User login
Identifying and Treating Nonalcoholic Fatty Liver Disease
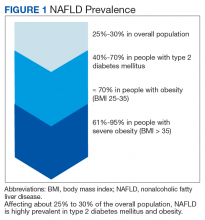
Nonalcoholic fatty liver disease (NAFLD) is a silent epidemic affecting nearly 1 in 3 Americans and is increasing within the Veterans Health Administration (VHA).1,2 NAFLD independently increases the risk of type 2 diabetes mellitus (
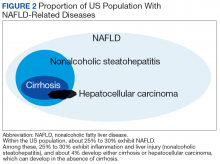
In most patients (80%), NAFLD progresses slowly over decades. The progression is related to continuing insulin resistance.15,16 Greater disease progression is seen in patients with T2DM or concomitant chronic liver disease (such as hepatitis C).10,11,16 Patients with NAFLD who develop advanced fibrosis or cirrhosis experience increased rates of overall mortality, liver-related events, and liver transplantation.1,9,17,18 Within the VHA, NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 and 70 years, respectively.19
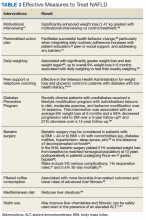
Although no pharmaceuticals are yet approved to treat NAFLD, even modest weight loss is beneficial. For example, weight loss > 4% improves fatty liver, ≥ 7% improves liver inflammation, and ≥ 10% decreases liver fibrosis (or scarring).21-23 In patients with a prior lack of success with weight loss, weight loss medications may be beneficial for short-term use.24 When comparing the effects of diet, exercise, obesity pharmacotherapy, and combinations for these approaches, intensive lifestyle modification with exercise had the greatest, most enduring benefit.25 Additionally, bariatric (weight loss) surgery has significantly improved health and liver-related outcomes for patients with NASH.26
In at-risk veterans, NAFLD has myriad negative effects on health and QOL. To improve its early identification and management in the VHA, we summarize strategies that all providers can use to screen and treat patients for this condition.
Screening for Advanced Fibrosis
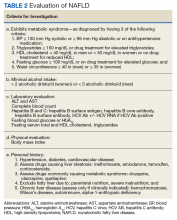
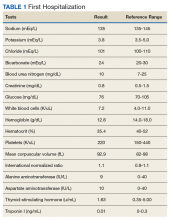
Advanced fibrosis in NAFLD is diagnosed by analyzing adequately sized liver biopsies.27,28 However, noninvasive approaches to quantify advanced fibrosis by imaging or use of a simple fibrosis prediction score also are available. Imaging modalities include measuring liver stiffness, using transient elastography (FibroScan, Waltham, MA) or magnetic resonance elastography.1,29-31 Fibrosis prediction scores use common clinical and laboratory data to predict the presence or absence of advanced fibrosis (Table 1).29
Does This Patient Have NAFLD?
To identify NAFLD, patients with metabolic syndrome and modest or no alcohol use are first assessed for liver injury with ALT, AST, and complete blood count (Figure 3; Case 1).16
Next, common underlying liver diseases that cause liver injury should be excluded by hepatitis B and C virus serology.11,16 Other underlying liver diseases are uncommon and should be assessed only if clinically indicated.
Evaluation of fasting glucose or hemoglobin A1c (HbA1c)can identify undiagnosed T2DM. NAFL, or simple steatosis, is independently associated with an increased risk of T2DM, cardiovascular and kidney disease, yet not overall mortality.16 Over 10 to 20 years, few patients (4%) with simple steatosis progress to cirrhosis.39
In NAFLD, simple steatosis can resolve, and NASH can significantly improve with 7% to 10% weight loss.16,23,40 Patients with simple steatosis on imaging and normal liver enzymes should be monitored with periodic liver enzymes and fibrosis prediction scores (eg, FIB-4) and encouraged to pursue intensive lifestyle intervention.16,33 Without weight loss and exercise interventions metabolic syndrome, T2DM, and NAFLD may progress.
Patients with combined liver steatosis and liver enzyme elevations may exhibit NASH and warrant an evaluation by a hepatologist or gastroenterologist for consideration of additional testing or liver biopsy.16
Encouraging Patients to Pursue Intensive Lifestyle InterventionS
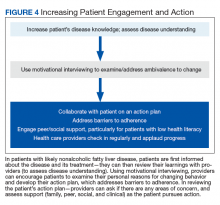
Most veterans wish to collaborate in their care (Table 3, Figure 4) yet experience many barriers, such as low health literacy, high rates of comorbidities, and ongoing drug/alcohol misuse.43,44
In addition to patient education, motivational interviewing significantly improves weight loss, resulting in a 3.3 lb (1.5 kg) increased weight loss in the intervention group vs the control group in weight loss studies.46
To start the conversation, the health care provider can explain that
- Why would you want to lose weight and exercise?
- How might you go about it in order to succeed?
- What are the 3 best reasons for you to do it?
- How important is it for you to make this change, and why? The provider can also ask the patient to quantify on a scale of 1 to 10: (a) How likely is it that they will make each required change? (b) How hard will each change be for them?
- The provider then summarizes the patient’s reasons for wanting change, how he/she can effect change, what their best reasons are, and how to successfully change. The provider then asks a final question:
- So what do you think you will do?
Most patients report feeling engaged, empowered, open, and understood with motivational interviewing. People are “persuaded by what they hear themselves say,” increasing motivation to change.47
This personalized action plan facilitates successful health behavior change.48 Action plans should integrate daily routines. For example, by placing the scale near the toothbrush, daily weighing is encouraged. Daily weighing is associated with significantly greater weight loss and less weight regain.49 In a 6-month, randomized controlled weight loss trial in men and women, daily weighing (using a scale that automatically transmitted weight data), with weekly e-mails and tailored feedback yielded an overall 9% weight loss and increased use of exercise and diet behaviors associated with weight loss in comparison with those who weighed themselves less than weekly.50 This simple daily measure seems to reinforce a patient’s action plan.
Adherence to an action plan significantly improves with patient education, peer or social support, and addressing barriers to adherence.51 For example, by providing support with weekly text messaging of “How are you?” and addressing the issues that patients reported in a large randomized treatment trial, adherence was significantly improved.52 In VHA patients with low health literacy, peer support or telephone coaching also has proven effective in increasing weight loss and glycemic control in patients with T2DM.53,54 Providing multidisciplinary team support during intensive lifestyle intervention, providers can partner with patients to address questions or issues and applaud progress.
Effective VHA interventions
In an ethnically diverse population of patients with prediabetes, up to 7% weight loss was observed in the Diabetes Prevention Program (DPP).55 In this study patients were randomized to placebo; metformin 850 mg twice daily; or a lifestyle-modification program in which they received one-on-one culturally sensitive, individualized lessons in diet, moderate exercise (≥ 150 minutes weekly), and behavior modification from case managers over 16 sessions. Lessons were reinforced in both group and individual sessions. This intervention was associated with an average of 6% weight loss at 6 months (half of participants attained 7% weight loss) and a 58% decrease in the rate of progression to T2DM over a nearly 3-year follow-up of this population with prediabetes compared with that of the placebo group.55 Over a 15-year follow-up, the intensive lifestyle intervention group sustained a 27% decrease in the incidence of T2DM compared with that of the placebo group.56 To emulate the success of the DPP in the VHA, a web-based DPP-like study in female veterans was performed with online coaching and daily weighing. This study achieved a 5.2% weight loss from baseline at 4 months.57
To improve outcomes, the VHA MOVE! Weight Management Program has been revised to include more sustained intervention (16 sessions) and multiple modes for participating—in person, by telephone, via video, via MOVE! Coach phone app, or any combination.58 Using shared decision making between patients with NAFLD and their providers, a customized MOVE! weight loss program can be developed to enable sustained intensive lifestyle intervention: hypocaloric diet, ≥ 150 minutes of moderate exercise weekly, and behavioral change.
In addition to intensive lifestyle intervention, a prospective study found that bariatric surgery significantly improved outcomes in patients with NASH, with most patients experiencing resolution of their NASH and nearly half exhibiting significantly improved fibrosis.26 In the VHA, bariatric surgery has yielded excellent long-term outcomes, with 21% sustained weight loss from baseline (vs matched nonsurgical population) at 10 years postoperatively in patients undergoing Roux-en-Y gastric bypass.59 Bariatric surgery also results in long-term remission of T2DM in most patients and significant improvement in hypertension and dyslipidemia.60 The risks of bariatric surgery include 3% serious complications, 1% reoperation rates, and 0.4% 30-day mortality.61,62 Bariatric surgery can be considered in patients with BMI > 40 or in patients with BMI > 35 who have comorbidities and do not have decompensated cirrhosis.63,64
Beyond weight loss, more favorable liver-related outcomes and lower rates of advanced liver fibrosis are observed in those consuming filtered coffee; a reduction in liver steatosis also is observed with adherence to a Mediterranean diet.65,66 In NAFLD, statins may improve liver chemistries and fibrosis; this class of medications can be used safely even in the presence of an elevated ALT.11,67
Conclusion
Nonalcoholic fatty liver disease independently increases the risk of T2DM, cardiovascular disease and kidney disease. With its rates increasing in the VHA, earlier identification and intervention is warranted in patients at high risk (ie, those with metabolic syndrome, obesity, and T2DM).2
NASH is more frequent in those with liver enzyme elevations or with an elevated FIB-4 and is associated with a long-term risk of cirrhosis. These patients merit referral to hepatology or gastroenterology for further evaluation and consideration of a liver biopsy to identify NASH. Patients with likely NAFLD without liver enzyme elevations can be further evaluated with FIB-4 scores to assess their probability of advanced liver fibrosis and potential need for referral to hepatology or gastroenterology.
Early NAFLD detection and intervention with intensive lifestyle modifications has the potential to avert progression to advanced fibrosis—and its associated increased overall and liver-related mortality, and impaired QOL.3,16,18 Although FIB-4 is a validated predictor of advanced fibrosis, this score is not yet used nationally to identify and risk stratify NAFLD in the VHA. Additionally, the very low use of VHA diet/exercise programs in eligible patients contributes to NAFLD progression.68 The cost-effective DPP has successfully yielded weight loss in patients with prediabetes and decreases in the incidence of T2DM through motivational interviewing and intensive lifestyle intervention.55
To improve NAFLD management, providers can successfully engage patients through motivational interviewing for intensive lifestyle intervention. Their resulting weight loss is enhanced with a personalized action plan, daily weighing, and peer support. When NAFLD is identified in patients with metabolic risk factors, the probability of advanced fibrosis is easily assessed in those with elevated FIB-4 scores who merit gastrointestinal referral.33,37
In all those identified with NAFLD, disease information should be provided to patients and their families. Intensive lifestyle modification targeting a ≥ 7% weight loss is recommended; motivational interviewing can increase commitment to change and yield a customized action plan for sustained weight loss. Working with the support and encouragement of their team of primary care providers, dieticians, and MOVE! coaches, patients can actively engage to improve their NAFLD and overall health.
1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263-2273.
2. Kanwal F, Kramer JR, Duan Z, et al. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
3. Golabi P, Otgonsuren M, Cable R, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14(1):18.
4. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
5. Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511-531.
6. Centers for Disease Control and Prevention. About Prediabetes & Type 2 Diabetes. https://www.cdc.gov/diabetes/prevention/prediabetes-type2/index.html. Updated June 11, 2018. Accessed November 7, 2018.
7. Littman AJ, Jacobson IG, Boyko EJ, Powell TM, Smith TC; Millennium Cohort Study Team. Weight change following US military service. Int J Obes (Lond). 2013;37(2):244-253.
8. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
9. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-854.
10. Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care. 2015;38(7):1347-1355.
11. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
12. Bril F, Barb D, Portillo‐Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132-1144.
13. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063-2072.
14. Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;27(2):199-210.
15. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-654.
16. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
17. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
18. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
19. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans, 2001-2013. Gastroenterology 2015;149(6):1471-1482.
20. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
21. Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4(1):e000139.
22. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255-266.
23. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
24. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
25. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808-1817.
26. Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388.
27. Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321.
28. Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60(2):565-567.
29. Tapper EB, Sengupta N, Hunink MG, Afdhal NH, Lai M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am J Gastroenterol. 2015;110(9):1298-1304.
30. Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non‐invasive diagnosis of advanced fibrosis in biopsy‐proven non‐alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41(12):1271-1280.
31. Tapper EB, Lok AS-F. Use of liver imaging and biopsy in clinical practice. N Engl J Med . 2017;377(8):756-768.
32. Sterling RK, Lissen E, Clumeck N; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-1325.
33. Imler T. Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/fibrosis-4-score. Published 2018. Accessed November 7, 2018.
34. Sun W, Cui H , Li N, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46(9):862-870.
35. Imler T, Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/nafld-fibrosis-score. Published 2018. Accessed November 7, 2018.
36. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441-1447.
37. Patel YA, Gifford EJ, Glass LM, et al. Identifying non-alcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9): 2259-2266.
38. Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234-240.
39. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413-1419.
40. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-129.
41. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
42. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab 2015;100(6):2231-2238.
43. Rodriguez V, Andrade AD, Garcia-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
44. Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56(2):320-325.
45. Veterans Health Administration. Non-alcoholic fatty liver: information for patients. https://www.hepatitis.va.gov/pdf/NAFL.pdf. Published September 2017. Accessed November 7, 2018.
46. Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
47. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. Guilford Press: NY, New York; 2013.
48. Leventhal H, Leventhal EA, Breland JY. Cognitive science speaks to the “common sense” of chronic illness management. Ann Behav Med. 2011;41(2):152-163.
49. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring). 2015;23(2):256-265.
50. Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters; daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet. 2015;115(4):511-518.
51. Charania MR, Marshall KJ, Lyles CM; HIV/AIDS Prevention Research Synthesis (PRS) Team. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of U.S.-based studies, 1996-2011. AIDS Behav. 2014;18(4):646-660.
52. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376(9755):1838-1845.
53. Dutton GR, Phillips JM, Kukkamalla M, Cherrington AL, Safford MM. Pilot study evaluating the feasibility and initial outcomes of a primary care weight loss intervention with peer coaches. Diabetes Educ. 2015:41(3):361-368.
54. Fisher EB, Coufal MM, Parada H, et al. Peer support in health care and prevention: Cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35(1):363-383.
55. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;(346):393-403.
56. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875.
57. Moin T, Ertl K, Schneider J, et al. Women veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res. 2015;17(5):e127.
58. US Department of Veterans Affairs. MOVE! Weight management program. https://www.move.va.gov/MOVE/index.asp. Updated October 5, 2018. Accessed November 7, 2018.
59. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046-1055.
60. Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143-1155.
61. Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications beforevs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792-799.
62. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445-454.
63. Brito JP, Montori VM, Davis AM; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317(6):635-636.
64. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897-901.
65. Saab S, Mallam D, Cox GA 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34(4):495-504.
66. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138-143.
67. Musso G, Gambino R, Cassader M, Pagano G. A meta‐analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79-104.
68. Patel Y, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven non-alcoholic fatty liver disease progression in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
Nonalcoholic fatty liver disease (NAFLD) is a silent epidemic affecting nearly 1 in 3 Americans and is increasing within the Veterans Health Administration (VHA).1,2 NAFLD independently increases the risk of type 2 diabetes mellitus (
In most patients (80%), NAFLD progresses slowly over decades. The progression is related to continuing insulin resistance.15,16 Greater disease progression is seen in patients with T2DM or concomitant chronic liver disease (such as hepatitis C).10,11,16 Patients with NAFLD who develop advanced fibrosis or cirrhosis experience increased rates of overall mortality, liver-related events, and liver transplantation.1,9,17,18 Within the VHA, NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 and 70 years, respectively.19
Although no pharmaceuticals are yet approved to treat NAFLD, even modest weight loss is beneficial. For example, weight loss > 4% improves fatty liver, ≥ 7% improves liver inflammation, and ≥ 10% decreases liver fibrosis (or scarring).21-23 In patients with a prior lack of success with weight loss, weight loss medications may be beneficial for short-term use.24 When comparing the effects of diet, exercise, obesity pharmacotherapy, and combinations for these approaches, intensive lifestyle modification with exercise had the greatest, most enduring benefit.25 Additionally, bariatric (weight loss) surgery has significantly improved health and liver-related outcomes for patients with NASH.26
In at-risk veterans, NAFLD has myriad negative effects on health and QOL. To improve its early identification and management in the VHA, we summarize strategies that all providers can use to screen and treat patients for this condition.
Screening for Advanced Fibrosis
Advanced fibrosis in NAFLD is diagnosed by analyzing adequately sized liver biopsies.27,28 However, noninvasive approaches to quantify advanced fibrosis by imaging or use of a simple fibrosis prediction score also are available. Imaging modalities include measuring liver stiffness, using transient elastography (FibroScan, Waltham, MA) or magnetic resonance elastography.1,29-31 Fibrosis prediction scores use common clinical and laboratory data to predict the presence or absence of advanced fibrosis (Table 1).29
Does This Patient Have NAFLD?
To identify NAFLD, patients with metabolic syndrome and modest or no alcohol use are first assessed for liver injury with ALT, AST, and complete blood count (Figure 3; Case 1).16
Next, common underlying liver diseases that cause liver injury should be excluded by hepatitis B and C virus serology.11,16 Other underlying liver diseases are uncommon and should be assessed only if clinically indicated.
Evaluation of fasting glucose or hemoglobin A1c (HbA1c)can identify undiagnosed T2DM. NAFL, or simple steatosis, is independently associated with an increased risk of T2DM, cardiovascular and kidney disease, yet not overall mortality.16 Over 10 to 20 years, few patients (4%) with simple steatosis progress to cirrhosis.39
In NAFLD, simple steatosis can resolve, and NASH can significantly improve with 7% to 10% weight loss.16,23,40 Patients with simple steatosis on imaging and normal liver enzymes should be monitored with periodic liver enzymes and fibrosis prediction scores (eg, FIB-4) and encouraged to pursue intensive lifestyle intervention.16,33 Without weight loss and exercise interventions metabolic syndrome, T2DM, and NAFLD may progress.
