mucosal impedance. Rather than directly measuring the pH or eosinophil counts, Dr. Patel and associates prospectively validated the diagnostic test performance of an add-on endoscopic mucosal impedance device that might enable the gastroenterologist to rule out GERD or rule in eosinophilic esophagitis during the index endoscopy with reasonable accuracy (AUC above 0.8 to rule out GERD or rule in eosinophilic esophagitis) while adding 2-3 minutes of procedure time. One patient was admitted for chest pain after use of the device but was discharged without complication, and the authors caution against use in severe fibrostenotic disease.
While work to refine a clinical prediction model with this technology is ongoing, the promise of diagnosing and following common esophageal conditions of GERD and eosinophilic esophagitis during endoscopy would have clear value in expediting care and enhancing compliance with treatment.
Eric D. Shah, MD, MBA, is assistant professor of medicine, director of gastrointestinal motility, esophageal, and swallowing disorders center, Geisel School of Medicine, Dartmouth College, Hanover, N.H. He has no disclosures.
Evaluating esophageal disorders such as GERD or eosinophilic esophagitis can be time consuming for patients in clinical practice and requires multiple visits to complete testing and obtain results. Other than visualizing complications of reflux such as erosive esophagitis or Barrett’s esophagus, there has been no immediate option to diagnose GERD in standard practice during routine endoscopy. Furthermore, the decision to pursue long-term medication or surgery for GERD relies on a brief pH assessment to be truly representative of a patient’s everyday symptoms. Follow-up of eosinophilic esophagitis requires repeated upper endoscopies with biopsies after every incremental change in medication or diet, which unsurprisingly, can reduce compliance with ongoing management for what is often a readily treatable condition.
Both GERD and eosinophilic esophagitis can be characterized by changes in esophageal mucosal impedance. Rather than directly measuring the pH or eosinophil counts, Dr. Patel and associates prospectively validated the diagnostic test performance of an add-on endoscopic mucosal impedance device that might enable the gastroenterologist to rule out GERD or rule in eosinophilic esophagitis during the index endoscopy with reasonable accuracy (AUC above 0.8 to rule out GERD or rule in eosinophilic esophagitis) while adding 2-3 minutes of procedure time. One patient was admitted for chest pain after use of the device but was discharged without complication, and the authors caution against use in severe fibrostenotic disease.
While work to refine a clinical prediction model with this technology is ongoing, the promise of diagnosing and following common esophageal conditions of GERD and eosinophilic esophagitis during endoscopy would have clear value in expediting care and enhancing compliance with treatment.
Eric D. Shah, MD, MBA, is assistant professor of medicine, director of gastrointestinal motility, esophageal, and swallowing disorders center, Geisel School of Medicine, Dartmouth College, Hanover, N.H. He has no disclosures.
Evaluating esophageal disorders such as GERD or eosinophilic esophagitis can be time consuming for patients in clinical practice and requires multiple visits to complete testing and obtain results. Other than visualizing complications of reflux such as erosive esophagitis or Barrett’s esophagus, there has been no immediate option to diagnose GERD in standard practice during routine endoscopy. Furthermore, the decision to pursue long-term medication or surgery for GERD relies on a brief pH assessment to be truly representative of a patient’s everyday symptoms. Follow-up of eosinophilic esophagitis requires repeated upper endoscopies with biopsies after every incremental change in medication or diet, which unsurprisingly, can reduce compliance with ongoing management for what is often a readily treatable condition.
Both GERD and eosinophilic esophagitis can be characterized by changes in esophageal mucosal impedance. Rather than directly measuring the pH or eosinophil counts, Dr. Patel and associates prospectively validated the diagnostic test performance of an add-on endoscopic mucosal impedance device that might enable the gastroenterologist to rule out GERD or rule in eosinophilic esophagitis during the index endoscopy with reasonable accuracy (AUC above 0.8 to rule out GERD or rule in eosinophilic esophagitis) while adding 2-3 minutes of procedure time. One patient was admitted for chest pain after use of the device but was discharged without complication, and the authors caution against use in severe fibrostenotic disease.
While work to refine a clinical prediction model with this technology is ongoing, the promise of diagnosing and following common esophageal conditions of GERD and eosinophilic esophagitis during endoscopy would have clear value in expediting care and enhancing compliance with treatment.
Eric D. Shah, MD, MBA, is assistant professor of medicine, director of gastrointestinal motility, esophageal, and swallowing disorders center, Geisel School of Medicine, Dartmouth College, Hanover, N.H. He has no disclosures.
.