Patients with combined liver steatosis and liver enzyme elevations may exhibit NASH and warrant an evaluation by a hepatologist or gastroenterologist for consideration of additional testing or liver biopsy.16
Encouraging Patients to Pursue Intensive Lifestyle InterventionS
Most veterans wish to collaborate in their care (Table 3, Figure 4) yet experience many barriers, such as low health literacy, high rates of comorbidities, and ongoing drug/alcohol misuse.43,44
In addition to patient education, motivational interviewing significantly improves weight loss, resulting in a 3.3 lb (1.5 kg) increased weight loss in the intervention group vs the control group in weight loss studies.46
To start the conversation, the health care provider can explain that
- Why would you want to lose weight and exercise?
- How might you go about it in order to succeed?
- What are the 3 best reasons for you to do it?
- How important is it for you to make this change, and why? The provider can also ask the patient to quantify on a scale of 1 to 10: (a) How likely is it that they will make each required change? (b) How hard will each change be for them?
- The provider then summarizes the patient’s reasons for wanting change, how he/she can effect change, what their best reasons are, and how to successfully change. The provider then asks a final question:
- So what do you think you will do?
Most patients report feeling engaged, empowered, open, and understood with motivational interviewing. People are “persuaded by what they hear themselves say,” increasing motivation to change.47
This personalized action plan facilitates successful health behavior change.48 Action plans should integrate daily routines. For example, by placing the scale near the toothbrush, daily weighing is encouraged. Daily weighing is associated with significantly greater weight loss and less weight regain.49 In a 6-month, randomized controlled weight loss trial in men and women, daily weighing (using a scale that automatically transmitted weight data), with weekly e-mails and tailored feedback yielded an overall 9% weight loss and increased use of exercise and diet behaviors associated with weight loss in comparison with those who weighed themselves less than weekly.50 This simple daily measure seems to reinforce a patient’s action plan.
Adherence to an action plan significantly improves with patient education, peer or social support, and addressing barriers to adherence.51 For example, by providing support with weekly text messaging of “How are you?” and addressing the issues that patients reported in a large randomized treatment trial, adherence was significantly improved.52 In VHA patients with low health literacy, peer support or telephone coaching also has proven effective in increasing weight loss and glycemic control in patients with T2DM.53,54 Providing multidisciplinary team support during intensive lifestyle intervention, providers can partner with patients to address questions or issues and applaud progress.
Effective VHA interventions
In an ethnically diverse population of patients with prediabetes, up to 7% weight loss was observed in the Diabetes Prevention Program (DPP).55 In this study patients were randomized to placebo; metformin 850 mg twice daily; or a lifestyle-modification program in which they received one-on-one culturally sensitive, individualized lessons in diet, moderate exercise (≥ 150 minutes weekly), and behavior modification from case managers over 16 sessions. Lessons were reinforced in both group and individual sessions. This intervention was associated with an average of 6% weight loss at 6 months (half of participants attained 7% weight loss) and a 58% decrease in the rate of progression to T2DM over a nearly 3-year follow-up of this population with prediabetes compared with that of the placebo group.55 Over a 15-year follow-up, the intensive lifestyle intervention group sustained a 27% decrease in the incidence of T2DM compared with that of the placebo group.56 To emulate the success of the DPP in the VHA, a web-based DPP-like study in female veterans was performed with online coaching and daily weighing. This study achieved a 5.2% weight loss from baseline at 4 months.57
To improve outcomes, the VHA MOVE! Weight Management Program has been revised to include more sustained intervention (16 sessions) and multiple modes for participating—in person, by telephone, via video, via MOVE! Coach phone app, or any combination.58 Using shared decision making between patients with NAFLD and their providers, a customized MOVE! weight loss program can be developed to enable sustained intensive lifestyle intervention: hypocaloric diet, ≥ 150 minutes of moderate exercise weekly, and behavioral change.
In addition to intensive lifestyle intervention, a prospective study found that bariatric surgery significantly improved outcomes in patients with NASH, with most patients experiencing resolution of their NASH and nearly half exhibiting significantly improved fibrosis.26 In the VHA, bariatric surgery has yielded excellent long-term outcomes, with 21% sustained weight loss from baseline (vs matched nonsurgical population) at 10 years postoperatively in patients undergoing Roux-en-Y gastric bypass.59 Bariatric surgery also results in long-term remission of T2DM in most patients and significant improvement in hypertension and dyslipidemia.60 The risks of bariatric surgery include 3% serious complications, 1% reoperation rates, and 0.4% 30-day mortality.61,62 Bariatric surgery can be considered in patients with BMI > 40 or in patients with BMI > 35 who have comorbidities and do not have decompensated cirrhosis.63,64
Beyond weight loss, more favorable liver-related outcomes and lower rates of advanced liver fibrosis are observed in those consuming filtered coffee; a reduction in liver steatosis also is observed with adherence to a Mediterranean diet.65,66 In NAFLD, statins may improve liver chemistries and fibrosis; this class of medications can be used safely even in the presence of an elevated ALT.11,67
Conclusion
Nonalcoholic fatty liver disease independently increases the risk of T2DM, cardiovascular disease and kidney disease. With its rates increasing in the VHA, earlier identification and intervention is warranted in patients at high risk (ie, those with metabolic syndrome, obesity, and T2DM).2
NASH is more frequent in those with liver enzyme elevations or with an elevated FIB-4 and is associated with a long-term risk of cirrhosis. These patients merit referral to hepatology or gastroenterology for further evaluation and consideration of a liver biopsy to identify NASH. Patients with likely NAFLD without liver enzyme elevations can be further evaluated with FIB-4 scores to assess their probability of advanced liver fibrosis and potential need for referral to hepatology or gastroenterology.
Early NAFLD detection and intervention with intensive lifestyle modifications has the potential to avert progression to advanced fibrosis—and its associated increased overall and liver-related mortality, and impaired QOL.3,16,18 Although FIB-4 is a validated predictor of advanced fibrosis, this score is not yet used nationally to identify and risk stratify NAFLD in the VHA. Additionally, the very low use of VHA diet/exercise programs in eligible patients contributes to NAFLD progression.68 The cost-effective DPP has successfully yielded weight loss in patients with prediabetes and decreases in the incidence of T2DM through motivational interviewing and intensive lifestyle intervention.55
To improve NAFLD management, providers can successfully engage patients through motivational interviewing for intensive lifestyle intervention. Their resulting weight loss is enhanced with a personalized action plan, daily weighing, and peer support. When NAFLD is identified in patients with metabolic risk factors, the probability of advanced fibrosis is easily assessed in those with elevated FIB-4 scores who merit gastrointestinal referral.33,37
In all those identified with NAFLD, disease information should be provided to patients and their families. Intensive lifestyle modification targeting a ≥ 7% weight loss is recommended; motivational interviewing can increase commitment to change and yield a customized action plan for sustained weight loss. Working with the support and encouragement of their team of primary care providers, dieticians, and MOVE! coaches, patients can actively engage to improve their NAFLD and overall health.
Nonalcoholic fatty liver disease (NAFLD) is a silent epidemic affecting nearly 1 in 3 Americans and is increasing within the Veterans Health Administration (VHA).1,2 NAFLD independently increases the risk of type 2 diabetes mellitus (
In most patients (80%), NAFLD progresses slowly over decades. The progression is related to continuing insulin resistance.15,16 Greater disease progression is seen in patients with T2DM or concomitant chronic liver disease (such as hepatitis C).10,11,16 Patients with NAFLD who develop advanced fibrosis or cirrhosis experience increased rates of overall mortality, liver-related events, and liver transplantation.1,9,17,18 Within the VHA, NAFLD is the third most common cause of cirrhosis and HCC, occurring at an average age of 66 and 70 years, respectively.19
Although no pharmaceuticals are yet approved to treat NAFLD, even modest weight loss is beneficial. For example, weight loss > 4% improves fatty liver, ≥ 7% improves liver inflammation, and ≥ 10% decreases liver fibrosis (or scarring).21-23 In patients with a prior lack of success with weight loss, weight loss medications may be beneficial for short-term use.24 When comparing the effects of diet, exercise, obesity pharmacotherapy, and combinations for these approaches, intensive lifestyle modification with exercise had the greatest, most enduring benefit.25 Additionally, bariatric (weight loss) surgery has significantly improved health and liver-related outcomes for patients with NASH.26
In at-risk veterans, NAFLD has myriad negative effects on health and QOL. To improve its early identification and management in the VHA, we summarize strategies that all providers can use to screen and treat patients for this condition.
Screening for Advanced Fibrosis
Advanced fibrosis in NAFLD is diagnosed by analyzing adequately sized liver biopsies.27,28 However, noninvasive approaches to quantify advanced fibrosis by imaging or use of a simple fibrosis prediction score also are available. Imaging modalities include measuring liver stiffness, using transient elastography (FibroScan, Waltham, MA) or magnetic resonance elastography.1,29-31 Fibrosis prediction scores use common clinical and laboratory data to predict the presence or absence of advanced fibrosis (Table 1).29
Does This Patient Have NAFLD?
To identify NAFLD, patients with metabolic syndrome and modest or no alcohol use are first assessed for liver injury with ALT, AST, and complete blood count (Figure 3; Case 1).16
Next, common underlying liver diseases that cause liver injury should be excluded by hepatitis B and C virus serology.11,16 Other underlying liver diseases are uncommon and should be assessed only if clinically indicated.
Evaluation of fasting glucose or hemoglobin A1c (HbA1c)can identify undiagnosed T2DM. NAFL, or simple steatosis, is independently associated with an increased risk of T2DM, cardiovascular and kidney disease, yet not overall mortality.16 Over 10 to 20 years, few patients (4%) with simple steatosis progress to cirrhosis.39
In NAFLD, simple steatosis can resolve, and NASH can significantly improve with 7% to 10% weight loss.16,23,40 Patients with simple steatosis on imaging and normal liver enzymes should be monitored with periodic liver enzymes and fibrosis prediction scores (eg, FIB-4) and encouraged to pursue intensive lifestyle intervention.16,33 Without weight loss and exercise interventions metabolic syndrome, T2DM, and NAFLD may progress.
Patients with combined liver steatosis and liver enzyme elevations may exhibit NASH and warrant an evaluation by a hepatologist or gastroenterologist for consideration of additional testing or liver biopsy.16
Encouraging Patients to Pursue Intensive Lifestyle InterventionS
Most veterans wish to collaborate in their care (Table 3, Figure 4) yet experience many barriers, such as low health literacy, high rates of comorbidities, and ongoing drug/alcohol misuse.43,44
In addition to patient education, motivational interviewing significantly improves weight loss, resulting in a 3.3 lb (1.5 kg) increased weight loss in the intervention group vs the control group in weight loss studies.46
To start the conversation, the health care provider can explain that
- Why would you want to lose weight and exercise?
- How might you go about it in order to succeed?
- What are the 3 best reasons for you to do it?
- How important is it for you to make this change, and why? The provider can also ask the patient to quantify on a scale of 1 to 10: (a) How likely is it that they will make each required change? (b) How hard will each change be for them?
- The provider then summarizes the patient’s reasons for wanting change, how he/she can effect change, what their best reasons are, and how to successfully change. The provider then asks a final question:
- So what do you think you will do?
Most patients report feeling engaged, empowered, open, and understood with motivational interviewing. People are “persuaded by what they hear themselves say,” increasing motivation to change.47
This personalized action plan facilitates successful health behavior change.48 Action plans should integrate daily routines. For example, by placing the scale near the toothbrush, daily weighing is encouraged. Daily weighing is associated with significantly greater weight loss and less weight regain.49 In a 6-month, randomized controlled weight loss trial in men and women, daily weighing (using a scale that automatically transmitted weight data), with weekly e-mails and tailored feedback yielded an overall 9% weight loss and increased use of exercise and diet behaviors associated with weight loss in comparison with those who weighed themselves less than weekly.50 This simple daily measure seems to reinforce a patient’s action plan.
Adherence to an action plan significantly improves with patient education, peer or social support, and addressing barriers to adherence.51 For example, by providing support with weekly text messaging of “How are you?” and addressing the issues that patients reported in a large randomized treatment trial, adherence was significantly improved.52 In VHA patients with low health literacy, peer support or telephone coaching also has proven effective in increasing weight loss and glycemic control in patients with T2DM.53,54 Providing multidisciplinary team support during intensive lifestyle intervention, providers can partner with patients to address questions or issues and applaud progress.
Effective VHA interventions
In an ethnically diverse population of patients with prediabetes, up to 7% weight loss was observed in the Diabetes Prevention Program (DPP).55 In this study patients were randomized to placebo; metformin 850 mg twice daily; or a lifestyle-modification program in which they received one-on-one culturally sensitive, individualized lessons in diet, moderate exercise (≥ 150 minutes weekly), and behavior modification from case managers over 16 sessions. Lessons were reinforced in both group and individual sessions. This intervention was associated with an average of 6% weight loss at 6 months (half of participants attained 7% weight loss) and a 58% decrease in the rate of progression to T2DM over a nearly 3-year follow-up of this population with prediabetes compared with that of the placebo group.55 Over a 15-year follow-up, the intensive lifestyle intervention group sustained a 27% decrease in the incidence of T2DM compared with that of the placebo group.56 To emulate the success of the DPP in the VHA, a web-based DPP-like study in female veterans was performed with online coaching and daily weighing. This study achieved a 5.2% weight loss from baseline at 4 months.57
To improve outcomes, the VHA MOVE! Weight Management Program has been revised to include more sustained intervention (16 sessions) and multiple modes for participating—in person, by telephone, via video, via MOVE! Coach phone app, or any combination.58 Using shared decision making between patients with NAFLD and their providers, a customized MOVE! weight loss program can be developed to enable sustained intensive lifestyle intervention: hypocaloric diet, ≥ 150 minutes of moderate exercise weekly, and behavioral change.
In addition to intensive lifestyle intervention, a prospective study found that bariatric surgery significantly improved outcomes in patients with NASH, with most patients experiencing resolution of their NASH and nearly half exhibiting significantly improved fibrosis.26 In the VHA, bariatric surgery has yielded excellent long-term outcomes, with 21% sustained weight loss from baseline (vs matched nonsurgical population) at 10 years postoperatively in patients undergoing Roux-en-Y gastric bypass.59 Bariatric surgery also results in long-term remission of T2DM in most patients and significant improvement in hypertension and dyslipidemia.60 The risks of bariatric surgery include 3% serious complications, 1% reoperation rates, and 0.4% 30-day mortality.61,62 Bariatric surgery can be considered in patients with BMI > 40 or in patients with BMI > 35 who have comorbidities and do not have decompensated cirrhosis.63,64
Beyond weight loss, more favorable liver-related outcomes and lower rates of advanced liver fibrosis are observed in those consuming filtered coffee; a reduction in liver steatosis also is observed with adherence to a Mediterranean diet.65,66 In NAFLD, statins may improve liver chemistries and fibrosis; this class of medications can be used safely even in the presence of an elevated ALT.11,67
Conclusion
Nonalcoholic fatty liver disease independently increases the risk of T2DM, cardiovascular disease and kidney disease. With its rates increasing in the VHA, earlier identification and intervention is warranted in patients at high risk (ie, those with metabolic syndrome, obesity, and T2DM).2
NASH is more frequent in those with liver enzyme elevations or with an elevated FIB-4 and is associated with a long-term risk of cirrhosis. These patients merit referral to hepatology or gastroenterology for further evaluation and consideration of a liver biopsy to identify NASH. Patients with likely NAFLD without liver enzyme elevations can be further evaluated with FIB-4 scores to assess their probability of advanced liver fibrosis and potential need for referral to hepatology or gastroenterology.
Early NAFLD detection and intervention with intensive lifestyle modifications has the potential to avert progression to advanced fibrosis—and its associated increased overall and liver-related mortality, and impaired QOL.3,16,18 Although FIB-4 is a validated predictor of advanced fibrosis, this score is not yet used nationally to identify and risk stratify NAFLD in the VHA. Additionally, the very low use of VHA diet/exercise programs in eligible patients contributes to NAFLD progression.68 The cost-effective DPP has successfully yielded weight loss in patients with prediabetes and decreases in the incidence of T2DM through motivational interviewing and intensive lifestyle intervention.55
To improve NAFLD management, providers can successfully engage patients through motivational interviewing for intensive lifestyle intervention. Their resulting weight loss is enhanced with a personalized action plan, daily weighing, and peer support. When NAFLD is identified in patients with metabolic risk factors, the probability of advanced fibrosis is easily assessed in those with elevated FIB-4 scores who merit gastrointestinal referral.33,37
In all those identified with NAFLD, disease information should be provided to patients and their families. Intensive lifestyle modification targeting a ≥ 7% weight loss is recommended; motivational interviewing can increase commitment to change and yield a customized action plan for sustained weight loss. Working with the support and encouragement of their team of primary care providers, dieticians, and MOVE! coaches, patients can actively engage to improve their NAFLD and overall health.
1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263-2273.
2. Kanwal F, Kramer JR, Duan Z, et al. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
3. Golabi P, Otgonsuren M, Cable R, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14(1):18.
4. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
5. Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511-531.
6. Centers for Disease Control and Prevention. About Prediabetes & Type 2 Diabetes. https://www.cdc.gov/diabetes/prevention/prediabetes-type2/index.html. Updated June 11, 2018. Accessed November 7, 2018.
7. Littman AJ, Jacobson IG, Boyko EJ, Powell TM, Smith TC; Millennium Cohort Study Team. Weight change following US military service. Int J Obes (Lond). 2013;37(2):244-253.
8. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
9. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-854.
10. Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care. 2015;38(7):1347-1355.
11. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
12. Bril F, Barb D, Portillo‐Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132-1144.
13. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063-2072.
14. Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;27(2):199-210.
15. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-654.
16. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
17. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
18. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
19. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans, 2001-2013. Gastroenterology 2015;149(6):1471-1482.
20. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
21. Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4(1):e000139.
22. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255-266.
23. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
24. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
25. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808-1817.
26. Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388.
27. Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321.
28. Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60(2):565-567.
29. Tapper EB, Sengupta N, Hunink MG, Afdhal NH, Lai M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am J Gastroenterol. 2015;110(9):1298-1304.
30. Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non‐invasive diagnosis of advanced fibrosis in biopsy‐proven non‐alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41(12):1271-1280.
31. Tapper EB, Lok AS-F. Use of liver imaging and biopsy in clinical practice. N Engl J Med . 2017;377(8):756-768.
32. Sterling RK, Lissen E, Clumeck N; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-1325.
33. Imler T. Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/fibrosis-4-score. Published 2018. Accessed November 7, 2018.
34. Sun W, Cui H , Li N, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46(9):862-870.
35. Imler T, Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/nafld-fibrosis-score. Published 2018. Accessed November 7, 2018.
36. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441-1447.
37. Patel YA, Gifford EJ, Glass LM, et al. Identifying non-alcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9): 2259-2266.
38. Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234-240.
39. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413-1419.
40. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-129.
41. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
42. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab 2015;100(6):2231-2238.
43. Rodriguez V, Andrade AD, Garcia-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
44. Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56(2):320-325.
45. Veterans Health Administration. Non-alcoholic fatty liver: information for patients. https://www.hepatitis.va.gov/pdf/NAFL.pdf. Published September 2017. Accessed November 7, 2018.
46. Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
47. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. Guilford Press: NY, New York; 2013.
48. Leventhal H, Leventhal EA, Breland JY. Cognitive science speaks to the “common sense” of chronic illness management. Ann Behav Med. 2011;41(2):152-163.
49. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring). 2015;23(2):256-265.
50. Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters; daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet. 2015;115(4):511-518.
51. Charania MR, Marshall KJ, Lyles CM; HIV/AIDS Prevention Research Synthesis (PRS) Team. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of U.S.-based studies, 1996-2011. AIDS Behav. 2014;18(4):646-660.
52. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376(9755):1838-1845.
53. Dutton GR, Phillips JM, Kukkamalla M, Cherrington AL, Safford MM. Pilot study evaluating the feasibility and initial outcomes of a primary care weight loss intervention with peer coaches. Diabetes Educ. 2015:41(3):361-368.
54. Fisher EB, Coufal MM, Parada H, et al. Peer support in health care and prevention: Cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35(1):363-383.
55. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;(346):393-403.
56. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875.
57. Moin T, Ertl K, Schneider J, et al. Women veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res. 2015;17(5):e127.
58. US Department of Veterans Affairs. MOVE! Weight management program. https://www.move.va.gov/MOVE/index.asp. Updated October 5, 2018. Accessed November 7, 2018.
59. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046-1055.
60. Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143-1155.
61. Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications beforevs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792-799.
62. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445-454.
63. Brito JP, Montori VM, Davis AM; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317(6):635-636.
64. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897-901.
65. Saab S, Mallam D, Cox GA 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34(4):495-504.
66. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138-143.
67. Musso G, Gambino R, Cassader M, Pagano G. A meta‐analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79-104.
68. Patel Y, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven non-alcoholic fatty liver disease progression in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263-2273.
2. Kanwal F, Kramer JR, Duan Z, et al. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
3. Golabi P, Otgonsuren M, Cable R, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14(1):18.
4. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
5. Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511-531.
6. Centers for Disease Control and Prevention. About Prediabetes & Type 2 Diabetes. https://www.cdc.gov/diabetes/prevention/prediabetes-type2/index.html. Updated June 11, 2018. Accessed November 7, 2018.
7. Littman AJ, Jacobson IG, Boyko EJ, Powell TM, Smith TC; Millennium Cohort Study Team. Weight change following US military service. Int J Obes (Lond). 2013;37(2):244-253.
8. Breland JY, Phibbs CS, Hoggatt KJ, et al. The obesity epidemic in the Veterans Health Administration: prevalence among key populations of women and men veterans. J Gen Intern Med. 2017;32(suppl 1):11-17.
9. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-854.
10. Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care. 2015;38(7):1347-1355.
11. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357.
12. Bril F, Barb D, Portillo‐Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132-1144.
13. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063-2072.
14. Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;27(2):199-210.
15. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643-654.
16. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
17. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
18. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.
19. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans, 2001-2013. Gastroenterology 2015;149(6):1471-1482.
20. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.
21. Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4(1):e000139.
22. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255-266.
23. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-378.
24. Apovian CM, Aronne LJ, Bessesen DH, et al; Endocrine Society. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362.
25. Haw JS, Galaviz KI, Straus AN, et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808-1817.
26. Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388.
27. Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321.
28. Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60(2):565-567.
29. Tapper EB, Sengupta N, Hunink MG, Afdhal NH, Lai M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am J Gastroenterol. 2015;110(9):1298-1304.
30. Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non‐invasive diagnosis of advanced fibrosis in biopsy‐proven non‐alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41(12):1271-1280.
31. Tapper EB, Lok AS-F. Use of liver imaging and biopsy in clinical practice. N Engl J Med . 2017;377(8):756-768.
32. Sterling RK, Lissen E, Clumeck N; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-1325.
33. Imler T. Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/fibrosis-4-score. Published 2018. Accessed November 7, 2018.
34. Sun W, Cui H , Li N, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46(9):862-870.
35. Imler T, Indiana University School of Medicine - GIHep calculators. http://gihep.com/calculators/hepatology/nafld-fibrosis-score. Published 2018. Accessed November 7, 2018.
36. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441-1447.
37. Patel YA, Gifford EJ, Glass LM, et al. Identifying non-alcoholic fatty liver disease advanced fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9): 2259-2266.
38. Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234-240.
39. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413-1419.
40. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-129.
41. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292.
42. Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab 2015;100(6):2231-2238.
43. Rodriguez V, Andrade AD, Garcia-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
44. Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56(2):320-325.
45. Veterans Health Administration. Non-alcoholic fatty liver: information for patients. https://www.hepatitis.va.gov/pdf/NAFL.pdf. Published September 2017. Accessed November 7, 2018.
46. Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
47. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. Guilford Press: NY, New York; 2013.
48. Leventhal H, Leventhal EA, Breland JY. Cognitive science speaks to the “common sense” of chronic illness management. Ann Behav Med. 2011;41(2):152-163.
49. Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring). 2015;23(2):256-265.
50. Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters; daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet. 2015;115(4):511-518.
51. Charania MR, Marshall KJ, Lyles CM; HIV/AIDS Prevention Research Synthesis (PRS) Team. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of U.S.-based studies, 1996-2011. AIDS Behav. 2014;18(4):646-660.
52. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376(9755):1838-1845.
53. Dutton GR, Phillips JM, Kukkamalla M, Cherrington AL, Safford MM. Pilot study evaluating the feasibility and initial outcomes of a primary care weight loss intervention with peer coaches. Diabetes Educ. 2015:41(3):361-368.
54. Fisher EB, Coufal MM, Parada H, et al. Peer support in health care and prevention: Cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35(1):363-383.
55. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;(346):393-403.
56. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875.
57. Moin T, Ertl K, Schneider J, et al. Women veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res. 2015;17(5):e127.
58. US Department of Veterans Affairs. MOVE! Weight management program. https://www.move.va.gov/MOVE/index.asp. Updated October 5, 2018. Accessed November 7, 2018.
59. Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046-1055.
60. Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143-1155.
61. Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications beforevs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792-799.
62. The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445-454.
63. Brito JP, Montori VM, Davis AM; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. JAMA. 2017;317(6):635-636.
64. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897-901.
65. Saab S, Mallam D, Cox GA 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34(4):495-504.
66. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138-143.
67. Musso G, Gambino R, Cassader M, Pagano G. A meta‐analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79-104.
68. Patel Y, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven non-alcoholic fatty liver disease progression in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268-278.
A Veteran With Acute Progressive Encephalopathy of Unknown Etiology
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
Meeting 21st Century Public Health Needs: Public Health Partnerships at the Uniformed Services University
The Uniformed Services University of the Health Sciences (USU) was established by Congress in 1972 under the Uniformed Services Health Professions Revitalization Act. The only medical school administered by the federal government, “America’s Medical School” as it is affectionately known, has a mission to educate, train, and comprehensively prepare uniformed services health professionals to support the US military and public health system.
The USU School of Medicine (SOM) matriculates about 170 students each year. Although the majority of the medical students receive commissions in the US Army, Navy, or Air Force and serve as military physicians in the Department of Defense (DoD), a small number of students each year are commissioned as officers in the US Public Health Service Commissioned Corps (PHS). The PHS is a uniformed service within the US Department of Health and Human Services (HHS) whose officers serve nationwide in more than 30 government agencies. However, unlike its sister DoD services, the PHS does not participate in the Health Professions Scholarship Program, so admission to USU represents the only direct accession to the PHS Commissioned Corps for prospective physicians.
Beginning with the first graduating class, more than 160 PHS physician officers have now been trained under agreements with PHS agencies and SOM, and numerous others have received training and experience from the other academic programs and research centers within USU. Ten of those graduates achieved the rank of Rear Admiral, the general officer or “flag” position of the PHS.
The benefits of the partnerships between USU, PHS, and the agencies served by PHS to public health outcomes are many. Specifically, investment in PHS students at the SOM has served to ease disparities experienced by American Indians and Alaskan Natives (AI/AN), combat the shortage of primary care physicians (PCPs), generate exceptional clinical researchers, and train health care professionals to be prepared and ready to respond to emerging threats to public health.
Addressing Health Care Disparities Experienced by AI/AN
Through numerous treaties, laws, court cases, and Executive Orders—and most recently reaffirmed by the reauthorization of the Indian Health Care Improvement Act as part of the Patient Protection and Affordable Care Act (2010)–the US federal government holds responsibility for the provision of medical services to AI/AN. The Indian Health Service (IHS) is the principal federal provider of health care services for the AI/AN population. The mission of the IHS is to raise the physical, mental, social, and spiritual health of the AI/AN population to the highest level. It seeks to accomplish this mission by assuring that comprehensive, culturally acceptable personal and public health services are available and accessible to all AI/AN people.
Agency partnerships at USU, like the one between the school and IHS, sponsor medical students to become PHS physicians who can combat health disparities, especially those experienced by AI/AN. AI/AN continue to be subjected to disparities in health status across a wide array of chronic conditions, with significantly higher mortality rates than those of white populations.1 These trends are driven by multifactorial etiologies, including social determinants of health,2 obesity and the metabolic syndrome,3 high rates of tobacco and alcohol use,4 and limited access to medical care.5
Recruitment and retention of health care providers (HCPs) has long been a challenge for the IHS.6 Despite many attractive factors, providing care in a setting of otherwise limited resources and the relative remoteness of most facilities may prove to be deterring factors to prospective applicants. Furthermore, promotion of quality providers to administrative roles and high turnover rates of contractors or temporary staff contribute to poor continuity of care in certain locations. Consequently, efforts are under way to increase provider retention and continuity of care for patients.
This effort is augmented by training officers for a career of service to the IHS within the PHS. After completion of medical school and a residency in primary care, IHS-sponsored graduates from USU serve as officers in the PHS, stationed at an IHS-designated high-priority site for 10 years.7 However, many stay with the IHS for much longer, like IHS Chief Medical Officer, RADM Michael Toedt (USU 1995). In fact, nearly all the officers commissioned in the past 20 years are still on active duty. Within the IHS, physicians focus on community-oriented practice and improving the health of small-town and rural residents at tribal or federally operated clinics and community hospitals. In addition to performing clinical duties, graduates frequently become leaders within the IHS, advocating for systemwide improvements, performing practice-based research, and improving the overall well-being of AI/AN communities.
Combating the PCP Shortage
It has been well documented that primary care is essential for the prevention and control of chronic disease.8 However, fewer US medical school graduates are choosing to practice in primary care specialties, and the number of PCPs is forecasted to be insufficient for the needs of the American population in the coming years.9,10 This deficit is predicted to be especially pronounced in rural and underserved communities.11
Training PHS officers at the USU can fill this growing need by cultivating PCPs committed to a career of service in areas of high need. PHS medical students who are sponsored to attend USU by the IHS select from 1 of 7 approved primary care residencies: emergency medicine, family medicine, general pediatrics, general internal medicine, general psychiatry, obstetrics/gynecology, and general surgery.7 PHS students are permitted to train at military or civilian graduate medical education programs; permission to pursue combination programs is granted on a case-by-case basis, with consideration for the needs of the agency. Previously, such authorizations have included internal medicine/pediatrics, internal medicine/psychiatry, and family medicine/preventive medicine. This requirement, understood at the time of matriculation, selects for students who are passionate about primary care and are willing to live and practice in rural, underserved areas during their 10-year service commitment to the agency.
During medical school, USU students participate in numerous training activities that prepare doctors for practice in isolated or resource-poor settings, including point-of-care ultrasonography and field exercises in stabilization and transport of critically ill patients. The motto of the SOM, “Learning to Care for Those in Harm’s Way,” thereby applies not only to battlefield medicine, but to those who practice medicine in austere environments of all kinds.
Generating Clinical Researchers
Although IHS currently funds most PHS students, sponsorship also is available through the National Institute of Allergy and Infectious Diseases (NIAID), one of the institutes of the National Institutes of Health (NIH) in Bethesda, Maryland. Students selected for this competitive program complete a residency in either internal medicine or pediatrics, then complete an NIH-sponsored fellowship in either infectious diseases or allergy and immunology. Similar to their IHS counterparts, they incur a debt of service—10 years in the PHS Commissioned Corps; however, their service obligation is served at NIH. This track supports the creation of the next generation of clinical researchers and physician-scientists, critical in this time of ever-increasing threats to public health and national security, like emerging infectious diseases and bioterrorism.
Emergency Response Preparations
Combined training with experts from DoD and HHS prepares junior medical officers to serve as leaders in responding to large-scale emergencies and disasters. According to a memorandum of December 11, 1981, then Surgeon General C. Everett Koop described the importance of this skill set, saying that USU students are “ready for instant mobilization to meet military [needs] and [respond to] national disasters.” He continued, “Students are taught the necessary leadership and management skills to command medical units and organizations in the delivery of health services...They are exposed to the problems of dealing with national medical emergencies such as floods, earthquakes, and mass immigrations to this country.”12 Fittingly, physician graduates of USU have recently led disaster response efforts for Hurricanes Harvey, Irma, and Maria and Typhoon Yutu.
Traditional medical school didactic coursework is supplemented by lectures on disaster response, emergency preparedness, and global health engagement. As training progresses, students translate their knowledge into action with practical fieldwork exercises in mass casualty triage, erection of field hospitals using preventive medicine principles, and containment of infectious disease outbreaks among displaced persons—under the close observation and guidance of military and public health subject matter experts from across the country. Medical students complete their clinical training at military treatment facilities around the country and have elective clerkship opportunities in operational medicine nationally and internationally. PHS graduates of USU are well prepared to interface with their military colleagues, building effective joint mission capacity.
Additional Training Opportunities
In addition to the 4-year, tuition-free MD program, the university offers 7 graduate degree programs in public health and residency programs in preventive medicine specialty areas. Continuing education opportunities and graduate certificates are available in global health, tropical medicine and hygiene, travelers’ health, international and domestic disaster response, and other fields of interest to any public health professional, military or civilian. Many programs are available to federal or uniformed service members at no cost, some incur a degree of service commitment. Furthermore, the university is home to multiple research centers, including the National Center for Disaster Medicine and Public Health, which strive to improve public health through research efforts and education.
Conclusion
Though the emerging public health needs of the nation are both varied and daunting, the USU/PHS partnership trains providers that will heed the call and face the modern public health needs head-on. USU remains an important source for commissioning PHS physicians and producing career officers. The unique training provided at USU educates and enables PHS physicians to ease disparities experienced by AI/AN, combat the shortage of PCPs, generate exceptional clinical researchers, and be prepared and ready to respond to emerging threats to public health.
1. Espey DK, Jim MA, Cobb N, et al. Leading causes of death and all-cause mortality in American Indians and Alaska natives. Am J Public Health . 2014;104(S3):S303-S311.
2. Kunitz SJ, Veazie M, Henderson JA. Historical trends and regional differences in all-cause and amenable mortality among American Indians and Alaska Natives since 1950. Am J Public Health. 2014;104(6)(suppl 3):S268-S277.
3. Sinclair KA, Bogart A, Buchwald D, Henderson JA. The prevalence of metabolic syndrome and associated risk factors in Northern Plains and Southwest American Indians. Diabetes Care. 2011;34(1):118-120.
4. Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(6)(suppl 3):S481-S489.
5. Warne D, Frizzell LB. American Indian health policy: historical trends and contemporary issues. Am J Public Health. 2014;104(6)(suppl 3):S263-S267.
6. Noren J, Kindig D, Sprenger A. Challenges to Native American health care. Public Health Rep. 1998;113(1):22-23.
7. Indian Health Services. Follow Your Path: The Uniformed Services University of the Health Sciences Participant Program Guide. https://www.ihs.gov/careeropps/includes/themes/responsive2017/display_objects/documents/USUHS-IHS-Participant-Program-Guide.pdf. Published October 2015. Accessed August 16, 2018.
8. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q . 2005;83(3):457-502.
9. Health Resources and Services Administration. Projecting the supply and demand for primary care practitioners through 2020. https://bhw.hrsa.gov/health-workforce-analysis/primary-care-2020. Accessed December 14, 2018.
10. Dill MJ, Salsberg ES. The complexities of physician supply and demand: projections through 2025. https://members.aamc.org/eweb/upload/The%20Complexities%20of%20Physician%20Supply.pdf. Published November 2008. Accessed December 14, 2018.
11. Wilson N, Couper I, De Vries E, Reid S, Fish T, Marais B. A critical review of interventions to redress the inequitable distribution of healthcare professionals to rural and remote areas. Rural Remote Health . 2009;9(2):1060.
12. Department of Health and Human Services. Memorandum. Continued PHS Participation at USUHS. https://profiles.nlm.nih.gov/ps/access/QQBBZV.pdf. Published December 11, 1981. Accessed December 14, 2018.
The Uniformed Services University of the Health Sciences (USU) was established by Congress in 1972 under the Uniformed Services Health Professions Revitalization Act. The only medical school administered by the federal government, “America’s Medical School” as it is affectionately known, has a mission to educate, train, and comprehensively prepare uniformed services health professionals to support the US military and public health system.
The USU School of Medicine (SOM) matriculates about 170 students each year. Although the majority of the medical students receive commissions in the US Army, Navy, or Air Force and serve as military physicians in the Department of Defense (DoD), a small number of students each year are commissioned as officers in the US Public Health Service Commissioned Corps (PHS). The PHS is a uniformed service within the US Department of Health and Human Services (HHS) whose officers serve nationwide in more than 30 government agencies. However, unlike its sister DoD services, the PHS does not participate in the Health Professions Scholarship Program, so admission to USU represents the only direct accession to the PHS Commissioned Corps for prospective physicians.