Source: American Gastroenterological Association
Each group showed a significantly different (P less than .01) pattern of mucosal impedance (MI), or disruption of mucosal integrity, along the esophageal axis, wrote Dhyanesh A. Patel, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates. Patients without GERD had higher MI values along all esophageal segments, while GERD was characterized by below-normal values in the distal esophagus only, and eosinophilic esophagitis led to low values throughout the esophagus.
The findings were validated in a separate patient cohort, and the only reported adverse event was an episode of mild chest pain. “This contour heatmap could easily be employed to establish a diagnosis during endoscopy, independent of biopsy or pH monitoring,” the investigators wrote in Gastroenterology. They cautioned that the balloon catheter cannot be safely used in patients with severe fibrostenotic disease.
Current definitive diagnostics for GERD leave much to be desired. Transnasal probes are imprecise and uncomfortable, and they can be insensitive if discomfort causes patients to vary normal activity or skip meals. Wireless ambulatory pH monitoring is more tolerable but unreliable and measures only acidity of refluxed material at a single point along the esophagus. These tests also “fail to account for day-to-day variability of reflux, as they only provide a 24- to 48-hour snapshot of a disease process that is chronic in nature,” the researchers wrote. Eosinophilic esophagitis is becoming more common and usually requires proximal and distal biopsies for diagnosis.
Mucosal impedance contour pattern testing is based on the fact that both GERD and eosinophilic esophagitis involve increased distance between esophageal epithelial cells. The amount of intercellular dilatation correlates inversely with MI values. In proof-of-concept studies, individuals with GERD, non-GERD, eosinophilic esophagitis, and achalasia had distinct MI patterns. However, these studies tested a single-channel catheter system that took only point measurements and was subject to interoperator variability. To improve on this concept, Dr. Patel and his associates mounted radial and axial sensors on a balloon catheter to measure MI at 180-degree intervals along a 10-cm esophageal segment.
They tested the new device prospectively in 69 patients undergoing esophagogastroduodenoscopy with or without pH monitoring (which was used as the standard). In all, 24 patients had GERD, 21 had eosinophilic esophagitis, and 24 had normal findings. By using the intercept and slope of the balloon MI measurements, the researchers detected GERD with an area under the receiver operating characteristic curve (AUC) of 0.67, eosinophilic esophagitis with an AUC of 0.84, and non-GERD with an AUC of 0.83.
These findings held up in a separate validation cohort of 36 patients (28 with GERD and eight with eosinophilic esophagitis) from three tertiary care centers. The probability of eosinophilic esophagitis was highest in patients with low distal MI values (that is, a low intercept) and a low slope (showing that MI values remained low proximally). A low distal MI intercept with a steeper positive slope suggested GERD, while a higher distal MI intercept with a steep slope signified non-GERD.
The system “potentially obviates the need for 24- to 48-hour ambulatory wireless pH monitoring or esophageal biopsies for histopathology,” the researchers concluded. “This can help reduce diagnostic and treatment latency and might allow for monitoring disease activity over time.”
The National Institutes of Health funded the external validation analysis. Diversatek Healthcare, which patented the device together with Vanderbilt University, gave research funding to four coinvestigators, including the senior author. Dr. Patel and the other five coinvestigators reported having no conflicts of interest.
SOURCE: Patel DA et al. Gastroenterology. 2019 Jan 31. doi: 10.1053/j.gastro.2019.01.253.
.
Source: American Gastroenterological Association
Each group showed a significantly different (P less than .01) pattern of mucosal impedance (MI), or disruption of mucosal integrity, along the esophageal axis, wrote Dhyanesh A. Patel, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and his associates. Patients without GERD had higher MI values along all esophageal segments, while GERD was characterized by below-normal values in the distal esophagus only, and eosinophilic esophagitis led to low values throughout the esophagus.
The findings were validated in a separate patient cohort, and the only reported adverse event was an episode of mild chest pain. “This contour heatmap could easily be employed to establish a diagnosis during endoscopy, independent of biopsy or pH monitoring,” the investigators wrote in Gastroenterology. They cautioned that the balloon catheter cannot be safely used in patients with severe fibrostenotic disease.
Current definitive diagnostics for GERD leave much to be desired. Transnasal probes are imprecise and uncomfortable, and they can be insensitive if discomfort causes patients to vary normal activity or skip meals. Wireless ambulatory pH monitoring is more tolerable but unreliable and measures only acidity of refluxed material at a single point along the esophagus. These tests also “fail to account for day-to-day variability of reflux, as they only provide a 24- to 48-hour snapshot of a disease process that is chronic in nature,” the researchers wrote. Eosinophilic esophagitis is becoming more common and usually requires proximal and distal biopsies for diagnosis.