Beginning with the first graduating class, more than 160 PHS physician officers have now been trained under agreements with PHS agencies and SOM, and numerous others have received training and experience from the other academic programs and research centers within USU. Ten of those graduates achieved the rank of Rear Admiral, the general officer or “flag” position of the PHS.
The benefits of the partnerships between USU, PHS, and the agencies served by PHS to public health outcomes are many. Specifically, investment in PHS students at the SOM has served to ease disparities experienced by American Indians and Alaskan Natives (AI/AN), combat the shortage of primary care physicians (PCPs), generate exceptional clinical researchers, and train health care professionals to be prepared and ready to respond to emerging threats to public health.
Addressing Health Care Disparities Experienced by AI/AN
Through numerous treaties, laws, court cases, and Executive Orders—and most recently reaffirmed by the reauthorization of the Indian Health Care Improvement Act as part of the Patient Protection and Affordable Care Act (2010)–the US federal government holds responsibility for the provision of medical services to AI/AN. The Indian Health Service (IHS) is the principal federal provider of health care services for the AI/AN population. The mission of the IHS is to raise the physical, mental, social, and spiritual health of the AI/AN population to the highest level. It seeks to accomplish this mission by assuring that comprehensive, culturally acceptable personal and public health services are available and accessible to all AI/AN people.
Agency partnerships at USU, like the one between the school and IHS, sponsor medical students to become PHS physicians who can combat health disparities, especially those experienced by AI/AN. AI/AN continue to be subjected to disparities in health status across a wide array of chronic conditions, with significantly higher mortality rates than those of white populations.1 These trends are driven by multifactorial etiologies, including social determinants of health,2 obesity and the metabolic syndrome,3 high rates of tobacco and alcohol use,4 and limited access to medical care.5
Recruitment and retention of health care providers (HCPs) has long been a challenge for the IHS.6 Despite many attractive factors, providing care in a setting of otherwise limited resources and the relative remoteness of most facilities may prove to be deterring factors to prospective applicants. Furthermore, promotion of quality providers to administrative roles and high turnover rates of contractors or temporary staff contribute to poor continuity of care in certain locations. Consequently, efforts are under way to increase provider retention and continuity of care for patients.
This effort is augmented by training officers for a career of service to the IHS within the PHS. After completion of medical school and a residency in primary care, IHS-sponsored graduates from USU serve as officers in the PHS, stationed at an IHS-designated high-priority site for 10 years.7 However, many stay with the IHS for much longer, like IHS Chief Medical Officer, RADM Michael Toedt (USU 1995). In fact, nearly all the officers commissioned in the past 20 years are still on active duty. Within the IHS, physicians focus on community-oriented practice and improving the health of small-town and rural residents at tribal or federally operated clinics and community hospitals. In addition to performing clinical duties, graduates frequently become leaders within the IHS, advocating for systemwide improvements, performing practice-based research, and improving the overall well-being of AI/AN communities.
Combating the PCP Shortage
It has been well documented that primary care is essential for the prevention and control of chronic disease.8 However, fewer US medical school graduates are choosing to practice in primary care specialties, and the number of PCPs is forecasted to be insufficient for the needs of the American population in the coming years.9,10 This deficit is predicted to be especially pronounced in rural and underserved communities.11
Training PHS officers at the USU can fill this growing need by cultivating PCPs committed to a career of service in areas of high need. PHS medical students who are sponsored to attend USU by the IHS select from 1 of 7 approved primary care residencies: emergency medicine, family medicine, general pediatrics, general internal medicine, general psychiatry, obstetrics/gynecology, and general surgery.7 PHS students are permitted to train at military or civilian graduate medical education programs; permission to pursue combination programs is granted on a case-by-case basis, with consideration for the needs of the agency. Previously, such authorizations have included internal medicine/pediatrics, internal medicine/psychiatry, and family medicine/preventive medicine. This requirement, understood at the time of matriculation, selects for students who are passionate about primary care and are willing to live and practice in rural, underserved areas during their 10-year service commitment to the agency.
During medical school, USU students participate in numerous training activities that prepare doctors for practice in isolated or resource-poor settings, including point-of-care ultrasonography and field exercises in stabilization and transport of critically ill patients. The motto of the SOM, “Learning to Care for Those in Harm’s Way,” thereby applies not only to battlefield medicine, but to those who practice medicine in austere environments of all kinds.
Generating Clinical Researchers
Although IHS currently funds most PHS students, sponsorship also is available through the National Institute of Allergy and Infectious Diseases (NIAID), one of the institutes of the National Institutes of Health (NIH) in Bethesda, Maryland. Students selected for this competitive program complete a residency in either internal medicine or pediatrics, then complete an NIH-sponsored fellowship in either infectious diseases or allergy and immunology. Similar to their IHS counterparts, they incur a debt of service—10 years in the PHS Commissioned Corps; however, their service obligation is served at NIH. This track supports the creation of the next generation of clinical researchers and physician-scientists, critical in this time of ever-increasing threats to public health and national security, like emerging infectious diseases and bioterrorism.
Emergency Response Preparations
Combined training with experts from DoD and HHS prepares junior medical officers to serve as leaders in responding to large-scale emergencies and disasters. According to a memorandum of December 11, 1981, then Surgeon General C. Everett Koop described the importance of this skill set, saying that USU students are “ready for instant mobilization to meet military [needs] and [respond to] national disasters.” He continued, “Students are taught the necessary leadership and management skills to command medical units and organizations in the delivery of health services...They are exposed to the problems of dealing with national medical emergencies such as floods, earthquakes, and mass immigrations to this country.”12 Fittingly, physician graduates of USU have recently led disaster response efforts for Hurricanes Harvey, Irma, and Maria and Typhoon Yutu.
Traditional medical school didactic coursework is supplemented by lectures on disaster response, emergency preparedness, and global health engagement. As training progresses, students translate their knowledge into action with practical fieldwork exercises in mass casualty triage, erection of field hospitals using preventive medicine principles, and containment of infectious disease outbreaks among displaced persons—under the close observation and guidance of military and public health subject matter experts from across the country. Medical students complete their clinical training at military treatment facilities around the country and have elective clerkship opportunities in operational medicine nationally and internationally. PHS graduates of USU are well prepared to interface with their military colleagues, building effective joint mission capacity.
Additional Training Opportunities
In addition to the 4-year, tuition-free MD program, the university offers 7 graduate degree programs in public health and residency programs in preventive medicine specialty areas. Continuing education opportunities and graduate certificates are available in global health, tropical medicine and hygiene, travelers’ health, international and domestic disaster response, and other fields of interest to any public health professional, military or civilian. Many programs are available to federal or uniformed service members at no cost, some incur a degree of service commitment. Furthermore, the university is home to multiple research centers, including the National Center for Disaster Medicine and Public Health, which strive to improve public health through research efforts and education.
Conclusion
Though the emerging public health needs of the nation are both varied and daunting, the USU/PHS partnership trains providers that will heed the call and face the modern public health needs head-on. USU remains an important source for commissioning PHS physicians and producing career officers. The unique training provided at USU educates and enables PHS physicians to ease disparities experienced by AI/AN, combat the shortage of PCPs, generate exceptional clinical researchers, and be prepared and ready to respond to emerging threats to public health.
The Uniformed Services University of the Health Sciences (USU) was established by Congress in 1972 under the Uniformed Services Health Professions Revitalization Act. The only medical school administered by the federal government, “America’s Medical School” as it is affectionately known, has a mission to educate, train, and comprehensively prepare uniformed services health professionals to support the US military and public health system.
The USU School of Medicine (SOM) matriculates about 170 students each year. Although the majority of the medical students receive commissions in the US Army, Navy, or Air Force and serve as military physicians in the Department of Defense (DoD), a small number of students each year are commissioned as officers in the US Public Health Service Commissioned Corps (PHS). The PHS is a uniformed service within the US Department of Health and Human Services (HHS) whose officers serve nationwide in more than 30 government agencies. However, unlike its sister DoD services, the PHS does not participate in the Health Professions Scholarship Program, so admission to USU represents the only direct accession to the PHS Commissioned Corps for prospective physicians.
Beginning with the first graduating class, more than 160 PHS physician officers have now been trained under agreements with PHS agencies and SOM, and numerous others have received training and experience from the other academic programs and research centers within USU. Ten of those graduates achieved the rank of Rear Admiral, the general officer or “flag” position of the PHS.
The benefits of the partnerships between USU, PHS, and the agencies served by PHS to public health outcomes are many. Specifically, investment in PHS students at the SOM has served to ease disparities experienced by American Indians and Alaskan Natives (AI/AN), combat the shortage of primary care physicians (PCPs), generate exceptional clinical researchers, and train health care professionals to be prepared and ready to respond to emerging threats to public health.
Addressing Health Care Disparities Experienced by AI/AN
Through numerous treaties, laws, court cases, and Executive Orders—and most recently reaffirmed by the reauthorization of the Indian Health Care Improvement Act as part of the Patient Protection and Affordable Care Act (2010)–the US federal government holds responsibility for the provision of medical services to AI/AN. The Indian Health Service (IHS) is the principal federal provider of health care services for the AI/AN population. The mission of the IHS is to raise the physical, mental, social, and spiritual health of the AI/AN population to the highest level. It seeks to accomplish this mission by assuring that comprehensive, culturally acceptable personal and public health services are available and accessible to all AI/AN people.
Agency partnerships at USU, like the one between the school and IHS, sponsor medical students to become PHS physicians who can combat health disparities, especially those experienced by AI/AN. AI/AN continue to be subjected to disparities in health status across a wide array of chronic conditions, with significantly higher mortality rates than those of white populations.1 These trends are driven by multifactorial etiologies, including social determinants of health,2 obesity and the metabolic syndrome,3 high rates of tobacco and alcohol use,4 and limited access to medical care.5
Recruitment and retention of health care providers (HCPs) has long been a challenge for the IHS.6 Despite many attractive factors, providing care in a setting of otherwise limited resources and the relative remoteness of most facilities may prove to be deterring factors to prospective applicants. Furthermore, promotion of quality providers to administrative roles and high turnover rates of contractors or temporary staff contribute to poor continuity of care in certain locations. Consequently, efforts are under way to increase provider retention and continuity of care for patients.
This effort is augmented by training officers for a career of service to the IHS within the PHS. After completion of medical school and a residency in primary care, IHS-sponsored graduates from USU serve as officers in the PHS, stationed at an IHS-designated high-priority site for 10 years.7 However, many stay with the IHS for much longer, like IHS Chief Medical Officer, RADM Michael Toedt (USU 1995). In fact, nearly all the officers commissioned in the past 20 years are still on active duty. Within the IHS, physicians focus on community-oriented practice and improving the health of small-town and rural residents at tribal or federally operated clinics and community hospitals. In addition to performing clinical duties, graduates frequently become leaders within the IHS, advocating for systemwide improvements, performing practice-based research, and improving the overall well-being of AI/AN communities.
Combating the PCP Shortage
It has been well documented that primary care is essential for the prevention and control of chronic disease.8 However, fewer US medical school graduates are choosing to practice in primary care specialties, and the number of PCPs is forecasted to be insufficient for the needs of the American population in the coming years.9,10 This deficit is predicted to be especially pronounced in rural and underserved communities.11
Training PHS officers at the USU can fill this growing need by cultivating PCPs committed to a career of service in areas of high need. PHS medical students who are sponsored to attend USU by the IHS select from 1 of 7 approved primary care residencies: emergency medicine, family medicine, general pediatrics, general internal medicine, general psychiatry, obstetrics/gynecology, and general surgery.7 PHS students are permitted to train at military or civilian graduate medical education programs; permission to pursue combination programs is granted on a case-by-case basis, with consideration for the needs of the agency. Previously, such authorizations have included internal medicine/pediatrics, internal medicine/psychiatry, and family medicine/preventive medicine. This requirement, understood at the time of matriculation, selects for students who are passionate about primary care and are willing to live and practice in rural, underserved areas during their 10-year service commitment to the agency.
During medical school, USU students participate in numerous training activities that prepare doctors for practice in isolated or resource-poor settings, including point-of-care ultrasonography and field exercises in stabilization and transport of critically ill patients. The motto of the SOM, “Learning to Care for Those in Harm’s Way,” thereby applies not only to battlefield medicine, but to those who practice medicine in austere environments of all kinds.
Generating Clinical Researchers
Although IHS currently funds most PHS students, sponsorship also is available through the National Institute of Allergy and Infectious Diseases (NIAID), one of the institutes of the National Institutes of Health (NIH) in Bethesda, Maryland. Students selected for this competitive program complete a residency in either internal medicine or pediatrics, then complete an NIH-sponsored fellowship in either infectious diseases or allergy and immunology. Similar to their IHS counterparts, they incur a debt of service—10 years in the PHS Commissioned Corps; however, their service obligation is served at NIH. This track supports the creation of the next generation of clinical researchers and physician-scientists, critical in this time of ever-increasing threats to public health and national security, like emerging infectious diseases and bioterrorism.
Emergency Response Preparations
Combined training with experts from DoD and HHS prepares junior medical officers to serve as leaders in responding to large-scale emergencies and disasters. According to a memorandum of December 11, 1981, then Surgeon General C. Everett Koop described the importance of this skill set, saying that USU students are “ready for instant mobilization to meet military [needs] and [respond to] national disasters.” He continued, “Students are taught the necessary leadership and management skills to command medical units and organizations in the delivery of health services...They are exposed to the problems of dealing with national medical emergencies such as floods, earthquakes, and mass immigrations to this country.”12 Fittingly, physician graduates of USU have recently led disaster response efforts for Hurricanes Harvey, Irma, and Maria and Typhoon Yutu.
Traditional medical school didactic coursework is supplemented by lectures on disaster response, emergency preparedness, and global health engagement. As training progresses, students translate their knowledge into action with practical fieldwork exercises in mass casualty triage, erection of field hospitals using preventive medicine principles, and containment of infectious disease outbreaks among displaced persons—under the close observation and guidance of military and public health subject matter experts from across the country. Medical students complete their clinical training at military treatment facilities around the country and have elective clerkship opportunities in operational medicine nationally and internationally. PHS graduates of USU are well prepared to interface with their military colleagues, building effective joint mission capacity.
Additional Training Opportunities
In addition to the 4-year, tuition-free MD program, the university offers 7 graduate degree programs in public health and residency programs in preventive medicine specialty areas. Continuing education opportunities and graduate certificates are available in global health, tropical medicine and hygiene, travelers’ health, international and domestic disaster response, and other fields of interest to any public health professional, military or civilian. Many programs are available to federal or uniformed service members at no cost, some incur a degree of service commitment. Furthermore, the university is home to multiple research centers, including the National Center for Disaster Medicine and Public Health, which strive to improve public health through research efforts and education.
Conclusion
Though the emerging public health needs of the nation are both varied and daunting, the USU/PHS partnership trains providers that will heed the call and face the modern public health needs head-on. USU remains an important source for commissioning PHS physicians and producing career officers. The unique training provided at USU educates and enables PHS physicians to ease disparities experienced by AI/AN, combat the shortage of PCPs, generate exceptional clinical researchers, and be prepared and ready to respond to emerging threats to public health.
1. Espey DK, Jim MA, Cobb N, et al. Leading causes of death and all-cause mortality in American Indians and Alaska natives. Am J Public Health . 2014;104(S3):S303-S311.
2. Kunitz SJ, Veazie M, Henderson JA. Historical trends and regional differences in all-cause and amenable mortality among American Indians and Alaska Natives since 1950. Am J Public Health. 2014;104(6)(suppl 3):S268-S277.
3. Sinclair KA, Bogart A, Buchwald D, Henderson JA. The prevalence of metabolic syndrome and associated risk factors in Northern Plains and Southwest American Indians. Diabetes Care. 2011;34(1):118-120.
4. Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(6)(suppl 3):S481-S489.
5. Warne D, Frizzell LB. American Indian health policy: historical trends and contemporary issues. Am J Public Health. 2014;104(6)(suppl 3):S263-S267.
6. Noren J, Kindig D, Sprenger A. Challenges to Native American health care. Public Health Rep. 1998;113(1):22-23.
7. Indian Health Services. Follow Your Path: The Uniformed Services University of the Health Sciences Participant Program Guide. https://www.ihs.gov/careeropps/includes/themes/responsive2017/display_objects/documents/USUHS-IHS-Participant-Program-Guide.pdf. Published October 2015. Accessed August 16, 2018.
8. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q . 2005;83(3):457-502.
9. Health Resources and Services Administration. Projecting the supply and demand for primary care practitioners through 2020. https://bhw.hrsa.gov/health-workforce-analysis/primary-care-2020. Accessed December 14, 2018.
10. Dill MJ, Salsberg ES. The complexities of physician supply and demand: projections through 2025. https://members.aamc.org/eweb/upload/The%20Complexities%20of%20Physician%20Supply.pdf. Published November 2008. Accessed December 14, 2018.
11. Wilson N, Couper I, De Vries E, Reid S, Fish T, Marais B. A critical review of interventions to redress the inequitable distribution of healthcare professionals to rural and remote areas. Rural Remote Health . 2009;9(2):1060.
12. Department of Health and Human Services. Memorandum. Continued PHS Participation at USUHS. https://profiles.nlm.nih.gov/ps/access/QQBBZV.pdf. Published December 11, 1981. Accessed December 14, 2018.
1. Espey DK, Jim MA, Cobb N, et al. Leading causes of death and all-cause mortality in American Indians and Alaska natives. Am J Public Health . 2014;104(S3):S303-S311.
2. Kunitz SJ, Veazie M, Henderson JA. Historical trends and regional differences in all-cause and amenable mortality among American Indians and Alaska Natives since 1950. Am J Public Health. 2014;104(6)(suppl 3):S268-S277.
3. Sinclair KA, Bogart A, Buchwald D, Henderson JA. The prevalence of metabolic syndrome and associated risk factors in Northern Plains and Southwest American Indians. Diabetes Care. 2011;34(1):118-120.
4. Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(6)(suppl 3):S481-S489.
5. Warne D, Frizzell LB. American Indian health policy: historical trends and contemporary issues. Am J Public Health. 2014;104(6)(suppl 3):S263-S267.
6. Noren J, Kindig D, Sprenger A. Challenges to Native American health care. Public Health Rep. 1998;113(1):22-23.
7. Indian Health Services. Follow Your Path: The Uniformed Services University of the Health Sciences Participant Program Guide. https://www.ihs.gov/careeropps/includes/themes/responsive2017/display_objects/documents/USUHS-IHS-Participant-Program-Guide.pdf. Published October 2015. Accessed August 16, 2018.
8. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q . 2005;83(3):457-502.
9. Health Resources and Services Administration. Projecting the supply and demand for primary care practitioners through 2020. https://bhw.hrsa.gov/health-workforce-analysis/primary-care-2020. Accessed December 14, 2018.
10. Dill MJ, Salsberg ES. The complexities of physician supply and demand: projections through 2025. https://members.aamc.org/eweb/upload/The%20Complexities%20of%20Physician%20Supply.pdf. Published November 2008. Accessed December 14, 2018.
11. Wilson N, Couper I, De Vries E, Reid S, Fish T, Marais B. A critical review of interventions to redress the inequitable distribution of healthcare professionals to rural and remote areas. Rural Remote Health . 2009;9(2):1060.
12. Department of Health and Human Services. Memorandum. Continued PHS Participation at USUHS. https://profiles.nlm.nih.gov/ps/access/QQBBZV.pdf. Published December 11, 1981. Accessed December 14, 2018.
Female Veterans’ Experiences With VHA Treatment for Military Sexual Trauma
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
Females are the fastest growing population to seek care at the Veterans Health Administration (VHA).1 Based on a 2014 study examining prevalence of military sexual trauma (MST), it is estimated that about one-third of females in the military screen positive for MST, and the rates are higher for younger veterans.2 Military sexual trauma includes both rape and any sexual activity that occurred without consent; offensive sexual remarks or advances can also represent MST. The issue of MST, therefore, is an important one to address adequately, especially for female veterans who are screened through the VHA system.
Since 1992, the VHA has been required to provide services for MST, defined as “sexual harassment that is threatening in character or physical assault of a sexual nature that occurred while the victim was in the military.”3 Despite this mandate, it has taken many years for all VHA hospitals to adopt recommended screening tools to identify survivors of MST and give them proper resources. Only half of VHA hospitals adopted screening 6 years after the policy change.4 In addition, the environment in which the survivors receive MST care may trigger posttraumatic stress symptoms as many of the other patients seeking care at the VHA hospital resemble the perpetrators.5 Thus, up to half of females who report a history of MST do not receive care for their MST through the VHA.6
Having a history of MST significantly increases the risks of developing mental health disorders, including posttraumatic stress disorder (PTSD), major depressive disorder, generalized anxiety disorder, and suicidal ideation.2 This group also has overall decreased quality of life (QOL). Female veterans have increased sexual dysfunction and dissatisfaction, which is heightened with a history of MST.7 Addressing MST requires treatment of all aspects of life affected by MST, such as mental health, sexual function, and QOL. The quality of treatment for MST through VHA hospitals deserves attention and likely still requires improvement with better incorporation of the patient’s perspective.
Qualitative research allows for incorporation of the patient’s perspective and is useful for exploring new ideas and themes.8 Current qualitative research using individual interviews of MST survivors focuses more on mental health treatment modalities through the VHA system and how resources are used within the system.9,10 While it is important to understand the quantity of these resources, their quality also should be explored. Research has identified unique gender-specific concerns such as female-only mental health groups.10 However, there has been less focus on how to improve current therapies and the treatment modalities (regardless of whether it is a community service or at the VHA system) females find most helpful. There is a gap in understanding the patient’s perspective and assessment of current MST treatments as well as the unmet needs both within and outside of the VHA system. Therefore, the purpose of this study is 2-fold: (1) examine the utilization of VHA services for MST, as well as outside services, through focusgroup sessions; and (2) to offer specific recommendations for improving MST treatment for female veterans from the patient’s perspective.
Methods
After obtaining institutional review board approval (16-H192), females who screened positive for a history of MST, using the validated MST screening questionnaire, were recruited from the Women’s Continuity Clinic, Urology clinic, and via a research flyer placed within key locations at the New Mexico Veterans Affairs (VA) Health Care System (NMVAHCS).11 Inclusion criteria were veterans aged > 18 years who could speak and understand English. Those who agreed to participate attended any 1 of 5 focus groups. Prior to initiation of the focus groups, the investigators generated a focus-group script, including specific questions or probes to explore treatment, unmet needs (such as other health conditions the veteran associated with MST that were not being addressed), and recommendations for care improvement.
Subjects granted consent privately prior to conduction of the focus group. Each participant completed a basic demographic (age, race, ethnicity) and clinical history (including pain conditions and therapy received for MST). These characteristics were evaluated with descriptive statistics, including means and frequencies.
The focus groups took place on the NM VAHCS Raymond G. Murphy VA Medical Center campus in a private conference room and were moderated by nonmedical research personnel experienced in focus-group moderation. Focus groups were recorded and transcribed. An iterative process was used with revisions to the script and probe questions as needed. Focus groups were planned for 2 hours but were allowed to continue at the participants’ discretion.
The de-identified transcripts were uploaded to the web-based qualitative engine Dedoose 6.2.21 software (Los Angeles, CA) and coded. Using grounded theory, the codes were grouped into themes and subsequently organized into emergent concepts.8,12 Following constant comparative methodology, ideas were compared and combined between each focus group.8,13 After completion of the focus groups, the generated ideas were organized and refined to create a conceptual framework that represented the collective ideas from the focus groups.
Results
Between January and June 2017, 5 focus groups with 17 participants were conducted; each session lasted about 3 hours. The average age was 52 ± 8.3 years, and were from a diverse racial and ethnic background. Most reported that > 20 years had passed since the first MST, and care-seeking for the first time was > 11 years after the trauma, although symptoms related to the MST most frequently began within 1 year of the trauma (Table 1).
Preliminary Themes
The Trauma
Focus-group participants noted improved therapies offered by the VA but challenges obtaining health care:
“…because I’m really trying to deal with it and just be happy and get my joy back and deal with the isolation.”
“Another way that the memories affected me was barricading myself in my own house, starting from the front door.”
Male-Dominated VA
Participants also noted that, along with screening improving the system, dedicated female staff and service connection are important:
“The Womens Clinic is nice, and it’s nice to know that I can go there and I’m not having to discuss everything with men all over the place.”
“The other thing... that would be really good for survivors of MST, is help with disability.”
While the focus-group participants found dedicated women’s clinics helpful and providing improved care, the overall VA environment remains male-dominated:
“Because it’s really hard to relax and be vulnerable and be in your body and in your emotions if there‘s a bunch of penises around. When I saw these guys on the floor I’m like, I ain’t going in there.”
This male-dominated sense also incorporated a feeling of being misunderstood by a system that has traditionally cared for male veterans:
“People don‘t understand. They think, oh, you‘re overreacting, but they don’t know what it feels like to be inside.”
“I wouldn’t say they treat you like a second citizen, but it’s like almost every appointment I go to that’s not in the Women’s Clinic, the secretaries or whatever will be like ‘Oh, are you looking for somebody, or...’
Assumption Females Are Not Veterans
“There was an older gentleman behind me, they were like ‘Are you checking him in?’ I said, ‘I’m sure he’ll check himself in, but I’m checking myself in.’”
Participants also reported that there is an assumption that you’re not a veteran when you’re female:
“All of the care should be geared to be the same. And we know we need to recognize that men have their issues, and women will have their issues. But we don’t need to just say ‘all women have this issue, throw them over there.’”
Self-Doubt
“The world doesn’t validate rape, you asked for it, it was what you were wearing, it was what you said.”
Ongoing efforts to have female-only spaces, therapy groups, and support networks were encouraged by all 5 focus groups. These themes, provided the foundation for emergent concepts regarding patients’ perceptions of their treatment for MST: (1) Improvement has been slow but measurable; (2) VA cares more about male veterans; (3) The isolation from MST is pervasive; (4) It’s hard to navigate the VA system or any health care when you’re traumatized; and (5) Sexual assault leaves lasting self-doubt that providers need to address.
Isolation
Because there are barriers to seeking care the overarching method for coping with the effects of MST was isolation.
Overcoming the isolation was essential to seeking any care. Participants reported years of living alone, avoiding social situations and contexts, and difficulty with basic tasks because of the isolation.
“That the coping skills, that the isolation is a coping skill and all these things, and that I had to do that to survive.”
Lack of family and provider support and the VHA’s perceived focus on male veterans perpetuated this sense of isolation. Additionally, feeding the isolation were other maladaptive behaviors, such as alcoholism, weight gain, and anger.
“I was always an athlete until my MST, and I still find myself drinking whisky and wanting to smoke pot. It’s not that I want to, I guess it gives me a sense of relief, because my MST made me an alcoholic.”
Participants reported that successful treatment of MST must include treatment of other maladaptive behaviors and specific provider-behavior changes.
At times, providers contribute to female MST survivors’ feeling undervalued:
“I had an hour session and she kept looking at her watch and blowing me off, and I finally said, okay, I’m done, good-bye, after 45 minutes.”
Validation
Participants’ suggestions to improve MST treatment, including goal sharing, validation, knowledge, and support:
“They should have staff awareness groups, or focus groups to teach them the same thing that the patients are receiving as far as how to handle yourself, how to interact with others. Don’t bring your sh** from home into your job. You’re an employee, don’t take it personal.” (
The need for provider-level support and validation likely stems from the sense that many females expressed that MST was their fault. As one participant said,
“It wasn’t violent for me. I froze. So that’s another reason that I feel guilty because it’s like I didn’t fight. I just froze and put up with it, so I feel like jeez it was my fault. I didn’t... Somehow I am responsible for this.”
Thus, the groups concluded that the most powerful support was provider validation:
“The most important for me was that I was told it was not my fault. Over and over and over. That is the most important thing that us females need to know. Because that is such a relief and that opened up so much more.”
At all of the focus groups, female veterans reported that physician validation of the assault was essential to healing. When providers communicated validation, the women experienced the most improvement in symptoms.
Therapies for MST
A variety of modalities was recommended as helpful in coping with symptoms associated with MST. One female noted her therapy dog allowed her to get her first Papanicolaou (Pap) smear in years:
“Pelvic exams are like the seventh circle of hell. Like, God, you’d think I was being abducted by aliens or something. Last time, up here, they let me bring my little dog, which was extraordinarily helpful for me.”
For others, more traditional therapy such as prolonged exposure therapy or cognitive behavioral therapy, was helpful.
“After my prolonged exposure therapy; it saved my life. I’m not suicidal, and the only thing that’s really, really affected is sometimes I still have to sleep with a night light. Over 80% of the symptoms that I had and the problems that I had were alleviated with the therapy.”
Other veterans noted alternative therapies as beneficial for overcoming trauma:
“Yoga has really helped me with dealing with chronic pain and letting go of things that no longer serve me, and remembering about the inhale, the exhale, there’s a pause between the exhale and an inhale, where that’s where I make my choices, my thoughts, catch it, check it, change it, challenge my thoughts, that’s really, really helped me.”
From these concepts, and the specific suggestions female veterans provided for improvement in care,
Discussion
This qualitative study of the quality of MST treatment with specific suggestions for improvement shows that the underlying force impacting health care in female survivors of MST is isolation. In turn, that isolation is perpetuated by personal beliefs, mental health, lack of support, and the VHA culture. While there was improvement in VHA care noted, female veterans offered many specific suggestions—simple ones that could be rapidly implemented—to enhance care. Many of these suggestions were targeted at provider-level behaviors such as validation, goal setting, knowledge (both about the military and about MST), and support.
Previous work showed that tangible (ie, words, being present) support rather than broad social support only generally helps reduces posttraumatic stress symptoms.15 These researchers found that tangible support moderated the relationship between number of lifetime traumas and PTSD. Schumm and colleagues also found that high social support predicted lower PTSD severity for inner-city women who experienced both child abuse and adult rape.16 A prior meta-analysis found social support was the strongest correlate of PTSD (effect size = 0.4).17
Our finding that female MST survivors desire verbal support from physicians may point to the inherent sense that validation helps healing, demonstrated by this meta-analysis. Importantly, the focus group participants did not specify the type of physician (psychiatrist, primary care provider, gynecologist, surgeon, etc) who needed to provide this support. Thus, we believe this suggestion is applicable to all physician interactions when the history of MST comes up. Physicians may be unaware of their profound impact in helping women recover from MST. This validation may also apply to survivors of other types of sexual trauma.
A second simple suggestion that arose from the focus groups was the need for broader options for MST therapy. Current data on the locations female veterans are treated for MST include specialty MST clinics, specialty PTSD clinics, psychosocial rehabilitation, and substance use disorder clinics, showing a wide range of settings.18 But female veterans are also asking for more services, including animal therapy, art therapy, yoga, and tai chi. While it may not be possible to offer every resource at every VHA facility, partnering with community services may help fulfill this veteran need.
The focus groups’ third suggestion for improvement in MST was better treatment for the health problems associated with sexual trauma, such as chronic pelvic pain, sexual dysfunction, and weight gain. It is important to note that the female veterans provided this list of associated health conditions from the broader facilitator question “What health problems do you think you have because of MST?” Females correctly identified common sequelae of sexual abuse, including pelvic pain and sexual dysfunction.14,19 Weight gain and obesity have been associated with childhood sexual trauma and abuse, but they are not well studied in MST and may be worth further exploration.20,21
Limitations
There are several inherent weaknesses in this study. The female veterans who agreed to participate in the focus group may not be representative of the entire population, particularly as survivors may be reluctant to talk about their MST experience. The participants in our focus groups were most commonly 2 decades past the MST and their experience with therapy may differ from that of women more recently traumatized and engaged in therapy. However, the fact that many of these females were still receiving some form of therapy 20 years after the traumatic event deserves attention.
Recall bias may have affected how female veterans described their experiences with MST treatment.
Strengths of the study included the inherent patient-centered approach and ability to analyze data not readily extracted from patient records or validated questionnaires. Additionally, this qualitative approach allows for the discovery of patient-driven ideas and concerns. Our focus groups also contained a majority of minority females (including Hispanic and American Indian) populations that are frequently underrepresented in research.
Conclusion
Our data show there is still substantial room for improvement in the therapies and in the physician-level care for MST. While each treatment experience was unique, the collective agreement was that multimodal therapy was beneficial. However, the isolation that often comes from MST makes accessing care and treatment challenging. A crucial component to combating this isolation is provider validation and support for the female’s experience with MST. The simple act of hearing “I believe you” from the provider can make a huge impact on continuing to seek care and overcoming the consequences of MST.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
1. Rossiter AG, Smith S. The invisible wounds of war: caring for women veterans who have experienced military sexual trauma. J Am Assoc Nurse Pract. 2014;26(7):364-369.
2. Klingensmith K, Tsai J, Mota N, et al. Military sexual trauma in US veterans: results from the national health and resilience in veterans study. J Clin Psychiatry. 2014;75(10):e1133-e1139.
3. US. Department of Veterans Affairs, Veteran Health Administration. Military sexual trauma. https://www.publichealth.va.gov/docs/vhi/military_sexual_trauma.pdf. Published January 2004. Accessed July 16, 2018.
4. Suris AM, Davis LL, Kashner TM, et al. A survey of sexual trauma treatment provided by VA medical centers. Psychiatr Serv. 1998;49(3):382-384.
5. Gilmore AK, Davis MT, Grubaugh A, et al. “Do you expect me to receive PTSD care in a setting where most of the other patients remind me of the perpetrator?”: home-based telemedicine to address barriers to care unique to military sexual trauma and veterans affairs hospitals. Contemp Clin Trials. 2016;48:59-64.
6. Calhoun PS, Schry AR, Dennis PA, et al. The association between military sexual trauma and use of VA and non-VA health care services among female veterans with military service in Iraq or Afghanistan. J Interpers Violence. 2018;33(15):2439-2464.
7. Rosebrock L, Carroll R. Sexual function in female veterans: a review. J Sex Marital Ther. 2017;43(3):228-245.
8. Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Glaser_1967.pdf. Published 1999. Accessed July 16, 2018.
9. Kelly MM, Vogt DS, Scheiderer EM, et al. Effects of military trauma exposure on women veterans’ use and perceptions of Veterans Health Administration care. J Gen Intern Med. 2008;23(6):741-747.
10. Kehle-Forbes SM, Harwood EM, Spoont MR, et al. Experiences with VHA care: a qualitative study of U.S. women veterans with self-reported trauma histories. BMC Women Health. 2017;17(1):38.
11. McIntyre LM, Butterfield MI, Nanda K. Validation of trauma questionnaire in Veteran women. J Gen Int Med;1999;14(3):186-189.
12. Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ. 2000;320:114-116.
13. Maykut PMR. Beginning Qualitative Research. A Philosophic and Practical Guide. London, England: The Falmer Press; 1994.
14. Cichowski SB, Rogers RG, Clark EA, et al. Military sexual trauma in female veterans is associated with chronic pain conditions. Mil Med. 2017;182(9):e1895-e1899.
15. Glass N, Perrin N, Campbell JC, Soeken K. The protective role of tangible support on post-traumatic stress disorder symptoms in urban women survivors of violence. Res Nurs Health. 2007;30(5):558-568.
16. Schumm JA, Briggs-Phillips M, Hobfoll SE. Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among Inner-city women. J Trauma Stress. 2006;19(6):825-836.
17. Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73.
18. Valdez C, Kimerling R, Hyun JK, et al. Veterans Health Administration mental health treatment settings of patients who report military sexual trauma. J Trauma Dissociation. 2011;12(3):232-243.
19. Maseroli E, Scavello I, Cipriani S, et al. Psychobiological correlates of vaginismus: an exploratory analysis. J Sex Med. 2017;14(11):1392-1402.
20. Imperatori C, Innamorati M, Lamis DA, et al. Childhood trauma in obese and overweight women with food addiction and clinical-level of binge eating. Child Abuse Negl. 2016;58:180-190.
21. Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26(8):1075-1082.
Adverse events a potential concern for chemotherapy-nivolumab in advanced gastric cancer
Adverse events limited the dose intensity or schedule of chemotherapy when added to nivolumab in patients with treatment-naive advanced gastric cancer, according to results from a small, phase 2 trial.
Fully 95% of patients delayed or reduced the dose of chemotherapy because of adverse events. Serious (grade 3 or higher) treatment-related adverse events affected 15% of patients and 13% of patients stopped treatment because of adverse events, said Narikazu Boku, MD, PhD, of National Cancer Center Hospital in Tokyo, together with his associates. The findings were published in Annals of Oncology.