Mucosal impedance contour pattern testing is based on the fact that both GERD and eosinophilic esophagitis involve increased distance between esophageal epithelial cells. The amount of intercellular dilatation correlates inversely with MI values. In proof-of-concept studies, individuals with GERD, non-GERD, eosinophilic esophagitis, and achalasia had distinct MI patterns. However, these studies tested a single-channel catheter system that took only point measurements and was subject to interoperator variability. To improve on this concept, Dr. Patel and his associates mounted radial and axial sensors on a balloon catheter to measure MI at 180-degree intervals along a 10-cm esophageal segment.
They tested the new device prospectively in 69 patients undergoing esophagogastroduodenoscopy with or without pH monitoring (which was used as the standard). In all, 24 patients had GERD, 21 had eosinophilic esophagitis, and 24 had normal findings. By using the intercept and slope of the balloon MI measurements, the researchers detected GERD with an area under the receiver operating characteristic curve (AUC) of 0.67, eosinophilic esophagitis with an AUC of 0.84, and non-GERD with an AUC of 0.83.
These findings held up in a separate validation cohort of 36 patients (28 with GERD and eight with eosinophilic esophagitis) from three tertiary care centers. The probability of eosinophilic esophagitis was highest in patients with low distal MI values (that is, a low intercept) and a low slope (showing that MI values remained low proximally). A low distal MI intercept with a steeper positive slope suggested GERD, while a higher distal MI intercept with a steep slope signified non-GERD.
The system “potentially obviates the need for 24- to 48-hour ambulatory wireless pH monitoring or esophageal biopsies for histopathology,” the researchers concluded. “This can help reduce diagnostic and treatment latency and might allow for monitoring disease activity over time.”
The National Institutes of Health funded the external validation analysis. Diversatek Healthcare, which patented the device together with Vanderbilt University, gave research funding to four coinvestigators, including the senior author. Dr. Patel and the other five coinvestigators reported having no conflicts of interest.
SOURCE: Patel DA et al. Gastroenterology. 2019 Jan 31. doi: 10.1053/j.gastro.2019.01.253.
FROM GASTROENTEROLOGY
More Reports from the Connective Tissue Oncology Society 2018 annual meeting in Rome, November 14-17
Early Results Find Olaratumab Combo With Doxorubicin Plus Ifosfamide Safe
Initial results of the phase 1b study of olaratumab plus doxorubicin and ifosfamide have shown the combination to be safe, reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues at CTOS 2018.
The phase 1 trial (NCT03283696) enrolled 16 patients with advanced or metastatic soft tissue sarcomas. Patients had received no prior lines of systemic therapy and had an ECOG performance status of 0-1. Adequate follow-up data were available for 10 patients.
Olaratumab (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4). This was followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin could be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had grade 4 febrile neutropenia and the other had grade 3 febrile neutropenia and grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of the data cutoff. Among 7 patients evaluated for tumor response, 3 patients had a partial response according to RECIST and 3 other patients had stabilized disease as best overall response, for a disease control rate of 86%.
Given that 8 of 10 evaluable patients have completed the dose-limiting toxicity period without dose-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
NOTE: Since CTOS 2018, olaratumab plus doxorubicin did not meet its phase 3 endpoint of overall survival (OS) advantage in the full study population or in the leiomyosarcoma subpopulation compared to doxorubicin alone.
Anthracycline-Based Regimen Excels in FIGO-1 Uterine Leiomyosarcoma
Patients with uterine leiomyosarcomas treated with anthracycline-based regimens experienced longer disease-free survival compared to patients treated with gemcitabine and docetaxel, according to a retrospective analysis reported at CTOS 2018.
Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues reviewed all patients with FIGO stage I uterine leiomyosarcomas at two Italian centers who underwent hysterectomy with or without oophorectomy and were then treated with adjuvant chemotherapy with anthracycline-based or gemcitabine-based regimens.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline-based therapy patients received was 4 (range 2-6) and the median number of cycles with gemcitabine and docetaxel was 5 (range 3-7). Disease-free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
These results suggest that future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, the investigators maintain.
Trabectedin and Concurrent Low-Dose Radiotherapy Feasible
Trabectedin concurrent with lowdose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions. Based on those results, trabectedin (Yondelis) at 1.5 mg/m2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues, reporting at CTOS 2018.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m2), 1 (1.3 mg/m2), and 2 (1.5 mg/m2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas, and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, or malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
Of the irradiated lesions, 71% had long-lasting dimensional responses: 4 completely responded, 8 responded partially, 4 were stable, and 1 progressed.