Nivolumab (Opdivo) is not approved for treating gastric cancer in the United States but is approved as third-line or later therapy in several other countries. In the phase 2 ATTRACTION-4 trial, 39 patients with previously untreated, unresectable, advanced, or recurrent gastric or gastroesophageal junction cancer received nivolumab (360 mg intravenously every 3 weeks), plus either S-1 (40 mg/m2 orally twice daily for 14 days followed by 7 days off) or capecitabine (1000 mg/m2 orally twice daily for 14 days followed by 7 days off), plus oxaliplatin (130 mg/m2 intravenously on day 1 every 3 weeks).
For the regimen containing S-1, the most common serious adverse events requiring delays or reductions in chemotherapy were thrombocytopenia (57%), neutropenia (48%), and nausea (19%), followed by diarrhea, vomiting, abdominal pain, peripheral sensory neuropathy, and fatigue (14% each). For the regimen containing capecitabine, the most common of these adverse events were neutropenia (44%), decreased appetite (28%), and palmar-plantar erythrodysesthesia syndrome (22%), followed by nausea, vomiting, diarrhea, and peripheral sensory neuropathy (17% each). There were no treatment-related deaths.
Efficacy endpoints were secondary and limited by small sample size. Nonetheless, regardless of which of the two chemotherapy regimens patients received, about two-thirds had a complete or partial treatment response, which is “numerically higher” than that reported for either chemotherapy regimen alone, the researchers wrote. After a median follow-up of 13.2 months, median overall survival was not reached, while median progression-free survival was 9.7 months for the S-1-based regimen and 10.6 months for the capecitabine-based regimen. Antitumor response appeared to be unrelated to programmed death–ligand 1 status.
Based on the findings, both nivolumab-chemotherapy regimens have “manageable safety profile and clinically relevant antitumor profile,” Dr. Boku and his coinvestigators stated. The second part of ATTRACTION-4 has recruited a larger group of patients and should shed more light on efficacy.
Ono Pharmaceutical and Bristol-Myers Squibb funded the work. Dr. Boku reported financial ties to both companies and to AstraZeneca and Chugai Pharmaceutical.
SOURCE: Boku N et al. Ann Oncol. 2018 Dec 19. doi: 10.1093/annonc/mdy540.
Although ATTRACTION-4 investigators called the safety profile of nivolumab plus chemotherapy in gastric cancer “manageable,” 95% of patients required chemotherapy dose delays or reductions because of treatment-emergent adverse events, noted Elizabeth Cartwright, MBBS, and Ian Chau, MD, in an editorial accompanying the study.
“Given the small safety population in the study, comparisons between arms cannot be made; nonetheless, the overall high rate of treatment-related adverse events across arms could impact patient care and standard chemotherapy dose intensity,” they wrote.
The small cohort sizes also limit conclusions regarding efficacy, they added. Although the data seem encouraging, the efficacy population of 38 patients “is more reflective of a safety run-in rather than a true randomized, phase 2 design, and the results are too preliminary to draw comparisons or conclusions against first-line standard-of-care chemotherapy.”
Finally, the study lacked quality-of-life data and was conducted exclusively in Japan and Korea. Gastric cancers from Asian and non-Asian patients show differences in the expression of genes related to immune function, which could affect treatment response, the experts wrote. Hence, they await results not only from the larger second part of ATTRACTION-4, but also from CheckMate 649, which “will provide a large, randomized, global parallel.”
Both editorialists are with Royal Marsden Hospital in London. Dr. Cartwright reported having no conflicts of interest. Dr. Chau reported ties to Bristol-Myers Squibb, which markets nivolumab in the United States, and to several other pharmaceutical companies. These comments are from their editorial (Ann Oncol. 2018 Dec 28. doi: 10.1093/annonc/mdy555).
Although ATTRACTION-4 investigators called the safety profile of nivolumab plus chemotherapy in gastric cancer “manageable,” 95% of patients required chemotherapy dose delays or reductions because of treatment-emergent adverse events, noted Elizabeth Cartwright, MBBS, and Ian Chau, MD, in an editorial accompanying the study.
“Given the small safety population in the study, comparisons between arms cannot be made; nonetheless, the overall high rate of treatment-related adverse events across arms could impact patient care and standard chemotherapy dose intensity,” they wrote.
The small cohort sizes also limit conclusions regarding efficacy, they added. Although the data seem encouraging, the efficacy population of 38 patients “is more reflective of a safety run-in rather than a true randomized, phase 2 design, and the results are too preliminary to draw comparisons or conclusions against first-line standard-of-care chemotherapy.”
Finally, the study lacked quality-of-life data and was conducted exclusively in Japan and Korea. Gastric cancers from Asian and non-Asian patients show differences in the expression of genes related to immune function, which could affect treatment response, the experts wrote. Hence, they await results not only from the larger second part of ATTRACTION-4, but also from CheckMate 649, which “will provide a large, randomized, global parallel.”
Both editorialists are with Royal Marsden Hospital in London. Dr. Cartwright reported having no conflicts of interest. Dr. Chau reported ties to Bristol-Myers Squibb, which markets nivolumab in the United States, and to several other pharmaceutical companies. These comments are from their editorial (Ann Oncol. 2018 Dec 28. doi: 10.1093/annonc/mdy555).
Although ATTRACTION-4 investigators called the safety profile of nivolumab plus chemotherapy in gastric cancer “manageable,” 95% of patients required chemotherapy dose delays or reductions because of treatment-emergent adverse events, noted Elizabeth Cartwright, MBBS, and Ian Chau, MD, in an editorial accompanying the study.
“Given the small safety population in the study, comparisons between arms cannot be made; nonetheless, the overall high rate of treatment-related adverse events across arms could impact patient care and standard chemotherapy dose intensity,” they wrote.
The small cohort sizes also limit conclusions regarding efficacy, they added. Although the data seem encouraging, the efficacy population of 38 patients “is more reflective of a safety run-in rather than a true randomized, phase 2 design, and the results are too preliminary to draw comparisons or conclusions against first-line standard-of-care chemotherapy.”
Finally, the study lacked quality-of-life data and was conducted exclusively in Japan and Korea. Gastric cancers from Asian and non-Asian patients show differences in the expression of genes related to immune function, which could affect treatment response, the experts wrote. Hence, they await results not only from the larger second part of ATTRACTION-4, but also from CheckMate 649, which “will provide a large, randomized, global parallel.”
Both editorialists are with Royal Marsden Hospital in London. Dr. Cartwright reported having no conflicts of interest. Dr. Chau reported ties to Bristol-Myers Squibb, which markets nivolumab in the United States, and to several other pharmaceutical companies. These comments are from their editorial (Ann Oncol. 2018 Dec 28. doi: 10.1093/annonc/mdy555).
Adverse events limited the dose intensity or schedule of chemotherapy when added to nivolumab in patients with treatment-naive advanced gastric cancer, according to results from a small, phase 2 trial.
Fully 95% of patients delayed or reduced the dose of chemotherapy because of adverse events. Serious (grade 3 or higher) treatment-related adverse events affected 15% of patients and 13% of patients stopped treatment because of adverse events, said Narikazu Boku, MD, PhD, of National Cancer Center Hospital in Tokyo, together with his associates. The findings were published in Annals of Oncology.
Nivolumab (Opdivo) is not approved for treating gastric cancer in the United States but is approved as third-line or later therapy in several other countries. In the phase 2 ATTRACTION-4 trial, 39 patients with previously untreated, unresectable, advanced, or recurrent gastric or gastroesophageal junction cancer received nivolumab (360 mg intravenously every 3 weeks), plus either S-1 (40 mg/m2 orally twice daily for 14 days followed by 7 days off) or capecitabine (1000 mg/m2 orally twice daily for 14 days followed by 7 days off), plus oxaliplatin (130 mg/m2 intravenously on day 1 every 3 weeks).
For the regimen containing S-1, the most common serious adverse events requiring delays or reductions in chemotherapy were thrombocytopenia (57%), neutropenia (48%), and nausea (19%), followed by diarrhea, vomiting, abdominal pain, peripheral sensory neuropathy, and fatigue (14% each). For the regimen containing capecitabine, the most common of these adverse events were neutropenia (44%), decreased appetite (28%), and palmar-plantar erythrodysesthesia syndrome (22%), followed by nausea, vomiting, diarrhea, and peripheral sensory neuropathy (17% each). There were no treatment-related deaths.
Efficacy endpoints were secondary and limited by small sample size. Nonetheless, regardless of which of the two chemotherapy regimens patients received, about two-thirds had a complete or partial treatment response, which is “numerically higher” than that reported for either chemotherapy regimen alone, the researchers wrote. After a median follow-up of 13.2 months, median overall survival was not reached, while median progression-free survival was 9.7 months for the S-1-based regimen and 10.6 months for the capecitabine-based regimen. Antitumor response appeared to be unrelated to programmed death–ligand 1 status.
Based on the findings, both nivolumab-chemotherapy regimens have “manageable safety profile and clinically relevant antitumor profile,” Dr. Boku and his coinvestigators stated. The second part of ATTRACTION-4 has recruited a larger group of patients and should shed more light on efficacy.
Ono Pharmaceutical and Bristol-Myers Squibb funded the work. Dr. Boku reported financial ties to both companies and to AstraZeneca and Chugai Pharmaceutical.
SOURCE: Boku N et al. Ann Oncol. 2018 Dec 19. doi: 10.1093/annonc/mdy540.
Adverse events limited the dose intensity or schedule of chemotherapy when added to nivolumab in patients with treatment-naive advanced gastric cancer, according to results from a small, phase 2 trial.
Fully 95% of patients delayed or reduced the dose of chemotherapy because of adverse events. Serious (grade 3 or higher) treatment-related adverse events affected 15% of patients and 13% of patients stopped treatment because of adverse events, said Narikazu Boku, MD, PhD, of National Cancer Center Hospital in Tokyo, together with his associates. The findings were published in Annals of Oncology.
Nivolumab (Opdivo) is not approved for treating gastric cancer in the United States but is approved as third-line or later therapy in several other countries. In the phase 2 ATTRACTION-4 trial, 39 patients with previously untreated, unresectable, advanced, or recurrent gastric or gastroesophageal junction cancer received nivolumab (360 mg intravenously every 3 weeks), plus either S-1 (40 mg/m2 orally twice daily for 14 days followed by 7 days off) or capecitabine (1000 mg/m2 orally twice daily for 14 days followed by 7 days off), plus oxaliplatin (130 mg/m2 intravenously on day 1 every 3 weeks).
For the regimen containing S-1, the most common serious adverse events requiring delays or reductions in chemotherapy were thrombocytopenia (57%), neutropenia (48%), and nausea (19%), followed by diarrhea, vomiting, abdominal pain, peripheral sensory neuropathy, and fatigue (14% each). For the regimen containing capecitabine, the most common of these adverse events were neutropenia (44%), decreased appetite (28%), and palmar-plantar erythrodysesthesia syndrome (22%), followed by nausea, vomiting, diarrhea, and peripheral sensory neuropathy (17% each). There were no treatment-related deaths.
Efficacy endpoints were secondary and limited by small sample size. Nonetheless, regardless of which of the two chemotherapy regimens patients received, about two-thirds had a complete or partial treatment response, which is “numerically higher” than that reported for either chemotherapy regimen alone, the researchers wrote. After a median follow-up of 13.2 months, median overall survival was not reached, while median progression-free survival was 9.7 months for the S-1-based regimen and 10.6 months for the capecitabine-based regimen. Antitumor response appeared to be unrelated to programmed death–ligand 1 status.
Based on the findings, both nivolumab-chemotherapy regimens have “manageable safety profile and clinically relevant antitumor profile,” Dr. Boku and his coinvestigators stated. The second part of ATTRACTION-4 has recruited a larger group of patients and should shed more light on efficacy.
Ono Pharmaceutical and Bristol-Myers Squibb funded the work. Dr. Boku reported financial ties to both companies and to AstraZeneca and Chugai Pharmaceutical.
SOURCE: Boku N et al. Ann Oncol. 2018 Dec 19. doi: 10.1093/annonc/mdy540.
FROM ANNALS OF ONCOLOGY
Key clinical point: Adverse events limited the dose intensity of chemotherapy when added to nivolumab in patients with treatment-naive advanced gastric cancer.
Major finding: Almost all (95%) patients required dose delays or reductions because of adverse events. Serious adverse events affected 15% of patients and in most cases led to treatment discontinuation.
Study details: A phase 2 trial of nivolumab, plus either S-1 or capecitabine, plus oxaliplatin in 39 patients with previously untreated, unresectable, advanced, or recurrent gastric or gastroesophageal junction cancer.
Disclosures: Ono Pharmaceutical and Bristol-Myers Squibb funded the work. Dr. Boku reported financial ties to both companies and to AstraZeneca and Chugai Pharmaceutical.
Source: Boku N et al. Ann Oncol. 2018 Dec 19. doi: 10.1093/annonc/mdy540.
Onset of pediatric status epilepticus may have a circadian pattern
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. The number of episodes is greatest between 10 a.m. and 11 a.m. and smallest between 10 p.m. and 11 p.m.
“Our findings may inform the increase in preventive monitoring, such as video monitoring or seizure-tracking devices for patients,” said Justice Clark, MPH, a program coordinator at Boston Children’s Hospital. “They may also inform chronotherapeutic strategies.”
Research suggests that various types of seizures cluster at different times of the day. Data about the circadian distribution of status epilepticus, however, are limited.
Ms. Clark and colleagues conducted a prospective observational study at 25 hospitals in the United States and Canada from June 2011 to January 2018. Eligible participants were between ages 1 month and 21 years, had focal or generalized convulsive status epilepticus, and had failed to respond to one benzodiazepine and one nonbenzodiazepine antiseizure medication. For patients with more than one episode of refractory status epilepticus during the study, the researchers included only the first episode.
The investigators examined whether the temporal distribution of pediatric refractory status epilepticus onset followed a circadian pattern using a cosinor analysis with a 12-hour cycle. They used the midline-estimating statistic of rhythm (MESOR) technique to estimate the mean number of refractory status epilepticus episodes per hour if onset was evenly distributed. The amplitude in this analysis was the difference in number of episodes per hour between the MESOR and the peak or the MESOR and the trough.
Ms. Clark and her colleagues included 300 patients in their analysis, each of whom had one episode. Approximately 45% of participants were female. The population’s median age was 4.2 years, and the median duration of status epilepticus was 120 minutes.
The MESOR was 12.5 episodes per hour, and the amplitude was 2.4 episodes per hour, indicating that the distribution was not even over 24 hours. The peak number of onsets was between 10 a.m. and 11 a.m., and the trough was between 10 p.m. and 11 p.m.
A secondary analysis examined the circadian distribution of time to treatment with rescue medications. The distribution of time to treatment with the first benzodiazepine did not differ significantly from a uniform distribution. The time to treatment with the first nonbenzodiazepine antiseizure medication, however, was not uniformly distributed. The longest time to treatment occurred between 3 a.m. and 4 a.m., and the shortest time was between 3 p.m. and 4 p.m. “Although fewer refractory status epilepticus episodes occurred at night, the time to antiseizure medication administration was the longest [during that period]. Thus, nighttime refractory status epilepticus episodes may be at higher risk for delayed treatment,” said Ms. Clark. A limitation of this analysis is that it was influenced by outliers, she added.
The Pediatric Epilepsy Research Foundation and the Epilepsy Research Fund supported the study.
SOURCE: Clark J et al. AES 2018. Abstract 3.426.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. The number of episodes is greatest between 10 a.m. and 11 a.m. and smallest between 10 p.m. and 11 p.m.
“Our findings may inform the increase in preventive monitoring, such as video monitoring or seizure-tracking devices for patients,” said Justice Clark, MPH, a program coordinator at Boston Children’s Hospital. “They may also inform chronotherapeutic strategies.”
Research suggests that various types of seizures cluster at different times of the day. Data about the circadian distribution of status epilepticus, however, are limited.
Ms. Clark and colleagues conducted a prospective observational study at 25 hospitals in the United States and Canada from June 2011 to January 2018. Eligible participants were between ages 1 month and 21 years, had focal or generalized convulsive status epilepticus, and had failed to respond to one benzodiazepine and one nonbenzodiazepine antiseizure medication. For patients with more than one episode of refractory status epilepticus during the study, the researchers included only the first episode.
The investigators examined whether the temporal distribution of pediatric refractory status epilepticus onset followed a circadian pattern using a cosinor analysis with a 12-hour cycle. They used the midline-estimating statistic of rhythm (MESOR) technique to estimate the mean number of refractory status epilepticus episodes per hour if onset was evenly distributed. The amplitude in this analysis was the difference in number of episodes per hour between the MESOR and the peak or the MESOR and the trough.
Ms. Clark and her colleagues included 300 patients in their analysis, each of whom had one episode. Approximately 45% of participants were female. The population’s median age was 4.2 years, and the median duration of status epilepticus was 120 minutes.
The MESOR was 12.5 episodes per hour, and the amplitude was 2.4 episodes per hour, indicating that the distribution was not even over 24 hours. The peak number of onsets was between 10 a.m. and 11 a.m., and the trough was between 10 p.m. and 11 p.m.
A secondary analysis examined the circadian distribution of time to treatment with rescue medications. The distribution of time to treatment with the first benzodiazepine did not differ significantly from a uniform distribution. The time to treatment with the first nonbenzodiazepine antiseizure medication, however, was not uniformly distributed. The longest time to treatment occurred between 3 a.m. and 4 a.m., and the shortest time was between 3 p.m. and 4 p.m. “Although fewer refractory status epilepticus episodes occurred at night, the time to antiseizure medication administration was the longest [during that period]. Thus, nighttime refractory status epilepticus episodes may be at higher risk for delayed treatment,” said Ms. Clark. A limitation of this analysis is that it was influenced by outliers, she added.
The Pediatric Epilepsy Research Foundation and the Epilepsy Research Fund supported the study.
SOURCE: Clark J et al. AES 2018. Abstract 3.426.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. The number of episodes is greatest between 10 a.m. and 11 a.m. and smallest between 10 p.m. and 11 p.m.
“Our findings may inform the increase in preventive monitoring, such as video monitoring or seizure-tracking devices for patients,” said Justice Clark, MPH, a program coordinator at Boston Children’s Hospital. “They may also inform chronotherapeutic strategies.”
Research suggests that various types of seizures cluster at different times of the day. Data about the circadian distribution of status epilepticus, however, are limited.
Ms. Clark and colleagues conducted a prospective observational study at 25 hospitals in the United States and Canada from June 2011 to January 2018. Eligible participants were between ages 1 month and 21 years, had focal or generalized convulsive status epilepticus, and had failed to respond to one benzodiazepine and one nonbenzodiazepine antiseizure medication. For patients with more than one episode of refractory status epilepticus during the study, the researchers included only the first episode.
The investigators examined whether the temporal distribution of pediatric refractory status epilepticus onset followed a circadian pattern using a cosinor analysis with a 12-hour cycle. They used the midline-estimating statistic of rhythm (MESOR) technique to estimate the mean number of refractory status epilepticus episodes per hour if onset was evenly distributed. The amplitude in this analysis was the difference in number of episodes per hour between the MESOR and the peak or the MESOR and the trough.
Ms. Clark and her colleagues included 300 patients in their analysis, each of whom had one episode. Approximately 45% of participants were female. The population’s median age was 4.2 years, and the median duration of status epilepticus was 120 minutes.
The MESOR was 12.5 episodes per hour, and the amplitude was 2.4 episodes per hour, indicating that the distribution was not even over 24 hours. The peak number of onsets was between 10 a.m. and 11 a.m., and the trough was between 10 p.m. and 11 p.m.
A secondary analysis examined the circadian distribution of time to treatment with rescue medications. The distribution of time to treatment with the first benzodiazepine did not differ significantly from a uniform distribution. The time to treatment with the first nonbenzodiazepine antiseizure medication, however, was not uniformly distributed. The longest time to treatment occurred between 3 a.m. and 4 a.m., and the shortest time was between 3 p.m. and 4 p.m. “Although fewer refractory status epilepticus episodes occurred at night, the time to antiseizure medication administration was the longest [during that period]. Thus, nighttime refractory status epilepticus episodes may be at higher risk for delayed treatment,” said Ms. Clark. A limitation of this analysis is that it was influenced by outliers, she added.
The Pediatric Epilepsy Research Foundation and the Epilepsy Research Fund supported the study.
SOURCE: Clark J et al. AES 2018. Abstract 3.426.
REPORTING FROM AES 2018
Key clinical point: The onset of pediatric refractory status epilepticus is not distributed uniformly across the day.
Major finding: Episodes peaked between 10 a.m. and 11 a.m.
Study details: A prospective, observational study conducted at 25 hospitals that included 300 patients.
Disclosures: The Pediatric Epilepsy Research Foundation and the Epilepsy Research Fund funded the study.
Source: Clark J et al. AES 2018. Abstract 3.426.
Mental health patients flocking to emergency departments
Emergency department visits in the United States climbed by 15% overall from 2006 to 2014. Over the same time period, ED visits by people with mental health issues soared by 44%, according to a report from the Agency for Health Care Research & Quality.
“The extent to which ERs are now flooded with patients with mental illness is unprecedented,” David R. Rubinow, MD, chairman of the department of psychiatry at the University of North Carolina, Chapel Hill, said in an interview with CNN.
This overflow is “having a really destructive effect on health care delivery in general,” Dr. Rubinow said. “There are ERs now that are repeatedly on diversion – which means they can’t see any more patients – because there are so many patients with mental illness or behavioral problems [who] are populating the ER.”
Physicians such as Mark D. Pearlmutter, MD, are convinced that EDs have become the medical refuge for many people with mental illness. “We are the safety net,” said Dr. Pearlmutter, an emergency physician affiliated with Steward Health Care in Brighton, Mass. Dr. Pearlmutter said some patients he has seen in the ED often have dual diagnoses, such as “substance abuse and depression, for example.”
As a result of this situation, patients with psychiatric needs might not receive the care that they really need, and care might be delayed for patients with other life-threatening conditions. “The ER is not a great place if you’re a mental health patient; the cardiac patients get put in front of you, and you could end up being there for a really long time, said David Morris, PhD, a psychologist at the O’Donnell Brain Institute in Dallas.
, Dr. Pearlmutter suggested. After all, increasingly, primary care physicians are providing mental health care.
Twists on New Year’s resolutions
Some people bring in each new year by shifting their perspectives – without making resolutions.
Tim Ferriss, an entrepreneur known for blogs and podcasts on work and life, engages in what he calls “past year reviews,” where accomplishments are tallied frequently throughout the year in terms of their positive or negative effect, with the latter being ruled out for the coming year. Over a few years, he hopes, the list of negatives will shrink and the positive items will increase, according to a post on the NBC News website.
Instead of making resolutions, Oprah Winfrey keeps a journal that is updated nightly with five things that spark gratitude. “I live in the present moment. I try to find the good that’s going on in any given situation,” Ms. Winfrey said in a 2017 interview. The practice has taught her to be careful in her personal wishes.
Melinda Gates starts the new year with a single word to provide guidance. Past examples include “gentle,” “spacious,” and, last year, “grace.” Her selections, she said, have helped her sharpen her focus on the really important aspects of her life.
“[Grace] even helped me find a beam of peace through the sadness of a friend’s funeral. When I was upset or distressed, I whispered to myself: ‘Grace.’ That’s the power of a well-chosen word of the year. It makes the year better – and it helps me be better, too, she wrote in a recent LinkedIn post.
20-somethings facing challenges
A recent article in the Guardian lamented a life that is not progressing as expected.
“I am 25 and a half, single, unable to pay my rent, and the closest thing I own to a car is a broken skateboard,” wrote Juliana Piskorz. “I’m in the throes of a quarter-life crisis.”
Ms. Piskorz, who said she suffers from anxiety attacks, said her experience of this crisis manifests itself by making her want to run away, start all over, or distract herself from reality.
She is not alone. According to LinkedIn, about three-quarters of people aged 25-33 share this kind of insecurity and doubt. Low self-esteem is an important culprit, according to James Arkell, MD, a psychiatrist affiliated with the Nightingale Hospital London. “Very often, 20-somethings I see here are beautiful, talented, and have the world on a plate, but they don’t like themselves and that’s got to be about society making them feel as if they have to keep up with these unrelenting standards.”
There are other reasons for millennial despair, Ms. Piskorz speculated.
“Our childhood visions for our lives ... are no longer realistic,” she wrote. “Due to unaffordable housing, less job security, and lower incomes, the traditional ‘markers’ of adulthood, such as owning a home, getting married, and having children, are being pushed back. This has left a vacuum between our teenage years and late 20s with many of us feeling we’re navigating a no man’s land with zero clue when we’ll reach the other side.”
Seeking optimism, Ms. Piskorz noted that, as a community, millennials share many positive characteristics that should serve them well.
“We are not afraid to talk about how we feel, although we should probably talk more,” she wrote. “We stand up for the causes that we think matter; we are not afraid to try new things, and we are not willing to live a life half lived.”
Apps monitor teen angst, depression
The smartphone, often seen as a tool that fuels angst, might be a resource that could identify teenagers in trouble.
According to an article in the Washington Post, research is underway on smartphone apps that can decipher the digital footprints left by users during their Internet ramblings.
“As teens scroll through Instagram or Snapchat, tap out texts, or watch YouTube videos, they also leave digital footprints that might offer clues to their psychological well-being,” wrote article author Lindsey Tanner, of the Associated Press. “Changes in typing speed, voice tone, word choice, and how often kids stay home could signal trouble.”
“We are tracking the equivalent of a heartbeat for the human brain,” said Alex Leow, MD, PhD, an app developer, and associate professor of psychiatry and bioengineering at the University of Illinois at Chicago.
The technology is not ready for deployment because of technical glitches and, more importantly, ethical issues concerning the recording and scrutiny of a user’s personal data being roadblocks. Still, with the permission of the user, mood-detecting apps might one day be a smartphone feature. “[Users] could withdraw permission at any time, said Nick B. Allen, PhD, a psychologist at the University of Oregon, Portland, who has helped create an app that is being tested on young people who have attempted suicide.
He said the biggest hurdle is figuring out “what’s the signal and what’s the noise – what is in this enormous amount of data that people accumulate on their phone that is indicative of a mental health crisis.”
Virtues of “intellectual humility”
Intellectual humility is neither a character flaw nor a sign of being a pushover.
Instead, wrote science reporter Brian Resnick in an article posted on Vox.com, “it’s a method of thinking. It’s about entertaining the possibility that you may be wrong and being open to learning from the experience of others. Intellectual humility is about being actively curious about your blind spots.”
In an effort to promote intellectual humility in psychology, two researchers, Tal Yarkoni, PhD, and Christopher F. Chabris, PhD, launched the Loss-of-Confidence project. The project is a safe space where researchers who doubt a previous finding in psychology can recalibrate. “I do think it’s a cultural issue that people are not willing to admit mistakes,” said Julia M. Rohrer, a PhD candidate and personality psychologist at the Max Planck Institute for Human Development in Berlin who joined the team in 2017. “Our broader goal is to gently nudge the whole scientific system and psychology toward a different culture where it’s okay, normalized, and expected for researchers to admit past mistakes and not get penalized for it.”
Put another way, the aim is to foster a culture where intellectually humble, honest, and curious people can thrive. For that to occur, “we all, even the smartest among us, need to better appreciate our cognitive blind spots,” Mr. Resnick wrote. “Our minds are more imperfect and imprecise than we’d often like to admit.”
In a recent paper, Ms. Rohrer and her associates said the Loss-of-Confidence project grew out of an online discussion in the wake of a post by Dana R. Carney, PhD, and associates on power poses. In that post, Dr. Carney explains why she changed her position on the value of power poses, concluding that the data gathered by her lab at the time leading to the power poses theory (Psychol Sci. 2010 Oct 21 [10]:1363-8) were real but flimsy. “My views have been updated to reflect the evidence,” she wrote. “As such, I do not believe that ‘power pose’ effects are real.”
In the Vox.com article, Mr. Resnick wrote that intellectual humility is needed for two reasons. “One is that our culture promotes and rewards overconfidence and arrogance. At the same time, when we are wrong – out of ignorance or error – and realize it, our culture doesn’t make it easy to admit it. Humbling moments too easily can turn into moments of humiliation.”
Emergency department visits in the United States climbed by 15% overall from 2006 to 2014. Over the same time period, ED visits by people with mental health issues soared by 44%, according to a report from the Agency for Health Care Research & Quality.
“The extent to which ERs are now flooded with patients with mental illness is unprecedented,” David R. Rubinow, MD, chairman of the department of psychiatry at the University of North Carolina, Chapel Hill, said in an interview with CNN.
This overflow is “having a really destructive effect on health care delivery in general,” Dr. Rubinow said. “There are ERs now that are repeatedly on diversion – which means they can’t see any more patients – because there are so many patients with mental illness or behavioral problems [who] are populating the ER.”
Physicians such as Mark D. Pearlmutter, MD, are convinced that EDs have become the medical refuge for many people with mental illness. “We are the safety net,” said Dr. Pearlmutter, an emergency physician affiliated with Steward Health Care in Brighton, Mass. Dr. Pearlmutter said some patients he has seen in the ED often have dual diagnoses, such as “substance abuse and depression, for example.”
As a result of this situation, patients with psychiatric needs might not receive the care that they really need, and care might be delayed for patients with other life-threatening conditions. “The ER is not a great place if you’re a mental health patient; the cardiac patients get put in front of you, and you could end up being there for a really long time, said David Morris, PhD, a psychologist at the O’Donnell Brain Institute in Dallas.
, Dr. Pearlmutter suggested. After all, increasingly, primary care physicians are providing mental health care.
Twists on New Year’s resolutions
Some people bring in each new year by shifting their perspectives – without making resolutions.
Tim Ferriss, an entrepreneur known for blogs and podcasts on work and life, engages in what he calls “past year reviews,” where accomplishments are tallied frequently throughout the year in terms of their positive or negative effect, with the latter being ruled out for the coming year. Over a few years, he hopes, the list of negatives will shrink and the positive items will increase, according to a post on the NBC News website.
Instead of making resolutions, Oprah Winfrey keeps a journal that is updated nightly with five things that spark gratitude. “I live in the present moment. I try to find the good that’s going on in any given situation,” Ms. Winfrey said in a 2017 interview. The practice has taught her to be careful in her personal wishes.
Melinda Gates starts the new year with a single word to provide guidance. Past examples include “gentle,” “spacious,” and, last year, “grace.” Her selections, she said, have helped her sharpen her focus on the really important aspects of her life.
“[Grace] even helped me find a beam of peace through the sadness of a friend’s funeral. When I was upset or distressed, I whispered to myself: ‘Grace.’ That’s the power of a well-chosen word of the year. It makes the year better – and it helps me be better, too, she wrote in a recent LinkedIn post.
20-somethings facing challenges
A recent article in the Guardian lamented a life that is not progressing as expected.
“I am 25 and a half, single, unable to pay my rent, and the closest thing I own to a car is a broken skateboard,” wrote Juliana Piskorz. “I’m in the throes of a quarter-life crisis.”
Ms. Piskorz, who said she suffers from anxiety attacks, said her experience of this crisis manifests itself by making her want to run away, start all over, or distract herself from reality.
She is not alone. According to LinkedIn, about three-quarters of people aged 25-33 share this kind of insecurity and doubt. Low self-esteem is an important culprit, according to James Arkell, MD, a psychiatrist affiliated with the Nightingale Hospital London. “Very often, 20-somethings I see here are beautiful, talented, and have the world on a plate, but they don’t like themselves and that’s got to be about society making them feel as if they have to keep up with these unrelenting standards.”
There are other reasons for millennial despair, Ms. Piskorz speculated.
“Our childhood visions for our lives ... are no longer realistic,” she wrote. “Due to unaffordable housing, less job security, and lower incomes, the traditional ‘markers’ of adulthood, such as owning a home, getting married, and having children, are being pushed back. This has left a vacuum between our teenage years and late 20s with many of us feeling we’re navigating a no man’s land with zero clue when we’ll reach the other side.”
Seeking optimism, Ms. Piskorz noted that, as a community, millennials share many positive characteristics that should serve them well.
“We are not afraid to talk about how we feel, although we should probably talk more,” she wrote. “We stand up for the causes that we think matter; we are not afraid to try new things, and we are not willing to live a life half lived.”
Apps monitor teen angst, depression
The smartphone, often seen as a tool that fuels angst, might be a resource that could identify teenagers in trouble.
According to an article in the Washington Post, research is underway on smartphone apps that can decipher the digital footprints left by users during their Internet ramblings.
“As teens scroll through Instagram or Snapchat, tap out texts, or watch YouTube videos, they also leave digital footprints that might offer clues to their psychological well-being,” wrote article author Lindsey Tanner, of the Associated Press. “Changes in typing speed, voice tone, word choice, and how often kids stay home could signal trouble.”
“We are tracking the equivalent of a heartbeat for the human brain,” said Alex Leow, MD, PhD, an app developer, and associate professor of psychiatry and bioengineering at the University of Illinois at Chicago.
The technology is not ready for deployment because of technical glitches and, more importantly, ethical issues concerning the recording and scrutiny of a user’s personal data being roadblocks. Still, with the permission of the user, mood-detecting apps might one day be a smartphone feature. “[Users] could withdraw permission at any time, said Nick B. Allen, PhD, a psychologist at the University of Oregon, Portland, who has helped create an app that is being tested on young people who have attempted suicide.
He said the biggest hurdle is figuring out “what’s the signal and what’s the noise – what is in this enormous amount of data that people accumulate on their phone that is indicative of a mental health crisis.”
Virtues of “intellectual humility”
Intellectual humility is neither a character flaw nor a sign of being a pushover.
Instead, wrote science reporter Brian Resnick in an article posted on Vox.com, “it’s a method of thinking. It’s about entertaining the possibility that you may be wrong and being open to learning from the experience of others. Intellectual humility is about being actively curious about your blind spots.”
In an effort to promote intellectual humility in psychology, two researchers, Tal Yarkoni, PhD, and Christopher F. Chabris, PhD, launched the Loss-of-Confidence project. The project is a safe space where researchers who doubt a previous finding in psychology can recalibrate. “I do think it’s a cultural issue that people are not willing to admit mistakes,” said Julia M. Rohrer, a PhD candidate and personality psychologist at the Max Planck Institute for Human Development in Berlin who joined the team in 2017. “Our broader goal is to gently nudge the whole scientific system and psychology toward a different culture where it’s okay, normalized, and expected for researchers to admit past mistakes and not get penalized for it.”
Put another way, the aim is to foster a culture where intellectually humble, honest, and curious people can thrive. For that to occur, “we all, even the smartest among us, need to better appreciate our cognitive blind spots,” Mr. Resnick wrote. “Our minds are more imperfect and imprecise than we’d often like to admit.”
In a recent paper, Ms. Rohrer and her associates said the Loss-of-Confidence project grew out of an online discussion in the wake of a post by Dana R. Carney, PhD, and associates on power poses. In that post, Dr. Carney explains why she changed her position on the value of power poses, concluding that the data gathered by her lab at the time leading to the power poses theory (Psychol Sci. 2010 Oct 21 [10]:1363-8) were real but flimsy. “My views have been updated to reflect the evidence,” she wrote. “As such, I do not believe that ‘power pose’ effects are real.”
In the Vox.com article, Mr. Resnick wrote that intellectual humility is needed for two reasons. “One is that our culture promotes and rewards overconfidence and arrogance. At the same time, when we are wrong – out of ignorance or error – and realize it, our culture doesn’t make it easy to admit it. Humbling moments too easily can turn into moments of humiliation.”
Emergency department visits in the United States climbed by 15% overall from 2006 to 2014. Over the same time period, ED visits by people with mental health issues soared by 44%, according to a report from the Agency for Health Care Research & Quality.
“The extent to which ERs are now flooded with patients with mental illness is unprecedented,” David R. Rubinow, MD, chairman of the department of psychiatry at the University of North Carolina, Chapel Hill, said in an interview with CNN.
This overflow is “having a really destructive effect on health care delivery in general,” Dr. Rubinow said. “There are ERs now that are repeatedly on diversion – which means they can’t see any more patients – because there are so many patients with mental illness or behavioral problems [who] are populating the ER.”