With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
The investigators concluded trabectedin with concurrent radiotherapy was feasible in patients with pulmonary metastatic soft tissue sarcoma regardless of their histologic subtype.
Early Results Find Olaratumab Combo With Doxorubicin Plus Ifosfamide Safe
Initial results of the phase 1b study of olaratumab plus doxorubicin and ifosfamide have shown the combination to be safe, reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues at CTOS 2018.
The phase 1 trial (NCT03283696) enrolled 16 patients with advanced or metastatic soft tissue sarcomas. Patients had received no prior lines of systemic therapy and had an ECOG performance status of 0-1. Adequate follow-up data were available for 10 patients.
Olaratumab (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4). This was followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin could be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had grade 4 febrile neutropenia and the other had grade 3 febrile neutropenia and grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of the data cutoff. Among 7 patients evaluated for tumor response, 3 patients had a partial response according to RECIST and 3 other patients had stabilized disease as best overall response, for a disease control rate of 86%.
Given that 8 of 10 evaluable patients have completed the dose-limiting toxicity period without dose-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
NOTE: Since CTOS 2018, olaratumab plus doxorubicin did not meet its phase 3 endpoint of overall survival (OS) advantage in the full study population or in the leiomyosarcoma subpopulation compared to doxorubicin alone.
Anthracycline-Based Regimen Excels in FIGO-1 Uterine Leiomyosarcoma
Patients with uterine leiomyosarcomas treated with anthracycline-based regimens experienced longer disease-free survival compared to patients treated with gemcitabine and docetaxel, according to a retrospective analysis reported at CTOS 2018.
Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues reviewed all patients with FIGO stage I uterine leiomyosarcomas at two Italian centers who underwent hysterectomy with or without oophorectomy and were then treated with adjuvant chemotherapy with anthracycline-based or gemcitabine-based regimens.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline-based therapy patients received was 4 (range 2-6) and the median number of cycles with gemcitabine and docetaxel was 5 (range 3-7). Disease-free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
These results suggest that future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, the investigators maintain.
Trabectedin and Concurrent Low-Dose Radiotherapy Feasible
Trabectedin concurrent with lowdose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions. Based on those results, trabectedin (Yondelis) at 1.5 mg/m2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues, reporting at CTOS 2018.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m2), 1 (1.3 mg/m2), and 2 (1.5 mg/m2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas, and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, or malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
Of the irradiated lesions, 71% had long-lasting dimensional responses: 4 completely responded, 8 responded partially, 4 were stable, and 1 progressed.
With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
The investigators concluded trabectedin with concurrent radiotherapy was feasible in patients with pulmonary metastatic soft tissue sarcoma regardless of their histologic subtype.
Early Results Find Olaratumab Combo With Doxorubicin Plus Ifosfamide Safe
Initial results of the phase 1b study of olaratumab plus doxorubicin and ifosfamide have shown the combination to be safe, reported Sebastian Bauer, MD, of the West German Cancer Center, University of Duisburg-Essen, Essen, Germany, and his colleagues at CTOS 2018.
The phase 1 trial (NCT03283696) enrolled 16 patients with advanced or metastatic soft tissue sarcomas. Patients had received no prior lines of systemic therapy and had an ECOG performance status of 0-1. Adequate follow-up data were available for 10 patients.
Olaratumab (Lartruvo), which binds platelet-derived growth factor receptor alpha (PDGFRα), was given at 15 mg/kg in combination with doxorubicin (75 mg/m2 on days 1-3) and ifosfamide (10 g/m2 on days 1-4). This was followed by mandatory granulocyte-colony-stimulating factor therapy in cycles 1-6 on a 21-day cycle. Doxorubicin could be administered by continuous infusion or bolus administration and with cardiac protection. Mesna dosing was at least 60% of the ifosfamide dose.
Two of the 10 patients had dose-limiting toxicities; one had grade 4 febrile neutropenia and the other had grade 3 febrile neutropenia and grade 3 mucositis. Common related adverse events occurring in over 30% of patients included fatigue, anemia, neutropenia, thrombocytopenia, constipation, and nausea. One patient discontinued study treatment due to progressive disease, and all others were on study treatment as of the data cutoff. Among 7 patients evaluated for tumor response, 3 patients had a partial response according to RECIST and 3 other patients had stabilized disease as best overall response, for a disease control rate of 86%.