Physicians such as Mark D. Pearlmutter, MD, are convinced that EDs have become the medical refuge for many people with mental illness. “We are the safety net,” said Dr. Pearlmutter, an emergency physician affiliated with Steward Health Care in Brighton, Mass. Dr. Pearlmutter said some patients he has seen in the ED often have dual diagnoses, such as “substance abuse and depression, for example.”
As a result of this situation, patients with psychiatric needs might not receive the care that they really need, and care might be delayed for patients with other life-threatening conditions. “The ER is not a great place if you’re a mental health patient; the cardiac patients get put in front of you, and you could end up being there for a really long time, said David Morris, PhD, a psychologist at the O’Donnell Brain Institute in Dallas.
, Dr. Pearlmutter suggested. After all, increasingly, primary care physicians are providing mental health care.
Twists on New Year’s resolutions
Some people bring in each new year by shifting their perspectives – without making resolutions.
Tim Ferriss, an entrepreneur known for blogs and podcasts on work and life, engages in what he calls “past year reviews,” where accomplishments are tallied frequently throughout the year in terms of their positive or negative effect, with the latter being ruled out for the coming year. Over a few years, he hopes, the list of negatives will shrink and the positive items will increase, according to a post on the NBC News website.
Instead of making resolutions, Oprah Winfrey keeps a journal that is updated nightly with five things that spark gratitude. “I live in the present moment. I try to find the good that’s going on in any given situation,” Ms. Winfrey said in a 2017 interview. The practice has taught her to be careful in her personal wishes.
Melinda Gates starts the new year with a single word to provide guidance. Past examples include “gentle,” “spacious,” and, last year, “grace.” Her selections, she said, have helped her sharpen her focus on the really important aspects of her life.
“[Grace] even helped me find a beam of peace through the sadness of a friend’s funeral. When I was upset or distressed, I whispered to myself: ‘Grace.’ That’s the power of a well-chosen word of the year. It makes the year better – and it helps me be better, too, she wrote in a recent LinkedIn post.
20-somethings facing challenges
A recent article in the Guardian lamented a life that is not progressing as expected.
“I am 25 and a half, single, unable to pay my rent, and the closest thing I own to a car is a broken skateboard,” wrote Juliana Piskorz. “I’m in the throes of a quarter-life crisis.”
Ms. Piskorz, who said she suffers from anxiety attacks, said her experience of this crisis manifests itself by making her want to run away, start all over, or distract herself from reality.
She is not alone. According to LinkedIn, about three-quarters of people aged 25-33 share this kind of insecurity and doubt. Low self-esteem is an important culprit, according to James Arkell, MD, a psychiatrist affiliated with the Nightingale Hospital London. “Very often, 20-somethings I see here are beautiful, talented, and have the world on a plate, but they don’t like themselves and that’s got to be about society making them feel as if they have to keep up with these unrelenting standards.”
There are other reasons for millennial despair, Ms. Piskorz speculated.
“Our childhood visions for our lives ... are no longer realistic,” she wrote. “Due to unaffordable housing, less job security, and lower incomes, the traditional ‘markers’ of adulthood, such as owning a home, getting married, and having children, are being pushed back. This has left a vacuum between our teenage years and late 20s with many of us feeling we’re navigating a no man’s land with zero clue when we’ll reach the other side.”
Seeking optimism, Ms. Piskorz noted that, as a community, millennials share many positive characteristics that should serve them well.
“We are not afraid to talk about how we feel, although we should probably talk more,” she wrote. “We stand up for the causes that we think matter; we are not afraid to try new things, and we are not willing to live a life half lived.”
Apps monitor teen angst, depression
The smartphone, often seen as a tool that fuels angst, might be a resource that could identify teenagers in trouble.
According to an article in the Washington Post, research is underway on smartphone apps that can decipher the digital footprints left by users during their Internet ramblings.
“As teens scroll through Instagram or Snapchat, tap out texts, or watch YouTube videos, they also leave digital footprints that might offer clues to their psychological well-being,” wrote article author Lindsey Tanner, of the Associated Press. “Changes in typing speed, voice tone, word choice, and how often kids stay home could signal trouble.”
“We are tracking the equivalent of a heartbeat for the human brain,” said Alex Leow, MD, PhD, an app developer, and associate professor of psychiatry and bioengineering at the University of Illinois at Chicago.
The technology is not ready for deployment because of technical glitches and, more importantly, ethical issues concerning the recording and scrutiny of a user’s personal data being roadblocks. Still, with the permission of the user, mood-detecting apps might one day be a smartphone feature. “[Users] could withdraw permission at any time, said Nick B. Allen, PhD, a psychologist at the University of Oregon, Portland, who has helped create an app that is being tested on young people who have attempted suicide.
He said the biggest hurdle is figuring out “what’s the signal and what’s the noise – what is in this enormous amount of data that people accumulate on their phone that is indicative of a mental health crisis.”
Virtues of “intellectual humility”
Intellectual humility is neither a character flaw nor a sign of being a pushover.
Instead, wrote science reporter Brian Resnick in an article posted on Vox.com, “it’s a method of thinking. It’s about entertaining the possibility that you may be wrong and being open to learning from the experience of others. Intellectual humility is about being actively curious about your blind spots.”
In an effort to promote intellectual humility in psychology, two researchers, Tal Yarkoni, PhD, and Christopher F. Chabris, PhD, launched the Loss-of-Confidence project. The project is a safe space where researchers who doubt a previous finding in psychology can recalibrate. “I do think it’s a cultural issue that people are not willing to admit mistakes,” said Julia M. Rohrer, a PhD candidate and personality psychologist at the Max Planck Institute for Human Development in Berlin who joined the team in 2017. “Our broader goal is to gently nudge the whole scientific system and psychology toward a different culture where it’s okay, normalized, and expected for researchers to admit past mistakes and not get penalized for it.”
Put another way, the aim is to foster a culture where intellectually humble, honest, and curious people can thrive. For that to occur, “we all, even the smartest among us, need to better appreciate our cognitive blind spots,” Mr. Resnick wrote. “Our minds are more imperfect and imprecise than we’d often like to admit.”
In a recent paper, Ms. Rohrer and her associates said the Loss-of-Confidence project grew out of an online discussion in the wake of a post by Dana R. Carney, PhD, and associates on power poses. In that post, Dr. Carney explains why she changed her position on the value of power poses, concluding that the data gathered by her lab at the time leading to the power poses theory (Psychol Sci. 2010 Oct 21 [10]:1363-8) were real but flimsy. “My views have been updated to reflect the evidence,” she wrote. “As such, I do not believe that ‘power pose’ effects are real.”
In the Vox.com article, Mr. Resnick wrote that intellectual humility is needed for two reasons. “One is that our culture promotes and rewards overconfidence and arrogance. At the same time, when we are wrong – out of ignorance or error – and realize it, our culture doesn’t make it easy to admit it. Humbling moments too easily can turn into moments of humiliation.”
Does reduced degradation of insulin by the liver cause type 2 diabetes?
LOS ANGELES –
That’s a hypothesis that Richard N. Bergman, PhD, and his colleagues are testing in his lab at the Sports Spectacular Diabetes and Obesity Wellness and Research Center at Cedars-Sinai Medical Center, Los Angeles.
“More than 50% of insulin secreted into the portal vein is degraded by the liver and never enters the systemic circulation,” Dr. Bergman said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “We have found that if you make an animal insulin resistant with a high fat diet, they degrade less of the insulin. Why is that? They deliver a higher fraction of the insulin into the systemic circulation. One of the answers is that the liver is a gateway for insulin delivery to the systemic circulation.” In fact, when he and his colleagues tested a population of normal dogs, they found wide variability in the ability of the liver to take up and degrade insulin (Diabetes. 2018 67[8]:1495-503).
“It ranged from 20% to 70%; I didn’t believe these data,” said Dr. Bergman, who is also chair in diabetes research at Cedars-Sinai. “We had to redo the study and the same thing was true. There’s a wide variation in what fraction of insulin that enters the liver is degraded. That led to the idea that this could be true in humans.”
To follow up on this concept, he and his colleagues used data from 100 African immigrants without diabetes to develop a model to estimate hepatic versus extrahepatic insulin clearance in humans (Diabetes. 2016;65[6]:1556-64). “This population was chosen because previous studies have suggested that individuals of African descent have reduced hepatic insulin clearance compared with Western subjects,” the authors wrote in the article. “Similarly, FSIGT [frequently sampled intravenous glucose tolerance test] data from two groups showed that African American women had much higher plasma insulin concentrations than European American women during periods of elevated endogenous secretion but not after intravenous insulin infusion, also suggesting reduced hepatic, but not extrahepatic, insulin clearance in African American subjects. Thus, this population was of special interest for applying a model that could quantify both hepatic and extrahepatic insulin clearance.”
The model was able to reproduce accurately the full plasma insulin profiles observed during the FSIGT and identify clear differences in parameter values among individuals. “The ability of the liver to degrade insulin is very variable across a normal human population,” Dr. Bergman said. “That means this may be a controlled variable.”
In a separate analysis of 23 African American and 23 European American women, Dr. Bergman, Francesca Piccinini, PhD, Barbara A. Gower, PhD, and colleagues found that hepatic but not extrahepatic insulin clearance is lower in the African American women, compared with their European American counterparts (Diabetes. 2017;66[10]:2564-70). Data from a cohort of children found the same thing (Diabetes Obes & Metab. 2018 Jul 18. doi: 10.1111/dom.13471).
“What does this mean that different ethnic groups have different clearance of insulin?” he asked. “It means that African Americans deliver a higher fraction of secreted insulin into the systemic circulation. We know that African Americans tend to be hyperinsulinemic. That isn’t necessarily due to oversecretion of insulin; it’s likely due primarily to reduced degradation of insulin. The question then is, can the reduced insulin clearance play a causal role in the pathogenesis of type 2 diabetes?”
He hypothesized that, in normal individuals, half of insulin secreted by the pancreas is exported into the systemic circulation and half is degraded. “We propose that in people at risk for diabetes, insulin is secreted by the pancreas but much less of it is degraded,” Dr. Bergman continued. “Insulin gets into the systemic circulation, so then you can get hyperinsulinemia, and insulin resistance. The resistance stresses the beta cells of the pancreas. Thus, the idea is that differences in clearance of insulin by the liver in some individuals may be pathogenic in the cause of diabetes.”
Dr. Bergman reported that he has done consulting/collaboration with Janssen, January, Novo Nordisk, and Zafgen. He has also received research grants from Astra Zeneca, Janssen, and the National Institutes of Health.
LOS ANGELES –
That’s a hypothesis that Richard N. Bergman, PhD, and his colleagues are testing in his lab at the Sports Spectacular Diabetes and Obesity Wellness and Research Center at Cedars-Sinai Medical Center, Los Angeles.
“More than 50% of insulin secreted into the portal vein is degraded by the liver and never enters the systemic circulation,” Dr. Bergman said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “We have found that if you make an animal insulin resistant with a high fat diet, they degrade less of the insulin. Why is that? They deliver a higher fraction of the insulin into the systemic circulation. One of the answers is that the liver is a gateway for insulin delivery to the systemic circulation.” In fact, when he and his colleagues tested a population of normal dogs, they found wide variability in the ability of the liver to take up and degrade insulin (Diabetes. 2018 67[8]:1495-503).
“It ranged from 20% to 70%; I didn’t believe these data,” said Dr. Bergman, who is also chair in diabetes research at Cedars-Sinai. “We had to redo the study and the same thing was true. There’s a wide variation in what fraction of insulin that enters the liver is degraded. That led to the idea that this could be true in humans.”
To follow up on this concept, he and his colleagues used data from 100 African immigrants without diabetes to develop a model to estimate hepatic versus extrahepatic insulin clearance in humans (Diabetes. 2016;65[6]:1556-64). “This population was chosen because previous studies have suggested that individuals of African descent have reduced hepatic insulin clearance compared with Western subjects,” the authors wrote in the article. “Similarly, FSIGT [frequently sampled intravenous glucose tolerance test] data from two groups showed that African American women had much higher plasma insulin concentrations than European American women during periods of elevated endogenous secretion but not after intravenous insulin infusion, also suggesting reduced hepatic, but not extrahepatic, insulin clearance in African American subjects. Thus, this population was of special interest for applying a model that could quantify both hepatic and extrahepatic insulin clearance.”
The model was able to reproduce accurately the full plasma insulin profiles observed during the FSIGT and identify clear differences in parameter values among individuals. “The ability of the liver to degrade insulin is very variable across a normal human population,” Dr. Bergman said. “That means this may be a controlled variable.”
In a separate analysis of 23 African American and 23 European American women, Dr. Bergman, Francesca Piccinini, PhD, Barbara A. Gower, PhD, and colleagues found that hepatic but not extrahepatic insulin clearance is lower in the African American women, compared with their European American counterparts (Diabetes. 2017;66[10]:2564-70). Data from a cohort of children found the same thing (Diabetes Obes & Metab. 2018 Jul 18. doi: 10.1111/dom.13471).
“What does this mean that different ethnic groups have different clearance of insulin?” he asked. “It means that African Americans deliver a higher fraction of secreted insulin into the systemic circulation. We know that African Americans tend to be hyperinsulinemic. That isn’t necessarily due to oversecretion of insulin; it’s likely due primarily to reduced degradation of insulin. The question then is, can the reduced insulin clearance play a causal role in the pathogenesis of type 2 diabetes?”
He hypothesized that, in normal individuals, half of insulin secreted by the pancreas is exported into the systemic circulation and half is degraded. “We propose that in people at risk for diabetes, insulin is secreted by the pancreas but much less of it is degraded,” Dr. Bergman continued. “Insulin gets into the systemic circulation, so then you can get hyperinsulinemia, and insulin resistance. The resistance stresses the beta cells of the pancreas. Thus, the idea is that differences in clearance of insulin by the liver in some individuals may be pathogenic in the cause of diabetes.”
Dr. Bergman reported that he has done consulting/collaboration with Janssen, January, Novo Nordisk, and Zafgen. He has also received research grants from Astra Zeneca, Janssen, and the National Institutes of Health.
LOS ANGELES –
That’s a hypothesis that Richard N. Bergman, PhD, and his colleagues are testing in his lab at the Sports Spectacular Diabetes and Obesity Wellness and Research Center at Cedars-Sinai Medical Center, Los Angeles.
“More than 50% of insulin secreted into the portal vein is degraded by the liver and never enters the systemic circulation,” Dr. Bergman said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. “We have found that if you make an animal insulin resistant with a high fat diet, they degrade less of the insulin. Why is that? They deliver a higher fraction of the insulin into the systemic circulation. One of the answers is that the liver is a gateway for insulin delivery to the systemic circulation.” In fact, when he and his colleagues tested a population of normal dogs, they found wide variability in the ability of the liver to take up and degrade insulin (Diabetes. 2018 67[8]:1495-503).
“It ranged from 20% to 70%; I didn’t believe these data,” said Dr. Bergman, who is also chair in diabetes research at Cedars-Sinai. “We had to redo the study and the same thing was true. There’s a wide variation in what fraction of insulin that enters the liver is degraded. That led to the idea that this could be true in humans.”
To follow up on this concept, he and his colleagues used data from 100 African immigrants without diabetes to develop a model to estimate hepatic versus extrahepatic insulin clearance in humans (Diabetes. 2016;65[6]:1556-64). “This population was chosen because previous studies have suggested that individuals of African descent have reduced hepatic insulin clearance compared with Western subjects,” the authors wrote in the article. “Similarly, FSIGT [frequently sampled intravenous glucose tolerance test] data from two groups showed that African American women had much higher plasma insulin concentrations than European American women during periods of elevated endogenous secretion but not after intravenous insulin infusion, also suggesting reduced hepatic, but not extrahepatic, insulin clearance in African American subjects. Thus, this population was of special interest for applying a model that could quantify both hepatic and extrahepatic insulin clearance.”
The model was able to reproduce accurately the full plasma insulin profiles observed during the FSIGT and identify clear differences in parameter values among individuals. “The ability of the liver to degrade insulin is very variable across a normal human population,” Dr. Bergman said. “That means this may be a controlled variable.”
In a separate analysis of 23 African American and 23 European American women, Dr. Bergman, Francesca Piccinini, PhD, Barbara A. Gower, PhD, and colleagues found that hepatic but not extrahepatic insulin clearance is lower in the African American women, compared with their European American counterparts (Diabetes. 2017;66[10]:2564-70). Data from a cohort of children found the same thing (Diabetes Obes & Metab. 2018 Jul 18. doi: 10.1111/dom.13471).
“What does this mean that different ethnic groups have different clearance of insulin?” he asked. “It means that African Americans deliver a higher fraction of secreted insulin into the systemic circulation. We know that African Americans tend to be hyperinsulinemic. That isn’t necessarily due to oversecretion of insulin; it’s likely due primarily to reduced degradation of insulin. The question then is, can the reduced insulin clearance play a causal role in the pathogenesis of type 2 diabetes?”
He hypothesized that, in normal individuals, half of insulin secreted by the pancreas is exported into the systemic circulation and half is degraded. “We propose that in people at risk for diabetes, insulin is secreted by the pancreas but much less of it is degraded,” Dr. Bergman continued. “Insulin gets into the systemic circulation, so then you can get hyperinsulinemia, and insulin resistance. The resistance stresses the beta cells of the pancreas. Thus, the idea is that differences in clearance of insulin by the liver in some individuals may be pathogenic in the cause of diabetes.”
Dr. Bergman reported that he has done consulting/collaboration with Janssen, January, Novo Nordisk, and Zafgen. He has also received research grants from Astra Zeneca, Janssen, and the National Institutes of Health.
EXPERT ANALYSIS FROM WCIRDC 2018
BMI changes in adolescence linked to later cancer risk
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
FROM ANNALS OF ONCOLOGY
Key clinical point:
Major finding: The pooled hazard ratio associated with a body mass index increase between age 10 and 18 years was 1.24 (95% CI: 1.11-1.39; P = .0002), compared with 1.06 (95% CI: 0.99-1.14; P = .10) for BMI change after age 18 years.
Study details: A prospective observational study of 133,526 women in the Nurses’ Health Study.
Disclosures: The study was supported by a grant from the National Institute of Health. The authors reported having no conflicts of interest.
Source: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Growing lesion
The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com