Given that 8 of 10 evaluable patients have completed the dose-limiting toxicity period without dose-limiting toxicities at the 15 mg/kg dose level of olaratumab, the study has proceeded to the next cohort. In those patients, an olaratumab loading dose of 20 mg/kg will be evaluated in cycle 1, followed by 15 mg/kg of olaratumab in subsequent cycles with the same doses of doxorubicin plus ifosfamide, the researchers wrote in their abstract.
NOTE: Since CTOS 2018, olaratumab plus doxorubicin did not meet its phase 3 endpoint of overall survival (OS) advantage in the full study population or in the leiomyosarcoma subpopulation compared to doxorubicin alone.
Anthracycline-Based Regimen Excels in FIGO-1 Uterine Leiomyosarcoma
Patients with uterine leiomyosarcomas treated with anthracycline-based regimens experienced longer disease-free survival compared to patients treated with gemcitabine and docetaxel, according to a retrospective analysis reported at CTOS 2018.
Roberta Sanfilippo, MD, of Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues reviewed all patients with FIGO stage I uterine leiomyosarcomas at two Italian centers who underwent hysterectomy with or without oophorectomy and were then treated with adjuvant chemotherapy with anthracycline-based or gemcitabine-based regimens.
Of 145 patients, 97 were treated with an anthracycline-based regimen and 48 with gemcitabine and docetaxel. The median number of cycles of anthracycline-based therapy patients received was 4 (range 2-6) and the median number of cycles with gemcitabine and docetaxel was 5 (range 3-7). Disease-free survival was 31 months in patients treated with anthracycline-based chemotherapy and 19 months in patients treated with gemcitabine and docetaxel.
These results suggest that future trials to assess the efficacy of adjuvant chemotherapy in uterine leiomyosarcoma should incorporate anthracyclines, the investigators maintain.
Trabectedin and Concurrent Low-Dose Radiotherapy Feasible
Trabectedin concurrent with lowdose radiotherapy is being examined as an option for patients with pulmonary metastatic soft tissue sarcoma (NCT02275286).
In a phase 1 study, long-lasting dimensional responses were seen in 71% of the irradiated lesions. Based on those results, trabectedin (Yondelis) at 1.5 mg/m2 will be the recommended dose for phase 2, according to Javier Martín-Broto, MD, of the Institute of Biomedicine Research (IBIS)-University Hospital Virgen del Rocio/CSIC/University of Seville, Spain, and his colleagues, reporting at CTOS 2018.
For the study, trabectedin was given along with radiotherapy (30 Gy) in 10 fractions (3 Gy/fraction). Three dose levels of trabectedin were administered: -1 (1.1 mg/m2), 1 (1.3 mg/m2), and 2 (1.5 mg/m2). Dose-limiting toxicity was defined as grade 3 or greater events excluding grade 3/4 neutropenia lasting less than 5 days, grade 3 transaminitis if it did not lead to trabectedin delay, and grade 3/4 nausea/vomiting due to inadequate prophylaxis.
Ten of the 18 patients enrolled had synovial sarcoma; 3 had undifferentiated pleomorphic sarcomas, and the other patients had either myxoid liposarcoma, dedifferentiated liposarcoma, G3 not otherwise specified sarcoma, leiomyosarcoma, or malignant peripheral nerve sheath tumor.
Patients received a median of 1 prior line of chemotherapy (range: 0-3). Twelve patients received trabectedin at dose level 1 and 6 patients at dose level 2. Grade 3/4 adverse events were neutropenia, seen in 8 patients; alanine aminotransferase (ALT) elevation, seen in 2 patients; gamma-glutamyl transferase (GGT) elevation, seen in 2 patients; anemia, seen in 2 patients; febrile neutropenia, seen in 1 patient; and pneumonitis, seen in 1 patient.
There were two dose-limiting toxicities: transient grade 4 ALT elevation at the level 1 dose and grade 4 neutropenia for more than 5 days at the level 2 dose.
Based on central radiological review of 17 evaluable patients, 2 patients achieved complete response, 3 had partial responses, 6 had stable disease, and 6 had progressive disease. The local review reported complete responses in 2 patients, partial responses in 5, stable disease in 4, and progressive disease in 6.
Of the irradiated lesions, 71% had long-lasting dimensional responses: 4 completely responded, 8 responded partially, 4 were stable, and 1 progressed.
With a median follow-up of 18 months, median progression-free survival was 2.83 months (95%CI: 2.3-3.3 months). Thirteen patients have died, with a median overall survival of 8.77 months (95%CI: 3.6-13.9) and a 12-month overall survival rate of 48%.
The investigators concluded trabectedin with concurrent radiotherapy was feasible in patients with pulmonary metastatic soft tissue sarcoma regardless of their histologic subtype.